Abstract
BACKGROUND AND OBJECTIVES:
Extremely preterm infants and infants born to adolescent mothers are at risk for adverse developmental. The objectives were to evaluate development and behavior outcomes of extremely low birth weight (ELBW) infants born to adolescent mothers <20 compared with adult mothers ≥20 years and to identify socioeconomic risk factors that affect outcomes.
METHODS:
Retrospective cohort analysis of 211 infants >27 weeks of adolescent mothers and 1723 infants of adult mothers at Neonatal Research Network centers from 2008 to 2011. Groups were compared and regression models were run to predict 18- to 22-month adverse outcomes. Primary outcomes were Bayley-III scores, neurodevelopmental impairment, and Brief Infant Toddler Social Emotional Assessment problem scores (BITSEA/P) ≥75th percentile.
RESULTS:
Adolescent mothers were more often single, Hispanic, less educated, and had public insurance. By 18 to 22 months, their children had significantly increased rates of having lived ≥3 places (21% vs 9%), state supervision (7% vs 3%), rehospitalization (56% vs 46%), and BITSEA/P ≥75th percentile (50% vs 32%) and nonsignificant Bayley-III language scores <85 (56% vs 49%, P = .07). In regression analysis, children of adolescent mothers were more likely to have BITSEA/P ≥75th percentile (relative risk 1.50, 95% confidence interval 1.08–2.07). Living ≥3 places and nonwhite race were predictors of adverse behavior. State supervision was an independent predictor of each Bayley-III composite <70 and neurodevelopmental impairment.
CONCLUSIONS:
ELBW infants of adolescent mothers experience high social and environmental risks that are associated with adverse behavior outcomes. These findings inform the need for comprehensive follow-up, coordinated care services, and behavior interventions for ELBW infants of adolescent mothers.
What’s Known on This Subject:
Infants born extremely premature and infants born to adolescent mothers are at risk for adverse developmental and behavior outcomes. There is limited research on the dual risk imparted to infants born extremely premature to adolescent mothers.
What This Study Adds:
Extremely premature infants of adolescent mothers have significantly increased rates of behavior problems. Nonwhite race and living in ≥3 places by 18 to 22 months of age are risk factors for adverse behavior outcomes among infants of adolescent mothers.
Infants born extremely preterm and to adolescent mothers are at increased risk for adverse developmental outcomes. Although survival of extremely low birth weight (ELBW) infants has improved, the rates of many neonatal morbidities and developmental impairment have remained unchanged or only minimally improved.1,2 ELBW infants have high rates of adverse cognitive and language outcomes that persist through school age.3–7 Infants of adolescent mothers also have adverse developmental outcomes on a variety of measures.8–10 There is limited research on the combined risk of extreme prematurity and having an adolescent mother. A large body of literature indicates that socioeconomic status (SES) influences development. The construct of lower SES is multifaceted and includes maternal race, age, ethnicity, marital status, education, income, and medical insurance. These factors have been consistently associated with adverse developmental outcomes11 and are imbedded within the context and risks for adolescent pregnancy.12
There are multiple coexisting socioeconomic and biologic factors that place extremely preterm infants of adolescent mothers at increased risk for adverse developmental and behavioral outcomes. Previous studies of extremely preterm infants have not explored the unique social risks of adolescent motherhood and previous studies of infants of adolescent mothers have not included extremely preterm infants. This study investigates the relationships between adolescents’ complex social environments and the developmental and behavioral outcomes of their extremely preterm infants at 18 to 22 months corrected age. The objectives of this study were (1) to evaluate the cognitive, language, and behavior outcomes of extremely preterm infants born to adolescent mothers (<20 years) compared with extremely preterm infants born to older mothers (≥20 years), and (2) to explore the unique social and home constructs of infants with adolescent mothers and the influences of these environmental factors on developmental and behavioral outcomes. It was hypothesized that extremely preterm infants born to adolescent mothers have (1) poorer Bayley Scales of Infant Development-3rd Edition (BSID-III) cognitive and language scores, (2) increased rates of neurodevelopmental impairment (NDI), and (3) increased behavior problems at 18 to 22 months, and that adolescent mothers would be more likely to have social and environmental factors that are associated with adverse outcomes than mothers ≥20 years.
Methods
This was a retrospective cohort analysis of data previously collected from the Eunice Kennedy Shriver National Institute of Child Health and Human Development’s (NICHD) Neonatal Research Network (NRN) Generic Database and Follow-Up studies. Infants born in participating NRN hospitals at <27 weeks’ gestation from January 1, 2008, to June 30, 2011, and who underwent comprehensive neurologic and developmental assessments at 18 to 22 months corrected age were included in the study. Infants with major congenital anomalies or syndromes associated with adverse developmental outcomes were excluded. All maternal and neonatal data and outcomes were prospectively collected in the NRN generic database by trained research personnel. Centers participating in the NRN each receive institutional review board approval for data collection.
At 18 to 22 months corrected age, a comprehensive neurodevelopmental assessment, including a complete neurologic examination and BSID-III, was performed by certified examiners.7,13 The BSID-III is a standardized test for children 1 to 42 months that includes scaled and composite scores, percentile ranks, and age equivalents (with confidence intervals [CIs]) for cognitive, language, and motor scores. Composite scores have a mean of 100 and an SD of 15. The language and motor composite scores are further divided into expressive and receptive language as well as gross and fine motor subsets. Each subset has a mean of 10 and an SD of 3, with individual age equivalents.13,14 The Brief Infant Social Emotional Assessment (BITSEA) is also conducted at 18 to 22 months. The BITSEA includes a Problem Scale (BITSEA/P) with 31 items and a Competence Scale (BITSEA/C) with 11 items. Each item has 3 response possibilities: 0, “not true/rarely true”; 1, “sometimes true/sometimes”; and 2, “very true/often.” Higher total scores on BITSEA/P reflect increased behavior and social problems and lower scores on BITSEA/C reflect lower levels of competence.15 The primary study outcomes were BSID-III composite cognitive and language scores. Secondary outcomes were BITSEA scores, NDI (moderate to severe cerebral palsy with Palisano Gross Motor Function Classification Scale ≥2, walks without assisted devices but with limitations walking outdoors), 18- to 22-month growth parameters and rates of rehospitalization.16 Infants were grouped according to maternal age (cohort 1 = infants/adolescent mother <20 years, cohort 2 = infant/adult mother ≥20 years). The cut point of 20 years was used be consistent with the Centers for Disease Control and Prevention’s definition of teen pregnancy and previous NRN reports.17,18
Baseline maternal, infant, and social characteristics and 18- to 22-month outcomes were compared between groups; χ2 tests were used for categorical variables and t tests for continuous variables. Regression models were used to compare relative risk (RR) of adverse outcomes at 18 to 22 months, controlling for infant and maternal characteristics that varied significantly between groups. The models were run on the total study cohort and adolescent mothers only. The following outcomes were analyzed: (1) BSID-III language composite <70 and <85, (2) BSID-III cognitive composite <70 and <85, (3) NDI, (4) BITSEA/P ≥ 75th percentile, and (5) BITSEA/C ≤15th percentile. In cases in which control variables were highly related or overlapped, only 1 control variable was included to avoid overestimation problems due to multicollinearity. All analyses were conducted by using SAS version 9.3 (SAS Institute, Inc, Cary, NC). The available sample sizes (n = 211 for adolescent mothers and n = 1723 for nonadolescent mothers) provide at least 80% power for detecting a small difference (h = 0.20) in proportions of children having adverse outcomes across the 2 groups at a P value of .05.19
Results
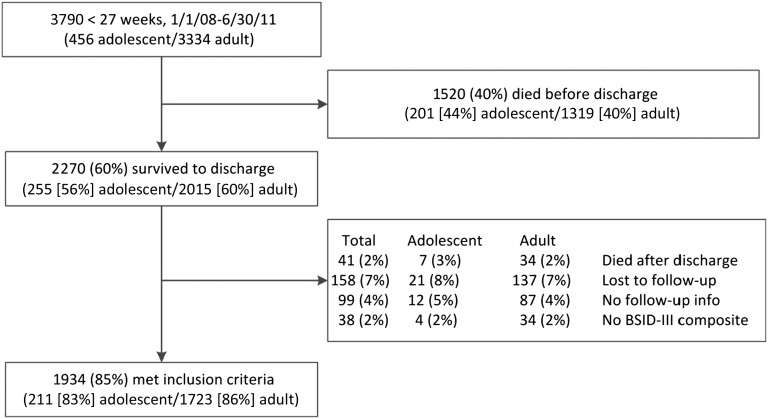
A total of 3790 infants born at <27 weeks’ gestational age were admitted to NRN centers between January 1, 2008, and June 30, 2011. Derivation of the study cohort is shown in Fig 1. There were no significant differences between rates of death before or after discharge, loss to follow-up, or insufficient follow-up. The analyses included 1934 infants with BSID-III scores at 18- to 22-month follow-up visit; 211 (10.9%) were infants of adolescent mothers.
