Supplemental Digital Content is available in the text.
Keywords: hydroxymethylglutaryl-CoA reductase inhibitors; natriuretic peptide, brain; primary prevention; troponin
Abstract
Background—
Cardiac troponin and B-type natriuretic peptide (BNP) concentrations are associated with adverse cardiovascular outcome in primary prevention populations. Whether statin therapy modifies this association is poorly understood.
Methods and Results—
We measured high-sensitivity cardiac troponin I (hsTnI) in 12 956 and BNP in 11 076 participants without cardiovascular disease in the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) trial before randomization to rosuvastatin 20 mg/d or placebo. Nearly 92% of participants had detectable circulating hsTnI, and 2.9% of men and 4.1% of women had levels above proposed sex-specific reference limits of 36 and 15 ng/L, respectively. hsTnI concentrations in the highest tertile were associated with a first major cardiovascular event (adjusted hazard ratio [aHR], 2.19; 95% confidence interval, 1.56–3.06; P for trend <0.001). BNP levels in the highest tertile were also associated a first cardiovascular event (aHR, 1.94; 95% confidence interval, 1.41–2.68; P for trend <0.001). The risk of all-cause mortality was elevated for the highest versus the lowest tertiles of hsTnI (aHR, 2.61; 95% confidence interval, 1.81–3.78; P for trend <0.001) and BNP (aHR, 1.45; 95% confidence interval, 1.03–2.04; P for trend 0.02). Rosuvastatin was equally effective in preventing a first cardiovascular event across categories of hsTnI (aHR range, 0.50–0.60) and BNP (aHR range, 0.42–0.67) with no statistically significant evidence of interaction (P for interaction=0.53 and 0.20, respectively).
Conclusions—
In a contemporary primary prevention population, baseline cardiac troponin I and BNP were associated with the risk of vascular events and all-cause mortality. The benefits of rosuvastatin were substantial and consistent regardless of baseline hsTnI or BNP concentrations.
Clinical Trial Registration—
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00239681.
Cardiac troponin is the preferred marker of myocardial cell necrosis.1 Recently, highly sensitive assays have become available that allow the detection of very low concentrations of circulating cardiac troponin in healthy individuals.2–5 Increasing troponin concentrations, even those well within the normal reference interval, are positively associated with adverse cardiovascular outcome in primary prevention populations. These adverse outcomes include myocardial infarction (MI), congestive heart failure, and cardiovascular death.2–6 Assays for B-type natriuretic peptide (BNP) are widely used for diagnosis and risk stratification of patients presenting with suspected heart failure.7,8 BNPs also have strong associations with adverse cardiovascular outcome in a variety of primary prevention and general populations.9–12
Clinical Perspective on p 1860
Little is known about therapies that might improve cardiovascular outcome in stable patients with troponin or BNP concentrations that identify them as being at increased cardiovascular risk. In particular, whether statin therapy might mitigate this risk has not been well studied.
To address this question, we measured circulating cardiac troponin I with a novel high-sensitivity assay (hsTnI) and BNP in a contemporary cohort of patients without known cardiovascular disease who were randomly allocated to active statin therapy (rosuvastatin 20 mg daily) or placebo in the Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER) trial. We sought to determine whether these markers of myocardial injury (hsTnI) and strain (BNP) were associated with adverse outcome in JUPITER and whether the effectiveness of statin therapy was modified by circulating hsTnI or BNP concentrations.
Methods
Study Population
JUPITER was a randomized, double-blind, placebo-controlled trial of rosuvastatin 20 mg/d conducted in 26 countries in men ≥50 years and women ≥60 years of age without a history of diabetes mellitus or cardiovascular disease and with a low-density lipoprotein (LDL) cholesterol <130 mg/dL and a high-sensitivity C-reactive protein (hsCRP) ≥2.0 mg/L. The trial design and primary results have been described previously.13 In brief, rosuvastatin was associated with a 44% (hazard ratio [HR], 0.56; 95% confidence interval [CI], 0.46–0.69; P<0.00001) reduction in the trial primary end point, a composite of nonfatal MI, nonfatal stroke, hospitalization for unstable angina, arterial revascularization, or death resulting from cardiovascular causes. Of the 17 802 participants included in the parent trial, 12 956 had baseline samples available for analysis. Institutional review boards approved the JUPITER protocol at each site, and each participant gave written informed consent.
Study End Points
This study took as its primary end point the prespecified primary end point in the JUPITER trial: the occurrence of a first major vascular event, defined as the composite of nonfatal MI, nonfatal stroke, hospitalization for unstable angina, arterial revascularization, or death resulting from cardiovascular causes. All trial end points were adjudicated by an independent committee of physicians blinded to treatment allocation. We also analyzed the association of baseline cardiac troponin and BNP concentrations with the individual components of the primary end point, as well as all-cause mortality, the composite of the primary end point plus all-cause mortality, and the occurrence of coronary heart disease, which is a composite of coronary revascularization, hospitalization for unstable angina, and all MI, including sudden death and nonfatal MI.
Laboratory Analysis
All biomarkers were measured in EDTA plasma samples in the BiomarCaRE laboratory in Hamburg, Germany. hsTnI concentrations were determined by the ARCHITECT STAT hsTnI immunoassay (Abbott Diagnostics; ARCHITECT i2000SR). Because plasma volume was limited, we first measured hsTnI and then diluted the remaining plasma sample 1:1 before measurement of BNP. In total, adequate sample was available in 12 956 participants for hsTnI and 11 057 participants for BNP. The limit of detection for the hsTnI assay is 1.9 ng/L. The assay supported a 10% coefficient of variation at a concentration of 5.2 ng/L. The intra-assay coefficient of variation was 4.96% to 5.73%, and the interassay coefficient of variation was 4.9% to 6.6%. The 99th percentile in a healthy reference population is 30 ng/L.14 However, other investigators have reported an upper reference limit of 15 ng/L for women and 36 ng/L for men,15 which are consistent with other sex-specific upper reference limits.5,16
For measurement of BNP, a chemiluminescent microparticle immunoassay was used (BNP, Abbott Diagnostics; ARCHITECT i2000SR). The limit of detection is 20 ng/L. Participants with BNP concentrations lower than this limit of detection (n=4990) were assigned a value of 19 ng/L for the determination of sex-specific tertiles and in analyses using natural logarithm (Ln)–transformed BNP concentrations as a linear variable. The intra-assay coefficient of variation for BNP was 5.47% to 5.38%, and the interassay coefficient of variation was 4.32% to 6.72%.
