Abstract
Background
Human papillomavirus (HPV) is a risk factor for head and neck cancers (HNC), yet HPV-associated tumors have better prognosis than HPV-negative tumors.
Methods
We evaluated whether pretreatment presence of antibodies to HPV capsids [virus-like particles (VLP)] or to HPV-16 oncoproteins E6 and E7 was a predictor of HPV-positive HNC and clinical outcomes. Sera from 156 HNC patients were tested for antibodies to HPV-16–derived antigens using ELISA. HPV-16 in tumors was evaluated by PCR and DNA sequencing.
Results
HPV-16 antibodies were found in 33% with HPV-16 VLP, 21% with HPV-16 E6, and 21% with E7. HPV-16 was detected in 26% of tumors. There was a strong correlation between detection of HPV-16 tumor DNA and antibodies to HPV-16 E6 or E7 (κ = 0.7) but not to HPV-16 VLP (κ = 0.4). Multivariate analyses showed significantly better disease-specific survival in seropositive HPV-16 VLP [hazard ratio (HR), 0.4; 95% confidence interval (95% CI), 0.1-0.9], HPV-16 E6 (HR, 0.1; 95% CI, 0.02-0.5), and HPV-16 E7 (HR, 0.3; 95% CI, 0.1-0.9) cases. Less disease recurrence occurred among those with antibodies to both E6 and E7 compared with those negative to both (P = 0.003). There was better disease-specific survival in patients who were E6 positive at baseline and remained positive at follow-up compared with individuals who were E6 negative at both time points (P = 0.03; κ = 0.9).
Conclusions
The presence of antibodies to HPV-16 E6 and E7 is associated with HPV in tumor cells and with better clinical outcomes. These findings suggest that the presence of E6/E7 antibodies before treatment is predictive of better clinical outcomes and that they may serve as biomarkers for selecting targeted therapeutic modalities developed for HPV-associated tumors.
Introduction
Incidence and survival for head and neck cancers (HNC) in the United States show little change over the past 30 years, with disease recurrence remaining high (1). Major risk factors for these cancers are tobacco and alcohol. Recently, a significant association has been established with high-risk human papillomavirus (HPV-HR) oncogenic types, which are detected in ~26% of HNC and constitute a risk factor independent of tobacco and alcohol (2, 3).
Oncogenic types of HPV encode two oncoproteins, E6 and E7, which bind to, inactivate, and tag for degradation tumor suppressor proteins p53 and pRb, can promote genomic instability, and interact with a number of other potential cellular targets for carcinogenesis (4, 5). In HNC, HPV is commonly identified as viral DNA in the tumor tissue. HPV infection also has been detected indirectly by the presence of antibodies to HPV antigens in sera (6-9). Studies by Smith et al. (6) and others (7-9) found agreement between the presence of HPV-16 in HNC tumors and serologic responses to HPV-16, further supporting an active role of HPV infection in HNC development.
A higher prevalence of HPV DNA positivity has been found in patients with advanced stage of disease or poorly differentiated tumors compared with those with early stage or well/moderately differentiated HNC at diagnosis (3, 10). Interestingly, despite the higher percentage of HPV-infected HNC cases with advanced disease characteristics, we (11) and others (3, 12, 13) have found that patients with HPV DNA-detected tumors have better prognosis and less disease recurrence compared to those with HPV-negative HNC, even after adjusting for other prognostic factors. This study investigated whether seropositivity to HPV type 16 capsid or HPV-16 E6 and E7 oncoproteins in newly diagnosed HNC cases was correlated with the presence of HPV in the tumor and with patient survival or recurrence, and thus could serve as a potential pretreatment biomarker test for targeted therapy. We also examined whether pretreatment HPV antibodies might be associated with clinical outcomes after treatment as has been shown for cervical cancer by comparing antibody status at diagnosis and at the initial posttreatment follow-up visit.
Materials and Methods
Patients
Participants included 156 consecutive, newly diagnosed cases with cancer of the oral cavity or oropharynx enrolled between March 2000 and December 2003 at the University of Iowa Hospitals and Clinics and the Iowa City Veterans Affairs Medical Center. Previously excluded were 7% who refused to provide a blood sample and 5% from whom blood was not available due to hardening of the veins or low blood volume. All participants signed an informed consent form.
Data Collection
Demographics and risk factors for the HNC cases were collected by a self-administered questionnaire at the clinic visit. Treatment and tumor-node-metastasis staging were obtained from the university Tumor Registry and chart reviews. Tumor staging was based on the 1997 American Joint Committee on Cancer criteria (14). Tumor site, grade, and histology were based on hospital pathology reports. Head and neck cancer sites were grouped into oral cavity versus oropharynx as suggested in the American Joint Committee on Cancer Staging Manual (lip and oral cavity versus oropharynx groupings excluding nasopharynx and hypopharynx; ref. 14). All histologic types of HNC were included in the study and were classified as squamous cell carcinoma and nonsquamous cell carcinoma (n = 16): 1 verrucous, 3 adenocarcinoma, 3 adenocystic carcinoma, 7 mucoepidermoid carcinoma, 1 carcinoma, and 1 polymorphous adenocarcinoma.
Baseline blood samples were obtained before treatment; serum was separated, aliquoted, and stored at −86°C until processed. Follow-up blood samples after the completion of treatment were available for 91 cases and were collected an average of 6 mo after the baseline blood draw (range, 2-24 mo).
Detection of HPV-Specific Antibodies
The presence of antibodies to antigens derived from HPV-specific proteins was evaluated using ELISA and has been described elsewhere (15). Virus-like particles (VLP) of HPV type 16 were prepared in a baculovirus expression system and used as antigens to detect antibodies to capsid proteins. The presence of antibodies to oncoproteins E6 and E7 derived from type 16 was assessed with a glutathione S-transferase capture ELISA system (16). Background reactivity of samples was determined for VLP antibodies in wells without antigen and for E6/E7 antibodies in wells coated with glutathione S-transferase antigen. The cutoff level, above which absorbance values were considered positive, was calculated separately for each antigen on a collection of 10 standard sera from regular blood donors known to be HPV antibody negative, included on each plate. Their absorbances were subtracted from corresponding values obtained in the presence of HPV-specific antigen. Absorbance values were recalculated into ratios by division by the particular cutoff value. An absorbance ratio >1.1 was considered positive.
