Abstract
Background
Exercise training enhances extracellular superoxide dismutase (EcSOD) expression in skeletal muscle and elicits positive health outcomes in individuals with diabetes. The goal of this study was to determine if enhanced skeletal muscle expression of EcSOD is sufficient to mitigate streptozotocin (STZ)-induced diabetic cardiomyopathy (DCM).
Methods and Results
Exercise training promotes EcSOD expression in skeletal muscle and provides protection against DCM; however, it is not known if enhanced EcSOD expression in skeletal muscle plays a functional role in this protection. Here, we show that skeletal muscle-specific EcSOD transgenic mice (TG) are protected from cardiac hypertrophy, fibrosis and dysfunction under the condition of type-1 diabetes induced by STZ injection. We also show that both exercise training and muscle-specific transgenic expression of EcSOD result in elevated EcSOD protein in the blood and heart without increased transcription in the heart, suggesting enhanced expression of EcSOD from skeletal muscle redistributes to the heart. Importantly, cardiac tissue in TG mice displayed significantly reduced oxidative stress, aberrant cell signaling and inflammatory cytokine expression compared with wild type mice under the same diabetic condition.
Conclusions
Enhanced expression of EcSOD in skeletal muscle is sufficient to mitigate STZ-induced DCM through attenuation of oxidative stress, aberrant cell signaling and inflammation, suggesting a cross-organ mechanism by which exercise training improves cardiac function in diabetes.
Keywords: diabetic cardiomyopathy, oxidative stress, antioxidant enzymes, exercise training, cardiomyocyte hypertrophy
There are ~26 million individuals with diabetes in the United States, and the disease is becoming more prevalent as predicted by global projections that by year 2030 every 4 of 100 people will be diabetic.1 Individuals with diabetes have a greater propensity (3–7 fold increase) in developing cardiovascular disease than non-diabetic populations,2 and the adverse cardiovascular events are the leading cause of diabetes-related death and disease.3 Diabetic cardiomyopathy (DCM) was first described in 1972 as chronic heart failure in diabetic patients and has since been diagnosed as left ventricular failure in the absence of atherosclerosis and hypertension.4 Ventricular failure is the phenotypic endpoint preempted by calcium irregularities,5 apoptosis,6 fibrosis,7 and oxidative stress.8,9 Since there is no cure for diabetes, finding effective modalities to prevent the development of and/or treat DCM is urgently needed to reduce the morbidity and mortality of the diabetic population.
Oxidative stress in the myocardium plays an important role in the pathogenesis of DCM.10 Both diabetic patients and animal models of diabetes display signs of oxidative damage in the heart tissue, such as lipid peroxidation, protein nitrosylation and altered endogenous antioxidant enzyme levels.11 Oxidative damage in the heart is often compounded by oxidative stress-induced signaling for apoptosis and fibrosis,12 which induce maladaptation leading to impaired structure and function. Although the primary source of oxidative stress in DCM is not fully understood (i.e., intracellular vs. extracellular), there is a clear link to hyperglycemia. More research is needed to identify ways to stymie oxidative stress in the heart to preserve the structural and functional integrity.
Since DCM is strongly associated with reduced endogenous antioxidant pools and activities,13 and increased oxidative stress in the myocardium,11 supplementing diabetic subjects with antioxidants was favorably considered as a therapy. This concept is supported by studies demonstrating that antioxidant therapy can improve certain features of cardiomyopathies in animal models of diabetes. Of note, overexpression of antioxidant enzymes in cardiomyocytes improves cardiac morphology and contractility, compared to wild type control mice under the condition of diabetes.14,15 However, randomized clinical trials in humans failed to demonstrate the effectiveness of antioxidants as therapy for heart failure,16 including diabetic patients.17 Despite the species differences between rodents and human patients, several biological reasons could potentially explain the failure of the antioxidant treatment in humans. In particular, a combination of lack of target specificity and continuous supply of the antioxidant with the supplementation approach may explain why genetic augmentation worked in animal models, but pharmacological treatment failed in humans. In addition, antioxidant supplementation in a pulsatile manner will not likely mimic the endogenous antioxidant defense under the condition of DCM, but may impair normal oxidant-mediated cell signaling that is important for cell physiology.18
Recent development in antioxidant research, in particular with regard to extracellular superoxide dismutase (EcSOD), prompted us to speculate that EcSOD expression in skeletal muscle provides protection to the heart against disease conditions, such as DCM. Firstly, EcSOD is a glycoprotein that is secreted and has high affinity for sulfated polysaccharides, such as heparin and heparin sulfate, through its heparin-binding domain on the C-terminus.19 Secondly, EcSOD is expressed and secreted by skeletal muscle and redistributed into the circulation,20 and can be taken up by tissue cells (i.e. endothelial cells and cardiomyocytes).21 Thirdly, EcSOD is potent in protection against skeletal muscle wasting in cardiac cachexia by reducing oxidative stress and cellular damage.20 Fourthly, exercise training and increased contractile activity promotes EcSOD expression in skeletal muscle.20 Finally and most importantly, exercise training improves cardiac phenotypes in both human patients and animal models of diabetes.22–24 It is therefore important to ascertain if enhanced EcSOD expression in skeletal muscle, such as under the condition of exercise training, would provide protection to the heart against DCM. Answering this question will improve our understanding of the biological importance of exercise training-induced expression of EcSOD in skeletal muscle and foster effective therapeutics for DCM.
Herein, we tested the hypothesis that enhanced EcSOD expression in skeletal muscle is sufficient to prevent DCM. We took advantage of the model of streptozotocin (STZ)-induced diabetes in our recently generated skeletal muscle-specific EcSOD transgenic mice (TG). We show that EcSOD TG mice have elevated EcSOD in the heart and other organs due to EcSOD redistribution through the circulation and are protected from oxidative stress, aberrant cell signaling, cardiac hypertrophy and fibrosis, and cardiac dysfunction.
Methods
Animals
Male wildtype, C57BL/6 mice were purchased from the Jackson Laboratory (Bar Harbor, ME). Additionally, EcSOD TG mouse line was generated at the University of Virginia Gene Targeting and Transgenic Facility as described.20 All animals were housed 3–4 mice/cage on a 12:12-h light-dark cycle. Food and water were provided ad labitum. Voluntary wheel running protocol was performed as described, but briefly involved 24/7 access to a running wheel for 4 weeks.25 All protocols were approved by the University of Virginia Animal Care and Use Committee.
EcSOD TG and wild type (WT) littermate control mice received a low-dose injection (50 mg/kg body weight) of STZ on 5 consecutive days [TG-STZ (n=11) and WT-STZ (n=12)]. Briefly, mice were fasted for 6-hr at 0900-hr each morning, and received a STZ injection at 1500-hr each afternoon. Non-diabetic controls [WT-CON (n=6), TG-CON (n=6)] received an equal-volume injection of citrate buffer each day. Blood glucose levels were assessed for all mice 72-hr following the final injection of STZ or citrate buffer. Briefly, tail vein blood glucose was assessed using a glucose meter (Ascensia, Bayer). The low-dose regimen of STZ had an equivalent success rate of inducing a diabetic phenotype (blood glucose >250 mg/dL) in TG-STZ and WT-STZ mice.
Ten weeks after the 5-day treatment with STZ and confirmation of hyperglycemia, all mice were assessed for cardiac function via electrocardiography and echocardiography. One week later, mice were euthanized by carbon dioxide followed by cervical dislocation. The heart was removed, and a small section of the left ventricle was placed in 10% formalin for histology. The remaining portions of the heart were minced, mixed, and separated into fractions for protein and mRNA analysis. The tibialis anterior (TA) and soleus skeletal muscle were harvested, weighed, and flash frozen in liquid nitrogen.
Electrocardiography
Electrocardiography was performed on fully conscious mice using the ECGenie (Mouse Specifics, Boston, MA) as described.20
Echocardiography
Mice were anesthetized in an induction chamber using isoflurane, and maintained anesthetized via an inhalation mask using 1.0% isoflurane mixed with oxygen at a flow rate of 200 mL/min in a supine position on a warm plate maintained at 37°C by a circulating water bath. Depilatory cream, 70% ethanol, and betadine were used to clear and clean the chest area. Warmed echo gel was applied, and the heart imaged with a 13 MHz linear transducer. Several 2D-guided M-mode recordings of the short-axis of the heart were obtained for determination of left ventricular end diastolic diameter, end systolic diameter, end diastolic wall thickness and end systolic wall thickness by a blinded operator. Fractional shortening and ejection fraction were calculated as described.22
Immunofluorescence
Fresh frozen sections were stained with anti-EcSOD (1:500), and anti-lectin (1:1000) and anti-α smooth muscle (1:1000) to visualize vasculature smooth muscle. Confocal images were acquired and analysis was performed,20 using ImageJ software.
