Abstract
BACKGROUND AND OBJECTIVES:
Community pharmacies may be positioned for an increased role in population health. We sought to develop a population-level measure of asthma medication fills and assess its relationship to asthma-related utilization.
METHODS:
We conducted a retrospective, ecological study (2010–2012). Medication data from a chain of pharmacies (n = 27) within 1 county were used to calculate a Pharmacy-level Asthma Medication Ratio (Ph-AMR), defined as controller fills divided by controller plus rescue fills. Higher values are superior because they indicate more controller compared with rescue fills. The outcome was the asthma-related utilization rate among children in the same census tract as the pharmacy, calculated by dividing all emergency visits and hospitalizations by the number of children in that tract. Covariates, including ecological measures of poverty and access to care, were used in multivariable linear regression.
RESULTS:
Overall, 35 467 medications were filled. The median Ph-AMR was 0.53 (range 0.38–0.66). The median utilization rate across included census tracts was 22.4 visits per 1000 child-years (range 1.3–60.9). Tracts with Ph-AMR <0.5 had significantly higher utilization rates than those with Ph-AMR ≥0.5 (26.1 vs 9.9; P = .001). For every 0.1 increase in Ph-AMR, utilization rates decreased by 9.5 (P = .03), after adjustment for underlying poverty and access. Seasonal variation in fills was evident, but pharmacies in high-utilizing tracts filled more rescue than controller medications at nearly every point during the study period.
CONCLUSIONS:
Ph-AMR was independently associated with ecological childhood asthma morbidity. Pharmacies may be a community-based leverage point for improving population-level asthma control through targeted interventions.
What’s Known on This Subject:
Disparities in asthma morbidity are exacerbated by underutilization of preventive controller medications. Community pharmacies are well positioned for an increased role in population health. The Asthma Medication Ratio, currently used at the patient-level, could be adapted for use at the pharmacy-level.
What This Study Adds:
A newly developed Pharmacy-level Asthma Medication Ratio was associated with population-level childhood asthma morbidity. Collaborative relationships between physicians, community pharmacists, and patients (and families) have the potential to promote testable interventions aimed at reducing asthma morbidity and cross-community disparities.
Although asthma is the most common chronic illness of childhood, the disease in experienced in different ways.1–3 Asthma morbidity is especially common among those living with poverty and limited access.4–6 Disparities are perpetuated by chronic underutilization of medications that can prevent asthma symptoms.7–11 Underutilization results from underprescribing by providers, underavailability of medications at pharmacies, and nonadherence by patients.12–15 Excess morbidity resulting from underuse of proven, evidence-based asthma controller medications puts undue strain on patients, communities, and the health care system.
Pharmacies are well positioned for an increased role in population health management. Collaborative relationships between physicians and community pharmacists could enhance utilization of asthma therapies and improve outcomes for those at highest risk.16–18 As front-line members of the health care delivery system, pharmacies and pharmacists offer the advantage of both consistent patient interaction and the ability to track prescription data over time. Existing provider- and patient-level measures could be translated for use by pharmacies to gauge community-level adherence to evidence-based asthma care.19–21 Moreover, tracking medication fills could highlight ways in which pharmacies could deliver proactive, as opposed to reactive, asthma care.22,23 New or redesigned processes could benefit from novel performance measures that illustrate the quality of care that reaches patients.24
Ultimately, we hope to grow collaborative, cross-institutional, and community-based interventions aimed at reducing asthma morbidity and disparities. As a first step, we sought to bring together hospital-based utilization data and prescription medication fill data from in-county pharmacies managed by a single nationwide chain. Specifically, using the established Asthma Medication Ratio (AMR) as a guide, we sought to calculate a Pharmacy-level AMR (Ph-AMR) and assess its relationship with community-level asthma morbidity. We then sought to illustrate how asthma-related controller and rescue medication fills at participating pharmacies varied over both time and place (ie, across low- and high-morbidity communities) to more effectively identify when and where promotion of controller medication distribution could occur.
Methods
Study Design
We conducted a retrospective analysis using 2 distinct data sets, 1 from a large, academic children’s hospital and 1 from a single chain of retail pharmacies. The 2 data sets provided information on the same geographic region; however, they were not able to be linked at the patient level.
The hospital-based data set included children, aged 2 to 17 years, who visited the emergency department (ED) or were hospitalized for asthma at Cincinnati Children’s Hospital Medical Center (CCHMC) facilities between January 31, 2010, and January 30, 2012. Subjects were identified using asthma-specific diagnoses (International Classification of Diseases, ninth Revision, Clinical Modification 493.XX).25 Children living outside of Hamilton County, Ohio, were excluded. Ohio Hospital Association data suggest that nearly 95% of in-county asthma-related hospitalizations occur at CCHMC facilities.26 ED-related market share data are less clear, but we expect that visits to other institutions would be minimal given CCHMC’s strong market penetration.
