Abstract
Much of our knowledge of the world depends on learning associations (e.g., face-name), for which the hippocampus (HPC) and prefrontal cortex (PFC) are critical. HPC-PFC interactions have rarely been studied in monkeys, whose cognitive/mnemonic abilities are akin to humans. Here, we show functional differences and frequency-specific interactions between HPC and PFC of monkeys learning object-pair associations, an animal model of human explicit memory. PFC spiking activity reflected learning in parallel with behavioral performance, while HPC neurons reflected feedback about whether trial-and-error guesses were correct or incorrect. Theta-band HPC-PFC synchrony was stronger after errors, was driven primarily by PFC to HPC directional influences, and decreased with learning. In contrast, alpha/beta-band synchrony was stronger after correct trials, was driven more by HPC, and increased with learning. Rapid object associative learning may occur in PFC, while HPC may guide neocortical plasticity by signaling success or failure via oscillatory synchrony in different frequency bands.
Introduction
Most neurophysiological studies of PFC-HPC interactions have examined spatial memory in rodents. It seems clear, especially in primates, that the HPC and PFC have broader roles including non-spatial explicit (declarative) memory. HPC damage causes deficits in non-spatial associative learning, if implicit memory (familiarity, priming) cannot be used1–3. Likewise, PFC damage impairs explicit non-spatial associative memories, sparing implicit memory4,5. Human imaging shows activation of both areas during associative memory6,7. In rodents, there is theta synchrony between the PFC and HPC during spatial memory performance8,9 and high-frequency ripple synchrony during subsequent sleep10, thought to reflect the HPC acquiring spatial information and then integrating it into cortical networks for long-term storage. A similar relationship is assumed for non-spatial memories11, but this has not been tested in primates. Here, we provide evidence that instead, in monkeys, non-spatial associations are acquired by the PFC. In contrast, HPC activity is consistent with the idea that it provides learning-related frequency-specific feedback to the PFC.
Results
Paired associate learning task and behavioral results
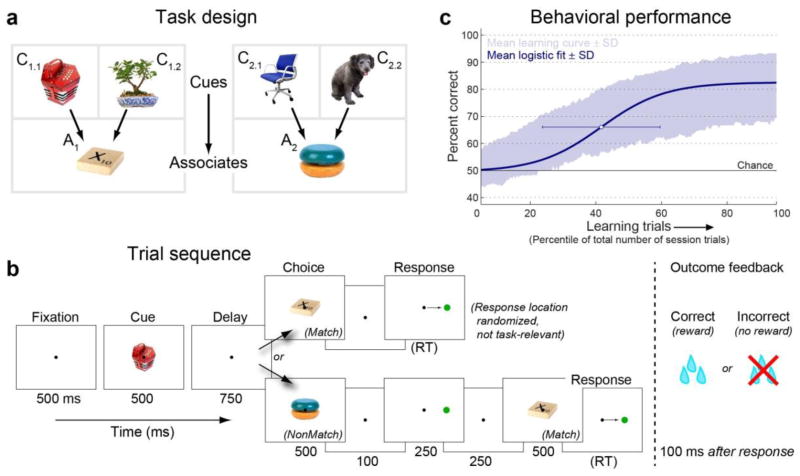
We trained two adult rhesus monkeys (Macaca mulatta) to perform an object paired-associate learning task. The monkeys learned—through trial-and-error—four novel associations between arbitrary pairs of objects in each experimental session (Fig. 1a and b). Each of four cue objects (C’s in Fig. 1a) was randomly paired with one of two associate objects (A1 or A2). This 4-to-2 mapping encourages prospective recall of the associate12 and distinguished neural activity to the cue from retrieval of its associate. Monkeys routinely learned associations within a few hundred trials (Fig. 1c; 313 of 348 associations learned to criterion), but measures of motivation, arousal, and motor function changed little with learning (Supplementary Fig. 1). Multiple microelectrodes were lowered daily into lateral prefrontal cortex and hippocampus (Fig. 2), and each recorded spiking and LFP signals while the monkeys performed the paired-associate learning task.
Figure 1. Paired-associate learning task.
(a) Task design. Each session, four objects were designated as cue objects; each was arbitrarily paired with one of two associate objects. (b) Task trial sequence. After central fixation, a cue object was followed by a short delay and a choice object. If it was that cue’s paired associate, monkeys had to saccade to a target (whose varied location was not task-relevant); otherwise, they were required to withhold response through another delay until the correct associate was presented. Correct choices were rewarded with juice; incorrect choices resulted in no reward and were signaled by a red error screen and 3-second “time-out”. Task period durations given in ms below panels (RT: reaction time). (c) Learning performance. Shaded area: mean ± SD of percent correct performance across all 348 associations (87 sessions), plotted as a function of the percentile of each session’s trials (mean ± SD trials per session: 1117 ± 125). Blue curve: average sigmoidal learning curve fit to each association. White dot: mean ± SD of fit curve centers.
Figure 2. Prefrontal and hippocampal recording locations.