Western blot analysis
For protein analysis, fractions of the left ventricle were immediately homogenized with glass homogenizers in protein loading buffer containing 50 mM Tris-HCl, pH 6.8, 1% sodium dodecyl sulfate (SDS), 10% glycerol, 20 mM dithiothreitol, 127 mM 2-mercaptoethanol, and 0.01% bromophenol blue, supplemented with protease inhibitors (Roche) and phosphatase inhibitors (Sigma-Aldrich). Homogenates were boiled for 5 min and centrifuged for 5 min at 13,000 rmp. The following antibodies were used to probe PVDF membranes: SOD3/EcSOD (1:1000), phosph-p38 MAPK (Thr180/Tyr182) (1:1000), total p38 (1:1000), calcineurin (1:1000), myostatin (1:1000), and glyceraldehyde 3-phosphate dehydrogenase (Gapdh; 1:1000). Membranes were analyzed and quantified using an Odyssey Infrared Imaging System (LI-COR Biosciences, Lincoln, NE, USA).25 Immunoblot analyses for oxidative stress markers was performed as follows: 4-hydroxinonenal (4-HNE) resulted in a definitive band at ~43 kDa and the density of this band alone was analyzed for each sample; carbonylation was assessed according the manufacturer’s instructions (OxyBlot, Millipore), and the end result a multiple band pattern across many molecular weights. The density of each band alone and the density of the entire column were assessed for each sample, and no significant difference was found between the two analysis methods.
Semi-quantitative RT-PCR
The analysis was performed as described,26 using primers for atrial natriuric peptide (ANP; F:5’-TGAAAAGCAAACTGAGGGC-3’; R:5’-CAGAGTGGGAGAGGCAAGAC-3’), brain natriuretic peptide (BNP; F:5’-CTGAAGGTGCTGTCCCAGAT; R:5’-ACTTCAGTGCGTTACAGCCC-3’), EcSOD (F:5’-TGATCCTGTCCCATACTAC; R:5’-AAGCCACACACATGCACATT), tumor necrosis factor α (TNF-α; F:5’-AGTCCGGGCAGGTCTACTTT; R:5’-GCACCTCAGGGAAGAGTCTG), interleukin 1 β (IL-1β; F:5’-CTCACAAGAGCACAAGC; R:5’-CTCAGTGCAGGCTATGACCA), and 18s (F:5’-AAACGGCTACCACATCCAAG; R:5’-CCCTCTTAATCATGGCCTCA).
Histology
Cardiomyocyte cross-sectional area was determined from paraffin-embedded sections stained with hematoxylin and eosin. Cardiac fibrosis was determined using a picosirius red staining. Multiple images were acquired from each stained heart section. Images were assessed using ImageJ software.
Statistical analysis
Data are represented as mean ± SEM. Differences among individual groups of mice are only reported when a significant interaction was observed by a two-way ANOVA (genotype × treatment) using JMP (SAS; NC, USA) statistical software. Data was required to pass normality (Shapiro-Wilk) and equal variance tests (Brown-Forsythe F test) prior to proceeding with the ANOVA analysis. All data passed the equal variance tests. When a significant interaction was observed, a Tukey’s HSD post-hoc analysis was performed. An α-level of 0.05 was used for all analyses.
Results
Skeletal muscle-specific EcSOD transgenic overexpression leads to elevated EcSOD level in the heart with redistribution through the circulation
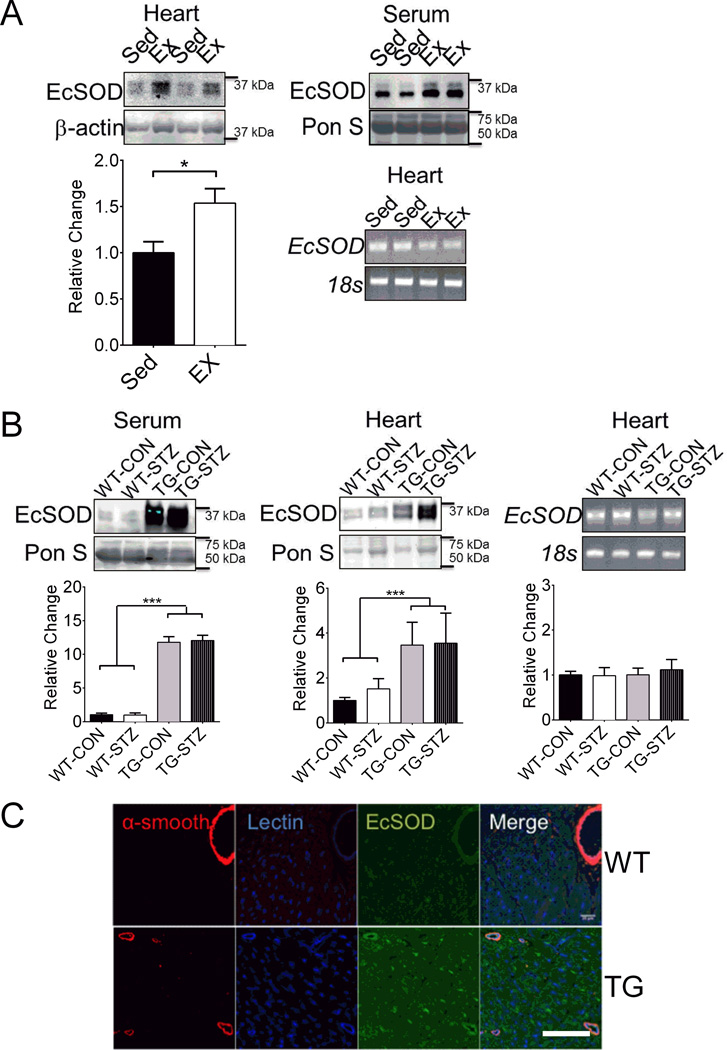
Previous studies have shown that exercise training provides protection against DCM,22,23 and ectopic expression of EcSOD is effective in counteracting oxidative stress in various tissues/organs.27,28 To ascertain the potential protective function of EcSOD in the heart and its origin under the condition of exercise training, we first tested the extent to which EcSOD protein level in the heart and serum was promoted by endurance exercise training in mice. Four weeks of voluntary wheel running significantly increased EcSOD protein level in the heart and serum in reference to the sedentary control mice. The increased EcSOD protein level in the heart did not appear to result from increased EcSOD gene transcription in the heart, as we did not detect significant increases in EcSOD mRNA (Figure 1A). These findings suggest that elevated EcSOD protein in the heart is a result of enhanced expression and secretion of EcSOD from other tissue(s) in the periphery, in this case skeletal muscle.
Figure 1. Enhanced expression of EcSOD in skeletal muscle redistributes to the heart.
(A) Four weeks of voluntary wheel running affected EcSOD expression in the hearts and serum of exercise trained WT (Ex; n=9) mice compared to WT sedentary (Sed; n=9) controls, and EcSOD levels in the heart were independent of mRNA expression. Student’s t-test, * denotes P<0.05. (B) EcSOD levels were 11-fold greater in the serum and 4-fold greater in the hearts of EcSOD TG mice, independent of STZ (n=12) compared to WT littermate controls, independent of STZ (n=12), and EcSOD levels in the heart were independent of mRNA expression. ANOVA, group effect for genotype, *** denotes P<0.001. (C) EcSOD protein was enriched near the endothelial cells in the heart, and had greater presence within individual cardiomyocytes in EcSOD TG mice compared to WT littermate controls. Scale bar is 25 µm. Graphical bars are means ± SE.
Since voluntary wheel running in mice enhances EcSOD protein expression in skeletal muscles,20 we speculated that enhanced expression of EcSOD in skeletal muscle could redistribute through the circulation to the heart. EcSOD TG had greater EcSOD levels in the serum and heart compared to WT mice (Figure 1B). To determine if the elevated EcSOD level in the heart was due to leaky expression of the transgene by the muscle creatine kinase (MCK) promoter, we examined the mRNA expression of EcSOD in the heart. There was no significant difference in EcSOD mRNA expression between EcSOD TG and WT mice (Figure 1B). Since the MCK promoter is not active in non-muscle tissues, these findings collectively suggest that elevated level of EcSOD protein in the heart of TG mice is mainly coming from skeletal muscle. We next examined the localization of EcSOD within the heart of EcSOD TG and WT mice (Figure 1C). Endogenous EcSOD appears to be enriched at the endothelium as evidenced by the signals from immunofluorescence staining of the ventricules in WT mice (Figure 1C). In EcSOD TG heart, significantly more EcSOD signals were detected near the endothelium as well as within the cardiomyocytes. These findings are consistent with the notion that EcSOD from the circulation can adhere to endothelial cells and can also be taken up by cardiac myocytes in the heart.
