Abstract
Osteoporosis and obesity are both major public health concerns. It has long been considered that these are distinct disorders rarely found in the same individual; however, emerging evidence supports an important interaction between adipose tissue and the skeleton. Whereas overweight per se may augment bone strength, animal studies suggest that the metabolic impairment that accompanies obesity is detrimental to bone. Obesity during childhood, a critical time for bone development, likely has profound and lasting effects on bone strength and fracture risk. This notion has received little attention in children and results are mixed, with studies reporting that bone strength development is enhanced or impaired by obesity. Whether obesity is a risk factor for osteoporosis or childhood bone health, in general, remains an important clinical question. Here, we will focus on clarifying the controversial relationships between childhood obesity and bone strength development, and provide insights into potential mechanisms that may regulate the effect of excess adiposity on bone.
Keywords: Obesity, Children, Fat, Bone, Inflammation, Insulin resistance
1. Introduction
Osteoporosis and obesity are both major public health concerns. Since the 1970s, obesity rates have doubled in adults aged 20 years or older and have tripled in children and adolescents aged 6–19 years (Flegal et al., 2002; Ogden et al., 2006). It was estimated recently that the direct costs associated with obesity in the US is approximately $80 billion per year, representing ~10% of the national health expenditures (Finkelstein et al., 2003). In the realm of osteoporosis, it is estimated that 1 in 2 women and 1 in 3 men over the age of 50 will experience an osteoporotic-related fracture in their lifetime (Johnell and Kanis, 2005). The estimated US healthcare costs associated with osteoporosis amounted to $18 billion in 2002 and is projected to approach $45 billion by 2020 (Melton, 2003). It has long been considered that obesity and osteoporosis are distinct disorders rarely found in the same individual; however, emerging evidence supports an important interaction between adipose tissue and the skeleton (Reid, 2002; Rosen and Bouxsein, 2006).
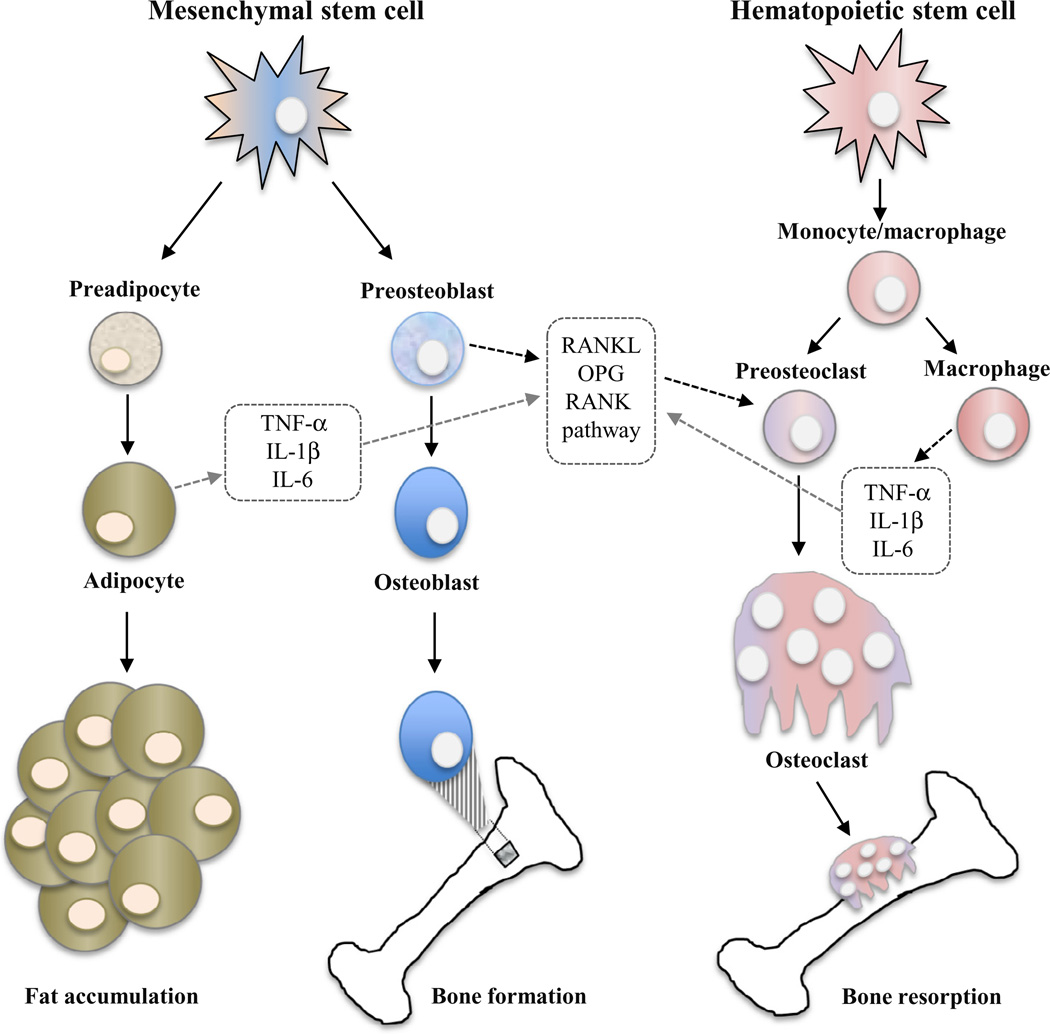
Adipose tissue was once considered just a passive reservoir for energy storage; however, it is now known to play a role in energy metabolism, neuroendocrine function and immune status. Likewise, analyses from cellular and molecular studies also suggest that adipose tissue plays a significant role in bone metabolism (Reid, 2002; Rosen and Bouxsein, 2006). Mechanisms involving bone and fat are intricate by nature, since both adipocytes and osteoblasts originate from mesenchymal stem cells in bone marrow, and factors that stimulate adipogenesis typically inhibit osteoblast differentiation (Rosen and Bouxsein, 2006) (Figure 1). What ultimately determines the fate of bone marrow stem cells is not fully understood and is the subject of ongoing investigation.
Fig. 1.
Interrelationships between fat accumulation, bone formation, and bone resorption. Mechanisms involving fat and bone are intricate by nature, since both adipocytes and osteoblasts originate from mesenchymal stem cells in bone marrow. Osteoclasts originate from monocyte/macrophage precursors of hematopoietic stem cells. Adipocytes and macrophages can secrete several cytokines such as TNF-α, IL-1β, and IL-6, which are capable of stimulating osteoclast activity and bone resorption via regulation of the RANKL/RANK/OPG pathway. OPG, a decoy receptor secreted by preosteoblasts, acts as a decoy receptor to RANKL, and therefore inhibits osteoclast activation and bone re-sorption. TNF-α: tumor necrosis factor-α; IL-1β: interleukin-1β; IL-6: interleukin-6.
Several intracellular signals and phenotype-specific transcription factors have been shown to influence the mesenchymal stem cell into either bone or fat cells. Simplistically, activation of the Wnt/β-Catenin pathway (Piters et al., 2008) and increased expression of transcription regulators such as the Runt-related transcription factor-2 (Runx2) (Ducy et al., 1997), osterix (Nakashima et al., 2002), and Msx2 (Satokata et al., 2000) have all been attributed to promote osteoblastogenesis. Estrogen has been shown to increase bone formation with associated inhibition of fat formation (Dang et al., 2002). On the other hand, members of the CCAAT/enhancer binding protein (C/EBP) family of transcription factors are characterized as regulators of adipogenesis (Tontonoz and Spiegelman, 2008). The transcription factor peroxisomal proliferator-activated receptor gamma (PPAR-γ) is seen as the master regular of fat formation within bone marrow, and activation of PPAR-γ favors differentiation of stem cells into adipocytes rather than osteoblasts (Rzonca et al., 2004).
Clinical investigations have established that a high body weight and obesity are positively correlated with bone mass and that a low body weight or loss of weight is associated with bone loss and fracture risk (Shapses et al., 2011). The greater bone mass in obesity may result from the greater mechanical load on bone due to excess weight or hormones produced by the excess adipose tissue. Furthermore, the regional distribution of fat may influence bone mass independently of obesity (Pollock et al., 2010, 2011a; Tarquini et al., 1997; Warming et al., 2003). Specific bone sites may also be affected differently depending on whether they are load-bearing or by the cortical:trabecular content of a particular bone (Laing et al., 2013; Pollock et al., 2007, 2011b). This weight–bone relationship is not gender specific and it is also found in children, although severe obesity at greater levels of adiposity observed typically only in Western countries may attenuate the positive effect on bone mass and/or bone quality in children (Shapses et al., 2011). Although obesity is associated with a higher bone mass, the impact of excess adiposity on bone quality, especially modification of the trabecular and cortical compartments, presents a more complicated picture that may actually lead to an increase in fracture risk. Thus, whether obesity is a risk factor for suboptimal bone strength development remains an important clinical question. In this review, we will focus on clarifying the controversial relationships between childhood obesity and bone health, and provide insights into potential mechanisms that may mediate the effect of excess fat accumulation on bone.
2. Evaluation of bone health in the developing skeleton
To maintain its functions, bone tissue is constantly turned over by processes referred to as modeling and remodeling, which involves bone resorption by osteoclasts and bone formation by osteoblasts (Einhorn, 1996). Modeling refers to alterations in the shape of bone, whereas remodeling refers to turnover of bone that does not alter its shape. The balance between modeling and remodeling differs between the growing and adult skeleton. In the former, modeling is the dominant mode, whereas in the latter, remodeling is dominant. Modeling, seen in early childhood up to early adulthood, is the process in which bones become larger, heavier, and denser; hence, osteoblastic activity exceeds osteoclastic activity. Bone remodeling begins to take over in adulthood, where bone mass undergoes constant and equal removal of old bone and renewal with newly formed bone (Parfitt, 1982). An equilibrium exists between bone resorption and formation until the fourth or fifth decade of life, when bone resorption begins to supersede the continually declining bone formation process (Frost, 1990). Any disorder or condition that alters bone formation or enhances bone resorption during the lifespan will lead to suboptimal bone turnover dynamics, presumably leading to a greater risk of osteoporotic fracture. Because bone integrity in childhood is likely to have biologically relevant effects on skeletal competence in advanced age (Baxter-Jones et al., 2011), it is important to understand the factors, including obesity, that influence bone in childhood.
Pediatric obesity has reached epidemic proportions globally (Wang and Lobstein, 2006). The dire consequence of childhood obesity is that it increases the risk for metabolic conditions such as hypertension, type 2 diabetes, and cardiovascular diseases (Weiss et al., 2004). Now, there is increasing concern that being overweight may be associated with suboptimal bone strength development, as there is mounting evidence linking childhood obesity to skeletal fractures (Table 1). Inevitably, the potential for increasing adiposity to negatively effect pediatric skeletal integrity is a subject of growing interest.
Table 1.
