Abstract
Optical imaging assays, especially fluorescence molecular assays, are minimally invasive if not completely noninvasive, and thus an ideal technique to be applied to live specimens. These fluorescence imaging assays are a powerful tool in biomedical sciences as they allow the study of a wide range of molecular and physiological events occurring in biological systems. Furthermore, optical imaging assays bridge the gap between the in vitro cell-based analysis of subcellular processes and in vivo study of disease mechanisms in small animal models. In particular, the application of Förster resonance energy transfer (FRET) and fluorescence lifetime imaging (FLIM), well-known techniques widely used in microscopy, to the optical imaging assay toolbox, will have a significant impact in the molecular study of protein-protein interactions during cancer progression. This review article describes the application of FLIM-FRET to the field of optical imaging and addresses their various applications, both current and potential, to anti-cancer drug delivery and cancer research.
Keywords: Cancer therapy, diagnostics, Förster resonance energy transfer (FRET), fluorescence lifetime imaging (FLIM), near infra-red (NIR), in vivo imaging, lifetime
INTRODUCTION
Förster resonance energy transfer (FRET) based imaging technology capitalizes on close proximity (2–10 nm) of two proteins to visualize protein-protein interactions, including receptor dimerization and receptor-ligand complex formation. The transfer of energy between two fluorophore molecules in close proximity and with significant spectral overlap, i.e. the emission wavelength of the donor fluorophore should overlap with the absorption wavelength of the acceptor, where energy from one fluorophore (donor) is transferred to another fluorophore (acceptor), is classified as FRET. Such transfer of energy is radiationless and involves a dipole-dipole interaction. German scientist Theoder Förster first described the theoretical concept of the molecular interactions involved in resonance energy transfer in the 1940s, setting the foundation for FRET microscopy, as we know it today. The most critical requirement for FRET to occur is the distance between the donor and the acceptor, with FRET only taking place if the donor and acceptor fluorophores are between 2–10 nm. FRET efficiency (E) has been shown to be inversely sixth power correlated to the distance between donor-acceptor pair. Thus, FRET provides an expression of distance, which is based on the Förster distance (R0), i.e. the distance at which half the excitation energy of the donor is transferred to the acceptor [1]. FRET has been effectively and extensively used to measure protein-protein interactions, such as, receptor dimerization/oligomerization, at the nanometer scale by labeling various proteins with different donor and acceptor fluorophores. Although E is dependent on various other factors such as spectral overlap between donor-acceptor fluorophore molecules, and the refractive index of the medium, its sheer dependence on the distance between donor-acceptor pair makes it a powerful approach to study intra- and inter-molecular interactions.
METHODOLOGY
There are various techniques by which one can detect if FRET has occurred in the context of microscopy. Based on the measurement of the fluorescence intensities of donor and acceptor molecules, intensity-based FRET is one of the most commonly employed FRET microscopy techniques and it relies on the phenomenon that when the donor is excited, the fluorescence intensity of donor will be reduced (“quenched”) and simultaneously the fluorescence intensity of acceptor will be increased (“sensitized”). Intensity-based FRET detection method requires a simpler setup, such as standard confocal or wide-field fluorescence microscopes, but there are some drawbacks to this method such as donor and acceptor bleedthrough, which requires careful correction measurements [2–4]. Also intensity-based E depends on the excitation intensity and the fluorophore concentration, and can determine whether a specific treatment or condition affects the proximity and the FRET signal between donor and acceptor molecules [5–9]. Another method to detect FRET is lifetime based, where in case of FRET occurrence the fluorescence lifetime of donor will be shortened. Although, both fluorescence lifetime imaging (FLIM) and intensity based FRET measurements are dependent on the acceptor: donor ratio, as shown previously [9–11], FLIM-FRET behaves independently of the donor concentration since fluorescence lifetime is inherent to each fluorophore and its surrounding environment in a concentration independent manner. This review is focused on FLIM for FRET applications, in particular in in vitro cell-based and in vivo cancer research. The imaging techniques and data analysis for FLIM are described in the following sections.
FLUORESCENCE LIFETIME IMAGING (FLIM)
A fluorescent molecule undergoes energy transitions between the ground state (S0) and excited state (S1), storing the absorbed light for a short time before emitting fluorescence. Fluorescence lifetime is the meantime for a fluorescent molecule to stay in S1 before returning to S0. It is an intrinsic characteristic of a fluorophore, and is independent of the fluorophore concentration but it depends on the molecular environment surrounding that fluorophore. Fluorescence lifetime is generally in the range of nanoseconds for most fluorophores. Generally, the fluorescence lifetime is described as the time when fluorescence intensity decreases to 1/e of its initial intensity [12]. FLIM is used to visualize and quantitate the exponential fluorescence decay of a wide variety of fluorophores. Occurrence of FRET leads to the quenching of the donor fluorescence and thus FRET can be determined by measuring the shortening of the fluorescence decay time of the donor in close proximity of an acceptor. Other methods such as time resolved anisotropy can also be a read out for FRET [13]. Moreover, FRET can also be measured by using a time resolved approach to determine the increase in the time of acceptor fluorescence upon donor excitation.
FLIM for FRET applications (FLIM-FRET) offers the opportunity of studying a wide variety of dynamic biological in vitro cell-based and in vivo protein-protein interactions and other proximity-based processes in a direct manner. The methods for FLIM have been broadly classified into two major categories: time-domain (TD) FLIM and frequency-domain (FD) FLIM. TD-FLIM uses a short pulsed light source (femtoseconds to picoseconds) to excite the sample and the subsequent fluorescence emission decay is recorded by high-speed detectors. FD-FLIM uses a modulated light source of a certain frequency to excite the sample and a gain-modulated detector to measure the phase shift and amplitude demodulation ratio of the emission relative to the excitation. Fluorophores with longer lifetimes have larger phase shift and smaller amplitude demodulation ratio. The different techniques for TD-FLIM and FD-FLIM are briefly described below and the comparison for these techniques is summarized in Table 1.
Table 1.
Comparison for TD-FLIM and FD-FLIM.
| Excitation Source | Detection | Advantages | Disadvantages | |
|---|---|---|---|---|
| Time-gated FLIM |
|
ICCD PMT |
|
|
| TCSPC FLIM |
|
PMT SPAD APD |
|
|
| Streak FLIM |
|
Streak camera |
|
|
| FD-FLIM |
|
ICCD, EMCCD, PMT, APD |
|
|
TIME DOMAIN (TD) FLIM
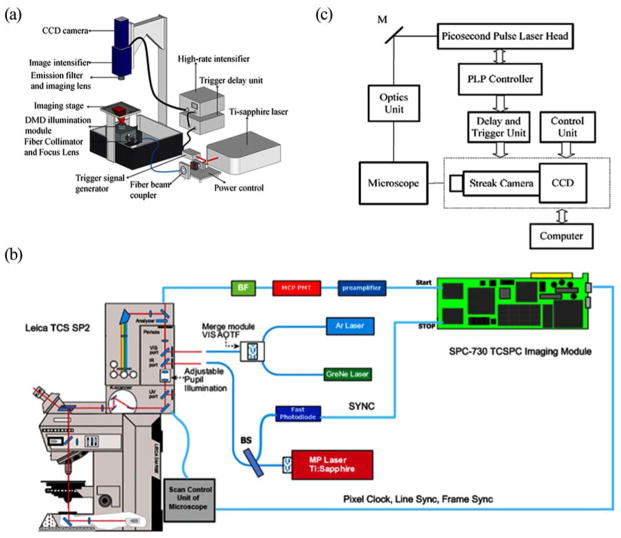
Generally, there are three main techniques for TD-FLIM, which are Time-gated detection, Time-correlated single photon counting (TCSPC) and streak camera [14–16]. Fig. (1) shows the concept for each technology.
Fig. (1).
The basic principle and configuration of TD-FLIM. (a) Schematic of Time-gated FLIM (from [20]. Reprinted permission from OSA). (b) System set-up of TCSPC-FLIM (from [23]. Reprinted with permission from SPIE). (c) Experimental setup of streak FLIM (Reprinted with permission from [24]. Copyright (2012) American Chemical Society).
TD-FLIM using Time-Gated Detection (Time-gated FLIM)
Time-gated FLIM systems consist of pulse laser source, image intensifier, CCD camera and gating-control electronics (See Fig. (1a)). By gating the image intensifier at different intervals with a specified time window along the fluorescence emission decay profile, one can obtain a series of time-gated fluorescence intensity images to estimate the donor fluorescence lifetime. Time-gated FLIM has been widely used in wide-field imaging, because the CCD chip allows simultaneous acquisition of dense spatial information [17]. This results in shorter acquisition times than the techniques based on spatial laser scanning (confocal/multiphoton laser scanning FLIM), and in less photobleaching [17–19], due to the reduced exposure time. However, compared to laser scanning FLIM, wide-field time-gated FLIM lacks optical sectioning ability. To overcome this problem, planar or structured illumination [20] and quasi-wide-field Nipkow (“spinning disc”) [21] are used to provide optical sectioning.
TD-FLIM Using Time-Correlated Single Photon Counting (TCSPC-FLIM)
TCSPC-FLIM systems consist of a pulse laser source, a photon-counting detector and a TCSPC module. In the “reversed start-stop” approach [23, 25], as shown in Fig. (1b), the laser beam from the pulse laser source is split into two beams by the beam splitter (BS). One beam goes into the imaging system to excite the sample. The fluorescence signal is detected by MCP-PMT (Micro Channel Plate Photomultiplier Tube) and amplified by an amplifier. Only one photon is detected to start a ramp of a time-to-amplitude converter (TAC) for recording the arrival time from the detection of a photon to the next laser pulse. The other beam is detected by a fast photodiode as a reference signal to stop the ramp in TCSPC. The output voltage of TAC is converted by an analog-to-digital converter (ADC) into a time channel address. The photon count in the corresponding time channel will be incrementally increased by one event. As a result of the photon count accumulation, the number of photon counts in the time channels represents the time-dependent fluorescence after an adequate number of excitation events. A short pulse laser is needed to generate multiple excitation events and to get reliable emission probabilities, since the fluorescence lifetime (~ns) is shorter than the dead time (~μs), i.e. the delay time during which the detector cannot collect a new arriving photon. The TCSPC-FLIM in a laser scanning system synchronizes both the photon-counting detector and the scanning clock to the excitation laser pulse, recording the arrival time (t) and location (x,y) to build up fluorescence decay curve for each pixel. TCSPC-FLIM can be used for low-light level detection and it can provide high signal-to-noise ratio (SNR) [26] and high temporal resolution (~25–30ps) [27], which is better than time-gated FLIM (~80ps) [14]. Moreover, TCSPC-FLIM has large dynamic range associated with photon counting techniques and linear recording characteristics. Long acquisition time is one major disadvantage of TCSPC-FLIM [28, 29], which may limit its application to fast and dynamic cellular biological processes. The photon count rate of TCSPC is limited by the detection electronics. To avoid the ‘pile-up’ effect that causes an error in the detection of fluorescence lifetime, the photon count rate needs to be smaller than the excitation rate (by a factor of 10–100) [30]. For the repetition rate of 80MHz laser, the maximum count rate is 8MHz. In a TCSPC-FLIM laser scanning system, the photobleaching and photodamage are nonlinearly increased with increasing exposure time of the laser scanning beam. Therefore, the acquisition time is limited by the photon count rate, the photostability and the brightness of the sample, as well as the scanning speed of the system.