Enhanced expression of EcSOD in skeletal muscle prevents DCM induced by STZ injection
To determine if EcSOD TG mice are protected from DCM, we subjected EcSOD TG and WT mice to a low-dose regimen of STZ injections. EcSOD and WT mice had equal severity in hyperglycemia following STZ injections (374±32 vs. 362±32 mg/dL; P=0.459). Left ventricular end diastolic diameter, a marker of diastolic dysfunction, showed an increased trend without statistical significance (Table 1) along with a significant increased left ventricular end systolic diameter, a marker of systolic dysfunction, in WT-STZ mice compared to WT-CON and TG-STZ mice 10 weeks after the 5-day injection treatment (Table 1). We used these two parameters to calculate ejection fraction and fractional shortening and showed that they were both significantly lower in WT-STZ mice compared to WT-CON, which were completely prevented in TG-STZ mice (Figure 2A–C). Fully conscious electrocardiograph analysis, a sensitive measurement of resting heart rate, showed that WT-STZ mice had significantly slower resting heart rate (bradycardia) than WT-Con, which was significantly attenuated in TG-STZ (Figure 2D). Consistently, electrocardiography waveform analysis showed that QRS-interval was lengthened in WT-STZ mice compared with WT-CON (Table 1), which was mitigated in TG-STZ mice. Together, these in vivo data strongly suggest that STZ treatment produced a phenotype of cardiac dysfunction, which was significantly attenuated in mice with skeletal muscle-specific EcSOD overexpression.
Table 1.
In vivo heart function of WT-CON, WT-STZ, TG-CON, TG-STZ mice
| WT-CON (n=6) | WT-STZ (n=11) | TG-CON (n=6) | TG-STZ (n=12) | |
|---|---|---|---|---|
| PR-interval (µs) | 23.8±0.7 | 27.5±0.8 | 23.0±0.5 | 24.5±0.5 |
| QRS-interval (µs) | 10.3±0.3 | 11.7±0.4* | 10.5±0.1 | 10.4±0.3 |
| QT-interval (µs) | 41.7±0.7 | 46.0±1.1 | 39.4±0.7 | 41.3±0.6 |
| LVEDD (mm) | 261±12 | 335±15 | 289±19 | 295±20 |
| LVESD (mm) | 135±14 | 212±7* | 147±10 | 164±13 |
| LVEDwt (mm) | 99±19 | 104±10 | 115±15 | 106±10 |
| LVESwt (mm) | 151±14 | 160±5 | 170±13 | 170±9 |
Values are means ± SEM
Left ventricular end diastolic- and systolic diameter (LVEDD and LVESD, respectively) Wall thickness (LVEDwt and LVESwt)
P<0.05 vs. WT-CON, TG-CON, TG-STZ
Figure 2. Enhanced EcSOD expression in skeletal muscle prevents STZ-induced impairment in cardiac function.
(A) Representative M-mode echocardiograph heart images. (B–C) Graphical representation showing ejection fraction and fractional shortening calculated from echocardiograph analysis. (D) Graphical representation of in vivo, fully conscious heart rate from electrocardiograph analysis. WT-Con, n=6; WT-STZ, n=11; TG-Con, n=6; TG-STZ, n=12. ANOVA, significant interaction, followed by Tukey’s HSD post-hoc test *, **, and *** denote P<0.05, P<0.01, and P<0.001, respectively. Bars are means ± SE.
Enhanced expression of EcSOD in skeletal muscle prevents cardiac hypertrophy and fibrosis induced by STZ injection
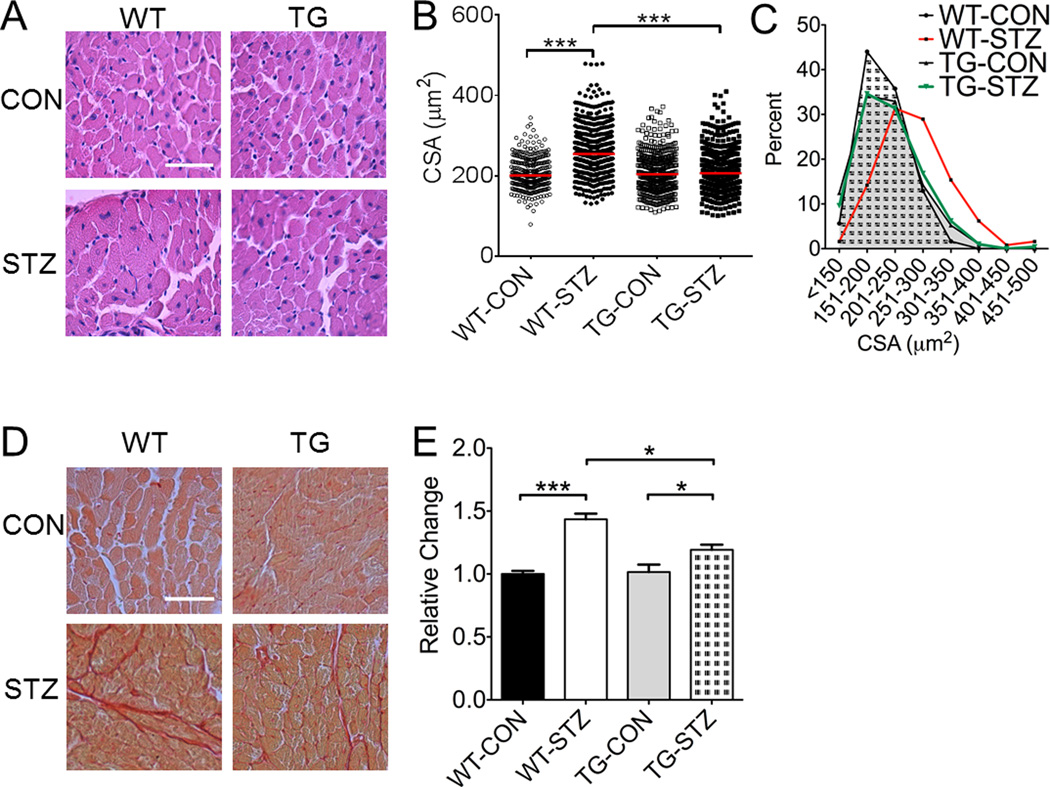
Cardiac dysfunction is often caused by abnormal morphological changes in the heart, such as hypertrophy and deposition of non-contractile, fibrotic components. To assess cardiac hypertrophy, we measured heart mass relative to body mass and cardiomyocyte cross-sectional area in all mice 11 weeks after STZ or citric acid injections and one week after in vivo functional analysis. Relative heart mass was significantly greater in WT-STZ mice compared to WT-CON mice, and TG-STZ mice did not show significant increase (vs. TG-CON) (Table 2). Kruskal-Wallis analysis of H&E stained ventricular muscle sections (Figure 3A) showed that the median cross-sectional area of individual cardiomyocytes was greater in WT-STZ mice compared to WT-CON (254 vs. 200 µm2), which was completely blocked in TG-STZ mice (206 µm2; Figure 3B). Chi-square analysis revealed that STZ treatment in littermate controls resulted in more large fibers, whereas STZ treatment in EcSOD TG mice did not (Figure 3C). Overall, STZ resulted in a rightward distribution shift in cardiomyocyte cross-sectional area for WT-STZ mice, which was significantly attenuated in TG-STZ mice. Picosirius red staining showed that there was enhanced deposition of collagen in interstitial space of the ventricular muscle sections in WT-STZ (vs. WT-CON), which was significantly attenuated in TG-STZ (vs. WT-STZ) consistent with the protection against cardiac fibrosis (Figure 3D–E). These data indicate that STZ-induced hyperglycemia is associated with DCM with pathological hypertrophy and cardiac fibrosis, and skeletal muscle-specific EcSOD overexpression mitigates these pathological processes.
Table 2.