Statistical Analysis
hsTnI concentrations were divided into increasing tertiles with the use of sex-specific cut points of 3.0 and 4.6 ng/L in men and 2.6 and 3.9 ng/L in women on the basis of the distribution of the biomarker within each sex. The limit of detection for hsTnI is 1.9 ng/L, but observed values <1.9 ng/L were used in calculating tertile cut points and in analyses with Ln-hsTnI used as a linear variable. BNP concentrations were divided into increasing tertiles with the use of sex-specific cut points of 20.0 and 28.6 ng/L in men and 20.0 and 44.4 ng/L in women on the basis of the distribution of the biomarker within each sex. No values were observed below the BNP limit of detection of 20 ng/L. Participants with a BNP concentration below the limit of detection were assigned a value of 19.9 ng/L for determination of tertile cut points and in analyses with Ln-BNP used as a linear variable. Baseline characteristics were compared across tertiles of hsTnI and BNP concentrations with Kruskal-Wallis rank-sum tests for continuous variables and χ2 tests for categorical variables. The risk and 95% CIs of the JUPITER primary end point, the individual components of the primary end point, all-cause mortality, the composite of any coronary heart disease event, and the composite of the primary end point or all-cause mortality according to increasing tertiles of hsTnI or BNP were calculated with Cox proportional hazards models adjusted for cardiovascular risk factors and study drug allocation. Four models were constructed with adjustments for the following covariates: model 1: age, race, sex, and study drug allocation; model 2: model 1 covariables plus body mass index, history of hypertension, current smoking, family history of MI, and total and high-density lipoprotein cholesterol; model 3: model 2 covariables plus hsCRP; and model 4: model 3 covariables plus Ln-BNP (for hsTnI effect estimates) or Ln-hsTnI (for BNP effect estimates). No violation of the proportional hazards assumption across tertiles of hsTnI (P=0.36) or BNP (P=0.91) was detected. The association per 1-SD of Ln-hsTnI and Ln-BNP was also calculated. Possible heterogeneity in the effects of statin therapy across biomarker tertiles was evaluated in a proportional hazards model that included tertile indicators, an indicator of rosuvastatin assignment, and an interaction term combining statin assignment and biomarker tertile. Incidence rates of the primary end point were calculated for patients randomly allocated to active or placebo rosuvastatin. Absolute risk reductions were defined as the incidence rate differences and 95% CIs between the active and placebo arms. Incidence rate differences (95% CI) were used to estimate the 5-year numbers needed to treat (NNTs) according to tertile of baseline hsTnI or BNP and rosuvastatin assignment.
Results
In total, 12 956 JUPITER participants had baseline samples available that were successfully analyzed for cardiac TnI, and 11 057 had samples successfully analyzed for BNP. Compared with the JUPITER participants for whom no blood sample was available, the cohort with blood samples available for hsTnI testing was more likely to be male and white, among other differences (Table I in the online-only Data Supplement). The median follow-up time in this cohort was 2.0 years (quartile 1–3 [Q1–Q3], 1.5–2.5 years).
The median hsTnI concentration was 3.4 ng/L (Q1–Q3, 2.6–5.0 ng/L) and was higher in men (3.6 ng/L; Q1–Q3, 2.7–5.3 ng/L) than in women [3.1 ng/L; Q1–Q3, 2.3–4.5 ng/L; P<0.0001). In total, 11 905 of 12 956 participants (91.9%) had hsTnI concentrations above the manufacturer’s limit of detection of 1.9 ng/L, and a higher proportion of men (7744 of 8260, 93.8%) than women (4161 of 4696, 88.6%) had values above this threshold (P<0.0001). The proportion of men with a value at or above the proposed upper reference limit of 36 ng/L was 2.9% (235 of 8260) compared with 4.1% (193 of 4696) of women above the proposed upper reference limit of 15 ng/L. The median BNP concentration was 22.3 ng/L (Q1–Q3, 19.0–42.7 ng/L) and was lower in men (19.0 ng/L; Q1–Q3, 19.0–35.3 ng/L) than in women (29.2 ng/L; Q1–Q3, 19.0–53.4 ng/L; P<0.0001).
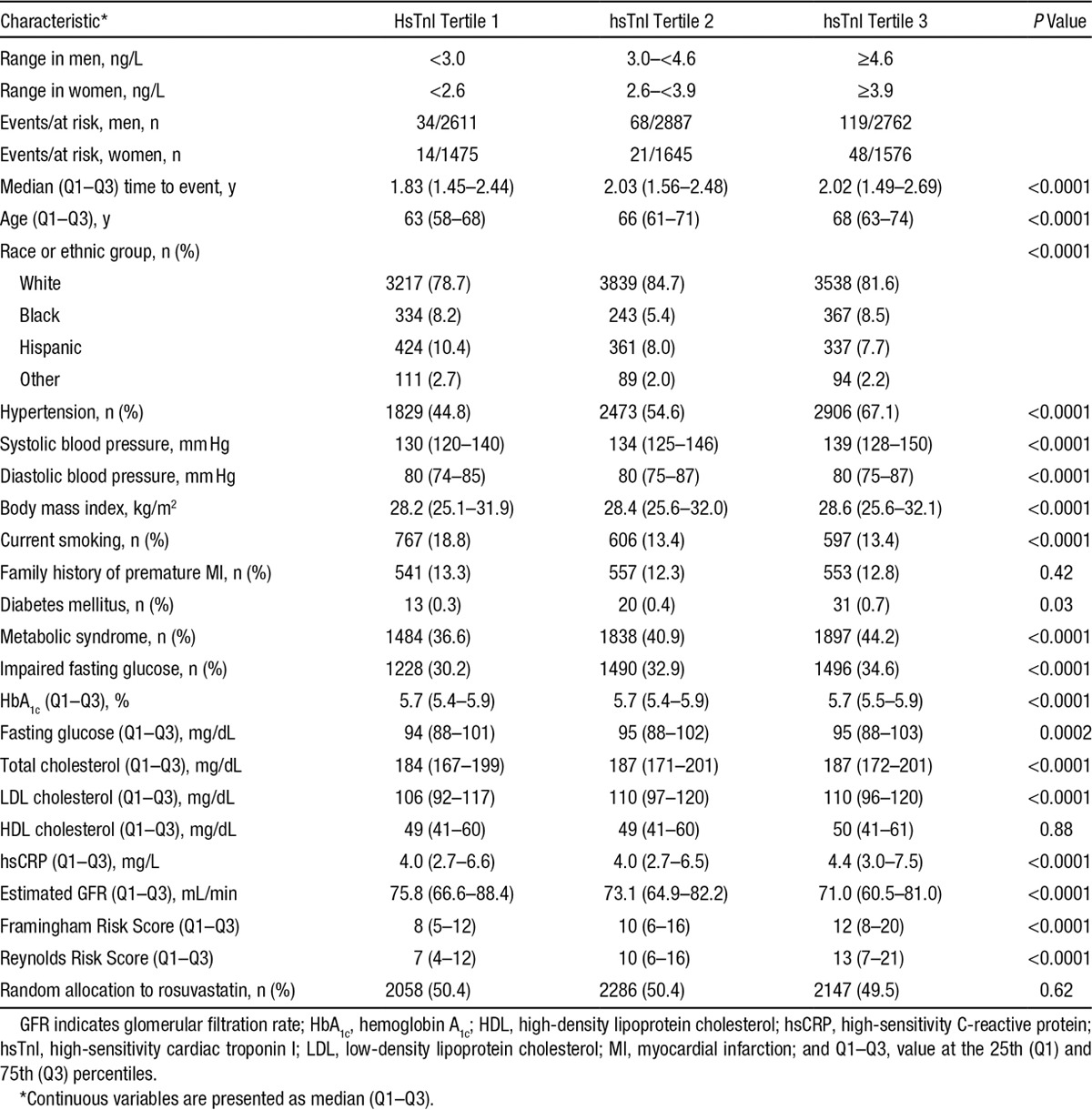
Baseline characteristics stratified by baseline tertile of hsTnI are presented in Table 1, and baseline characteristics stratified by baseline tertile of BNP are presented in Table II in the online-only Data Supplement. Age, systolic and diastolic blood pressures, the prevalence of hypertension, total cholesterol, LDL cholesterol, the Framingham Risk Scores, and the Reynolds Risk Score were all positively correlated with baseline hsTnI and BNP. The metabolic syndrome, impaired fasting glucose, fasting glucose, and hsCRP were positively correlated with hsTnI and inversely correlated with BNP. Both biomarkers were inversely correlated with estimated glomerular filtration rate. In sex-stratified analyses of a variety of baseline biomarkers, hsTnI and BNP were correlated with one another (ρ=0.30 in men and 0.25 in women; Tables III and IV in the online-only Data Supplement).