A separate administrative data set was provided by a single national retail chain with 27 in-county pharmacies in 27 distinct census tracts. Census tracts are relatively homogenous areas of ∼4000 people defined by local municipalities and the US Census.27 The included chain is among the local leaders for in-county market share with an estimated 45% (B. Turner, personal communication, 2014). This data set comprised all 35 467 asthma medications filled for deidentified children aged 2 to 17 years during the same time period as the utilization events. Included medications classified as controllers were inhaled corticosteroids (flunisolide, mometasone, triamcinolone, ciclesonide, fluticasone, budesonide, beclomethasone), combined inhaled corticosteroids/long-acting β-agonists (fluticasone/salmeterol, mometasone/formoterol, budesonide/formoterol), and leukotriene receptor antagonists (montelukast). Included rescue medications were inhaled short-acting β-agonists (albuterol, levalbuterol).
Calculation of the Ph-AMR
We calculated a Ph-AMR for each included pharmacy. The Ph-AMR was calculated similarly to the AMR, a National Committee for Quality Assurance measure for patients with persistent asthma, by dividing all controller by summed controller and rescue fills.28 It is generally calculated over a 1-year period. This yields a ratio ranging from 0 to 1 where 1 is ideal (ie, all controller and no rescue fills).
After classifying medications as either controller or rescue, we calculated a Ph-AMR for each pharmacy by dividing all dispensed controller medications by the sum of all controller plus all rescue medications. The resulting Ph-AMR was analyzed, first as a dichotomous variable, split at 0.5. This cut point was chosen given previous evidence at the patient level demonstrating that an AMR <0.5 is associated with increased utilization (eg, ED visitation or hospitalization) and lower quality of life.19,21,29,30 Second, we explored a potential dose effect using quintiles, placing each pharmacy into 1 of 5 groups based on ascending Ph-AMR. Finally, Ph-AMR was treated as a continuous variable.
Outcomes
Our primary outcome was ecological child asthma morbidity, defined by rates of asthma utilization for each included census tract. We opted to use the population living in the same census tract as the pharmacy as a proxy for that pharmacy’s market share. The pharmacies’ 27 distinct tracts were composed of a combined 129 161 individuals and 25 599 children.27
Asthma utilization rates were calculated using hospital data. All ED visits and hospitalizations were connected to the patient’s home address at the time of the utilization event. This address was geocoded and mapped to the corresponding 2010 census tract. We then divided the sum of all in-tract utilization events by the total number of children, aged 2 to 17 years, living within that tract. This was converted to a rate, measured per 1000 children per year, or per 1000 child-years.
Covariates
Many area-level factors have been shown to be relevant to asthma morbidity. To maintain a stable, parsimonious model, we focused on both poverty and access to care because they were seen as particularly relevant to both utilization and the filling of prescribed medications.13 Thus, we extracted data elements available to us from the US Census’ 2008–2012 American Community Survey. Poverty was defined as the percentage of in-tract individuals living below the federal poverty line. Limited car access was defined as the percentage of tract households with no car.27 As a further measure of medical care access, we used the Health Resource and Services Administration–defined Medically Underserved Areas (MUA).31 To ensure that census tracts were composed of similar age distributions, we also calculated the proportion of children within each tract using data from the 2010 U.S. Census (number of children aged 2–17 years divided by total tract population).27 The MUA variable was binary, whereas each of the other variables was continuous.
Data Analysis
Descriptive statistics were used to illustrate demographic characteristics of patients in the hospital-based data set and variation in Ph-AMR across included pharmacies. The Wilcoxon rank sum test, analysis of variance, and unadjusted linear regression models were used to assess bivariate relationships between categories of Ph-AMR (dichotomized, in quintiles, and continuous, respectively) and tract-level utilization rates, and covariates. Multivariable linear regression was then used to assess associations between the Ph-AMR and utilization after adjustment for covariates.
We used time-series analyses to further assess relationships between controller and rescue medication fills. First, we created a chart that depicted medication fills, by week, across all pharmacies. Cross-correlation functions assessed any potential time-delay or seasonal trend in fills. A linear mixed model with autoregressive moving average covariance structure was applied to a time-varying Ph-AMR to further evaluate seasonal effects. We created 2 additional charts, splitting pharmacies based on the utilization rate of the tract in which each pharmacy was located. Fills at pharmacies in the 13 “healthy” or “low-utilizing” tracts were on 1 chart and pharmacies in the 14 “sick” or “high-utilizing” tracts were on a second. These charts were used to assess differences in numbers of controller and rescue fills over time.
Geocoding and mapping were pursued using ArcGIS software (version 10.1, Redlands, CA). Statistical analyses were performed using SAS software (version 9.3, Cary, NC).
This study was approved by the CCHMC Institutional Review Board.
