Abstract
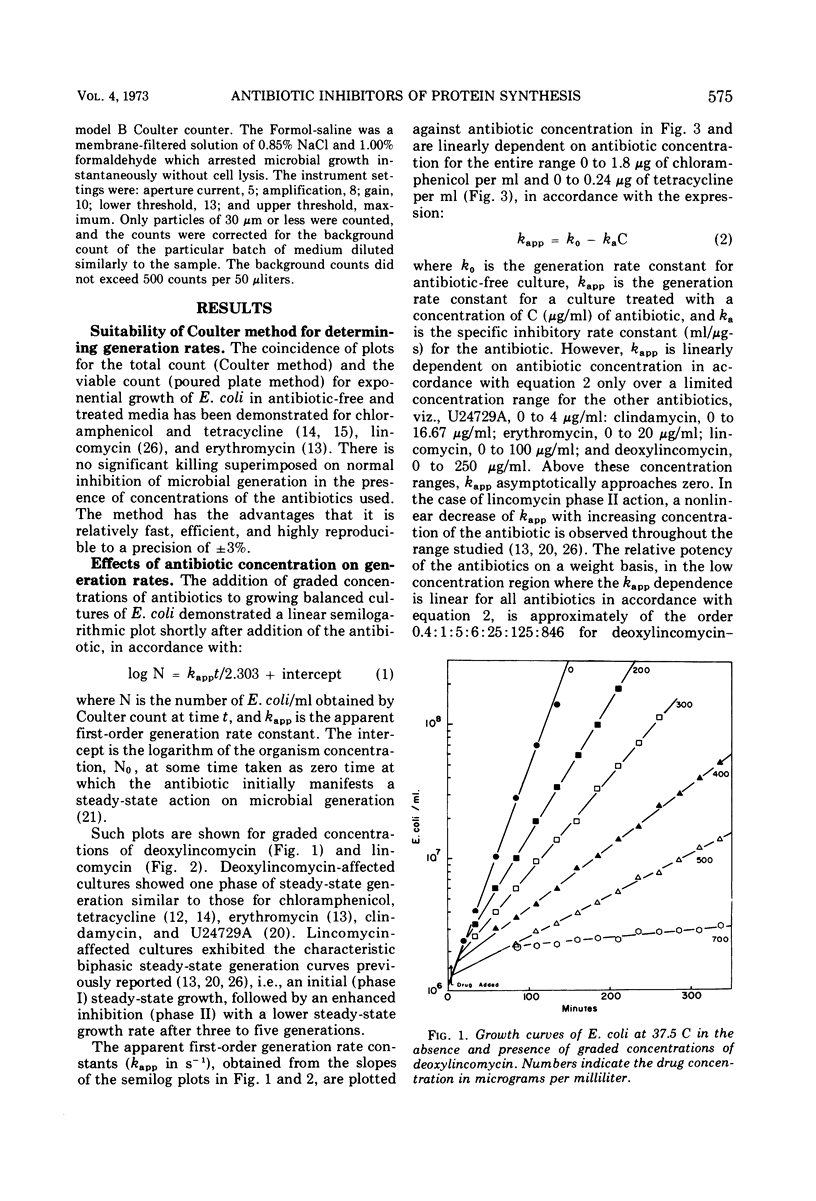
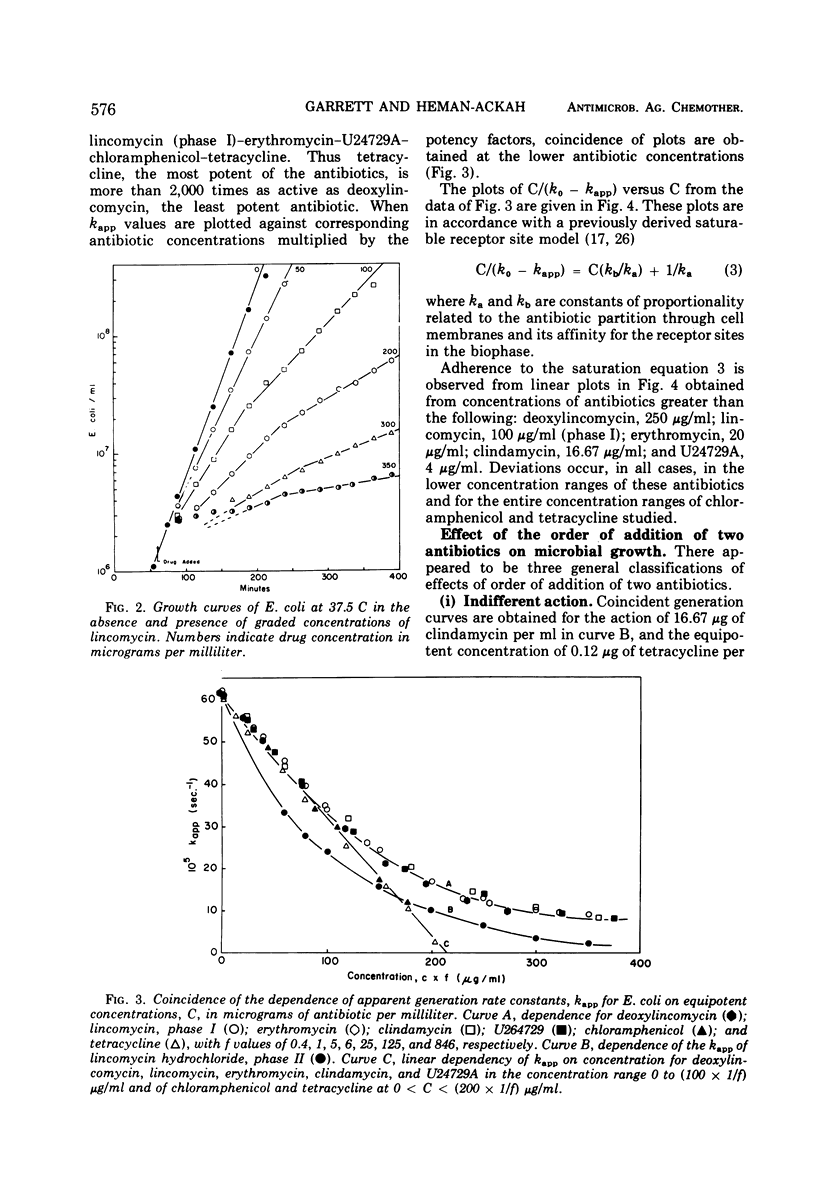
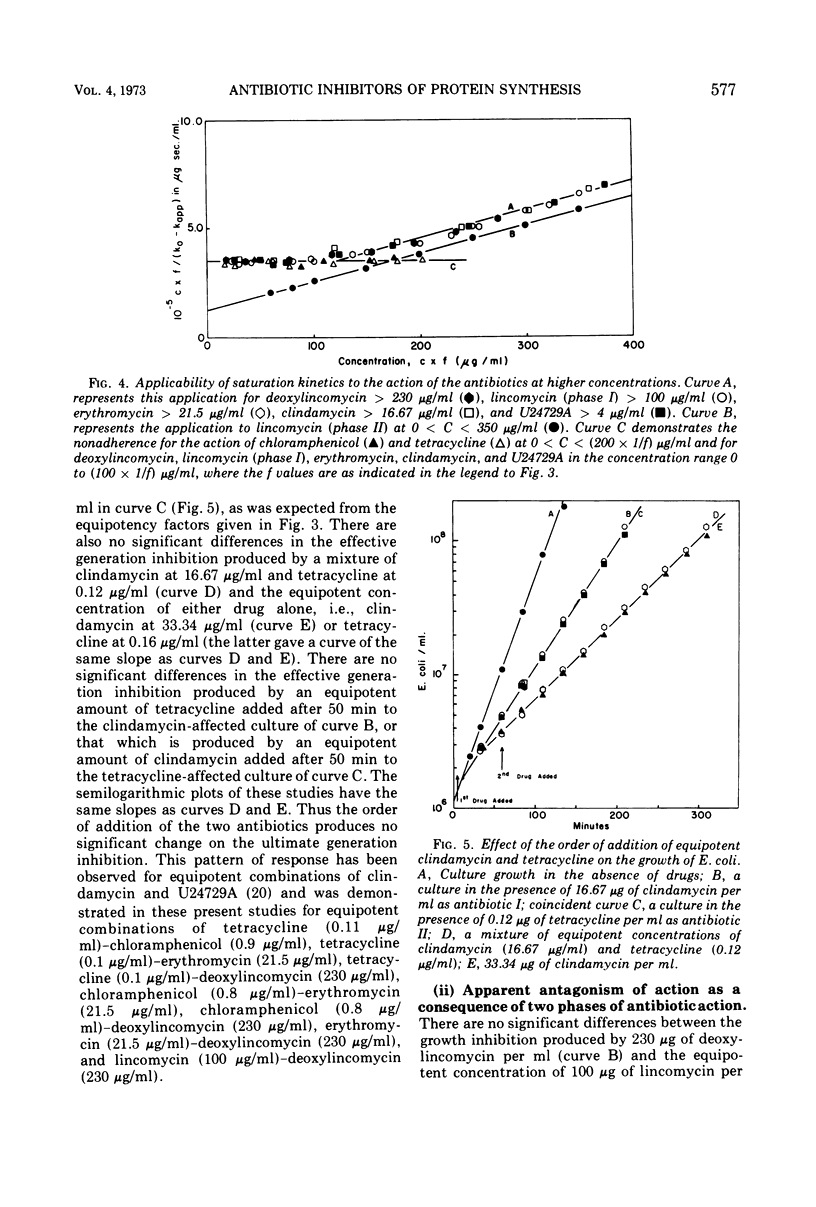
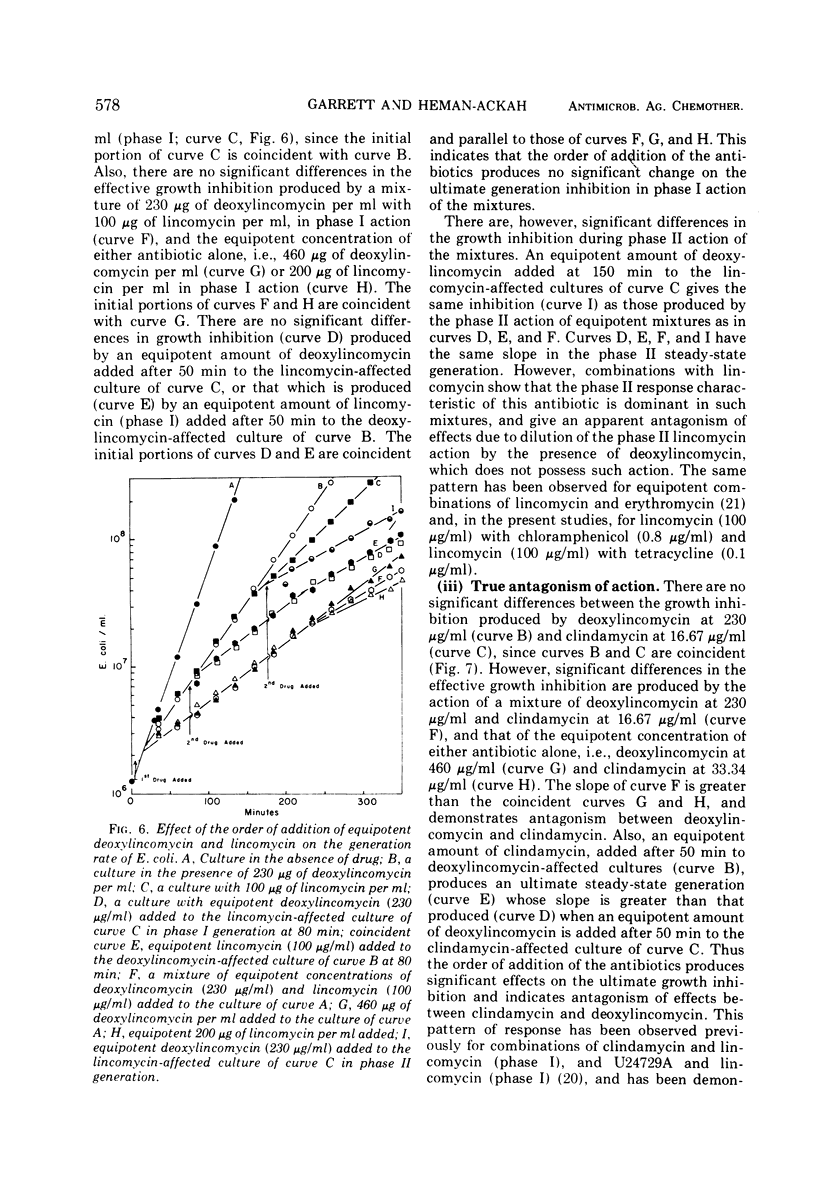
The generation rate constants for the steady-state growth of antibiotic-inhibited Escherichia coli have the same formal dependency on concentration for deoxylincomycin, lincomycin (phase I), erythromycin, clindamycin, and U24729A. They may be kinetically classified as a group A, in which the first three compounds comprise a subgroup A1 and the latter two a subgroup A2. Generation rate constants initially decrease linearly with concentration but asymptotically approach zero at higher concentrations. With tetracycline or chloramphenicol, the generation rate decreases linearly with all concentrations, and these compounds may be kinetically classified as group B. Combining an antibiotic from group A with one from group B gives a response equal to that obtained with equivalent amounts of each antibiotic alone, and there are no significant effects from the order of antibiotic addition. However, combinations of an A1 with an A2 antibiotic are antagonistic, and there are significant effects from the order of addition. The dependencies of generation rate constants in the presence of these antibiotics can be rationalized by a receptor site model that considers varying degrees of the rate of drug transfer and drug inactivation in the organism.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambrus J. L., Ambrus C. M. Principles of combination chemotherapy. Am J Pharm Sci Support Public Health. 1970 May-Jun;142(3):116–126. [PubMed] [Google Scholar]