Body mass, heart, tibialis anterior, soleus muscle mass of WT-CON, WT-STZ, TG-CON, TG-STZ mice
| WT-CON (n=6) | WT-STZ (n=11) | TG-CON (n=6) | TG-STZ (n=12) | |
|---|---|---|---|---|
| Body mass (g) | 24.1±0.9 | 23.6±1.2 | 24.8±0.9 | 25.7±1.0 |
| Heart mass (mg) | 118.9±4.3 | 149.9±5.6 | 121.3±1.9 | 130.0±8.1 |
| H/BM (mg/g) | 4.9±0.1 | 6.3±0.3* | 4.9±0.2 | 5.0±0.1 |
| TA mass (mg) | 38.4±1.9 | 27.7±3.7 | 42.3±2.4 | 40.1±3.0 |
| TA/BM (mg/g) | 1.59±0.04 | 1.14±0.11* | 1.71±0.08 | 1.55±0.09 |
| Sol mass (mg) | 7.3±0.2 | 7.5±0.5 | 7.5±0.4 | 8.2±0.4 |
| Sol/BM (mg/g) | 0.31±0.02 | 0.32±0.02 | 0.30±0.01 | 0.32±0.01 |
Values are means ± SEM
BM, body mass; H, heart; TA, tibialis anterior muscle; Sol, soleus muscle.
P<0.05 vs. WT-CON, TG-CON, TG-STZ
Figure 3. Enhanced EcSOD expression in skeletal muscle mitigates STZ-induced cardiac hypertrophy and fibrosis.
(A) Representative heart images stained with hemotoxylin and eosin to identify individual cardiomyocytes. (B) Graphic representation of individual cardiomyocyte cross-sectional area. (C) Cardiomyocyte size frequency distributions based on cross-sectional area. WT-CON, black bar with dotted area; WT-STZ, red bar; TG-CON, grey area; TG-STZ, green bar. Note the rightward distribution shift of the WT-STZ fibers relative to all other groups. Among these mice there was an abnormally large amount of large fibers (>300 µm). (D) Representative heart images stained for fibrotic tissue deposition by sirius red. (E) Graphical representation of collagen staining. WT-Con, n=6; WT-STZ, n=11; TG-Con, n=6; TG-STZ, n=12. ANOVA, significant interaction, followed by Tukey’s HSD post-hoc test ** and *** denote P<0.01 and P<0.001.
DCM cachexia is prevented with overexpression of EcSOD
An important clinical outcome of cardiomyopathy is skeletal muscle catabolic wasting and cachexia. We have recently reported that enhanced expression of EcSOD in skeletal muscle is sufficient to prevent chronic heart failure induced catabolic wasting.20 Hyperglycemia induced by diabetes may also directly impair skeletal muscle function. To determine the effects of skeletal muscle-specific EcSOD overexpression on DCM-induced catabolic muscle wasting, we examined the masses of the fast-twitch tibialis anterior muscle, and slow-twitch soleus muscle. Following 11 weeks of hyperglycemia, relative TA muscle masses were less in WT-STZ mice compared to WT-CON, which was attenuated in TG-STZ mice (vs. WT-STZ) (Table 2). This catabolic muscle wasting was not observed in the slow-twitch soleus muscles (Table 2). These findings are completely consistent with previous findings in other models of catabolic muscle wasting.29
Enhanced skeletal muscle expression of EcSOD results in reduced oxidative stress in the heart under the condition of diabetes induced by STZ injections
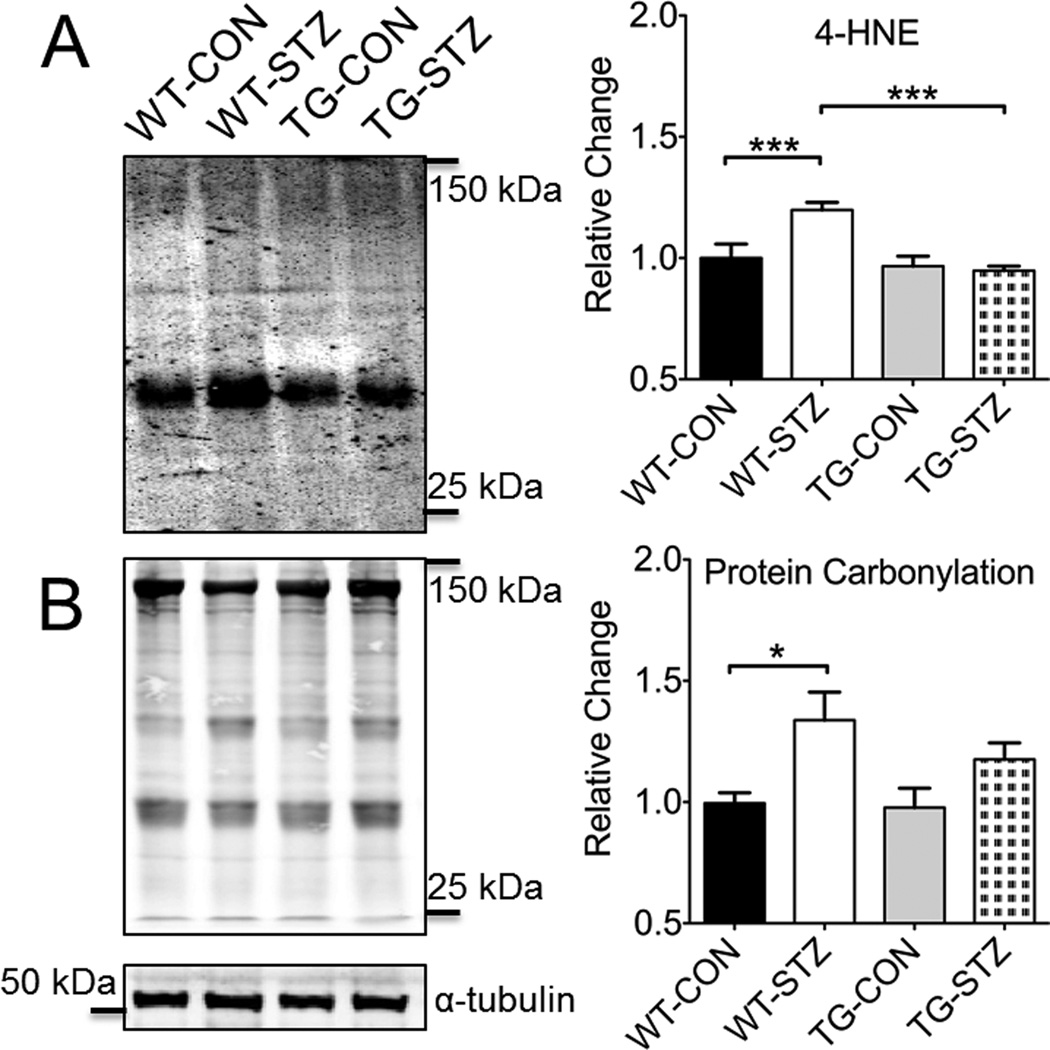
Oxidative stress is clearly involved in the pathogenesis of DCM. To determine if enhanced skeletal muscle expression of EcSOD may result in reduced oxidative stress in the heart under the diabetic condition, we examined oxidative stress markers in the heart. 4-hydroxynoneal, a by-product of lipid peroxidation, was greater in WT-STZ compared to WT-CON, which was mitigated in TG-STZ mice (vs. WT-STZ) (Figure 4A). Protein carbonylation, which generally defines the post-translation modification of amino acid side chains to aldehyde and ketone derivatives (i.e., carbonyls), was greater in WT-STZ compared to WT-CON (Figure 4B), but not different among other groups. These findings collectively are consistent with the notion that enhanced EcSOD expression in skeletal muscle is sufficient to protect the heart from oxidative stress and damage induced by diabetic hyperglycemia.
Figure 4. Enhanced EcSOD expression in skeletal muscle protects against STZ-induced oxidative damage and aberrant signaling in the heart.
Representative immunoblots and graphical representation of statistical analysis is shown for 4-HNE (A) and protein carbonylation (B). Staining was normalized by tubulin and is shown relative to WT-CON. WT-Con, n=6; WT-STZ, n=11; TG-Con, n=6; TG-STZ, n=12. ANOVA, significant interaction, followed by Tukey’s HSD post-hoc test * and *** denotes P<0.05 and P<0.001. Bars are means ± SE.