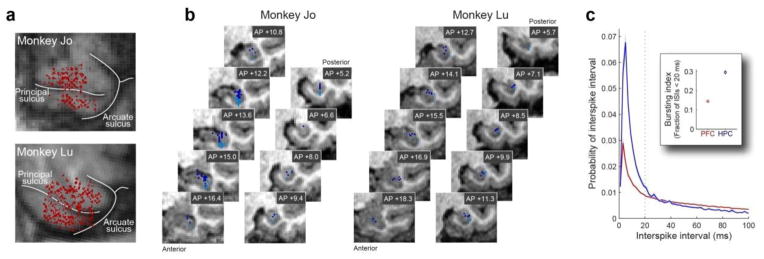
(a) Prefrontal recording sites in two monkeys, “Jo” and “Lu”, projected onto a tangential MRI slice. Each dot represents a location where neurons were recorded. Recordings spanned a broad region of dorsolateral and ventrolateral PFC (including parts of areas 46, 45, and 8). (b) Hippocampal recording sites, plotted on an anterior-to-posterior series of coronal MRI slices (labeled with mm relative to interaural line along the anterior-posterior axis). Dark blue: locally-projecting subregions (dentate gyrus, CA3, CA2). Light blue: output subregions (CA1, subiculum). Recordings spanned all subregions of the anterior ~3/4 of the hippocampal formation. (c) Hippocampal neurons exhibit characteristic bursty firing. Population mean (± SEM) interspike interval (ISI) histograms showing the probability of each ISI averaged across all PFC (red) and HPC (blue) putative principal neurons (putative fast-spiking interneurons were discriminated based on spike waveform and firing rate and excluded from this plot). Inset: population mean (± SEM) spike bursting index39—the fraction of each neuron’s ISIs < 20 ms. Both plots illustrate the distinctive burstiness of hippocampal neurons in comparison to neocortical neurons.
PFC and HPC neurons reflect learned associations and trial outcomes
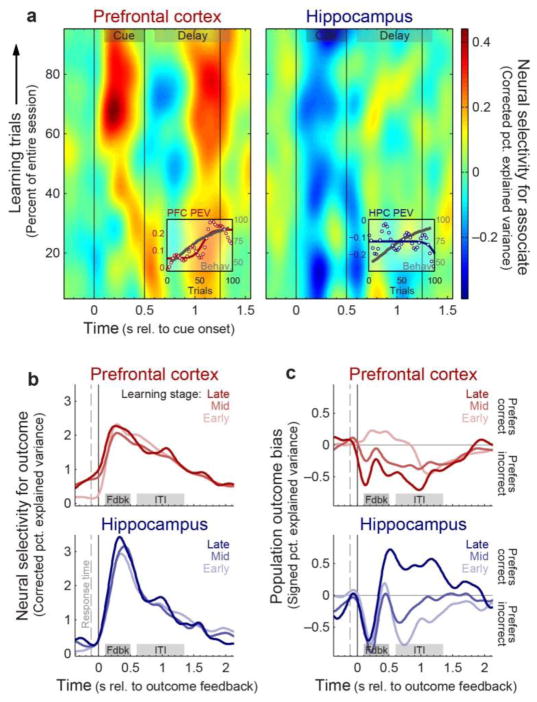
With learning, PFC neurons increasingly showed activity after the cue that anticipated its paired associate, with an across-trial progression similar to the improvement in performance (example single neuron: Supplementary Fig. 2a and b; population summary: Fig. 3a; Spearman’s ρ= 0.59 with cue-epoch spike rate, p = 0.04, 2-sided permutation test). However, while HPC neuronal activity conveyed sensory signals reflecting the cue object (Supplementary Fig. 3), it did not reflect learning of the paired associate and showed no correlation with behavior (ρ = −0.21, p = 0.73). Instead, HPC reflected the trial outcome after the feedback (reward vs. no-reward) about whether the behavioral response was correct or incorrect. This effect was stronger in the HPC than the PFC (example single neuron: Supplementary Fig. 2c and d; population summary: Fig. 3b; p = 0.049; 2-way area × learning-stage permutation ANOVA), and within HPC, stronger in the output subregions (CA1, subiculum) than locally-projecting subregions (CA3, dentate gyrus; p ≤ 10−4; Supplementary Fig. 4a). With learning, HPC activity shifted from stronger activation after incorrect to correct outcomes (Fig. 3c; p = 0.027, 2-sided permutation test on early vs. late learning stages [first vs. last third of learning trials], with number of incorrect vs. correct trials matched across learning; see Methods). This shift was present in HPC output subregions (p = 0.005; Supplementary Fig. 4b), but not local-projection subregions (p = 0.83; subregion × learning-stage interaction: p = 0.04), which may be related to a corresponding shift in communication between the HPC and PFC described below.
Figure 3. Prefrontal neurons reflect learned associations; hippocampal neurons reflect trial outcome.