FIGURE 1.
Study flowchart.
Table 1 includes comparisons of maternal and infant characteristics among children born to adolescent and adult mothers. The mean age of the adolescent cohort was 17.7 ± 1.3 years (3% 13–14 years, 32% 15–16 years, 64% 17–19 years) and the mean age of the older cohort was 28.6 ± 5.8 years. Adolescent mothers were significantly more likely to have singleton births and less likely to have cesarean delivery, rupture of membranes (ROM) >18 hours before delivery, receive antenatal steroids, or have hypertension/preeclampsia than adult mothers. Adolescent mothers were more likely gravida 1, had lower education levels, were more likely to be Hispanic and less likely to be white than adult mothers. Adolescent mothers were more often single. Infants had similar gender, birth weight, gestational age, and rates of common neonatal morbidities.
TABLE 1.
Maternal and Infant Characteristics
| Characteristic | Adolescent, <20 y | Adult, ≥20 y | P |
|---|---|---|---|
| Maternal characteristics | |||
| Gravida, mean ± SD | 3.0 ± 2.1 | 1.5 ± 0.9 | <.001 |
| Gravida, n (%) | <.001a | ||
| 1 | 138 (65) | 450 (26) | <.001 |
| 2 | 50 (24) | 407 (24) | .99 |
| 3+ | 23 (11) | 864 (50) | <.001 |
| Chorioamnionitis, n (%) | 41 (19) | 341 (20) | .89 |
| Singleton pregnancy, n (%) | 179 (85) | 1323 (77) | .008 |
| Prenatal care (at least 1 visit), n (%) | 200 (95) | 1656 (96) | .31 |
| Cesarean delivery, n (%) | 108 (51) | 1149 (67) | < .001 |
| PPROM (ROM >18 h), n (%) | 46 (22) | 494 (29) | .04 |
| Antenatal steroids, n (%) | 177 (84) | 1544 (90) | .009 |
| PIH/preeclampsia, n (%) | 23 (11) | 400 (23) | < .001 |
| Highest grade completed, n (%) | <.001a | ||
| Less than high school | 122 (58) | 233 (14) | < .001 |
| High school graduate | 48 (23) | 360 (21) | .53 |
| Some college | 14 (7) | 339 (20) | < .001 |
| College graduate | 1 (0) | 327 (19) | < .001 |
| Unknown | 26 (12) | 464 (27) | < .001 |
| Race/ethnicity, n (%) | <.001a | ||
| White | 57 (27) | 629 (37) | .007 |
| African American | 88 (42) | 620 (36) | .10 |
| Hispanic/Latino | 49 (23) | 248 (14) | < .001 |
| Other | 6 (3) | 105 (6) | .06 |
| Unknown | 11 (5) | 121 (7) | .33 |
| Single, n (%) | 156 (74) | 779 (45) | < .001 |
| Infant characteristics | |||
| Male, % | 105 (50) | 820 (48) | .55 |
| Birth weight, g, mean ± SD | 782.4 ± 173.7 | 763.3 ± 151.6 | .09 |
| Gestational age, wk, mean ± SD | 24.9±1.0 | 25.0±1.0 | .35 |
| Postnatal steroids, n (%) | 39 (18) | 328 (19) | .84 |
| Early-onset sepsis (+ blood culture ≤72 h), n (%) | 2 (1) | 41 (2) | .18 |
| Late-onset sepsis (+ blood culture >72 h), n (%) | 76 (36) | 536 (31) | .15 |
| NEC (Bell classification IIA or higher), n (%) | 25 (12) | 167 (10) | .32 |
| IVH (Grade III-IV), n (%) | 40 (19) | 258 (15) | .12 |
| Cystic PVL, n (%) | 10 (5) | 97 (6) | .60 |
| ROP, n (%) | 145 (70) | 1280 (75) | .12 |
| BPD (supplemental oxygen at 36weeks), n (%) | 112 (54) | 954 (56) | .60 |
| Days of ventilation, mean ± SD | 27.9 ± 24.9 | 28.3 ± 25.2 | .83 |
| Days in hospital, mean ± SD | 116.4 ± 50.0 | 116.4 ± 44.7 | .99 |
Significance test of overall effect across all categories of variable. BPD, bronchopulmonary dysplasia; IVH, intraventricular hemorrhage; NEC, necrotizing enterocolitis; PIH, pregnancy induced hypertension; PPROM, preterm premature rupture of membranes; ROP, retinopathy of prematurity.
Social and environmental characteristics at 18- to 22-month follow-up are shown in Table 2. Infants and their adolescent mothers were more likely to have public insurance or be uninsured and less likely to have private insurance. Infants of adolescent mothers were significantly more likely to have received special services from a visiting nurse or social worker, to be under state supervision, and to have lived more places since discharge. Adolescent mothers were less likely to be married and to not be the primary caretaker at 18 to 22 months. Infants of adolescent mothers were more likely to have grandparents or nonrelatives as primary caretakers or to be in congregate care. Infants of adolescent mothers were significantly less likely to live in 2-parent households and were more likely to live in households with biologic parent(s) in an extended family or in households without a biologic parent.
TABLE 2.
