Abstract
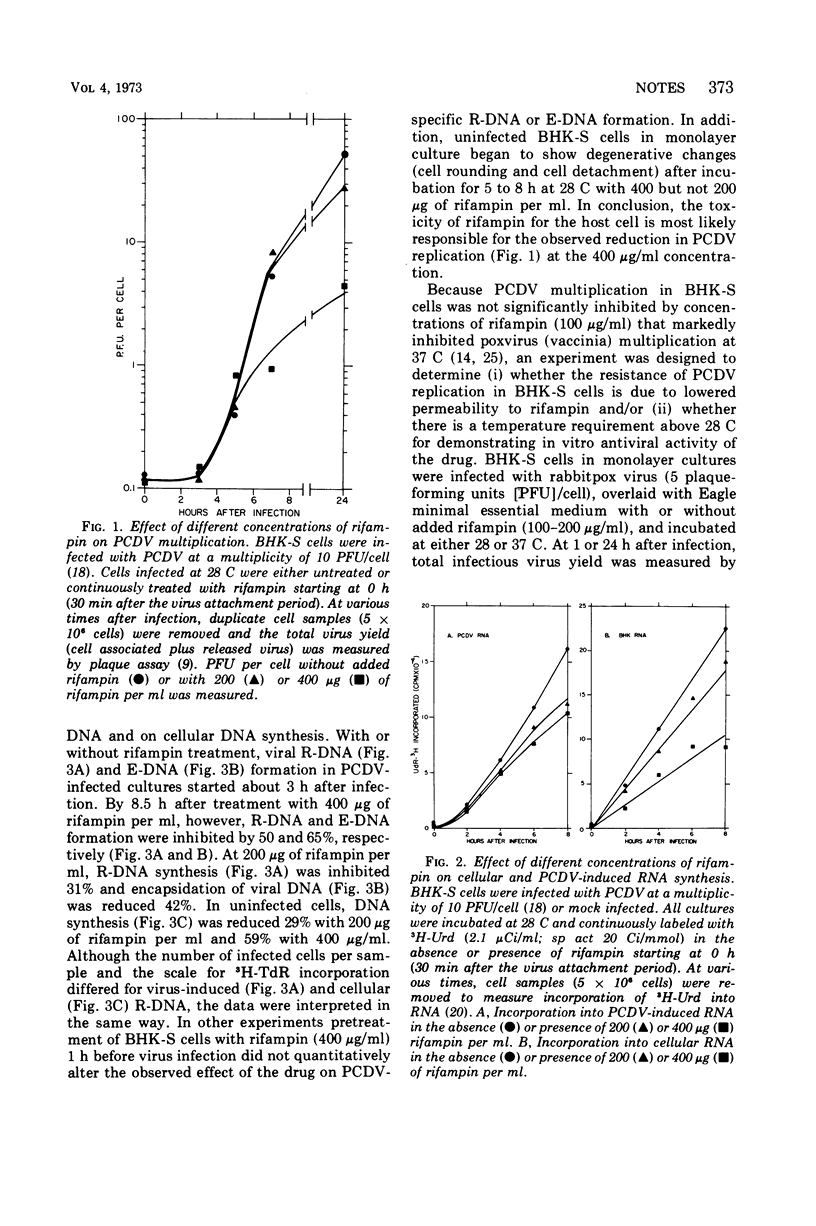
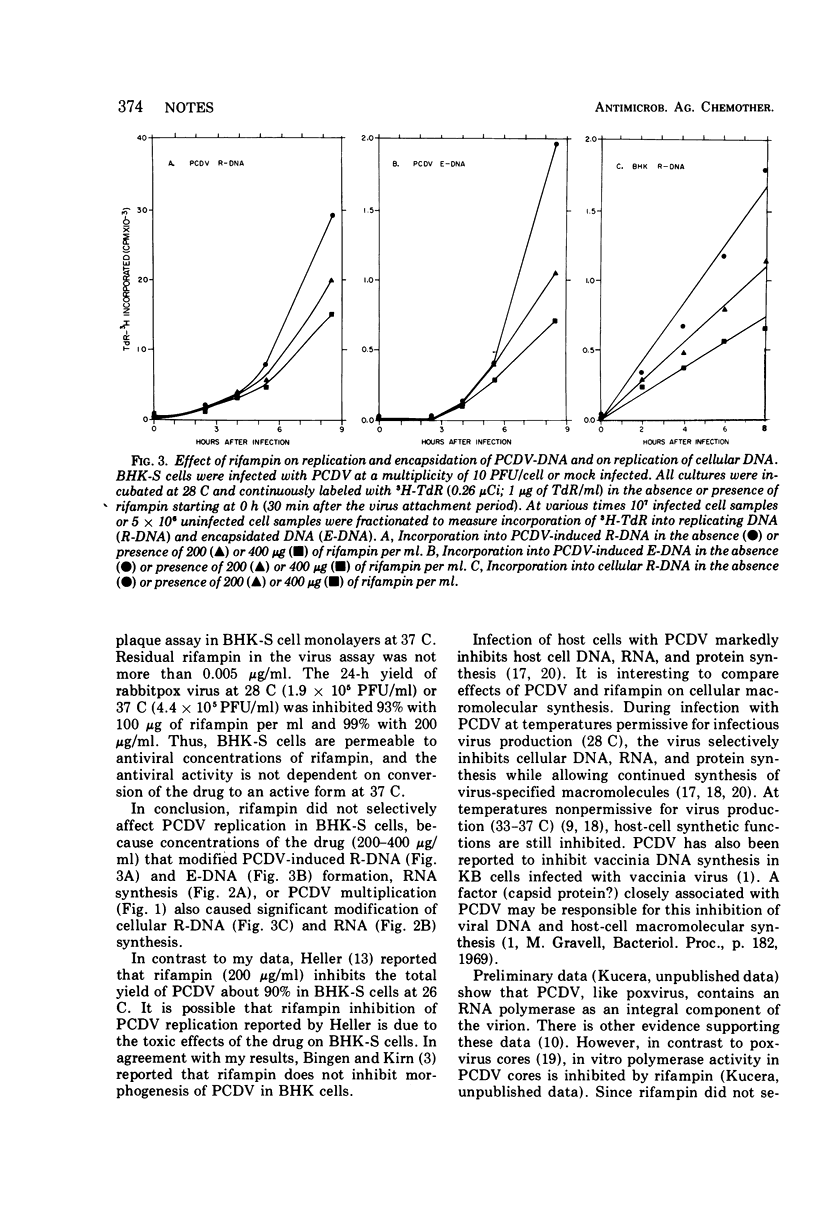
Resistance of frog virus multiplication to rifampin suggests that components peculiar to cytoplasmic deoxyribonucleic acid replicating viruses (e.g., poxvirus) are not equally sensitive to rifampin.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubertin A. M., Guir J., Kirn A. The inhibition of vaccinia virus DNA synthesis in KB cells infected with frog virus 3. J Gen Virol. 1970 Aug;8(2):105–111. doi: 10.1099/0022-1317-8-2-105. [DOI] [PubMed] [Google Scholar]
- Bellett A. J. The iridescent virus group. Adv Virus Res. 1968;13:225–246. doi: 10.1016/s0065-3527(08)60254-7. [DOI] [PubMed] [Google Scholar]
- Dardiri A. H., Bachrach H. L., Heller E. Inhibition by rifampin of African swine fever virus replication in tissue culture. Infect Immun. 1971 Jul;4(1):34–36. doi: 10.1128/iai.4.1.34-36.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENNER F., WOODROOFE G. M. The reactivation of poxviruses. II. The range of reactivating viruses. Virology. 1960 May;11:185–201. doi: 10.1016/0042-6822(60)90061-1. [DOI] [PubMed] [Google Scholar]
- Granoff A. Viruses of amphibia. Curr Top Microbiol Immunol. 1969;50:107–137. doi: 10.1007/978-3-642-46169-9_4. [DOI] [PubMed] [Google Scholar]
- Gravell M., Cromeans T. L. Mechanisms involved in nongenetic reactivation of frog polyhedral cytoplasmic deoxyribovirus: evidence for an RNA polymerase in the virion. Virology. 1971 Oct;46(1):39–49. doi: 10.1016/0042-6822(71)90004-3. [DOI] [PubMed] [Google Scholar]
- Gravell M., Naegele R. F. Nongenetic reactivation of frog polyhedral cytoplasmic deoxyribovirus (PCDV). Virology. 1970 Jan;40(1):170–174. doi: 10.1016/0042-6822(70)90390-9. [DOI] [PubMed] [Google Scholar]