(a) Mean percent of variance explained (PEV) by learned associate objects in PFC (left; n = 319 neurons) and HPC (right; n = 199) spiking activity, plotted across time after cue onset and learning trials. Bias correction results in negative values for some trials/times where values are less than expected based on selectivity for random combinations of cue objects (see Methods). Gray bars: analytical epochs focusing on cue and delay periods. Insets: behavioral (gray) and neural “learning curves”—mean cue-epoch PEV across trials. Only PFC shows learning of associates in parallel with behavior. (b) Mean percent of variance in PFC (top) and HPC (bottom) neurons explained by trial outcome (correct vs. incorrect), plotted across time after outcome feedback (reward vs. no-reward) for early, middle, and late learning stages (light-to-dark colors). Gray bars: analytical epochs focusing on transient responses to outcome feedback and sustained activity during the inter-trial interval (ITI). Outcome is represented more strongly in HPC in the outcome feedback epoch. (c) Mean bias (signed PEV; see Methods) in PFC (top) and HPC (bottom) neurons for correct (positive values) vs. incorrect (negative) outcomes, as a function of time and learning stages. HPC shifts from incorrect to correct outcomes with learning. Though there is a significant area × learning-stage interaction (p = 0.03), PFC shows no significant change with learning (p = 0.3).
The learning-related change in HPC outcome bias could reflect either a sign-flip in the preference of individual neurons from incorrect to correct trials, or just a relative modulation of neurons whose outcome preference is consistent throughout learning (broadly analogous to the distinction between “global remapping” and “rate remapping” in rodent hippocampal place cells13). To distinguish between these possibilities, we trained a linear (logistic regression) classifier to discriminate correct vs. incorrect trials based on HPC activity early in learning, and asked whether the trained classifier weights transferred to predict trial outcome late in learning. The preference-flip model predicts early-learning-derived weights and late-learning activity will have largely opposing outcome preferences, and will thus produce prediction accuracy near (or below) chance. We instead found that an early-learning trained classifier predicted the outcome on 79% of late-learning trials, comparable to 89% for a classifier both trained and tested on (distinct) late-learning trials. This provides evidence that the observed shift with learning reflects modulation of a largely invariant hippocampal neural code for trial outcome (cf. Supplementary Fig. 2d).
Band-specific PFC-HPC synchrony reflects trial outcome and learning
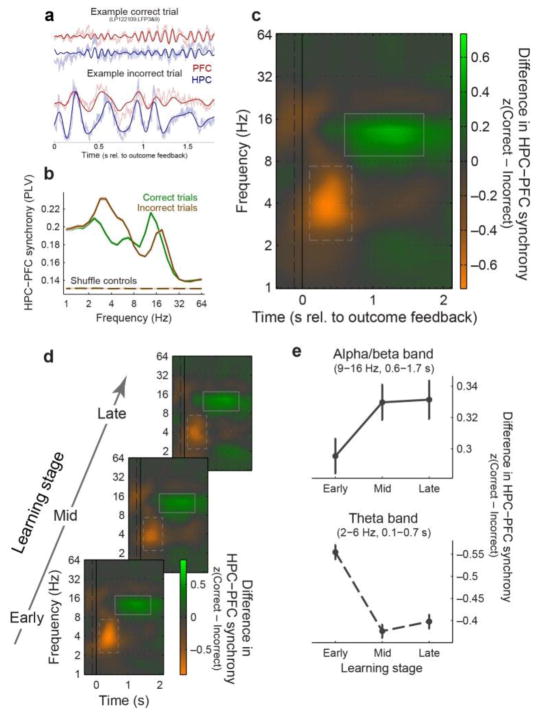
We examined outcome-related neural communication using synchrony (phase-locking) between local field potentials (LFPs; Fig. 3a) recorded after the behavioral response and feedback. This revealed PFC-HPC synchrony in two frequency bands: a shorter latency theta-band (~2–6 Hz) synchrony and longer latency alpha/low-beta band (~9–16 Hz) synchrony. Alpha/beta synchrony was stronger after correct trials; theta synchrony was stronger after incorrect trials (Fig. 4a–c). Though PFC-HPC synchrony was present before the behavioral response, it did not robustly reflect trial outcome (Supplementary Fig. 5). Theta synchrony following incorrect outcomes decreased with learning (Fig. 4d and e; p ≤ 10−4), while alpha/beta synchrony following correct outcomes increased with learning (p = 5×10−4, 2-sided permutation test on early vs. late learning). While the theta effect was similar across HPC subregions, the alpha/beta increase with learning only occurred for synchrony between PFC and HPC output subregions (Supplementary Fig. 6). Thus, with learning, there was a shift in PFC-HPC synchrony from theta toward higher frequencies, paralleling the shift in HPC spiking activity from incorrect to correct trials (see above).
Figure 4. Hippocampal-prefrontal oscillatory synchrony carries learning-related information about trial outcome.
(a) Example LFPs from a pair of sites in HPC (blue) and PFC (red) following a correct (top) and an incorrect (bottom) trial. Lighter colors: raw LFPs; darker colors: LFPs filtered within the 9–16 Hz (top) and 2–6 Hz (bottom) bands. (b) Mean synchrony (± SEM) between HPC and PFC LFPs, plotted as a function of frequency, following correct (green) and incorrect (brown) outcomes. Synchrony is computed as the across-trial phase-locking value (PLV), calculated within the feedback and ITI epochs (100–1725 ms after outcome feedback), and is averaged across all 970 electrode pairs and sessions. Dashed curves: mean synchrony (± SEM) expected by chance (based on shuffling HPC and PFC signals across trials), which is nearly identical across trial outcome and frequency. (c) Mean z-scored difference in HPC-PFC synchrony (dPLV) between correct and incorrect trials, plotted as spectrograms across time and frequency. Theta-band (dashed rectangle) and alpha/beta-band (solid rectangle) synchrony were stronger for incorrect and correct outcomes, respectively. (d) Mean dPLV as a function of learning (bottom to top: early, middle, and late learning stages). (e) Summary of synchrony learning effects—mean dPLV (± SEM) pooled within the alpha/beta-band (top) and theta-band (bottom; note that higher values in this plot reflect stronger negative PLV differences) regions of interest, as a function of learning stage. Theta (incorrect) synchrony decreases with learning, while alpha/beta (correct) increases.