- Celma M. L., Monro R. E., Vazquez D. Substrate and antibiotic binding sites at the peptidyl transferase centre of E. coli ribosomes. FEBS Lett. 1970 Feb 16;6(3):273–277. doi: 10.1016/0014-5793(70)80076-x. [DOI] [PubMed] [Google Scholar]
- Cundliffe E., McQuillen K. Bacterial protein synthesis: the effects of antibiotics. J Mol Biol. 1967 Nov 28;30(1):137–146. doi: 10.1016/0022-2836(67)90249-5. [DOI] [PubMed] [Google Scholar]
- Das H. K., Goldstein A., Kanner L. C. Inhibition by chlorampenicol of the growth of nascent protein chains in Escherichia coli. Mol Pharmacol. 1966 Mar;2(2):158–170. [PubMed] [Google Scholar]
- ELION G. B., SINGER S., HITCHINGS G. H. Antagonists of nucleic acid derivatives. VIII. Synergism in combinations of biochemically related antimetabolites. J Biol Chem. 1954 Jun;208(2):477–488. [PubMed] [Google Scholar]
- Freeman K. B. Inhibition of mitochondrial and bacterial protein synthesis by chloramphenicol. Can J Biochem. 1970 Apr;48(4):479–485. doi: 10.1139/o70-077. [DOI] [PubMed] [Google Scholar]
- GARRETT E. R., MILLER G. H. KINETICS AND MECHANISMS OF ACTION OF ANTIBIOTICS ON MICROORGANISMS. 3. INHIBITORY ACTION OF TETRACYCLINE AND CHLORAMPHENICOL ON ESCHERICHIA COLI ESTABLISHED BY TOTAL AND VIABLE COUNTS. J Pharm Sci. 1965 Mar;54:427–431. doi: 10.1002/jps.2600540318. [DOI] [PubMed] [Google Scholar]