Results
During the study period, there were 554 asthma-related ED visits and 203 hospitalizations from the included census tracts (N = 27). These 757 utilization events involved 474 unique children who were 58% male, 53% African American, and 57% publicly insured, with a median age of 6.2 years (Table 1). The median utilization rate across the 27 tracts was 22.4 events per 1000 child-years (range 1.3–60.9).
TABLE 1.
Data Available for Characteristics of Individual Patients From the 27 Included Census Tracts That Had an Asthma-Related Emergency Department Visit or Hospital Admission Between January 31, 2010 and January 30, 2012 (n = 474)
| N (or Median) | %a (or IQR) | |
|---|---|---|
| Age (y) | 6.2 | 3.6–10.0 |
| Male gender | 277 | 58.4 |
| Race | ||
| Black/African American | 249 | 52.5 |
| White | 168 | 35.4 |
| Other | 47 | 9.9 |
| Unknown or missing | 10 | 2.1 |
| Hispanic ethnicity | 20 | 4.2 |
| Insurance | ||
| Public | 269 | 56.8 |
| Private | 191 | 40.3 |
| Unknown or missing | 14 | 3.0 |
Percentages may not add to exactly 100 due to rounding.
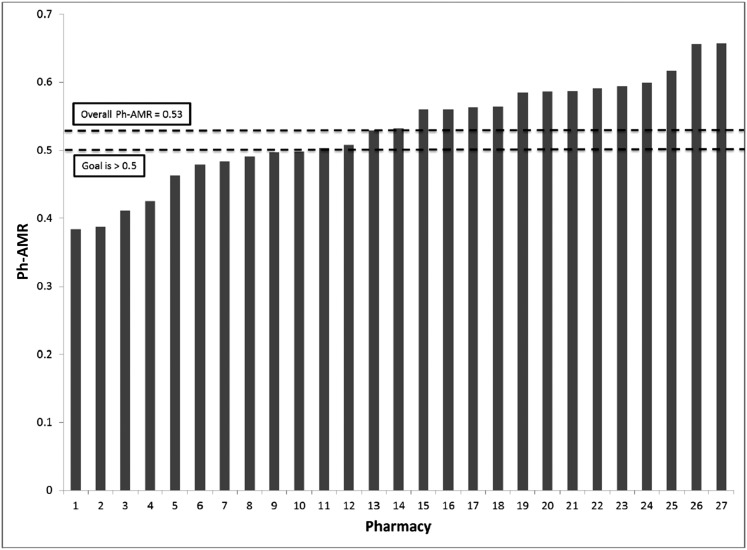
Included pharmacies dispensed 18 842 controller and 16 625 rescue medications. There was considerable variation in Ph-AMR across individual pharmacies (median Ph-AMR of 0.53, range 0.38–0.66; 10 pharmacies had a Ph-AMR <0.5) (Fig 1).
FIGURE 1.
Ph-AMR for a single chain of 27 Hamilton County, Ohio, pharmacies. Goal of >0.5 extrapolated from what has been found to be optimal at the patient level.
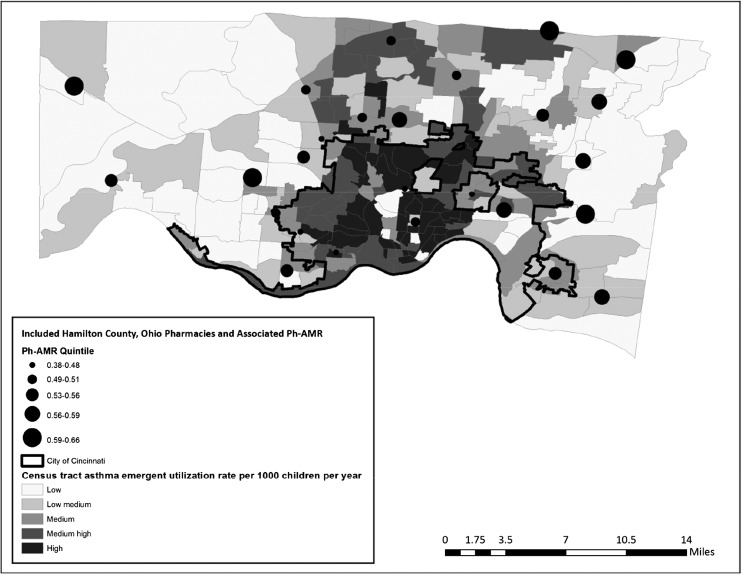
Pharmacies were well distributed geographically, both in Cincinnati’s urban core and outlying suburbs (Fig 2). Across included tracts, the median percentage of individuals living below the federal poverty line was 12.2% (range 2.3%–42.2%). The median percentage of households with no car was 8.5% (range 1.5%–25.6%).
FIGURE 2.
Ph-AMR and asthma emergent utilization based on census tract, both measured for time period between January 31, 2010, and January 30, 2012.
