Abstract
Objective
To assess subclinical kidney injury in severely obese adolescents by measuring biomarkers of early kidney disease and to assess changes in the levels of these biomarkers following bariatric procedure.
Methods
22 severely obese adolescents undergoing bariatric surgery with no microalbuminuria and normal kidney function were selected. Urinary NGAL, IL-18, and KIM-1 were measured at baseline, 6, and 12 months post-operatively. Biomarker levels were compared to 44 age-gender-matched lean controls.
Results
Obese subjects had a mean baseline BMI of 48 kg/m2 that decreased by 34% at 1 year follow-up. Urine NGAL, IL-18 and KIM-1 were significantly elevated in obese compared to lean controls at baseline. The obese cohort had a further significant increase in NGAL and KIM-1 at 6 months, followed by decline at 1 year. The overall change in levels of all three biomarkers through 1 year after surgery, however, was not significant compared to baseline.
Conclusions
Adolescent severe obesity is associated with increased urinary excretion of novel biomarkers of kidney injury, despite no microalbuminuria or decreased kidney function. This subclinical kidney injury persists 1 year after significant weight loss induced by bariatric surgery, suggesting that close long-term follow up of kidney status is warranted in these adolescents.
Keywords: Childhood obesity, adolescents, chronic kidney disease, urinary biomarkers, bariatric surgery
Introduction
Childhood obesity is becoming a worldwide epidemic.1-3 The prevalence of severe obesity (SO), defined as an absolute BMI ≥35 kg/m2 or > 120th percent of the 95th percentile4, is increasing and now affects 4-6% of U.S. children and adolescents.5, 6 It is also well-documented that obesity during adolescence is associated with a higher prevalence of chronic kidney disease (CKD) in adulthood.7-9 Proposed mechanisms of obesity-induced chronic kidney injury include kidney hyperfiltration, inflammation, oxidative stress, metabolic disorder (reduced insulin sensitivity) and other comorbidities, especially cardiovascular disease.10-14 Recent analysis of kidney status from the Teen Longitudinal Assessment of Bariatric Surgery (Teen-LABS) study, a large multicenter cohort of adolescents undergoing bariatric surgery15, showed that prior to surgery, 17% had micro/macroalbuminuria and 3% had decreased kidney function with estimated glomerular filtration rate (eGFR) less than 60 ml/min/1.73m2. While the results of this study indicate that a significant number of SO adolescents had kidney abnormalities, the vast majority of these patients still had normal kidney status defined as normal eGFR and no proteinuria.15
Over the last decade, novel sensitive and specific biomarkers of early structural and inflammatory kidney injury (e.g. neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule 1 (KIM-1) and interleukin-18 (IL-18) have been identified and characterized, especially as markers of acute kidney injury (AKI).16, 17 Focus of current research has been on understanding the role of these and other biomarkers in high-risk populations for CKD development and progression. For example, recent systematic review identified 13 biomarkers independently predicting either onset or progression of diabetic nephropathy in adults.18 A report from the Chronic Renal Insufficiency Cohort (CRIC) study showed that urine NGAL was significant risk factor for progression of established CKD but it only modestly improved prediction of outcome events.19 While above studies focused on evaluation of biomarkers in older adults, their role as markers of early CKD in SO adolescents has not been extensively studied. Thus we conducted a pilot study to measure urinary NGAL, KIM-1, and IL-18 prior to bariatric surgery and at 6 months and 1 year post-operatively. We selected adolescents with normal eGFR and no microalbuminuria to test the hypothesis that in SO adolescents, urinary excretion of biomarkers of sub-clinical kidney injury would be increased despite otherwise normal kidney status.
Methods and procedures
Study Design and Patients
This analysis used specimens and data that had been collected and stored by the Pediatric Obesity Tissue Repository (POTR) at Cincinnati Children’s Hospital Medical Center (CCHMC) under an IRB approved protocol. This analysis included twenty-eight patients younger than 20 years who underwent either Roux-en-Y gastric bypass (RYGB, n=6) or a vertical sleeve gastrectomy (VSG, n=22) procedures at CCHMC between 2010 and 2012. These subjects had voluntarily provided spot urine and serum specimens at baseline, 6 months, and 12 months after surgery for research use. Specimens were collected in the operating room (baseline) or in the Clinical & Translational Research Center (post-operatively). Blood was processed for serum storage only; both serum and urine samples were split into 1 mL aliquots and stored at −80°C. Baseline specimens from 6 subjects were found to have occult albuminuria and these subjects were thus excluded from the analysis by design, leaving 22 subjects (4 with RYGB and 18 with VSG) for final analysis. All demographic information and clinical data were abstracted from the CCHMC electronic medical record.