DNA Extraction and HPV Detection
Tumor tissue was available for 145 cases. Extraction of DNA from paraffin-embedded tissue sections and HPV detection methods has previously been described (17). Briefly, 4 μL of DNA extracted from the biopsy specimens were PCR amplified with MY09 and MY11 primers (18) to detect HPV and with primers that amplify a portion of the β-globin gene (19) to verify the presence of intact DNA and the adequacy of PCR amplifications. Ten microliters of the PCR reaction were examined after electrophoresis in agarose gel for the presence of HPV and β-globin PCR products. The PCR product was transferred onto a nylon membrane and dot blot hybridized with a 32P-labeled probe for HPV-16. All samples that were positive in dot blot hybridization underwent heminested PCR amplification with MY09 and GP5+ or GP5+ and GP6+ primers (20). PCR products of the expected size were sequenced to determine HPV types. These techniques allowed identification of low copy numbers for a broad range of high-risk HPV types. Laser-capture microdissection procedures were done and verified that the HPV DNA was in the tumor cells and not in adjacent nontumorous epithelium as previously described (6).
Data Analysis
The Wilcoxon rank-sum test was used to compare continuous variables between patient groups. Multiple logistic regression was used to estimate the age-adjusted odds ratios (OR) for HPV seropositivity. The κ statistic measured the agreement between HPV DNA detected in tumors and HPV antibodies present in sera. Date of death or last known to be alive data were obtained from the National Cancer Institute Iowa Surveillance, Epidemiology, and End Results Cancer and hospital tumor registries. Cause of death was based on ICD-10 codes from death certificates. Disease recurrence was followed through the tumor registry, pathology reports, and by patient contact.
Survival was measured in years from the date of diagnosis until death or until the date the patient was last known to be alive. Patients who died of causes other than oral cancer were considered censored observations in the disease-specific survival analyses. Time to recurrence was measured in years from the date of diagnosis until disease recurrence or until the date the patient was last known to be alive. Patients who were never disease-free were excluded from the “time-to-disease recurrence” analyses, and patients dying before disease recurrence were treated as censored observations.
Time-to-event measures were estimated by the Kaplan-Meier method and survival curves were compared using the log-rank test. Hazard ratios (HR) measuring the association between seropositivity or HPV tumor status and survival or recurrence were estimated from the Cox proportional hazards models and were adjusted for age (continuous variable), gender, alcohol (dose-duration), tobacco (dose-duration), tumor site (oropharynx versus oral cavity), stage of disease at diagnosis (I/II versus III/IV), tumor grade (well/moderately differentiated versus poor/undifferentiated), tumor histology (squamous cell carcinoma versus other carcinomas), and treatment. Although not statistically significant in age-adjusted or age/other-adjusted models, age was included in the final HRs for the prognostic outcomes. Alcohol and tobacco were never significant in the survival analyses and were excluded from the final models (data not shown). Results of analyses, which included only squamous cell carcinoma cases, were similar to those presented for all carcinomas; therefore, all histologic types were combined for analyses. Treatment was dichotomized as surgery versus no surgery and radiation versus no radiation. Chemotherapy was examined but not found to be prognostically relevant and was not a confounder. The final HRs included all risk and pathologic factors except alcohol and tobacco.
The proportional hazards assumption was assessed and examination of changes in HR estimates was used to assess confounding. Ninety-five percent confidence intervals (95% CI) for ORs and HRs were based on normal approximations. P values from Cox regression were based on the likelihood ratio test. All reported P values were two-sided and statistically significant at P < 0.05. Statistical analyses were done using SAS version 8.2 (2001). Survival curves were created using SPlus 2000 (1999).
Results
The mean age of the HNC patients (N = 156) was 60 years (range, 21-91 years) and 61% were males. Most cases used both tobacco and alcohol (63%); 19% used one or the other on a regular basis for at least one year; and 18% reported never using either substance. Thirty-one percent (n = 48) of the tumors were located in the oropharynx and 69% (n = 108) in the oral cavity. Advanced stage of disease (III/IV) was diagnosed in 70% of the patients. Treatment regimens included surgery (74%), radiation (62%), and chemotherapy (18%), with 52% of the patients receiving some type of combination of these therapies (data not shown).
Antibodies to HPV-16
Antibodies to at least one of the following antigens were detected in 41% of patients: HPV-16 VLP (33%), HPV-16 E6 (21%), HPV-16 E7 (21%), and HPV-16 E6 and/or E7 (25%). Among seropositive cases, 49% were detected with more than one HPV antibody. Seropositive patients were significantly younger than those without HPV antibodies (56 versus 62 years; P < 0.01). The prevalence of HPV-16 VLP was statistically significantly higher among younger cases (≤60), current tobacco users, oropharyngeal tumors, advanced stage, and positive nodal involvement (Table 1). ORs for HPV-16 E6 and/or E7 seropositivity also were significantly elevated in younger age, but in contrast to HPV-16 VLP, male gender and former, not current, tobacco users had significantly elevated OR. The prevalence of HPV-16 E6 and/or E7 seropositivity was also much higher in oropharyngeal tumors, advanced stage, nodal involvement, poorly/undifferentiated tumors, and squamous cell carcinoma.