Social and Environmental Characteristics at 18 to 22 Months
| Characteristic | Adolescent, <20 y | Adult, ≥20 y | P |
|---|---|---|---|
| Medical insurance, n (%) | < .001a | ||
| Public | 159 (75) | 832 (48) | < .001 |
| Private | 25 (12) | 766 (44) | < .001 |
| Uninsured | 23 (11) | 80 (5) | < .001 |
| Special services received at 18 mo, n (%) | |||
| Visiting nurse | 113 (54) | 784 (46) | .02 |
| Social worker | 43 (20) | 248 (14) | .02 |
| Home nurse | 12 (6) | 121 (7) | .48 |
| Physical therapy | 118 (56) | 980 (57) | .80 |
| Occupational therapy | 74 (35) | 619 (36) | .81 |
| Early intervention | 145 (69) | 1147 (67) | .58 |
| State supervision, n (%) | 15 (7) | 60 (3) | .01 |
| No. places lived since discharge, mean ± SD | 1.9±0.9 | 1.5±0.7 | < .001 |
| No. places lived since discharge, n (%) | <.001a | ||
| 1 | 78 (37) | 1011 (59) | < .001 |
| 2 | 88 (42) | 543 (32) | .003 |
| 3+ | 43 (21) | 159 (9) | < .001 |
| Caretaker married, n (%) | 54 (26) | 938 (55) | < .001 |
| Primary caretaker not mother at 18-mo visit | 41 (19) | 119 (7) | < .001 |
| Primary caretaker, n (%) | <.001a | ||
| Mother | 170 (81) | 1599 (93) | < .001 |
| Father | 6 (3) | 31 (2) | .30 |
| Maternal grandparent(s) | 15 (7) | 18 (1) | < .001 |
| Paternal grandparent(s) | 2 (1) | 3 (0) | .04 |
| Other relative | 2 (1) | 6 (0) | .20 |
| Nonrelative | 14 (7) | 59 (3) | .02 |
| Congregate care | 1 (0) | 0 (0) | .004 |
| Still hospitalized | 1 (0) | 2 (0) | .21 |
| Living arrangements, n (%) | < .001a | ||
| Single biologic parent | 37 (18) | 292 (17) | .81 |
| 2-parent household (with biologic parent) | 59 (28) | 1069 (62) | < .001 |
| Biologic parent in extended family | 82 (39) | 280 (16) | < .001 |
| Household without biologic parent | 31 (15) | 74 (4) | < .001 |
| Total no. of people in house, mean ± SD | 4.4 ± 2.1 | 4.3 ± 1.5 | .28 |
| Day care/child care, n (%) | |||
| Traditional day care | 19 (9) | 195 (11) | .31 |
| Special day care | 4 (2) | 61 (4) | .21 |
| Home care | 18 (9) | 214 (12) | .10 |
| Babysitter/au pair | 27 (13) | 319 (19) | .04 |
| Primary Language, n (%) | .31a | ||
| English | 189 (90) | 1487 (87) | .22 |
| Spanish | 19 (9) | 178 (10) | .54 |
| Other | 3 (1) | 53 (3) | .18 |
| Bilingual home, n (%) | 61 (29) | 437 (25) | .28 |
Significance test of overall effect across all categories of variable.
Table 3 shows 18- to 22-month child outcomes. There were no significant differences between groups in BSID-III cognitive, language, and motor composite scores (mean score, composite <70 or <85) or NDI. Infants of both adolescent and older mothers had high rates of language composite scores <85 (adolescent 56%, adult 49%, P = .07) and receptive language scores <7 (adolescent 47%, adult 40%, P = .08). Infants of adolescent mothers had higher BITSEA/P scores (mean 14.8 vs 12.1, P < .001) and BITSEA/P scores ≥75th percentile (50% vs 32%, P < .001) than infants of adult mothers. Infants of adolescent mothers had similar weight and length but significantly smaller head circumferences and increased rehospitalization compared with infants of older mothers at 18 to 22 months.
TABLE 3.
Outcomes at 18- to 22-Month Follow-up
| Characteristic | Adolescent, <20 y | Adult, ≥20 y | P |
|---|---|---|---|
| Age at follow-up, mo, mean ± SD | 20.3 ± 2.7 | 20.4 ± 2.7 | .71 |
| Neurologic examination, n (%) | |||
| Normal | 142 (67) | 1097 (64) | .31 |
| Moderate to severe cerebral palsy | 14 (7) | 102 (6) | .68 |
| Vision impairment (<20:200 bilateral), n (%) | 4 (2) | 20 (1) | .36 |
| Hearing impairment (require amplification), n (%) | 5 (2) | 58 (3) | .44 |
| Neurodevelopmental impairment, n (%) | 38 (18) | 311 (18) | 1.00 |
| Bayley III | |||
| Cognitive composite score, mean ± SD | 88.0 ± 15.7 | 89.1 ± 15.5 | .34 |
| Language composite score, mean ± SD | 83.0 ± 17.0 | 84.5 ± 17.2 | .22 |
| Expressive language score, mean ± SD | 7.6 ± 2.8 | 7.6 ± 3.0 | .76 |
| Receptive language score, mean ± SD | 7.1 ± 2.6 | 7.4 ± 3.0 | .24 |
| Motor composite score, mean ± SD | 87.0 ± 17.3 | 87.6 ± 16.7 | .62 |
| Fine motor score, mean ± SD | 8.5 ± 2.9 | 8.6 ± 3.0 | .74 |
| Gross motor score, mean ± SD | 7.6 ± 2.8 | 7.5 ± 2.8 | .68 |
| Cognitive composite score <85, n (%) | 58 (27) | 495 (29) | .69 |
| Language composite score <85, n (%) | 117 (56) | 831 (49) | .07 |
| Expressive language score <7, n (%) | 81 (40) | 623 (38) | .47 |
| Receptive language score <7, n (%) | 93 (47) | 666 (40) | .08 |
| BITSEA | |||
| Social emotional problems, mean ± SD | 14.8 ± 7.7 | 12.1 ± 7.2 | <.001 |
| Social emotional competencies, mean ± SD | 16.6 ± 3.4 | 16.6 ± 3.7 | .98 |
| Social emotional problems >75%ile, n (%) | 101 (50) | 543 (32) | <.001 |
| Social emotional competencies <15%ile, n (%) | 54 (27) | 440 (27) | .98 |
| Weight, g, mean ± SD | 10.8 ± 1.7 | 10.7 ± 1.5 | .46 |
| Length, cm, mean ± SD | 80.9 ± 6.7 | 81.5 ± 4.7 | .10 |
| Head circumference, cm, mean ± SD | 46.4 ± 2.1 | 46.8 ± 2.3 | .03 |
| Rehospitalization | |||
| Rehospitalized since discharge, n (%) | 119 (56) | 796 (46) | .005 |
| No. of times, mean ± SD | 1.2 ± 1.8 | 1.1 ± 1.9 | .20 |
Table 4 shows regression analysis of the total study cohort controlling for infant and maternal characteristics that varied across groups. Children of adolescent mothers were significantly more likely to have BITSEA/P scores ≥75th percentile (RR 1.47, 95% CI 1.06–2.04). Cognitive, language and motor scores and NDI did not vary significantly by maternal age after controlling for other factors. Cesarean delivery was associated with increased risk of language composite <70 and public insurance with increased risks of cognitive and language composites <85, BITSEA/P ≥75th percentile and BITSEA/C ≤25th percentile. Nonwhite race imparted an increased risk of language composite <85 and decreased risk of motor composite <70. Antenatal steroids were protective with lower rates of adverse cognitive, language, and BITSEA/P scores. Living in ≥3 places since discharge increased risks of BITSEA/P ≥75th percentile, whereas living in 2 places was associated with lower rates of adverse language scores. State supervision imparted increased risks of adverse cognitive, language, and motor scores and NDI. In regression analysis of adolescent mothers only (Table 5), cesarean delivery and prolonged ROM were associated with increased likelihood of language composite <70. Antenatal steroids were protective, resulting in lower rates of language and motor composites <70, NDI, and BITSEA/P scores ≥75th percentile. Household composition, public insurance, or being cared for by a babysitter was not predictive of any composite BSID-III, NDI, or BITSEA scores. Adverse BITSEA/P scores were significantly more likely among infants with nonwhite race (RR 2.43, 95% CI 1.13–5.22) and having lived in more than 3 places since discharge (RR 3.04, 95% CI 1.19–7.73). State supervision was an independent predictor of each BSID-III composite score <70 (cognitive RR 7.27, 95% CI 1.80–29.41, language RR 8.83, 95% CI 2.00–38.86, motor RR 6.45 95% CI 1.58–26.29) and NDI (RR 4.74, 95% CI 1.27–17.72).
TABLE 4.
