Abstract
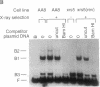
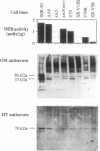
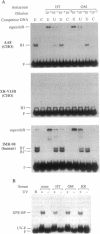
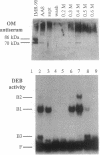
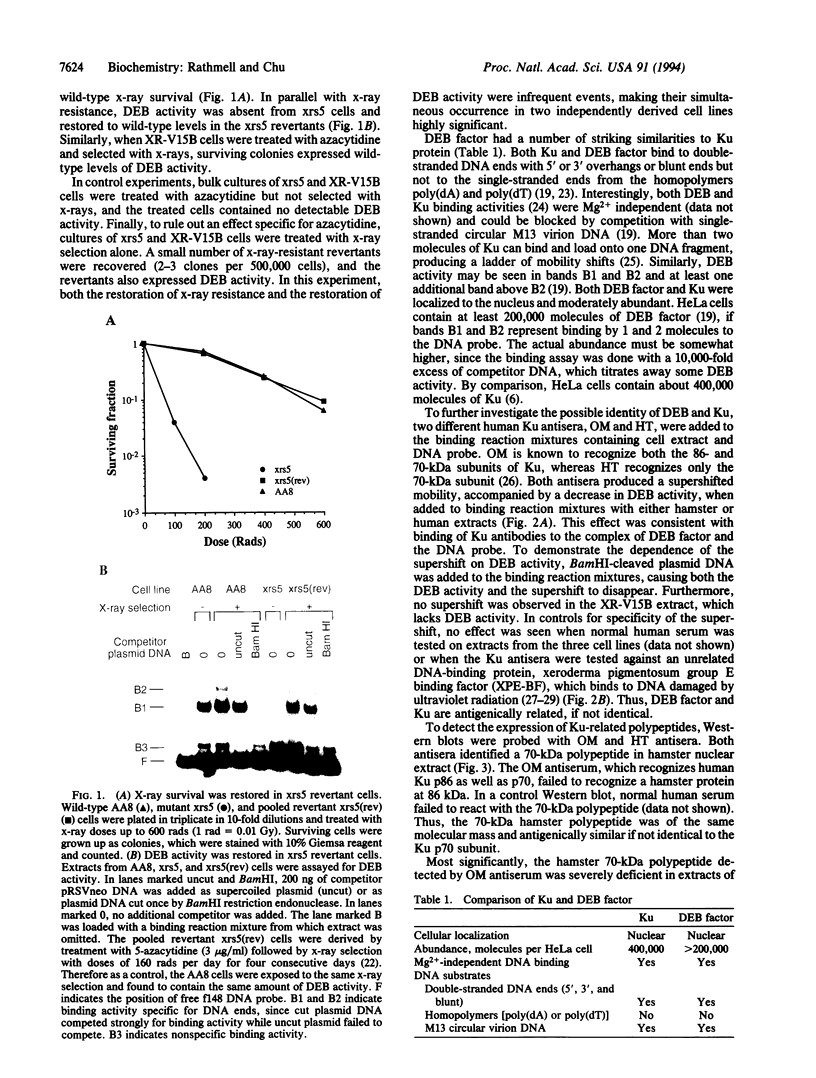
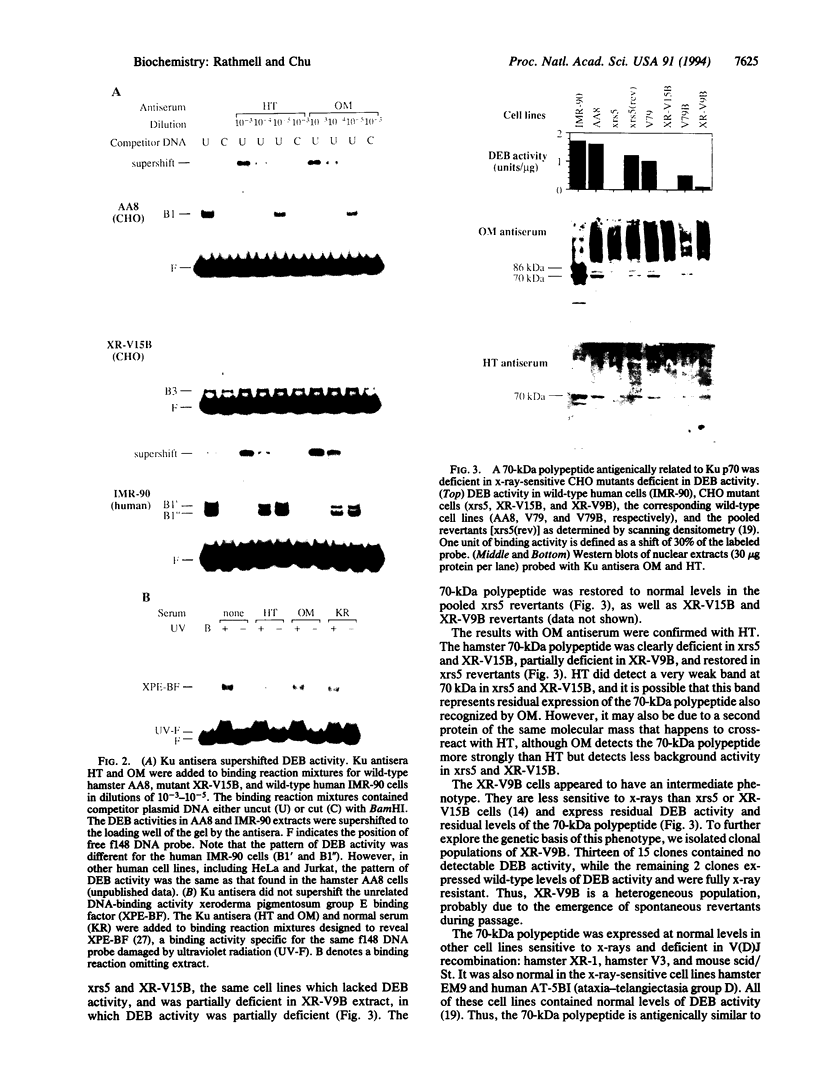
The Ku autoantigen is a well-characterized heterodimer of 70 and 86 kDa that binds to DNA ends, but its cellular function has been obscure. An electrophoretic mobility-shift assay and Ku antisera were used to show that Ku or a closely related protein was deficient in three mutant hamster cell lines from x-ray-sensitive complementation group 5, which is characterized by defects in DNA double-strand break repair and V(D)J recombination. Furthermore, Ku protein expression was restored when the cells reverted to x-ray resistance. The Ku p86 gene maps to human chromosome 2q33-35, and group 5 cells are rescued by almost precisely the same region, 2q34-36. Thus, biochemical and genetic evidence suggests that Ku is involved in pathways for DNA recombination and repair. By its association with a DNA-dependent protein kinase activated by DNA ends, Ku may also initiate a signaling pathway induced by DNA damage, perhaps for cell cycle arrest.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biedermann K. A., Sun J. R., Giaccia A. J., Tosto L. M., Brown J. M. scid mutation in mice confers hypersensitivity to ionizing radiation and a deficiency in DNA double-strand break repair. Proc Natl Acad Sci U S A. 1991 Feb 15;88(4):1394–1397. doi: 10.1073/pnas.88.4.1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blier P. R., Griffith A. J., Craft J., Hardin J. A. Binding of Ku protein to DNA. Measurement of affinity for ends and demonstration of binding to nicks. J Biol Chem. 1993 Apr 5;268(10):7594–7601. [PubMed] [Google Scholar]
- Cai Q. Q., Plet A., Imbert J., Lafage-Pochitaloff M., Cerdan C., Blanchard J. M. Chromosomal location and expression of the genes coding for Ku p70 and p80 in human cell lines and normal tissues. Cytogenet Cell Genet. 1994;65(4):221–227. doi: 10.1159/000133635. [DOI] [PubMed] [Google Scholar]
- Chen D. J., Marrone B. L., Nguyen T., Stackhouse M., Zhao Y., Siciliano M. J. Regional assignment of a human DNA repair gene (XRCC5) to 2q35 by X-ray hybrid mapping. Genomics. 1994 May 15;21(2):423–427. doi: 10.1006/geno.1994.1287. [DOI] [PubMed] [Google Scholar]
- Chu G., Chang E. Cisplatin-resistant cells express increased levels of a factor that recognizes damaged DNA. Proc Natl Acad Sci U S A. 1990 May;87(9):3324–3327. doi: 10.1073/pnas.87.9.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu G., Chang E. Xeroderma pigmentosum group E cells lack a nuclear factor that binds to damaged DNA. Science. 1988 Oct 28;242(4878):564–567. doi: 10.1126/science.3175673. [DOI] [PubMed] [Google Scholar]