Census tracts with pharmacies that had a Ph-AMR <0.5 had significantly higher utilization rates than tracts with pharmacies that had a Ph-AMR ≥0.5 (26.1 vs 9.9 events per 1000 child-years; P = .001). Similarly, low Ph-AMR tracts had higher rates of poverty (16.6% vs 8.5%; P = .02) and households without cars (13.8% vs 6.8%; P = .07). All 3 tracts that were located in an MUA were also located in tracts with pharmacies that had a Ph-AMR <0.5 (Fisher’s exact test P < .05).
Ph-AMR quintile was associated with population-level emergent utilization rates in a graded fashion (Table 2). The utilization rate in the group with the lowest Ph-AMR was 28.4 events per 1000 child-years; the quintile with the highest Ph-AMR had a utilization rate of 7.1 per 1000 children (P = .001). Similar trends were noted for relationships between Ph-AMR quintile and poverty and limited car access (P = .05 and P = .02, respectively). The proportion of children did not differ significantly between included census tracts.
TABLE 2.
Ph-AMR Quintile and Associations With Census Tract–Level Covariatesa and the Primary Outcome, Census Tract Emergent Utilization Rate per 1000 Children
| Ph-AMR Quintile | Child Population (%) | Poverty Rate (%)b | No Car Rateb (%) | Utilization Ratec (per 1000 children/y) |
|---|---|---|---|---|
| 0.38–0.48 | 19.6 | 18.9 | 17.3 | 28.4 |
| 0.49–0.51 | 18.8 | 15.2 | 10.8 | 22.4 |
| 0.53–0.56 | 19.9 | 11.8 | 8.0 | 10.0 |
| 0.56–0.59 | 20.3 | 5.9 | 5.9 | 10.4 |
| 0.59–0.66 | 20.5 | 5.9 | 5.0 | 7.1 |
Weighted averages, using population denominators available from the US Census.
P ≤ .05 for relationship between Ph-AMR and census tract–level variable.
P < .01 for relationship between Ph-AMR and census tract–level variable.
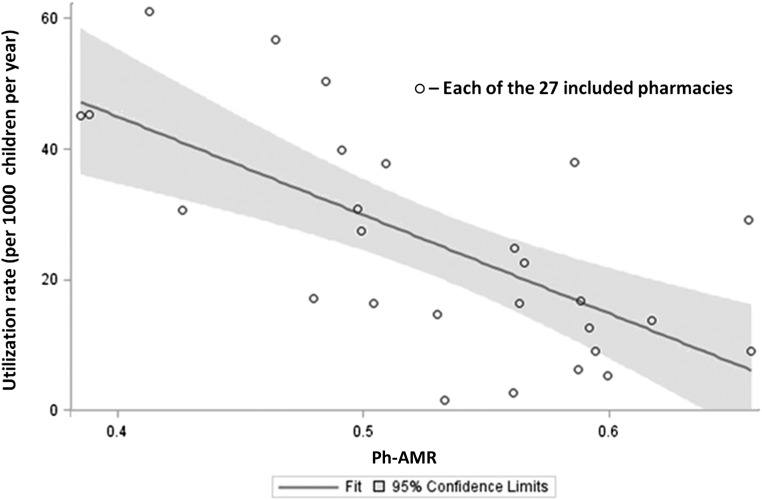
Figure 3 illustrates the linear relationship between Ph-AMR, on a continuous scale, and utilization rates (R2 = 0.45, P < .0001). When the model included poverty, car access and MUA, the R2 rose to 0.66, with the model accounting for 66% of the variation (Ph-AMR P = .03).
FIGURE 3.
Linear regression fit plot for Ph-AMR and asthma-related emergent utilization rate for the census tract in which the pharmacy is located. Unadjusted linear regression; R2 for this model is 0.45 (P = .0001).