Table 1.
Baseline Characteristics of the Study Population
The unadjusted incidence of the composite primary end point increased with increasing hsTnI category, from 0.56 per 100 person-years in the first tertile to 1.74 events per 100 person-years in the third tertile (P<0.0001; Table 2 and Figure 1A). Participants with an hsTnI lower than the limit of detection (1.9 ng/L) had an incidence of the primary end point of 0.14 per 100 person-years. After adjustment for age, race, sex, study drug assignment, cardiovascular risk factors, and hsCRP, the highest tertile of hsTnI was at more than double the risk of the composite cardiovascular outcome (model 3 HR, 2.19; 95% CI, 1.56–3.06; P for trend <0.0001). Adjusting for BNP led to a modest attenuation of this estimate (HR, 1.86; 95% CI, 1.25–2.76; P for trend=0.001). As a sensitivity analysis, we excluded those with hsTnI concentrations in the abnormal range, and the adjusted risk of the primary end point for those in the highest compared with the lowest tertile was essentially unchanged (model 3 HR, 2.25; 95% CI, 1.59–3.17; P for trend <0.0001). When analyzed as a linear variable, hsTnI demonstrated an independent association with the primary outcome (model 3 HR per 1-SD Ln-hsTnI, 1.12; 95% CI, 1.05–1.21; P=0.002).
Table 2.
Risk of a First Major Cardiovascular Event* According to Tertile of hsTnI or BNP Measured at Baseline
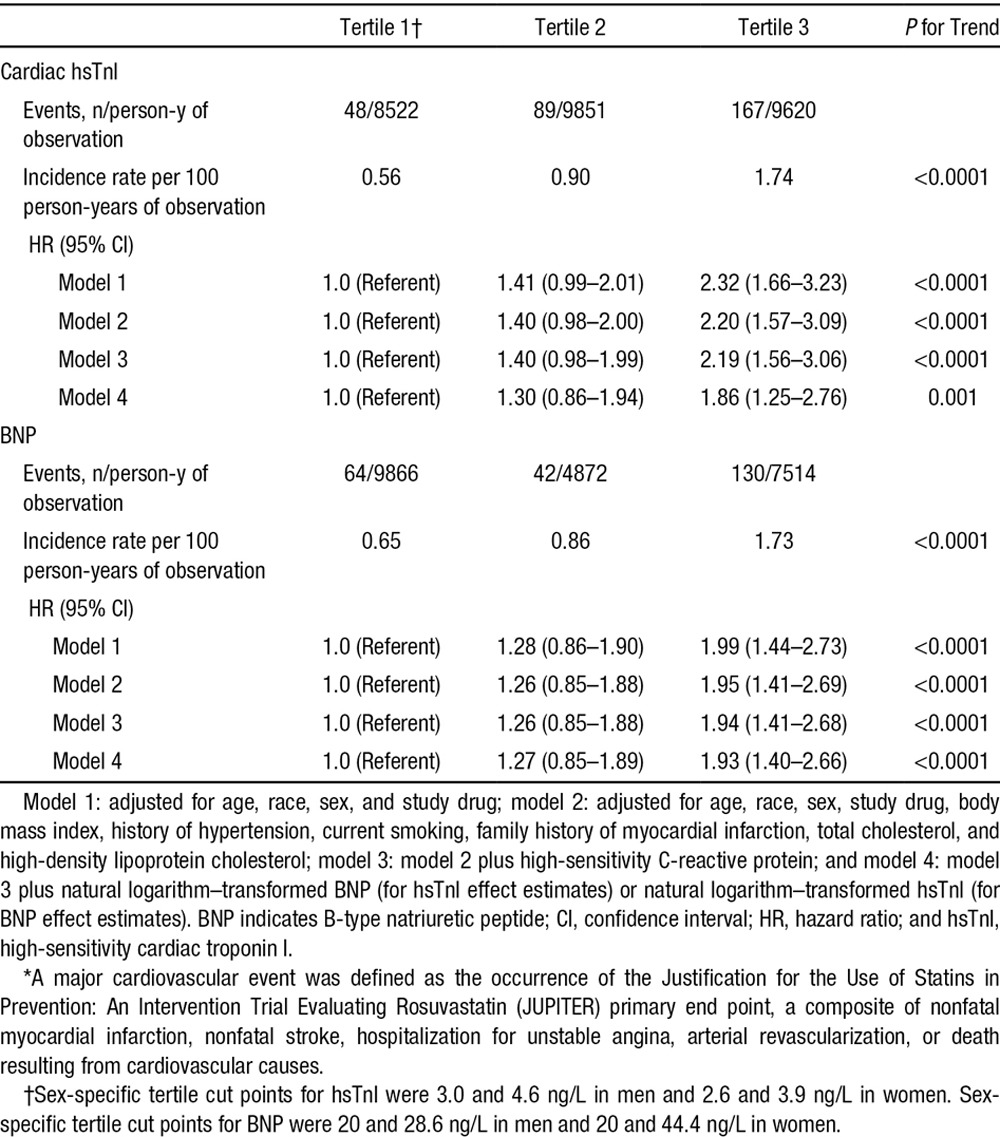
Figure 1.
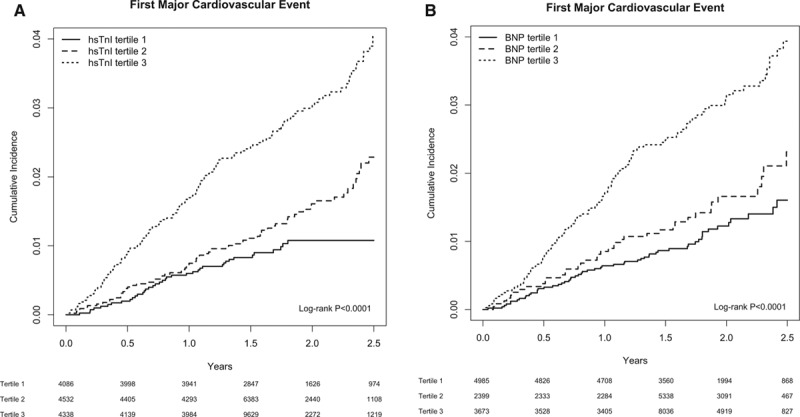
Cumulative incidence of a first major cardiovascular event according to baseline tertile of high-sensitivity cardiac troponin I (hsTnI tertile; A) or B-type natriuretic peptide (BNP tertile; B). A first major cardiovascular event is defined as the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) primary end point (nonfatal myocardial infarction, nonfatal stroke, hospitalization for unstable angina, arterial revascularization, or death resulting from cardiovascular causes). The sex-specific tertile cut points for hsTnI were 3.0 and 4.6 ng/L in men and 2.6 and 3.9 ng/L in women. The sex-specific tertile cut points for BNP were 20 and 28.6 ng/L in men and 20 and 44.4 ng/L in women. For the Kaplan–Meier analysis and log-rank P values presented here, follow-up was limited to 2.5 years, when 25% and 20% of patients remained in the hsTnI and BNP analyses, respectively.