- Collins A. R. Mutant rodent cell lines sensitive to ultraviolet light, ionizing radiation and cross-linking agents: a comprehensive survey of genetic and biochemical characteristics. Mutat Res. 1993 Jan;293(2):99–118. doi: 10.1016/0921-8777(93)90062-l. [DOI] [PubMed] [Google Scholar]
- Falzon M., Fewell J. W., Kuff E. L. EBP-80, a transcription factor closely resembling the human autoantigen Ku, recognizes single- to double-strand transitions in DNA. J Biol Chem. 1993 May 15;268(14):10546–10552. [PubMed] [Google Scholar]
- Giaccia A. J., Lewis A. D., Denko N. C., Cholon A., Evans J. W., Waldren C. A., Stamato T. D., Brown J. M. The hypersensitivity of the Chinese hamster ovary variant BL-10 to bleomycin killing is due to a lack of glutathione S-transferase-alpha activity. Cancer Res. 1991 Aug 15;51(16):4463–4469. [PubMed] [Google Scholar]
- Gottlieb T. M., Jackson S. P. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell. 1993 Jan 15;72(1):131–142. doi: 10.1016/0092-8674(93)90057-w. [DOI] [PubMed] [Google Scholar]
- Griffith A. J., Blier P. R., Mimori T., Hardin J. A. Ku polypeptides synthesized in vitro assemble into complexes which recognize ends of double-stranded DNA. J Biol Chem. 1992 Jan 5;267(1):331–338. [PubMed] [Google Scholar]
- Hafezparast M., Kaur G. P., Zdzienicka M., Athwal R. S., Lehmann A. R., Jeggo P. A. Subchromosomal localization of a gene (XRCC5) involved in double strand break repair to the region 2q34-36. Somat Cell Mol Genet. 1993 Sep;19(5):413–421. doi: 10.1007/BF01233246. [DOI] [PubMed] [Google Scholar]
- Hwang B. J., Chu G. Purification and characterization of a human protein that binds to damaged DNA. Biochemistry. 1993 Feb 16;32(6):1657–1666. doi: 10.1021/bi00057a033. [DOI] [PubMed] [Google Scholar]
- Iijima S., Teraoka H., Date T., Tsukada K. DNA-activated protein kinase in Raji Burkitt's lymphoma cells. Phosphorylation of c-Myc oncoprotein. Eur J Biochem. 1992 Jun 1;206(2):595–603. doi: 10.1111/j.1432-1033.1992.tb16964.x. [DOI] [PubMed] [Google Scholar]
- Jeggo P. A., Hafezparast M., Thompson A. F., Broughton B. C., Kaur G. P., Zdzienicka M. Z., Athwal R. S. Localization of a DNA repair gene (XRCC5) involved in double-strand-break rejoining to human chromosome 2. Proc Natl Acad Sci U S A. 1992 Jul 15;89(14):6423–6427. doi: 10.1073/pnas.89.14.6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeggo P. A., Holliday R. Azacytidine-induced reactivation of a DNA repair gene in Chinese hamster ovary cells. Mol Cell Biol. 1986 Aug;6(8):2944–2949. doi: 10.1128/mcb.6.8.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeggo P. A., Kemp L. M. X-ray-sensitive mutants of Chinese hamster ovary cell line. Isolation and cross-sensitivity to other DNA-damaging agents. Mutat Res. 1983 Dec;112(6):313–327. doi: 10.1016/0167-8817(83)90026-3. [DOI] [PubMed] [Google Scholar]