We also examined within-area LFP phase-locking and power. While within-PFC synchrony followed a similar pattern to between-area synchrony (Supplementary Fig. 7), intra-hippocampal synchrony exhibited a distinct pattern in which theta synchrony increased—rather than decreased—with learning. This indicates the observed learning-related synchrony changes do not simply reflect state changes with global effects. LFP power, reflecting local synchrony, exhibited a pattern broadly similar to cross-area synchrony (Supplementary Fig. 8), as would be expected from an interacting system with causal links between local and long-range synchrony. Outcome selectivity in cross-area synchrony could not, however, be fully attributed to local power differences, as it remained significant even when band-specific power was balanced across correct and incorrect trials (P ≤ 10−4 for both frequency bands, 1-sample bootstrap test; Supplementary Fig. 9a).
Band-specific directionality of PFC-HPC causal influence
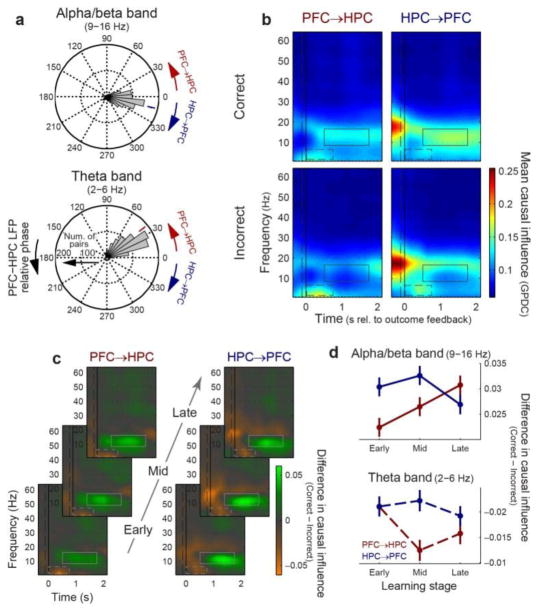
Theta and alpha/beta synchrony differed in the direction of putative causal influence. For theta frequencies, the phase of HPC LFPs lagged behind PFC (Fig. 5a; mean rel. phase = 39°; p ≤ 10−4; bootstrap test vs. zero phase lag), consistent with a PFC to HPC directionality; the reverse was true for alpha/beta frequencies (−13.5°; p ≤ 10−4). We confirmed this using generalized partial directed coherence (GPDC), a frequency-domain analog of Granger causality that measures the degree to which signals can predict each other’s future values. It also revealed oscillatory interactions in the theta and alpha/beta bands (Fig. 5b), with stronger theta influence (dashed lines) from PFC to HPC (p = 0.01) and stronger alpha/beta influence (solid lines) from the HPC to PFC (p ≤ 10−4, direction factor in 2-way causal direction × trial outcome permutation ANOVA). These differences remained significant when band-specific LFP power was balanced across correct and incorrect trials (P ≤ 10−4 for both frequency bands, direction factor in 2-way causal direction × trial outcome permutation ANOVA); Supplementary Fig. 9b), indicating that the observed directionality cannot be explained by differences in local power. As above, theta and alpha/beta interactions were stronger for incorrect and correct trials, respectively (p ≤ 10−4 for both; Fig. 5b). With learning, there were significant decreases in incorrect-reflecting PFC to HPC theta influences (Fig. 5c and d; p = 0.021) and correct-reflecting HPC to PFC alpha/beta influences (p = 0.04, 2-sided permutation test on early vs. late learning) suggesting these interactions may be most important during the early stages of learning. In contrast, initially weak PFC to HPC alpha/beta influences reflecting correct outcomes increased with learning (p ≤ 10−4), eventually becoming even stronger than the HPC to PFC direction (p = 10−3; interaction in 2-way causal-direction × learning-stage permutation ANOVA).
Figure 5. Stronger theta PFC→HPC directional influence; stronger HPC→PFC alpha/beta influence.
(a) Angular histograms of mean PFC-HPC LFP phase lag (n = 970 electrode pairs), pooled within alpha/beta-band (top) and theta-band (bottom) regions of interest. Colored tick marks indicate mean across all pairs. HPC leads for alpha/beta frequencies, while PFC leads for theta. (b) Frequency-domain directional influences (generalized partial directed coherence; GPDC) from PFC to HPC (left; n = 970 electrode pairs) and from HPC to PFC (right; n = 970), following correct (top) and incorrect (bottom) trials. GPDC is plotted as spectrograms across time and frequency. Overall, alpha/beta-band influences (solid rectangles) are stronger from HPC to PFC and for correct, while theta-band influences (dashed rectangles) are stronger from PFC to HPC and for incorrect trials. (c) Cross-area directional influences across learning. Selectivity for trial outcome is quantified by the difference in directional strength (GPDC) between correct and incorrect outcomes, and is plotted separately for each direction (PFC→HPC, left; HPC→PFC, right) and learning stage (bottom to top). (d) Summary of learning effects: mean difference (correct – incorrect) in causal influence (± s.e.m.) pooled in the alpha/beta-band (top) and theta-band (bottom) regions of interest, as a function of learning stage. With learning, theta interactions showed a decreasing trend, whereas alpha/beta interactions shifted from a HPC→PFC to PFC→HPC directionality.