Pediatric studies evaluating relationship of adiposity and skeletal fractures.
| Study | Population | Obesity definition/adiposity measure |
Bone measure/site and fracture assessment |
Methodological/statistical approach |
Overall study results |
|---|---|---|---|---|---|
| Goulding et al. (1998) | 200 females with (n = 100) and without (n = 100) distal forearm fractures (average time of 47 days post-fracture); age: 3–15 years |
|
|
To compare bone density of forearm fracture cases versus controls, matched for age and height. |
Young females with forearm fractures had greater BMI, higher levels of fat mass, and lower aBMD at all skeletal sites than controls. |
| Goulding et al. (2000) | 170 females with (n = 82) and without (n = 88) prior distal forearm fracture; age: 3–15 years at baseline |
|
|
To determine risk factors for occurrence of new skeletal fractures over a 4-year period. |
In young females, previous forearm fracture, low total body aBMD, low spine aBMD, and high BMI each increase the risk of new skeletal fracture within 4 years. |
| Goulding et al. (2001) | 200 males with (n = 100) and without (n = 100) distal forearm fractures (average time of 4 weeks post-fracture); age: 3–19 years |
|
|
To determine factors associated with forearm fractures in young males. |
Low total body aBMD, low spine aBMD, high BMD, and high adiposity each increased the risk of forearm fracture in young males. |
| Skaggs et al. (2001) | 100 females with (n = 50) and without (n = 50) distal forearm fracture (average time of 4-weeks post- fracture); age: 4–15 years |
|
At the radius, the following CT-derived bone parameters were assessed at the distal site:
|
To compare bone density and bone size of forearm fracture cases versus controls, matched for age, height, weight, and pubertal stage. |
Young females with forearm fractures had a significantly smaller bone size (total CSA) and tend to be more overweight than controls. However, differences in vBMD between groups were not observed |
| Davidson et al. (2003) | 50 males; age: 4–17 years |
|
|
To compare the relative likelihood of forearm fractures between obese (≥95th BMI percentile) and non-obese (<85th BMI percentile) male children and adolescents; matched for age, pubertal stage, and height. |
Obese male children and adolescents were shown to have 1.7 times greater risk of forearm fracture compared to their non- obese counterparts. |
| Goulding et al. (2005) | 90 children and adolescents having experienced ≥2 forearm fractures; age: 5–19 y |
|
|
To determine factors associated with multiple forearm fractures during childhood. |
A greater number of forearm fractures was associated with a higher BMI and lower radial aBMD and BMC. |
| Taylor et al. (2006) | 355 children and adolescents; Age: 12.2 ± 2.8 y |
|
|
Nonoverweight (5–95th BMI percentile) versus overweight (>95th BMI percentile) |
Overweight/obese children and adolescents were found to have greater prevalence of skeletal fractures than nonoverweight peers. |
| Pollack et al. (2008) | 3,232 children and adolescents; age: 9–15 y |
|
|
To compare the relative likelihood of skeletal fractures between overweight/obese and normal weight children and adolescents. |
The risk of skeletal fractures of the lower and upper extremities (due to motor vehicle crashes) was two and half times as great in overweight/obese versus normal-weight children. |
| Dimitri et al. (2009) | 103 children and adolescents; age: 11.7 ± 2.8 y |
|
|
To determine relations between total fat mass and bone parameters in four groups of children and adolescents: obese (≥99th BMI percentile) with and without prior fracture and normal-weight (<85th BMI percentile) with and without prior fracture; statistical control for gender, age, and weight or gender, height and weight or lean mass. |
There was a consistent pattern of differences in BMC, bone area, and aBMD at the skeletal sites according to both obesity and prior skeletal fracture. Specifically, children and adolescents who fracture have smaller bones; the difference is substantially increased in those children and adolescents who have a history of skeletal fracture and are obese. |
| Kessler et al. (2013) | 913,178 children and adolescents; age: 2–19 y |
BMI |
|
To compare the relative likelihood of lower extremity fractures between obese, overweight, and normal weight children and adolescents. |
The odds of foot, ankle, leg, and knee fractures were significantly higher in the overweight and obese children relative to their normal weight peers. There was no association between weight status and increased risk of fractures of the femur and hip. |
| Fornari et al. (2013) | 1,345 children and adolescents; age: 5.0 ± 2.5 y |
BMI |
|
To determine whether obesity status is a risk factor in the type and severity of upper extremity fracture (lateral condyle versus supracondylar humerus). |
Obesity places a child at greater risk for sustaining a lateral condyle fracture versus supracondylar fracture. When these lateral condyle fractures occur, they are often more severe injuries compared to those in nonobese children. |
| Sabhaney et al. (2014) | 2,213 children and adolescents; age: 2–17 y |
BMI |
|
To compare the relative likelihood of skeletal fractures between obese, overweight, and normal weight children and adolescents. |
The odds of fracture were significantly higher in the lower extremity injuries among overweight relative to normal weight children, though an increased odds of lower extremity fracture was not observed among obese children compared with normal weight children. There was no significant association between BMI and odds of fracture with upper extremity injuries or odds of complex fractures. |
BMI, body mass index; DXA, dual-energy X-ray absorptiometry; pQCT, peripheral quantitative computed tomography; CT/QCT, axial quantitative computed tomography; aBMD, areal bone mineral density; BMC, bone mineral content; vBMD, volumetric BMD; CSA, cross-sectional area; trab, trabecular.
Determination of body mass index (BMI) is recommended for classification of overweight and obesity. For children and adolescents, BMI is age- and sex-specific to reference curves such as those published by the Centers for Disease Control and Prevention (CDC) (Kuczmarski et al., 2000) and the World Health Organization (WHO) (de Onis et al., 2007). According to the CDC, a BMI above the 85th and 95th percentiles are considered to be overweight and obese, respectively. The WHO defines obese as a BMI > 3 standard deviations above the WHO growth standard median.
With the emergence of dual energy X-ray absorptiometry (DXA) in the 1990s as a clinical instrument to assess bone mineral density (BMD) and soft tissue mass, great strides were made in furthering our understanding of factors influencing bone health in adults and bone mineral accrual in the growing skeleton. BMD and bone mineral content (BMC) can be measured with precision by DXA at the lumbar spine, total hip, wrist, and whole body. BMD has been applied to establish normal patterns that differ between different race/ethnic groups and that permit the precise and accurate quantification of bone mass according to age, group, and sex (Kanis, 2002). DXA has proven to be a valuable two-dimensional bone imaging technique, but because DXA calculates areal, and not volumetric, BMD and since the bone area does not increase at the same proportion as bone volume during growth, the true BMD of children and adolescents might be overestimated for large bones and underestimated for small bones. Therefore, BMC is recommended as the bone measure to assess bone mass status in children and adolescents (Gordon et al., 2008).
Recently, peripheral quantitative computed tomography (pQCT) was proposed as an imaging tool for the study of both trabecular and cortical bones in adolescents and adults. This proposal is based on the capacity of this method to evaluate volumetric bone density and bone geometry, providing a better understanding of bone strength (Zemel et al., 2008). Although the contrast resolution of pQCT is lower than that of magnetic resonance imaging, the low costs of this method favor its use for the evaluation of large population samples. However, studies are needed to determine reference ranges and measurement sites and to standardize devices and software. Since children and adolescents are in the phase of growth, the exact and most consistent site of measurement needs to be determined (Zemel et al., 2008).
3. Clinical evidence for obesity and bone relationships during childhood
In adults, a high body mass index (BMI) or being obese has long been thought to be protective against osteoporosis and related fractures (Reid, 2002, 2008). Contrary to the notion that obesity may also protect children from fractures, a recent study reviewing medical records of 913,178 children aged 2–19 years reported that moderately obese and extremely obese children had an increased odds ratio (OR, 1.23 and 1.42, respectively, with 95% CI, 1.12–1.35 and 1.26–1.61, respectively) of fracture compared to normal weight children (Kessler et al., 2013). A higher fracture risk in obese children may be the result of greater forces generated during a fall, a lifestyle contraindicative to strong bones, and/or excess fat tissue that impairs bone strength development (Frost, 1997). Given the current state of increasing pediatric obesity along with reports of high fracture rates in obese children, it is vital to understand the effects of adiposity on the skeleton since achieving optimal bone mass and size during growth will presumably reduce the risk of osteoporotic fractures later in life (Hui et al., 2003).
An obligatory level of fat is required for the initiation of skeletal maturation; however, excess adiposity, while associated with increased bone size, may have an adverse effect on bone quality. Across the pubertal years, body fat has been associated with larger bones in boys, and larger and denser bones in girls (Streeter et al., 2013). However, accelerated skeletal growth and greater bone size may not translate into reduced fracture risk (Kessler et al., 2013). In fact, a recent fracture study in females aged 4–15 years reported that those who sustained a fracture were more overweight and had a smaller cross-sectional area at the non-fractured forearm compared to the non-fracture group (Skaggs et al., 2001). Since differences in bone mass and size are apparent between males and females during puberty (Gilsanz et al., 1997), it is possible that gender may affect the association between obesity and bone outcomes. In a study of adolescents, total body fat mass was associated with bone mass in females but not males (Sayers and Tobias, 2010). In a case–control study of children with and without fractures, it was found in girls that the prevalence of overweight/obesity was greater in those presenting with fracture at either upper or lower limb; however, in boys, the prevalence was greater only in those with fracture at the lower limb (Valerio et al., 2012). Race may be another important factor in the bone–fat relationship (Pollock et al., 2011b, 2011c). The National Health and Nutrition Examination Survey, 2003–2006, estimated that the overweight prevalence among black females, aged 12–19 years, was almost double the rate for white females (28% versus 15%) (Ogden et al., 2008). If the effect of obesity on bone is independent of race, skeletal health in this population could be a public health concern.
Determining whether excess adiposity is either beneficial or detrimental to the growing skeleton has been challenging. Whereas some studies report greater bone mass in overweight children and adolescents compared to their healthy weight peers (Clark et al., 2006; Ducher et al., 2009; Leonard et al., 2004), others conclude that obesity is linked to lower bone mass or that extra weight from fat mass had no effect on bone mass (Petit et al., 2005; Pollock et al., 2007, 2011b; Wosje et al., 2009). Discrepancies in these childhood bone–fat investigations may be attributed, in part, to the methodological limitations when comparing bone mass between overweight and healthy weight children of the same age. At any given age, a wide variation exists among children in stature, body composition, rate of growth, and timing and tempo of biological maturation. Since overweight compared to healthy weight children of the same age are generally further advanced in maturation, their skeletal development is likewise more advanced, because of increased hormonal activity than their healthy weight peers. Another limitation of the aforementioned pediatric bone–fat investigations is that biochemical parameters indicative of metabolic abnormalities in the overweight children were not reported. Comorbidities associated with adult obesity, such as hypertension, dyslipidemia, and insulin resistance, also occur in children and adolescents (Weiss et al., 2004), and more importantly, the prevalence of these metabolic abnormalities are increasing alongside childhood obesity (Johnson et al., 2009). Since adult investigations have linked lower bone mass to metabolic syndrome (Hwang and Choi, 2010; von Muhlen et al., 2007) as well as individual components of the metabolic syndrome such as hypercholesterolemia (Tanko et al., 2003) and hyperglycemia (Petit et al., 2010), it is possible that discrepancies in prior pediatric bone–fat studies could have been attributed to metabolic abnormalities present in some but not all overweight subjects. This notion seems plausible given that elevated glucose concentrations have been shown in vitro to inhibit bone mass accrual (Balint et al., 2001) combined with additional evidence suggesting that oxidized lipids direct un-differentiated mesenchymal stem cells toward adipocyte rather than osteoblast differentiation (Parhami et al., 1997).