Lean control subjects for this investigation were identified from the Cincinnati Genomic Control Cohort (CGCC) and were matched to the SO bariatric subjects for age (+/− 1 year) and gender. Two controls were selected for each SO subject. The control cohort excluded subjects with any of the following criteria: presence of known genetic diseases or severe chronic medical conditions, such as chromosomal abnormality, unwillingness to complete family and personal health history or allow storage or genetic testing of samples, and adopted, without full contact with biological parent(s) to be able to obtain family history information. Importantly for this analysis, we also excluded any subjects with known kidney injury or disease, including, but not limited to IgA nephropathy, kidney stones, abnormal bladder, urinary reflux and ureteral reimplantation. Control urine samples were collected from 2007-2010 and stored at −80°C until measurement in 2013.
Biomarker measurements
Levels of serum cystatin C, urine albumin, urine creatinine, NGAL, IL-18, and KIM-1were tested in CCHMC nephrology biomarker laboratory. The urine NGAL ELISA was performed using a commercially available assay (NGAL ELISA Kit 036; Bioporto, Grusbakken, Denmark) that specifically detects human NGAL.20 The intra-assay coefficient of variation (CV) value was 2.1% and inter-assay variation was 9.1%. Urine IL-18 was measured using commercially available ELISA kits (Medical & Biological Laboratories Co., Nagoya, Japan) per manufacturer’s instructions. The inter-assay CV for IL-18 was 7.3% and intra-assay was 7.5%.21 The urine KIM-1 ELISA was constructed using commercially available reagents (Duoset DY1750, R & D Systems, Inc., Minneapolis, MN) as described previously.22 Intra and inter-assay CVs for KIM-1 were 2% and 7.8%, respectively. Cystatin C was measured by a particle-enhanced nephelometric immunoassay on a BNII clinical nephelometer (Siemens, Munich, Germany). Microalbumin and urine creatinine were measured on a Dimension Xpand plus HM clinical analyzer (Siemens, Munich, Germany). We used a reference range for NGAL, IL-18, and KIM-1 from a CCHMC population of healthy children and adolescents to define abnormal biomarker levels (above 95th percentile according to gender and age 15-18 years).23 All biomarker measurements were performed in one batch in a period of one week.
Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as (fasting glucose (mg/dL) × insulin (uU/ml)) / 405. Kidney function was assessed by calculating cystatin C-based eGFR, where eGFR = 77.24 × (Cystatin-C)−1.2623 according to the Larsson formula as recommended by the assay manufacturer (Dade Behring, Deerfield, Illinois). Microalbuminuria was defined as having a urine Albumin to Creatinine Ratio (ACR) ≥30 mg/gm and <300 mg/gm; macroalbuminuria was defined as ACR ≥300 mg/gm.24
Normal weight was defined as BMI< 85th percentile for age and gender in subjects < 20 years and BMI < 25 kg/m2 in subjects ≥ 20 years. Overweight was defined as BMI ≥85th percentile < 95th percentile for age and gender in subjects < 20 years and BMI≥ 25 kg/m2 < 30 kg/m2 in subjects ≥ 20 years. Obesity is defined as BMI ≥ 95th percentile for age and gender in subjects < 20 years and BMI ≥30 kg/m2 in subjects ≥ 20 years.25
Statistical analysis
Descriptive statistics were calculated to summarize subject characteristics. Frequencies and percentages are reported for categorical measures. Data were tested for normal distribution with the Kolmogorov–Smirnov test. Median and interquartile ranges were calculated for skewed continuous variables. The data were logarithmically transformed, when appropriate. Differences between lean controls vs. obese at baseline were tested for significance using t-test or Mann-Whitney Rank Sum Test. Repeated measure ANOVA was performed to test differences in biomarkers levels over time. Statistical analysis was performed using the SAS9.4. All reported p-values are two-sided and considered statistically significant at ≤ 0.05.