- Garrett E. R., Heman-Ackah S. M., Perry G. L. Kinetics and mechanisms of action of drugs on microorganisms. XI. Effect of erythromycin and its supposed antagonism with lincomycin on the microbial growth of Escherichia coli. J Pharm Sci. 1970 Oct;59(10):1448–1456. doi: 10.1002/jps.2600591017. [DOI] [PubMed] [Google Scholar]
- Garrett E. R., Miller G. H., Brown M. R. Kinetics and mechanisms of action of antibiotics on microorganisms. V. Chloramphenicol and tetracycline affected Escherichia coli generation rates. J Pharm Sci. 1966 Jun;55(6):593–600. doi: 10.1002/jps.2600550613. [DOI] [PubMed] [Google Scholar]
- Garrett E. R., Nolte H. Kinetics and mechanisms of drug action on microorganisms. XIV. The action of fluorouracil, other uracils and derived nucleosides on the microbial kinetics of Escherichia coli. Chemotherapy. 1972;17(2):81–108. doi: 10.1159/000220843. [DOI] [PubMed] [Google Scholar]
- Garrett E. R., Wright O. K. Kinetics and mechanisms of action of drugs on microorganisms. VII. Quantitative adherence of sulfonamide action on microbial growth to a receptor-site model. J Pharm Sci. 1967 Dec;56(12):1576–1585. doi: 10.1002/jps.2600561209. [DOI] [PubMed] [Google Scholar]
