Abstract
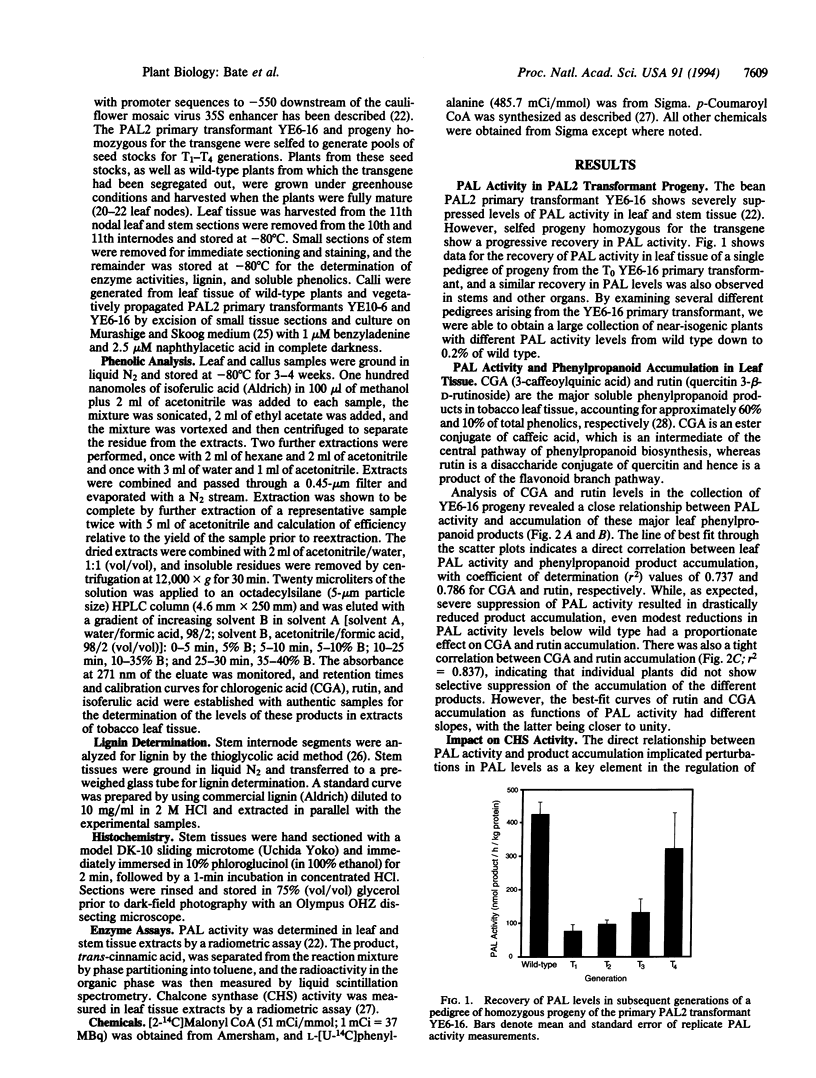
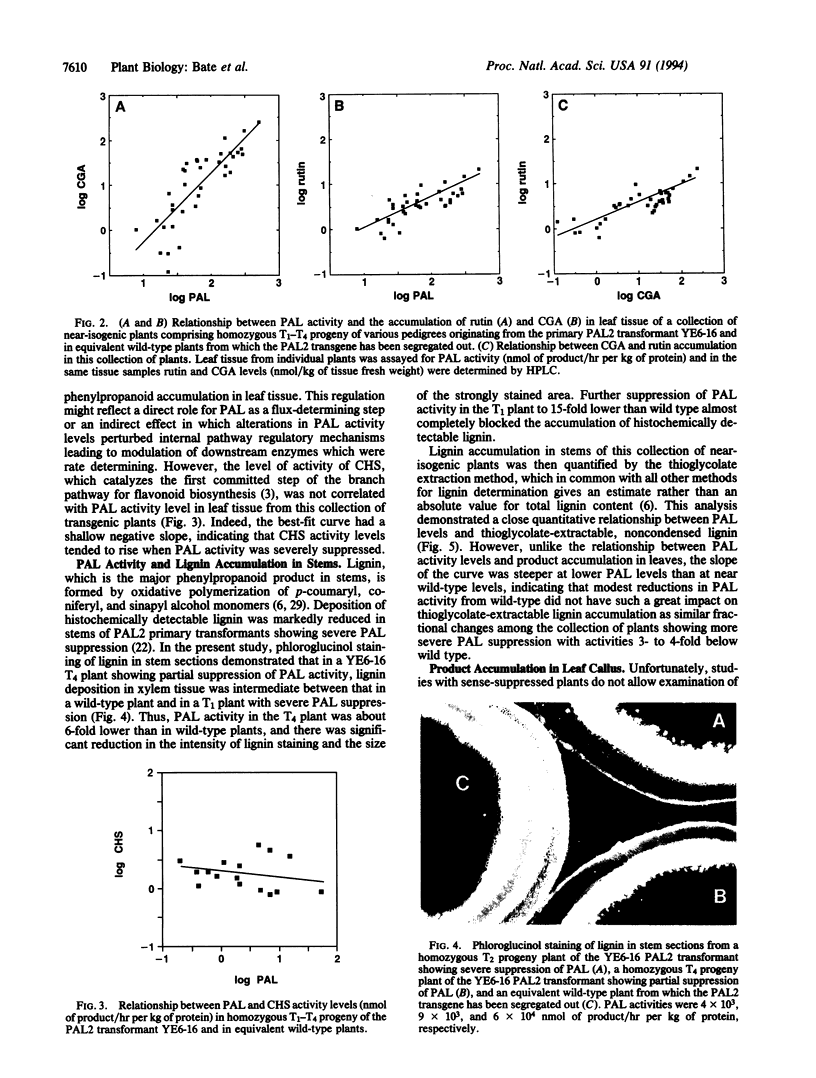
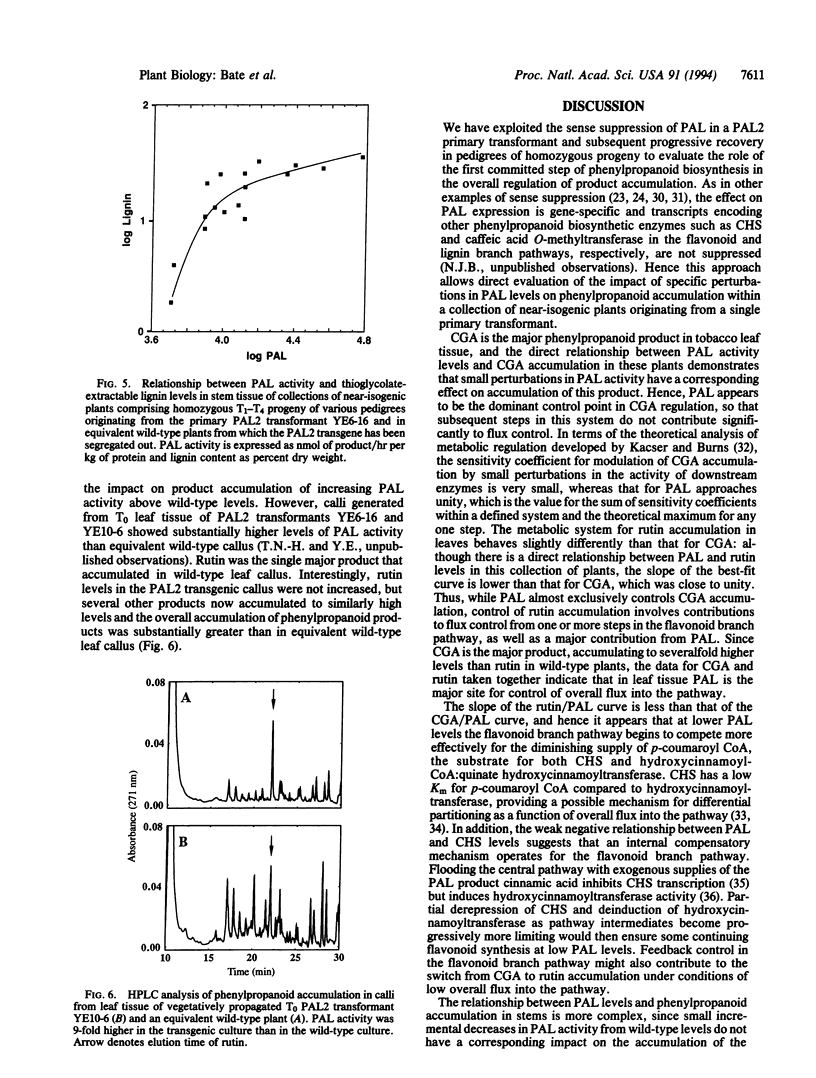
Phenylalanine ammonia-lyase (PAL) catalyzes the first step in phenylpropanoid synthesis. The role of PAL in pathway regulation was investigated by measurement of product accumulation as a function of enzyme activity in a collection of near-isogenic transgenic tobacco plants exhibiting a range of PAL levels from wild type to 0.2% of wild type. In leaf tissue, PAL level is the dominant factor regulating accumulation of the major product chlorogenic acid and overall flux into the pathway. In stems, PAL at wild-type levels contributes, together with downstream steps, in the regulation of lignin deposition and becomes the dominant, rate-determining step at levels 3- to 4-fold below wild type. The metabolic impact of elevated PAL levels was investigated in transgenic leaf callus that overexpressed PAL. Accumulation of the flavonoid rutin, the major product in wild-type callus, was not increased, but several other products accumulated to similarly high levels. These data indicate that PAL is a key step in the regulation of overall flux into the pathway and, hence, accumulation of major phenylpropanoid products, with the regulatory architecture of the pathway poised so that downstream steps control partitioning into different branch pathways.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bevan M., Shufflebottom D., Edwards K., Jefferson R., Schuch W. Tissue- and cell-specific activity of a phenylalanine ammonia-lyase promoter in transgenic plants. EMBO J. 1989 Jul;8(7):1899–1906. doi: 10.1002/j.1460-2075.1989.tb03592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruce R. J., West C. A. Elicitation of lignin biosynthesis and isoperoxidase activity by pectic fragments in suspension cultures of castor bean. Plant Physiol. 1989 Nov;91(3):889–897. doi: 10.1104/pp.91.3.