Regression Analysis of Biologic and Social Risk Factors on Development and Behavior Outcomes at 18- to 22-Month Follow-up for Entire Study Cohort (Adult and Adolescent Mothers)
| Characteristic | Cognitive Composite <70 | Cognitive Composite <85 | Language Composite <70 | Language Composite <85 | Motor Composite <70 | NDI | BITSEA/P ≥ 75th percentile | BITSEA/C ≤15th percentile |
|---|---|---|---|---|---|---|---|---|
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Adolescent mother, <20 y old | 1.42 (0.88–2.29) | 0.83 (0.58–1.17) | 0.97 (0.64–1.47) | 1.15 (0.83–1.59) | 1.00 (0.62–1.59) | 1.01 (0.67–1.52) | 1.57 (1.14–2.18)* | 0.84 (0.58–1.20) |
| Biologic factors | ||||||||
| Multiple birth | 0.75 (0.50,1.13) | 0.98 (0.75–1.29) | 0.90 (0.66–1.24) | 1.12 (0.87–1.43) | 0.76 (0.52–1.10) | 0.76 (0.55–1.05) | 1.03 (0.79–1.34) | 0.96 (0.72–1.27) |
| Cesarean delivery | 1.36 (0.95–1.94) | 1.06 (0.84–1.34) | 1.39 (1.05–1.84)* | 1.10 (0.89–1.36) | 1.20 (0.88–1.65) | 1.19 (0.91–1.56) | 1.15 (0.91–1.44) | 1.10 (0.87–1.41) |
| PPROM (ROM >18 h) | 0.79 (0.54–1.16) | 0.88 (0.69–1.13) | 0.94 (0.71–1.26) | 0.99 (0.80–1.24) | 0.88 (0.63–1.23) | 0.86 (0.65–1.15) | 1.12 (0.89–1.41) | 0.86 (0.67–1.11) |
| PIH/Preeclampsia | 0.89 (0.59–1.33) | 1.07 (0.82–1.40) | 0.77 (0.56–1.06) | 0.92 (0.71–1.18) | 1.05 (0.73–1.49) | 0.93 (0.68–1.27) | 0.94 (0.72–1.22) | 1.06 (0.80–1.39) |
| Antenatal steroids | 0.94 (0.57–1.52) | 0.72 (0.51–1.00)* | 0.66 (0.46–0.96)* | 0.84 (0.61–1.17) | 0.75 (0.49–1.15) | 0.94 (0.63–1.39) | 0.64 (0.46–0.89)* | 1.21 (0.84–1.76) |
| Social Factors | ||||||||
| Household composition | ||||||||
| Single biologic parent | 0.86 (0.55–1.35) | 1.04 (0.77–1.40) | 0.82 (0.57–1.17) | 1.12 (0.84–1.47) | 1.10 (0.74–1.63) | 0.99 (0.70–1.40) | 1.15 (0.86–1.53) | 1.30 (0.96–1.76) |
| 2-parent household (with biologic parent) | REF | REF | REF | REF | REF | REF | REF | REF |
| Biologic parent in extended family | 0.78 (0.50–1.23) | 1.01 (0.75–1.34) | 0.78 (0.55–1.11) | 1.00 (0.77–1.30) | 0.91 (0.61–1.35) | 0.79 (0.55–1.12) | 1.22 (0.93–1.61) | 1.14 (0.85–1.53) |
| Household without biologic parent | 0.94 (0.47–1.91) | 1.31 (0.79–2.18) | 0.84 (0.46–1.55) | 1.04 (0.64–1.70) | 0.82 (0.42–1.63) | 1.00 (0.56–1.79) | 1.18 (0.71–1.98) | 1.60 (0.95–2.71) |
| Public insurance | 1.10 (0.77–1.56) | 1.34 (1.06–1.70)* | 1.15 (0.87–1.51) | 1.54 (1.24–1.91)* | 1.34 (0.97–1.84) | 1.16 (0.88–1.53) | 1.92 (1.53–2.42)* | 1.41 (1.11–1.80)* |
| Nonwhite race | 0.79 (0.56–1.12) | 1.02 (0.80–1.30) | 1.10 (0.83–1.46) | 1.41 (1.13–1.76)* | 0.63 (0.46–0.86)* | 0.79 (0.60–1.04) | 1.21 (0.96–1.53) | 1.11 (0.86–1.43) |
| Cared for by babysitter | 0.72 (0.45–1.13) | 0.88 (0.66–1.18) | 0.65 (0.45–0.92)* | 0.86 (0.66–1.10) | 0.71 (0.48–1.07) | 0.81 (0.58–1.13) | 0.88 (0.67–1.16) | 0.64 (0.47–0.86)* |
| No. of places lived since discharge | ||||||||
| 1 | REF | REF | REF | REF | REF | REF | REF | REF |
| 2 | 0.89 (0.62–1.26) | 0.87 (0.69–1.10) | 0.64 (0.48–0.85)* | 0.81 (0.65–1.00)* | 0.83 (0.60–1.14) | 0.82 (0.62–1.08) | 1.23 (0.98–1.54) | 1.09 (0.86–1.37) |
| ≥3 | 0.99 (0.60–1.64) | 0.90 (0.63–1.28) | 0.77 (0.51–1.17) | 0.93 (0.67–1.30) | 0.99 (0.63–1.56) | 0.86 (0.57–1.30) | 1.64 (1.18–2.29)* | 0.99 (0.70–1.43) |
| State supervision | 2.16 (1.06–4.42)* | 1.84 (1.05–3.22)* | 1.91 (1.02–3.59)* | 1.08 (0.61–1.91) | 2.44 (1.27–4.69)* | 2.02 (1.10–3.71)* | 0.55 (0.30–1.02) | 1.03 (0.56–1.89) |
significant; PIH, pregnancy induced hypertension; PPROM, preterm premature rupture of membranes; REF, reference parameter.
TABLE 5.
Regression Analysis of Biologic and Social Risk Factors on Development and Behavior Outcomes at 18- to 22-Month Follow-up for Infants of Adolescent Mothers Only
| Characteristic | Cognitive Composite <70 | Cognitive Composite <85 | Language Composite <70 | Language Composite <85 | Motor Composite <70 | NDI | BITSEA/P > 75th percentile | BITSEA/C ≤ 15th percentile |
|---|---|---|---|---|---|---|---|---|
| RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | RR (95% CI) | |
| Biologic factors | ||||||||
| Multiple birth | 0.48 (0.10–2.33) | 1.26 (0.43–3.67) | 0.59 (0.15–2.33) | 0.56 (0.20–1.63) | 0.72 (0.18–2.94) | 0.37 (0.10–1.43) | 0.52 (0.20–1.39) | 0.91 (0.31–2.70) |
| Cesarean delivery | 1.41 (0.52–3.86) | 1.38 (0.62–3.04) | 5.50 (1.98–15.27)* | 0.94 (0.47–1.91) | 0.97 (0.36–2.61) | 1.31 (0.56–3.08) | 0.73 (0.37–1.44) | 0.58 (0.27–1.26) |
| PPROM (ROM > 18 h) | 1.72 (0.58–5.04) | 2.39 (0.98–5.82) | 4.09 (1.32–12.67)* | 1.30 (0.58–2.92) | 2.86 (0.97–8.39) | 1.84 (0.71–4.75) | 0.80 (0.36–1.80) | 1.12 (0.46–2.72) |
| PIH/Preeclampsia | 0.58 (0.10–3.20) | 0.49 (0.12–1.94) | 0.30 (0.06–1.56) | 1.03 (0.34–3.12) | 0.65 (0.11–3.98) | 0.49 (0.11–2.23) | 1.49 (0.51–4.37) | 0.85 (0.24–3.00) |
| Antenatal steroids | 0.83 (0.22–3.22) | 0.42 (0.16–1.09) | 0.26 (0.08–0.81)* | 0.43 (0.17–1.13) | 0.31 (0.10–0.96)* | 0.32 (0.11–0.90)* | 0.34 (0.14–0.86)* | 0.59 (0.23–1.54) |
| Social factors | ||||||||
| Household composition | ||||||||
| Single biologic parent | 0.31 (0.06–1.61) | 0.60 (0.20–1.87) | 0.56 (0.14–2.16) | 0.79 (0.29–2.17) | 0.73 (0.18–3.02) | 0.56 (0.17–1.86) | 1.64 (0.65–4.10) | 0.67 (0.24–1.82) |
| 2-parent household (with biologic parent) | REF | REF | REF | REF | REF | REF | REF | REF |
| Biologic parent in extended family | 0.40 (0.12–1.38) | 0.56 (0.22–1.39) | 0.41 (0.13–1.34) | 0.85 (0.24–1.25) | 0.68 (0.21–2.17) | 0.51 (0.18–1.43) | 1.22 (0.57–2.60) | 0.48 (0.20–1.13) |
| Household without biologic parent | 1.13 (0.33–3.86) | 0.74 (0.23–2.44) | 0.64 (0.16–2.57) | 0.72 (0.24–2.20) | 0.71 (0.18–2.89) | 1.35 (0.43–4.27) | 0.98 (0.34–2.88) | 1.14 (0.37–3.50) |
| Public insurance | 1.26 (0.41–3.84) | 1.27 (0.53–3.03) | 0.71 (0.26–1.99) | 1.12 (0.52–2.44) | 1.16 (0.39–3.44) | 1.26 (0.48–3.30) | 1.03 (0.48–2.18) | 1.94 (0.80–4.71) |
| Nonwhite race | 0.70 (0.24–2.01) | 0.90 (0.37–2.18) | 1.32 (0.43–4.08) | 1.77 (0.79–3.99) | 0.77 (0.27–2.20) | 0.86 (0.34–2.15) | 2.43 (1.13–5.22)* | 1.58 (0.67–3.76) |
| Cared for by babysitter | 0.56 (0.09–3.35) | 1.56 (0.53–4.57) | 0.34 (0.05–2.21) | 1.46 (0.55–3.83) | 1.09 (0.25–4.72) | 1.08 (0.30–3.91) | 1.99 (0.77–5.11) | 1.91 (0.72–5.06) |
| Number of places lived since discharge | ||||||||
| 1 | REF | REF | REF | REF | REF | REF | REF | REF |
| 2 | 1.29 (0.44–3.75) | 0.86 (0.38–1.96) | 0.36 (0.13–1.06) | 0.91 (0.44–1.90) | 0.60 (0.21–1.74) | 1.18 (0.47–2.97) | 0.97 (0.49–1.95) | 1.28 (0.58–2.81) |
| ≥3 | 1.09 (0.27–4.38) | 1.23 (0.45–3.40) | 1.02 (0.31–3.35) | 1.71 (0.67–4.37) | 0.97 (0.29–3.26) | 1.67 (0.54–5.11) | 3.04 (1.19–7.73)* | 1.23 (0.45–3.36) |
| State supervision | 7.27 (1.80–29.41)* | 13.95 (2.84–68.58)* | 8.83 (2.00–38.86)* | 6.21 (1.17–33.02)* | 6.45 (1.58–26.29)* | 4.74 (1.27–17.72)* | 1.55 (0.37–6.51) | 3.37 (0.80–14.18) |
significant; PIH, pregnancy induced hypertension; PPROM, preterm premature rupture of membranes; REF, reference parameter.
Discussion