In an effort to provide better insight into the childhood bone and fat relationship, our research work has focused solely on overweight and obese children with and without metabolic abnormalities (i.e., cardiometabolic risk factors such as high waist circumference, blood pressure, fasting glucose, triglycerides, and low HDL-cholesterol). In our pediatric investigations (Pollock et al., 2010, 2011a, 2011d), metabolic abnormalities were characterized as cardiometabolic risk factors (CMR) and defined according to the National Cholesterol Education Program Adult Treatment Panel III definition modified for age (“American Academy of Pediatrics: Report of the Expert Panel on Blood Cholesterol Levels in Children and Adolescents,” 1992; Cook et al., 2008; Daniels and Greer, 2008). Specifically, these risk factors were defined accordingly: (1) waist circumference ≥90th percentile for age and gender; (2) HDL cholesterol ≤40 mg/dL; (3) triglycerides ≥110 mg/dL; (4) systolic or diastolic blood pressure ≥90th percentile for age, gender, and height; and (5) fasting glucose ≥100 mg/dL. In overweight (OW) adolescents aged 14–18 years, we compared total body bone mass, as measured by DXA, between those with and without cardiometabolic risk factors (CMR) (Figure 2) (Pollock et al., 2011a). We found that compared to OW + Healthy group, total body bone mass was 5.4% lower in the OW + 1CMR group and 6.3% lower in the OW +≥2CMR group. Given that the CMR groups were carrying significantly greater total body fat mass loads (an average of 9-lbs of fat mass in the OW + 1CMR group and 22-lbs of fat mass in the OW +≥2CMR group) than the OW + Healthy group, it appears that excess weight from the fat mass provides no additional weight-loading benefit to bone mass. These findings suggest that if cardiometabolic risk factors are present alongside being overweight, it could have a negative effect on bone. More importantly, it is possible to hypothesize that if the extra weight from fat mass provides no additional influence to bone, an overweight adolescent with metabolic abnormalities may be more susceptible to fracture because traumatic impact forces generally scale with body weight (Davidson et al., 2003; Kim et al., 2013). However, further work is required to establish this connection.
Fig. 2.
Mean (±SE) total body bone mineral content (BMC), bone area, and areal bone mineral density (aBMD) in overweight adolescents with no cardiometabolic risk factors (CMR) (healthy group, n = 55), overweight adolescents with one CMR (1 CMR group, n = 46), and overweight adolescents with two or more CMR (≥2 CMR group, n = 42). a Overall P-value on the basis of analysis of covariance, adjusted for age, sex, race, height and fat-free soft tissue mass. bP < 0.05, significantly different from healthy group (least squares difference adjustment for multiple comparisons). (From J Pediatr, Vol. 158, N.K. Pollock et al., Adolescent obesity, bone mass, and cardiometabolic risk factors, pp. 727–734. Copyright 2011, with permission from Elsevier.)
Even in adults, the associations of metabolic syndrome with bone mass have not been extensively explored, and the results have been mixed. Whereas some authors have reported greater bone mass in adults with metabolic syndrome (Kinjo et al., 2007), others have shown the opposite relationship (Hwang and Choi, 2010; von Muhlen et al., 2007). These conflicting reports may be due, in part, to the heterogeneous samples studied (e.g. age, co-morbid disease status and medication use) and potentially of greater importance, the lack of consideration of the generally larger body size in metabolic syndrome patients compared to controls. If the latter is not considered, the effect of body size on bone mass could lead to misinterpretations when comparing individuals of different stature and body composition.
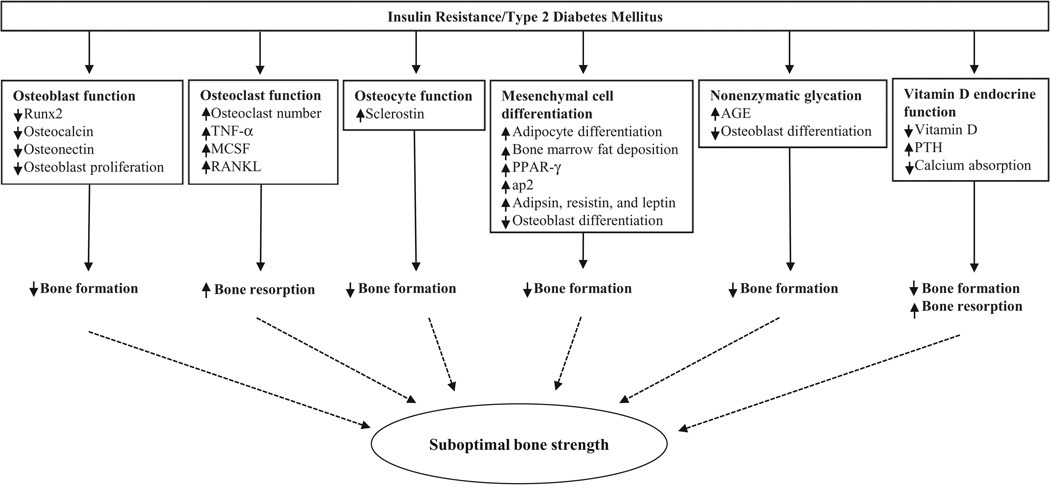
Recent studies have challenged the traditionally accepted view that obesity is beneficial to the growing skeleton (Petit et al., 2005; Pollock et al., 2007, 2011b; Wosje et al., 2009); however, it is apparent that a phenotype, beyond total fat accumulation, is needed to explain the complex relationship between developing bone and obesity. Given that the relationship between type 2 diabetes and osteoporotic fractures is increasingly being recognized (Schwartz and Sellmeyer, 2007), it is possible that insulin resistance may be the culprit linking poor bone health and childhood obesity. In our adolescent study (Pollock et al., 2011a), homeostasis model assessment of insulin resistance (HOMA-IR), a surrogate measure of insulin resistance based on fasting insulin and glucose, explained a significant proportion of the variance in total body bone mass. Thus, it is plausible that insulin resistance may explain, in part, the association between obesity and suboptimal bone mass. Evidence to support this notion was first reported in a cohort of overweight Hispanic-American children in which total body BMC was inversely associated with markers of insulin resistance, as determined by oral glucose tolerance test (Afghani et al., 2005). Similarly, in a recent study of overweight prepubertal children (Pollock et al., 2010), our group observed lower bone mass in the children with pre-diabetes compared to those with normal glucose levels. Taken together, these studies indicate that abnormal glucose regulation has a negative effect on the growing skeleton. The mechanism for the potential negative effect of insulin resistance on bone development is currently unknown; however, some hypotheses include decreased osteoblast function, increased osteoclast and osteocyte functions, excessive nonenzymatic glycation, and alterations in vitamin D endocrine function (Figure 3).
Fig. 3.
Possible pathways leading to suboptimal bone strength due to uncontrolled high glucose levels in conditions of insulin resistance and type 2 diabetes mellitus. The mechanism for how impaired glucose metabolism leads to suboptimal bone strength is still elusive. However, some hypotheses include decreased osteoblast function (i.e., decreasing Runx2, osteocalcin and osteonectin expressions and suppressing osteoblast proliferation), increased osteoclast function (i.e., inducing production of TNF-α, MCSF and RANKL, all of which are osteoblast-derived activators of osteoclast proliferation and differentiation), increased osteocyte function (i.e., increasing sclerostin expression), excessive nonenzymatic glycation (i.e., increasing AGE in the organic bone matrix and suppressing osteoblast differentiation), and alterations in vitamin D endocrine function (i.e., low circulating levels of vitamin D, thereby stimulating production of PTH and reducing calcium absorption). Runx2: Runt-related transcription factor; TNF-α: tumor necrosis factor-α; MCSF: macrophage-colony stimulating factor; RANKL: receptor activator of nuclear factor-κB ligand; PPAR-γ: peroxisomal proliferator-activated receptor gamma; ap2: adipocyte fatty acid binding protein; AGE: advanced glycosylation end-products; PTH: parathyroid hormone.
It is also possible that obesity during childhood promotes both low bone mass accrual and risk for diabetes through events that are mechanistically associated. Recent animal data have uncovered the presence of a “bone-fat-pancreas” axis that regulates energy homeostasis, coordinates energy partitioning between bone and adipose tissue, and impacts insulin sensitivity.
In mice lacking the gene for osteocalcin, a bone-derived protein and well-known biomarker of bone formation, Lee et al. (2007) observed phenotypes of glucose intolerance, insulin resistance, and visceral obesity. When recombinant osteocalcin was administered to the animals, improvements in glucose tolerance and insulin secretion were observed (Lee et al., 2007). In a mouse model lacking the gene for the insulin receptor in osteoblast (Ob-ΔIR), Fulzele et al. (2010) demonstrated lower postnatal bone mass acquisition in the Ob-ΔIR mice compared to controls. Along with decreased bone formation, the Ob-ΔIR mice displayed marked peripheral adiposity and insulin resistance that was accompanied by decreased osteocalcin concentrations. When recombinant osteocalcin was administered in this study, the researchers observed improvements in glucose tolerance and insulin sensitivity (Fulzele et al., 2010). In support of the animal studies linking osteocalcin and insulin sensitivity (Fulzele et al., 2010; Lee et al., 2007; Yoshikawa et al., 2011), we reported associations between higher insulin sensitivity and greater osteocalcin levels in cohorts of obese children (Pollock et al., 2011d) and obese adults (Gower et al., 2013).
These novel relationships described above between osteocalcin and glucose-insulin metabolism appear to be regulated via leptin (Hinoi et al., 2008), an adipocyte-derived hormone that is strongly and positively correlated with fat mass levels (Hamrick and Ferrari, 2008). Animal studies reveal that leptin treatment elicits a bimodal response, where low doses of leptin can stimulate bone formation and prevent bone loss, but higher concentrations of leptin actually suppress bone formation and increase bone resorption (Martin et al., 2007). Thus, one way in which high levels of adiposity may inhibit the accumulation of bone mass during growth is via hyperleptinemia. Though the basic mechanisms underlying this biphasic effect are unclear, they may involve leptin receptor downregulation, which is known to occur with increases in endogenous leptin due to increased food intake or adiposity (Martin et al., 2000). Given that obesity, insulin resistance, and type 2 diabetes are related disorders of energy metabolism, further investigation of the bone-fat-pancreas axis is warranted.
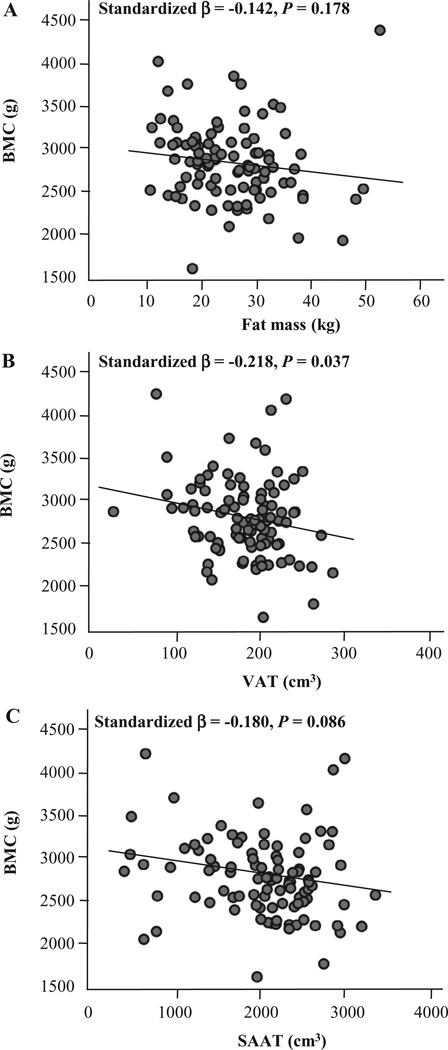
Another significant finding in our pediatric studies was that the influence of adiposity on bone mass may depend on the manner in which the fat mass accumulates (Pollock et al., 2010, 2011a). A limitation of previous pediatric investigations of fat-bone relationships, which were inconclusive, was that most did not investigate the separate influence of total and central adiposity on bone. Given that metabolic abnormalities are more strongly associated with central rather than total adiposity (Fox et al., 2007), it is possible that this type of fat accumulation could affect developing bone distinctly, which could be another potential explanation for the conflicting pediatric data of the bone-fat relationship. In our study of overweight pre-pubertal children (Pollock et al., 2010), we found that both total adiposity (total body fat mass measured by DXA) and central adiposity (visceral adipose tissue measured by magnetic resonance imaging) have significant but opposing relations with total body bone mass. Whereas total body fat had a positive association with bone mass, visceral fat had a negative relationship with bone mass. In our regression analyses, however, it was visceral fat, rather than total body fat, that was the conclusive independent predictor of bone mass. Similarly, this relationship between visceral fat and bone mass was also observed in our adolescent study (Figure 4) (Pollock et al., 2011a). Taken together, it seems that increased central adiposity, which is more clinically relevant for metabolic abnormalities than increased total body adiposity, could play an adverse role in bone health.