Enhanced skeletal muscle expression of EcSOD prevents aberrant cell signaling and inflammation in the heart
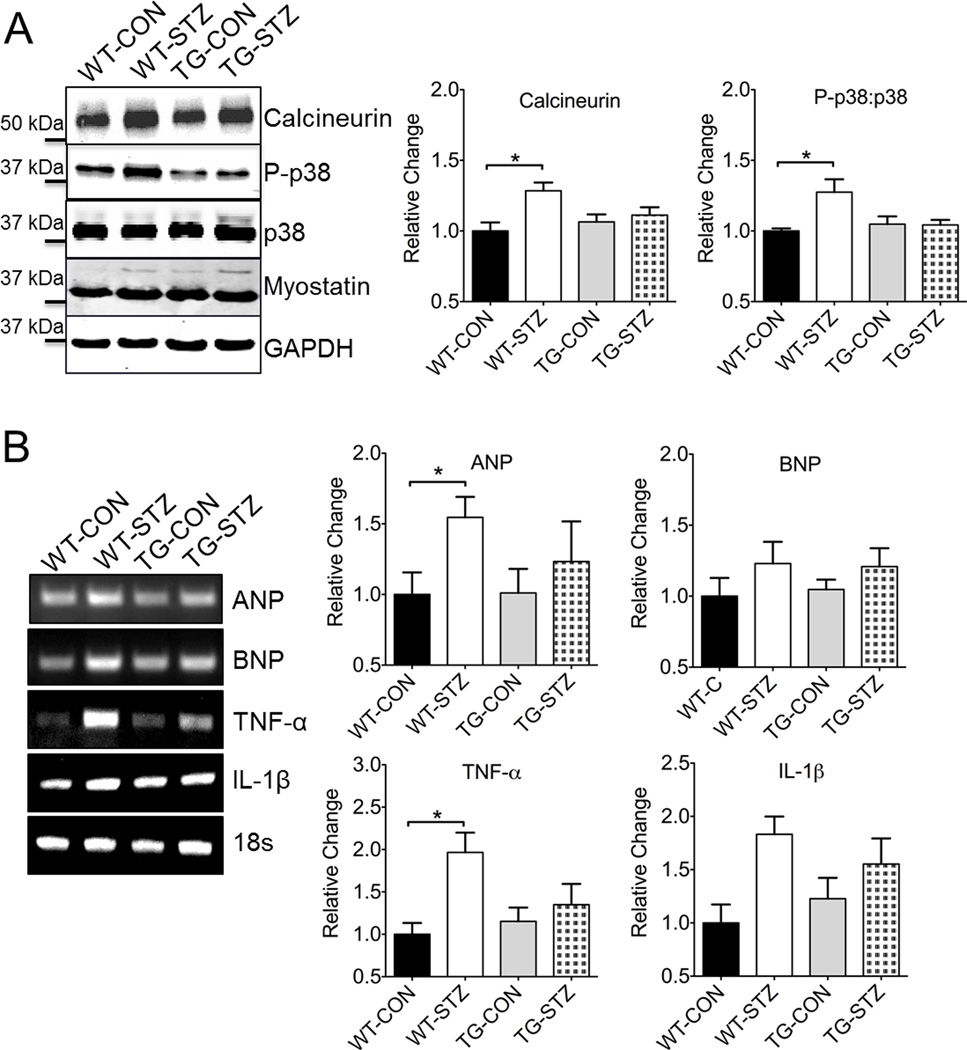
Cell signaling in response to hyperglycemia and oxidative stress is clearly an important part of maladaptive remodeling in the heart during the development of DCM. To further examine the mechanism of EcSOD-mediated protection in the heart, we probed for proteins associated with signaling in cardiac hypertrophy (Figure 5A). Activation of calcineurin is involved in cardiac hypertrophy in both animals and humans.30,31 Here, we observed a modest but significant increase in calcineurin expression in the heart of WT-STZ mice compared to WT-CON mice (Figure 5A). We did not observe any significant difference in myostatin expression across groups (Figure 5A). We observed an activation (phosphorylation) of p38 mitogen-activated protein kinase (MAPK), a stress pathway that is critical for the inflammatory process in maladaptive remodeling of the heart, in WT-STZ compared to WT-CON mice (Figure 5A), which coincided with greater expression of the inflammatory cytokine tumor necrosis factor-α (TNF-α) (Figure 5B). Finally, we tested in diabetic cardiomyopathy in our hands was associated with the more tradition expression of hypertrophy molecules, atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP). We observed a significant elevation of ANP gene expression in the heart of WT-STZ mice compared to WT-CON mice (Figure 5B). We did not observed any significant difference in BNP gene expression across groups. These findings suggest a complex mechanism of diabetic cardiomyopathy, but support that premise of EcSOD derived from skeletal muscle as having some protective function in this disease model of type-1 diabetes.
Figure 5. Enhanced EcSOD expression in skeletal muscle protects against STZ-induced aberrant signaling in the heart.
(A) STZ-induced activation of calcineurin and p38 MAPK in hearts is mitigated in EcSOD TG mice. (B) EcSOD alleviates STZ-induced TNF-α and ANP mRNA expression. WT-Con, n=6; WT-STZ, n=11; TG-Con, n=6; TG-STZ, n=12. ANOVA, significant interaction, followed by Tukey’s HSD post-hoc test * denotes P<0.05. Bars are means ± SE.
Discussion
In this study, we showed that STZ-induced diabetes elicited DCM with clear evidence of ventricular dysfunction and pathological remodeling. Cardiac dysfunction, hypertrophy, fibrosis, and oxidative stress were all effectively mitigated in skeletal muscle-specific EcSOD transgenic mice. This, to our knowledge, is the first report of genetically enhanced gene expression in skeletal muscle showing protection against DCM. Since elevated EcSOD protein level in the heart in these transgenic mice is due to redistribution of EcSOD from skeletal muscle via the circulation, these findings support that enhanced skeletal muscle expression of EcSOD is sufficient to mitigate oxidative stress and aberrant cell signaling and preserves cardiac function in vivo. Since exercise training improves cardiac function in both human patients and animal models of diabetes,22,23 and exercise training leads to elevated EcSOD levels in the heart in the absence of enhanced ectopic mRNA expression, we speculate that induced EcSOD expression in skeletal muscle underlies at least in part the beneficial effects of exercise training in diabetic populations.
The most exciting aspect of our findings is the protection of the heart from enhanced expression of EcSOD in skeletal muscle. This presents an interesting feature of the integrated biological system in our body and raises the possibility of using this feature to circumvent disease conditions of other tissues/organs. In fact, organ-organ crosstalk is emerging as an important area of research, which will likely advance biomedical science with profound clinical relevance. For example, interleukin-6 (IL-6) has been shown mediate brown adipose tissue (BAT)-facilitated glucose sensitivity in the heart,32 and skeletal muscle-derived IL-6 can significantly influence lipid metabolism in the liver.33 Myostatin, a potent regulator of muscle mass, secreted from the failing heart, is involved in heart failure-induced skeletal muscle wasting.34 More recently, irisin secreted from skeletal muscles has been shown to promote “browning” of adipose tissue.35 Up to date, there has been no evidence for an antioxidant functioning as a mediator of organ-organ crosstalk. Since skeletal muscle is the largest internal organ in the body, it represents a potentially important, continuous source of EcSOD to mitigate oxidative stress in peripheral tissues/organs (e.g., the heart) under disease conditions. Our findings support the notion that enhanced expression of EcSOD in skeletal muscle induced by exercise training contributes to the improved cardiac phenotypes in diabetes.22,23 The findings also support augmenting EcSOD expression in skeletal muscle or other tissue/organs as therapeutic intervention for DCM. Continued research efforts in this exciting new field will undoubtedly lead to the discovery of other potential factors.
DCM features structural remodeling of the heart (i.e., hypertrophy and fibrosis) and eventual contractile dysfunction. Preempting the development of DCM is the elevated blood glucose levels (hyperglycemia), which is positively correlated with cardiovascular cell death.36 Myocardial hypertrophy initially occurs as an adaptive response to mitigate the negative impacts of the pathological insults. When the pathological insults persist, excessive extracellular matrix (ECM) deposition occurs as a result of increased production and/or reduced degradation of ECM components. In fact, hyperglycemia per se is sufficient to increase cardiac fibroblast proliferation leading to interstitial and perivascular fibrosis.37 As a consequence, the myocardium increases its stiffness and reduces its compliance, which together with apoptotic loss of cardiomyocytes leads to systolic and diastolic dysfunction. Our findings that EcSOD TG mice are resistant to both cardiac hypertrophy and fibrosis independent of improved glycemic control strongly suggest that elevated EcSOD protein in the heart mitigates the pathological signal that is upstream of the hypertrophy and fibrosis cascades in the myocardium under the diabetic condition. We will in the future determine if enhanced skeletal muscle expression of EcSOD would reverse the pathological changes and cardiac dysfunction in DCM.
The development of ventricular dysfunction in diabetes is a complex process with many contributing factors. Left ventricular (LV) diastolic dysfunction often occurs in isolation, or prior to LV systolic dysfunction in DCM in humans,38 although some studies also showed prevalence of LV systolic dysfunction.39 Here, we showed clear evidence of LV systolic dysfunction concurrent with a trend of LV diastolic dysfunction in STZ-injected WT mice, which were significantly ameliorated in EcSOD TG mice (Figure 2). Since hyperglycemia-induced impairment of calcium handling in cardiomyocytes is a major contributor to LV systolic dysfunction,5 our findings suggest that elevated EcSOD in the heart may mitigate hyperglycemia-induced impairment of calcium handling.