TD-FLIM Using Streak Camera (Streak FLIM)
Streak FLIM systems consist of a pulse laser, a pulse picker, a streak camera and a CCD detector [24, 31–33]. The pulse picker is used to obtain the repetition rates for streak-FLIM detection. The streak camera can generate a stack of 2D streak images, in which each line consists of the fluorescence decay profiles for each pixel along the axis x. Each image includes time (t) for one line of the pixels along the axis y. The advantage of streak FLIM is that it has very high temporal resolution (~1ps–20ps) from (Universal streak camera C10910-05, HAMAMATSU, Inc.), fast acquisition times, and the capability of obtaining the complete fluorescence decay profiles. However, this technique is very expensive and difficult to operate and thus, has not been widely used in biological applications.
FREQUENCY DOMAIN (FD) FLIM
FD-FLIM systems consist of a modulated source (Continuous wave (CW) laser or pulse laser, or LED), and a modulated detector. Both source and detector can be modulated at the same frequency (called Homodyne), or at slightly different frequencies (called Heterodyne). The phase lifetime (τφ) can be obtained from the phase shift (Δφ) at each modulation frequency (ω). The modulation lifetime (τm) can be obtained from the amplitude demodulation ratio (m). The expression is given by
Generally, biological samples with different lifetime components need different modulation frequencies. For mono-exponential decay, the τφ and τm are the same at all modulation frequencies. For multi-exponential decay, the τφ is shorter than τm [34]. ω ε [2–10GHz] and ω ε [2–200MHz] are used to measure the fluorescence lifetime in the picosecond range and 1–10ns range, respectively [29].
The FD-FLIM has been used in wide-field imaging and laser scanning imaging. The advantage of FD-FLIM is that it is easy to implement and it is cheaper, since CW lasers can be used for nanosecond range measurements. Furthermore, FD-FLIM does not require the deconvolution of the instrumental response and fluorescence decay curve. TD-FLIM can provide better SNR due to the shorter dead time and to the lack of saturation of the detection electronics. However, FD-FLIM shows reduced sensitivity to low SNR and weak fluorescence signals, affecting its application to low signal cellular biological processes [26].
FLIM-FRET ANALYSIS
As mentioned earlier, when FRET occurs, the donor expresses the fluorescence decay behaviors of quenched and unquenched components. Therefore, the fluorescence decay curve of donor under FRET conditions usually expresses bi-exponential behavior [11]. The general expression for calculating E based on fluorescence lifetime is given by E=1−τ DA/τD, τDA is the lifetime of quenched donor measured from the mixture of donor and acceptor; τD is the natural lifetime of the donor measured from donor-alone. The data analysis for TD-FLIM and FD-FLIM are mathematically equivalent due to the inter-conversion by Fourier transform. In this review, the methods for FLIM-FRET analysis are classified into two major categories: fitting method and non-fitting method. The different approaches for fitting method and non-fitting method are briefly described and the comparison for these methods is summarized in Table 2.
Table 2.
Comparison for fitting method and non-fitting method.
| Name | Advantage | Disadvantage | |
|---|---|---|---|
| Fitting method | Pixel-wise nonlinear |
|
|
| Global analysis |
|
|
|
| Maximum likelihood analysis |
|
|
|
| Bayesian analysis |
|
|
|
| Non-fitting method | Rapid lifetime determination |
|
|
| Minimal fraction of interacting donor |
|
|
|
| Phasor analysis |
|
|
FITTING METHOD
Fitting methods extract the lifetimes of the different fluorescence components and the corresponding amplitudes by fitting the measured fluorescence decay curves to an appropriate model. To fit the measured data correctly, the instrument response function (IRF) of the system is used to convolve with the fitting model. IRF is treated as the decay curve recorded by the FLIM system for an infinitely short fluorescence lifetime. For in vitro cell-based applications, the IRF can be obtained from a mono-compound with known (very short lifetime), scattering medium, second harmonic generation, or a piece of paper. For in vivo applications, depending on the optical properties in the tissue, the IRF can be obtained in two ways. If the tissue is slightly turbid, such as a nude mouse model, the IRF can be obtained using the same method as for the in vitro cell-based application. If the tissue is strongly scattering, such as a rat model, the measured decay curve is modified by the tissue transfer function. Thus, using a fitting model convolved with the IRF-alone is not an accurate analysis approach. To overcome this problem, when fluorescence lifetime is 1.5 times larger than the full width at half maximum (FWHM) of IRF, the fluorescence lifetimes can be obtained by fitting the decay tail without deconvolving IRF [35–37]. However, this approach has not been used for FRET applications. In order to alleviate the effect of optical properties in strongly scattering tissues, IRF can be replaced by decay curve measured at the excitation wavelength which can accurately combine the effect of optical properties with the model. This approach has been used for FRET applications [38]. Based on the different fitting models, fitting methods include pixel-wise nonlinear analysis, global analysis, maximum likelihood analysis, and Bayesian analysis.
Pixel-wise Nonlinear Analysis
Pixel-wise nonlinear analysis [39, 40] is the most common method used in FLIM analysis. It is a gold-standard for other methods to determine their accuracy. Pixel-wise nonlinear analysis uses the bi-exponential fluorescence decay model to fit the measured data and generates four main parameters, which are the lifetime of donor molecules not involved in FRET (non-FRETing donors), and the lifetime of donor molecules involved in FRET (FRETing donors) and the corresponding fractional amplitudes of the lifetimes, respectively. The E can be extracted from these parameters from the equation introduced above. The nonlinear least-squares method is used for this analysis to interactively minimize the reduced goodness-of-fit parameter. This analysis usually is performed pixel-by-pixel, leading to a slow speed in the execution of the algorithm. The E is normally obtained with a large standard deviation, due to the SNR generated from each pixel. Thus, to obtain more accurate results, only photon counts larger than 1000 should be used for bi-exponential fitting. To overcome this limitation, active illumination has been developed to increase SNR and enhance the dynamic range [22, 41].
Global Analysis
Global analysis [42–44] uses all the pixels under the assumption that the lifetimes of FRETing and non-FRETing donors are invariant, and therefore the FRET efficiencies are constant over all pixels, but also allows fractional amplitudes of the lifetimes to vary on each pixel. Due to the assumption, the algorithm is accelerated significantly. A global goodness-of-fit parameter is minimized by the whole dataset simultaneously. Generally, the global analysis is faster than the pixel-wise nonlinear analysis. However, the common global analysis is difficult to estimate more complex decay models under FRET conditions, such as the donor with a multi-exponential decay (more than one lifetime components), i.e., enhanced cyan fluorescent protein (ECFP) is a donor with two lifetime components. To overcome this challenge, a rapid global fitting [45] has been developed. It is able to fit the data to more complex decay models and it is possible to get quantitative information from large datasets with high speed.
MAXIMUM LIKELIHOOD ANALYSIS
Both pixel-wise nonlinear analysis and global analysis are based on nonlinear least-squares method to minimize the reduced goodness-of-fit parameter. Maximum likelihood analysis [46] utilizes the integration of the intensity within several windows of time to determine the lifetime of the fluorophore. An iterative solver is used to estimate the lifetimes using the measurement data. Compared to nonlinear least-squares method, this method is able to provide similar results at the normal signals level with faster speed and more accurate results at low signal level.
Bayesian Analysis
Bayesian analysis [47–49] estimates lifetimes and fractional amplitudes of the measurement data based on a statistical fitting model. The fitting results are determined by the prior distribution and posterior distribution of the model parameters. Bayesian analysis provides high precise estimation at low photon counts or significant background levels within accuracy of 20% at 50 photon counts [49]. However, how valid the estimation is depends on the validity of the prior distribution. Thus, the prior distribution is crucial for Bayesian analysis. Maximum entropy [47, 49] and Gaussian estimation [48] have been used to determine prior distribution.
NON-FITTING METHOD
The non-fitting method allows the recovering of the lifetime values and amplitude fractions directly, including the rapid lifetime determination, minimal fraction of interacting donor and phasor analysis. These methods have been used for in vitro cell-based FRET applications, and they provide great potential for in vivo FRET applications with fast acquisition speed.
Rapid Lifetime Determination (RLD)
RLD is a non-fitting technique to calculate the fluorescence lifetime using the integrated fluorescence intensity during different time-gate windows. It has been widely used for fast-FLIM imaging based on time-gated FLIM [18, 19, 50]. The primary application has been used for fluorophores exhibiting mono-exponential decays by taking only two time gates. It also extends to bi-exponential decay model for FRET analysis by taking four time gates in time-gated FLIM. The fluorescence image at the first time-gate image (D0) was obtained with a time-gate window of ΔT. Similarly, the fluorescence images at other time gates (D1, D2 and D3) were acquired with the same ΔT. The lifetime can be obtained from τ1 = −ΔT / ln( y2); τ2 = −ΔT / ln(x2), where τ1 and τ2 are the short lifetime and long lifetime of the donor, in the presence of acceptor, x and y are determined by D0, D1, D2 and D3. (refer to more details in [50, 51]). However, since the fluorescence decay curve is greatly under sampled, it is impossible to estimate the lifetimes from multi-exponential decay curves. Thus, it makes difficult to evaluate the amplitude fractions of quenched and unquenched donors under FRET conditions [28].
Minimal Fraction of Interacting Donor Approach
Minimal fraction of interacting donor (mfD) [52, 53] is used to get the minimal percentage of donor involved in FRET. The mfD calculation is expressed as [52] mfD = 1−<τ> / <τ>D. <τ> is mean lifetime of the donor, in the presence of the acceptor. <τ>D is the mean lifetime of donor alone. For time-gated FLIM, both <τ> and <τ>D are calculated based on the following equation using several time gates (~5 time-gate images) [52], where Δti is the time delay of the ith measured fluorescence and Ii is the fluorescence intensity of each pixel in the ith image. The advantage of mfD method is that it can directly extract the quantitative information related to the relative concentration of interacting donor with fast acquisition time, without fitting procedure, because it only depends upon experimental parameters, such as time gate selection and measured fluorescence intensity. Moreover, due to the independence of donor behavior (or E), it can be used for both single- and multi-lifetime decay models, which is a difficult task for the general decay curve fitting methods. The disadvantage of mfD method is that it is not suitable for the high donor fraction under FRET conditions.
Phasor Analysis
Phasor analysis [54–56] is a non-fitting method, and can be used for both TD-FLIM and FD-FLIM. The measured fluorescence decay curve is transferred to a new coordinate system (phasor plot) with g and s coordinates. When the measured data is multi-exponential components, the coordinate g and s are given by [54] , where τk is lifetime, fk is intensity-weighted fractions for τk, ω is the angular frequency of laser repetition or light modulation [54]. In the phasor plot of FLIM images, non-FRETing donors and FRETing donors are distributed in specific regions on the semicircle, without considering autofluorescence. On this semicircle, a phasor corresponding to the FRETing donor is close to the point (1,0), while a phasor corresponding to the non-FRETing donor is close to the point (0,0). Thus, it is easy to recognize the population of the molecules undergoing FRET. The large benefit of phasor-plot is that FLIM data analysis provides an intuitive interface for beginners. The phasor approach has the potential to analyze large FLIM datasets, since it does not require exponential analysis for each pixel in the image. In time-gated FLIM, only 4 time gates are needed for calculating two lifetime components by phasor analysis [55]. However, it is not sensitive to low photon counts (less than 100 counts) [49].