Results
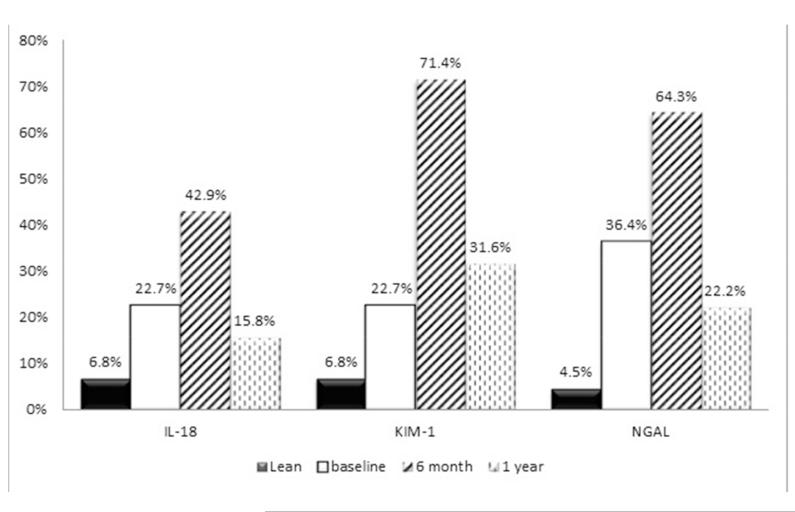
The comparison of demographic, clinical, and laboratory characteristics of lean controls and the obese subjects at baseline prior to bariatric procedure is shown in Table 1. By design, the SO and lean groups were well-matched for age, gender, and race. The SO subjects had significantly higher prevalence of hypertension than control group; one subject had type I diabetes. The SO subjects had significantly higher levels of all three studied urinary biomarkers than controls at baseline. Twenty-three percent of SO subjects had KIM-1 and IL-18 levels above the 95th percentiles of normal value and 36% had NGAL levels above the 95th percentiles of normal values for these biomarkers prior to surgery (Figure).
Table 1. Demographic and Clinical Characteristics.
| Characteristics | Lean (n = 44) | Severely Obese Baseline (n=22) |
P value |
|---|---|---|---|
| Age (years) - - median (Q1,Q3) | 16.5 (14.5, 17.0) | 16.5 (15.0, 17.4) | 0.109 |
| Female – n (%) | 34 (77.2%) | 17 (77.2%) | 1.000 |
| Race/Ethnicity – n (%) | |||
| Non-Hispanic White | 35 (79.5%) | 17 (77.3%) | 0.831 |
| Non-Hispanic Black | 8 (18.2%) | 5 (22.7%) | 0.662 |
| Hispanic | 1 (2.3%) | 0 | 0.486 |
| Diabetes (type I) – n (%) | 0 | 1 (4.5%) | 0.154 |
| Hypertension – n (%) | 3 (6.8%) | 4 (18.2%) | 0.158 |
| 2 BMI, kg/m2 - median (Q1,Q3) | 20.1 (19.1, 22.1) | 48.4 (42.0, 51.7) | <0.001 |
| Urine IL-18 (pg/mL) - median (Q1,Q3) | 24.5(14.0, 46.4) | 78.3 ( 39.7, 246.5) | <0.001 |
| Urine NGAL (ng/mL) - median (Q1,Q3) | 17.8 (6.8, 28.0) | 31.0 (22.2, 162.3) | 0.006 |
| Urine KIM-1 (pg/mL) - median (Q1,Q3) | 370.7 (243.2, 689.5) | 849.2 (337.2, 1189.8) | 0.045 |
BMI, body mass index; HOMA-IR, homeostasis model assessment of insulin resistance; eGFR, estimated glomerular filtration rate; IL-18, interleukin-18; NGAL, neutrophil gelatinase-associated lipocalin, KIM-1, kidney injury molecule 1
Figure. Percentage of subjects with biomarker levels above 95th percentile of normal values.
N=8 missing at 6 months; n=3 missing at 12 months)
Changes in clinical characteristics and urinary biomarkers over time in SO subjects are shown in Table 2. There was a significant decrease in BMI in all bariatric subjects. Four achieved normal weight and 4 subjects improved such that their BMI categorized them as overweight at 1-year follow up. Significant improvement compared to the baseline was found for HOMA-IR. Hypertension resolved in all subjects. Cystatin C based mean eGFR remained normal and stable at 1 year after the procedure in the cohort. One female subject developed microalbuminuria (174 mg/gm) at 1 year follow up. The BMI of this subject decreased from 41.6 kg/m2 at baseline to 23.5 kg/m2 at 1 year after surgery; her eGFR was however unchanged (165 ml/min/1.73m2 at baseline and 169 ml/min/1.73m2 at 1-year follow up).