Herein, changes in intracellular calcium handling may have led to aberrant signaling and maladaptation in the diabetic heart. Calcineurin, a calcium-regulated phosphatase that is activated during prolonged increases of intracellular calcium concentrations,30 has been shown to dephosphorylate nuclear factor of activated T cell (NFAT) and lead to its nuclear translocation and activation of genes involved in cardiac hypertrophy.40 Here, we observed a significant increase in calcineurin protein expression in DCM, which was blunted in EcSOD TG mice. Elevated calcineurin protein expression in the heart has been observed previously under the condition of human heart failure,31 suggesting that increased calcineurin expression is involved in cardiac hypertrophy. Since the main function of EcSOD is scavenging superoxide radical, our finding that EcSOD TG mice are resistant to pathological hypertrophy induced by diabetes suggests that oxidative stress may directly, or indirectly, lead to impaired calcium handling and aberrant calcium-calcineurin signaling in DCM.
Numerous studies have shown that redox-sensitive, stress signaling pathways, such as c-jun N-terminal protein kinase (JNK) and p38 mitogen-activated protein kinase (p38 MAPK), are activated by diabetic conditions.41 In particular, the p38 MAPK pathway has been shown to regulate the expression of inflammatory cytokines in diabetic cardiomyopathy, and pharmacological or genetic inhibition of p38 MAPK mitigates STZ-induced systolic dysfunction, hypertrophy, fibrosis, oxidative stress and inflammation.12 These findings support that p38 MAPK activity is functionally important for the induction of adverse cardiac remodeling and dysfunction under the diabetic condition. We showed that p38 MAPK activation was greater in WT mice compared to EcSOD TG in response to STZ injection. Together with the findings that EcSOD TG diabetic mice were protected from pathological hypertrophy along with significant abrogation of fibrotic tissue deposition, we speculate that p38 MAPK plays an important role in fibrosis as previously described.12
Oxidative stress is accepted as an important player in the development of cardiac hypertrophy and fibrosis in DCM.8 Previous studies have demonstrated that STZ-induced diabetes are associated with increases in many known markers of oxidative stress in the heart, including superoxide anion production, protein nitrosylation, and lipid peroxidation.42,43 Here, we examined four markers of oxidative damage, of which two were significantly elevated in diabetic heart and the other two showed increased trends. While the etiology of ROS in DCM is still being elucidated, mitochondria, xanthine oxidase, NADPH oxidase, monoamine oxidase-A, and uncoupled nitric oxide synthases might all play a role in the increased production of ROS.44,45
An increasing number of studies have shown the efficacy of enhancing antioxidant defense in counteracting the pathophysiology of DCM. For example, Shen et al. showed that enhanced expression of mitochondrial manganese superoxide dismutase reduces oxidative stress, improves mitochondrial morphology, and attenuates cardiac contractility in a mouse model of type 1 diabetes.15 Overexpression of catalase or GPx1also improves cardiac morphology, mitochondrial structure and myofibrillar structure, as well as cardiomyocyte contractility.46,47 However, clinical application of antioxidant therapies have not been very successful. Administration of exogenous SOD in vivo is limited by the large size (restricting cell permeability) and short half-life. Vitamin C or E supplementation as a “pharmacotherapy” have failed to show clear cardiovascular benefit.48 These unfavorable outcomes may be related to one or all of the following limitations: low activity of exogenous antioxidants, lack of targeting to the intended action site and mis-targeting that impairs physiological function. Our findings that elevated EcSOD level in EcSOD TG mice is localized at the endothelial cells and within cardiomyocytes in the heart suggest that targeted scavenging of oxidative stress in endothelial cells and/or cardiomyocytes is a promising intervention against pathogenesis of DCM.
Several studies utilizing genetic, pharmacological, and nutritional approaches to mitigate oxidative stress demonstrate in proof-of-principle that maintaining a healthy redox status in the heart can provide protection against cardiac dysfunction.9 In this study, we chose to focus on EcSOD as it appears to be critically involved in heart health, as EcSOD knockout mice have exacerbated cardiac hypertrophy.49 The true novelty of this study is that EcSOD expressed in skeletal muscle could attenuate oxidative stress in the heart, most likely through redistribution of EcSOD through the circulation. Here, we show that enhanced EcSOD expression in skeletal muscle by exercise training in mice leads to increased EcSOD levels in the blood and heart, without increased ectopic EcSOD expression in the heart, consistent with previous gene array findings.50 Collectively, our findings support the notion that enhanced expression of EcSOD in skeletal muscle acts through the circulation to prevent oxidative stress in peripheral tissues; a finding well in line with the concept of organ-organ cross talk. It is important in the future to determine whether skeletal muscle-derived EcSOD is required for the exercise training-mediated protection against DCM.
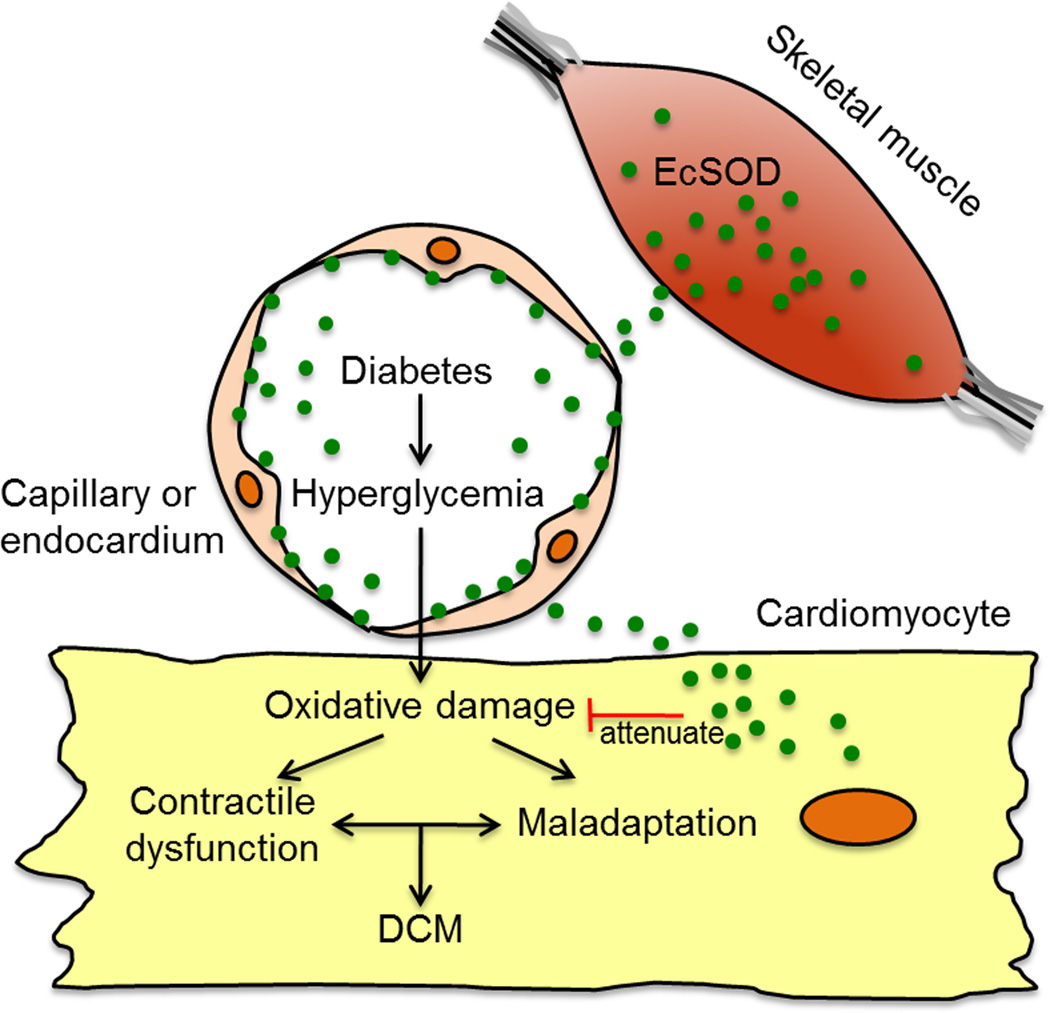
Overall, the present study provides novel evidence that enhanced EcSOD expression in skeletal muscle through its redistribution to the heart is sufficient to reduce oxidative stress and aberrant cell signaling and hence ameliorates pathological changes and cardiac dysfunction in STZ-induced DCM (Figure 6). The STZ model of diabetes in rodents is not without its limitations, as the manifesting condition is severe with a complete loss of insulin control. Indeed, the severity of the phenotype might contribute to the development of DCM, which is absent in other models of diabetes, such as the Akita mouse. It is worth speculating that the protective effects observed herein may in fact be bolstered in a more appropriate model. Finally, because of EcSOD’s antioxidant capabilities and cellular location, our findings raise the possibility of enhancing EcSOD levels systemically by pharmacological, genetic and/or exercise intervention as therapeutic approaches for diabetic patients with DCM.
