Abstract
Bone-seeking radiopharmaceuticals are frequently used as diagnostic agents in nuclear medicine, because they can detect bone disorders before anatomical changes occur. Furthermore, their effectiveness in the palliation of metastatic bone cancer pain has been demonstrated in the clinical setting. With the aim of developing superior bone-seeking radiopharmaceuticals, many compounds have been designed, prepared, and evaluated. Here, several well-designed bone-seeking compounds used for diagnostic and therapeutic use, having the concept of radiometal complexes conjugated to carrier molecules to bone, are reviewed.
1. Introduction
For many years, 99mTc-bisphosphonate complexes, 99mTc-methylenediphosphonate (99mTc-MDP, Figure 1(a)) and 99mTc-hydroxymethylenediphosphonate (99mTc-HMDP, Figure 1(b)), have been clinically used for nuclear medical imaging of metastatic bone cancer [1–4], because their high sensitivity can detect bone metastases before occurrence of anatomical changes. Bone metastases are characterized as osteolytic, osteosclerotic, or mixed type of osteolytic and osteosclerotic; namely, osteolytic or osteosclerotic changes occur in lesion sites of bone metastases. These anatomical changes could cause pathologic fractures and severe pain.
Figure 1.
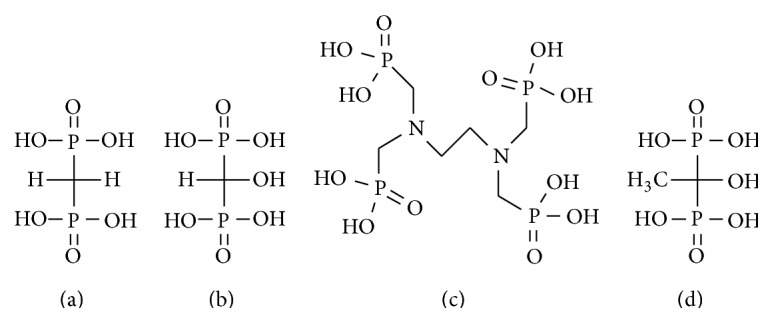
Chemical structures of bisphosphonates analogs (a) MDP, (b) HMDP, (c) EDTMP, and (d) HEDP.
It has been known that strontium (Sr) acts as calcium mimic and accumulates in high osteoblastic activity lesions since strontium is one of the alkaline earth metals [5]. 89Sr has a physical half-life of 50.5 days and emits beta particles with a maximum energy of 1.46 MeV (Table 1). Strontium-89 chloride (89SrCl2, Metastron) was the first radiopharmaceutical approved for the palliation of metastatic bone pain by the US Food and Drug Administration (FDA). 89SrCl2 for the palliation of metastatic bone pain for breast cancer patients and prostate cancer patients showed a pain relief rate of 57–92%. These studies are summarized in reviews [6–9].
Table 1.
Properties of radionuclides.
| Radionuclide | Half-life | Maximum energy of beta particles (MeV) | Main energy of gamma ray (keV, %) | Maximum range (mm) | Main production |
|---|---|---|---|---|---|
| Tc-99m | 6.02 h | None | 141 (89%) | — |
98Mo(n, γ)99Mo 99Mo/99mTc generator |
| Ga-68 | 67.6 m | None | 1077 (3%) 1899 (89%)* |
— |
69Ga(p, 2n)68Ge 68Ge/68Ga generator |
| Sr-89 | 50.5 d | 1.46 | 910 (0.01%) | 7 | 88Sr(n, γ)89Sr |
| Sm-153 | 1.9 d | 0.81 | 103 (28%) | 4 | 152Sm(n, γ)153Sm |
| Re-186 | 3.8 d | 1.07 | 137 (9%) | 5 |
186W(p, n)186Re 185Re(n, γ)186Re |
| Re-188 | 17.0 h | 2.12 | 155 (15%) | 10 |
186W(n, γ)187W 187W(n, γ)188W 188W/188Re generator |
| Ra-223 | 11.4 d | 7.53† | 154 (6%), 270 (14%) | <0.1 |
227Ac/227Th generator |
*Positron energy.