Table 2. Changes in clinical characteristics and urinary biomarkers in obese subjects after bariatric procedure.
| Characteristics | Severely Obese (n = 22) | |||
|---|---|---|---|---|
| Baseline | ^6 months | #12 months | P for trend | |
| BMI, kg/m2 - median (Q1,Q3) | 48.4 (42.0, 51.7)*† | 34.3 (29.5, 39.2)‡ | 32.2 (28.6, 37.1) | <0.001 |
| HOMA-IR - median (Q1,Q3) | 6.6 (5.1, 10.0)*† | 1.5 (1.2, 2.2) | 1.3 (1.1, 1.9) | <0.001 |
| eGFR (mL/min/1.73m2) - median (Q1,Q3) | 113.7 (102.1, 136.0) | 109.2 (98.3, 121.0) | 111.4 (96.2, 161.5) | 0.346 |
| Urine Albumin to Creatinine Ratio, mg/gm – median (Q1,Q3) | 6.3 (4.6, 8.4) | 8.1 (5.2, 12.8) | 7.9 (5.5, 13.5) | 0.176 |
| Urine IL-18 (pg/mL) - median (Q1,Q3) | 78 ( 40, 247) | 143 (78, 278) | 53 (39, 143) | 0.344 |
| Urine NGAL (ng/mL) - median (Q1,Q3) | 31 (22, 162)* | 106 (55, 251)‡ | 43 (23, 95) | 0.092 |
| Urine KIM-1 (pg/mL) - median (Q1,Q3) | 849 (337, 1190)* | 2690 (1346, 3169) | 1048 (667, 2497) | 0.009 |
Significant difference between baseline and 6 month,
Significant difference between baseline and 12 months
Significant difference between 6 months and 12 months
n=8 missing
n=3 missing
BMI, body mass index; HOMA-IR, homeostasis model assessment of insulin resistance; eGFR, estimated glomerular filtration rate; IL-18, interleukin-18; NGAL, neutrophil gelatinase-associated lipocalin, KIM-1, kidney injury molecule 1
In the 16 subjects with specimens at all 3 timepoints, levels of all three biomarkers, especially KIM-1 and NGAL, increased from baseline to 6 months postoperatively before declining to levels similar to baseline by 1 year after surgery (Table 2). There was no significant difference in biomarker levels between subjects who underwent RYGB versus VSG at 1-year of follow up (p>0.05 for all biomarkers). No statistical difference in biomarker levels was found among subjects with normal weight, overweight or obesity at 1 year follow up (p>0.05). A separate analysis comparing baseline pre-procedure biomarker levels with 1-year follow up levels in subjects achieving normal weight, transitioned to overweight or whose BMI improved but remained in the obese range showed no significant decrease in the biomarker levels in either group (p>0.05). The percentage of subjects with abnormally high biomarker levels remained elevated at 1 year after surgery for all studied biomarkers (Figure).
Discussion
To develop SO during childhood and to carry this burden into adulthood may result in early kidney damage that is potentially greater than that seen in adult-onset obesity9. Indeed, our data supports our hypothesis that in SO adolescents without functional impairment, there is a clinically ‘silent’ kidney injury pattern detectable with novel biomarkers.
All three biomarkers of early kidney tubular injury (NGAL, KIM-1 and IL18) were significantly elevated in the SO group prior to and during the 1 year following weight loss surgery as compared to lean adolescents. Elevation of these markers is concerning since prior work has established that these biomarkers are clearly associated with the response to a variety of kidney insults.26-28 Mechanistic studies following acute kidney injury (AKI) showed that NGAL acts to stimulate proliferation and epithelialization and to inhibit apoptosis in tubule cells.29 Over expression of IL-18 may promote proximal tubule epithelial cell injury and activation in the process of renal tubulointerstitial fibrosis.16, 17 KIM-1 is believed to participate in the regeneration process after epithelial injury through phagocytosis. 30 Importantly, with the resolution of acute kidney insult, these novel subclinical biomarkers of kidney injury decline to pre-injury concentrations in the urine, demonstrating that these biomarkers are not permanently altered by a kidney insult but instead may be useful for tracking the resolution of the insult.29 Interestingly, one small adult study have reported development of clinical AKI within 2-3 days after bariatric surgery which was associated with an increase in NGAL levels on the first postoperative day. 31 We did not measure urinary biomarkers in the perioperative period however we did not observe clinically evident AKI in any of our subjects either.
These biomarkers have also been associated with obesity. Catalán et al. reported higher NGAL protein expression in the visceral fat depot of obese patients compared to lean subjects. They also demonstrated a significant positive association between NGAL gene expression levels and inflammatory markers. Those findings suggested NGAL’s potential involvement in the low-grade chronic inflammation accompanying obesity.32 We did not measure NGAL or other biomarkers in serum and can’t rule out that serum levels could be elevated secondary to systemic inflammation found in obesity. However, previous mechanistic studies showed that elevated urinary levels of NGAL, IL-18 and KIM-1are directly caused by production in renal tubules.14, 33, 34 Thus, these proteins are biologically plausible urinary biomarkers for subclinical kidney injury associated with obesity. The significantly elevated levels of all three urinary biomarkers suggests that there is on-going inflammation (reflected by NGAL and IL-18) as well as more chronic fibrotic changes (suggested by KIM-1) in kidneys of SO adolescents.