This study was designed to assess the dual developmental risk for extremely premature infants born to adolescent mothers and to explore the developmental influences of the complex social environments of adolescent mothers. There has been limited research examining the developmental risk of prematurity and having an adolescent mother. In 1980, Field et al9 examined 4 mother-infant dyads within a population of low SES black women: preterm infant-adolescent mother (one-half randomized to home-based parent-training intervention arm, one-half control arm), preterm infant-adult mother, term infant-adolescent mother, and term infant-adult mother. At 8 months of age, preterm infants of adolescent mothers in the control arm had the lowest Bayley Mental Development Index (MDI) scores (101) compared with preterm infants with adolescent mothers in the intervention arm (MDI 110), preterm infants with older mothers (MDI 111), and term infants with any aged mother (MDI 113.5). In a report of NRN data focusing on neurodevelopment outcomes relative to higher maternal age, Vohr et al17 found an increased incidence of NDI or death in ELBW infants of adolescent mothers at 18 to 22 months corrected age (68% <20 years old versus 60% 30–39 years old). The mean BSID-II MDI of ELBW infants of mothers <20 was 77.6 ± 17.0 and 33.8% had an MDI <70.
This is the first report of BSID-III cognitive and language outcomes of extremely preterm infants born to adolescent mothers. Although the BSID-III is currently administered in many follow-up studies of preterm infants, several reports suggest it underestimates cognitive, language, and motor delays.4 As such, thresholds for reporting results in the literatures have shifted from scores <70 to scores <85, or both. Our findings did not support our first 2 hypotheses that cognitive and language scores are lower and NDI rates are higher for preterm infants of adolescent mothers compared with infants of older mothers. Our analysis showed similar BSID-III cognitive scores for both age groups: 27% of infants to adolescent mothers and 29% of infants of older mothers had cognitive composites <85. Infants of both adolescent and older mothers had high rates of language composite scores <85 (adolescent 56%, adult 49%, P = .07) and receptive language scores <7 (adolescent 47%, adult 40%, P = .08). Interestingly, infants of adolescent mothers had significantly smaller head circumferences. Previous review of NICHD data demonstrates a significant relationship between poor head growth and increased rates of cerebral palsy, adverse Bayley MDI, and NDI.20 Similarly, Cheong et al21 found strong correlations with head circumference and brain volume, and microcephaly at age 2 was associated with adverse neurodevelopmental outcomes and cerebral palsy. The similarities between cognitive and language outcomes of ELBW infants of adolescent and older mothers may reflect the high use of early intervention services at 18 to 22 months (69%) and close developmental follow-up for all extremely preterm infants in our NRN centers. The developmental outcomes of infants with adolescent mothers also may have been buffered by the increased need and utilization of visiting nurse and social work services. Nonetheless, a significant number of extremely preterm infants born to adolescent mothers are at risk for developmental delays. This reiterates the need for close follow-up and aggressive early intervention for this high-risk population.
Our findings support our third hypothesis that infants of adolescent mothers have increased behavioral problems compared with infants of older mothers. Infants of adolescent mothers had increased mean BITSEA/P social and emotional scores (mean 14.8 vs 12.1, P < .001, and BITSEA/P scores ≥75th percentile 50% vs 32%, P < .001). In regression analysis of total study population, having an adolescent mother was an independent predictor of elevated BITSEA/P scores. Historically, infants of adolescent mothers exhibit more hyperactivity, hostility, resistance, and lack of impulse control and adjustment skills.8,22 Infant temperament, maternal depression, unwanted pregnancy, and quality of home environment have been found to be statistical predictors of behavioral problems, inattention, and aggression.23–25 These same risk factors are embedded in adolescent motherhood.9,26 Also, language delays in the first 10 months have been associated with psychopathology at school age,25 and low levels of adult speech at 1 year predict later diagnosis of childhood behavior and psychiatric disorders.26–28 Our regression analysis of the adolescent cohort revealed that nonwhite race and living in 3 or more places predicted elevated BITSEA/P scores. We speculate that nonwhite race and living in 3 or more places may be reflective of unstable home environments that contribute to behavioral issues.
Our findings support our final hypothesis that adolescent mothers have more social and environmental factors that are associated with adverse outcomes than older mothers. A previous report of adolescent mothers found that 73% were single, 57% had not completed high school, 76% had Medicaid, 33% screened positive for depression, and 33% had been reported to child protection service for maltreatment or neglect.12 We also found increased rates of environmental risks for adolescent mothers, with 74% single, 58% with less than high school education, 86% public insurance/uninsured, 73% nonwhite race, 21% living in more than 3 locations, and 7% with state supervision (The NRN database provides a full spectrum of a variety of home compositions of ELBW infants born to adolescent mothers. Black et al12 previously reported that among adolescent mothers, 25% of households consisted of 3 generations: grandmother-mother-child. Our data also show that adolescent mothers are more likely to live within an extended family (39% vs 16%, P < .001) or in a household without a biologic parent (15% vs 4%, P < .001) than older mothers. The effect of coresidence with a grandmother on development has been inconsistent. Most research on the role of family structure suggests that the presence of adults other than the young mother can mitigate the deleterious health, developmental, and psychosocial effects on the child.29
Regression analyses were performed to determine the RR of adverse outcome imparted by social and environmental factors. Among all extremely preterm infants, nonwhite race, which was more common among adolescent mothers, was associated with language composite score <85. In regression analysis among adolescent mothers only, none of the household compositions, nonwhite race, public insurance, number of places lived, or being cared for by a babysitter were significant predictors of Bayley scores or NDI. State supervision was more prevalent among infants of adolescent mothers and was an independent predictor of each Bayley score <70 and NDI.