NEAR INFRARED FLUOROPHORES
For several decades now, optical imaging for detecting and tracking biomarkers of interest has attracted researchers and clinicians because of its applications in biology and medicine. In recent years, in vivo optical imaging in the near infrared (NIR) wavelengths of 650–900 nm, or “tissue transmission window”, has sparked interest due to the major advantages of NIR fluorophores over their visible range counterparts [57]. In particular, fluorophores with excitation and emission events within the NIR spectrum have less tissue absorbance, as compared to visible light. The major absorbing components in a living, typical tissue including cancerous tissues are water, lipids and hemoglobin [58, 59]. The minimal tissue absorbance of fluorophores that emit in the long wavelength band, allows for deeper penetration of light, making whole animal imaging with deeper organ resolution feasible. Another advantage of NIR probes is reduced autofluorescence present at NIR wavelengths compared to visible ones, allowing for enhanced signal-to-background ratios. The penetration depth of visible fluorophores is limited to 1–2 mm in vivo while it has been predicted that NIR fluorophores can have penetration depth up to several centimeters [60]. Along with these advantages, the tremendous development of monochromatic light sources (lasers), which can deliver high power per wavelength compared to white-light illuminators, and availability of highly sensitive detectors, make NIR macroscopic fluorescence imaging of high value for in vivo pre-clinical cancer research by allowing live small animal imaging without requiring animal opening and dissection.
Theoretically, fluorophores can be any chemical moiety that absorbs light at one wavelength and emits it at a longer wavelength. These include small organic dyes, fluorescent proteins and larger inorganic quantum dots and can be naturally found or synthetically manufactured. The main goal of fluorophore development is to improve SNR, enhance brightness and reduce cellular toxicity and damage. In the next section of this review, we give a brief introduction to these fluorophores, which can be employed in various FLIM-FRET imaging biological applications especially in the NIR spectrum. Detailed information on fluorophores with non-NIR wavelengths can be found elsewhere [61]. An ideal NIR FRET pair will include a strongly bright donor, a donor-acceptor pair with significant spectral overlap, high targeting specificity for enhanced target-to-background ratio and high stability in target environment.
FLUORESCENT PROTEINS
Green fluorescent protein (GFP) derived from jellyfish, Aequorea victoria, and its various homologs from other marine animals are universally used as fluorescent labels, with researchers focusing on the identification and development of novel uorescent proteins with enhanced photophysical properties. Further, the cloning of GFP allowed researchers to engineer chimeric fluorescent proteins and fluorescence labeling of target proteins. The discovery and extraction of GFP from jellyfish in the 1960s revolutionized cell biology and imaging, and led to the Nobel Prize award to Osamu Shimomura, Martin Chalfie, and Roger Tsien in 2008 for their work on GFP applications in biomedical research. GFP and its several multicolor variants, commonly referred as fluorescent proteins (FPs), have been subjected to tremendous developments in the last two decades [62, 63]. Existing FPs cover the entire visible spectrum, providing researchers with numerous combinations for multicolor labeling to study various molecular interactions.
A distinct class of FPs is photoactivatable FPs which can be turned on and off by light of a specific wavelength. This phenomenon of switching off and on or changing colors of activated photoactivatable FPs in different spectrums, introduces unique possibilities of tracking biomolecules or proteins of interest in living systems. Examples of such reversible photoactivatable FPs include none (off-state color) -to-red (on-state color) activatable kindling FPs such as FP595 and its mutant variants, such as KFP1, derived from Anthozoa (Anemonia sulcata). Irreversible photoactivatable FPs, which undergo one-time photoconversion from non-fluorescent to bright fluorescent state, cannot be reverted back to the initial nonfluorescence state. These irreversible photoactivatable FPs have applications in in vivo photolabeling and visualization as they allow for the accumulation of contrasting activated signals. Example of irreversible photoactivatable FPs includes PS-CFP (photoswitchable cyan fluorescent protein) and its enhanced versions which switch from cyan (initial color) to green fluorescent state (switched color) in response to irradiation by a 405-nm UV-violet laser line.
Another set of FPs include genetically encoded sensors allowing researchers to monitor the enzymatic activity, concentrations and conformational changes of various bio-molecules during physiologically significant cellular processes in vitro cell-based and in vivo. One class of such sensors includes intrinsic fluorescent proteins which can sense a physiological change, without modifications other than primary sequence mutagenesis, such as GFP sensors of pH. Yellow fluorescent protein (YFP), a GFP variant, is intrinsically sensitive to both pH and chloride ions. Another class of sensors includes FPs linked to one or more protein domain, where the embedded domain can sense the physiological change and fluoresce variably. FRET based biosensors generally consists of an acceptor and a donor fluorophore along with sensor and ligand domains. A physiological change such as phosphorylation will induce conformational change in sensor domain, allowing the ligand domain to bind to the former, thus bringing acceptor and donor in close proximity for FRET. FRET based biosensors can be intermolecular or intramolecular depending on the biological application. Timer FPs, that can change fluorescence with time, and fast maturing/fast degrading FPs, that are coupled with promoter activity allowing monitoring of activation or inactivation of specific promoters, are other FPs that expand the field of imaging to real time studies in vitro cell-basedas well as in vivo.
The options of visible FPs are endless with various genetically encoded variants available which allow for studies of different biological processes. However, these visible FPs have limited applications in in vivo live animal imaging in which a NIR FP would be more suitable. It was not until the last decade that bright, red-shifted FPs have been developed. Shaner et al. reported a series of red-shifted fluorescent mutants of DrBphP bacteriophytochrome from Discosoma sp., named IFP1.4 with higher quantum yield and photostability compared to mRFP, the first monomer derived from Discosoma sp. These monomeric far-red shifted FPs, termedmCherry, mPlum, mTomato and mRaspberry had emission maxima as high as 649 nm, however, the in vivo imaging of this series of red-shifted proteins was still suboptimal [64].
Shcherbo et al. isolated a bright, red shifted variant called Katushka, derived from the sea anemone Entacmaea quadri-color, with emission maxima of >620 nm [65]. The first infrared FPs, termed IFPs, were derived in 2009 from Deinococcus radiodurans, with excitation and emission maximum of 684 and 708 nm respectively [66]. A bright and stable NIR FP variant of phytchrome RpBphP2, derived from Rhodopseudomonas palustris was recently reported Filonov and collegeoues [67]. This NIR FP, named iRFP has excitation and emission maxima at 690 nm and 713 nm respectively. In comparison to IFPs, iRFP has higher brightness, intracellular stability and photostability, and most importantly, a higher signal-to-background ratio in an in vivo mouse model, broadening the possibilities of noninvasive animal imaging [68]. Several NIR FPs are in research and development all over the world, such as very recently developed irfP670, irfP682, irfP702 and irfP720 which enables multicolor imaging with spectral distinction in live mice [69]. The ideal NIR FRET pair should be spectrally distinct with high brightness in mammalian cells and tissues.
ORGANIC FLUOROPHORES
The most commonly used fluorescent organic dyes are excited by ultraviolet and blue light, and include fluorescein, rhodamine, acridine orange, propidium iodide (PI), 4′,6-diamidino-2-phenylindole (DAPI) and Hoechst to name a few. DAPI and Hoeshst fluorescent probes are popular as nuclear counterstains that fluoresce brightly upon selectively binding to the double stranded DNA. Their selectivity for DNA and high fluorescence make them useful in multicolor immunofluorescence microscopy and high-content screening methods based on quantitation of DNA content. PI also binds to DNA but it binds to double stranded RNA as well and is often utilized for nuclear staining in multiple labeling experiments. Sulfonated derivatives of rhodamine, another fluorescent organic dye, called Alexa Fluors (AF) are gaining increasing popularity due to greater photostability and better water solubility. AF dyes range widely in excitation and emission spectrum allowing for multiple labeling experiments and are frequently applied to FRET experiments.
Most common organic fluorophores functional at NIR wavelengths are: cyanines, porphyrins, squaraines and BODIPYs. Cyanine dye family includes Cy2, Cy3, Cy5 and their derivatives, and exhibits high photostability and enhanced water solubility at visible range. Cy7 (7 methine protons), a cyanine family member with longer polymethine bridge emits in NIR range but has low quantum yield. Indocyanine green (ICG), belonging to the same family, is the only FDA approved long wavelength NIR fluorescent dye for direct human administration in medical diagnostics such as retinal angiography and hepatic functions [70, 71]. ICG has been used to image tumors and shown to accumulate in tumors, due to the enhanced permeability and retention effect (EPR) [72]. Various ICG NIR analogues have been synthesized for better tumor-to-background contrast and solubility. H-ICG is an example of such NIR dye which is sensitive to pH changes at a physiologically relevant range. A hydroxyl-sulfonylbutyl arm from the commercially available ICG dye was removed to produce a non-specific pH indicator that is useful at physiological range [73]. Recently, the same group developed a highly selective NIR-pH activatable probe which specifically targets an integrin overexpressed in endothelial cells during tumor angiogenesis. It has negligible fluorescence at pH over 6 but high fluorescence for pH below 5, making it ideal for imaging acidic tumor lysosomes or other cell organelles [74]. Further development of such NIR pH indicators will enhance the optical imaging of physiological processes in biomedical research. The DyLight Fluor family of fluorescent dyes is synthesized by addition of sulfonate group to rhodamine and cyanine dyes. Compared to parent rhodamine and cyanine dyes, DyLight fluors have reportedly better photostability and brightness with comparable excitation and emission spectra. Atto dyes, another derivative of rhodamines, exhibit better photostability, brightness and slightly improved signal lifetime (0.6–4.1 ns), compared to DyLight family with reduced background. However the lifetime of NIR fluorophores is shorter than their visible counterparts, making it harder to perform FLIM. The development of NIR fluorophores with longer lifetimes has great potential for future in vivo FLIM applications. Optical properties of selected fluorescent dyes which operate in NIR range are mentioned in Table 3.
Table 3.
| Fluorophore | Absorption maxima (nm) | Emission maxima (nm) | Acceptor extinction coefficient (EC) | Relative Quantum Efficiency | Brightness |
|---|---|---|---|---|---|
| AF 700 | 693 | 719 | 192,000 | 0.37 | 0.70 |
| AF 790 | 778 | 808 | 260,000 | 0.63 | 1.60 |
| Atto 700 | 699 | 720 | 120,000 | 0.21 | 0.25 |
| CF 790 | 784 | 811 | 210,000 | 0.58 | 1.20 |
| IRDye 700DX | 689 | 702 | 165,000 | 0.44 | 0.61 |
| IRDye 800CW | 776 | 800 | 240,000 | 0.54 | 1.21 |
| IRDye 820 | 820 | 850 | 202,000 | - | - |
| ICG | 785 | 822 | 204,000 | 0.13 | - |
| DL 680 | 678 | 708 | 140,000 | 0.29 | 0.26 |
| DL 800 | 771 | 798 | 270,000 | 0.34 | 0.80 |
Pyrrolopyrrole cyanine (PPCy) are a new class of cyanine NIR fluorophores with narrow absorption spectrum, long fluorescence lifetimes, low photobleaching and high quantum yield, however, their aqueous soluble derivative have not been reported [75]. Porphyrins and their metal complexes, with absorption in 650–800 nm and emission in 700–800 nm range, are one of the most intensively studied NIR-dyes. Tanaka et al. developed an NIR fluorophore by increasing the number of large porphyrin rings and demonstrated an absorption band shift from 411 nm for basic porphyrin to 953 nm for an octadecaphyrin, placing the fluorophore in NIR window [76]. Taking a different approach, Srinivasan and group introduced bis-metal complexed hexaphyrins with ‘confused’ pyrrolic units which led to absorption band shift from 325 nm to 755 nm [77]. However, most reports using these fluorescent dyes focus on their electrical properties rather than their optical absorbance in living systems.
