Abstract
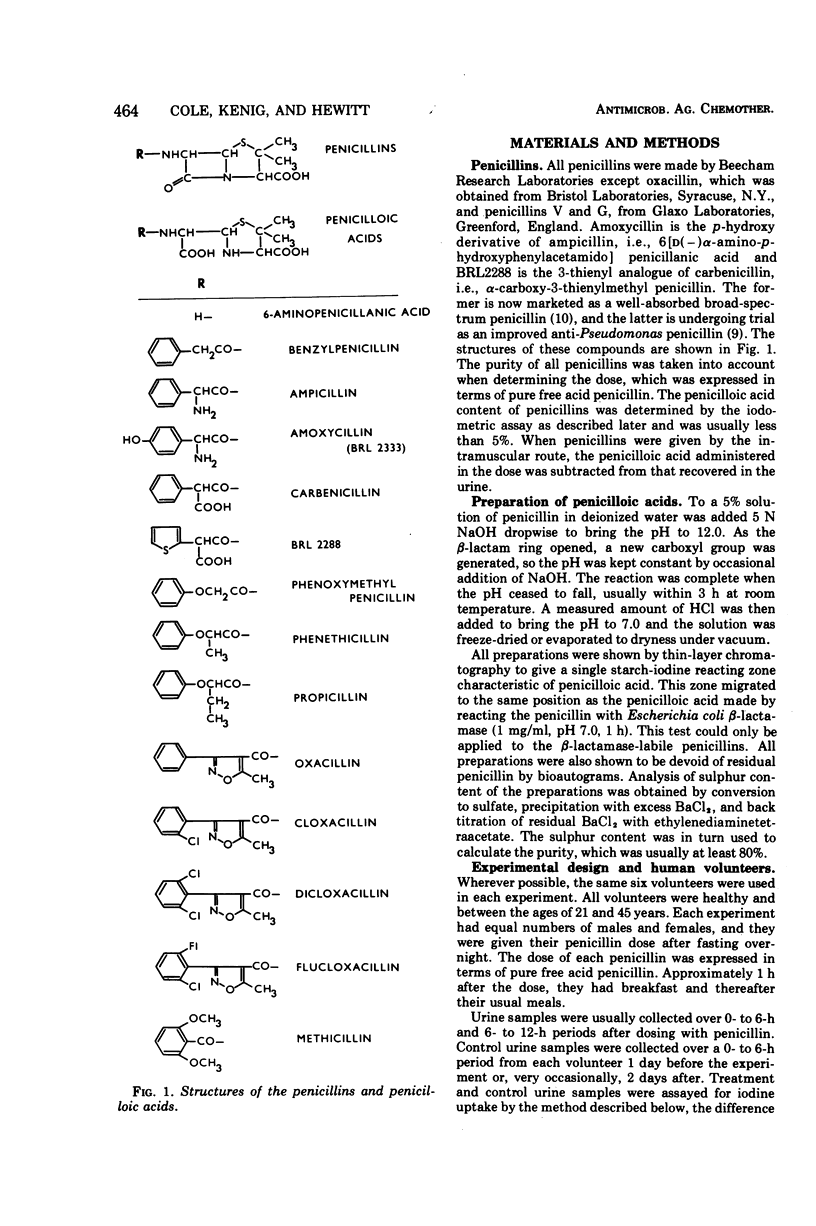
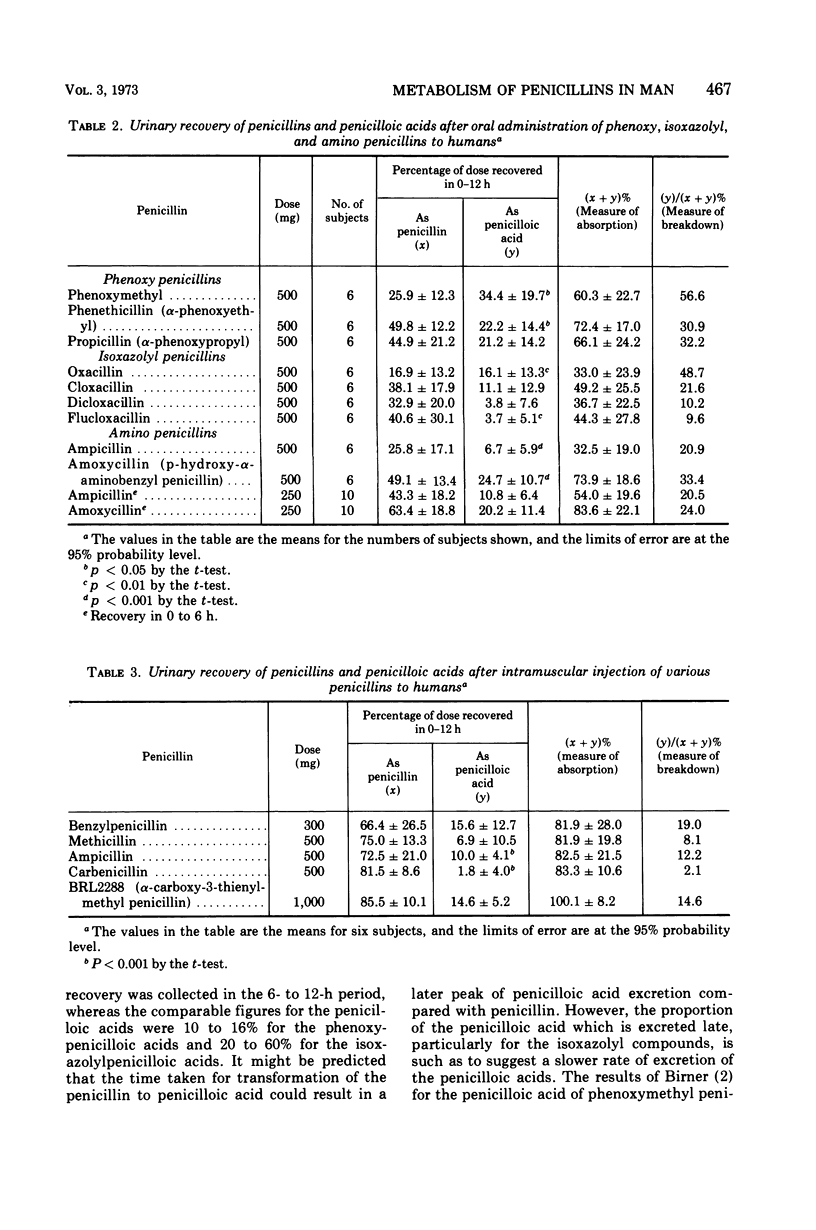
Penicillins can be metabolized to penicilloic acids in man, the extent being dependent on the penicillin structure. In the phenoxy penicillin series, phenoxymethyl penicillin was found to be particularly unstable, but the higher homologues were more stable. In the isoxazolyl series, oxacillin was unstable, and progressive insertion of halogen in the phenyl ring increased stability. Ampicillin and amoxycillin showed some instability, ampicillin possibly being the more stable. After intramuscular administration, carbenicillin was very stable in the body, ampicillin was fairly stable, and benzyl penicillin was unstable. It is important to take into account the penicilloic acid content of urine when estimating total absorption of a penicillin. Increased stability in the body as well as slower renal clearance can lead to high concentrations in the serum. Penicilloic acids seemed to be more slowly cleared from the body than penicillins. The liver is probably the site of inactivation.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birner J. Determination of phenoxymethyl penicilloic acid and phenoxyethyl penicilloic acid in urine in the presence of the parent penicillins. J Pharm Sci. 1970 Jun;59(6):757–760. doi: 10.1002/jps.2600590606. [DOI] [PubMed] [Google Scholar]
- Cole M., Sutherland R. The role of penicillin acylase in the resistance of gram-negative bacteria to penicillins. J Gen Microbiol. 1966 Mar;42(3):345–356. doi: 10.1099/00221287-42-3-345. [DOI] [PubMed] [Google Scholar]