- Kapp D. S., Smith K. C. Chemical nature of chain breaks produced in DNA by x-irradiation in vitro. Radiat Res. 1970 Apr;42(1):34–49. [PubMed] [Google Scholar]
- Kastan M. B., Onyekwere O., Sidransky D., Vogelstein B., Craig R. W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991 Dec 1;51(23 Pt 1):6304–6311. [PubMed] [Google Scholar]
- Knuth M. W., Gunderson S. I., Thompson N. E., Strasheim L. A., Burgess R. R. Purification and characterization of proximal sequence element-binding protein 1, a transcription activating protein related to Ku and TREF that binds the proximal sequence element of the human U1 promoter. J Biol Chem. 1990 Oct 15;265(29):17911–17920. [PubMed] [Google Scholar]
- Lees-Miller S. P., Chen Y. R., Anderson C. W. Human cells contain a DNA-activated protein kinase that phosphorylates simian virus 40 T antigen, mouse p53, and the human Ku autoantigen. Mol Cell Biol. 1990 Dec;10(12):6472–6481. doi: 10.1128/mcb.10.12.6472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori T., Akizuki M., Yamagata H., Inada S., Yoshida S., Homma M. Characterization of a high molecular weight acidic nuclear protein recognized by autoantibodies in sera from patients with polymyositis-scleroderma overlap. J Clin Invest. 1981 Sep;68(3):611–620. doi: 10.1172/JCI110295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimori T., Hardin J. A., Steitz J. A. Characterization of the DNA-binding protein antigen Ku recognized by autoantibodies from patients with rheumatic disorders. J Biol Chem. 1986 Feb 15;261(5):2274–2278. [PubMed] [Google Scholar]
- Mimori T., Ohosone Y., Hama N., Suwa A., Akizuki M., Homma M., Griffith A. J., Hardin J. A. Isolation and characterization of cDNA encoding the 80-kDa subunit protein of the human autoantigen Ku (p70/p80) recognized by autoantibodies from patients with scleroderma-polymyositis overlap syndrome. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1777–1781. doi: 10.1073/pnas.87.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn L., Kunkel S., Nabel G. J. Tumor necrosis factor alpha and interleukin 1 stimulate the human immunodeficiency virus enhancer by activation of the nuclear factor kappa B. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2336–2340. doi: 10.1073/pnas.86.7.2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paillard S., Strauss F. Analysis of the mechanism of interaction of simian Ku protein with DNA. Nucleic Acids Res. 1991 Oct 25;19(20):5619–5624. doi: 10.1093/nar/19.20.5619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pergola F., Zdzienicka M. Z., Lieber M. R. V(D)J recombination in mammalian cell mutants defective in DNA double-strand break repair. Mol Cell Biol. 1993 Jun;13(6):3464–3471. doi: 10.1128/mcb.13.6.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson S. R., Dvir A., Anderson C. W., Dynan W. S. DNA binding provides a signal for phosphorylation of the RNA polymerase II heptapeptide repeats. Genes Dev. 1992 Mar;6(3):426–438. doi: 10.1101/gad.6.3.426. [DOI] [PubMed] [Google Scholar]
- Rathmell W. K., Chu G. A DNA end-binding factor involved in double-strand break repair and V(D)J recombination. Mol Cell Biol. 1994 Jul;14(7):4741–4748. doi: 10.1128/mcb.14.7.4741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves W. H. Antibodies to the p70/p80 (Ku) antigens in systemic lupus erythematosus. Rheum Dis Clin North Am. 1992 May;18(2):391–414. [PubMed] [Google Scholar]
- Reeves W. H., Sthoeger Z. M. Molecular cloning of cDNA encoding the p70 (Ku) lupus autoantigen. J Biol Chem. 1989 Mar 25;264(9):5047–5052. [PubMed] [Google Scholar]
- Suwa A., Hirakata M., Takeda Y., Jesch S. A., Mimori T., Hardin J. A. DNA-dependent protein kinase (Ku protein-p350 complex) assembles on double-stranded DNA. Proc Natl Acad Sci U S A. 1994 Jul 19;91(15):6904–6908. doi: 10.1073/pnas.91.15.6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taccioli G. E., Rathbun G., Oltz E., Stamato T., Jeggo P. A., Alt F. W. Impairment of V(D)J recombination in double-strand break repair mutants. Science. 1993 Apr 9;260(5105):207–210. doi: 10.1126/science.8469973. [DOI] [PubMed] [Google Scholar]
- Yaneva M., Wen J., Ayala A., Cook R. cDNA-derived amino acid sequence of the 86-kDa subunit of the Ku antigen. J Biol Chem. 1989 Aug 15;264(23):13407–13411. [PubMed] [Google Scholar]
- Zdzienicka M. Z., Tran Q., van der Schans G. P., Simons J. W. Characterization of an X-ray-hypersensitive mutant of V79 Chinese hamster cells. Mutat Res. 1988 Nov;194(3):239–249. doi: 10.1016/0167-8817(88)90025-9. [DOI] [PubMed] [Google Scholar]
- Zdzienicka M. Z., van Wessel N., van der Schans G. P. A fourth complementation group among ionizing radiation-sensitive Chinese hamster cell mutants defective in DNA double-strand break repair. Radiat Res. 1992 Sep;131(3):309–314. [PubMed] [Google Scholar]
- Zhang W. W., Yaneva M. On the mechanisms of Ku protein binding to DNA. Biochem Biophys Res Commun. 1992 Jul 15;186(1):574–579. doi: 10.1016/s0006-291x(05)80847-2. [DOI] [PubMed] [Google Scholar]