Certain squaraine water-soluble derivatives have been synthesized with absorption maxima at 787 nm and emission maxima at 812 nm. Umezawa et al. synthesized water soluble NIR squaraine derivatives, named KSQ-4, with emission maxima at 817 nm. KSQ-4 conjugated with bovine serum albumin (BSA) showed a strong absorption peak at 787 nm and emission peak at 812 nm, suggestive of its potential in biological applications [78].
Similar chemical modifications to the BODIPY structure have been demonstrated to tune the emission wavelengths from visible to NIR range. For example, Tasior and O’Shea synthesized water soluble BODIPY based dyes with conjugated side groups such as sulfonate terminated alkyl chains, and demonstrated that they had a emission maxima shift to 717–730 nm, however, these compounds had very low quantum yield [81]. Clearly, several organic fluorophores that span the entire NIR spectrum are now available but for these fluorophores to be employed in vivo, several parameters including photostability, quantum yield, solubility and signal-to-background ratio should be considered. Quantum dots carry the potential to solve many of the above mentioned problems related to in vivo imaging.
QUANTUM DOTS
Quantum dots (QDs), or quantum confined particles are unique nanometer-sized semiconductor crystals or particles that can act as fluorescent labels when covalently linked to biorecognition targets such as peptides, antibodies or nucleic acids. In comparison to traditional organic fluorophore, e.g., organic dyes and fluorescent proteins, QDs have unique optical and electronic properties, such as high quantum yield and photostability. The most wanted advantage being that they have wide absorption spectrum while narrow, symmetric emission spectrum, making them highly desirable in multiplexing where multiple colors and intensities are combined [82]. Most QDs have excitation and emission maxima in visible range, with some reports on synthesis and in vivo testing of NIR-QDs [83, 84].
Gao and colleagues were first to report the use of QD-tagged cancer cells and QDs-antibody conjugates as probes for multicolor imaging of prostate cancer in living animals. They successfully demonstrated uniform and specific binding of prostate specific membrane antigen (PMSA) antigen and PMSA QDs-antibody conjugates in subcutaneous prostate tumors, opening infinite possibilities for multiplex imaging of various targets in vivo. However, the QD probes were functional at 535–630 nm, making optical imaging of core organs such as liver and spleen very limited [85]. The same year Kim et al. synthesized QDs with a wide absorption spectrum of 500–800 nm and a narrow emission spectrum of 850 nm. Upon assessment as real-time surgical aids, these particles accumulate in sentinel lymph nodes of mice and pigs, making them useful in the diagnosis and treatment of various cancers [84].
Since then, several groups are utilizing QDs for fluorescence imaging and immunohistochemistry (IHC) of fixed cells and tissue specimens. Weng et al. synthesized immunoliposome-based nanoparticles (QD-ILs) by inserting anti-human epidermal growth factor-2 (HER2) single-chain variable fragment (scFv) in human breast cancer cells, and demonstrated efficient receptor-mediated endocytosis and anti-cancer activity by drug loaded QD-ILs, further reciting the importance of QDs in cancer diagnosis and treatment [86]. Park et al. synthesized QDs with 745 nm emission and demonstrated uniform biodistribution of these NIR-QDs in mice upon tail vein injection [83]. Very recently, Li and colleagues utilized NIR fluorescence imaging with QDs operating in second NIR window (1000–1350 nm) for monitoring in vivo lymphatic drainage and vascular networks with high spatial and temporal resolution [87], indicating the promising future of NIR-QDs in tracking angiogenesis mediated by tumors and testing anti-angiogenic drugs.
APPLICATIONS OF FLIM-FRET IN CANCER
Currently, optical imaging techniques such as FLIM-FRET are utilized in oncology for several in vitro cell-based, ex vivo and in vivo applications ranging from fundamental basic cancer research to the pre-clinical and clinical evaluation of therapeutic targets. The applications of FRET mainly answer the questions ‘when and where’ protein-protein interaction events take place in a cell in vitro and since recently ex vivo and in vivo. As mentioned earlier, FRET has been used to measure distance between two molecules, detect degree of interaction between proteins, as well as to detect the location and interactions of genes and cellular structures including integrin and membrane proteins. In vivo, visualization of deep-seated tumors by visible-fluorophores is obstructed by high absorbance by skin melanin and hemoglobin. A fluorophore with excitation and emission maxima in a NIR window of ~650–900 nm will have lower absorbance and less light scattering by biological tissue. However, even the most recently characterized far-red fluorescent proteins still have excitation wavelengths on the shorter range of the NIR spectrum. Utilizing NIR fluorophores will allow us to improve signal penetration through biological tissues and hence enhance tumor-to-background signals, essential for non-invasive in vivo optical imaging. Performing FRET in the NIR spectrum for in vivo imaging will provide with a better way to cross in vitro cell-based and in vivo observations, and understand the complex biomolecular microenvironment for successful clinical developments. Properties of select currently available FRET pairs, functional in NIR spectrum are overviewed in Table 4. In the next section, we will discuss different in vitro cell-based, ex vivo and in vivo applications of FLIM-FRET in cancer research, and also highlight recent advances in NIR-FLIM-FRET imaging and draw on the potential to utilize NIR-FLIM-FRET for cancer diagnosis and therapy.
Table 4.
Overview of selected NIR FRET couples of fluorescent protein. Modified from reference [84]. Non-fluorescent* [85].
| FRET pairs | Donor Excitation peak (nm) | Acceptor Emmision peak (nm) | Donor Quantum Yield | Acceptor EC | Forster distance (nm) | |
|---|---|---|---|---|---|---|
| Donor | Acceptor | |||||
| Alexa647 | Alexa680 | 650 | 702 | 0.33 | 184,000 | - |
| Alexa647 | Alexa700 | 650 | 723 | 0.33 | 192,000 | - |
| Alexa647 | Alexa750 | 650 | 780 | 0.33 | 240,000 | - |
| Alexa647 | QSY 21 | 650 | NF* | 0.33 | 90,000 | 6.9 |
| AF700 | AF750 | 723 | 780 | 0.37 | 240,000 | 7.76 |
| Cybate | Cypate | 720 | 760 | ~0.17 | - | 6.13 |
| Cy5 | Cy5.5 | 649 | 694 | >0.28 | 250,000 | >8.0 |
IN VITRO CELL-BASED FLIM-FRET
FLIM-FRET analysis has been used to obtain information about a number of molecular processes including metabolic or signaling pathways and interactions or formations of protein complexes. Nowadays this technique is frequently applied during various multiplex analysis including drug discovery processes and cell signaling events. In an elegant study published over a decade ago, authors used FLIM-FRET as a measure to trace the catalytic activity of fluorescently tagged protein kinase Cα (PKCα) in live and fixed cultured cells. They even applied the approach to image differential activity of PKCα in pathological patient samples as a diagnostic biomarker for breast cancer [90]. Ahmed et al. used FLIM-FRET to compare the direct interactions between GFP-labeled wild type and mutated FGFR2 (fibroblast growth factor receptor 2) and monomeric red fluorescent protein (mRFP)-labeled FRS2 (FGFR substrate 2) in HEK293T cells. An altered cellular signaling mechanism was observed following a single point mutation using FLIM-FRET [91]. Recently, we used FLIM to detect NIR-FRET of transferrin receptor-tfn complex in vitro cell-based. Using Alexa Fluor (AF) dyes, AF700 (donor) and AF750 (acceptor) in T47D human breast cancer cells, we demonstrated an increased uptake of tfn by cancer cells as compared to normal mammary epithelial cells, demonstrating the potential of NIR-FLIM-FRET in quantifying cellular ligand or drug internalization in vitro cell-based [11].
In recent years, genetically encoded FRET biosensors, both intramolecular (unimolecular) and intermolecular (bimolecular), have been used to visualize intracellular signaling in live cells which can help connect to in vivo disease signaling. For example, FLIM-FRET was used to detect protein-protein interactions in living HeLa cells co-expressing either unfused-free enhanced-GFP (donor) and unfused-free mCherry (acceptor), or GFP linked to mCherry via a linker [92]. Caron and group generated a G-protein transglutaminase type 2 (TG2) FRET-biosensor that allowed them to assess the conformational changes of TG2 in live cells, as measured by FLIM. Authors demonstrated in live cells that this FRET biosensor can measure the effects of various biological stresses such as changes in calcium levels, point mutations and specific chemical inhibitors on the TG2. Using FLIM-FRET, this biosensor can provide information regarding conformation and/or localization of TG2 or any other enzyme upon exposure to specific inhibitors or cell stress, and can be amenable to expression in an in vivo mouse model [93]. FLIM-FRET has been widely used to quantitatively image EGFR signal propagation in vitro cell-based. GFP tagged EGFR was visualized to be laterally stimulated with EGF attached to beads using FLIM-FRET [94]. Very recently, FLIM-FRET was utilized to reveal the dynamic conformational transitions of EGFR in living CHO mammalian cells upon binding to the respective ligand. Ziomkiewicz and group covalently attached Atto390 dye, functional in visible range, to EGFR and using FLIM-FRET to demonstrate a reorientation of ectodomain I of EGFR upon binding to the native ligand EGF, in live cells in vitro cell-based [95]. Waterhouse et al. applied FLIM-FRET to assess EGFR and human epidermal growth factor-2 (HER2) dimerization, which is an important predictive biomarker in targeted therapy [96]. Using a pair of FRET fluorescence antibodies from different species, a change in dimerization status of EGFR/HER2 in paraffin embedded cancer cells with various treatments was observed, opening the doors for the assay to be applied to tumor tissues to access any receptor dimerization or complex status in situ.
Nonetheless, as during the development of any therapy, including cancer therapy, one of the deadliest diseases, differences between in vitro cell-based and in vivo environment should be taken into account before extrapolating the in vitro cell-based findings to a clinical setting. Importantly, endogenous promoters should be selected to reflect true in vivo concentrations. Moreover, in vitro cell-based tumor cells lack the complex microenvironment involving extracellular matrix, endothelial cells, immune cells and other soluble secreted factors present in vivo [97]. Cells in such microenvironment divide less frequent, around 40–300 days [98], as opposed to cells in culture that divide every 24–72 hours, which may lead to ever changing signaling pathways. All these factors and constantly changing complex microenvironment may be the reason why many in vitro cell-based observations do not correlate with in vivo observations, suggesting the importance of intravital and potentially non-invasive in vivo imaging for successful therapeutic developments. Recently using FRET, differences between in vitro cell-based and in vivo mechanism of action of the drug docetaxel, a widely used chemotherapeutic drug belonging to taxane family were demonstrated. In a colorectal tumor mouse model, mitotic progression and apoptosis induction was studied using intravital imaging to track photo marked tumor cells during multiple imaging sessions. Using a caspase-3 FRET probe with CFP (donor)-YFP (acceptor) pair, it was demonstrated that mitotic cell death caused docetaxel-induced apoptosis in vitro cell-based while in vivo anti-cancer effects of docetaxel were not exerted through mitotic perturbation [99]. This further illustrates the need and requirement for intravital or non-invasive in vivo imaging techniques to study and validate cancer therapy and diagnostics.