The unadjusted incidence of the composite primary end point increased with increasing BNP category, from 0.65 per 100 person-years in the first tertile to 1.73 events per 100 person-years in the third tertile (P<0.0001; Table 2 and Figure 1B). In models adjusted for age, race, sex, and study drug, the risk of the primary end point was approximately double among those in the highest compared with the lowest tertile of BNP (HR, 1.99; 95% CI, 1.44–2.73; P for trend <0.0001; Table 2). This estimate did not change substantially after adjustment for cardiovascular risk factors and for hsTnI (model 4 HR, 1.93; 95% CI, 1.40–2.66; P for trend <0.0001). When analyzed as a linear variable, BNP demonstrated an independent association with the primary outcome (model 3 HR per 1-SD Ln-BNP, 1.38; 95% CI, 1.23–1.53; P<0.0001)
We observed no significant difference in the relationship between hsTnI or BNP and the JUPITER primary end point when analyses were stratified by random allocation to rosuvastatin or placebo (Figure I in the online-only Data Supplement; P for interaction=0.53 and 0.20, respectively).
The association of hsTnI and BNP with the JUPITER primary end point was consistent across a number of key risk subgroups, including age ≥70 or <70 years, men and women, white and nonwhite participants, and those with favorable and unfavorable lipid or metabolic traits (Figure 2). Similarly consistent associations were observed for BNP (Figure 3).
Figure 2.
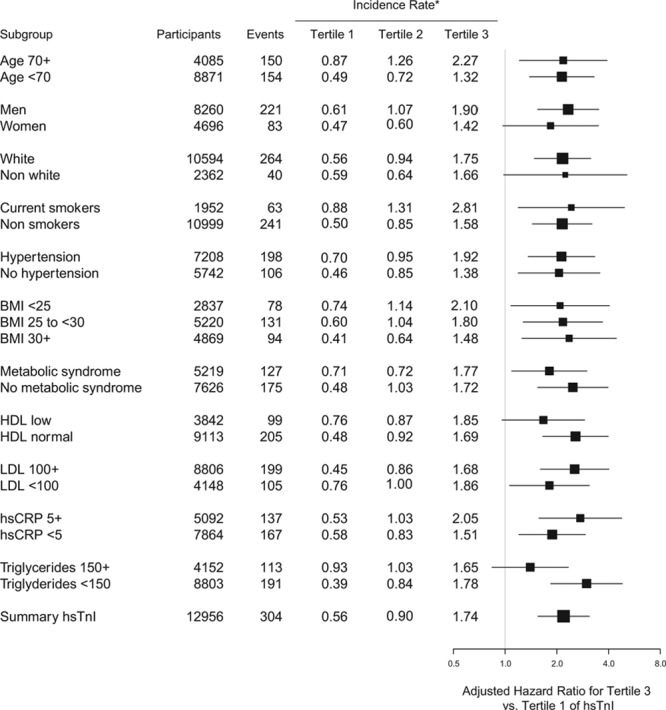
Adjusted hazard ratios and 95% confidence intervals of a first major cardiovascular event for the highest vs lowest tertile of high-sensitivity cardiac troponin I (hsTnI) stratified by a number of key risk subgroups. Risk estimates are adjusted for age, sex, drug, race, hypertension, smoking, body mass index (BMI), total cholesterol, high-density lipoprotein (HDL) cholesterol, family history of coronary heart disease, and high-sensitivity C-reactive protein (hsCRP) at baseline. LDL indicates low-density lipoprotein. *Incidence rates are per 100 person-years of observation.
Figure 3.
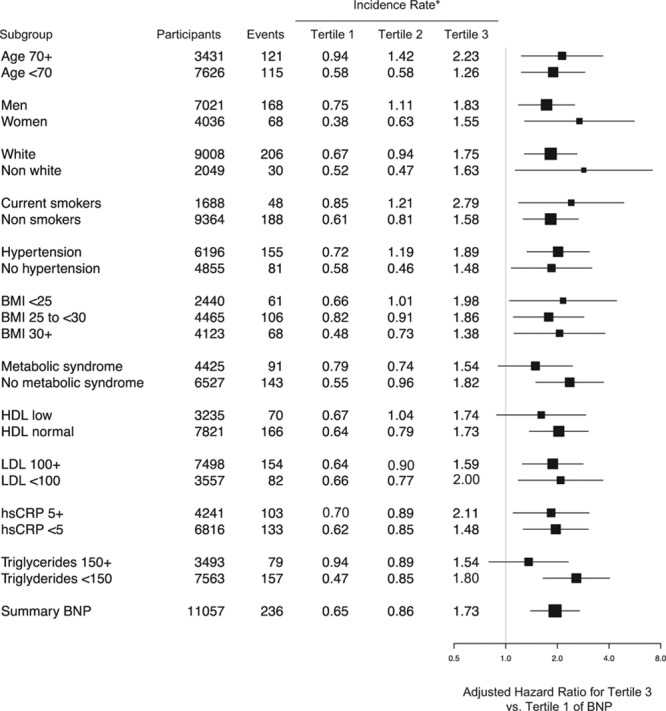
Adjusted hazard ratios and 95% confidence intervals of a first major cardiovascular event for the highest vs lowest tertile of B-type natriuretic peptide (BNP) stratified by a number of key risk subgroups. Risk estimates for are adjusted for age, sex, drug, race, hypertension, smoking, body mass index (BMI), total cholesterol, high-density lipoprotein (HDL) cholesterol, family history of coronary heart disease, and high-sensitivity C-reactive protein (hsCRP) at baseline. LDL indicates low-density lipoprotein. *Incidence rates are per 100 person-years.
The independent associations of hsTnI with each of the individual components of the primary end point, as well as death and coronary heart disease, are displayed in Figure 4. Participants with hsTnI values in the top tertile were at increased risk of cardiovascular mortality (HR, 2.45; 95% CI, 0.97–6.18; P for trend=0.03), nonfatal MI (HR, 3.08; 95% CI, 1.52–6.26; P for trend=0.0008), nonfatal stroke (HR, 1.84; 95% CI, 0.93–3.64; P for trend=0.04), and hospitalization for unstable angina (HR, 3.36; 95% CI, 1.21–9.30; P for trend=0.01). We also observed a statistically robust relationship between hsTnI and all-cause mortality (HR, 2.61; 95% CI, 1.81–3.78; P for trend <0.0001) and between the composite of the primary end point plus all-cause mortality (HR, 2.42; 95% CI, 1.86–3.15; P for trend <0.0001). BNP demonstrated independent associations with MI, stroke, arterial revascularization, all-cause mortality, coronary heart disease, and the composite of the primary end point plus all-cause mortality (Figure 5).
Figure 4.