† α energy (Ra-223 has multiple decay to stable nuclide in which 4α particles are generated during each decay, resulting in high energy deposition (28.2 MeV), with 95% of the energy from the α emissions [67].).
‡Ra-223 could be produced from 227Ac/227Th generator and purified using Ac-resin to immobilize 227Ac and 227Th [33].
Samarium-153 (153Sm) has a physical half-life of 46.3 hours and emits beta particles with a maximum energy of 0.81 MeV (20%), 0.71 MeV (49%), and 0.64 MeV (30%) and a 28% abundance of gamma rays with energy of 103 keV (Table 1). 153Sm-ethylenediaminetetramethylene phosphonic acid (EDTMP, Quadramet) is a complex of 153Sm and EDTMP (Figure 1(c)), which has high affinity for bone mineral. 153Sm-EDTMP was approved and has been widely used in the United States for palliation of metastatic bone pain. The biodistribution of 153Sm-EDTMP is similar to that of bone scintigraphic agents such as 99mTc-MDP (methylene diphosphonate) [10]. Accordingly, it was reported that the dosimetry of 153Sm-EDTMP could be predicted using 99mTc-MDP bone scintigraphy [11]. 153Sm-EDTMP showed a pain relief rate in 62–84% of patients with metastatic bone pain. These studies are also summarized in reviews [6–8]. Meanwhile, in a study of comparison between the effects of the 89SrCl2 and 153Sm-EDTMP to patients with bone metastases, there was no statistical difference in response rates [12].
Zoledronic acid (Zometa), which is a bisphosphonate compound, has been widely used for the prevention of skeletal complications. Lam et al. combined zoledronic acid and 153Sm-EDTMP to treat hormone-refractory prostate cancer patients [13]. It was concluded that zoledronic acid treatment does not influence 153Sm-EDTMP skeletal uptake and combined treatment is feasible and safe.
The therapeutic bone-seeking radiopharmaceutical radium-223 chloride (223RaCl2) was approved by FDA and European Medicines Agency (EMA) in 2013 based on data from a phase III randomized trial (the Alpharadin in Symptomatic Prostate Cancer Patients: ALSYMPCA). Surprisingly, 223RaCl2 significantly improved overall survival in patients with castration-resistant prostate cancer with bone metastases in the ALSYMPCA study [14, 15]. In addition, because it is the first radiopharmaceutical emitting alpha particles approved for clinical use, 223RaCl2 is currently attracting much attention.
99mTc-MDP, 99mTc-HMDP, 89SrCl2, 153Sm-EDTMP, and 223RaCl2 are milestones in the development of bone-seeking radiopharmaceuticals for clinical use (Table 2). Although developing superior bone-seeking compounds is difficult, we reviewed the promising well-designed bone-seeking compounds for diagnosis and therapy of bone metastases in basic research.
Table 2.
Radiopharmaceuticals approved for bone metastases by FDA or EMA.
| Radiopharmaceutical | Standard dose | Use |
|---|---|---|
| 99mTc-MDP | 370–740 MBq | Diagnosis |
| 99mTc-HMDP | 370–740 MBq | Diagnosis |
| [18F]NaF (IASOflu) | 18.5–74 MBq | Diagnosis |
| 89SrCl2 (Metastron) | 148 MBq 1.5–2.2 MBq/kg |
Therapy |
| 153Sm-EDTMP (Quadramet) | 37 MBq/kg | Therapy |
| 223RaCl2 (Xofigo) | 50 kBq/kg | Therapy |
2. 99mTc-Complex-Conjugated Bisphosphonate Compounds Designed to Overcome Drawbacks of 99mTc-MDP and 99mTc-HMDP Complexes
Although 99mTc-MDP and 99mTc-HMDP are considered to be the gold standards for bone scintigraphy agents, they have not yet been optimized from a chemical and pharmaceutical perspective, because these complexes are not well-defined single-chemical species but are mixtures of short-chain and long-chain oligomers [16]. Moreover, the phosphonate groups in 99mTc-MDP and 99mTc-HMDP are used both as ligands for coordination and as carriers to hydroxyapatite (HA) in bone [17], which may decrease the inherent affinity of MDP and HMDP for bone. To overcome these drawbacks, a more logical design strategy has been proposed on the basis of the conjugation of a stable radiometal complex to a carrier molecule to bone. This drug design allows the ligand and carrier function to work independently and effectively.