We found no significant differences in common neonatal morbidities associated with adverse outcomes between the 2 groups. Ninety-five percent of adolescent mothers in this study received prenatal care, and adolescent mothers had fewer biologic risk factors, including cesarean delivery, multiple deliveries, prolonged ROM, and hypertension or preeclampsia. These lower rates of maternal biologic risk factors and similar rates of neonatal risks demonstrate the important influence of social factors on development of infants with adolescent mothers.
There are several limitations to this study, including the retrospective study design. The BITSEA is a parent questionnaire and not a formal behavioral assessment. The BITSEA results therefore can be biased by parental reporting. It is also difficult to analyze the specific individual risk imparted by environmental risk factors even after controlling for overlapping variables. These risks do not occur in isolation and are likely additive in nature. An alternative form of analysis may yield different information on the effects of multiple SES risks on each other and on infant development. A major strength of this study is that this is the first study designed to assess the dual risk of extreme prematurity and adolescent mother. It has the benefit of using prospectively collected data on an extremely large cohort of infants within the NRN. Finally, it is the first study reporting Bayley-III unique cognitive and language scores for infants of adolescent mothers.
Conclusions
Extremely preterm infants of adolescent mothers remain a high-risk population for adverse development outcomes, especially social-emotional and behavior problems. State supervision, as an indicator of abuse and neglect, is prevalent among adolescent mothers and is an independent risk factor for poor outcomes in all developmental domains. The increased use of special services, including social work and visiting nurse, among infants of adolescent mothers, and high rates of early intervention for all extremely preterm infants may mitigate some of the adverse developmental effects of having an adolescent mother. These findings inform the need for comprehensive follow-up and coordinated care services for extremely preterm infants and their adolescent mothers. Future research should focus on behavior intervention strategies for extremely preterm infants of adolescent mothers and to minimize the effects of unstable living environments and factors that contribute to state supervision of infants.
Acknowledgments
The National Institutes of Health and NICHD provided grant support for the Neonatal Research Network’s Generic Database (January 1, 2008, to June 30, 2011) and Follow-up Study (October 1, 2009, to February 28, 2013) through cooperative agreements. Although NICHD staff did have input into the study design, conduct, analysis, and manuscript drafting, the comments and views of the authors do not necessarily represent the views of NICHD.
Data collected at participating sites of the NICHD NRN were transmitted to RTI International, the data coordinating center (DCC) for the network, which stored, managed, and analyzed the data for this study. On behalf of the NRN, Dr Abhik Das (DCC principal investigator) and Mr Douglas Kendrick (DCC statistician) had full access to all the data in the study and take responsibility for the integrity of the data and accuracy of the data analysis.
We are indebted to our medical and nursing colleagues and the infants and their parents who agreed to take part in this study. The following investigators, in addition to those listed as authors, participated in this study:
NRN Steering Committee Chair: Michael S. Caplan, MD, University of Chicago, Pritzker School of Medicine; Richard A. Polin, MD, Division of Neonatology, College of Physicians and Surgeons, Columbia University (2011–present).
Alpert Medical School of Brown University and Women & Infants Hospital of Rhode Island (U10 HD27904): Abbot R. Laptook, MD; Angelita M. Hensman, RN, BSN; William Oh, MD; Martin Keszler, MD; Robert Burke, MD; Melinda Caskey, MD; Katharine Johnson, MD; Barbara Alksninis, PNP; Theresa M. Leach, MEd, CAES; Bonnie E. Stephens, MD; Victoria E. Watson, MS, CAS; Suzy Ventura; Kristin M. Basso, BSN, MA; Elisa Vieira, RN, BSN; Andrea Halbrook.
Case Western Reserve University, Rainbow Babies & Children's Hospital (U10 HD21364, M01 RR80): Michele C. Walsh, MD, MS; Avroy A. Fanaroff, MD; Anna Marie Hibbs, MD; Deanne E. Wilson-Costello, MD; Nancy S. Newman, BA, RN; Bonnie S. Siner, RN; Monika Bhola, MD; Gulgun Yalcinkaya, MD; Harriet G. Friedman, MA.
Children's Mercy Hospital (U10 HD68284): William E. Truog, MD; Eugenia K. Pallotto, MD, MSCE; Howard W. Kilbride, MD; Cheri Gauldin, RN, MSN, CCRC; Anne Holmes, RN, MSN, MBA-HCM, CCRC; Kathy Johnson, RN, CCRC.
Cincinnati Children's Hospital Medical Center, University Hospital, and Good Samaritan Hospital (U10 HD27853, M01 RR8084): Kurt Schibler, MD; Edward F. Donovan, MD; Kate Bridges, MD; Barbara Alexander, RN; Cathy Grisby, BSN, CCRC; Jody Hessling, RN; Estelle E. Fischer, MHSA, MBA; Lenora D. Jackson, CRC; Kristin Kirker, CRC; Holly L. Mincey, RN, BSN; Greg Muthig, BS; Teresa L. Gratton, PA; Jean J. Steichen, MD; Kimberly Yolton, PhD.
Duke University School of Medicine, University Hospital, University of North Carolina, and Duke Regional Hospital (U10 HD40492, M01 RR30, UL1 TR83): Ronald N. Goldberg, MD; C. Michael Cotten, MD, MHS; Ricki F. Goldstein, MD; Kimberley A. Fisher, PhD, FNP-BC, IBCLC; Kathy J. Auten, MSHS; Katherine A. Foy, RN; Sandra Grimes, RN, BSN; Joanne Finkle, RN, JD; Kathryn E. Gustafson, PhD; Melody B. Lohmeyer, RN, MSN; Matthew M. Laughon, MD, MPH; Carl L. Bose, MD; Janice Bernhardt, MS, RN; Gennie Bose, RN; Janice K. Wereszczak, CPNP-AC/PC.
Emory University, Children’s Healthcare of Atlanta, Grady Memorial Hospital, and Emory University Hospital Midtown (U10 HD27851, UL1 TR454): Barbara J. Stoll, MD; David P. Carlton, MD; Ellen C. Hale, RN, BS, CCRC; Ira Adams-Chapman, MD; Maureen Mulligan LaRossa, RN; Sheena L. Carter, PhD.
Eunice Kennedy Shriver National Institute of Child Health and Human Development: Stephanie Wilson Archer, MA.
Indiana University, University Hospital, Methodist Hospital, Riley Hospital for Children, and Eskenazi Health (U10 HD27856, M01 RR750, UL1 TR6): Brenda B. Poindexter, MD, MS; Gregory M. Sokol, MD; Anna M. Dusick, MD (deceased); Ann B. Cook, MS; Dianne E. Herron, RN; Faithe Hamer, BS; Carolyn Lytle, MD, MPH; Lucy C. Miller, RN, BSN, CCRC; Heike M. Minnich, PsyD, HSPP; Leslie Dawn Wilson, BSN, CCRC.
Nationwide Children’s Hospital and the Ohio State University Medical Center (U10 HD68278): Leif D. Nelin, MD; Sudarshan R. Jadcherla, MD; Patricia Luzader, RN; Christine A. Fortney, PhD, RN; Gail E. Besner; Nehal A. Parikh, MD.