Table 1. Demographic and disease characteristics by anti–HPV-16 VLP and anti–HPV-16 E6 and/or E7 antibody status.
| Characteristic | HPV-16 VLP seropositive* |
HPV-16 VLP seronegative |
Age-adjusted OR (95% CI) |
HPV-16 E6/E7 seropositive† |
HPV-16 E6/E7 seronegative |
Age-adjusted OR (95% CI) |
|---|---|---|---|---|---|---|
| n = 52 (%) | n = 104 (%) | n = 39 (%) | n = 117 (%) | |||
| Age (y) | ||||||
| ≤60 | 40 (77) | 54 (52) | 3.1 (1.5-6.5) | 32 (82) | 62 (53) | 4.1 (1.7-9.9) |
| >60 | 12 (23) | 50 (48) | 1.0 | 7 (18) | 55 (47) | 1.0 |
| Gender | ||||||
| Male | 32 (62) | 63 (61) | 0.9 (0.5-1.9) | 30 (77) | 65 (56) | 2.5 (1.1-5.7) |
| Female | 20 (38) | 41 (39) | 1.0 | 9 (23) | 52 (44) | 1.0 |
| Tobacco status | ||||||
| Never | 8 (15) | 34 (33) | 1.0 | 7 (18) | 35 (30) | 1.0 |
| Former | 15 (29) | 33 (32) | 2.2 (0.8-6.2) | 15 (38) | 33 (28) | 3.4 (1.1-10.2) |
| Current | 29 (56) | 37 (36) | 3.0 (1.2-7.6) | 17 (44) | 49 (42) | 1.5 (0.5-4.1) |
| Alcohol status | ||||||
| Never | 10 (19) | 33 (32) | 1.0 | 7 (18) | 36 (31) | 1.0 |
| Former | 13 (25) | 25 (24) | 1.7 (0.7-4.7) | 11 (28) | 27 (23) | 2.2 (0.7-6.5) |
| Current | 29 (56) | 46 (44) | 1.8 (0.8-4.3) | 21 (54) | 54 (46) | 1.6 (0.6-4.4) |
| Tumor site | ||||||
| Oropharynx | 26 (50) | 22 (21) | 3.4 (1.6-7.1) | 32 (82) | 16 (14) | 27.0 (10.0-72.7) |
| Oral cavity | 26 (50) | 82 (79) | 1.0 | 7 (18) | 101 (86) | 1.0 |
| Stage of disease | ||||||
| 0, I, II | 7 (13) | 38 (38) | 1.0 | 2 (5) | 44 (38) | 1.0 |
| III, IV | 45 (87) | 64 (62) | 3.8 (1.6-9.4) | 37 (95) | 72 (62) | 11.5 (2.6-50.7) |
| Tumor grade‡ | ||||||
| Well/moderate | 38 (75) | 81 (79) | 1.0 | 20 (54) | 99 (85) | 1.0 |
| Poor/undifferentiated | 13 (25) | 22 (21) | 1.0 (0.4-2.1) | 17 (46) | 18 (15) | 4.0 (1.7-9.3) |
| Nodal involvement | ||||||
| Yes | 37 (71) | 50 (49) | 2.4 (1.1-4.9) | 36 (92) | 50 (45) | 13.9 (4.0-47.8) |
| No | 15 (29) | 53 (51) | 1.0 | 3 (8) | 62 (55) | 1.0 |
| Histology | ||||||
| SCC | 48 (92) | 92 (88) | 1.7 (0.5-5.6) | 39 (100) | 101 (86) | 5.3 (0.7-41.7)§ |
| Non-SCC | 4 (8) | 12 (12) | 1.0 | 0 (0) | 16 (14) | 1.0 |
NOTE: Number of patients, percentages (%) based on available data.
Abbreviation: SCC, squamous cell carcinoma.
HPV-16 VLP defined as seropositive for HPV-16, compared with individuals HPV-16 VLP seronegative.
HPV-16 E6/E7 seropositive defined as seropositive for HPV-16 E6 and/or HPV-16 E7, compared with individuals seronegative for HPV-16 E6 and E7.
Well/moderately differentiated versus poor/undifferentiated.
Logit estimator used to determine OR and CI.
HPV Concordance between Serology and DNA
HPV-16 DNA was found in 26% (n = 37) of the tumors. In the oral cavity 12% were HPV-16 positive, and in the oropharynx 60% were HPV-16 positive. Table 2 presents the prevalence and degree of concordance between HPV antibodies and HPV-16 DNA tumor status. Two cases were excluded from these analyses because their tumor results were detected with HPV-33 (as was their serology) and that HPV type was not the focus of this analysis. Among the HPV-16 DNA–positive cases, 62% had antibodies to HPV-16 VLPs (κ = 0.36). The HPV-16 VLP–negative patients whose tumors were HPV-16 positive included nine who had HPV-16 E6 and/or E7 antibodies present and five who had no HPV-16 antibodies. In the HPV tumor–negative cases, 23% were HPV-16 VLP positive. Antibodies to HPV-16 E6 and/or E7 were found in 76% of HPV-16 tumor–positive individuals, but in only 5% of the HPV tumor–negative cases with a much higher level of concordance (κ = 0.74) than for HPV-16 VLP. Among the five HPV tumor–negative cases, four had high absorbance ratios (>1.5-15.0), three were male, and one was female with no current diagnosis of another HPV-related premalignancy or cancer.
Table 2. Concordance between tumor HPV DNA and anti-HPV antibodies.
| Antibodies | HPV-16 DNA negative |
HPV-16 DNA positive |
κ statistic (95% CI) |
|---|---|---|---|
| n = 106 (%) | n = 37 (%) | ||
| HPV-16 VLP | |||
| Negative | 82 (77) | 14 (38) | 0.36 (0.20-0.53) |
| Positive | 24 (23) | 23 (62) | |
| HPV-16 E6 | |||
| Negative | 103 (97) | 12 (32) | 0.70 (0.56-0.84) |
| Positive | 3 (3) | 25 (68) | |
| HPV-16 E7 | |||
| Negative | 103 (97) | 13 (35) | 0.68 (0.54-0.82) |
| Positive | 3 (3) | 24 (65) | |
| HPV-16 E6 and/or E7 | |||
| Negative | 101 (95) | 9 (24) | 0.74 (0.61-0.87) |
| Positive | 5 (5) | 28 (76) |
NOTE: HPV DNA results from paraffin-embedded tissues available for 145 patients at the time of analysis; two cases were excluded because their tumors were detected with HPV-33.
Overall and Disease-Specific Survival and Disease Recurrence
Among the 156 newly diagnosed cases, there were 45 deaths and 27 recurrences, and 16 patients were never disease-free. Among the deaths, 69% were due to HNC. The median follow-up time among those alive at last contact was 2.3 years (range, 0.1-4.2 years) and 93% had at least 1 year of follow-up data. There were three cases in which the cause of death was unknown and one case in which recurrence status was unknown. The 2-year rates were 74% for overall survival, 80% disease-specific survival, and 24% disease recurrence.