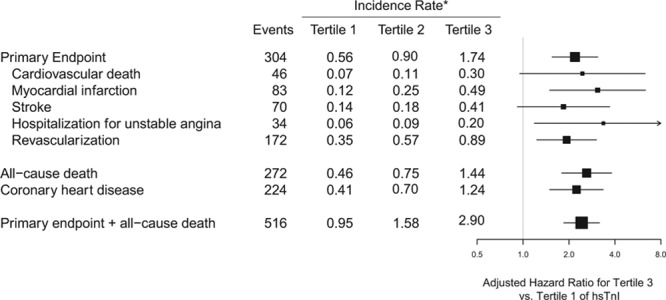
Adjusted hazard ratios and 95% confidence intervals for the highest vs lowest tertile of high-sensitivity cardiac troponin I (hsTnI) for the individual components of the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) composite primary end point, as well as all-cause death, coronary heart disease, and the composite of the primary end point or all-cause death. Risk estimates are adjusted for age, sex, drug, race, hypertension, smoking, body mass index, total cholesterol, high-density lipoprotein cholesterol, family history of coronary heart disease, and high-sensitivity C-reactive protein at baseline. *Incidence rates are per 100 person-years. Total N=12956.
Figure 5.
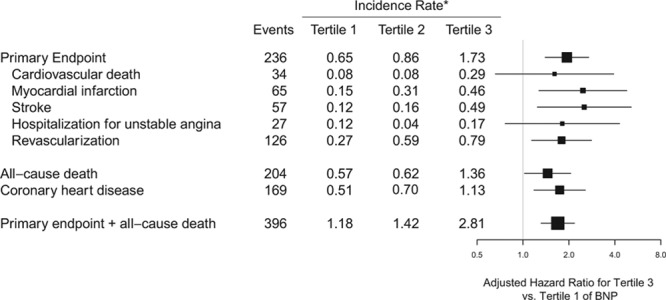
Adjusted hazard ratios and 95% confidence intervals for the highest vs lowest tertile of B-type natriuretic peptide (BNP) for the individual components of the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) composite primary end point, as well as all-cause death, coronary heart disease, and the composite of the primary end point or all-cause death. Risk estimates are adjusted for age, sex, drug, race, hypertension, smoking, body mass index, total cholesterol, high-density lipoprotein cholesterol, family history of coronary heart disease, and high-sensitivity C-reactive protein at baseline. *Incidence rates are per 100 person-years. Total n=11 057.
Rosuvastatin was equally effective in preventing the occurrence of the primary end point across different baseline concentrations of either hsTnI or BNP (Table 3 and Figure II in the online-only Data Supplement). For example, rosuvastatin therapy was associated with a 42% reduction in the adjusted relative risk of the primary end point in the first tertile of hsTnI and a 50% reduction in the third tertile (P for interaction=0.53). These results are consistent with the overall effect of rosuvastatin on the primary end point in this subcohort of JUPITER (HR, 0.55; 95% CI, 0.43–0.69; Figure II in the online-only Data Supplement) and in the trial as a whole.13
Table 3.
Risk of the JUPITER Primary End Point for Rosuvastatin Versus Placebo Stratified by Tertile of Baseline hsTnI, Tertile of BNP, or Framingham Risk Score
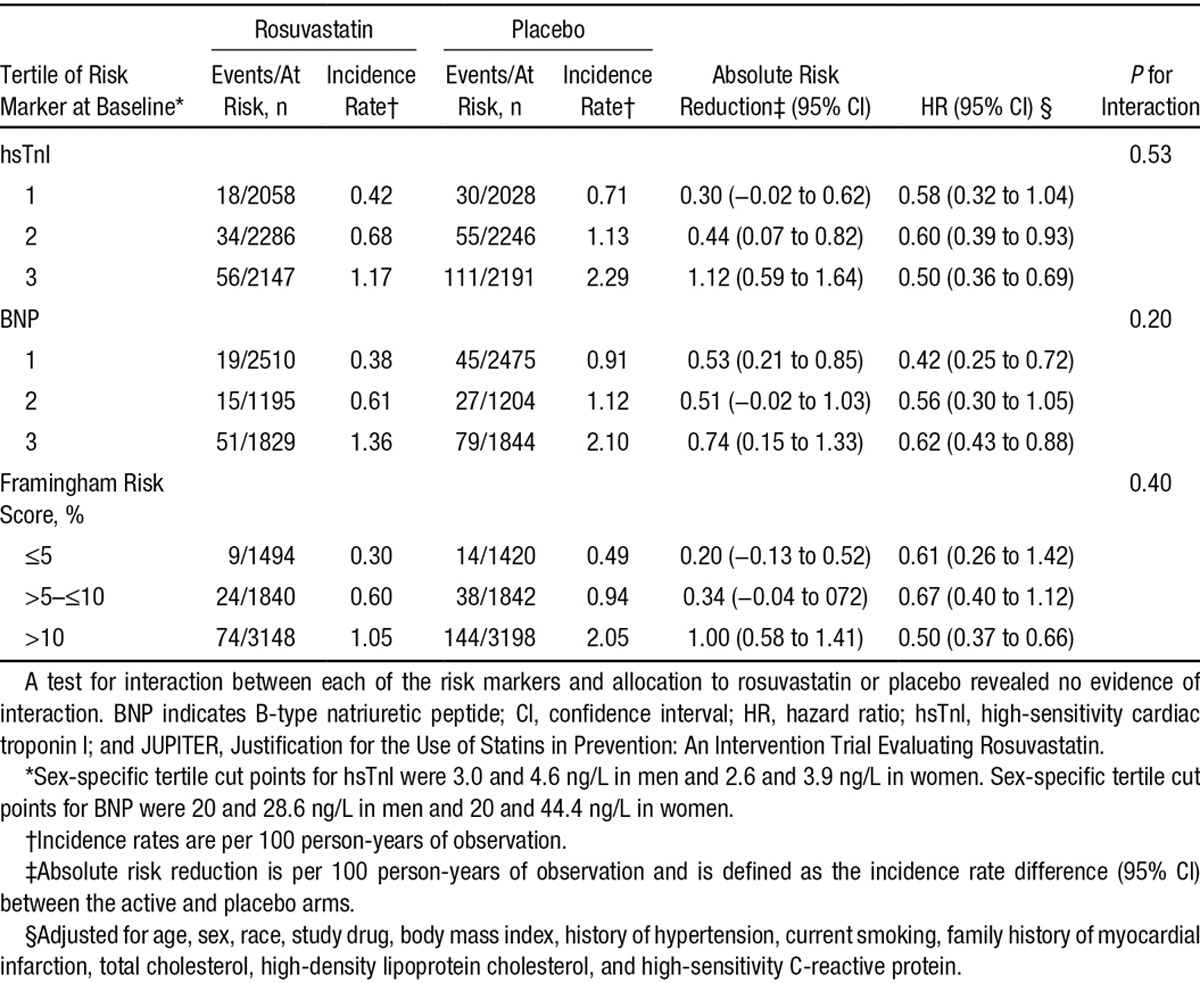
The absolute risk of the JUPITER primary end point appeared to increase across categories of hsTnI (Table 3). The absolute risk reduction, calculated as the difference in the incidence rates of the primary end point between the active and placebo arms, was 0.30 (95% CI, −0.02 to 0.62) in the lowest tertile of hsTnI, yielding an estimated 5-year NNT of 67. In the highest tertile of hsTnI, the absolute risk reduction of the primary end point between active and placebo was 1.12 (95% CI, 0.59–1.64), for an estimated 5-year NNT of 18. These results are similar to those observed when the cohort is stratified by Framingham Risk Score (Table 3), which had an NNT of 103 for the lowest risk scores (≤5%) and 20 for the highest (>10%). BNP did not appear to have as strong a relationship with absolute risk, with absolute risk reductions ranging from 0.53 (95% CI, 0.21–0.85) for the lowest tertile to 0.74 (95% CI, 0.15–1.33) in the highest tertile (Table 3). NNTs for BNP ranged from 38 for the lowest tertile to 27 for the highest tertile.