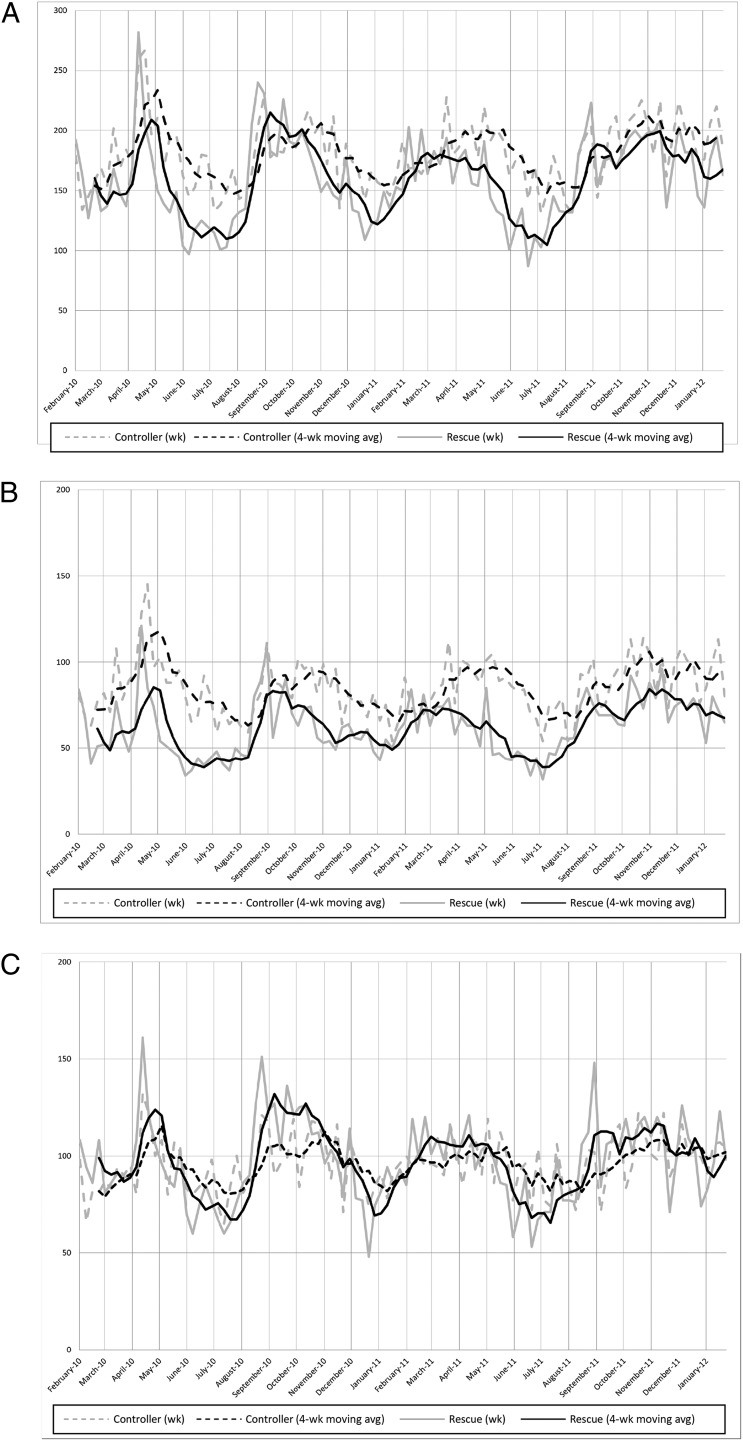
Figure 4 illustrates our time-series analyses. Dotted lines represent controller fills, and solid lines represent rescue medication fills. The gray lines are weekly data, and the black lines are 4-week moving averages. In Fig 4A, we depict medication fills at all pharmacies. There is significant variation across time with peaks of both controller and rescue medication fills occurring in spring and fall. The cross-correlation function of controller and rescue medication fills illustrates alignment for the timing of fill peaks and valleys consistent with the presumed seasonal variation. A Ph-AMR calculated at various time points demonstrates that spring and summer were found to have significantly higher Ph-AMR values than winter (on average, the Ph-AMR is 2.2% higher in spring, P = .05; and 3.1% higher in summer, P = .005), suggesting that controllers are filled more regularly during these seasons.
FIGURE 4.
Time-series illustrating absolute number of all controller and rescue medication fills by week over a 2-year period for (A) all pharmacies, (B) pharmacies within a census tract with emergent utilization rate below the median; and (C) pharmacies within a census tract with emergent utilization rate above the median. avg, average.
Figure 4B presents data just for the 13 pharmacies within low-utilizing, “healthy” tracts (ie, below the median). Similar seasonal trends are noted, although the controller line is higher than the rescue line at nearly all points over time. Figure 4C presents data for the 14 pharmacies within census tracts that have utilization rates above the median. Here, the rescue line appears higher than the controller line at nearly all points. Indeed, pharmacies in “healthy,” low-utilizing neighborhoods dispensed more controller medications; pharmacies in “sick,” high-utilizing neighborhoods dispensed more rescue medications (P < .0001).
Discussion
There is a strong relationship between a newly developed pharmacy-level metric that quantifies the ratio of dispensed asthma controller and rescue medications and asthma emergent care utilization. A 0.1 increase in a pharmacy’s Ph-AMR was associated with a reduction of 9.5 events per 1000 in the corresponding census tract’s utilization rate. If, in fact, these relationships are causal, as supported by the previous literature, improved Ph-AMR rates, and associated use of proven controller therapies, would substantially reduce asthma morbidity and its associated cost.