Although not statistically significant in age-adjusted models, age was included in the final HRs for the prognostic outcomes. Alcohol and tobacco were never significant in the survival analyses and were excluded from the final models. Results of analyses, which included only squamous cell carcinoma cases, were similar to those presented for all carcinomas; therefore, all histologic types were combined for analyses. In multivariate analyses, adjusted for age or age/other risk factors, stage of disease was the most significant factor associated with survival. Advanced-stage patients had poorer prognosis (overall: HR, 5.5; 95% CI, 1.8-17.0; P = 0.003; disease-specific: HR, 10.0; 95% CI, 2.1-48.8; P = 0.004) but were not significantly more likely to have disease recurrence (HR, 1.8; 95% CI, 0.5-6.2; P = 0.35) than early-stage individuals. Patients with oropharyngeal cancer had greater survival than those with oral cavity cancer (overall: HR, 0.3; 95% CI, 0.1-0.8; P = 0.02; disease-specific: HR, 0.2; 95% CI, 0.1-0.6; P = 0.03) but not less disease recurrence (HR, 0.3; 95% CI, 0.1-1.7; P = 0.16). Age was not a significant risk factor in the adjusted analyses. There were no risk factors significantly associated with disease recurrence.
Prognosis by Antibody Status to HPV-16 VLP, E6, and E7
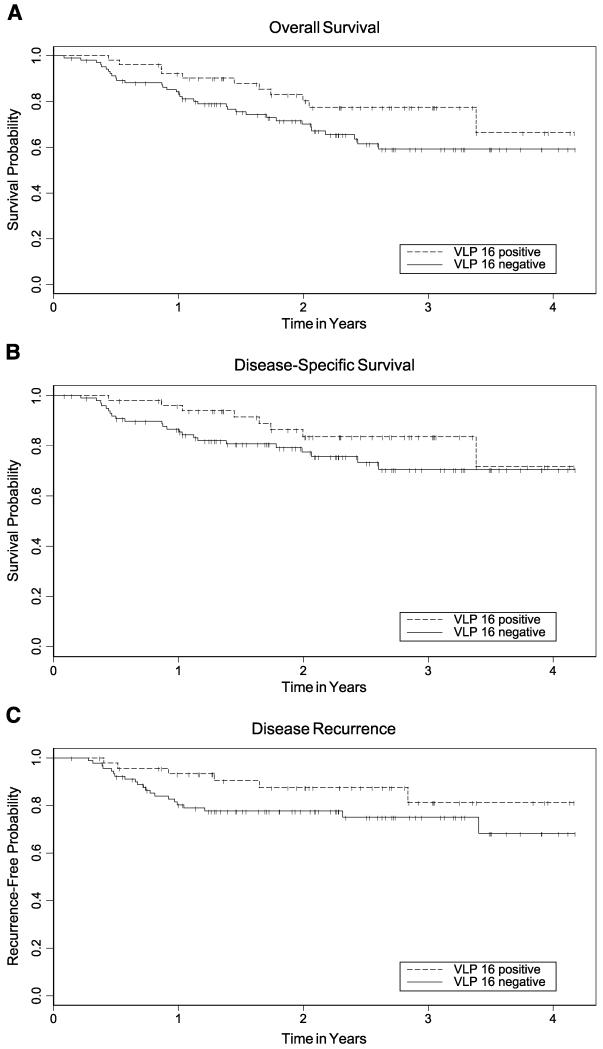
Table 3 presents the adjusted HRs from multivariate analyses evaluating the associations of HPV DNA and HPV antibodies with survival and recurrence. HPV-16 VLP seropositivity (Table 3; Fig. 1) was significantly associated with better overall and disease-specific survival (both P < 0.05), but not recurrence, compared with HPV-16 VLP–seronegative cases. However, HPV-16 VLP seropositivity was predictive of better disease prognosis only if E6 and E7 antibodies also were present (HR, 0.3; 95% CI, 0.1-1.1).
Table 3. HRs for mortality or disease recurrence by presence of anti-HPV antibodies.
| Antibodies/tumor DNA | Overall mortality, HR (95% CI) |
Disease-specific mortality, HR (95% CI) |
Disease recurrence, HR (95% CI) |
|---|---|---|---|
| HPV-16 VLP | 0.4 (0.2-0.8) | 0.4 (0.1-0.9) | 0.6 (0.2-1.5)* |
| HPV-16 E6 | 0.1 (0.04-0.5) | 0.1 (0.02-0.5) | 0.3 (0.1-1.5)† |
| HPV-16 E7 | 0.4 (0.1-0.9) | 0.3 (0.1-0.9) | 0.2 (0.02-1.4)† |
| HPV-16 E6 and/or E7 | 0.3 (0.1-0.7) | 0.3 (0.1-0.9) | 0.5 (0.1-1.7)* |
| HPV-16 E6 and E7 | 0.2 (0.1-0.6) | 0.1 (0.03-0.7) | — ‡ |
| HPV DNA+ | 0.2 (0.1-0.6) | 0.2 (0.1-0.7) | 0.6 (0.2-2.0)* |
NOTE: HRs adjusted for age, gender, stage of disease, tumor grade, histology, surgery, and radiation; reference group includes those negative for the HPV antibody examined.
P > 0.20.
P ≥ 0.10.
HR not estimable due to 0 recurrences among patients with anti–HPV-16 E6 and E7 antibodies present.
Figure 1.
Kaplan-Meier estimates for overall survival (A), disease-specific survival (B), and time-to-recurrence (C) by anti–HPV-16 VLP. Dashed line, anti–HPV-16 VLP–positive patients; solid line, anti–HPV-16 VLP antibody–negative patients; vertical tick marks, censored observations. Based on the log-rank test in unadjusted analyses, P = 0.09 for overall survival, P = 0.18 for disease-specific survival, and P = 0.13 for disease recurrence.