Discussion
In this prospective examination of 12 956 men and women with normal cholesterol levels and no prior cardiovascular disease, we demonstrated a statistically robust association between baseline concentrations of circulating cardiac TnI, as measured by a high-sensitivity assay, and the occurrence of major vascular events and death. This association was consistent across a variety of subgroups and was observed for a number of clinically important end points, including all-cause mortality, cardiovascular mortality, MI, stroke, and hospitalization for unstable angina. Similarly robust associations were observed for BNP. Rosuvastatin offered similar relative reductions in the risk of major vascular events regardless of baseline hsTnI or BNP concentrations. In the highest category of baseline hsTnI, rosuvastatin therapy was associated with the most substantial reduction in the absolute risk of cardiovascular events and therefore the lowest NNTs.
We believe these results have clinical implications for a number of reasons. First, the men and women enrolled in JUPITER had no prior evidence of cardiovascular disease or diabetes mellitus and were required to have an LDL cholesterol <130 mg/dL to be eligible. Nonetheless, nearly 94% of men and 89% of women had detectable concentrations of circulating cardiac troponin. These results are comparable to those obtained from a population based study in a European cohort5 and are consistent with reports from clinical trials of patients with atrial fibrillation and stable coronary artery disease.17,18 hsTnI concentrations in JUPITER participants are lower than those in populations with prevalent cardiovascular disease. In primary prevention or general populations, the prevalence of circulating cardiac troponin T ranges from 30% among healthy middle-aged women2 to ≈70% among men and women with a mean age >70 years.4
Most of the men and women in JUPITER had circulating hsTnI concentrations that were lower than thresholds that have been proposed as a diagnostic cutoff for MI in the appropriate clinical context.5,15 In this contemporary primary prevention population, 2.9% of men and 4.1% of women had TnI concentrations above proposed sex-specific upper reference limits.5,15,16 Nonetheless, we observed a clear gradient of risk among those with hsTnI concentrations below these thresholds such that the men and women with a hsTnI in the top tertile (≥4.6 ng/L in men and ≥3.9 ng/L in women) were at 2.2-times higher risk of a first major vascular event, including cardiovascular mortality, with an absolute rate of 2.3 events per 100 person-years of observation in the placebo arm. These observations are consistent with those in patients with existing coronary artery disease and in those with atrial fibrillation, among whom hsTnI was correlated with the risk of stroke/systemic embolism, cardiac mortality, and major bleeding.17,18
Rosuvastatin was equally effective in reducing the relative risk of major vascular events across categories of hsTnI and BNP. These results are similar to those seen for hsCRP in a previously published analysis of all 17 802 JUPITER participants.19 In this study, hsTnI appeared to be more closely related to the absolute risk of the primary end point than did BNP. The absolute benefit of rosuvastatin increased with increasing hsTnI level, and the calculated NNT for 5 years to prevent 1 primary end point in the highest tertile of hsTnI (NNT=18) compares favorably with the estimated NNT for a Framingham Risk Score >10% (NNT=20) or the highest tertile of BNP (NNT=27). When the results reported here are viewed in the context of previously published analyses of the NNT in JUPITER, hsTnI appears as useful as other accepted markers of high absolute risk such as advanced age, a history of hypertension, or low high-density lipoprotein cholesterol in helping to identify a group of patients with an NNT <20.20 This number compares favorably with other interventions in primary prevention populations such β-blockers or diuretics for hypertension or aspirin.20–23 The absolute risk reductions observed with statin therapy in secondary prevention populations, however, are more substantial.24 For example, the NNT for only 1 year in the Cholesterol and Recurrent Events (CARE) trial, a secondary prevention trial of pravastatin 40 mg in patients with prior MI and LDL between 115 and 174 ng/L, was 33 (95% CI, 20–99).24,25
We were unable to determine whether rosuvastatin therapy altered circulating hsTnI or BNP concentrations because of a lack of follow-up blood samples. However, secondary analyses of the Controlled Rosuvastatin Multinational Trial in HF (CORONA) study reported no difference in high-sensitivity cardiac troponin T concentrations after 3 months of rosuvastatin 10 mg/d.26
We observed similarly robust associations between baseline BNP concentrations and the occurrence of major vascular events. Both cardiac troponin and BNP appear to provide independent information on future cardiovascular risk in JUPITER, observations that are consistent with those from other primary prevention cohorts that have measured both cardiac troponin and natriuretic peptides.3,4,27
The cause for the ongoing troponin release in otherwise stable individuals is incompletely understood. Age, male sex, black race, renal function, diabetes mellitus, hypertension, left ventricular hypertrophy, and a history of heart failure have all been reported as determinants of circulating cardiac troponin T concentrations,2–4,6 and data from a secondary prevention cohort suggest that the determinants of cardiac troponin T and TnI are similar.18 In the Apixaban for Reduction in Stroke and other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) data, the determinants of hsTnI concentration were similar to those reported for cardiac troponin T but also included permanent or persistent atrial fibrillation.17 The mechanisms of troponin release in patients enrolled in JUPITER and other stable patients are likely to represent a combination of a number of different pathophysiological processes, including myocardial cell necrosis, apoptosis, hemodynamic stress, and metabolic abnormalities. Some have proposed increased rates of myocardial cell turnover or changes in cell wall permeability.28,29
Strengths of our study include the relatively large sample size of individuals without known pre-existing vascular disease, its prospective nature, the number of women, and the random allocation of statin therapy in the parent trial. These strengths, plus the relatively large number of vascular events and deaths, allow the estimation of the association of each hsTnI and BNP with adverse outcome and the assessment of whether statin therapy alters that relationship. Limitations include the fact that participants were followed up for a median of 2 years (maximum, 5 years), so the longer-term implications of hsTnI and BNP concentrations cannot be determined in this cohort. In addition, although we had adequate plasma for the hsTnI assay, the lack of adequate plasma for BNP means that many participants have no BNP value or have a value that is below the limit of detection in a diluted sample.
Conclusions
In this population of patients with normal LDL cholesterol concentrations and no known pre-existing cardiovascular disease, cardiac TnI can be detected in 92% and exceeds sex-specific reference limits in 3% of participants. We observed a statistically robust relationship between baseline measures of myocardial injury (cardiac TnI) and myocardial strain (BNP) and the risk of a vascular events, as well as death, in the entire cohort and in a variety of high- and low-risk patient subgroups. The benefits of rosuvastatin compared with placebo were substantial and consistent regardless of baseline hsTnI or BNP concentrations. Cardiac TnI appeared to be able to identify individuals at particularly high absolute risk of vascular events, a group among whom rosuvastatin offered a substantial reduction in the absolute risk of these clinically important events.
Sources of Funding
JUPITER was an investigator-initiated study supported by AstraZeneca. This work has been supported by the European Union Seventh Framework Programme (FP7/2007-2013) under grant agreement No. HEALTH-F2-2011-278913 (BiomarCaRE). Abbott Diagnostics provided reagents for TnI and BNP.