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkind Y., Edwards R., Mavandad M., Hedrick S. A., Ribak O., Dixon R. A., Lamb C. J. Abnormal plant development and down-regulation of phenylpropanoid biosynthesis in transgenic tobacco containing a heterologous phenylalanine ammonia-lyase gene. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9057–9061. doi: 10.1073/pnas.87.22.9057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kacser H., Burns J. A. The control of flux. Symp Soc Exp Biol. 1973;27:65–104. [PubMed] [Google Scholar]
- Kishore G. M., Somerville C. R. Genetic engineering of commercially useful biosynthetic pathways in transgenic plants. Curr Opin Biotechnol. 1993 Apr;4(2):152–158. doi: 10.1016/0958-1669(93)90116-e. [DOI] [PubMed] [Google Scholar]
- Kreuzaler F., Ragg H., Heller W., Tesch R., Witt I., Hammer D., Hahlbrock K. Flavanone synthase from Petroselinum hortense. Molecular weight, subunit composition, size of messenger RNA, and absence of pantetheinyl residue. Eur J Biochem. 1979 Aug 15;99(1):89–96. doi: 10.1111/j.1432-1033.1979.tb13235.x. [DOI] [PubMed] [Google Scholar]
- Lamb C. J., Lawton M. A., Dron M., Dixon R. A. Signals and transduction mechanisms for activation of plant defenses against microbial attack. Cell. 1989 Jan 27;56(2):215–224. doi: 10.1016/0092-8674(89)90894-5. [DOI] [PubMed] [Google Scholar]
- Lamb C. J. trans-Cinnamic acid as a mediator of the light-stimulated increase in hydroxycinnamoyl-CoA: quinate hydroxycinnamoyl transferase. FEBS Lett. 1977 Mar 15;75(1):37–40. doi: 10.1016/0014-5793(77)80047-1. [DOI] [PubMed] [Google Scholar]
- Lewis N. G., Yamamoto E. Lignin: occurrence, biogenesis and biodegradation. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:455–496. doi: 10.1146/annurev.pp.41.060190.002323. [DOI] [PubMed] [Google Scholar]