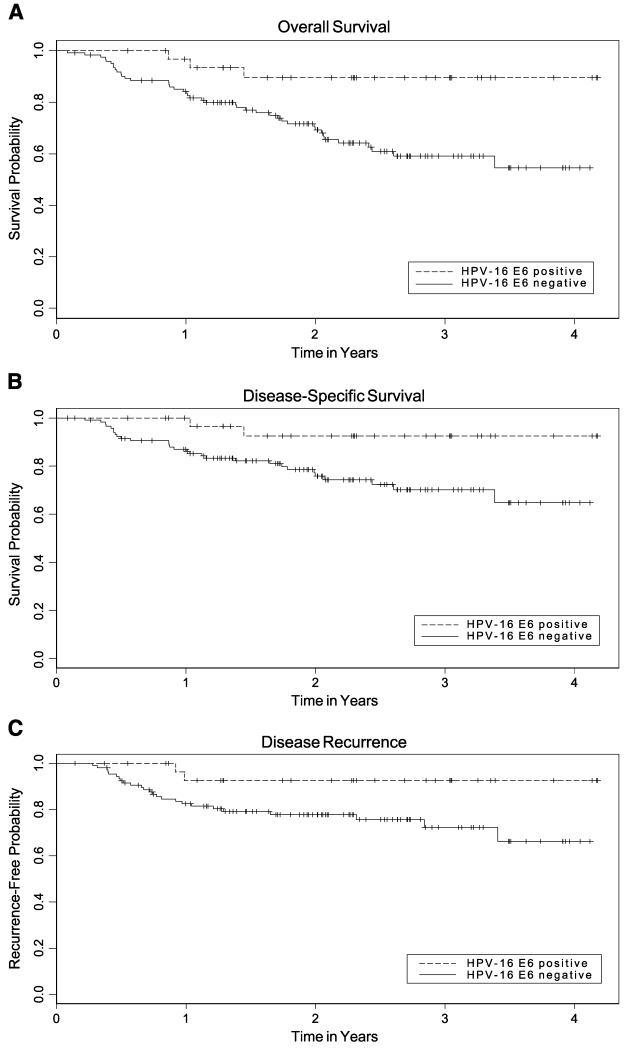
HPV-16 E6 seropositivity was statistically significantly associated with even lower mortality than HPV-16 VLP due to any cause (HR, 0.1; 95% CI, 0.04-0.5; P = 0.002) or to disease (HR, 0.1; 95% CI, 0.02-0.5; P = 0.004) compared to E6-seronegative cases (Table 3; Fig. 2). These findings also were observed for cases that were seropositive for both E6 and E7 compared with those seronegative for both antigens (overall: HR, 0.2; 95% CI, 0.1-0.6; P = 0.005; disease-specific: HR, 0.1; 95% CI, 0.03-0.6; P = 0.008). HPV-16 E7 seropositivity status also was an independent prognostic factor associated with better overall (HR, 0.4; 95% CI, 0.1-0.9; P = 0.03) and disease-specific survival (HR, 0.3; 95% CI, 0.1-0.9; P = 0.04). Disease recurrence was not significantly associated with E6 or E7 (both P > 0.10). However, patients who were seropositive to both E6 and E7 had statistically significantly less disease recurrence than individuals who were E6 and E7 seronegative (P = 0.003) after adjustment for stage. Because the E6- and E7-seropositive group had no recurrences, the adjusted HR could not be estimated.
Figure 2.
Kaplan-Meier estimates for overall survival (A), disease-specific survival (B), and time-to-recurrence (C) by HPV-16 E6 seropositivity. Dashed line, anti–HPV-16 E6 – positive patients; solid line, anti–HPV-16 E6–negative patients; vertical tick marks, censored observations. Based on the log-rank test in unadjusted analyses, P = 0.01 for overall survival, P = 0.02 for disease-specific survival, and P = 0.04 for disease recurrence.
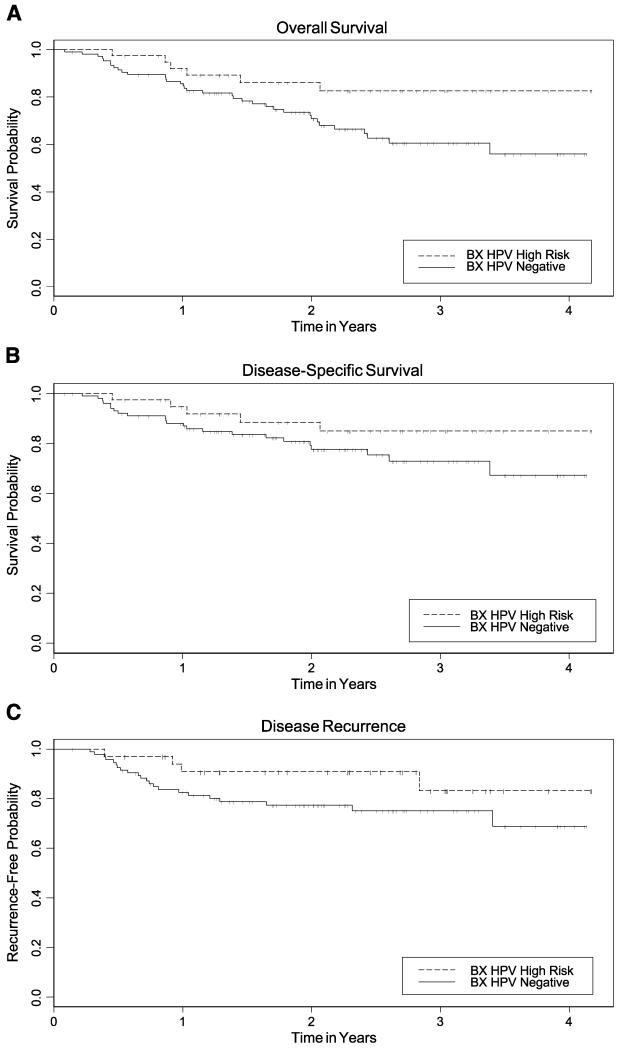
Prognosis by HPV-16 DNA Status
In multivariate analyses, HPV-16 DNA–positive tumors had improved overall (HR, 0.2; 95% CI, 0.1-0.6; P = 0.03) and disease-specific survival (HR, 0.2; 95% CI, 0.1-0.7; P = 0.01) compared with HPV-16–negative tumors (Table 3; Fig. 3). Similar to conclusions for the HPV antibodies, disease recurrence was not statistically significantly associated with HPV DNA status (HR, 0.6; 95% CI, 0.2-2.0; P = 0.4). Those with an HPV-16–positive tumor who received radiation had better disease-specific survival (HR, 0.3; 95% CI, 0.1-0.98; P < 0.05) and less recurrence (HR, 0.1; 95% CI, 0.02-0.9; P = 0.04; data not shown). The same was true for those receiving radiation who were E6 positive (disease-specific HR, 0.2; 95% CI, 0.04-0.7; P = 0.02) and E7 positive (HR, 0.3; 95% CI, 0.1-1.1; P = 0.07). The recurrence-free HR was not estimable for E6-positive/radiation-treated cases because there were no failures (0% versus 28%; P = 0.0001). E7-positive cases had significantly better recurrence-free survival than E7-negative patients (HR, 0.1; 95% CI, 0.02-0.9; P = 0.04) with radiation.