Disclosures
Dr Everett reports research support from Roche Diagnostics and Novartis Pharmaceuticals and serves as consultant to SOCAR research. Dr Glynn reports research support from Novartis and the National Institutes of Health. Dr Ridker has received investigator-initiated research grants from the National Institutes of Health, the American Heart Association, the Leducq Foundation, the Reynolds Foundation, AstraZeneca, Novartis, Amgen, and Pfizer; has served as a consultant to Pfizer, ISIS, Amgen, Vascular Biogenics, and BostonHeart; and is listed as the coinventor of patents held by the Brigham and Women’s Hospital that relate to the use of inflammatory biomarkers in cardiovascular disease. Dr Blankenberg has received honoraria from Abbott Diagnostics, SIEMENS, Thermo Fisher, and Roche Diagnostics and is a consultant for Thermo Fisher. The sponsor played no role in the design or conduct of this study; in the management, analysis, or interpretation of the data; or in the preparation, review, or approval of the manuscript or in the decision to submit the manuscript for publication. Dr Zeller reports no conflicts.
Supplementary Material
Footnotes
Guest Editor for this article was Gregory Y.H. Lip, MD.
The online-only Data Supplement is available with this article at http://circ.ahajournals.org/lookup/suppl/doi:10.1161/CIRCULATIONAHA.114.014522/-/DC1.
CLINICAL PERSPECTIVE
Circulating concentrations of cardiac troponin and B-type natriuretic peptide (BNP) as markers of myocardial necrosis and strain, respectively, have shown strong, consistent associations with adverse cardiovascular outcomes, including myocardial infarction, stroke, and cardiovascular death. Little is known about therapies that might improve outcomes for patients with cardiac troponin or BNP concentrations that place them at increased cardiovascular risk. In this study, we sought to determine whether statin therapy might be particularly effective in patients with higher concentrations of either cardiac biomarker. We measured cardiac troponin I with a novel, high-sensitivity assay (hsTnI) and BNP in 12 956 men and women enrolled in the Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER) trial, a randomized, double-blind, placebo-controlled trial of rosuvastatin 20 mg/d in patients without pre-existing cardiovascular disease. Patients with either hsTnI or BNP concentrations in the highest tertile were at approximately double the risk of a first major vascular event, even after adjustment for a wide variety of cardiovascular risk factors. This relationship was consistent across a broad array of high- and low-risk subgroups and was also seen for all-cause mortality. The effects of rosuvastatin were substantial and were consistent across baseline hsTnI or BNP concentrations, with a 40% to 50% reduction in the risk of a major vascular event for all participants. Because hsTnI appeared to identify individuals at high absolute risk of vascular events, rosuvastatin use in participants with higher hsTnI concentrations led to a substantial reduction in the absolute risk of these clinically important events.
References
- 1.Thygesen K, Alpert JS, White HD Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction. Universal definition of myocardial infarction. Eur Heart J. 2007;28:2525–2538. doi: 10.1093/eurheartj/ehm355. doi: 10.1093/eurheartj/ehm355. [DOI] [PubMed] [Google Scholar]
- 2.Everett BM, Cook NR, Magnone MC, Bobadilla M, Kim E, Rifai N, Ridker PM, Pradhan AD. Sensitive cardiac troponin T assay and the risk of incident cardiovascular disease in women with and without diabetes mellitus: the Women’s Health Study. Circulation. 2011;123:2811–2818. doi: 10.1161/CIRCULATIONAHA.110.009928. doi: 10.1161/CIRCULATIONAHA.110.009928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Lemos JA, Drazner MH, Omland T, Ayers CR, Khera A, Rohatgi A, Hashim I, Berry JD, Das SR, Morrow DA, McGuire DK. Association of troponin T detected with a highly sensitive assay and cardiac structure and mortality risk in the general population. JAMA. 2010;304:2503–2512. doi: 10.1001/jama.2010.1768. doi: 10.1001/jama.2010.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.deFilippi CR, de Lemos JA, Christenson RH, Gottdiener JS, Kop WJ, Zhan M, Seliger SL. Association of serial measures of cardiac troponin T using a sensitive assay with incident heart failure and cardiovascular mortality in older adults. JAMA. 2010;304:2494–2502. doi: 10.1001/jama.2010.1708. doi: 10.1001/jama.2010.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zeller T, Tunstall-Pedoe H, Saarela O, Ojeda F, Schnabel RB, Tuovinen T, Woodward M, Struthers A, Hughes M, Kee F, Salomaa V, Kuulasmaa K, Blankenberg S MORGAM Investigators. High population prevalence of cardiac troponin I measured by a high-sensitivity assay and cardiovascular risk estimation: the MORGAM Biomarker Project Scottish Cohort. Eur Heart J. 2014;35:271–281. doi: 10.1093/eurheartj/eht406. doi: 10.1093/eurheartj/eht406. [DOI] [PubMed] [Google Scholar]
- 6.Saunders JT, Nambi V, de Lemos JA, Chambless LE, Virani SS, Boerwinkle E, Hoogeveen RC, Liu X, Astor BC, Mosley TH, Folsom AR, Heiss G, Coresh J, Ballantyne CM. Cardiac troponin T measured by a highly sensitive assay predicts coronary heart disease, heart failure, and mortality in the Atherosclerosis Risk in Communities Study. Circulation. 2011;123:1367–1376. doi: 10.1161/CIRCULATIONAHA.110.005264. doi: 10.1161/CIRCULATIONAHA.110.005264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA Breathing Not Properly Multinational Study Investigators. Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med. 2002;347:161–167. doi: 10.1056/NEJMoa020233. doi: 10.1056/NEJMoa020233. [DOI] [PubMed] [Google Scholar]
- 8.Thygesen K, Mair J, Mueller C, Huber K, Weber M, Plebani M, Hasin Y, Biasucci LM, Giannitsis E, Lindahl B, Koenig W, Tubaro M, Collinson P, Katus H, Galvani M, Venge P, Alpert JS, Hamm C, Jaffe AS Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care. Recommendations for the use of natriuretic peptides in acute cardiac care: a position statement from the Study Group on Biomarkers in Cardiology of the ESC Working Group on Acute Cardiac Care. Eur Heart J. 2012;33:2001–2006. doi: 10.1093/eurheartj/ehq509. doi: 10.1093/eurheartj/ehq509. [DOI] [PubMed] [Google Scholar]
- 9.Wang TJ, Larson MG, Levy D, Benjamin EJ, Leip EP, Omland T, Wolf PA, Vasan RS. Plasma natriuretic peptide levels and the risk of cardiovascular events and death. N Engl J Med. 2004;350:655–663. doi: 10.1056/NEJMoa031994. doi: 10.1056/NEJMoa031994. [DOI] [PubMed] [Google Scholar]