Recently, Goknar et al reported elevated urinary KIM-1 in obese children in comparison to lean children but no difference was found in urinary NGAL levels.35 The difference in the results between this study and ours is likely due to the fact that subjects in their study were younger than those in this current study (11.73 years vs. 16.5 years) and had less severe obesity and presumably a lower “pound-year” obesity burden, which might predict a lesser degree of kidney injury.
As expected, bariatric surgery led to a dramatic decrease in BMI and HOMA-IR post-operatively. Hypertension also resolved after weight loss. However, abnormally elevated NGAL, KIM-1, and IL-18 in the urine did not decrease by 1 year after surgery as compared to baseline. The fact that many subjects have increased biomarker levels at 6 months post-surgery as compared to baseline is worthy of further investigation. This finding might well represent a physiologic response to increased metabolic demands or a response to stored fat soluble toxins associated with massive mobilization of 50kg or more adipose tissue during the period of rapid postoperative weight reduction. Indeed it is relevant that others have observed progression of liver disease associated with rapid weight loss during the initial 6-12 months after bariatric surgery, and have speculated that large scale lipolysis, with consequent mobilization of large quantities of long-chain fatty acids from visceral adipose tissue for metabolism in the liver may precipitate progressive steatohepatitis due to the stress of an acute and large metabolic load.36
There are a few possible explanations why biomarker levels remained elevated at 1 year post bariatric procedure. First, it is possible that recovery from obesity-induced kidney injury will take longer than one year post surgery, and measurements taken at 18 or 24 months may demonstrate lower biomarker values. Second, despite dramatic weight loss over a relatively short period, a majority of these patients remained obese leaving open the possibility that ongoing obesity-related kidney injury could be responsible for persistently elevated biomarker values at one year. Sugerman et al reported that about two third of 30 extremely obese adolescents with mean BMI of 52 kg/m2 continued to experience weight loss between 1 and 5 years post bariatric procedure. Thus with continued weight loss, further changes may be occurring in the kidney. 37 Finally, and least desirable, it is possible that kidney injury that occurred pre-operatively may not be fully reversible, even with major weight reduction as was seen in our study subjects who have improved from severe obesity to either normal weight or overweight category but have continued to have elevated biomarker levels at 1-year after surgery. Thus, to better understand the effect of severe obesity and changes in kidney status associated with weight loss, longer follow up is needed.
Recent analysis of kidney status from one of the largest cohorts of adults after bariatric surgery enrolled in the Swedish Obese Subjects (SOS) study38 showed that after median follow up 10 years, albuminuria developed in 246 participants in the control group and in 126 in the bariatric surgery group, corresponding to incidence rates of 20.4 and 9.4 per 1000 person years, respectively. While the study clearly demonstrates a lower incidence of microalbuminuria after surgery and discusses possible mechanisms associated with the positive effect of surgery on kidney injury, it does not address the question of why some of these patients still developed albuminuria. One of the possible reasons for this could be preexisting subclinical kidney injury manifesting by increased levels of urinary biomarkers prior to the development of albuminuria.
In conclusion, despite pilot nature and short follow up of a relatively small cohort, this study indicates that substantial number of SO adolescents have increased urinary excretion of novel biomarkers of structural and inflammatory kidney injury, despite the absence of microalbuminuria or decreased eGFR. This is concerning since progression to overt renal impairment (low eGFR and or proteinuria) is insidious and often not detected until late stage. In addition, persistence of sub-clinical kidney injury in some patients one year after procedure despite significant weight loss suggests that close long-term follow up of kidney status is warranted in SO adolescents irrespective of whether or not bariatric surgery has been used for weight management. The follow up studies should focus on confirming the results of this pilot study in a large cohort of SO adolescents and on assessing the role of urinary biomarkers in predicting development of clinical CKD with proteinuria and decreased kidney function.
What is already known on this subject
Untreated severe obesity of adolescents is associated with abnormal kidney function and high prevalence of micro/macroalbuminuria
What this study adds
This study demonstrated that adolescents with severe obesity have sub-clinical kidney injury in the presence of normal kidney function and absence of microalbuminuria
The study also determined that sub-clinical kidney injury persists 1 year after significant weight loss induced by bariatric surgery
Acknowledgements
The authors would like to thank the following individuals their technical expertise and coordinating effort to accomplish this research: Qing Ma, Christopher Haffner, and Lindsey Shaw.