Figure 3.
Kaplan-Meier estimates for overall survival (A), disease-specific survival (B), and time-to-recurrence (C) by HPV DNA tumor status (Bx, biopsy). Dashed line, HPV DNA–positive tumors; solid line, HPV-negative tumors; vertical tick marks, censored observations. Based on the log-rank test in unadjusted analyses, P = 0.04 for overall survival, P = 0.17 for disease-specific survival, and P = 0.13 for disease recurrence.
Prevalence of HPV Antibodies at Diagnosis and After Treatment
We evaluated antibody status at baseline compared with the initial follow-up. There were strong correlations with pretreatment HPV seropositivity compared with 6 months posttreatment, with HPV-16 E6 having the highest measure of agreement (κ = 0.90) compared with HPV-16 VLP (κ = 0.85) or E7 (κ = 0.80). After adjusting for stage of disease and surgery, there was significantly better disease-specific survival in patients who were E6 positive at both baseline and follow-up compared with individuals who were E6 negative at both time points (P = 0.03; data not shown). There was no significant difference in disease-specific survival for HPV-16 VLP or E7 antibody. Disease recurrence was not significantly related to any HPV antibody by pretreatment/posttreatment status.
Discussion
This is the first study to examine concordance between pretreatment HPV antibodies and posttreatment HPV tumor tissue results and to compare prognostic findings between these two measures in HNC. The purpose was to determine whether a simple laboratory medical test, HPV serology, performed before treatment could predict HPV in HNC tumors and potentially serve as a biomarker of treatment and survival. No large studies have examined this predictive potential of HPV serologic measures.
In a previous study of HNC (6), we examined HPV-16 VLP, E6, and E7 antibodies in cases and controls for risk factors associated with disease. We also compared these antibody findings with HPV-16 tumor tissue and found significant concordance between serology and DNA tumor findings of HPV. In this current HNC case/case comparison study of HPV-16–positive or HPV-16–negative patients, we have established that pretreatment HPV-seropositive HNC cases, especially those with E6 and E7 antibodies, have significantly better overall and disease-specific survival when compared with those who are seronegative to these same antibodies. They also have better survival like that found for HPV-positive tumor tissue. These findings open up the possibility of identifying patients likely to have HPV-associated HNC before treatment to provide the most appropriate therapy modalities.
In agreement with our previous findings based solely on HPV detection in tumors (11), E6- or E7-seropositive cases showed advanced disease characteristics, yet had significantly better survival compared with seronegative individuals independent of age, gender, stage of disease, grade, histology, or treatment. The most likely reason that E6 and E7 seropositivity was strongly related to survival and lack of recurrence is that they were in high agreement with, and thus a reliable indicator of, HPV-16 presence and expression in the tumors (6-9). In addition, E6 or E7 antibody–positive cases have similar overall and disease-specific survival as that based on tumor DNA status. Taken together, the correlation of HPV E6 and E7 antibody response with HPV-associated HNC provides yet another strong line of evidence in support of the causal role of the HPV E6 and E7 genes in the development of HPV-related HNC.
HPV-16 VLP seropositivity alone showed lower concordance with HPV DNA in tumor tissues than did E6 and E7 antibodies. Furthermore, anti-VLP antibodies were not significantly predictive of survival outcomes after controlling for the presence of E6 and E7 antibodies. Thus, testing for VLP antibodies to identify HPV-positive tumors seems to be of questionable value because they are less sensitive HNC markers compared with E6 or E7 seroresponse. This conclusion is not surprising in view of the fact that VLP immunity reflects current as well as past HPV infection.
Because it is part of the ICD-O WHO classification of sites in the oral cavity, we included cancer of the lip. Two cases were in late stage and a third case was in early stage. All were HPV negative in the tumor and by serologic measures, but this is not surprising because only ~15% of oral cavity cancers are detected with an HPV oncogenic type. Few epidemiologic studies have examined subsites of HNC, but instead examined subsite groups as we have done in this investigation because there are too few cases in each of the subsites to evaluate separately. Analyses of HPV status by subsite will require additional numbers of cases.
Because the E6 and E7 proteins are expressed and immunogenic in HPV-related HNC, they may be promising targets for the development of an antigen-specific immunotherapeutic vaccine to prevent the progression and recurrence or even to induce the regression of invasive head and neck tumors. Recent vaccine trials using VLPs have shown promising success in preventing HPV infection (21, 22). In the uterine cervix, a crucial step in the progression of HPV-HR–infected cells to cancer is thought to be dysregulation of the expression of the E6 and E7 oncogenes, most frequently by disruption of the viral plasmid and E6/E7 gene integration. However, HPV-positive cancers of the cervix do not express viral late proteins or produce viral particles because the late part of the genome is commonly deleted, interrupted, or inactivated in the integrated HPV fragments; current vaccines that prevent new infection will likely not be helpful in established disease. In contrast, our data show that the E6/E7 proteins are highly immunogenic and represent a significant potential target. A number of therapeutic vaccines based on the E6/E7 proteins are currently being tested in experimental systems (23, 24).