- Hartmann G., Honikel K. O., Knüsel F., Nüesch J. The specific inhibition of the DNA-directed RNA synthesis by rifamycin. Biochim Biophys Acta. 1967;145(3):843–844. doi: 10.1016/0005-2787(67)90147-5. [DOI] [PubMed] [Google Scholar]
- Heller E., Argaman M., Levy H., Goldblum N. Selective inhibition of vaccinia virus by the antibiotic rifampicin. Nature. 1969 Apr 19;222(5190):273–274. doi: 10.1038/222273a0. [DOI] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Poxvirus DNA-dependent RNA polymerase. Proc Natl Acad Sci U S A. 1967 Jul;58(1):134–141. doi: 10.1073/pnas.58.1.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kates J., Dahl R., Mielke M. Synthesis and intracellular localization of vaccinia virus deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Virol. 1968 Sep;2(9):894–900. doi: 10.1128/jvi.2.9.894-900.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucera L. S. Effects of temperature on frog polyhedral cytoplasmic deoxyribovirus multiplication: thermosensitivity of initiation, replication, and encapsidation of viral DNA. Virology. 1970 Nov;42(3):576–589. doi: 10.1016/0042-6822(70)90304-1. [DOI] [PubMed] [Google Scholar]
- Kucera L. S., Granoff A. Viruses and renal carcinoma of Rana pipiens. VI. Interrelationships of macromolecular synthesis and infectious virus production in frog virus 3-infected BHK 21/13 cells. Virology. 1968 Feb;34(2):240–249. doi: 10.1016/0042-6822(68)90233-x. [DOI] [PubMed] [Google Scholar]
- Maes R., Granoff A. Viruses and renal carcinoma of Rana pipiens. IV. Nucleic acid synthesis in frog virus 3-infected BHK 21/13 cells. Virology. 1967 Nov;33(3):491–502. doi: 10.1016/0042-6822(67)90125-0. [DOI] [PubMed] [Google Scholar]
- Mcauslan B. R. Rifampicin inhibition of vaccinia replication. Biochem Biophys Res Commun. 1969 Oct 8;37(2):289–295. doi: 10.1016/0006-291x(69)90733-5. [DOI] [PubMed] [Google Scholar]