EX VIVO FLIM-FRET
In animals, FLIM-FRET has been used to monitor the interactions between ligand and receptors, receptor dimerization and receptor-mediated signaling pathways. For example, using FLIM-FRET, differences in the binding of PKCα and pro-inflammatory chemokine receptor CXCR4 at the surface and core of xenograft tumor tissues were discovered [100]. Similarly, FRET-based sensors have been used to directly visualize biomolecular interactions within cells of living animals and subsequently for successful development of chemotherapeutics. Keese et al. were one of the first researchers to apply genetically encoded FRET-based sensors in a tumor mouse model. FLIM was used to detect chemotherapy-induced apoptosis in liver and peritoneum metastasis in a small intravital animal model. Murine colorectal cancer cells were engineered to stably express FRET-based caspase-3 activity sensor, which were then injected into mice to induce peritoneal and liver metastasis [101]. This syngeneic mouse tumor model allowed in vitro cell-based, post mortem in vivo and ex vivo analysis of chemotherapy-induced apoptosis by visually monitoring the caspase-3 activity in the tumor. Further, tumor cells could be reisolated from mice and propagated in culture, and apoptosis induction could be evaluated ex vivo in these cells using fluorescence lifetime analysis. Using such functional imaging technique employing stably expressed FRET-based sensors opens up the field of technical platform to study cancer physiology.
Recently, we used FLIM to detect NIR-FRET of transferrin receptor-tfn complex ex vivo [22], as shown in Fig. (2). Mice were injected with AF750-tfn (AF-750 labeled tfn) and/or AF 700-tfn at molar acceptor to donor (A:D) ratios of 0:1 and 2:1 via tail vein and sacrificed after 6-hour or 24-hour post tail vein injections. Based on the expression of transferrin receptor in different organs, five organs, i.e. liver, kidney, spleen, brain, and heart, were harvested from each mouse and subjected to FLIM-FRET ex vivo imaging. These organs are also most commonly used for pharmacokinetics studies. Our biodistribution results of FRET NIR pair suggests that upon injection of fluorophore labeled tfn into nude mice, the labeled tfn can pass freely through various tissues and reach epithelial- and phagocytic cells-rich organs, such as liver, and spleen and kidney, rapidly. During circulation, tfn is potentially taken up by phagocytic cell-rich organs, such as liver and spleen leading to their later clearance by phagocytosis. Although FRET signal 6 hour post injection is detected mainly in liver, spleen and brain tissues with high transferrin receptor expression, the FRET signal after 24 hour post injection remains predominantly in the liver indicating the importance of this organ in the metabolism and catabolism of tail-vein injected NIR-tfn.
Fig. (2).
(a) The imaging layout of five organs (Liver, kidney, spleen, brain and heart) distribution in one field of view (FOV, 35mm × 12mm) (from [22]. Reprinted permission from OSA). (b) Comparison of fluorescence intensity (FI) and FRET Donor fraction (FD %) from 6-hour and 24-hour injection at A:D = 0:1 and A:D = 2:1. (from [22]. Reprinted permission from OSA). (c) Fluorescence intensity based analysis of ex vivo distribution. The photon counts were calculated for five organs (Liver, kidney, spleen, brain and heart) from the fluorescence images.
IN VIVO FLIM-FRET
Characterization of tumor biomarkers is critical in engineering a treatment strategy for any cancer ranging from metastatic breast cancer to benign thyroid tumor. Ardeshir-pour and colleagues utilized the fluorescence lifetime of a NIR-florescent probe to characterize the status of HER2 receptor, an important biomarker linked to poor prognosis of many cancers including breast, uterine and ovarian cancer. A HER2 specific affibody molecule was linked to the NIR fluorescent dye to monitor the binding of optical probe to HER2+ cancer. A change in the fluorescence lifetime of HER2-DyLight750 conjugate NIR probe was observed when bound to the HER2 receptor-positive cancer cells (BT-474, high HER2 expressing human breast cancer cells), relative to unbound probe in a mouse xenograft model cells [102]. The fluorescence lifetime model was non-sensitive to the intensity of excitation light or the fluorophore concentration, giving the model major advantage over those applications utilizing fluorescence intensity measurements. This method may be very useful in the “image and treat” concept, allowing the doctors to tailor the treatment plan to cater a particular patient based on the biomarker expression levels.
Improving target to non-target ratio for in vivo cancer imaging using antibodies is another major challenge. Due to slow blood clearance of antibodies, a prolonged retention and accumulation of fluorescence antibodies in vivo is observed which leads to undesirable low tumor target-to-background ratio. Combining FRET quenching methods with an avidin ‘chase’ to clear the unbound, circulating antibody was demonstrated to decrease the background signals [71]. Injections of NIR fluorophore conjugated trastuzumab, a humanized anti-HER2 antibody, along with FRET quencher (QSY-21) conjugated with avidin in tumor bearing mice reduced background signals. This was due to potential preferential clearing of unbound fluorescence antibody to the liver after the avidin additions as the avidin-antibody complex is rapidly removed from circulation in vivo. In addition to avidin chase, FRET quenching using QSY-21 led to enhanced internalization of target-bound-antibodies, further improving the target signals [71]. Such dual quench-and-chase strategy can be applied to improve target to non-target ratio in in vivo molecular cancer imaging.
Solomon et al. exploited the unique characteristics of sensor-fluorescent probes and applied them to fluorescence lifetime based imaging to detect changes in FRET in vivo. Utilizing an enzyme-sensitive fluorescence probe in murine orthotopic breast cancer model, the researchers demonstrate upregulated enzyme activity and fluorescence intensity at the tumor site due to enhanced enzymatic activity [103]. A good tumor-normal contrast was achieved in around 4–6 hours post-injection of enzyme-sensitive fluorescence probe but it is unclear whether this enhanced fluorescence intensity was due to enhanced tumoral accumulation of fluorescent protolytic probes activated at extra-tumor (non-tumor) site.
McGinty et al. employed FLIM based detection methods to determine FRET between genetically expressed eGFP (donor) and mCherry (acceptor) FP pair in the mouse leg muscles in vivo. Using FLIM-FRET, a clear distinction between the emission of free donor (in presence of acceptor) and donor linked to the acceptor via a tandem eGFP-mCherry FRET construct was demonstrated in hind leg muscles of live animal [104]. Although it provided with an in vivo application to study bio-molecular interactions in their native state, due to the use of visible fluorophore, the exact fluorescence localization was potentially compromised by biological tissue autofluorescence and limited to imaging small volumes due poor signal penetration depth through animal tissues. The absorption and scattering of the fluorescence radiation is also a challenge since this method operates in visible spectrum range. Utilizing engineered FRET probes which operate in red and NIR spectrum may mitigate these challenges.
More recently, FLIM-FRET Src biosensors were used as another example of intravital approach to study complex three-dimensional (3D) tumor microenvironment in its native state, thereby allowing for the development of innovative drug delivery approaches. Using FLIM-FRET Src biosensor to analyze and quantitate drug-targeting efficacy in a pancreatic mouse model, a spatially distinct gradient of Src activity was observed within the live tumor cells, with enhanced activity observed within invading cells compared to cells at the tumor cortex. Upon in vivo treatment with an anti-invasive Src inhibitor, dasatinib, a switch in the Src activity at the invasive borders was observed, which is in accordance with impaired metastatic capacity. Moreover, as seen in Fig. (3), a differential regulation of Src activity in response to the drug was observed between cells closer to the host vasculature relative to cells distal to the vasculature, suggestive of presence of drug penetrance threshold in vivo [105]. The ability to investigate 3D heterogeneous tumor microenvironment using FLIM-FRET biosensors to obtain spatial and temporal map of drug response provides with an effective drug delivery tool to monitor drug response in vivo, which was otherwise intractable by standard techniques.
Fig. (3).
Intravital FLIM-FRET imaging for quantification of drug delivery in vivo with respect to tumor vasculature. A. Representative in vivo fluorescence images of cancer cells with biosensors, host tumor vasculature and host extracellular matrix components (purple) and corresponding lifetime map demonstrating the ability to monitor drug perfusion via tumor blood supply. B. Representative in vivo fluorescence images of cancer cells in presence of drug treatment and corresponding lifetime map demonstrating the proximity between cells and local vasculature. C. In vivo quantitative data demonstrating the ability of FLIM-FRET to successfully determine the response of cancer cells with biosensors to dasatinib drug (Adapted from [101]).
Xiong and colleagues were able to use self-luminescing NIR nanoparticles in vivo to image lymphatic networks and vasculatures in tumor carrying living mice using BRET (Bioluminescence Resonance Energy Transfer)-FRET based detection methods [106]. Although this method operates in NIR window, it can still not discriminate between the active uptake and non-specific accumulation of biomolecules at the site due to EPR effect. Similar limitations due to biologically heterogeneous tissues or organs are observed with other optical techniques, such as fluorescence tomography. A significant depth-dependent sensitivity is observed with dual-tracer MRI-guided fluorescence tomography technique making visualization and quantification of small deep-seated tumors infeasible [107]. Lu et al. utilized far-red fluorescence gene reporters to image deep-seated orthotopic primary human prostate cancer in a mouse model. Human prostate cancer cells were genetically constructed to stably express far-red fluorescence gene reporters IFP1.4 and iRFP, and orthotopically implanted in mice [108]. In vivo gene reporter fluorescence tomography (GRFT) and positron emission tomography (PET) imaging was able to reconstruct the location of the prostate cancer, but the image reconstruction in this study was prepared with assumption of a homogeneous optical distribution and it will be useful to improve reconstruction quality by using precise anatomical information.
In vivo NIR-FLIM-FRET as a ‘Perfect’ Non-invasive Tool
Due to its dependence on the molecular distance, FRET has become an indispensable tool in biology and an important application of FRET is to devise a non-invasive specific imaging approach to detect the internalization of drug candidates into target cells in living animals. Using NIR-FLIM-FRET, a new phase of optical imaging, we may be able to overcome the challenges of in vivo optical imaging since FRET component will allow us to specifically address receptor-ligand internalization while NIR-FLIM-FRET will provide us with opportunity to improve the signal penetration in biologically heterogeneous tissues.