Funding Source: This research was completed in the laboratory of Dr. Devarajan and was supported by grants from the NIH P50 DK096418 (Dr. Devarajan), K24DK090070 (Dr. Mitsnefes) U01DK072493 (Dr. Inge) and UM1DK072493 (Dr. Inge).
Footnotes
Potential Conflicts of Interest: All authors have indicated they have no relationships relevant to this article to disclose
Financial Disclosure: Dr Inge was the recipient of an Ethicon Endosurgery investigator initiated research grant award.
Clinical Trial Registry Identifier: Adolescent Bariatrics: Assessing Health Benefits and Risk (also known as Teen-Longitudinal Assessment of Bariatric Surgery [Teen-LABS], NCT00474318
Author contributions:
Nianzhou Xiao made substantial contributions to study conception and design, to analysis and interpretation of data, drafting the manuscript, and approved the final version
Prasad Devarajan made substantial contributions to study conception and design, critical revision of the article for important intellectual content, and approved the final version
Thomas H. Inge made substantial contributions to study conception and design, recruitment of participants, collection of data, analysis and interpretation of data, critical revision of the article for important intellectual content, and approved the final version
Todd M Jenkins made substantial contributions to study design, analysis and interpretation of data, revising the article critically for important intellectual content, and approved the final version
Michael Bennett made substantial contributions to analysis and interpretation of data, drafting the manuscript, and approved the final version
Mark M Mitsnefes made substantial contributions to study conception and design, to analysis and interpretation of data, drafting the manuscript, and approved the final version
References
- 1.Wang Y, Lobstein T. Worldwide trends in childhood overweight and obesity. International journal of pediatric obesity: IJPO: an official journal of the International Association for the Study of Obesity. 2006;1:11–25. doi: 10.1080/17477160600586747. [DOI] [PubMed] [Google Scholar]
- 2.Abdelkafi Koubaa A, Younes K, Gabsi Z, Bouslah A, Maalel I, Maatouk El May W, Dahmen H, Bel Abed N, Bchir N, Gabsi A, Tekaya MS, Jebara H. [risk factors of children overweight and obesity] La Tunisie medicale. 2012;90:387–393. [PubMed] [Google Scholar]
- 3.Dietz WH. Overweight in childhood and adolescence. The New England journal of medicine. 2004;350:855–857. doi: 10.1056/NEJMp048008. [DOI] [PubMed] [Google Scholar]
- 4.Kelly AS, Barlow SE, Rao G, Inge TH, Hayman LL, Steinberger J, Urbina EM, Ewing LJ, Daniels SR. American Heart Association Atherosclerosis H, Obesity in the Young Committee of the Council on Cardiovascular Disease in the Young CoNPA, Metabolism, Council on Clinical C. Severe obesity in children and adolescents: Identification, associated health risks, and treatment approaches: A scientific statement from the american heart association. Circulation. 2013;128:1689–1712. doi: 10.1161/CIR.0b013e3182a5cfb3. [DOI] [PubMed] [Google Scholar]
- 5.Claire Wang Y, Gortmaker SL, Taveras EM. Trends and racial/ethnic disparities in severe obesity among us children and adolescents, 1976-2006. International journal of pediatric obesity: IJPO: an official journal of the International Association for the Study of Obesity. 2011;6:12–20. doi: 10.3109/17477161003587774. [DOI] [PubMed] [Google Scholar]
- 6.Koebnick C, Smith N, Coleman KJ, Getahun D, Reynolds K, Quinn VP, Porter AH, Der-Sarkissian JK, Jacobsen SJ. Prevalence of extreme obesity in a multiethnic cohort of children and adolescents. The Journal of pediatrics. 2010;157:26–31 e22. doi: 10.1016/j.jpeds.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Serra A, Romero R, Lopez D, Navarro M, Esteve A, Perez N, Alastrue A, Ariza A. Renal injury in the extremely obese patients with normal renal function. Kidney international. 2008;73:947–955. doi: 10.1038/sj.ki.5002796. [DOI] [PubMed] [Google Scholar]