- Garrett E. R., Wright O. K., Miller G. H., Smith K. L. Quantification and prediction of the biological activities of chloramphenicol analogs by microbial kinetics. J Med Chem. 1966 Mar;9(2):203–208. doi: 10.1021/jm00320a011. [DOI] [PubMed] [Google Scholar]
- Heman-Ackah S. M., Garrett E. R. Kinetics and mechanisms of action of drugs on microorganisms. XV. Effect of erythromycin on the action of lincomycin (Phase II) and the 7 (S)-chloro analogs of lincomycin against Escherichia coli. J Pharm Sci. 1972 Apr;61(4):545–551. doi: 10.1002/jps.2600610410. [DOI] [PubMed] [Google Scholar]
- Heman-Ackah S. M., Garrett E. R. Kinetics and mechanisms of drug action on microorganisms. 13. Comparative studies on action of lincomycin, clindamycin, and U 24729A against Escherichia coli. J Med Chem. 1972 Feb;15(2):152–163. doi: 10.1021/jm00272a009. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Kaji A. Relationship between sites 1,2 and acceptor, donor sites for the binding of aminoacyl tRNA to ribosomes. Eur J Biochem. 1970 May 1;14(1):41–46. doi: 10.1111/j.1432-1033.1970.tb00258.x. [DOI] [PubMed] [Google Scholar]
- Last J. A. Studies on the binding of tetracycline to ribosomes. Biochim Biophys Acta. 1969 Dec 16;195(2):506–514. doi: 10.1016/0005-2787(69)90657-1. [DOI] [PubMed] [Google Scholar]
- Maxwell I. H. Studies of the binding of tetracycline to ribosomes in vitro. Mol Pharmacol. 1968 Jan;4(1):25–37. [PubMed] [Google Scholar]