Discussion
These results suggest different roles and interactions between the PFC and HPC during object associative learning. Only PFC neurons showed neural correlates of learning the paired associates. The HPC was more engaged when feedback was given about whether the trial was correct or incorrect. Early in learning, incorrect outcomes activated HPC neurons and promoted cross-area theta synchrony with a stronger influence from the PFC to the HPC. Correct outcomes, in contrast, promoted alpha/beta-band synchrony that was initially stronger in the HPC to PFC direction. But as learning progressed, correct outcomes increasingly evoked PFC to HPC alpha/beta-band influences and HPC neuronal spiking. This shift in HPC outcome coding (and other properties; Supplementary Fig. 10) distinguishes it from unipolar positive and negative reward-prediction-error signals in the midbrain dopaminergic nuclei14 and lateral habenula15, respectively. It may, however, reflect a functional shift in the importance of negative and positive feedback. Early in trial-and-error learning, errors provide critical information about which pairs of objects should not be associated. But once associations are learned, errors are more likely to simply reflect lapses in response inhibition or attention rather than true errors of associative choice. In contrast, positive feedback following correct trials16, and the likely resulting dopamine release in hippocampus17, have been shown to preferentially enhance long-term consolidation of new learning. Thus, the shift in HPC bias from incorrect to correct outcomes as learning progresses is consistent with a transition from neural signals that support acquisition to those that promote consolidation.
The HPC is critical for formation of explicit memories. Rodent neurophysiological studies suggest it acquires spatial memories and consolidates them in the neocortex, including PFC8,10 The primate HPC shows rapid activity changes related to spatial associative learning18. But the HPC is also known to be critical for non-spatial memory in rodents11,19 and especially in primates, where it plays a general role in explicit memory formation1,3. Lesion studies have suggested that perirhinal cortex—part of the medial temporal lobe system3 that includes the HPC—may be more critical for object associative learning than the HPC19,20, and neural correlates of object associations have been seen in perirhinal, prefrontal, and inferotemporal cortex12,21–23. However, these studies examined associations that were familiar or learned gradually (over days or weeks), situations known to favor neocortical representation. Our results suggest that rapid acquisition of object associations also occurs in the neocortex, not the HPC, perhaps particularly the PFC given its importance for behavioral flexibility. Object associations may lack the context required for explicit HPC representation24.
Both the HPC and PFC signal trial outcome, differentiating between correct and error trials25,26. Our results suggest this information is communicated between HPC and PFC via synchrony at different frequencies: theta for incorrect and alpha/beta for correct. Human and animal studies suggest that oscillatory activity is associated with memory encoding and retrieval27–29, as well as other cognitive processes30–32. Higher frequency (gamma) oscillations are thought to underlie the transient formation of local neuronal ensembles, while lower frequencies may recruit larger networks due to their longer integration times31,33,34. Thus, the lower frequency (theta and alpha/beta) synchrony we observed may reflect formation of larger networks connecting PFC, HPC, and likely many other cortical and subcortical structures. Further experimentation will be necessary to delineate the extent of these networks and dissect out how each of their nodes function in learning. Human EEG also shows theta oscillations with a frontal source reflecting conflict or error35; our results suggest these oscillations are propagated to hippocampus during learning. We did not, however, observe the sustained bouts of theta oscillations typically seen in locomoting rodents8,9,36; it remains an open question whether our theta-band synchrony reflects a distinct or related phenomenon.
But what computational role might these particular frequencies have? Growing evidence suggests beta oscillations are ideal for maintaining active cell assemblies and their associated cognitive states37. This hypothesis is consistent with the idea that alpha/beta oscillations might have a role in maintaining neural representations active during correct associations. Studies of synaptic plasticity have also shown that low-frequency synaptic stimulation fosters long-term depression, while high-frequency stimulation fosters long-term potentiation38, with the crossover point at ~8–10 Hz. PFC-HPC theta interactions may therefore have weakened synapses active during incorrect associations, while alpha/beta interactions strengthened those active for the correct associations.
In sum, these observations show that rapid formation of non-spatial associations may occur within the PFC, not the HPC. The main role of the HPC appears to be signalling trial outcome, signals which are communicated with PFC via band-specific oscillatory synchrony and may be involved in guiding neocortical learning. The results also provide further support for the idea that synchrony in different frequency bands may have functionally different roles in neural communication30,34.
Methods and any associated references are available in the Supplementary Materials.