- 8.Vivante A, Golan E, Tzur D, Leiba A, Tirosh A, Skorecki K, Calderon-Margalit R. Body mass index in 1.2 million adolescents and risk for end-stage renal disease. Archives of internal medicine. 2012;172:1644–1650. doi: 10.1001/2013.jamainternmed.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inge TH, King WC, Jenkins TM, Courcoulas AP, Mitsnefes M, Flum DR, Wolfe BM, Pomp A, Dakin GF, Khandelwal S, Zeller MH, Horlick M, Pender JR, Chen JY, Daniels SR. The effect of obesity in adolescence on adult health status. Pediatrics. 2013;132:1098–1104. doi: 10.1542/peds.2013-2185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miyauchi K, Takiyama Y, Honjyo J, Tateno M, Haneda M. Upregulated il-18 expression in type 2 diabetic subjects with nephropathy: Tgf-beta1 enhanced il-18 expression in human renal proximal tubular epithelial cells. Diabetes research and clinical practice. 2009;83:190–199. doi: 10.1016/j.diabres.2008.11.018. [DOI] [PubMed] [Google Scholar]
- 11.Savino A, Pelliccia P, Chiarelli F, Mohn A. Obesity-related renal injury in childhood. Hormone research in paediatrics. 2010;73:303–311. doi: 10.1159/000308161. [DOI] [PubMed] [Google Scholar]
- 12.Eknoyan G. Obesity and chronic kidney disease. Nefrologia: publicacion oficial de la Sociedad Espanola Nefrologia. 2011;31:397–403. doi: 10.3265/Nefrologia.pre2011.May.10963. [DOI] [PubMed] [Google Scholar]
- 13.Laville M. [renal consequences of obesity] Nephrologie & therapeutique. 2011;7:80–85. doi: 10.1016/j.nephro.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 14.van Timmeren MM, van den Heuvel MC, Bailly V, Bakker SJ, van Goor H, Stegeman CA. Tubular kidney injury molecule-1 (kim-1) in human renal disease. The Journal of pathology. 2007;212:209–217. doi: 10.1002/path.2175. [DOI] [PubMed] [Google Scholar]
- 15.Nianzhou Xiao M, Jenkins Todd M, Nehus Edward, Inge Thomas H, Michalsky Marc P, Harmon Carroll M., Helmrath Michael A., Brandt Mary L., Courcoulas Anita, Moxey-Mims Marva, Mitsnefes Mark M, for the Teen-LABS Consortium Kidney function in severely obese adolescents undergoing bariatric surgery. Obesity. 2014 doi: 10.1002/oby.20870. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devarajan P. The use of targeted biomarkers for chronic kidney disease. Advances in chronic kidney disease. 2010;17:469–479. doi: 10.1053/j.ackd.2010.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsigou E, Psallida V, Demponeras C, Boutzouka E, Baltopoulos G. Role of new biomarkers: Functional and structural damage. Critical care research and practice. 2013;2013:361078. doi: 10.1155/2013/361078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hellemons ME, Kerschbaum J, Bakker SJ, Neuwirt H, Mayer B, Mayer G, de Zeeuw D, Lambers Heerspink HJ, Rudnicki M. Validity of biomarkers predicting onset or progression of nephropathy in patients with type 2 diabetes: A systematic review. Diabetic medicine: a journal of the British Diabetic Association. 2012;29:567–577. doi: 10.1111/j.1464-5491.2011.03437.x. [DOI] [PubMed] [Google Scholar]
- 19.Liu KD, Yang W, Anderson AH, Feldman HI, Demirjian S, Hamano T, He J, Lash J, Lustigova E, Rosas SE, Simonson MS, Tao K, Hsu CY. Urine neutrophil gelatinase-associated lipocalin levels do not improve risk prediction of progressive chronic kidney disease. Kidney international. 2013;83:909–914. doi: 10.1038/ki.2012.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bennett M, Dent CL, Ma Q, Dastrala S, Grenier F, Workman R, Syed H, Ali S, Barasch J, Devarajan P. Urine ngal predicts severity of acute kidney injury after cardiac surgery: A prospective study. Clinical journal of the American Society of Nephrology: CJASN. 2008;3:665–673. doi: 10.2215/CJN.04010907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krawczeski CD, Goldstein SL, Woo JG, Wang Y, Piyaphanee N, Ma Q, Bennett M, Devarajan P. Temporal relationship and predictive value of urinary acute kidney injury biomarkers after pediatric cardiopulmonary bypass. J Am Coll Cardiol. 2011;58:2301–2309. doi: 10.1016/j.jacc.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chaturvedi S, Farmer T, Kapke GF. Assay validation for kim-1: Human urinary renal dysfunction biomarker. Int J Biol Sci. 2009;5:128–134. doi: 10.7150/ijbs.5.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bennett MRNE, Haffner C, Qing Ma, Devarajan P. Pediatric reference ranges for acute kidney injury biomarkers. Pediatric Nephrology. 2014 doi: 10.1007/s00467-014-2989-y. Accepted for publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Levey AS, Eckardt KU, Tsukamoto Y, Levin A, Coresh J, Rossert J, De Zeeuw D, Hostetter TH, Lameire N, Eknoyan G. Definition and classification of chronic kidney disease: A position statement from kidney disease: Improving global outcomes (kdigo) Kidney Int. 2005;67:2089–2100. doi: 10.1111/j.1523-1755.2005.00365.x. [DOI] [PubMed] [Google Scholar]