Fig. 4.
Relationships between total body bone mineral content (BMC) and total and central adiposity in overweight adolescents. A, Fat mass versus total body BMC; B, visceral adipose tissue (VAT) versus total body BMC; and C, subcutaneous abdominal adipose tissue (SAAT) versus total body BMC. Relationships are adjusted for age, sex, race, height and fat-free soft tissue mass. N = 143. (From J Pediatr, Vol. 158, N.K. Pollock et al., Adolescent obesity, bone mass, and cardiometabolic risk factors, pp. 727–734. Copyright 2011, with permission from Elsevier.)
4. Biologic mechanisms for the bone-fat connection
Osteoblasts and bone marrow adipocytes share a common mesenchymal stromal cell progenitor and, consequently, the abundance of mature cells is not only dependent on the availability of that common progenitor but also on the direction of differentiation toward one lineage, since signals that skew differentiation toward one phenotype can suppress the development of the other (Figure 1). Bone marrow adipocytes share many features of extramedullary adipocytes (i.e., the adipocytes residing in adipose tissue) (Shockley et al., 2009); and therefore, factors that modulate metabolic function and cell fate in peripheral adipose depots may also affect marrow adipocytes. Historically, adipocytes in the marrow were regarded as passive “placeholders” to fill the space not needed for hematopoiesis (Gimble et al., 1996). However, more recently, it has been recognized that these cells play an active role in the marrow, most likely as a source of paracrine factors, as well as being a receptacle for energy storage (Gimble et al., 1996). Consequently, marrow adipocytes may secrete bone-modulating adipokines, such as leptin and adiponectin, directly into the local microenvironment, which influence the function of osteoblasts (Lecka-Czernik, 2012). In addition, recent evidence suggests that bone marrow pre-adipocytes also secrete factors into the bone marrow microenvironment that modulate the lineage commitment of mesenchymal progenitors (Abdallah and Kassem, 2012), influencing the abundance of adipocytes relative to osteoblasts. As a repository of energy storage and expenditure, marrow adipocytes have been found to share characteristics with brown adipocytes, which are primarily involved in thermogenesis and energy dissipation (Krings et al., 2012). Increased marrow adiposity during puberty, when bone mass accrual reaches a peak (Kawai and Rosen, 2010), also supports the notion that marrow adipocytes may act as an energy reservoir. However, although marrow adiposity is associated with increased energy availability, it may also result in a reduction in bone mass, bone formation (Di Iorgi et al., 2008; Justesen et al., 2001; Verma et al., 2002) and bone mineral density (BMD) (Bredella et al., 2011; Shen et al., 2007), with the latter being a strong predictor of fracture risk (Johnell et al., 2005).
4.1. Obesity, inflammation, and insulin resistance
Obesity is frequently associated with chronic systemic inflammation and metabolic alterations, which predispose to the development of type 2 diabetes and cardiovascular disease (Van Gaal et al., 2006). Activation of the immune system and chronic low-grade inflammation may link excessive fat accumulation to obesity-related metabolic dysregulation (Elenkov et al., 2005; Esser et al., 2014; Stofkova, 2009). The activation of the immune system in obesity is not only reflected by higher circulating concentrations of proinflammatory cytokines, but also by infiltration of macrophages and other immune cells in adipose tissue, liver, muscle and pancreas. Immune cell populations shift toward a proinflammatory profile with a production of pro-inflammatory cytokines, which both affect insulin signaling in peripheral tissues and induce β-cell dysfunction and subsequent insulin secretion defect (Esser et al., 2014).
At the level of adipose tissue, systemic inflammation may be initiated by dysfunction of adipocytes (Esser et al., 2014). Adipocyte dysfunction may be caused by intracellular “toxins”, such as ceramides or other lipids (Vandanmagsar et al., 2011), which accumulate in adipose tissue during weight gain. However, not all obese individuals develop chronic systemic inflammation, metabolic or cardiovascular diseases (Stefan et al., 2008). Therefore, there must be factors that protect a subgroup of obese individuals against these obesity-related traits. It has been suggested that heterogeneity in body composition, fat distribution and adipose tissue function maybe more important to determine the individual cardiometabolic risk than body fat mass.
Weight gain leads to the accumulation of adipose tissue by an increase of adipocyte volume (hypertrophy) or number (hyperplasia). The expansion of adipose tissue significantly influences adipocyte biology and subsequently impairs whole-body glucose homeostasis. Hypertrophy of adipocytes is considered a key event associated with a loss of insulin sensitivity both in lean and obese conditions (Cotillard et al., 2014; Salans et al., 1968). Individuals with larger adipocytes typically have elevated proinflammatory factors including leptin, interleukin-1 (IL-6), IL-8, monocyte chemoattractant protein-1 (MCP-1) (Skurk et al., 2007), reduced levels of the insulin-sensitivity related adipokine adiponectin and IL-10 (Kloting et al., 2010; Skurk et al., 2007), and increased basal and catecholamine-stimulated lipolysis (Laurencikiene et al., 2011). In obese individuals, adipocyte hypertrophy seems to be associated with deleterious effects on inflammation and glucose metabolism.
4.2. Inflammation and bone
A recent development in obesity research is the concept that increased adiposity is characterized by greater systemic inflammation. The basis for this notion is that inflammatory-related factors, including various cytokines and acute phase proteins, are increased in obesity, particularly abdominal obesity (Trujillo and Scherer, 2006). As adipocytes and macrophages within fat can secrete cytokines and acute phase proteins, it is considered that increasing visceral fat accumulation contributes to greater production of these inflammatory-related factors, thus leading to chronic low-grade systemic inflammation, a well-known risk factor for cardiovascular disease and diabetes (Kershaw and Flier, 2004).
Our understanding of bone turnover has increased considerably with the discovery of the key molecular drivers of osteoclastogenesis, receptor activator of nuclear factor-κΒ ligand (RANKL) and osteoprotegerin (OPG) (Vega et al., 2007). Osteoblasts have been revealed to regulate osteoclasts activity through the expression of RANKL and OPG (Figure 1). RANKL is expressed on the osteoblast cell surface and binds to its receptor, RANK, on the surface of hematopoietic precursor cells to stimulate osteoclast differentiation and maturation in the presence of macrophage colony stimulation factor (Yasuda et al., 1998). OPG, a decoy receptor secreted by osteoblasts, binds RANKL to prevent activation of RANK, and therefore prevents osteoclast activation and bone resorption (Lacey et al., 1998). Clinically, it has been demonstrated in postmenopausal women that elevated osteoclastic activity and increased bone resorption is positively associated with upregulation of RANKL (Cao et al., 2005; Eghbali-Fatourechi et al., 2003).
Proinflammatory cytokines including TNF-α, IL-1, and IL-6 are key mediators in the process of osteoclast differentiation and bone resorption. Chronic inflammation and increased proinflammatory cytokines induce bone resorption and bone loss in patients with periodontitis (Van Dyke and Serhan, 2003), pancreatitis (Mann et al., 2003), inflammatory bowel disease (Bernstein et al., 2003), and rheumatoid arthritis (Romas et al., 2002). It has also been established that upregulated proinflammatory cytokines are primary mediators of osteopenia or osteoporosis. The accelerated bone loss at menopause is linked to increased production of proinflammatory cytokines including TNF-α, IL 1, and IL-6 (Mundy, 2007). These proinflammatory cytokines are capable of stimulating osteoclast activity through the regulation of the RANKL/RANK/OPG pathway (Khosla, 2001; Pfeilschifter et al., 2002). In mice lacking IL-1β and TNF genes (Vargas et al., 1996) or over-expressing TNF-α decoy receptor (Ammann et al., 1997), ovariectomy did not cause bone loss. Blocking the action of IL-1 with an IL-1 receptor antagonist or the signaling of TNF- with a TNF-binding protein decreased osteoclast formation and bone resorption in ovariectomized mice (Kimble et al., 1995). The significant increase in the development of osteoarthritis in obese human subjects is another evidence that chronic inflammation influences bone metabolism (Anandacoomarasamy et al., 2008).
4.3. Insulin resistance, type 2 diabetes, and bone
Although type 1 diabetes is known to have negative effects on bone metabolism (Albright and Reifenstein, 1948; Schwartz et al., 2001), it was not until recently that type 2 diabetes, which is increasing globally as a consequence of the obesity epidemic, is considered a potential risk for skeletal fractures (Holmberg et al., 2006; Melton et al., 2008). The low bone mass typically associated with type 1 diabetes is thought to contribute to the greater number of skeletal fractures observed in type 1 diabetes patients than in controls (Buysschaert et al., 1992; Hofbauer et al., 2007). Type 2 diabetes patients, on the other hand, are generally characterized with high BMC and BMD (Buysschaert et al., 1992; van Daele et al., 1995), although reduced bone size and structure, assessed by 3-dimensional bone imaging, have recently been reported in type 2 diabetic adult men (Petit et al., 2010). This finding, along with animal work (Ahmad et al., 2003), suggests that increased bone fragility in type 2 diabetes may not be discerned as much from bone mass as other aspects of skeletal strength such as bone size and structure. The exact underlying mechanism for how impaired glucose metabolism leads to suboptimal bone strength is still elusive; however, some hypotheses include decreased osteoblast function, increased osteoclast and osteocyte functions, excessive nonenzymatic glycation, and alterations in vitamin D endocrine function (Figure 3).
Co-culture and animal studies have revealed that uncontrolled high glucose levels may have direct and indirect deleterious effects on osteoblast function and bone formation. At the cellular level, a recent in vitro study in human osteoblast-like MG-63 cells demonstrated that a hyperglycemic condition suppressed cell growth, mineralization, and expression of various osteogenic markers including Runx2, type I collagen, osteocalcin and osteonectin, but inversely promoted expression of adipogenic markers such as PPAR-γ, adipocyte fatty acid binding protein, resistin, and adipsin (Wang et al., 2010). Consistent with the in vitro findings, a histomorphometric analysis in streptozotocin-induced diabetic mice showed increases in osteoclast numbers and expression of osteoclastogenic mediators, including TNF-α, macrophage colony stimulating factor (MCSF), and RANKL (Kayal et al., 2007). Moreover, there were upregulations of PPAR-γ, adipocyte fatty acid binding protein, and resistin mRNAs, as well as increases in lipid-dense adipocytes in the bone marrow of tibias of these streptozotocin-induced diabetic mice (Botolin et al., 2005). It is thus plausible that, in addition to direct interference with osteoblast function and bone formation, insulin resistance and type 2 diabetes may also induce lipid accumulation in the marrow of long bones, thereby leading to expansion of the bone marrow cavity and thinning of the cortical envelope to make the bone more fragile. In addition, the osteoblast to adipocyte shift may also reduce the number of differentiated osteoblasts available for bone formation.