Asthma is a manageable chronic illness with an evidence base highlighting the positive impact daily controller medications can have on symptom prevention. Still, morbidity persists, disproportionately affecting patients living in high-poverty and low-access communities.4 Underutilization of controllers likely contributes to observed disparities and is especially common among underserved children.9,10,13 Lintzenich et al found that just 52% of children aged 2 to 18 years were prescribed a controller medication after discharge from the hospital—patients who would qualify for and benefit from such medications.7 Given that many of these same patients may not follow up with a primary care physician,7 alternative opportunities (and locations) for care delivery should be explored.
Pharmacies with low Ph-AMRs were in tracts that had utilization rates 4 times those of pharmacies with high Ph-AMRs. Likewise, pharmacies in high-morbidity tracts dispensed more rescue than controller medications at nearly all points over the study period. Given their expertise and in-community location, pharmacies are well positioned to facilitate evidence-based management of chronic asthma, especially within communities at highest risk. Indeed, collaborations between hospitals and pharmacies, physicians and pharmacists, would be an important step toward the development and testing of potential interventions aimed at improving care delivery and avoiding or preventing of emergent care visits. Multidisciplinary teams that include community pharmacists could facilitate the provision of comprehensive patient- and population-level care. Such teams could break down barriers that have historically fragmented health care. The Ph-AMR would allow pharmacies to track such collaborations, functioning as a pharmacy-level performance measure and complementing other measures used by the Pharmacy Quality Alliance.32
The AMR is currently used by physicians and managed care organizations to improve adherence to evidence-based guidelines and has shown consistent associations with symptom control.19–21,24,30 Guided by the AMR and motivated by deep disparities across our community,4 we developed the Ph-AMR as a means of harnessing geographic variation to inform allocation of resources to those pharmacies and areas in most need of improvement.33,34 The Ph-AMR could identify pharmacies that are underdispensing controller medications while also facilitating a deeper understanding among pharmacies of their population in ways that would prompt the design of innovative interventions. In partnership with hospitals, pharmacies may also use underlying asthma utilization rates to highlight high-risk communities. Pharmacies could then more aggressively provide medication delivery or counseling services, flag patients who refill a disproportionate number of rescue medications compared with controllers, or more regularly communicate with physicians regarding those patients who do not seem to be responding to current therapy. Such pharmacy-driven asthma intervention programs have been met with success.22,35,36 One program in Asheville, North Carolina, focused on targeted asthma education, scheduled consultations, and pharmacy-led monitoring and led to improvements in asthma control and reductions in asthma utilization.22
Identification of problem spots, targeting of resources, and tracking of performance are all especially relevant with the goal of improved population health and contained cost. Health care delivery organizations would benefit from a deeper understanding of where interventions could be put in place that favor chronic prevention over response to acute exacerbation.37 As Hacker and Walker note, population health requires an enhanced “capacity to assess, monitor, and prioritize” impactful risk factors.17 This capacity is augmented by using existing data, such as that kept by pharmacies, in innovative and additive ways. The Ph-AMR and the following of fill patterns enables physicians, pharmacists, and accountable care organizations to continuously monitor actionable trends. Such measurement strategies could highlight areas where health promotion could be prioritized; pharmacies could focus on dispensing controller medications as a means toward proactive prevention, toward appropriate anticipation of seasonal morbidity peaks rather than as a reaction to seasonal variation in demand. Moreover, the Ph-AMR could be used to identify high-risk areas and then the patient-level AMR could be used, in concert, to track specific high-risk patients.
This study is not without limitations. First, analyses relied on a single pharmacy chain’s administrative data. Nevertheless, included pharmacies have ∼45% of the market share and are well distributed across the county. Second, those that use this pharmacy chain may differ from those who do not. Data from other chains and in other regions are needed to validate and generalize our findings. Third, we do not know the true market for each individual pharmacy. We believe the census tract is a conservative estimate of market share. Still, a family’s choice of pharmacy may be independently related to where they live and/or where they seek medical care. Fourth, we believe that included covariates address some but likely not all confounding. The number of pharmacies included in the analyses precluded us from adjusting for many more variables. Residual factors such as health-care-seeking behaviors, exposure to tobacco smoke, exposure to indoor and outdoor pollutants or allergens, and influenza rates all may be associated with the outcome, but such data were not available to us. We did not adjust for race/ethnicity given that we expected race/ethnicity was likely related to both predictor and outcome through the other assessed covariates (eg, poverty, access).3 Fifth, the data sets used were distinct and were unable to be matched at the patient level. Finally, the sample through which we calculated our population-level outcome was drawn from a single hospital; we cannot account for children who seek care elsewhere. However, the vast majority of in-county children use CCHMC for hospital care; we expect that a similarly high percentage makes use of our emergency facilities.
In the future, we will test the Ph-AMR against additional outcomes (eg, acute asthma-related primary care visits). We also will assess whether an analogous medication ratio can be developed at the community-level using insurance claims data. Additionally, we expect to translate our findings into the implementation and evaluation of targeted interventions aimed at increasing controller medication fills in those areas at highest risk.