In cervical cancer, tumor recurrence is higher among cases that have an elevated HPV-16 E6 or E7 antibody titer at follow-up (25, 26). Thus, better disease-specific survival and lower risk of recurrence in HNC patients were expected and observed among those who were seropositive at baseline who converted to seronegativity for E6 and/or E7 antibodies (25, 27). However, survival differences may not be apparent until some time after treatment when reassessment of HPV titer levels may predict prognosis. In cervical cancer, HPV antibodies have been shown to remain positive for 16 to 27 months after surgical removal of the lesion before reverting to seronegativity (25). Although our serology data were limited to short-term follow-up results at this time, among those who were seropositive at baseline, HPV antibody levels dropped after treatment; this was accompanied by increased survival. Longer follow-up and a larger number of seropositive cases are needed to determine whether the time frame for seroconversion in HNC is similar to that of cervical cancer and predictive of prognosis.
The ability to identify patients with tumors that are HPV driven at diagnosis may be useful in determining specific treatment modalities that work more effectively on HPV-associated tumors. Two reports have shown that HPV-16–positive HNCs may be more susceptible to radiation treatment (12, 27). We also found that those with an HPV-16–positive tumor who received radiation had better disease-specific survival as well as those receiving radiation who were E6 positive. Our data suggest that serologic status to the viral oncogenes may be useful for selecting a particular treatment. Research is warranted to determine whether molecular targeted agents could be developed for use on these HPV-associated tumors.
Several editorials on useful cancer prognostic factors indicate that despite years of research and significant funding, few valid prognostic markers exist (29, 30). The methods used in this study are in accord with many of the guidelines specified in these editorials. The serology test for E6/E7 is highly standardized using a routine ELISA and has high validity and reliability. Few patients refused or were unable to provide blood, thus reducing the potential bias toward patients with available specimens. In addition, assessment using a serology test for HPV detection has many advantages over a test that requires DNA isolation from a biopsy: It can be performed before treatment, is less invasive to patients, faster, less expensive, and may serve as a method to monitor treatment response over time. In addition, as recommended for ideal prognostic markers, all tumor tissue was available for these patients with adequate quantity for HPV testing using well-established, reliable methods. Further, the archived material had good clinical and pathologic annotation. In contrast to most prognostic or predictive marker investigations, our study is based on prospectively collected serology specimens in a well-defined population. We collected detailed risk factor data for all cases and reliable follow-up information from the Iowa National Cancer Institute Surveillance, Epidemiology, and End Results Cancer Registry database on survival and recurrence based on stringent quality control and high completeness (>99%) of these prognostic data. Tracking patient outcomes is more readily surmountable in patient populations that have an established high follow-up cancer registry network. Longer follow-up is needed in a larger number of HNC cases (because only a portion have an HPV-associated cancer and because of the large portion of cancer deaths) to confirm our prognostic findings about survival and recurrence based on E6 and E7 serologic evidence. Because this is the first study to compare the HPV serology assays with tumor tissue findings in association with survival, power calculations could not be determined. The elucidation of the specific molecular pathways involved also is needed to improve clinical outcomes associated with HNC because alternative mechanisms may help evade inhibition of growth or apoptosis in response to therapy, and prognosis among the HPV-independent tumors remains poor.
Acknowledgments
Grant support: NIH grants DE13110 (E.M. Smith, J.M. Ritchie, D.H.W., T.H. Haugen, and L.P. Turek) and DE1311-S1 (E.M. Smith, J.M. Ritchie, T.H. Haugen, E. Hamsikova, and L.P. Turek), Fogarty International Research Collaboration Award TW01500 (E.M. Smith, E. Hamsikova, and L.P. Turek), and Veterans Affairs Merit Review Funds (T.H. Haugen and L.P. Turek).
Footnotes
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Ries LA, Eisner MP, Kosary CL, et al., editors. SEER cancer statistics review, 1975-2001. National Cancer Institute; Bethesda (MD): 2004. http://seer.cancer.gov/csr/1975_2001. [Google Scholar]
- 2.Smith EM, Ritchie JM, Summersgill KF, et al. Human papillomavirus in oral exfoliated cells and risk of head and neck cancer. J Natl Cancer Inst. 2004;96:449–55. doi: 10.1093/jnci/djh074. [DOI] [PubMed] [Google Scholar]
- 3.Gillison ML, Koch WM, Capone RB, et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 4.Fehrmann F, Laimins LA. Human papillomaviruses: targeting differentiating epithelial cells for malignant transformation. Oncogene. 2003;22:5201–7. doi: 10.1038/sj.onc.1206554. [DOI] [PubMed] [Google Scholar]
- 5.Munger K, Howley PM. Human papillomavirus immortalization and transformation functions. Virus Res. 2002;89:213–28. doi: 10.1016/s0168-1702(02)00190-9. [DOI] [PubMed] [Google Scholar]
- 6.Smith EM, Ritchie JM, Pawlita M, et al. Human papillomavirus seropositivity and risks of head and neck cancer. Int J Cancer. 2006;120:825–32. doi: 10.1002/ijc.22330. [DOI] [PubMed] [Google Scholar]
- 7.Herrero R, Castellsague X, Pawlita M, et al. Human papillomavirus and oral cancer: the international agency for research on cancer multicenter study. J Natl Cancer Inst. 2003;95:1772–83. doi: 10.1093/jnci/djg107. [DOI] [PubMed] [Google Scholar]