- Liang X. W., Dron M., Schmid J., Dixon R. A., Lamb C. J. Developmental and environmental regulation of a phenylalanine ammonia-lyase-beta-glucuronidase gene fusion in transgenic tobacco plants. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9284–9288. doi: 10.1073/pnas.86.23.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loake G. J., Choudhary A. D., Harrison M. J., Mavandad M., Lamb C. J., Dixon R. A. Phenylpropanoid pathway intermediates regulate transient expression of a chalcone synthase gene promoter. Plant Cell. 1991 Aug;3(8):829–840. doi: 10.1105/tpc.3.8.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napoli C., Lemieux C., Jorgensen R. Introduction of a Chimeric Chalcone Synthase Gene into Petunia Results in Reversible Co-Suppression of Homologous Genes in trans. Plant Cell. 1990 Apr;2(4):279–289. doi: 10.1105/tpc.2.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsholme E. A., Crabtree B. Metabolic aspects of enzyme activity regulation. Symp Soc Exp Biol. 1973;27:429–460. [PubMed] [Google Scholar]
- Robbins M. P., Bolwell G. P., Dixon R. A. Metabolic changes in elicitor-treated bean cells. Selectivity of enzyme induction in relation to phytoalexin accumulation. Eur J Biochem. 1985 May 2;148(3):563–569. doi: 10.1111/j.1432-1033.1985.tb08877.x. [DOI] [PubMed] [Google Scholar]
- Tien M. Properties of ligninase from Phanerochaete chrysosporium and their possible applications. Crit Rev Microbiol. 1987;15(2):141–168. doi: 10.3109/10408418709104456. [DOI] [PubMed] [Google Scholar]
- de Carvalho F., Gheysen G., Kushnir S., Van Montagu M., Inzé D., Castresana C. Suppression of beta-1,3-glucanase transgene expression in homozygous plants. EMBO J. 1992 Jul;11(7):2595–2602. doi: 10.1002/j.1460-2075.1992.tb05324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Krol A. R., Mur L. A., Beld M., Mol J. N., Stuitje A. R. Flavonoid genes in petunia: addition of a limited number of gene copies may lead to a suppression of gene expression. Plant Cell. 1990 Apr;2(4):291–299. doi: 10.1105/tpc.2.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]