Figure 6. Schematic illustration of the role of enhanced EcSOD expression in skeletal muscle in protection against DCM.
Enhanced EcSOD expression in skeletal muscle leads to increased distribution of EcSOD via the circulation to cardiomyocytes through either capillary endothelium or endocardial endothelium. Increased EcSOD in the cardiomyocytes mitigates hyperglycemia-induced oxidative stress hence preventing contractile dysfunction and maladaptation and blocking the pathogenesis of DCM.
Acknowledgments
We sincerely thank Drs. Shayn M. Peirce-Cottler and Kyle L. Hoehn for their providing technical support for this study.
Sources of Funding
This work was supported by the National Institutes of Health [AR050429 to Z.Y., T32-HL007284 to J.A.C.], the American Heart Association [12POST12030231 to J.A.C.], the Nakatomi Foundation [M.O.], and the American Physiological Society [V.A.L.].
Footnotes
Disclosures
None.
References
- 1.Wild S, Roglic G, Green A, Sicree R, King H. Global Prevalence of Diabetes: Estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 2.Hayat Sa, Patel B, Khattar RS, Malik Ra. Diabetic cardiomyopathy: mechanisms, diagnosis and treatment. Clin Sci (Lond) 2004;107:539–557. doi: 10.1042/CS20040057. [DOI] [PubMed] [Google Scholar]
- 3.Role of cardiovascular risk factors in prevention and treatment of macrovascular disease in diabetes. American Diabetes Association. Diabetes Care. 1989;12:573–579. doi: 10.2337/diacare.12.8.573. [DOI] [PubMed] [Google Scholar]
- 4.Rubler S, Dlugash J, Yuceoglu YZ, Kumral T, Branwood AW, Grishman A. New type of cardiomyopathy associated with diabetic glomerulosclerosis. Am J Cardiol. 1972;30:595–602. doi: 10.1016/0002-9149(72)90595-4. [DOI] [PubMed] [Google Scholar]
- 5.Choi KM, Zhong Y, Hoit BD, Grupp IL, Hahn H, Dilly KW, Guatimosim S, Lederer WJ, Matlib MA. Defective intracellular Ca(2+) signaling contributes to cardiomyopathy in Type 1 diabetic rats. Am J Physiol Heart Circ Physiol. 2002;283:H1398–H1408. doi: 10.1152/ajpheart.00313.2002. [DOI] [PubMed] [Google Scholar]
- 6.Poornima IG, Parikh P, Shannon RP. Diabetic cardiomyopathy: the search for a unifying hypothesis. Circ Res. 2006;98:596–605. doi: 10.1161/01.RES.0000207406.94146.c2. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu M, Umeda K, Sugihara N, Yoshio H, Ino H, Takeda R, Okada Y, Nakanishi I. Collagen remodelling in myocardia of patients with diabetes. J Clin Pathol. 1993;46:32–36. doi: 10.1136/jcp.46.1.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 9.Maritim aC, Sanders Ra, Watkins JB. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 10.Giugliano D, Ceriello A, Paolisso G. Oxidative stress and diabetic vascular complications. Diabetes Care. 1996;19:257–267. doi: 10.2337/diacare.19.3.257. [DOI] [PubMed] [Google Scholar]
- 11.Cai L, Kang YJ. Oxidative stress and diabetic cardiomyopathy: a brief review. Cardiovasc Toxicol. 2001;1:181–193. doi: 10.1385/ct:1:3:181. [DOI] [PubMed] [Google Scholar]
- 12.Westermann D, Rutschow S, Linthout SVan, Linderer a, Bücker-Gärtner C, Sobirey M, Riad a, Pauschinger M, Schultheiss H-P, Tschöpe C. Inhibition of p38 mitogen-activated protein kinase attenuates left ventricular dysfunction by mediating pro-inflammatory cardiac cytokine levels in a mouse model of diabetes mellitus. Diabetologia. 2006;49:2507–2513. doi: 10.1007/s00125-006-0385-2. [DOI] [PubMed] [Google Scholar]
- 13.Turkseven S, Kruger A, Mingone CJ, Kaminski P, Inaba M, Rodella LF, Ikehara S, Wolin MS, Abraham NG. Antioxidant mechanism of heme oxygenase-1 involves an increase in superoxide dismutase and catalase in experimental diabetes. Am J Physiol Heart Circ Physiol. 2005;289:H701–H707. doi: 10.1152/ajpheart.00024.2005. [DOI] [PubMed] [Google Scholar]
- 14.Hamblin M, Smith HM, Hill MF. Dietary supplementation with vitamin E ameliorates cardiac failure in type I diabetic cardiomyopathy by suppressing myocardial generation of 8-iso-prostaglandin F2alpha and oxidized glutathione. J Card Fail. 2007;13:884–892. doi: 10.1016/j.cardfail.2007.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shen X, Zheng S, Metreveli NS, Epstein PN. Protection of cardiac mitochondria by overexpression of MnSOD reduces diabetic cardiomyopathy. Diabetes. 2006;55:798–805. doi: 10.2337/diabetes.55.03.06.db05-1039. [DOI] [PubMed] [Google Scholar]
- 16.Flaherty JT, Pitt B, Gruber JW, Heuser RR, Rothbaum DA, Burwell LR, George BS, Kereiakes DJ, Deitchman D, Gustafson N. Recombinant human superoxide dismutase (h-SOD) fails to improve recovery of ventricular function in patients undergoing coronary angioplasty for acute myocardial infarction. Circulation. 1994;89:1982–1991. doi: 10.1161/01.cir.89.5.1982. [DOI] [PubMed] [Google Scholar]
- 17.Celik T, Yuksel C, Iyisoy A. Alpha tocopherol use in the management of diabetic cardiomyopathy: lessons learned from randomized clinical trials. J Diabetes Complications. 2010;24:286–288. doi: 10.1016/j.jdiacomp.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 18.Wang X, Jian C, Zhang X, Huang Z, Xu J, Hou T, Shang W, Ding Y, Zhang W, Ouyang M, Wang Y, Yang Z, Zheng M, Cheng H. Superoxide flashes: elemental events of mitochondrial ROS signaling in the heart. J Mol Cell Cardiol. 2012;52:940–948. doi: 10.1016/j.yjmcc.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 19.Chu Y, Iida S, Lund DD, Weiss RM, DiBona GF, Watanabe Y, Faraci FM, Heistad DD. Gene transfer of extracellular superoxide dismutase reduces arterial pressure in spontaneously hypertensive rats: role of heparin-binding domain. Circ Res. 2003;92:461–468. doi: 10.1161/01.RES.0000057755.02845.F9. [DOI] [PubMed] [Google Scholar]
- 20.Okutsu M, Call JA, Lira VA, Zhang M, Donet JA, French BA, Martin KS, Peirce-Cottler SM, Rembold CM, Annex BH, Yan Z. Extracellular Superoxide Dismutase Ameliorates Skeletal Muscle Abnormalities, Cachexia and Exercise Intolerance in Mice with Congestive Heart Failure. Circ Heart Fail. 2014;7:519–530. doi: 10.1161/CIRCHEARTFAILURE.113.000841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chu Y, Piper R, Richardson S, Watanabe Y, Patel P, Heistad DD. Endocytosis of extracellular superoxide dismutase into endothelial cells: role of the heparin-binding domain. Arterioscler Thromb Vasc Biol. 2006;26:1985–1990. doi: 10.1161/01.ATV.0000234921.88489.5c. [DOI] [PubMed] [Google Scholar]
- 22.Shao C, Wehrens X, Wyatt T. Exercise training during diabetes attenuates cardiac ryanodine receptor dysregulation. J Appl Physiol. 2009:1280–1292. doi: 10.1152/japplphysiol.91280.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atalay M, Laaksonen DE. Review article DIABETES, OXIDATIVE STRESS AND PHYSICAL EXERCISE. Diabetes. 2002:1–14. [PMC free article] [PubMed] [Google Scholar]
- 24.Korte FS, Mokelke EA, Sturek M, McDonald KS. Exercise improves impaired ventricular function and alterations of cardiac myofibrillar proteins in diabetic dyslipidemic pigs. J Appl Physiol. 2005;98:461–467. doi: 10.1152/japplphysiol.00551.2004. [DOI] [PubMed] [Google Scholar]
- 25.Akimoto T, Ribar TJ, Williams RS, Yan Z. Skeletal muscle adaptation in response to voluntary running in Ca2+/calmodulin-dependent protein kinase IV-deficient mice. Am J Physiol Cell Physiol. 2004;287:C1311–C1319. doi: 10.1152/ajpcell.00248.2004. [DOI] [PubMed] [Google Scholar]