- 25.Ogden CL, Flegal KM. Changes in terminology for childhood overweight and obesity. National health statistics reports. 2010:1–5. [PubMed] [Google Scholar]
- 26.Mishra J, Dent C, Tarabishi R, Mitsnefes MM, Ma Q, Kelly C, Ruff SM, Zahedi K, Shao M, Bean J, Mori K, Barasch J, Devarajan P. Neutrophil gelatinase-associated lipocalin (ngal) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–1238. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 27.Wagener G, Jan M, Kim M, Mori K, Barasch JM, Sladen RN, Lee HT. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006;105:485–491. doi: 10.1097/00000542-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 28.Han WK, Waikar SS, Johnson A, Betensky RA, Dent CL, Devarajan P, Bonventre JV. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney international. 2008;73:863–869. doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mishra J, Mori K, Ma Q, Kelly C, Yang J, Mitsnefes M, Barasch J, Devarajan P. Amelioration of ischemic acute renal injury by neutrophil gelatinase-associated lipocalin. Journal of the American Society of Nephrology: JASN. 2004;15:3073–3082. doi: 10.1097/01.ASN.0000145013.44578.45. [DOI] [PubMed] [Google Scholar]
- 30.Ichimura T, Asseldonk EJ, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. The Journal of clinical investigation. 2008;118:1657–1668. doi: 10.1172/JCI34487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Koukoulaki M, Spyropoulos C, Hondrogiannis P, Papachristou E, Mitsi E, Kalfarentzos F, Goumenos DS. Neutrophil gelatinase-associated lipocalin as a biomarker of acute kidney injury in patients with morbid obesity who underwent bariatric surgery. Nephron extra. 2013;3:101–105. doi: 10.1159/000354892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Catalan V, Gomez-Ambrosi J, Rodriguez A, Ramirez B, Rotellar F, Valenti V, Silva C, Gil MJ, Salvador J, Fruhbeck G. Six-transmembrane epithelial antigen of prostate 4 and neutrophil gelatinase-associated lipocalin expression in visceral adipose tissue is related to iron status and inflammation in human obesity. European journal of nutrition. 2012 doi: 10.1007/s00394-012-0464-8. [DOI] [PubMed] [Google Scholar]
- 33.Paragas N, Qiu A, Zhang Q, Samstein B, Deng SX, Schmidt-Ott KM, Viltard M, Yu W, Forster CS, Gong G, Liu Y, Kulkarni R, Mori K, Kalandadze A, Ratner AJ, Devarajan P, Landry DW, D’Agati V, Lin CS, Barasch J. The ngal reporter mouse detects the response of the kidney to injury in real time. Nature medicine. 2011;17:216–222. doi: 10.1038/nm.2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Melnikov VY, Ecder T, Fantuzzi G, Siegmund B, Lucia MS, Dinarello CA, Schrier RW, Edelstein CL. Impaired il-18 processing protects caspase-1-deficient mice from ischemic acute renal failure. The Journal of clinical investigation. 2001;107:1145–1152. doi: 10.1172/JCI12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goknar N, Oktem F, Ozgen IT, Torun E, Kucukkoc M, Demir AD, Cesur Y. Determination of early urinary renal injury markers in obese children. Pediatric nephrology. 2014 doi: 10.1007/s00467-014-2829-0. [DOI] [PubMed] [Google Scholar]
- 36.Rabl C, Campos GM. The impact of bariatric surgery on nonalcoholic steatohepatitis. Seminars in liver disease. 2012;32:80–91. doi: 10.1055/s-0032-1306428. [DOI] [PubMed] [Google Scholar]
- 37.Sugerman HJ, Sugerman EL, DeMaria EJ, Kellum JM, Kennedy C, Mowery Y, Wolfe LG. Bariatric surgery for severely obese adolescents. J Gastrointest Surg. 2003;7:102–108. doi: 10.1016/S1091-255X(02)00125-7. [DOI] [PubMed] [Google Scholar]
- 38.Carlsson LM, Romeo S, Jacobson P, Burza MA, Maglio C, Sjoholm K, Svensson PA, Haraldsson B, Peltonen M, Sjostrom L. The incidence of albuminuria after bariatric surgery and usual care in swedish obese subjects (sos): A prospective controlled intervention trial. Int J Obes (Lond) 2015;39:169–175. doi: 10.1038/ijo.2014.72. [DOI] [PMC free article] [PubMed] [Google Scholar]