- 8.Mork J, Lie AK, Glattre E, et al. Human papillomavirus infection as a risk factor for squamous-cell carcinoma of the head and neck. New Engl J Med. 2001;344:1125–31. doi: 10.1056/NEJM200104123441503. [DOI] [PubMed] [Google Scholar]
- 9.Zumbach K, Hoffmann M, Kahn T, et al. Antibodies against oncoproteins E6 and E7 of human papillomavirus types 16 and 18 in patients with head-and-neck squamous-cell carcinoma. Int J Cancer. 2000;85:815–8. doi: 10.1002/(sici)1097-0215(20000315)85:6<815::aid-ijc14>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10.Smith EM, Ritchie JM, Summersgill KF, et al. Age, sexual behavior and human papillomavirus infection in oral cavity and oropharyngeal cancers. Int J Cancer. 2004;108:766–72. doi: 10.1002/ijc.11633. [DOI] [PubMed] [Google Scholar]
- 11.Ritchie JM, Smith EM, Summersgill KF, et al. Human papillomavirus infection as a prognostic factor in carcinomas of the oral cavity and oropharynx. Int J Cancer. 2003;104:336–44. doi: 10.1002/ijc.10960. [DOI] [PubMed] [Google Scholar]
- 12.Schwartz SR, Yueh B, McDougall JK, Daling JR, Schwartz SM. Human papillomavirus infection and survival in al squamous cell cancer: a population-based study. Otolaryngol Head Neck Surg. 2001;125:1–9. doi: 10.1067/mhn.2001.116979. [DOI] [PubMed] [Google Scholar]
- 13.Li W, Thompson CH, O’Brien CJ, et al. Human papillomavirus positivity predicts favourable outcome for squamous carcinoma of the tonsil. Int J Cancer. 2003;106:553–8. doi: 10.1002/ijc.11261. [DOI] [PubMed] [Google Scholar]
- 14.Fleming ID, Cooper JS, Henson DE, et al., editors. AJCC cancer staging manual. 5th ed. Lippincott-Raven; Philadelphia (PA): 1997. [Google Scholar]
- 15.Parker TM, Smith EM, Ritchie RM, et al. Head and neck cancer associated with herpes simplex virus 1 and 2 and other risk factors. Oral Oncol. 2006;42:288–96. doi: 10.1016/j.oraloncology.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 16.Sehr P, Zumbach K, Pawlita M. A generic capture ELISA for recombinant proteins fused to glutathione S-transferase: validation for HPV serology. J Immunol Methods. 2001;253:153–62. doi: 10.1016/s0022-1759(01)00376-3. [DOI] [PubMed] [Google Scholar]
- 17.Summersgill KF, Smith EM, Kirchner HL, Haugen TH, Turek LP. p53 polymorphism, human papillomavirus infection in the oral cavity, and oral cancer. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:334–9. doi: 10.1067/moe.2000.107359. [DOI] [PubMed] [Google Scholar]
- 18.Ting Y, Manos MM. Detection and typing of genital human papillomaviruses. In: Innis MA, editor. PCR protocols, a guide to methods and applications. Academic Press; Berkeley (CA): 1990. pp. 356–67. [Google Scholar]
- 19.Chehab FF, Doherty M, Cai SP, Kan YW, Cooper S, Ruben EM. Detection of sickle cell anaemia and thalassaemias [letter] [published erratum appears in Nature 1987 Oct 22–28;329:678. Nature. 1987;329:293–4. doi: 10.1038/329293b0. [DOI] [PubMed] [Google Scholar]
- 20.de Roda Husman A-M, Walboomers JMM, van den Brule AJC, Meijer CJ, Snijders PJ. The use of general primers GP5 and GP6 elongated at their 3′ ends with adjacent highly conserved sequences improves human papillomavirus detection by PCR. J Gen Virol. 1995;76:1057–62. doi: 10.1099/0022-1317-76-4-1057. [DOI] [PubMed] [Google Scholar]
- 21.Poland GA, Jacobson RM, Koutsky LA, et al. Immunogenicity and reactogenicity of a novel vaccine for human papillomavirus 16: a 2-year randomized controlled clinical trial. Mayo Clin Proc. 2005;80:601–10. doi: 10.4065/80.5.601. [DOI] [PubMed] [Google Scholar]
- 22.Villa LL, Costa RL, Petta CA, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: a randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–8. doi: 10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 23.Greenstone GR, Nieland JD, de Visser KE, et al. Chimeric papillomavirus virus-like particles elicit antitumor immunity against the E7 oncoprotein particles in an HPV16 tumor model. Proc Natl Acad Sci U S A. 2001;95:1800–5. doi: 10.1073/pnas.95.4.1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Devaraj K, Gillison ML, Wu T-C. Development of HPV vaccines for HPV-associated head and neck squamous cell carcinoma. Crit Rev Oral Biol Med. 2003;14:345–62. doi: 10.1177/154411130301400505. [DOI] [PubMed] [Google Scholar]
- 25.Hamsikova E, Ludvikova V, Tachezy R, Kovanik J, Brouskova L, Vonka V. Longitudinal follow-up of antibody response to selected antigens of HPV and herpesvirus in patients with invasive cervical carcinoma. Int J Cancer. 2000;86:351–5. doi: 10.1002/(sici)1097-0215(20000501)86:3<351::aid-ijc8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 26.Baay MF, Duk JM, Burger MP, Debruijn HWA, Stolz E, Herbrink P. Humoral immune response against proteins E6 and E7 in cervical carcinoma patients positive for human papilloma virus type 16 during treatment and follow-up. Eur J Clin Microbiol Infect Dis. 1999;18:126–32. doi: 10.1007/s100960050240. [DOI] [PubMed] [Google Scholar]
- 27.Carter JJ, Galloway DA. Humoral immune response to human papillomavirus infection. Clin Dermatol. 1997;15:249–59. doi: 10.1016/s0738-081x(97)00068-0. [DOI] [PubMed] [Google Scholar]
- 28.Friesland S, Mellin H, Munck-Wikland E, et al. Human papilloma virus (HPV) and p53 immunostaining in advanced tonsillar carcinoma—relation to radiotherapy response and survival. Anticancer Res. 2001;21:529–34. [PubMed] [Google Scholar]
- 29.McShane LM, Altman DG, Sauerbrei W. Identification of clinically useful cancer prognostic factors: What are we missing? J Natl Cancer Inst. 2005;97:1023–4. doi: 10.1093/jnci/dji193. [DOI] [PubMed] [Google Scholar]
- 30.McShane LM, Altman DG, Sauerbrei W, Taube SE, Gion M, Clark GM, The Statisticis Subcomittee of the NCI-EORTC Working Group on Cancer Diagnostics Reporting recommendations for tumor marker prognostic studies (REMARK) J Natl Cancer Inst. 2005;97:1180–4. doi: 10.1093/jnci/dji237. [DOI] [PubMed] [Google Scholar]