Uncontrolled high glucose levels in conditions of insulin resistance and type 2 diabetes lead to accumulation of advanced glycosylation end-products (AGE) in the organic bone matrix by a process known as nonenzymatic glycation (Monnier et al., 1984; Vashishth, 2007). Hemoglobin A1c is a common example of an early-stage glycation product. The production of AGE are associated with osteoblast apoptosis and inhibition of osteoblast differentiation, thus leading to reduced osteoblast function (Alikhani et al., 2007). In contrast to normal enzymatic cross-linking in collagen (with pyridinoline, for example), which gives bone its toughness and scaffolding properties, AGE crosslinks lead to biomechanically more brittle bone that has lost its toughness and is less able to deform before fracturing (Tang et al., 2009). Pentosidine, when measured in urine, was associated with a 42% increase in clinical fracture incidence in type 2 diabetes (Schwartz et al., 2009), and when measured in serum was increased in patients with type 2 diabetes who had vertebral fractures (Yamamoto et al., 2008). These data suggest that increased levels of AGE may render bone more fragile in conditions of insulin resistance and type 2 diabetes.
Bone mass and strength at any particular time reflects the bone turnover balance between bone formation and resorption. Increased AGEs may weaken bone by decreasing bone formation. There is evidence suggesting that AGEs interfere with normal osteoblast development (Kume et al., 2005) and function (Sanguineti et al., 2008). In addition, reduced bone formation also works in the opposite direction to further increase AGE, as, for example, with high bisphosphonate dosages (Tang et al., 2009). Clinical data have shown that bone turnover dynamics is reduced in type 2 diabetes, with a disproportionate reduction in bone formation (Dobnig et al., 2006;
Gerdhem et al., 2005). Bone formation markers measured in serum have generally been reduced in conditions of prediabetes (Pollock et al., 2011d) and type 2 diabetes (Kanazawa et al., 2009; Shu et al., 2012), although it still has not been established definitively that abnormal glucose metabolism is characterized by low bone formation. It is thought that the link between bone formation and glucose metabolism is regulated via leptin, an adipocyte-derived hormone. It has been shown that as leptin levels increase, there is a subsequent decrease in bone formation, which in turn depresses insulin sensitivity and secretion (Hinoi et al., 2009). Other data suggest that sclerostin, an osteocyte-derived protein with anti-anabolic effects on bone formation, is increased in type 2 diabetes (Gennari et al., 2012), although no relationships were found between circulating sclerostin levels and serum markers of bone formations. Interestingly, the known inverse relationship between parathyroid hormone (PTH) and sclerostin was not observed (Gennari et al., 2012), perhaps suggesting that there may be an absence in type 2 diabetes of the usual inhibitory effect of PTH on sclerostin production.
Circulating vitamin D levels are inversely correlated to insulin resistance and significantly lower in patients with type 2 diabetes (Joergensen et al., 2010; Parikh et al., 2012). Vitamin D is an essential factor of bone and muscle activities because vitamin D deficiency stimulates the production of PTH, which is a negative regulator of osteoblast functioning but a positive regulator of osteoclast functioning which then in turn reduces bone formation and increases bone resorption, respectively (Bouillon, 1991; Fowlkes et al., 2011). Type 2 diabetes-related vitamin D deficiency may also lead to reduced muscle strength because it lowers the rate of calcium absorption by the intestine and thereby reduces the activity of muscle (Gao et al., 2008; McNair et al., 1979), which may be a risk factor of bone fractures through increasing the rate of falling.
The mechanisms for suboptimal bone strength and increased fracture risk in obesity-related diabetes appear to include both material and structural abnormalities. These abnormalities could be interrelated since distorted collagen is also likely poorly mineralized. An overly glycated collagen matrix, confounded by reduced osteo-blast differentiation, in the setting of increased cortical porosity, may lead to compromised biomechanical competence. Because the extent and the nature of collagen crosslinking are significant contributors to bone matrix quality, it is important to conduct additional studies to determine independent contributions to collagen and, ultimately, bone strength.
5. Conclusion
The effect of obesity on childhood bone development has emerged as an important area of investigation, as obese children have been a population over-represented in fracture groups of children. Although it has become clear that body mass is a significant determinant of bone mass and bone quality in children and adolescents, the influence of fat (particularly excessive amounts of fat) on bone during critical stages of bone strength development remains uncertain. Peak bone strength is a major determinant of bone health and fracture risk in later life and so there is a clear need for longitudinal studies covering pediatric populations to identify key factors regulating bone development and the relative changes in the relationship between fat and bone during growth. This knowledge will provide critical information needed to maximize potential therapeutic interventions to counter the linked risks of obesity and osteoporosis, both major public health concerns.
Acknowledgements
Studies contributing to data reviewed herein were supported by Grants R01 DK60692, R01 HL 87923-03S1, P01 AG036675, and P30 DK056336 from the National Institutes of Health.
References
- Abdallah BM, Kassem M. New factors controlling the balance between osteoblastogenesis and adipogenesis. Bone. 2012;50(2):540–545. doi: 10.1016/j.bone.2011.06.030. [DOI] [PubMed] [Google Scholar]
- Afghani A, Cruz ML, Goran MI. Impaired glucose tolerance and bone mineral content in overweight latino children with a family history of type 2 diabetes. Diabetes Care. 2005;28(2):372–378. doi: 10.2337/diacare.28.2.372. [DOI] [PubMed] [Google Scholar]
- Ahmad T, Ohlsson C, Saaf M, Ostenson CG, Kreicbergs A. Skeletal changes in type-2 diabetic Goto-Kakizaki rats. J. Endocrinol. 2003;178(1):111–116. doi: 10.1677/joe.0.1780111. [DOI] [PubMed] [Google Scholar]
- Albright F, Reifenstein E. The Parathyroid Glands and Metabolic Bone Disease; Selected Studies. Baltimore, MD: Williams & Williams; 1948. [Google Scholar]
- Alikhani M, Alikhani Z, Boyd C, MacLellan CM, Raptis M, Liu R, et al. Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone. 2007;40(2):345–353. doi: 10.1016/j.bone.2006.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Academy of Pediatrics. National cholesterol education program: report of the expert panel on blood cholesterol levels in children and adolescents. Pediatrics. 1992;89(3 Pt 2):525–584. [PubMed] [Google Scholar]
- Ammann P, Rizzoli R, Bonjour JP, Bourrin S, Meyer JM, Vassalli P, et al. Transgenic mice expressing soluble tumor necrosis factor-receptor are protected against bone loss caused by estrogen deficiency. J. Clin. Invest. 1997;99(7):1699–1703. doi: 10.1172/JCI119333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anandacoomarasamy A, Caterson I, Sambrook P, Fransen M, March L. The impact of obesity on the musculoskeletal system. Int. J. Obes. (Lond) 2008;32(2):211–222. doi: 10.1038/sj.ijo.0803715. [DOI] [PubMed] [Google Scholar]
- Balint E, Szabo P, Marshall CF, Sprague SM. Glucose-induced inhibition of in vitro bone mineralization. Bone. 2001;28(1):21–28. doi: 10.1016/s8756-3282(00)00426-9. [DOI] [PubMed] [Google Scholar]
- Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J. Bone Miner. Res. 2011;26(8):1729–1739. doi: 10.1002/jbmr.412. [DOI] [PubMed] [Google Scholar]
- Bernstein CN, Leslie WD, Taback SP. Bone density in a population-based cohort of premenopausal adult women with early onset inflammatory bowel disease. Am. J. Gastroenterol. 2003;98(5):1094–1100. doi: 10.1111/j.1572-0241.2003.07415.x. [DOI] [PubMed] [Google Scholar]
- Botolin S, Faugere MC, Malluche H, Orth M, Meyer R, McCabe LR. Increased bone adiposity and peroxisomal proliferator-activated receptor-gamma2 expression in type I diabetic mice. Endocrinology. 2005;146(8):3622–3631. doi: 10.1210/en.2004-1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouillon R. Diabetic bone disease. Calcif. Tissue Int. 1991;49(3):155–160. doi: 10.1007/BF02556109. [DOI] [PubMed] [Google Scholar]
- Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, et al. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring) 2011;19(1):49–53. doi: 10.1038/oby.2010.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysschaert M, Cauwe F, Jamart J, Brichant C, De Coster P, Magnan A, et al. Proximal femur density in type 1 and 2 diabetic patients. Diabete Metab. 1992;18(1):32–37. [PubMed] [Google Scholar]
- Cao JJ, Wronski TJ, Iwaniec U, Phleger L, Kurimoto P, Boudignon B, et al. Aging increases stromal/osteoblastic cell-induced osteoclastogenesis and alters the osteoclast precursor pool in the mouse. J. Bone Miner. Res. 2005;20(9):1659–1668. doi: 10.1359/JBMR.050503. [DOI] [PubMed] [Google Scholar]
- Clark EM, Ness AR, Tobias JH. Adipose tissue stimulates bone growth in prepubertal children. J. Clin. Endocrinol. Metab. 2006;91(7):2534–2541. doi: 10.1210/jc.2006-0332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook S, Auinger P, Li C, Ford ES. Metabolic syndrome rates in United States adolescents, from the National Health and Nutrition Examination Survey, 1999–2002. J. Pediatr. 2008;152(2):165–170. doi: 10.1016/j.jpeds.2007.06.004. [DOI] [PubMed] [Google Scholar]
- Cotillard A, Poitou C, Torcivia A, Bouillot JL, Dietrich A, Kloting N, et al. Adipocyte size threshold matters: link with risk of type 2 diabetes and improved insulin resistance after gastric bypass. J. Clin. Endocrinol. Metab. 2014;99(8):E1466–E1470. doi: 10.1210/jc.2014-1074. [DOI] [PubMed] [Google Scholar]
- de Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO growth reference for school-aged children and adolescents. Bull. World Health Organ. 2007;85(9):660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang ZC, van Bezooijen RL, Karperien M, Papapoulos SE, Lowik CW. Exposure of KS483 cells to estrogen enhances osteogenesis and inhibits adipogenesis. J. Bone Miner. Res. 2002;17(3):394–405. doi: 10.1359/jbmr.2002.17.3.394. [DOI] [PubMed] [Google Scholar]
- Daniels SR, Greer FR. Lipid screening and cardiovascular health in childhood. Pediatrics. 2008;122(1):198–208. doi: 10.1542/peds.2008-1349. [DOI] [PubMed] [Google Scholar]