- 26.Yan Z, Choi S, Liu X, Zhang M, Schageman JJ, Lee SY, Hart R, Lin L, Thurmond FA, Williams RS. Highly coordinated gene regulation in mouse skeletal muscle regeneration. J Biol Chem. 2003;278:8826–8836. doi: 10.1074/jbc.M209879200. [DOI] [PubMed] [Google Scholar]
- 27.Konkalmatt PR, Wang F, Piras BA, Xu Y, O’Connor DM, Beyers RJ, Epstein FH, Annex BH, Hossack JA, French BA. Adeno-associated virus serotype 9 administered systemically after reperfusion preferentially targets cardiomyocytes in the infarct border zone with pharmacodynamics suitable for the attenuation of left ventricular remodeling. J Gene Med. 2012;14:609–620. doi: 10.1002/jgm.2673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saqib A, Prasad K-MR, Katwal AB, Sanders JM, Lye RJ, French BA, Annex BH. Adeno-associated virus serotype 9-mediated overexpression of extracellular superoxide dismutase improves recovery from surgical hind-limb ischemia in BALB/c mice. J Vasc Surg. 2011;54:810–818. doi: 10.1016/j.jvs.2011.03.278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yu Z, Li P, Zhang M, Hannink M, Stamler JS, Yan Z. Fiber type-specific nitric oxide protects oxidative myofibers against cachectic stimuli. PLoS One. 2008;3:e2086. doi: 10.1371/journal.pone.0002086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maillet M, Davis J, Auger-Messier M, York A, Osinska H, Piquereau J, Lorenz JN, Robbins J, Ventura-Clapier R, Molkentin JD. Heart-specific deletion of CnB1 reveals multiple mechanisms whereby calcineurin regulates cardiac growth and function. J Biol Chem. 2010;285:6716–6724. doi: 10.1074/jbc.M109.056143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lim HW, Molkentin JD. Calcineurin and human heart failure. Nat Med. 1999;5:246–247. doi: 10.1038/6430. [DOI] [PubMed] [Google Scholar]
- 32.Stanford KI, Middelbeek RJW, Townsend KL, An D, Nygaard EB, Hitchcox KM, Markan KR, Nakano K, Hirshman MF, Tseng Y-H, Goodyear LJ. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J Clin Invest. 2013;123:215–223. doi: 10.1172/JCI62308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pedersen BK, Febbraio MA. Muscles, exercise and obesity: skeletal muscle as a secretory organ. Nat Rev Endocrinol. 2012;8:457–465. doi: 10.1038/nrendo.2012.49. [DOI] [PubMed] [Google Scholar]
- 34.Heineke J, Auger-Messier M, Xu J, Sargent M, York A, Welle S, Molkentin JD. Genetic deletion of myostatin from the heart prevents skeletal muscle atrophy in heart failure. Circulation. 2010;121:419–425. doi: 10.1161/CIRCULATIONAHA.109.882068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZ, Kajimura S, Zingaretti MC, Vind BF, Tu H, Cinti S, Højlund K, Gygi SP, Spiegelman BM. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature. 2012;481:463–468. doi: 10.1038/nature10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laakso M. Glycemic control and the risk for coronary heart disease in patients with non-insulin-dependent diabetes mellitus. The Finnish studies. Ann Intern Med. 1996;124:127–130. doi: 10.7326/0003-4819-124-1_part_2-199601011-00009. [DOI] [PubMed] [Google Scholar]
- 37.Tokudome T, Horio T, Yoshihara F, Suga S-I, Kawano Y, Kohno M, Kangawa K. Direct effects of high glucose and insulin on protein synthesis in cultured cardiac myocytes and DNA and collagen synthesis in cardiac fibroblasts. Metabolism. 2004;53:710–715. doi: 10.1016/j.metabol.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 38.Palmieri V, Capaldo B, Russo C, Iaccarino M, Pezzullo S, Quintavalle G, Minno GDi, Riccardi G, Celentano A. Uncomplicated type 1 diabetes and preclinical left ventricular myocardial dysfunction: insights from echocardiography and exercise cardiac performance evaluation. Diabetes Res Clin Pract. 2008;79:262–268. doi: 10.1016/j.diabres.2007.09.014. [DOI] [PubMed] [Google Scholar]
- 39.Yu C-M, Lin H, Yang H, Kong S-L, Zhang Q, Lee SW-L. Progression of systolic abnormalities in patients with “isolated” diastolic heart failure and diastolic dysfunction. Circulation. 2002;105:1195–1201. doi: 10.1161/hc1002.105185. [DOI] [PubMed] [Google Scholar]
- 40.Crabtree GR, Olson EN. NFAT signaling: choreographing the social lives of cells. Cell. 2002;109(Suppl):S67–79. doi: 10.1016/s0092-8674(02)00699-2. [DOI] [PubMed] [Google Scholar]
- 41.Ruwhof C, Laarse A van der. Mechanical stress-induced cardiac hypertrophy: mechanisms and signal transduction pathways. Cardiovasc Res. 2000;47:23–37. doi: 10.1016/s0008-6363(00)00076-6. [DOI] [PubMed] [Google Scholar]
- 42.Song Y, Wang J, Li Y, Du Y, Arteel GE, Saari JT, Kang YJ, Cai L. Cardiac metallothionein synthesis in streptozotocin-induced diabetic mice, and its protection against diabetes-induced cardiac injury. Am J Pathol. 2005;167:17–26. doi: 10.1016/S0002-9440(10)62949-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malhotra A, Kang BPS, Vashistha H, Yadav VS, Meggs LG. Overexpression of Gsalpha compensates for myocyte loss in diabetic cardiomyopathy. Can J Physiol Pharmacol. 2008;86:122–130. doi: 10.1139/y08-015. [DOI] [PubMed] [Google Scholar]
- 44.Rajesh M, Mukhopadhyay P, Bátkai S, Mukhopadhyay B, Patel V, Haskó G, Szabó C, Mabley JG, Liaudet L, Pacher P. Xanthine oxidase inhibitor allopurinol attenuates the development of diabetic cardiomyopathy. J Cell Mol Med. 2009;13:2330–2341. doi: 10.1111/j.1582-4934.2008.00564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li J, Zhu H, Shen E, Wan L, Arnold JMO, Peng T. Deficiency of rac1 blocks NADPH oxidase activation, inhibits endoplasmic reticulum stress, and reduces myocardial remodeling in a mouse model of type 1 diabetes. Diabetes. 2010;59:2033–2042. doi: 10.2337/db09-1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ye G, Metreveli NS, Donthi RV, Xia S, Xu M, Carlson EC, Epstein PN. Catalase protects cardiomyocyte function in models of type 1 and type 2 diabetes. Diabetes. 2004;53:1336–1343. doi: 10.2337/diabetes.53.5.1336. [DOI] [PubMed] [Google Scholar]
- 47.Matsushima S, Kinugawa S, Ide T, Matsusaka H, Inoue N, Ohta Y, Yokota T, Sunagawa K, Tsutsui H. Overexpression of glutathione peroxidase attenuates myocardial remodeling and preserves diastolic function in diabetic heart. Am J Physiol Heart Circ Physiol. 2006;291:H2237–H2245. doi: 10.1152/ajpheart.00427.2006. [DOI] [PubMed] [Google Scholar]
- 48.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians’ Health Study II randomized controlled trial. JAMA. 2008;300:2123–2133. doi: 10.1001/jama.2008.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Deel ED van, Lu Z, Xu X, Zhu G, Hu X, Oury TD, Bache RJ, Duncker DJ, Chen Y. Extracellular superoxide dismutase protects the heart against oxidative stress and hypertrophy after myocardial infarction. Free Radic Biol Med. 2008;44:1305–1313. doi: 10.1016/j.freeradbiomed.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Giusti B, Marini M, Rossi L, Lapini I, Magi A, Capalbo A, Lapalombella R, Tullio S di, Samaja M, Esposito F, Margonato V, Boddi M, Abbate R, Veicsteinas A. Gene expression profile of rat left ventricles reveals persisting changes following chronic mild exercise protocol: implications for cardioprotection. BMC Genomics. 2009;10:342. doi: 10.1186/1471-2164-10-342. [DOI] [PMC free article] [PubMed] [Google Scholar]