- 10.Di Angelantonio E, Chowdhury R, Sarwar N, Ray KK, Gobin R, Saleheen D, Thompson A, Gudnason V, Sattar N, Danesh J. B-type natriuretic peptides and cardiovascular risk: systematic review and meta-analysis of 40 prospective studies. Circulation. 2009;120:2177–2187. doi: 10.1161/CIRCULATIONAHA.109.884866. doi: 10.1161/CIRCULATIONAHA.109.884866. [DOI] [PubMed] [Google Scholar]
- 11.Blankenberg S, Zeller T, Saarela O, Havulinna AS, Kee F, Tunstall-Pedoe H, Kuulasmaa K, Yarnell J, Schnabel RB, Wild PS, Münzel TF, Lackner KJ, Tiret L, Evans A, Salomaa V MORGAM Project. Contribution of 30 biomarkers to 10-year cardiovascular risk estimation in 2 population cohorts: the MONICA, Risk, Genetics, Archiving, And Monograph (MORGAM) biomarker project. Circulation. 2010;121:2388–2397. doi: 10.1161/CIRCULATIONAHA.109.901413. doi: 10.1161/CIRCULATIONAHA.109.901413. [DOI] [PubMed] [Google Scholar]
- 12.Everett BM, Berger JS, Manson JE, Ridker PM, Cook NR. B-type natriuretic peptides improve cardiovascular disease risk prediction in a cohort of women. J Am Coll Cardiol. 2014;64:1789–1797. doi: 10.1016/j.jacc.2014.04.089. doi: 10.1016/j.jacc.2014.04.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM, Jr, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, MacFadyen JG, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-reactive protein. N Engl J Med. 2008;359:2195–2207. doi: 10.1056/NEJMoa0807646. doi: 10.1056/NEJMoa0807646. [DOI] [PubMed] [Google Scholar]
- 14.Keller T, Zeller T, Ojeda F, Tzikas S, Lillpopp L, Sinning C, Wild P, Genth-Zotz S, Warnholtz A, Giannitsis E, Möckel M, Bickel C, Peetz D, Lackner K, Baldus S, Münzel T, Blankenberg S. Serial changes in highly sensitive troponin I assay and early diagnosis of myocardial infarction. JAMA. 2011;306:2684–2693. doi: 10.1001/jama.2011.1896. doi: 10.1001/jama.2011.1896. [DOI] [PubMed] [Google Scholar]
- 15.Apple FS, Ler R, Murakami MM. Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem. 2012;58:1574–1581. doi: 10.1373/clinchem.2012.192716. doi: 10.1373/clinchem.2012.192716. [DOI] [PubMed] [Google Scholar]
- 16.Aw TC, Phua SK, Tan SP. Measurement of cardiac troponin I in serum with a new high-sensitivity assay in a large multi-ethnic Asian cohort and the impact of gender. Clin Chim Acta. 2013;422:26–28. doi: 10.1016/j.cca.2013.03.034. doi: 10.1016/j.cca.2013.03.034. [DOI] [PubMed] [Google Scholar]
- 17.Hijazi Z, Siegbahn A, Andersson U, Granger CB, Alexander JH, Atar D, Gersh BJ, Mohan P, Harjola VP, Horowitz J, Husted S, Hylek EM, Lopes RD, McMurray JJ, Wallentin L ARISTOTLE Investigators. High-sensitivity troponin I for risk assessment in patients with atrial fibrillation: insights from the Apixaban for Reduction in Stroke and other Thromboembolic Events in Atrial Fibrillation (ARISTOTLE) trial. Circulation. 2014;129:625–634. doi: 10.1161/CIRCULATIONAHA.113.006286. doi: 10.1161/CIRCULATIONAHA.113.006286. [DOI] [PubMed] [Google Scholar]
- 18.Omland T, Pfeffer MA, Solomon SD, de Lemos JA, Røsjø H, Šaltytė Benth J, Maggioni A, Domanski MJ, Rouleau JL, Sabatine MS, Braunwald E PEACE Investigators. Prognostic value of cardiac troponin I measured with a highly sensitive assay in patients with stable coronary artery disease. J Am Coll Cardiol. 2013;61:1240–1249. doi: 10.1016/j.jacc.2012.12.026. doi: 10.1016/j.jacc.2012.12.026. [DOI] [PubMed] [Google Scholar]
- 19.Ridker PM, MacFadyen J, Libby P, Glynn RJ. Relation of baseline high-sensitivity C-reactive protein level to cardiovascular outcomes with rosuvastatin in the Justification for Use of statins in Prevention: an Intervention Trial Evaluating Rosuvastatin (JUPITER). Am J Cardiol. 2010;106:204–209. doi: 10.1016/j.amjcard.2010.03.018. doi: 10.1016/j.amjcard.2010.03.018. [DOI] [PubMed] [Google Scholar]
- 20.Ridker PM, MacFadyen JG, Fonseca FA, Genest J, Gotto AM, Kastelein JJ, Koenig W, Libby P, Lorenzatti AJ, Nordestgaard BG, Shepherd J, Willerson JT, Glynn RJ, Group JS. Number needed to treat with rosuvastatin to prevent first cardiovascular events and death among men and women with low low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein: Justification for the Use of Statins in Prevention: An Intervention Trial Evaluating Rosuvastatin (JUPITER). Circ Cardiovasc Qual Outcomes. 2009;2:616–623. doi: 10.1161/CIRCOUTCOMES.109.848473. [DOI] [PubMed] [Google Scholar]
- 21.Berger JS, Roncaglioni MC, Avanzini F, Pangrazzi I, Tognoni G, Brown DL. Aspirin for the primary prevention of cardiovascular events in women and men: a sex-specific meta-analysis of randomized controlled trials. JAMA. 2006;295:306–313. doi: 10.1001/jama.295.3.306. doi: 10.1001/jama.295.3.306. [DOI] [PubMed] [Google Scholar]
- 22.Pearce KA, Furberg CD, Psaty BM, Kirk J. Cost-minimization and the number needed to treat in uncomplicated hypertension. Am J Hypertens. 1998;11:618–629. doi: 10.1016/s0895-7061(97)00488-3. [DOI] [PubMed] [Google Scholar]
- 23.Wiysonge CS, Bradley H, Mayosi BM, Maroney R, Mbewu A, Opie LH, Volmink J. Beta-blockers for hypertension. Cochrane Database Syst Rev. 2007:CD002003. doi: 10.1002/14651858.CD002003.pub2. [DOI] [PubMed] [Google Scholar]
- 24.Kumana CR, Cheung BM, Lauder IJ. Gauging the impact of statins using number needed to treat. JAMA. 1999;282:1899–1901. doi: 10.1001/jama.282.20.1899. [DOI] [PubMed] [Google Scholar]
- 25.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels: Cholesterol and Recurrent Events Trial Investigators. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 26.Gravning J, Askevold ET, Nymo SH, Ueland T, Wikstrand J, McMurray JJ, Aukrust P, Gullestad L, Kjekshus J CORONA Study Group. Prognostic effect of high-sensitive troponin T assessment in elderly patients with chronic heart failure: results from the CORONA trial. Circ Heart Fail. 2014;7:96–103. doi: 10.1161/CIRCHEARTFAILURE.113.000450. doi: 10.1161/CIRCHEARTFAILURE.113.000450. [DOI] [PubMed] [Google Scholar]
- 27.Wang TJ, Wollert KC, Larson MG, Coglianese E, McCabe EL, Cheng S, Ho JE, Fradley MG, Ghorbani A, Xanthakis V, Kempf T, Benjamin EJ, Levy D, Vasan RS, Januzzi JL. Prognostic utility of novel biomarkers of cardiovascular stress: the Framingham Heart Study. Circulation. 2012;126:1596–1604. doi: 10.1161/CIRCULATIONAHA.112.129437. doi: 10.1161/CIRCULATIONAHA.112.129437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.White HD. Pathobiology of troponin elevations: do elevations occur with myocardial ischemia as well as necrosis? J Am Coll Cardiol. 2011;57:2406–2408. doi: 10.1016/j.jacc.2011.01.029. doi: 10.1016/j.jacc.2011.01.029. [DOI] [PubMed] [Google Scholar]
- 29.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabé-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Frisén J. Evidence for cardiomyocyte renewal in humans. Science. 2009;324:98–102. doi: 10.1126/science.1164680. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]