- Davidson PL, Goulding A, Chalmers DJ. Biomechanical analysis of arm fracture in obese boys. J. Paediatr. Child Health. 2003;39(9):657–664. doi: 10.1046/j.1440-1754.2003.00243.x. [DOI] [PubMed] [Google Scholar]
- Di Iorgi N, Rosol M, Mittelman SD, Gilsanz V. Reciprocal relation between marrow adiposity and the amount of bone in the axial and appendicular skeleton of young adults. J. Clin. Endocrinol. Metab. 2008;93(6):2281–2286. doi: 10.1210/jc.2007-2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimitri P, Wales J, Bishop N. Fat and bone in children – differential effects of obesity on bone size and mass according to fracture history. J. Bone Miner. Res. 2009;25:527–536. doi: 10.1359/jbmr.090823. [DOI] [PubMed] [Google Scholar]
- Dobnig H, Piswanger-Solkner JC, Roth M, Obermayer-Pietsch B, Tiran A, Strele A, et al. Type 2 diabetes mellitus in nursing home patients: effects on bone turnover, bone mass, and fracture risk. J. Clin. Endocrinol. Metab. 2006;91(9):3355–3363. doi: 10.1210/jc.2006-0460. [DOI] [PubMed] [Google Scholar]
- Ducher G, Bass SL, Naughton GA, Eser P, Telford RD, Daly RM. Overweight children have a greater proportion of fat mass relative to muscle mass in the upper limbs than in the lower limbs: implications for bone strength at the distal forearm. Am. J. Clin. Nutr. 2009;90(4):1104–1111. doi: 10.3945/ajcn.2009.28025. [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89(5):747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Eghbali-Fatourechi G, Khosla S, Sanyal A, Boyle WJ, Lacey DL, Riggs BL. Role of RANK ligand in mediating increased bone resorption in early postmenopausal women. J. Clin. Invest. 2003;111(8):1221–1230. doi: 10.1172/JCI17215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einhorn T. The Bone Organ System: Form and Function. San Diego, CA: Academic Press; 1996. [Google Scholar]
- Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP. Cytokine dysregulation, inflammation and well-being. Neuroimmunomodulation. 2005;12(5):255–269. doi: 10.1159/000087104. [DOI] [PubMed] [Google Scholar]
- Esser N, Legrand-Poels S, Piette J, Scheen AJ, Paquot N. Inflammation as a link between obesity, metabolic syndrome and type 2 diabetes. Diabetes Res. Clin. Pract. 2014;105(2):141–150. doi: 10.1016/j.diabres.2014.04.006. [DOI] [PubMed] [Google Scholar]
- Finkelstein EA, Fiebelkorn IC, Wang G. National medical spending attributable to overweight and obesity: how much, and who’s paying? Health Aff. (Millwood) 2003:W3-219–W3-226. doi: 10.1377/hlthaff.w3.219. Suppl Web Exclusives. [DOI] [PubMed] [Google Scholar]
- Flegal KM, Carroll MD, Ogden CL, Johnson CL. Prevalence and trends in obesity among US adults, 1999–2000. JAMA. 2002;288(14):1723–1727. doi: 10.1001/jama.288.14.1723. [DOI] [PubMed] [Google Scholar]
- Fornari ED, Suszter M, Roocroft J, Bastrom T, Edmonds EW, Schlechter J. Childhood obesity as a risk factor for lateral condyle fractures over supracondylar humerus fractures. Clin. Orthop. Relat. Res. 2013;471:1193–1198. doi: 10.1007/s11999-012-2566-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowlkes JL, Bunn RC, Cockrell GE, Clark LM, Wahl EC, Lumpkin CK, et al. Dysregulation of the intrarenal vitamin D endocytic pathway in a nephropathy-prone mouse model of type 1 diabetes. Exp. Diabetes Res. 2011;2011:269378. doi: 10.1155/2011/269378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox CS, Massaro JM, Hoffmann U, Pou KM, Maurovich-Horvat P, Liu CY, et al. Abdominal visceral and subcutaneous adipose tissue compartments: association with metabolic risk factors in the Framingham Heart Study. Circulation. 2007;116(1):39–48. doi: 10.1161/CIRCULATIONAHA.106.675355. [DOI] [PubMed] [Google Scholar]
- Frost HM. Skeletal structural adaptations to mechanical usage (SATMU): 2. Redefining Wolff’s law: the remodeling problem. Anat. Rec. 1990;226(4):414–422. doi: 10.1002/ar.1092260403. [DOI] [PubMed] [Google Scholar]
- Frost HM. Obesity, and bone strength and “mass”: a tutorial based on insights from a new paradigm. Bone. 1997;21(3):211–214. doi: 10.1016/s8756-3282(97)00124-5. [DOI] [PubMed] [Google Scholar]
- Fulzele K, Riddle RC, DiGirolamo DJ, Cao X, Wan C, Chen D, et al. Insulin receptor signaling in osteoblasts regulates postnatal bone acquisition and body composition. Cell. 2010;142(2):309–319. doi: 10.1016/j.cell.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y, Ordas R, Klein JD, Price SR. Regulation of caspase-3 activity by insulin in skeletal muscle cells involves both PI3-kinase and MEK-1/2. J. Appl. Physiol. 2008;105(6):1772–1778. doi: 10.1152/japplphysiol.90636.2008. (1985) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gennari L, Merlotti D, Valenti R, Ceccarelli E, Ruvio M, Pietrini MG, et al. Circulating sclerostin levels and bone turnover in type 1 and type 2 diabetes. J. Clin. Endocrinol. Metab. 2012;97(5):1737–1744. doi: 10.1210/jc.2011-2958. [DOI] [PubMed] [Google Scholar]
- Gerdhem P, Isaksson A, Akesson K, Obrant KJ. Increased bone density and decreased bone turnover, but no evident alteration of fracture susceptibility in elderly women with diabetes mellitus. Osteoporos. Int. 2005;16(12):1506–1512. doi: 10.1007/s00198-005-1877-5. [DOI] [PubMed] [Google Scholar]
- Gilsanz V, Kovanlikaya A, Costin G, Roe TF, Sayre J, Kaufman F. Differential effect of gender on the sizes of the bones in the axial and appendicular skeletons. J. Clin. Endocrinol. Metab. 1997;82(5):1603–1607. doi: 10.1210/jcem.82.5.3942. [DOI] [PubMed] [Google Scholar]
- Gimble JM, Robinson CE, Wu X, Kelly KA. The function of adipocytes in the bone marrow stroma: an update. Bone. 1996;19(5):421–428. doi: 10.1016/s8756-3282(96)00258-x. [DOI] [PubMed] [Google Scholar]
- Gordon CM, Bachrach LK, Carpenter TO, Crabtree N, El-Hajj Fuleihan G, Kutilek S, et al. Dual energy X-ray absorptiometry interpretation and reporting in children and adolescents: the 2007 ISCD Pediatric Official Positions. J. Clin. Densitom. 2008;11(1):43–58. doi: 10.1016/j.jocd.2007.12.005. [DOI] [PubMed] [Google Scholar]
- Goulding A, Cannan R, Williams SM, Gold EJ, Taylor RW, Lewis-Barned NJ. Bone mineral density in girls with forearm fractures. J. Bone Miner. Res. 1998;13:143–148. doi: 10.1359/jbmr.1998.13.1.143. [DOI] [PubMed] [Google Scholar]
- Goulding A, Jones IE, Taylor RW, Manning PJ, Williams SM. More broken bones: a 4-year double cohort study of young girls with and without distal forearm fractures. J. Bone Miner. Res. 2000;15:2011–2018. doi: 10.1359/jbmr.2000.15.10.2011. [DOI] [PubMed] [Google Scholar]
- Goulding A, Jones IE, Taylor RW, Williams SM, Manning PJ. Bone mineral density and body composition in boys with distal forearm fractures: a dual-energy x-ray absorptiometry study. J. Pediatr. 2001;139:509–515. doi: 10.1067/mpd.2001.116297. [DOI] [PubMed] [Google Scholar]
- Goulding A, Grant AM, Williams SM. Bone and body composition of children and adolescents with repeated forearm fractures. J. Bone Miner. Res. 2005;20:2090–2096. doi: 10.1359/JBMR.050820. [DOI] [PubMed] [Google Scholar]
- Gower BA, Pollock NK, Casazza K, Clemens TL, Goree LL, Granger WM. Associations of total and undercarboxylated osteocalcin with peripheral and hepatic insulin sensitivity and beta-cell function in overweight adults. J. Clin. Endocrinol. Metab. 2013;98(7):E1173–E1180. doi: 10.1210/jc.2013-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamrick MW, Ferrari SL. Leptin and the sympathetic connection of fat to bone. Osteoporos. Int. 2008;19(7):905–912. doi: 10.1007/s00198-007-0487-9. [DOI] [PubMed] [Google Scholar]
- Hinoi E, Gao N, Jung DY, Yadav V, Yoshizawa T, Myers MG, Jr., et al. The sympathetic tone mediates leptin’s inhibition of insulin secretion by modulating osteocalcin bioactivity. J. Cell Biol. 2008;183(7):1235–1242. doi: 10.1083/jcb.200809113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinoi E, Gao N, Jung DY, Yadav V, Yoshizawa T, Kajimura D, et al. An osteoblast-dependent mechanism contributes to the leptin regulation of insulin secretion. Ann. N. Y. Acad. Sci. 2009;1173(Suppl. 1):E20–E30. doi: 10.1111/j.1749-6632.2009.05061.x. [DOI] [PubMed] [Google Scholar]
- Hofbauer LC, Brueck CC, Singh SK, Dobnig H. Osteoporosis in patients with diabetes mellitus. J. Bone Miner. Res. 2007;22(9):1317–1328. doi: 10.1359/jbmr.070510. [DOI] [PubMed] [Google Scholar]
- Holmberg AH, Johnell O, Nilsson PM, Nilsson J, Berglund G, Akesson K. Risk factors for fragility fracture in middle age. A prospective population-based study of 33,000 men and women. Osteoporos. Int. 2006;17(7):1065–1077. doi: 10.1007/s00198-006-0137-7. [DOI] [PubMed] [Google Scholar]
- Hui SL, Dimeglio LA, Longcope C, Peacock M, McClintock R, Perkins AJ, et al. Difference in bone mass between black and white American children: attributable to body build, sex hormone levels, or bone turnover? J. Clin. Endocrinol. Metab. 2003;88(2):642–649. doi: 10.1210/jc.2002-020653. [DOI] [PubMed] [Google Scholar]
- Hwang DK, Choi HJ. The relationship between low bone mass and metabolic syndrome in Korean women. Osteoporos. Int. 2010;21(3):425–431. doi: 10.1007/s00198-009-0990-2. [DOI] [PubMed] [Google Scholar]
- Joergensen C, Gall MA, Schmedes A, Tarnow L, Parving HH, Rossing P. Vitamin D levels and mortality in type 2 diabetes. Diabetes Care. 2010;33(10):2238–2243. doi: 10.2337/dc10-0582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnell O, Kanis J. Epidemiology of osteoporotic fractures. Osteoporos. Int. 2005;16(Suppl. 2):S3–S7. doi: 10.1007/s00198-004-1702-6. [DOI] [PubMed] [Google Scholar]
- Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, et al. Predictive value of BMD for hip and other fractures. J. Bone Miner. Res. 2005;20(7):1185–1194. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- Johnson WD, Kroon JJ, Greenway FL, Bouchard C, Ryan D, Katzmarzyk PT. Prevalence of risk factors for metabolic syndrome in adolescents: National Health and Nutrition Examination Survey (NHANES), 2001–2006. Arch. Pediatr. Adolesc. Med. 2009;163(4):371–377. doi: 10.1001/archpediatrics.2009.3. [DOI] [PubMed] [Google Scholar]
- Justesen J, Stenderup K, Ebbesen EN, Mosekilde L, Steiniche T, Kassem M. Adipocyte tissue volume in bone marrow is increased with aging and in patients with osteoporosis. Biogerontology. 2001;2(3):165–171. doi: 10.1023/a:1011513223894. [DOI] [PubMed] [Google Scholar]
- Kanazawa I, Yamaguchi T, Yamamoto M, Yamauchi M, Kurioka S, Yano S, et al. Serum osteocalcin level is associated with glucose metabolism and atherosclerosis parameters in type 2 diabetes mellitus. J. Clin. Endocrinol. Metab. 2009;94(1):45–49. doi: 10.1210/jc.2008-1455. [DOI] [PubMed] [Google Scholar]