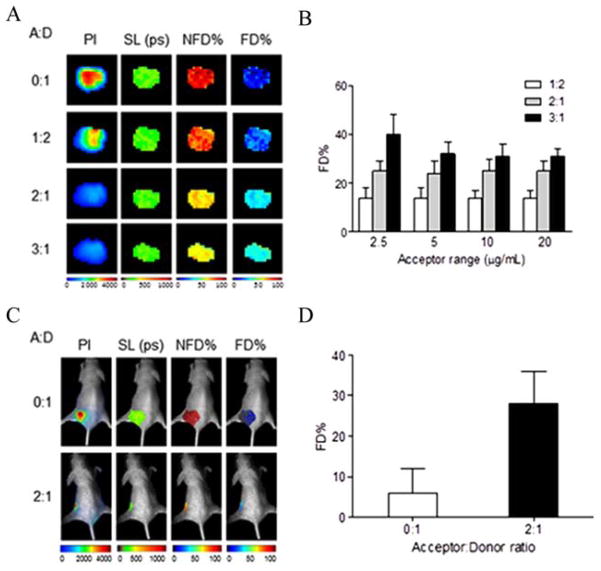
Recently, our group has demonstrated live non-invasive in vivo imaging of NIR-tfn using FLIM-FRET [11]. This study was first of its kind to exploit the dimeric nature of transferrin receptor to evaluate the internalization of tfn using NIR-FLIM-FRET. We used two commercially available AF dyes, AF700 (donor) and AF750 (acceptor) with significant spectral overlap of donor emission and acceptor excitation, and have a R0 of 7.76 nm, making this pair suitable for FRET imaging. Since this NIR FRET pair operates in NIR spectrum range, it overcomes the challenges of non-invasively quantifying visible fluorescence signals through living tissues in animal models due to high degree of autofluorescence and poor signal penetration depth through biologically heterogeneous tissues. In the mentioned study, mice with palpable tumors were injected with tfn conjugated to NIR AF dyes and subjected to FLIM-FRET Fig. (4). A decreased fluorescence lifetime due to FRET was detectable in live mice in vivo one hour post-injection, making this technique unique as it can non-invasively determine non-bound (non-internalized) tfn versus bound (internalized) tfn, providing us with a method to determine whether any drug conjugated with tfn is internalized into the target tissue or organ. Furthermore, quantifying the proportion of AF700-tfn (donor) in FRETing state compared to that in non-FRETing state will allow us to quantitate the efficacy of drug-tfn conjugate uptake [11]. Overall this study demonstrated the sensitivity and accuracy of NIR-FLIM-FRET as a unique non-invasive imaging tool to be used in vivo. Conventional FLIM microscopy systems operate in visible range limiting the imaging capabilities due to autofluorescence, reduced SNR and light scattering and preventing a straightforward in vitro cell-based validation of NIR-FRET-FLIM systems for in vivo applications; thus most in vivo NIR-FLIM studies do not report the in vitro cell-based microscopic imaging validation of in vivo FLIM measurements [109]. Previous studies have used upgraded confocal microscopy systems with NIR laser and detectors or used two-photon excitations at NIR wavelengths [102, 110, 111]. For example, Ardeshirpour and group used a specially adapted Olympus FV1000 inverted laser scanning two-photon microscope for evaluating in vitro cell-based the lifetime of their NIR probe, which was used in in vivo FLIM imaging experiments [102]. However, the microscope depth resolution was significantly reduced compared to confocal microscope. The modification of microscopy systems with the addition of a NIR-FLIM module would enable in the future the establishment and use of quantitative lifetime-based molecular assays at multiple scales, ranging from in vitro cell-based microscopic to in vivo macroscopic approaches.
Fig. (4).
NIR fluorescence lifetime FRET in vitro cell-based and in vivo [10]. (A) Cancer cells were internalized with AF700-tfn (donor) or AF750-tfn (acceptor) in increasing A:D ratios of 0:1, 1:2, 2:1, and 3:1. Pixel intensity (PI) of donor fluorophores decreases in intensity as the ratio of acceptor molecules (AF750-tfn) increase due to quenching. Short component lifetimes (SL) measured in picoseconds (ps), indicates a high sensitivity and uniform detection. Non-FRETing Donor% (NFD%) and FRETing Donor% (FD%) columns indicate the relative abundance of NFD populations and FD populations respectively. (B) The FD% in relation to acceptor levels show an independent relationship as seen in FRET results using visible FRET detected by confocal microscopy in cancer cells. (C) Decreased fluorescence lifetime is detected due to FRET in a mouse tumor model using A:D ratios of 0:1 (upper row) or 2:1 (lower row) 1 h post-injection. (D) FD% increases in the presence of acceptor NIR-tfn, indicative of FRET within a live mouse model.
CONCLUSION AND OUTLOOK
In conclusion, although the in vivo FLIM-FRET technique is still in its infancy it has already captivated researchers due to its unique live imaging ability. Frequent measurements or time kinetic measurements are possible to perform using FLIM-FRET in a non-invasive fashion. FLIM-FRET imaging is a powerful, quantitative and non-invasive tool in not only imaging and screening the presence of human xenograft tumors in vivo, but also allowing for the optimization of targeted delivery systems based on receptor-ligand mediated uptake into cancer cells and xenograft tumors. The ultimate goal is to apply NIR-FLIM-FRET imaging in situ in cancer patients by the virtue of using ligand-therapeutic agent complex as Trojan’s horse, where their delivery at the tumor site can lead to the specific delivery of therapeutic compounds. Development of such targeted therapy using receptor-ligand complex in tumors will allow for delivery of therapeutic molecules at the tumor site increasing the responsiveness of the patients, thus saving valuable time and reducing the mortality.
Acknowledgments
This work was partly funded by the National Institutes of Health grant R21 CA161782-01 and National Science Foundation CAREER AWARD CBET-1149407.
Footnotes
CONFLICT OF INTEREST
The authors confirm that this article content has no conflict of interest.
References
- 1.Sekar RB, Periasamy A. Fluorescence resonance energy transfer (FRET) microscopy imaging of live cell protein localizations. J Cell Biol. 2003;160:629–33. doi: 10.1083/jcb.200210140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elangovan M, Wallrabe H, Chen Y, Day R. Characterization of one-and two-photon excitation fluorescence resonance energy transfer microscopy. Methods. 2003;29:58–73. doi: 10.1016/s1046-2023(02)00283-9. [DOI] [PubMed] [Google Scholar]
- 3.Wallrabe H, Elangovan M, Burchard A, Periasamy A, Barroso M. Confocal FRET microscopy to measure clustering of ligand-receptor complexes in endocytic membranes. Biophys J. 2003;85:559–71. doi: 10.1016/S0006-3495(03)74500-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wallrabe H, Stanley M, Periasamy A, Barroso M. One- and two-photon fluorescence resonance energy transfer microscopy to establish a clustered distribution of receptor-ligand complexes in endocytic membranes. J Biomed Opt. 2003;8:339–46. doi: 10.1117/1.1584444. [DOI] [PubMed] [Google Scholar]
- 5.Wallrabe H, Chen Y, Periasamy A, Barroso M. Issues in confocal microscopy for quantitative FRET analysis. Microsc Res Tech. 2006;69:196–206. doi: 10.1002/jemt.20281. [DOI] [PubMed] [Google Scholar]
- 6.Wallrabe H, Bonamy G, Periasamy A, Barroso M. Receptor Complexes Cotransported via Polarized Endocytic Pathways Form Clusters with Distinct Organizations. Mol Biol Cell. 2007;18(6):2226–43. doi: 10.1091/mbc.E06-08-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barroso MM. Quantum dots in cell biology. J Histochem Cytochem. 2011;59:237–51. doi: 10.1369/0022155411398487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Periasamy A, Wallrabe H, Chen Y, Barroso M. Quantitation of Protein - Protein Interactions: Confocal FRET Microscopy. Methods Cell Biol. 2008;89:569–98. doi: 10.1016/S0091-679X(08)00622-5. [DOI] [PubMed] [Google Scholar]
- 9.Talati R, Vanderpoel A, Eladdadi A, Anderson K, Abe K, Barroso M. Automated selection of regions of interest for intensity-based FRET analysis of transferrin endocytic trafficking in normal vs. cancer cells. Methods. 2014;66:139–52. doi: 10.1016/j.ymeth.2013.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Renz M, Daniels BR, Vámosi G, Arias IM, Lippincott-Schwartz J. Plasticity of the asialoglycoprotein receptor deciphered by ensemble FRET imaging and single-molecule counting PALM imaging. Proc Natl Acad Sci USA. 2012;109:E2989–97. doi: 10.1073/pnas.1211753109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abe K, Zhao L, Periasamy A, Intes X, Barroso M. Non-invasive in vivo imaging of near infrared-labeled transferrin in breast cancer cells and tumors using fluorescence lifetime FRET. PLoS One. 2013;8:e80269. doi: 10.1371/journal.pone.0080269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wallrabe H, Periasamy A. Imaging protein molecules using FRET and FLIM microscopy. Curr Opin Biotechnol. 2005;16:19–27. doi: 10.1016/j.copbio.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 13.Sisamakis E, Valeri A, Kalinin S, Rothwell PJ, Seidel CA. Accurate single-molecule FRET studies using multiparameter fluorescence detection. Methods Enzymol. 2010;475:455–514. doi: 10.1016/S0076-6879(10)75018-7. [DOI] [PubMed] [Google Scholar]
- 14.Suhling K, French PMW, Phillips D. Time-resolved fluorescence microscopy. Photochem Photobiol Sci. 2005;4:13–22. doi: 10.1039/b412924p. [DOI] [PubMed] [Google Scholar]
- 15.Becker W. Fluorescence lifetime imaging--techniques and applications. J Microsc. 2012;247:119–36. doi: 10.1111/j.1365-2818.2012.03618.x. [DOI] [PubMed] [Google Scholar]
- 16.Sun Y, Rombola C, Jyothikumar V, Periasamy A. Forster Resonance Resonance Energy Transfer F o Microscopy and Spectroscopy for Localizing Protein - Protein Interactions in Living Cells. Cytom Part A. 2013;83:780–93. doi: 10.1002/cyto.a.22321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elson D, Requejo-isidro J, Munro I, et al. Time-domain fluorescence lifetime imaging applied to biological tissue. Photochem Photobiol Sci. 2004;3:795–801. doi: 10.1039/b316456j. [DOI] [PubMed] [Google Scholar]
- 18.Kumar S, Alibhai D, Margineanu A, et al. FLIM FRET technology for drug discovery: automated multiwell-plate high-content analysis, multiplexed readouts and application in situ. Chemphyschem. 2011;12:609–26. doi: 10.1002/cphc.201000874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alibhai D, Kelly DJ, Warren S, et al. Automated fluorescence lifetime imaging plate reader and its application to Förster resonant energy transfer readout of Gag protein aggregation. J Biophotonics. 2013;6:398–408. doi: 10.1002/jbio.201200185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mertz J. Optical sectioning microscopy with planar or structured illumination. Nat Methods. 2011;8:811–9. doi: 10.1038/nmeth.1709. [DOI] [PubMed] [Google Scholar]
- 21.Talbot CB, McGinty J, Grant DM, et al. High speed unsupervised fluorescence lifetime imaging confocal multiwell plate reader for high content analysis. J Biophotonics. 2008;1:514–21. doi: 10.1002/jbio.200810054. [DOI] [PubMed] [Google Scholar]