Supplementary Material
Acknowledgments
This work was supported by NIMH Conte Center grant P50-MH094263-03 (E.K.M.), NIMH fellowship F32-MH081507 (S.L.B.), and The Picower Foundation. We thank E. Antzoulatos, A. Bastos, J. Donoghue, N. Kopell, S. Kornblith, R. Loonis, M. Lundqvist, M. Moazami, V. Puig, J. Rose, J. Roy, A. Salazar-Gómez, L. Tran, and M. Wilson for helpful comments and suggestions, and D. Altschul, B. Gray, M. Histed, D. Ouellette, and the MIT veterinary staff for technical assistance.
Footnotes
Author contributions S.L.B. and E.K.M. designed the experiments. S.L.B. trained the monkeys, performed the experiments, and analyzed the data. S.L.B. and E.K.M. wrote the paper.
Author information The authors declare no competing financial interests.
References
- 1.Scoville WB, Milner B. Loss Of Recent Memory After Bilateral Hippocampal Lesions. J Neurol Neurosurg Psychiatry. 1957;20:11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen NJ, Squire LR. Preserved Learning and Retention of Pattern-Analyzing Skill in Amnesia: Dissociation of Knowing How and Knowing That. Science. 1980;210:207–210. doi: 10.1126/science.7414331. [DOI] [PubMed] [Google Scholar]
- 3.Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 4.Gutnikov SA, Ma YY, Gaffan D. Temporo-frontal Disconnection Impairs Visual-visual Paired Association Learning but not Configural Learning in Macaca Monkeys. Eur J Neurosci. 1997;9:1524–1529. doi: 10.1111/j.1460-9568.1997.tb01507.x. [DOI] [PubMed] [Google Scholar]
- 5.Farovik A, Dupont LM, Arce M, Eichenbaum H. Medial Prefrontal Cortex Supports Recollection, But Not Familiarity, in the Rat. J Neurosci. 2008;28:13428–13434. doi: 10.1523/JNEUROSCI.3662-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sperling RA, et al. Encoding novel face-name associations: A functional MRI study. Hum Brain Mapp. 2001;14:129–139. doi: 10.1002/hbm.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim H. Neural activity that predicts subsequent memory and forgetting: A meta-analysis of 74 fMRI studies. NeuroImage. 2011;54:2446–2461. doi: 10.1016/j.neuroimage.2010.09.045. [DOI] [PubMed] [Google Scholar]
- 8.Jones MW, Wilson MA. Theta Rhythms Coordinate Hippocampal–Prefrontal Interactions in a Spatial Memory Task. PLoS Biol. 2005;3:e402. doi: 10.1371/journal.pbio.0030402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hyman JM, Zilli EA, Paley AM, Hasselmo ME. Medial prefrontal cortex cells show dynamic modulation with the hippocampal theta rhythm dependent on behavior. Hippocampus. 2005;15:739–749. doi: 10.1002/hipo.20106. [DOI] [PubMed] [Google Scholar]
- 10.Siapas AG, Wilson MA. Coordinated interactions between hippocampal ripples and cortical spindles during slow-wave sleep. Neuron. 1998;21:1123–1128. doi: 10.1016/s0896-6273(00)80629-7. [DOI] [PubMed] [Google Scholar]
- 11.Eichenbaum H, Dudchenko P, Wood E, Shapiro M, Tanila H. The hippocampus, memory, and place cells: Is it spatial memory or a memory space? Neuron. 1999;23:209–226. doi: 10.1016/s0896-6273(00)80773-4. [DOI] [PubMed] [Google Scholar]
- 12.Rainer G, Rao SC, Miller EK. Prospective coding for objects in primate prefrontal cortex. J Neurosci. 1999;19:5493–5505. doi: 10.1523/JNEUROSCI.19-13-05493.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Colgin LL, Moser EI, Moser MB. Understanding memory through hippocampal remapping. Trends Neurosci. 2008;31:469–477. doi: 10.1016/j.tins.2008.06.008. [DOI] [PubMed] [Google Scholar]
- 14.Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- 15.Matsumoto M, Hikosaka O. Lateral habenula as a source of negative reward signals in dopamine neurons. Nature. 2007;447:1111–1115. doi: 10.1038/nature05860. [DOI] [PubMed] [Google Scholar]
- 16.Abe M, et al. Reward Improves Long-Term Retention of a Motor Memory through Induction of Offline Memory Gains. Curr Biol. 2011;21:557–562. doi: 10.1016/j.cub.2011.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bethus I, Tse D, Morris RGM. Dopamine and Memory: Modulation of the Persistence of Memory for Novel Hippocampal NMDA Receptor-Dependent Paired Associates. J Neurosci. 2010;30:1610–1618. doi: 10.1523/JNEUROSCI.2721-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wirth S, et al. Single neurons in the monkey hippocampus and learning of new associations. Science. 2003;300:1578–1581. doi: 10.1126/science.1084324. [DOI] [PubMed] [Google Scholar]
- 19.Bunsey M, Eichenbaum H. Conservation of memory function in rats and humans. Nature. 1996;379:255–257. doi: 10.1038/379255a0. [DOI] [PubMed] [Google Scholar]
- 20.Murray EA, Gaffan D, Mishkin M. Neural substrates of visual stimulus-stimulus association in rhesus monkeys. J Neurosci. 1993;13:4549–4561. doi: 10.1523/JNEUROSCI.13-10-04549.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sakai K, Miyashita Y. Neural organization for the long-term memory of paired associates. Nature. 1991;354:152–155. doi: 10.1038/354152a0. [DOI] [PubMed] [Google Scholar]