- Kanis JA. Diagnosis of osteoporosis and assessment of fracture risk. Lancet. 2002;359(9321):1929–1936. doi: 10.1016/S0140-6736(02)08761-5. [DOI] [PubMed] [Google Scholar]
- Kawai M, Rosen CJ. PPARgamma: a circadian transcription factor in adipogenesis and osteogenesis. Nat. Rev. Endocrinol. 2010;6(11):629–636. doi: 10.1038/nrendo.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayal RA, Tsatsas D, Bauer MA, Allen B, Al-Sebaei MO, Kakar S, et al. Diminished bone formation during diabetic fracture healing is related to the premature resorption of cartilage associated with increased osteoclast activity. J. Bone Miner. Res. 2007;22(4):560–568. doi: 10.1359/jbmr.070115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J. Clin. Endocrinol. Metab. 2004;89(6):2548–2556. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- Kessler J, Koebnick C, Smith N, Adams A. Childhood obesity is associated with increased risk of most lower extremity fractures. Clin. Orthop. Relat. Res. 2013;471(4):1199–1207. doi: 10.1007/s11999-012-2621-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khosla S. Minireview: the OPG/RANKL/RANK system. Endocrinology. 2001;142(12):5050–5055. doi: 10.1210/endo.142.12.8536. [DOI] [PubMed] [Google Scholar]
- Kim JE, Hsieh MH, Soni BK, Zayzafoon M, Allison DB. Childhood obesity as a risk factor for bone fracture: a mechanistic study. Obesity (Silver Spring) 2013;21(7):1459–1466. doi: 10.1002/oby.20355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble RB, Matayoshi AB, Vannice JL, Kung VT, Williams C, Pacifici R. Simultaneous block of interleukin-1 and tumor necrosis factor is required to completely prevent bone loss in the early postovariectomy period. Endocrinology. 1995;136(7):3054–3061. doi: 10.1210/endo.136.7.7789332. [DOI] [PubMed] [Google Scholar]
- Kinjo M, Setoguchi S, Solomon DH. Bone mineral density in adults with the metabolic syndrome: analysis in a population-based U.S. sample. J. Clin. Endocrinol. Metab. 2007;92(11):4161–4164. doi: 10.1210/jc.2007-0757. [DOI] [PubMed] [Google Scholar]
- Kloting N, Fasshauer M, Dietrich A, Kovacs P, Schon MR, Kern M, et al. Insulin-sensitive obesity. Am. J. Physiol. Endocrinol. Metab. 2010;299(3):E506–E515. doi: 10.1152/ajpendo.00586.2009. [DOI] [PubMed] [Google Scholar]
- Krings A, Rahman S, Huang S, Lu Y, Czernik PJ, Lecka-Czernik B. Bone marrow fat has brown adipose tissue characteristics, which are attenuated with aging and diabetes. Bone. 2012;50(2):546–552. doi: 10.1016/j.bone.2011.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, et al. CDC growth charts: United States. Adv. Data. 2000;314:1–27. [PubMed] [Google Scholar]
- Kume S, Kato S, Yamagishi S, Inagaki Y, Ueda S, Arima N, et al. Advanced glycation end-products attenuate human mesenchymal stem cells and prevent cognate differentiation into adipose tissue, cartilage, and bone. J. Bone Miner. Res. 2005;20(9):1647–1658. doi: 10.1359/JBMR.050514. [DOI] [PubMed] [Google Scholar]
- Lacey DL, Timms E, Tan HL, Kelley MJ, Dunstan CR, Burgess T, et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell. 1998;93(2):165–176. doi: 10.1016/s0092-8674(00)81569-x. [DOI] [PubMed] [Google Scholar]
- Laing EM, Tripp RA, Pollock NK, Baile CA, Della-Fera MA, Rayalam S, et al. Adenovirus 36, adiposity, and bone strength in late-adolescent females. J. Bone Miner. Res. 2013;28(3):489–496. doi: 10.1002/jbmr.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurencikiene J, Skurk T, Kulyte A, Heden P, Astrom G, Sjolin E, et al. Regulation of lipolysis in small and large fat cells of the same subject. J. Clin. Endocrinol. Metab. 2011;96(12):E2045–E2049. doi: 10.1210/jc.2011-1702. [DOI] [PubMed] [Google Scholar]
- Lecka-Czernik B. Marrow fat metabolism is linked to the systemic energy metabolism. Bone. 2012;50(2):534–539. doi: 10.1016/j.bone.2011.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee NK, Sowa H, Hinoi E, Ferron M, Ahn JD, Confavreux C, et al. Endocrine regulation of energy metabolism by the skeleton. Cell. 2007;130(3):456–469. doi: 10.1016/j.cell.2007.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard MB, Shults J, Wilson BA, Tershakovec AM, Zemel BS. Obesity during childhood and adolescence augments bone mass and bone dimensions. Am. J. Clin. Nutr. 2004;80(2):514–523. doi: 10.1093/ajcn/80.2.514. [DOI] [PubMed] [Google Scholar]
- Mann ST, Stracke H, Lange U, Klor HU, Teichmann J. Alterations of bone mineral density and bone metabolism in patients with various grades of chronic pancreatitis. Metabolism. 2003;52(5):579–585. doi: 10.1053/meta.2003.50112. [DOI] [PubMed] [Google Scholar]
- Martin A, David V, Malaval L, Lafage-Proust MH, Vico L, Thomas T. Opposite effects of leptin on bone metabolism: a dose-dependent balance related to energy intake and insulin-like growth factor-I pathway. Endocrinology. 2007;148(7):3419–3425. doi: 10.1210/en.2006-1541. [DOI] [PubMed] [Google Scholar]
- Martin RL, Perez E, He YJ, Dawson R, Jr., Millard WJ. Leptin resistance is associated with hypothalamic leptin receptor mRNA and protein downregulation. Metabolism. 2000;49(11):1479–1484. doi: 10.1053/meta.2000.17695. [DOI] [PubMed] [Google Scholar]
- McNair P, Madsbad S, Christensen MS, Christiansen C, Faber OK, Binder C, et al. Bone mineral loss in insulin-treated diabetes mellitus: studies on pathogenesis. Acta Endocrinol. (Copenh.) 1979;90(3):463–472. doi: 10.1530/acta.0.0900463. [DOI] [PubMed] [Google Scholar]
- Melton LJ., 3rd Adverse outcomes of osteoporotic fractures in the general population. J. Bone Miner. Res. 2003;18(6):1139–1141. doi: 10.1359/jbmr.2003.18.6.1139. [DOI] [PubMed] [Google Scholar]
- Melton LJ, 3rd, Leibson CL, Achenbach SJ, Therneau TM, Khosla S. Fracture risk in type 2 diabetes: update of a population-based study. J. Bone Miner. Res. 2008;23(8):1334–1342. doi: 10.1359/JBMR.080323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier VM, Kohn RR, Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc. Natl. Acad. Sci. U.S.A. 1984;81(2):583–587. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundy GR. Osteoporosis and inflammation. Nutr. Rev. 2007;65(12 Pt 2):S147–S151. doi: 10.1111/j.1753-4887.2007.tb00353.x. [DOI] [PubMed] [Google Scholar]
- Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, et al. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108(1):17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Flegal KM. High body mass index for age among US children and adolescents, 2003–2006. JAMA. 2008;299(20):2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- Parfitt AM. The coupling of bone formation to bone resorption: a critical analysis of the concept and of its relevance to the pathogenesis of osteoporosis. Metab. Bone Dis. Relat. Res. 1982;4(1):1–6. doi: 10.1016/0221-8747(82)90002-9. [DOI] [PubMed] [Google Scholar]
- Parhami F, Morrow AD, Balucan J, Leitinger N, Watson AD, Tintut Y, et al. Lipid oxidation products have opposite effects on calcifying vascular cell and bone cell differentiation. A possible explanation for the paradox of arterial calcification in osteoporotic patients. Arterioscler. Thromb. Vasc. Biol. 1997;17(4):680–687. doi: 10.1161/01.atv.17.4.680. [DOI] [PubMed] [Google Scholar]
- Parikh S, Guo DH, Pollock NK, Petty K, Bhagatwala J, Gutin B, et al. Circulating 25-hydroxyvitamin D concentrations are correlated with cardiometabolic risk among American black and white adolescents living in a year-round sunny climate. Diabetes Care. 2012;35(5):1133–1138. doi: 10.2337/dc11-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit MA, Beck TJ, Shults J, Zemel BS, Foster BJ, Leonard MB. Proximal femur bone geometry is appropriately adapted to lean mass in overweight children and adolescents. Bone. 2005;36(3):568–576. doi: 10.1016/j.bone.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Petit MA, Paudel ML, Taylor BC, Hughes JM, Strotmeyer ES, Schwartz AV, et al. Bone mass and strength in older men with type 2 diabetes: the osteoporotic fractures in men study. J. Bone Miner. Res. 2010;25(2):285–291. doi: 10.1359/jbmr.090725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr. Rev. 2002;23(1):90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- Piters E, Boudin E, Van Hul W. Wnt signaling: a win for bone. Arch. Biochem. Biophys. 2008;473(2):112–116. doi: 10.1016/j.abb.2008.03.006. [DOI] [PubMed] [Google Scholar]
- Pollack KM, Xie D, Arbogast KB, Durbin DR. Body mass index and injury risk among US children 9–15 years old in motor vehicle crashes. Inj. Prev. 2008;14:366–371. doi: 10.1136/ip.2008.019208. [DOI] [PubMed] [Google Scholar]
- Pollock NK, Laing EM, Baile CA, Hamrick MW, Hall DB, Lewis RD. Is adiposity advantageous for bone strength? A peripheral quantitative computed tomography study in late adolescent females. Am. J. Clin. Nutr. 2007;86(5):1530–1538. doi: 10.1093/ajcn/86.5.1530. [DOI] [PubMed] [Google Scholar]
- Pollock NK, Bernard PJ, Wenger K, Misra S, Gower BA, Allison JD, et al. Lower bone mass in prepubertal overweight children with prediabetes. J. Bone Miner. Res. 2010;25(12):2760–2769. doi: 10.1002/jbmr.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock NK, Bernard PJ, Gutin B, Davis CL, Zhu H, Dong Y. Adolescent obesity, bone mass, and cardiometabolic risk factors. J. Pediatr. 2011a;158(5):727–734. doi: 10.1016/j.jpeds.2010.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock NK, Laing EM, Hamrick MW, Baile CA, Hall DB, Lewis RD. Bone and fat relationships in postadolescent black females: a pQCT study. Osteoporos. Int. 2011b;22(2):655–665. doi: 10.1007/s00198-010-1266-6. [DOI] [PubMed] [Google Scholar]
- Pollock NK, Laing EM, Taylor RG, Baile CA, Hamrick MW, Hall DB, et al. Comparisons of trabecular and cortical bone in late adolescent black and white females. J. Bone Miner. Metab. 2011c;29(1):44–53. doi: 10.1007/s00774-010-0186-z. [DOI] [PubMed] [Google Scholar]
- Pollock NK, Bernard PJ, Gower BA, Gundberg CM, Wenger K, Misra S, et al. Lower uncarboxylated osteocalcin concentrations in children with prediabetes is associated with β-cell function. J. Clin. Endocrinol. Metab. 2011d;96(7):E1092–E1099. doi: 10.1210/jc.2010-2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid IR. Relationships among body mass, its components, and bone. Bone. 2002;31(5):547–555. doi: 10.1016/s8756-3282(02)00864-5. [DOI] [PubMed] [Google Scholar]
- Reid IR. Relationships between fat and bone. Osteoporos. Int. 2008;19(5):595–606. doi: 10.1007/s00198-007-0492-z. [DOI] [PubMed] [Google Scholar]
- Romas E, Gillespie MT, Martin TJ. Involvement of receptor activator of NFkappaB ligand and tumor necrosis factor-alpha in bone destruction in rheumatoid arthritis. Bone. 2002;30(2):340–346. doi: 10.1016/s8756-3282(01)00682-2. [DOI] [PubMed] [Google Scholar]