- 22.Zhao L, Abe K, Rajoria S, Pian Q, Barroso M, Intes X. Spatial light modulator based active wide-field illumination for ex vivo and in vivo quantitative NIR FRET imaging. Biomed Opt Express. 2014;5:944. doi: 10.1364/BOE.5.000944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhao L, Chen D, Qi J, Qu J. Multispectral autofluorescence lifetime imaging of RPE cells using two-photon excitation. Proc SPIE. 2010;7569:75692B–5. [Google Scholar]
- 24.Pliss A, Zhao L, Ohulchanskyy TY, Qu J, Prasad PN. Fluorescence lifetime of fluorescent proteins as an intracellular environment probe sensing the cell cycle progression. ACS Chem Biol. 2012;7:1385–92. doi: 10.1021/cb300065w. [DOI] [PubMed] [Google Scholar]
- 25.Becker W, Bergmann a, Hink Ma, König K, Benndorf K, Biskup C. Fluorescence lifetime imaging by time-correlated single-photon counting. Microsc Res Tech. 2004;63:58–66. doi: 10.1002/jemt.10421. [DOI] [PubMed] [Google Scholar]
- 26.Gratton E, Breusegem S, Sutin J, Ruan Q, Barry N. Fluorescence lifetime imaging for the two-photon microscope: time-domain and frequency-domain methods. J Biomed Opt. 2003;8:381–90. doi: 10.1117/1.1586704. [DOI] [PubMed] [Google Scholar]
- 27.Shrestha S, Applegate BE, Park J, Xiao X, Pande P, Jo Ja. High-speed multispectral fluorescence lifetime imaging implementation for in vivo applications. Opt Lett. 2010;35:2558–60. doi: 10.1364/OL.35.002558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebrecht R, Don Paul C, Wouters FS. Fluorescence lifetime imaging microscopy in the medical sciences. Protoplasma. 2014;251:293–305. doi: 10.1007/s00709-013-0598-4. [DOI] [PubMed] [Google Scholar]
- 29.Borst JW, Visser AJWG. Fluorescence lifetime imaging microscopy in life sciences. Meas Sci Technol. 2010;21:102002. [Google Scholar]
- 30.Katsoulidou V, Bergmann A, Becker W. How fast can TCSPC FLIM be made? Proc SPIE. 2007;6771:67710B–7. [Google Scholar]
- 31.Krishnan RV, Masuda a, Centonze VE, Herman B. Quantitative imaging of protein-protein interactions by multiphoton fluorescence lifetime imaging microscopy using a streak camera. J Biomed Opt. 2003;8:362–7. doi: 10.1117/1.1577574. [DOI] [PubMed] [Google Scholar]
- 32.Biskup C, Zimmer T, Benndorf K. FRET between cardiac Na+ channel subunits measured with a confocal microscope and a streak camera. Nat Biotechnol. 2004;22:220–4. doi: 10.1038/nbt935. [DOI] [PubMed] [Google Scholar]
- 33.Biskup C, Zimmer T, Kelbauskas L, et al. Multi-Dimensional Fluorescence Lifetime and FRET Measurements. Microsc Res Tech. 2007;70:442–51. doi: 10.1002/jemt.20431. [DOI] [PubMed] [Google Scholar]
- 34.Colyer RA, Lee C, Gratton E. A Novel Fluorescence Lifetime Imaging System That Optimizes Photon Efficiency. Microsc Res Tech. 2008;71:201–13. doi: 10.1002/jemt.20540. [DOI] [PubMed] [Google Scholar]
- 35.Ma G, Mincu N, Lesage F, Gallant P, McIntosh L. System IRF impact on fluorescence lifetime fitting in turbid medium. Proc SPIE. 2005;5699:263–73. [Google Scholar]
- 36.Rice WL, Kumar ATN. Preclinical whole body time domain fluorescence lifetime multiplexing of fluorescent proteins. J Biomed Opt. 2014;19:046005. doi: 10.1117/1.JBO.19.4.046005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kumar ATN, Chung E, Raymond SB, et al. Feasibility of in vivo imaging of fluorescent proteins using lifetime contrast. Opt Lett. 2009;34:2066–8. doi: 10.1364/ol.34.002066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Venugopal V, Chen J, Barroso M, Intes X. Quantitative tomographic imaging of intermolecular FRET in small animals. Biomed Opt Express. 2012;3:3161–75. doi: 10.1364/BOE.3.003161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Boens N, Qin W, Basaric N, et al. Fluorescence Lifetime Standards for Time and Frequency Domain Fluorescence Spectroscopy. Anal Chem. 2007;79:2137–49. doi: 10.1021/ac062160k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones PB, Herl L, Berezovska O, Kumar ATN, Bacskai BJ, Hyman BT. Time-domain fluorescent plate reader for cell based protein-protein interaction and protein conformation assays. J Biomed Opt. 2006;11:054024. doi: 10.1117/1.2363367. [DOI] [PubMed] [Google Scholar]
- 41.Zhao L, Abe K, Barroso M, Intes X. Active wide-field illumination for high-throughput fluorescence lifetime imaging. Opt Lett. 2013;38:3976–9. doi: 10.1364/OL.38.003976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Verveer PJ, Squire a, Bastiaens PI. Global analysis of fluorescence lifetime imaging microscopy data. Biophys J. 2000;78:2127–37. doi: 10.1016/S0006-3495(00)76759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grecco HE, Roda-Navarro P, Verveer PJ. Global analysis of time correlated single photon counting FRET-FLIM data. Opt Express. 2009;17:6493–508. doi: 10.1364/oe.17.006493. [DOI] [PubMed] [Google Scholar]
- 44.Barber P, Ameer-Beg S, Gilbey J, et al. Multiphoton time-domain fluorescence lifetime imaging microscopy: practical application to protein-protein interactions using global analysis. J R Soc Interface. 2009;6:S93–S105. [Google Scholar]
- 45.Warren SC, Margineanu A, Alibhai D, et al. Rapid global fitting of large fluorescence lifetime imaging microscopy datasets. PLoS One. 2013;8:e70687. doi: 10.1371/journal.pone.0070687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Collier BB, McShane MJ. Time-resolved measurements of luminescence. J Lumin. 2013;144:180–90. [Google Scholar]
- 47.Barber PR, Ameer-Beg SM, Pathmananthan S, Rowley M, Coolena CC. A Bayesian method for single molecule, fluorescence burst analysis. Biomed Opt Express. 2010;1:1148–58. doi: 10.1364/BOE.1.001148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lichten Ca, Swain PS. A Bayesian method for inferring quantitative information from FRET data. BMC Biophys. 2011;4:10. doi: 10.1186/2046-1682-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rowley MI, Barber PR, Coolen ACC, Vojnovic B. Bayesian analysis of fluorescence lifetime imaging data. Proc SPIE Vol. 2011;7903:790325–12. [Google Scholar]
- 50.Elangovan M, Day RN, Periasamy a. Nanosecond fluorescence resonance energy transfer-fluorescence lifetime imaging microscopy to localize the protein interactions in a single living cell. J Microsc. 2002;205:3–14. doi: 10.1046/j.0022-2720.2001.00984.x. [DOI] [PubMed] [Google Scholar]
- 51.Sharman KK, Periasamy a, Ashworth H, Demas JN. Error analysis of the rapid lifetime determination method for double-exponential decays and new windowing schemes. Anal Chem. 1999;71:947–52. doi: 10.1021/ac981050d. [DOI] [PubMed] [Google Scholar]
- 52.Padilla-Parra S, Audugé N, Coppey-Moisan M, Tramier M. Quantitative FRET analysis by fast acquisition time domain FLIM at high spatial resolution in living cells. Biophys J. 2008;95:2976–88. doi: 10.1529/biophysj.108.131276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Padilla-Parra S, Auduge N, Coppey-Moisan M, Tramier M. Non fitting based FRET-FLIM analysis approaches applied to quantify protein-protein interactions in live cells. Biophys Rev. 2011;3:63–70. doi: 10.1007/s12551-011-0047-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Digman Ma, Caiolfa VR, Zamai M, Gratton E. The phasor approach to fluorescence lifetime imaging analysis. Biophys J. 2008;94:L14–6. doi: 10.1529/biophysj.107.120154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fereidouni F, Esposito a, Blab Ga, Gerritsen HC. A modified phasor approach for analyzing time-gated fluorescence lifetime images. J Microsc. 2011;244:248–58. doi: 10.1111/j.1365-2818.2011.03533.x. [DOI] [PubMed] [Google Scholar]
- 56.Stringari C, Cinquin A, Cinquin O, Digman MA, Donovan PJ, Gratton E. Phasor approach to fluorescence lifetime microscopy distinguishes different metabolic states of germ cells in a live tissue. Proc Natl Acad Sci U S A. 2011;108:13582–7. doi: 10.1073/pnas.1108161108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Intes X, Chance B. Non-PET functional imaging techniques: optical. Radiol Clin North Am. 2005;43:221–34. doi: 10.1016/j.rcl.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 58.Lim Y, Kim S, Nakayama A, Stott N, Bawendi M, Frangioni J. Selection of Quantum Dot Wavelengths for Biomedical Assays and Imaging. Mol Imaging. 2003;2:50–64. doi: 10.1162/15353500200302163. [DOI] [PubMed] [Google Scholar]
- 59.Chance B. Near-infrared images using continuous, phase-modulated, and pulsed light with quantitation of blood and blood oxygenation. Ann N Y Acad Sci. 1998;838:29–45. doi: 10.1111/j.1749-6632.1998.tb08185.x. [DOI] [PubMed] [Google Scholar]
- 60.Ntziachristos V, Bremer C, Weissleder R. Fluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imaging. Eur Radiol. 2003;13:195–208. doi: 10.1007/s00330-002-1524-x. [DOI] [PubMed] [Google Scholar]
- 61.Lavis LD, Raines RT. Bright ideas for chemical biology. ACS Chem Biol. 2008;3:142–55. doi: 10.1021/cb700248m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Li CPL, Parodi Giusino U. Contribution to the nosological classification of parietal dysplasias of the gallbladder. Minerva Chir. 1967;22:344–57. [PubMed] [Google Scholar]
- 63.Fisler RE, Cohen A, Ringer Sa, Lieberman E. Neonatal outcome after trial of labor compared with elective repeat cesarean section. Birth. 2003;30:83–8. doi: 10.1046/j.1523-536x.2003.00225.x. [DOI] [PubMed] [Google Scholar]
- 64.Shaner NC, Campbell RE, Steinbach Pa, Giepmans BNG, Palmer AE, Tsien RY. Improved monomeric red, orange and yellow fluorescent proteins derived from Discosoma sp. red fluorescent protein. Nat Biotechnol. 2004;22:1567–72. doi: 10.1038/nbt1037. [DOI] [PubMed] [Google Scholar]
- 65.Shcherbo D, Merzlyak EM, Chepurnykh TV, et al. Bright far-red fluorescent protein for whole-body imaging. Nat Methods. 2007;4:741–6. doi: 10.1038/nmeth1083. [DOI] [PubMed] [Google Scholar]