RTI International (U10 HD36790): Abhik Das, PhD; Dennis Wallace, PhD; Jamie E. Newman, PhD, MPH; Jeanette O’Donnell Auman, BS; Margaret M. Crawford, BS, CCRP; Marie G. Gantz, PhD; Carolyn M. Petrie Huitema, MS, CCRP; Kristin M. Zaterka-Baxter, RN, BSN, CCRP.
Stanford University, Dominican Hospital, El Camino Hospital, and Lucile Packard Children's Hospital (U10 HD27880, M01 RR70, UL1 TR93): Krisa P. Van Meurs, MD; David K. Stevenson, MD; Susan R. Hintz, MD, MS, Epi; Marian M. Adams, MD; M. Bethany Ball, BS, CCRC; Andrew W. Palmquist, RN, BSN; Melinda S. Proud, RCP; Barbara Bentley, PhD; Elizabeth Bruno, PhD; Maria Elena DeAnda, PhD; Anne M. DeBattista, RN, PNP; Lynne C. Huffman, MD; Jean G. Kohn, MD, MPH; Casey E. Krueger, PhD; Brian Tang, MD; Hali E.Weiss, MD.
Tufts Medical Center, Floating Hospital for Children (U10 HD53119, M01 RR54): Ivan D. Frantz III, MD; John M. Fiascone, MD; Brenda L. MacKinnon, RNC; Ellen Nylen, RN, BSN; Anne Furey, MPH; Elisabeth C. McGowan, MD; Cecelia E Sibley, PT, MHA; Ana K. Brussa, MS, OTR/L.
University of Alabama at Birmingham Health System and Children’s Hospital of Alabama (U10 HD34216, M01 RR32): Waldemar A. Carlo, MD; Namasivayam Ambalavanan, MD; Myriam Peralta-Carcelen, MD, MPH; Monica V. Collins, RN, BSN, MaEd; Shirley S. Cosby, RN, BSN; Fred J. Biasini, PhD; Kristen C. Johnston, MSN, CRNP; Kathleen G. Nelson, MD; Cryshelle S. Patterson, PhD; Vivien A. Phillips, RN, BSN; Sally Whitley, MA, OTR-L, FAOTA; Amanda D. Soong, MD; Carin Kiser, MD; Leigh Ann Smith, CRNP; Sara Kryzwanski, MS; Richard V. Rector, PhD; Sarah Ryan, PhD; Kristy Domnanovich, PhD; Leslie Rodrigues, PhD.
University of California-Los Angeles, Mattel Children's Hospital, Santa Monica Hospital, Los Robles Hospital and Medical Center, and Olive View Medical Center (U10 HD68270): Uday Devaskar, MD; Meena Garg, MD; Isabell B. Purdy, PhD, CPNP; Teresa Chanlaw, MPH; Rachel Geller, RN, BSN.
University of California–San Diego Medical Center and Sharp Mary Birch Hospital for Women and Newborns (U10 HD40461): Neil N. Finer, MD; Yvonne E. Vaucher, MD, MPH; David Kaegi, MD; Maynard R. Rasmussen, MD; Kathy Arnell, RNC; Clarence Demetrio, RN; Martha G. Fuller, RN, MSN; Chris Henderson, RCP, CRTT; Wade Rich, BSHS, RRT; Radmila West, PhD.
University of Iowa and Mercy Medical Center (U10 HD53109, M01 RR59): Edward F. Bell, MD; Dan L. Ellsbury, MD; John A. Widness, MD; Tarah T. Colaizy, MD, MPH; Karen J. Johnson, RN, BSN; Donia B. Campbell, RNC-NIC; Jacky R. Walker, RN; Michael J. Acarregui, MD; Diane L. Eastman, RN, CPNP, MA.
University of New Mexico Health Sciences Center (U10 HD53089, UL1 TR41): Kristi L. Watterberg, MD; Robin K. Ohls, MD; Janell F. Fuller, MD; Conra Backstrom Lacy, RN; Rebecca A. Montman, BSN, RNC; Jean R. Lowe, PhD; Andrea Freeman Duncan, MD; Sandra Brown, BSN; Theresa Wussow, BSN; Carol Hartenberger, BSN, MPH; Julie Rohr, MSN, RNC, CNS.
University of Pennsylvania, Hospital of the University of Pennsylvania, Pennsylvania Hospital, and Children's Hospital of Philadelphia (U10 HD68244): Barbara Schmidt, MD, MSc; Haresh Kirpalani, MB, MSc; Sara B. DeMauro, MD, MSCE; Aasma S. Chaudhary, BS, RRT; Soraya Abbasi, MD; Toni Mancini, RN, BSN, CCRC; Dara M. Cucinotta, RN; Judy C. Bernbaum, MD; Marsha Gerdes, PhD; Hallam Hurt, MD.
University of Rochester Medical Center, Golisano Children’s Hospital, and the University of Buffalo Women's and Children's Hospital of Buffalo (U10 HD68263, U10 HD40521, M01 RR44, UL1 TR42): Dale L. Phelps, MD; Gary J. Myers, MD; Carl T. D’Angio, MD; Linda J. Reubens, RN, CCRC; Erica Burnell, RN; Diane Hust, MS, RN, CS; Rosemary L. Jensen; Emily Kushner, MA; Deanna Maffett, RN; Joan Merzbach, LMSW; Holly I.M. Wadkins, MA; Kelley Yost, PhD; Julianne Hunn, BS; Satyan Lakshminrusimha, MD; Anne Marie Reynolds, MD, MPH; Osman Farooq, MD; Ashley Williams, MS Ed; Karen Wynn, RN.
University of Texas Health Science Center at Houston Medical School, Children's Memorial Hermann Hospital, and Lyndon Baines Johnson General Hospital/Harris County Hospital District (U10 HD21373): Kathleen A. Kennedy, MD, MPH; Jon E. Tyson, MD, MPH; Katrina Burson, RN, BSN; Patricia W. Evans, MD; Charles Green, PHD; Margarita Jiminez, MD, MPH; Georgia E. McDavid, RN; M. Layne Poundstone, RN, BSN; Peggy Robichaux, RN, BSN; Saba Siddiki, MD; Patti L. Pierce Tate, RCP; Sharon L. Wright, MT (ASCP).
University of Texas Southwestern Medical Center at Dallas, Parkland Health & Hospital System, and Children's Medical Center Dallas (U10 HD40689, M01 RR633): Pablo J. Sánchez, MD; Roy J. Heyne, MD; Luc P. Brion, MD; LiJun Chen, RN, PhD; Alicia Guzman; Melissa H. Leps, RN; Nancy A. Miller, RN; Diana M. Vasil, RNC, NIC; Lizette E. Torres, RN; Sally S. Adams, MS, RN, CPNP; Linda A. Madden, RN, CPNP; Elizabeth Heyne, PsyD PA-C; Janet S. Morgan, RN; Catherine Twell Boatman, MS, CIMI.
University of Utah University Hospital, Intermountain Medical Center, LDS Hospital, and Primary Children's Medical Center (U10 HD53124, M01 RR64, UL1 TR105): Roger G. Faix, MD; Bradley A. Yoder, MD; Anna Bodnar, MD; Karen A. Osborne, RN, BSN, CCRC; Shawna Baker, RN; Karie Bird, RN, BSN; Jill Burnett, RNC, BSN; Jennifer J. Jensen, RN, BSN; Cynthia Spencer, RNC, BSN; Mike Steffen, PhD; Kimberlee Weaver-Lewis, RN, MS; Sarah Winter, MD; Karen Zanetti, RN.
Wayne State University, University of Michigan, Hutzel Women’s Hospital, and Children’s Hospital of Michigan (U10 HD21385): Seetha Shankaran, MD; Athina Pappas, MD; John Barks, MD; Martha Carlson, MD; Angela Argento, PhD; Rebecca Bara, RN, BSN; Laura A. Goldston, MA; Mary Johnson, RN, BSN; Mary Christensen, RT; Stephanie Wiggins, MS.