Conclusions
This newly developed Ph-AMR was strongly associated with population-level childhood asthma morbidity. Pharmacies located in high-utilizing census tracts were more likely to dispense rescue medications than controller medications. Collaborative relationships among physicians, community pharmacists, and patients (and families) have the potential to promote testable interventions aimed at reducing asthma morbidity and cross-community disparities. In this way, pharmacies could play a substantial role as a population health leverage point for assessing and improving asthma control within a community.
Acknowledgments
We thank Ms Margaret Jones, Dr Karen Jerardi, and Ms Hadley Sauers for reviewing the manuscript. None of those mentioned had pertinent financial disclosures or conflicts of interest to report.
Footnotes
Dr Beck conceptualized and designed the study, carried out the initial analysis, drafted the initial manuscript, and revised the manuscript; Drs Bradley and Heaton provided a pharmacy perspective for the design of the initial analysis and revised the manuscript; Dr Huang advised on the initial study analysis, carried out subsequent analyses, and reviewed and revised the manuscript; Dr Simmons conceptualized and designed the study, supervised data collection, and revised the manuscript; Dr Kahn conceptualized and designed the study, supervised data collection, and critically reviewed and revised the manuscript; and all authors approved the final manuscript as submitted.
These data were presented in abstract form at the American Public Health Association Annual Meeting & Exposition; November 18, 2014; New Orleans, LA.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Support for this work was provided through the Cincinnati Children’s Research Foundation Procter Scholar Award and through the National Institutes of Health (NIH 1K23AI112916). Funders played no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript.
POTENTIAL CONFLICT OF INTEREST: Dr Bradley received funding from Kroger Pharmacy during the time of manuscript preparation, but not during data collection, as part of pharmacy residency training. The other authors have indicated they have no potential conflicts to disclose.
COMPANION PAPER: A companion to this article can be found on page 1133, and online at www.pediatrics.org/cgi/doi/10.1542/peds.2015-0809.
References
- 1.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980–2007. Pediatrics. 2009;123(suppl 3):S131–S145 [DOI] [PubMed] [Google Scholar]
- 2.Akinbami LJ, Moorman JE, Liu X. Asthma prevalence, health care use, and mortality: United States, 2005–2009. Natl Health Stat Rep. 2011;32:1–14 [PubMed] [Google Scholar]
- 3.Beck AF, Huang B, Simmons JM, et al. Role of financial and social hardships in asthma racial disparities. Pediatrics. 2014;133(3):431–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beck AF, Moncrief T, Huang B, et al. Inequalities in neighborhood child asthma admission rates and underlying community characteristics in one US county. J Pediatr. 2013;163(2):574–580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Williams DR, Sternthal M, Wright RJ. Social determinants: taking the social context of asthma seriously. Pediatrics. 2009;123(suppl 3):S174–S184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wright RJ, Subramanian SV. Advancing a multilevel framework for epidemiologic research on asthma disparities. Chest. 2007;132(suppl 5):757S–769S [DOI] [PubMed] [Google Scholar]
- 7.Lintzenich A, Teufel RJ, II, Basco WT, Jr. Under-utilization of controller medications and poor follow-up rates among hospitalized asthma patients. Hosp Pediatr. 2011;1(1):8–14 [DOI] [PubMed] [Google Scholar]
- 8.Adams RJ, Fuhlbrigge A, Finkelstein JA, et al. Impact of inhaled antiinflammatory therapy on hospitalization and emergency department visits for children with asthma. Pediatrics. 2001;107(4):706–711 [DOI] [PubMed] [Google Scholar]
- 9.Finkelstein JA, Lozano P, Farber HJ, Miroshnik I, Lieu TA. Underuse of controller medications among Medicaid-insured children with asthma. Arch Pediatr Adolesc Med. 2002;156(6):562–567 [DOI] [PubMed] [Google Scholar]
- 10.Wilson SE, Leonard A, Moomaw C, Schneeweiss S, Eckman MH. Underuse of controller medications among children with persistent asthma in the Ohio medicaid population: evolving differences with new medications. Ambul Pediatr. 2005;5(2):83–89 [DOI] [PubMed] [Google Scholar]
- 11.Gottlieb DJ, Beiser AS, O’Connor GT. Poverty, race, and medication use are correlates of asthma hospitalization rates. A small area analysis in Boston. Chest. 1995;108(1):28–35 [DOI] [PubMed] [Google Scholar]