- 66.Shu X, Royant A, Lin MZ, Aguilera Ta, Lev-Ram V, Steinbach Pa, et al. Mammalian expression of infrared fluorescent proteins engineered from a bacterial phytochrome. Science (80- ) 2009;324:804–7. doi: 10.1126/science.1168683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Filonov GS, Piatkevich KD, Ting L-M, Zhang J, Kim K, Verkhusha VV. Bright and stable near-infrared fluorescent protein for in vivo imaging. Nat Biotechnol. 2011;29:757–61. doi: 10.1038/nbt.1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Filonov GS, Krumholz A, Xia J, Yao J, Wang LV, Verkhusha VV. Deep-tissue photoacoustic tomography of genetically encoded iRFP probe. Angew Chem Int Ed Engl. 2012;51:1448–51. doi: 10.1002/anie.201107026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shcherbakova DM, Verkhusha VV. Near-infrared fluorescent proteins for multicolor in vivo imaging. Nat Methods. 2013;10:751–4. doi: 10.1038/nmeth.2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang X, Bloch S, Akers W, Achilefu S. Near-infrared Molecular Probes for In vivo Imaging. Curr Protoc Cytom. 2012:1–28. doi: 10.1002/0471142956.cy1227s60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ogawa M, Kosaka N, Choyke PL, Kobayashi H. In vivo molecular imaging of cancer with a quenching near-infrared fluorescent probe using conjugates of monoclonal antibodies and indocyanine green. Cancer Res. 2009;69:1268–72. doi: 10.1158/0008-5472.CAN-08-3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Intes X, Ripoll J, Chen Y, Nioka S, Yodha G, Chance B. In vivo continuous-wave optical breast imaging enhanced with Indocyanine Green. Med Phys. 2003;30:1039. doi: 10.1118/1.1573791. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Z, Achilefu S. Design, synthesis and evaluation of near-infrared fluorescent pH indicators in a physiologically relevant range. Chem Commun (Camb) 2005;47:5887–9. doi: 10.1039/b512315a. [DOI] [PubMed] [Google Scholar]
- 74.Lee H, Akers W, Bhushan K, et al. Near-infrared pH-activatable fluorescent probes for imaging primary and metastatic breast tumors. Bioconjug Chem. 2011;22:777–84. doi: 10.1021/bc100584d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Escobedo JO, Rusin O, Lim S, Strongin RM. NIR dyes for bioimaging applications. Curr Opin Chem Biol. 2010;14:64–70. doi: 10.1016/j.cbpa.2009.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanaka Y, Shin J-Y, Osuka A. Facile Synthesis of Largemeso-Pentafluorophenyl-Substituted Expanded Porphyrins. Eur J Org Chem. 2008;2008:1341–9. [Google Scholar]
- 77.Srinivasan A, Ishizuka T, Osuka A, Furuta H. Doubly N-confused hexaphyrin: a novel aromatic expanded porphyrin that complexes bis-metals in the core. J Am Chem Soc. 2003;125:878–9. doi: 10.1021/ja029018v. [DOI] [PubMed] [Google Scholar]
- 78.Umezawa K, Citterio D, Suzuki K. Water-soluble NIR fluorescent probes based on squaraine and their application for protein labeling. Anal Sci. 2008;24:213–7. doi: 10.2116/analsci.24.213. [DOI] [PubMed] [Google Scholar]
- 79.Tynan CJ, Clarke DT, Coles BC, Rolfe DJ, Martin-fernandez ML, Webb SED. Multicolour Single Molecule Imaging in Cells with Near Infra-Red Dyes. PLoS One. 2012;7:e36265. doi: 10.1371/journal.pone.0036265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.James NS, Chen Y, Joshi P, et al. Evaluation of polymethine dyes as potential probes for near infrared fluorescence imaging of tumors: part - 1. Theranostics. 2013;3:692–702. doi: 10.7150/thno.5922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Tasior M, O’Shea DF. BF2-chelated tetraarylazadipyrromethenes as NIR fluorochromes. Bioconjug Chem. 2010;21:1130–3. doi: 10.1021/bc100051p. [DOI] [PubMed] [Google Scholar]
- 82.Shao L, Gao Y, Yan F. Semiconductor quantum dots for biomedicial applications. Sensors (Basel) 2011;11:11736–51. doi: 10.3390/s111211736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Park J, Dvoracek C, Lee KH, et al. CuInSe/ZnS core/shell NIR quantum dots for biomedical imaging. Small. 2011;7:3148–52. doi: 10.1002/smll.201101558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kim S, Lim YT, Soltesz EG, et al. Near-infrared fluorescent type II quantum dots for sentinel lymph node mapping. Nat Biotechnol. 2004;22:93–7. doi: 10.1038/nbt920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Gao X, Cui Y, Levenson RM, Chung LWK, Nie S. In vivo cancer targeting and imaging with semiconductor quantum dots. Nat Biotechnol. 2004;22:969–76. doi: 10.1038/nbt994. [DOI] [PubMed] [Google Scholar]
- 86.Weng KC, Noble CO, Papahadjopoulos-Sternberg B, Chen FF, Drummond DC, Kirpotin DB, et al. Targeted tumor cell internalization and imaging of multifunctional quantum dot-conjugated immunoliposomes in vitro and in vivo. Nano Lett. 2008;8:2851–7. doi: 10.1021/nl801488u. [DOI] [PubMed] [Google Scholar]
- 87.Li C, Zhang Y, Wang M, et al. In vivo real-time visualization of tissue blood flow and angiogenesis using Ag2S quantum dots in the NIR-II window. Biomaterials. 2014;35:393–400. doi: 10.1016/j.biomaterials.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 88.Ishikawa-Ankerhold HC, Ankerhold R, Drummen GPC. Advanced fluorescence microscopy techniques--FRAP, FLIP, FLAP, FRET and FLIM. Molecules. 2012;17:4047–132. doi: 10.3390/molecules17044047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Zhang Z, Fan J, Cheney PP, Berezin MY, Edwards WBAW. Activatable Molecular Systems Using Homologous Near-Infrared Fluorescent Probes for Monitoring Enzyme Activities In vitro, In Cellulo, and In vivo. Mol Pharm. 2009;6:1–26. doi: 10.1021/mp800264k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ng T, Shima D, Squire A, et al. PKC α regulates β 1 integrin-dependent cell motility through association and control of integrin traffic. EMBO Journal. 1999;18:3909–23. doi: 10.1093/emboj/18.14.3909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ahmed Z, Schüller AC, Suhling K, Tregidgo C, Ladbury JE. Extracellular point mutations in FGFR2 elicit unexpected changes in intracellular signalling. Biochem J. 2008;413:37–49. doi: 10.1042/BJ20071594. [DOI] [PubMed] [Google Scholar]
- 92.Llères D, Swift S, Lamond AI. Detecting protein-protein interactions in vivo with FRET using multiphoton fluorescence lifetime imaging microscopy (FLIM) Curr Protoc Cytom. 2007;42:10.1–12.10.19. doi: 10.1002/0471142956.cy1210s42. [DOI] [PubMed] [Google Scholar]
- 93.Janssen A, Beerling E, Medema R, van Rheenen J. Intravital FRET imaging of tumor cell viability and mitosis during chemotherapy. PLoS One. 2013;8:e64029. doi: 10.1371/journal.pone.0064029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Verveer PJ, Wouters FS, Reynolds AR. Quantitative Imaging of Lateral ErbB1 Receptor Signal Propagation in the Plasma Membrane. Science (80- ) 2000;290:1567–70. doi: 10.1126/science.290.5496.1567. [DOI] [PubMed] [Google Scholar]
- 95.Ziomkiewicz I, Loman A, Klement R, et al. Dynamic conformational transitions of the EGF receptor in living mammalian cells determined by FRET and fluorescence lifetime imaging microscopy. Cytometry A. 2013;83:794–805. doi: 10.1002/cyto.a.22311. [DOI] [PubMed] [Google Scholar]
- 96.Waterhouse BR, Gijsen M, Barber PR, Tullis IDC, Vojnovic B, Kong A. Assessment of EGFR / HER2 dimerization by FRET-FLIM utilizing Alexa-conjugated secondary antibodies in relation to targeted therapies in cancers. Oncotarget. 2011;2:728–36. doi: 10.18632/oncotarget.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ritsma L, Ponsioen B, van Rheenen J. Intravital imaging of cell signaling in mice. IntraVital. 2012;1:2–10. [Google Scholar]
- 98.Mitchison TJ. The proliferation rate paradox in antimitotic chemotherapy. Mol Biol Cell. 2012;23:1–6. doi: 10.1091/mbc.E10-04-0335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Janssen A, Beerling E, Medema R, van Rheenen J. Intravital FRET imaging of tumor cell viability and mitosis during chemotherapy. PLoS One. 2013;8:e64029. doi: 10.1371/journal.pone.0064029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kelleher MT, Fruhwirth G, Patel G, et al. The potential of optical proteomic technologies to individualize prognosis and guide rational treatment for cancer patients. Target Oncol. 2009;4:235–52. doi: 10.1007/s11523-009-0116-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Keese M, Yagublu V, Schwenke K, Post S, Bastiaens P. Fluorescence lifetime imaging microscopy of chemotherapy-induced apoptosis resistance in a syngenic mouse tumor model. Int J Cancer. 2010;126:104–13. doi: 10.1002/ijc.24730. [DOI] [PubMed] [Google Scholar]
- 102.Ardeshirpour Y, Chernomordik V, Zielinski R, et al. In vivo fluorescence lifetime imaging monitors binding of specific probes to cancer biomarkers. PLoS One. 2012;7:e31881. doi: 10.1371/journal.pone.0031881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Solomon M, Guo K, Sudlow GP, et al. Detection of enzyme activity in orthotopic murine breast cancer by fluorescence lifetime imaging using a fluorescence resonance energy transfer-based molecular probe. J Biomed Opt. 2011;16:066019. doi: 10.1117/1.3594153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.McGinty J, Stuckey DW, Soloviev VY, et al. In vivo fluorescence lifetime tomography of a FRET probe expressed in mouse. Biomed Opt Express. 2011;2:1907–17. doi: 10.1364/BOE.2.001907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nobis M, McGhee EJ, Morton JP, et al. Intravital FLIM-FRET imaging reveals dasatinib-induced spatial control of src in pancreatic cancer. Cancer Res. 2013;73:4674–86. doi: 10.1158/0008-5472.CAN-12-4545. [DOI] [PubMed] [Google Scholar]
- 106.Xiong L, Shuhendler AJ, Rao J. Self-luminescing BRET-FRET near-infrared dots for in vivo lymph-node mapping and tumour imaging. Nat Commun. 2012;3:1193. doi: 10.1038/ncomms2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Davis SC, Samkoe KS, Tichauer KM, et al. Dynamic dual-tracer MRI-guided fluorescence tomography to quantify receptor density in vivo. Proc Natl Acad Sci U S A. 2013;110:9025–30. doi: 10.1073/pnas.1213490110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Lu Y, Darne CD, Tan I-C, et al. In vivo imaging of orthotopic prostate cancer with far-red gene reporter fluorescence tomography and in vivo and ex vivo validation. J Biomed Opt. 2013;18:101305. doi: 10.1117/1.JBO.18.10.101305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Nothdurft R, Sarder P, Bloch S, Culver J, Achilefu S. Fluorescence lifetime imaging microscopy using near-infrared contrast agents. J Microsc. 2012;247:202–7. doi: 10.1111/j.1365-2818.2012.03634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Becker W, Shcheslavskiy V. Fluorescence lifetime imaging with near-infrared dyes. Proc SPIE. 2013;8588:85880R. [Google Scholar]
- 111.Ushakov DS, Caorsi V, Manning HB, et al. Response of rigor cross-bridges to stretch detected by fluorescence lifetime imaging microscopy of myosin essential light chain in skeletal muscle fibers. J Biol Chem. 2011;286:842–50. doi: 10.1074/jbc.M110.149526. [DOI] [PMC free article] [PubMed] [Google Scholar]