- 22.Erickson CA, Desimone R. Responses of macaque perirhinal neurons during and after visual stimulus association learning. J Neurosci. 1999;19:10404–10416. doi: 10.1523/JNEUROSCI.19-23-10404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Messinger A, Squire LR, Zola SM, Albright TD. Neuronal representations of stimulus associations develop in the temporal lobe during learning. Proc Natl Acad Sci. 2001;98:12239–12244. doi: 10.1073/pnas.211431098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eichenbaum H, Sauvage M, Fortin N, Komorowski R, Lipton P. Towards a functional organization of episodic memory in the medial temporal lobe. Neurosci Biobehav Rev. 2012;36:1597–1608. doi: 10.1016/j.neubiorev.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wirth S, et al. Trial Outcome and Associative Learning Signals in the Monkey Hippocampus. Neuron. 2009;61:930–940. doi: 10.1016/j.neuron.2009.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Histed MH, Pasupathy A, Miller EK. Learning Substrates in the Primate Prefrontal Cortex and Striatum: Sustained Activity Related to Successful Actions. Neuron. 2009;63:244–253. doi: 10.1016/j.neuron.2009.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jutras MJ, Fries P, Buffalo EA. Gamma-Band Synchronization in the Macaque Hippocampus and Memory Formation. J Neurosci. 2009;29:12521–12531. doi: 10.1523/JNEUROSCI.0640-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Düzel E, Penny WD, Burgess N. Brain oscillations and memory. Curr Opin Neurobiol. 2010;20:143–149. doi: 10.1016/j.conb.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Fell J, Axmacher N. The role of phase synchronization in memory processes. Nat Rev Neurosci. 2011;12:105–118. doi: 10.1038/nrn2979. [DOI] [PubMed] [Google Scholar]
- 30.Fries P. A mechanism for cognitive dynamics: neuronal communication through neuronal coherence. Trends Cogn Sci. 2005;9:474–480. doi: 10.1016/j.tics.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 31.Buschman TJ, Miller EK. Top-Down Versus Bottom-Up Control of Attention in the Prefrontal and Posterior Parietal Cortices. Science. 2007;315:1860–1862. doi: 10.1126/science.1138071. [DOI] [PubMed] [Google Scholar]
- 32.Siegel M, Warden MR, Miller EK. Phase-dependent neuronal coding of objects in short-term memory. Proc Natl Acad Sci. 2009;106:21341–21346. doi: 10.1073/pnas.0908193106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kopell N, Ermentrout GB, Whittington MA, Traub RD. Gamma rhythms and beta rhythms have different synchronization properties. Proc Natl Acad Sci. 2000;97:1867–1872. doi: 10.1073/pnas.97.4.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Buzsaki G, Draguhn A. Neuronal Oscillations in Cortical Networks. Science. 2004;304:1926–1929. doi: 10.1126/science.1099745. [DOI] [PubMed] [Google Scholar]
- 35.Luu P, Tucker DM, Makeig S. Frontal midline theta and the error-related negativity: neurophysiological mechanisms of action regulation. Clin Neurophysiol. 2004;115:1821–1835. doi: 10.1016/j.clinph.2004.03.031. [DOI] [PubMed] [Google Scholar]
- 36.Buzsáki G, Moser EI. Memory, navigation and theta rhythm in the hippocampal-entorhinal system. Nat Neurosci. 2013;16:130–138. doi: 10.1038/nn.3304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Engel AK, Fries P. Beta-band oscillations—signalling the status quo? Curr Opin Neurobiol. 2010;20:156–165. doi: 10.1016/j.conb.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 38.Dudek SM, Bear MF. Homosynaptic long-term depression in area CA1 of hippocampus and effects of N-methyl-D-aspartate receptor blockade. Proc Natl Acad Sci. 1992;89:4363–4367. doi: 10.1073/pnas.89.10.4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skaggs WE, et al. EEG Sharp Waves and Sparse Ensemble Unit Activity in the Macaque Hippocampus. J Neurophysiol. 2007;98:898–910. doi: 10.1152/jn.00401.2007. [DOI] [PubMed] [Google Scholar]
- 40.Nelson MJ, Pouget P, Nilsen EA, Patten CD, Schall JD. Review of signal distortion through metal microelectrode recording circuits and filters. J Neurosci Methods. 2008;169:141–157. doi: 10.1016/j.jneumeth.2007.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller EK, Gochin PM, Gross CG. Habituation-like decrease in the responses of neurons in inferior temporal cortex of the macaque. Vis Neurosci. 1991;7:357–362. doi: 10.1017/s0952523800004843. [DOI] [PubMed] [Google Scholar]
- 42.Xiang JZ, Brown MW. Neuronal responses related to long-term recognition memory processes in prefrontal cortex. Neuron. 2004;42:817–829. doi: 10.1016/j.neuron.2004.05.013. [DOI] [PubMed] [Google Scholar]
- 43.Yanike M, Wirth S, Smith AC, Brown EN, Suzuki WA. Comparison of Associative Learning-Related Signals in the Macaque Perirhinal Cortex and Hippocampus. Cereb Cortex. 2009;19:1064–1078. doi: 10.1093/cercor/bhn156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manly BFJ. Randomization, bootstrap, and Monte Carlo methods in biology. Chapman & Hall/CRC; 2007. [Google Scholar]