- Mielck J. B., Garrett E. R. Kinetics and mechanisms of drug action on microorganisms. IX. Inhibitory action of lincomycin on Escherichia coli by microbial kinetics. Chemotherapy. 1969;14(6):337–355. doi: 10.1159/000220643. [DOI] [PubMed] [Google Scholar]
- Miller G. H., Khalil S. A., Martin A. N. Structure-activity relationships of tetracyclines. I. Inhibition of cell division and protein and nucleic acid syntheses in Escherichia coli W. J Pharm Sci. 1971 Jan;60(1):33–40. doi: 10.1002/jps.2600600104. [DOI] [PubMed] [Google Scholar]
- Seydel J. K., Wempe E., Miller G. H., Miller L. Kinetics and mechanisms of action of trimethoprim and sulfonamides, alone or in combination, upon Escherichia coli. Chemotherapy. 1972;17(4):217–258. doi: 10.1159/000220857. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Teraoka H., Tamaki M. Peptidyl puromycin synthesis; effect of several antibiotics which act on 50 S ribosomal subunits. FEBS Lett. 1971 Feb 12;13(1):65–67. doi: 10.1016/0014-5793(71)80666-x. [DOI] [PubMed] [Google Scholar]
- Weisblum B., Davies J. Antibiotic inhibitors of the bacterial ribosome. Bacteriol Rev. 1968 Dec;32(4 Pt 2):493–528. [PMC free article] [PubMed] [Google Scholar]
- Wilhelm J. M., Oleinick N. L., Corcoran J. W. Interaction of antibiotics with ribosomes: structure-function relationships and a possible common mechanism for the antibacterial action of the macrolides and lincomycin. Antimicrob Agents Chemother (Bethesda) 1967;7:236–250. [PubMed] [Google Scholar]