- Rosen CJ, Bouxsein ML. Mechanisms of disease: is osteoporosis the obesity of bone? Nat. Clin. Pract. Rheumatol. 2006;2(1):35–43. doi: 10.1038/ncprheum0070. [DOI] [PubMed] [Google Scholar]
- Rzonca SO, Suva LJ, Gaddy D, Montague DC, Lecka-Czernik B. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology. 2004;145(1):401–406. doi: 10.1210/en.2003-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabhaney V, Boutis K, Yang G, Barra L, Tripathi R, Tran TT, et al. Bone fractures in children: is there an association with obesity? J. Pediatr. 2014;165:313–318. doi: 10.1016/j.jpeds.2014.04.006. e311. [DOI] [PubMed] [Google Scholar]
- Salans LB, Knittle JL, Hirsch J. The role of adipose cell size and adipose tissue insulin sensitivity in the carbohydrate intolerance of human obesity. J. Clin. Invest. 1968;47(1):153–165. doi: 10.1172/JCI105705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanguineti R, Storace D, Monacelli F, Federici A, Odetti P. Pentosidine effects on human osteoblasts in vitro. Ann. N. Y. Acad. Sci. 2008;1126:166–172. doi: 10.1196/annals.1433.044. [DOI] [PubMed] [Google Scholar]
- Satokata I, Ma L, Ohshima H, Bei M, Woo I, Nishizawa K, et al. Msx2 deficiency in mice causes pleiotropic defects in bone growth and ectodermal organ formation. Nat. Genet. 2000;24(4):391–395. doi: 10.1038/74231. [DOI] [PubMed] [Google Scholar]
- Sayers A, Tobias JH. Fat mass exerts a greater effect on cortical bone mass in girls than boys. J. Clin. Endocrinol. Metab. 2010;95(2):699–706. doi: 10.1210/jc.2009-1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz AV, Sellmeyer DE. Diabetes, fracture, and bone fragility. Curr. Osteoporos. Rep. 2007;5(3):105–111. doi: 10.1007/s11914-007-0025-x. [DOI] [PubMed] [Google Scholar]
- Schwartz AV, Sellmeyer DE, Ensrud KE, Cauley JA, Tabor HK, Schreiner PJ, et al. Older women with diabetes have an increased risk of fracture: a prospective study. J. Clin. Endocrinol. Metab. 2001;86(1):32–38. doi: 10.1210/jcem.86.1.7139. [DOI] [PubMed] [Google Scholar]
- Schwartz AV, Garnero P, Hillier TA, Sellmeyer DE, Strotmeyer ES, Feingold KR, et al. Pentosidine and increased fracture risk in older adults with type 2 diabetes. J. Clin. Endocrinol. Metab. 2009;94(7):2380–2386. doi: 10.1210/jc.2008-2498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapses SA, Pollock NK, Lewis RD. Obesity, adipose tissue, and bone. In: Anderson J, Garner SC, Klemmer PJ, editors. Diet, Nutrients, and Bone Health. Vol. 1. New York: CRC Press: Taylor & Francis Group; 2011. pp. 325–368. [Google Scholar]
- Shen W, Chen J, Punyanitya M, Shapses S, Heshka S, Heymsfield SB. MRI-measured bone marrow adipose tissue is inversely related to DXA-measured bone mineral in Caucasian women. Osteoporos. Int. 2007;18(5):641–647. doi: 10.1007/s00198-006-0285-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shockley KR, Lazarenko OP, Czernik PJ, Rosen CJ, Churchill GA, Lecka-Czernik B. PPARgamma2 nuclear receptor controls multiple regulatory pathways of osteoblast differentiation from marrow mesenchymal stem cells. J. Cell. Biochem. 2009;106(2):232–246. doi: 10.1002/jcb.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu A, Yin MT, Stein E, Cremers S, Dworakowski E, Ives R, et al. Bone structure and turnover in type 2 diabetes mellitus. Osteoporos. Int. 2012;23(2):635–641. doi: 10.1007/s00198-011-1595-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs DL, Loro ML, Pitukcheewanont P, Tolo V, Gilsanz V. Increased body weight and decreased radial cross-sectional dimensions in girls with forearm fractures. J. Bone Miner. Res. 2001;16(7):1337–1342. doi: 10.1359/jbmr.2001.16.7.1337. [DOI] [PubMed] [Google Scholar]
- Skurk T, Alberti-Huber C, Herder C, Hauner H. Relationship between adipocyte size and adipokine expression and secretion. J. Clin. Endocrinol. Metab. 2007;92(3):1023–1033. doi: 10.1210/jc.2006-1055. [DOI] [PubMed] [Google Scholar]
- Stefan N, Kantartzis K, Machann J, Schick F, Thamer C, Rittig K, et al. Identification and characterization of metabolically benign obesity in humans. Arch. Intern. Med. 2008;168(15):1609–1616. doi: 10.1001/archinte.168.15.1609. [DOI] [PubMed] [Google Scholar]
- Stofkova A. Leptin and adiponectin: from energy and metabolic dysbalance to inflammation and autoimmunity. Endocr. Regul. 2009;43(4):157–168. [PubMed] [Google Scholar]
- Streeter AJ, Hosking J, Metcalf BS, Jeffery AN, Voss LD, Wilkin TJ. Body fat in children does not adversely influence bone development: a 7-year longitudinal study (EarlyBird 18) Pediatr. Obes. 2013;8(6):418–427. doi: 10.1111/j.2047-6310.2012.00126.x. [DOI] [PubMed] [Google Scholar]
- Tang SY, Allen MR, Phipps R, Burr DB, Vashishth D. Changes in non-enzymatic glycation and its association with altered mechanical properties following 1-year treatment with risedronate or alendronate. Osteoporos. Int. 2009;20(6):887–894. doi: 10.1007/s00198-008-0754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanko LB, Bagger YZ, Nielsen SB, Christiansen C. Does serum cholesterol contribute to vertebral bone loss in postmenopausal women? Bone. 2003;32(1):8–14. doi: 10.1016/s8756-3282(02)00918-3. [DOI] [PubMed] [Google Scholar]
- Tarquini B, Navari N, Perfetto F, Piluso A, Romano S, Tarquini R. Evidence for bone mass and body fat distribution relationship in postmenopausal obese women. Arch. Gerontol. Geriatr. 1997;24(1):15–21. doi: 10.1016/s0167-4943(96)00723-6. [DOI] [PubMed] [Google Scholar]
- Taylor ED, Theim KR, Mirch MC, Ghorbani S, Tanofsky-Kraff M, Adler-Wailes DC, et al. Orthopedic complications of overweight in children and adolescents. Pediatrics. 2006;117:2167–2174. doi: 10.1542/peds.2005-1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARgamma. Annu. Rev. Biochem. 2008;77:289–312. doi: 10.1146/annurev.biochem.77.061307.091829. [DOI] [PubMed] [Google Scholar]
- Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr. Rev. 2006;27(7):762–778. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- van Daele PL, Stolk RP, Burger H, Algra D, Grobbee DE, Hofman A, et al. Bone density in non-insulin-dependent diabetes mellitus. The Rotterdam Study. Ann. Intern. Med. 1995;122(6):409–414. doi: 10.7326/0003-4819-122-6-199503150-00002. [DOI] [PubMed] [Google Scholar]
- von Muhlen D, Safii S, Jassal SK, Svartberg J, Barrett-Connor E. Associations between the metabolic syndrome and bone health in older men and women: the Rancho Bernardo Study. Osteoporos. Int. 2007;18(10):1337–1344. doi: 10.1007/s00198-007-0385-1. [DOI] [PubMed] [Google Scholar]
- Valerio G, Galle F, Mancusi C, Di Onofrio V, Guida P, Tramontano A, et al. Prevalence of overweight in children with bone fractures: a case control study. BMC Pediatr. 2012;12:166. doi: 10.1186/1471-2431-12-166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke TE, Serhan CN. Resolution of inflammation: a new paradigm for the pathogenesis of periodontal diseases. J. Dent. Res. 2003;82(2):82–90. doi: 10.1177/154405910308200202. [DOI] [PubMed] [Google Scholar]
- Van Gaal LF, Mertens IL, De Block CE. Mechanisms linking obesity with cardiovascular disease. Nature. 2006;444(7121):875–880. doi: 10.1038/nature05487. [DOI] [PubMed] [Google Scholar]
- Vandanmagsar B, Youm YH, Ravussin A, Galgani JE, Stadler K, Mynatt RL, et al. The NLRP3 inflammasome instigates obesity-induced inflammation and insulin resistance. Nat. Med. 2011;17(2):179–188. doi: 10.1038/nm.2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vargas SJ, Naprta A, Glaccum M, Lee SK, Kalinowski J, Lorenzo JA. Interleukin-6 expression and histomorphometry of bones from mice deficient in receptors for interleukin-1 or tumor necrosis factor. J. Bone Miner. Res. 1996;11(11):1736–1744. doi: 10.1002/jbmr.5650111117. [DOI] [PubMed] [Google Scholar]
- Vashishth D. The role of the collagen matrix in skeletal fragility. Curr. Osteoporos. Rep. 2007;5(2):62–66. doi: 10.1007/s11914-007-0004-2. [DOI] [PubMed] [Google Scholar]
- Vega D, Maalouf NM, Sakhaee K. CLINICAL Review #: the role of receptor activator of nuclear factor-kappaB (RANK)/RANK ligand/osteoprotegerin: clinical implications. J. Clin. Endocrinol. Metab. 2007;92(12):4514–4521. doi: 10.1210/jc.2007-0646. [DOI] [PubMed] [Google Scholar]
- Verma S, Rajaratnam JH, Denton J, Hoyland JA, Byers RJ. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J. Clin. Pathol. 2002;55(9):693–698. doi: 10.1136/jcp.55.9.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Zhang X, Zheng J, Yang J. High glucose stimulates adipogenic and inhibits osteogenic differentiation in MG-63 cells through cAMP/protein kinase A/extracellular signal-regulated kinase pathway. Mol. Cell. Biochem. 2010;338(1–2):115–122. doi: 10.1007/s11010-009-0344-6. [DOI] [PubMed] [Google Scholar]
- Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. Int. J. Pediatr. Obes. 2006;1(1):11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- Warming L, Ravn P, Christiansen C. Visceral fat is more important than peripheral fat for endometrial thickness and bone mass in healthy postmenopausal women. Am. J. Obstet. Gynecol. 2003;188(2):349–353. doi: 10.1067/mob.2003.93. [DOI] [PubMed] [Google Scholar]
- Weiss R, Dziura J, Burgert TS, Tamborlane WV, Taksali SE, Yeckel CW, et al. Obesity and the metabolic syndrome in children and adolescents. N. Engl. J. Med. 2004;350(23):2362–2374. doi: 10.1056/NEJMoa031049. [DOI] [PubMed] [Google Scholar]
- Wosje KS, Khoury PR, Claytor RP, Copeland KA, Kalkwarf HJ, Daniels SR. Adiposity and TV viewing are related to less bone accrual in young children. J. Pediatr. 2009;154(1):79–85. doi: 10.1016/j.jpeds.2008.06.031. e72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Yamaguchi T, Yamauchi M, Yano S, Sugimoto T. Serum pentosidine levels are positively associated with the presence of vertebral fractures in postmenopausal women with type 2 diabetes. J. Clin. Endocrinol. Metab. 2008;93(3):1013–1019. doi: 10.1210/jc.2007-1270. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, et al. Osteoclast differentiation factor is a ligand for osteoprotegerin/ osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc. Natl. Acad. Sci. U.S.A. 1998;95(7):3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa Y, Kode A, Xu L, Mosialou I, Silva BC, Ferron M, et al. Genetic evidence points to an osteocalcin-independent influence of osteoblasts on energy metabolism. J. Bone Miner. Res. 2011;26(9):2012–2025. doi: 10.1002/jbmr.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemel B, Bass S, Binkley T, Ducher G, Macdonald H, McKay H, et al. Peripheral quantitative computed tomography in children and adolescents: the 2007 ISCD Pediatric Official Positions. J. Clin. Densitom. 2008;11(1):59–74. doi: 10.1016/j.jocd.2007.12.006. [DOI] [PubMed] [Google Scholar]