- 45.Torrence C, Compo G. A practical guide to wavelet analysis. Bull Am Meteorol Soc. 1998;79:61–78. [Google Scholar]
- 46.Cui J, Xu L, Bressler SL, Ding M, Liang H. BSMART: A Matlab/C toolbox for analysis of multichannel neural time series. Neural Netw. 2008;21:1094–1104. doi: 10.1016/j.neunet.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: Open Source Software for Advanced Analysis of MEG, EEG, and Invasive Electrophysiological Data. Comput Intell Neurosci. 2011;2011:1–9. doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bokil H, Andrews P, Kulkarni JE, Mehta S, Mitra PP. Chronux: A platform for analyzing neural signals. J Neurosci Methods. 2010;192:146–151. doi: 10.1016/j.jneumeth.2010.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gallistel CR, Fairhurst S, Balsam P. The learning curve: Implications of a quantitative analysis. Proc Natl Acad Sci U S A. 2004;101:13124–13131. doi: 10.1073/pnas.0404965101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zar JH. Biostatistical analysis. Prentice-Hall/Pearson; 2010. [Google Scholar]
- 51.Olejnik S, Algina J. Generalized Eta and Omega Squared Statistics: Measures of Effect Size for Some Common Research Designs. Psychol Methods. 2003;8:434–447. doi: 10.1037/1082-989X.8.4.434. [DOI] [PubMed] [Google Scholar]
- 52.Meyers EM, Kreiman G. Visual Population Codes. MIT Press; 2012. pp. 517–538. [Google Scholar]
- 53.Kalcher J, Pfurtscheller G. Discrimination between phase-locked and non-phase-locked event-related EEG activity. Electroencephalogr Clin Neurophysiol. 1995;94:381–384. doi: 10.1016/0013-4694(95)00040-6. [DOI] [PubMed] [Google Scholar]
- 54.Ding M, Bressler SL, Yang W, Liang H. Short-window spectral analysis of cortical event-related potentials by adaptive multivariate autoregressive modeling: data preprocessing, model validation, and variability assessment. Biol Cybern. 2000;83:35–45. doi: 10.1007/s004229900137. [DOI] [PubMed] [Google Scholar]
- 55.Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999;8:194–208. doi: 10.1002/(SICI)1097-0193(1999)8:4<194::AID-HBM4>3.0.CO;2-C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vinck M, van Wingerden M, Womelsdorf T, Fries P, Pennartz CMA. The pairwise phase consistency: A bias-free measure of rhythmic neuronal synchronization. NeuroImage. 2010;51:112–122. doi: 10.1016/j.neuroimage.2010.01.073. [DOI] [PubMed] [Google Scholar]
- 57.Fisher NI. Statistical analysis of circular data. Univ. Press; 1995. [Google Scholar]
- 58.Baccalá LA, Sameshima K, Takahashi DY. Generalized partial directed coherence; Digital Signal Processing, 2007 15th International Conference on; 2007. pp. 163–166. [Google Scholar]
- 59.Granger CW. Investigating causal relations by econometric models and cross-spectral methods. Econ J Econ Soc. 1969:424–438. [Google Scholar]
- 60.Morf M, Vieira A, Lee DT, Kailath T. Recursive multichannel maximum entropy spectral estimation. Geosci Electron IEEE Trans. 1978;16:85–94. [Google Scholar]
- 61.Geweke J. Measurement of linear dependence and feedback between multiple time series. J Am Stat Assoc. 1982;77:304–313. [Google Scholar]
- 62.Hess Eckhard H, Polt James M. Pupil size as related to interest value of visual stimuli. Science. 1960;132:349–350. doi: 10.1126/science.132.3423.349. [DOI] [PubMed] [Google Scholar]
- 63.Kennerley SW, Wallis JD. Reward-Dependent Modulation of Working Memory in Lateral Prefrontal Cortex. J Neurosci. 2009;29:3259–3270. doi: 10.1523/JNEUROSCI.5353-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nassar MR, et al. Rational regulation of learning dynamics by pupil-linked arousal systems. Nat Neurosci. 2012;15:1040–1046. doi: 10.1038/nn.3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shepherd SV, Lanzilotto M, Ghazanfar AA. Facial Muscle Coordination in Monkeys during Rhythmic Facial Expressions and Ingestive Movements. J Neurosci. 2012;32:6105–6116. doi: 10.1523/JNEUROSCI.6136-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Funahashi S, Bruce CJ, Goldman-Rakic PS, et al. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. J Neurophysiol. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- 67.Cromer JA, Machon M, Miller EK. Rapid association learning in the primate prefrontal cortex in the absence of behavioral reversals. J Cogn Neurosci. 2011;23:1823–1828. doi: 10.1162/jocn.2010.21555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Merletti R, Di Torino P. Standards for reporting EMG data. J Electromyogr Kinesiol. 1999;9:3–4. [Google Scholar]
- 69.Schoffelen JM. Neuronal Coherence as a Mechanism of Effective Corticospinal Interaction. Science. 2005;308:111–113. doi: 10.1126/science.1107027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.