- ENGLISH A. R., HUANT H. T., SOBIN B. A. 6-Aminopenicillanic acid in urine after oral administration of penicillins. Proc Soc Exp Biol Med. 1960 Jul;104:405–406. doi: 10.3181/00379727-104-25853. [DOI] [PubMed] [Google Scholar]
- Kind A. C., Beaty H. N., Fenster L. F., Kirby W. M. Inactivation of penicillins by the isolated rat liver. J Lab Clin Med. 1968 May;71(5):728–735. [PubMed] [Google Scholar]
- Rosenblatt J. E., Kind A. C., Brodie J. L., Kirby W. M. Mechanisms responsible for the blood level differences of isoxazolyl penicillins: oxacillin, cloxacillin, and dicloxacillin. Arch Intern Med. 1968 Apr;121(4):345–348. [PubMed] [Google Scholar]
- Standiford H. C., Jordan M. C., Kirby W. M. Clinical pharmacology of carbenicillin compared with other penicillins. J Infect Dis. 1970 Sep;122(Suppl):S9–13. doi: 10.1093/infdis/122.supplement_1.s9. [DOI] [PubMed] [Google Scholar]
- Sutherland R., Burnett J., Rolinson G. N. -carboxy-3-thienylmethylpenicillin (BRL 2288), a new semisynthetic penicillin: in vitro evaluation. Antimicrob Agents Chemother (Bethesda) 1970;10:390–395. [PubMed] [Google Scholar]
- Sutherland R., Croydon E. A., Rolinson G. N. Amoxycillin: a new semi-synthetic penicillin. Br Med J. 1972 Jul 1;3(5817):13–16. doi: 10.1136/bmj.3.5817.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMAS R. Colorimetric detection of penicillins and cephalosporins on paper. Nature. 1961 Sep 16;191:1161–1163. doi: 10.1038/1911161a0. [DOI] [PubMed] [Google Scholar]