Yale University, Yale-New Haven Children’s Hospital, and Bridgeport Hospital (U10 HD27871, UL1-RR024139, UL1 TR142): Richard A. Ehrenkranz, MD; Harris Jacobs, MD; Christine G. Butler, MD; Patricia Cervone, RN; Sheila Greisman, RN; Monica Konstantino, RN, BSN; JoAnn Poulsen, RN; Janet Taft, RN, BSN; Joanne Williams, RN, BSN; Elaine Romano, MSN.
Footnotes
Dr Hoffman conceptualized and designed the study, drafted the initial manuscript, and approved the final manuscript as submitted; Dr Bann performed all the statistical analysis; Dr Higgins reviewed and approved the research protocol and final manuscript; and Dr Vohr mentored and assisted Dr Hoffman in conceptualizing and designing the study, revising the manuscript, and approving of the final manuscript as submitted.
FINANCIAL DISCLOSURE: The authors have indicated they have no financial relationships relevant to this article to disclose.
FUNDING: Eunice Kennedy Shriver National Institute of Child Health and Human Development Research Network. Funded by the National Institutes of Health (NIH).
POTENTIAL CONFLICT OF INTEREST: The authors have indicated they have no potential conflicts of interest to disclose.
References
- 1.Fanaroff AA, Hack M, Walsh MC. The NICHD neonatal research network: changes in practice and outcomes during the first 15 years. Semin Perinatol. 2003;27(4):281–287 [DOI] [PubMed] [Google Scholar]
- 2.Hack M, Fanaroff AA. Outcomes of children of extremely low birthweight and gestational age in the 1990s. Semin Neonatol. 2000;5(2):89–106 [DOI] [PubMed] [Google Scholar]
- 3.Vohr BR, Wright LL, Poole WK, McDonald SA. Neurodevelopmental outcomes of extremely low birth weight infants <32 weeks’ gestation between 1993 and 1998. Pediatrics. 2005;116(3):635–643 [DOI] [PubMed] [Google Scholar]
- 4.Anderson PJ, De Luca CR, Hutchinson E, Roberts G, Doyle LW, Victorian Infant Collaborative Group . Underestimation of developmental delay by the new Bayley-III Scale. Arch Pediatr Adolesc Med. 2010;164(4):352–356 [DOI] [PubMed] [Google Scholar]
- 5.Censullo M. Developmental delay in healthy premature infants at age two years: implications for early intervention. J Dev Behav Pediatr. 1994;15(2):99–104 [PubMed] [Google Scholar]
- 6.Barre N, Morgan A, Doyle LW, Anderson PJ. Language abilities in children who were very preterm and/or very low birth weight: a meta-analysis. J Pediatr. 2011;158(5):766–774.e1 [DOI] [PubMed]
- 7.Vohr BR, Stephens BE, Higgins RD, Bann CM, Hintz SR, Das A, et al. Are outcomes of extremely preterm infants improving? Impact of Bayley assessment on outcomes. J Pediatr. 2012;161(2):222–228.e3 [DOI] [PMC free article] [PubMed]
- 8.Baldwin W, Cain VS. The children of teenage parents. Fam Plann Perspect. 1980;12(1):34–39, 42–43 [PubMed]
- 9.Field TM, Widmayer SM, Stringer S, Ignatoff E. Teenage, lower-class, black mothers and their preterm infants: an intervention and developmental follow-up. Child Dev. 1980;51(2):426–436 [PubMed] [Google Scholar]
- 10.Hann DM, Osofsky JD, Culp AM. Relating the adolescent mother–child relationship to preschool outcomes. Infant Ment Health J. 1996;17(4):302–309 [Google Scholar]
- 11.Fernald A, Marchman VA, Weisleder A. SES differences in language processing skill and vocabulary are evident at 18 months. Dev Sci. 2013;16(2):234–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Black MM, Nitz K. Grandmother co-residence, parenting, and child development among low income, urban teen mothers. J Adolesc Health. 1996;18(3):218–226 [DOI] [PubMed] [Google Scholar]
- 13.Albers CA, Grieve AJ. Test Review of Bayley, N. (2006). Bayley Scales of Infant and Toddler Development-Third Edition. San Antonio, TX: Harcourt Assessment. J Psychoed Assess. 2007;25(2):180–198 [Google Scholar]
- 14.Bayley N. Bayley Scales of Infant Development. 3rd ed. San Antonio, TX: Psychological Corporation; 2006 [Google Scholar]
- 15.Karabulut A, Ozkan S, Bozkurt AI, Karahan T, Kayan S. Perinatal outcomes and risk factors in adolescent and advanced age pregnancies: comparison with normal reproductive age women. J Obstet Gynaecol. 2013;33(4):346–350 [DOI] [PubMed] [Google Scholar]
- 16.Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. 1997;39(4):214–223 [DOI] [PubMed] [Google Scholar]
- 17.Vohr BR, Tyson JE, Wright LL, Perritt RL, Li L, Poole WK, et al. Maternal age, multiple birth, and extremely low birth weight infants. J Pediatr. 2009;154(4):498–503.e2 [DOI] [PMC free article] [PubMed]
- 18.Martin J. Births: final data for 2013. National Vital Statistics Reports. 2015. Available at: www.cdc.gov/nchs/data/nvsr/nvsr64/nvsr64_01.pdf. Accessed March 20, 2015 [PubMed]
- 19.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum Associates; 1988 [Google Scholar]
- 20.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117(4):1253–1261 [DOI] [PubMed] [Google Scholar]
- 21.Cheong JL, Hunt RW, Anderson PJ, et al. Head growth in preterm infants: correlation with magnetic resonance imaging and neurodevelopmental outcome. Pediatrics 2008;121(6). Available at: www.pediatrics.org/cgi/content/full/121/6/e1534 [DOI] [PubMed]
- 22.Brooks-Gunn J, Furstenberg F. The children of adolescent mothers: physical, academic, and psychological outcomes. Dev Rev. 1986;6(3):224–251 [Google Scholar]
- 23.Keenan K, Shaw DS. The development of aggression in toddlers: a study of low-income families. J Abnorm Child Psychol. 1994;22(1):53–77 [DOI] [PubMed] [Google Scholar]
- 24.Robson AL, Pederson DR. Predictors of individual differences in attention among low birth weight children. J Dev Behav Pediatr. 1997;18(1):13–21 [DOI] [PubMed] [Google Scholar]
- 25.Skovgaard AM, Olsen EM, Christiansen E, Houmann T, Landorph SL, Jørgensen T, CCC 2000 Study Group . Predictors (0-10 months) of psychopathology at age 1 1/2 years—a general population study in The Copenhagen Child Cohort CCC 2000. J Child Psychol Psychiatry. 2008;49(5):553–562 [DOI] [PubMed] [Google Scholar]
- 26.Culp RE. Adolescent and older mothers: comparison between prenatal maternal variables and newborn interaction measures. Infant Behav Dev. 1988;11(3):353–362 [Google Scholar]
- 27.Allely CS, Purves D, McConnachie A, et al. Parent-infant vocalisations at 12 months predict psychopathology at 7 years. Res Dev Disabil. 2013;34(3):985–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marwick H, Doolin O, Allely CS, et al. Predictors of diagnosis of child psychiatric disorder in adult-infant social-communicative interaction at 12 months. Res Dev Disabil. 2013;34(1):562–572 [DOI] [PubMed] [Google Scholar]
- 29.Pope SK, Whiteside L, Brooks-Gunn J, et al. Low-birth-weight infants born to adolescent mothers. Effects of coresidency with grandmother on child development. JAMA. 1993;269(11):1396–1400 [PubMed] [Google Scholar]