- 12.Amstislavski P, Matthews A, Sheffield S, Maroko AR, Weedon J. Medication deserts: survey of neighborhood disparities in availability of prescription medications. Int J Health Geogr. 2012;11:48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bollinger ME, Mudd KE, Boldt A, Hsu VD, Tsoukleris MG, Butz AM. Prescription fill patterns in underserved children with asthma receiving subspecialty care. Ann Allergy Asthma Immunol. 2013;111(3):185–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fischer MA, Stedman MR, Lii J, et al. Primary medication non-adherence: analysis of 195,930 electronic prescriptions. J Gen Intern Med. 2010;25(4):284–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mansour ME, Lanphear BP, DeWitt TG. Barriers to asthma care in urban children: parent perspectives. Pediatrics. 2000;106(3):512–519 [DOI] [PubMed] [Google Scholar]
- 16.Pollack CE, Armstrong K. Accountable care organizations and health care disparities. JAMA. 2011;305(16):1706–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hacker K, Walker DK. Achieving population health in accountable care organizations. Am J Public Health. 2013;103(7):1163–1167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stine NW, Chokshi DA, Gourevitch MN. Improving population health in US cities. JAMA. 2013;309(5):449–450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schatz M, Nakahiro R, Crawford W, Mendoza G, Mosen D, Stibolt TB. Asthma quality-of-care markers using administrative data. Chest. 2005;128(4):1968–1973 [DOI] [PubMed] [Google Scholar]
- 20.Vernacchio L, Trudell EK, Muto JM. Correlation of care process measures with childhood asthma exacerbations. Pediatrics. 2013;131(1):e136–e143 [DOI] [PubMed] [Google Scholar]
- 21.Andrews AL, Simpson AN, Basco WT, Jr, Teufel RJ, II. Asthma medication ratio predicts emergency department visits and hospitalizations in children with asthma. Medicare Medicaid Res Rev. 2013;3(4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bunting BA, Cranor CW. The Asheville Project: long-term clinical, humanistic, and economic outcomes of a community-based medication therapy management program for asthma. J Am Pharm Assoc (2003). 2006;46(2):133–147 [DOI] [PubMed]
- 23.van Boven JF, Hiddink EG, Stuurman-Bieze AG, Schuiling-Veninga CC, Postma MJ, Vegter S. The pharmacists’ potential to provide targets for interventions to optimize pharmacotherapy in patients with asthma. Int J Clin Pharmacol. 2013;35(6):1075–1082 [DOI] [PubMed] [Google Scholar]
- 24.Schatz M, Zeiger RS. Improving asthma outcomes in large populations. J Allergy Clin Immunol. 2011;128(2):273–277 [DOI] [PubMed] [Google Scholar]
- 25.International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). Available at: http://www.cdc.gov/nchs/icd/icd9cm.htm. Accessed May 25, 2014
- 26.Bosnjakovic E. INSIGHT Database. Columbus, OH: Ohio Hospital Association; 2009 [Google Scholar]
- 27.American FactFinder. 2013. Available at: http://factfinder2.census.gov/faces/nav/jsf/pages/searchresults.xhtml?refresh=t. Accessed July 12, 2013
- 28.Measures HEDIS. 2014. Available at: http://www.ncqa.org. Accessed May 25, 2014
- 29.Yong PL, Werner RM. Process quality measures and asthma exacerbations in the medicaid population. J Allergy Clin Immunol. 2009;124(5):961–966 [DOI] [PubMed] [Google Scholar]
- 30.Schatz M, Zeiger RS, Vollmer WM, et al. The controller-to-total asthma medication ratio is associated with patient-centered as well as utilization outcomes. Chest. 2006;130(1):43–50 [DOI] [PubMed] [Google Scholar]
- 31.Find Shortage Areas. MUA/P by State and County. 2014. Available at: http://muafind.hrsa.gov. Accessed December 29, 2014
- 32.Performance Measures PQA. 2014. Available at: http://pqaalliance.org/measures/default.asp. Accessed June 15, 2014
- 33.Hardt NS, Muhamed S, Das R, Estrella R, Roth J. Neighborhood-level hot spot maps to inform delivery of primary care and allocation of social resources. Perm J. 2013;17(1):4–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miranda ML, Ferranti J, Strauss B, Neelon B, Califf RM. Geographic health information systems: a platform to support the “triple aim.” Health Aff (Millwood). 2013;32(9):1608–1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.González-Martin G, Joo I, Sánchez I. Evaluation of the impact of a pharmaceutical care program in children with asthma. Patient Educ Couns. 2003;49(1):13–18 [DOI] [PubMed] [Google Scholar]
- 36.Stergachis A, Gardner JS, Anderson MT, Sullivan SD. Improving pediatric asthma outcomes in the community setting: does pharmaceutical care make a difference? J Am Pharm Assoc (Wash). 2002;42(5):743–752 [DOI] [PubMed] [Google Scholar]
- 37.Williams DR, Costa MV, Odunlami AO, Mohammed SA. Moving upstream: how interventions that address the social determinants of health can improve health and reduce disparities. J Public Health Manag Pract. 2008;14(suppl):S8–S17 [DOI] [PMC free article] [PubMed] [Google Scholar]