- Moss B., Rosenblum E. N., Grimley P. M. Assembly of vaccinia virus particles from polypeptides made in the presence of rifampicin. Virology. 1971 Jul;45(1):123–134. doi: 10.1016/0042-6822(71)90119-x. [DOI] [PubMed] [Google Scholar]
- Nagaya A., Pogo B. G., Dales S. Biogenesis of vaccinia: separation of early stages from maturation by means of rifampicin. Virology. 1970 Apr;40(4):1039–1051. doi: 10.1016/0042-6822(70)90150-9. [DOI] [PubMed] [Google Scholar]
- Pennington T. H., Follett E. A. Inhibition of poxvirus maturation by rifamycin derivatives and related compounds. J Virol. 1971 Jun;7(6):821–829. doi: 10.1128/jvi.7.6.821-829.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogo B. G. Biogenesis of vaccinia: effect of rifampicin on transcription. Virology. 1971 Jun;44(3):576–581. doi: 10.1016/0042-6822(71)90371-0. [DOI] [PubMed] [Google Scholar]
- Subak-Sharpe J. H., Timbury M. C., Williams J. F. Rifampicin inhibits the growth of some mammalian viruses. Nature. 1969 Apr 26;222(5191):341–345. doi: 10.1038/222341a0. [DOI] [PubMed] [Google Scholar]
- Vaheri A., Hanafusa H. Effect of rifampicin and a derivative on cells transformed by Rous sarcoma virus. Cancer Res. 1971 Dec;31(12):2032–2036. [PubMed] [Google Scholar]
- Wehrli W., Nüesch J., Knüsel F., Staehelin M. Action of rifamycins on RNA polymerase. Biochim Biophys Acta. 1968 Mar 18;157(1):215–217. doi: 10.1016/0005-2787(68)90285-2. [DOI] [PubMed] [Google Scholar]
- Zwillenberg L. O., Wolf K. Ultrastructure of lymphocystis virus. J Virol. 1968 Apr;2(4):393–399. doi: 10.1128/jvi.2.4.393-399.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- di Mauro E., Synder L., Marino P., Lamberti A., Coppo A., Tocchini-Valentini G. P. Rifampicin sensitivity of the components of DNA-dependent RNA polymerase. Nature. 1969 May 10;222(5193):533–537. doi: 10.1038/222533a0. [DOI] [PubMed] [Google Scholar]