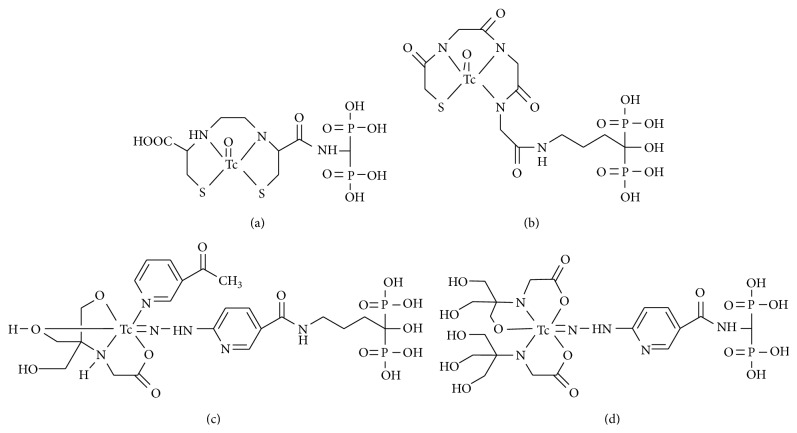
In 2002, Verbeke et al. described 99mTc-l,l-ethylenedicysteine (EC) complex, a renal tracer agent known to have rapid renal excretion, conjugated to bisphosphonate (99mTc-EC-AMDP, Figure 2(a)) [18]. 99mTc-EC-AMDP showed faster blood clearance and a higher bone/blood ratio compared with 99mTc-MDP in animal experiments.
Figure 2.
Chemical structures of 99mTc-complex-conjugated bisphosphonate compounds: (a) 99mTc-EC-AMDP, (b) 99mTc-MAG3-HBP, (c) 99mTc-HYNIC-HBP, and (d) 99mTc-HYNIC-AMDP.
In 2006, we reported 99mTc-mercaptoacetylglycylglycylglycine- (MAG3-) conjugated bisphosphonate (99mTc-MAG3-HBP, Figure 2(b)) and 99mTc-6-hydrazinonicotinic acid (HYNIC) with tricine and 3-acetylpyridine as coligands conjugated to bisphosphonate (99mTc-HYNIC-HBP, Figure 2(c)) [19]. In in vitro HA binding experiments, the binding rates of 99mTc-MAG3-HBP and 99mTc-HYNIC-HBP to HA were higher than those of 99mTc-HMDP. In a biodistribution study in rats, 99mTc-MAG3-HBP and 99mTc-HYNIC-HBP showed higher accumulation in bone compared with 99mTc-HMDP reflecting the in vitro findings. The blood clearance of 99mTc-MAG3-HBP was delayed because of the high rate of protein binding in blood and the bone/blood ratio of 99mTc-MAG3-HBP was lower than that of 99mTc-HMDP. In contrast, the blood clearance of 99mTc-HYNIC-HBP was as rapid as that of 99mTc-HMDP and its bone/blood ratio was higher.
Liu et al. reported findings on 99mTc-HYNIC-conjugated bisphosphonate (99mTc-HYNIC-AMDP, Figure 2(d)) in 2011 [20]. The authors found that 99mTc-HYNIC-AMDP had a higher bone uptake and higher bone/blood and bone/muscle ratios at an early time point after injection as compared with 99mTc-MDP. In that study, 99mTc-HYNIC-AMDP showed favorable biodistribution as a bone-seeking agent, but the bone accumulation of 99mTc-MDP, a bone scintigraphy agent as a control, appeared to be too low. Two tricine molecules are used as coligands in 99mTc-HYNIC-AMDP. However, as it has been reported, the complex [99mTc](HYNIC)(tricine)2 is not stable and exists in multiple forms, and the pharmacokinetics could be affected by the exchange reaction between tricine and protein in the plasma and tissues [21–23]. The pharmacokinetics of 99mTc-HYNIC-AMDP may be improved by exchanging one tricine molecule for another molecule, such as one of the pyridine derivatives.
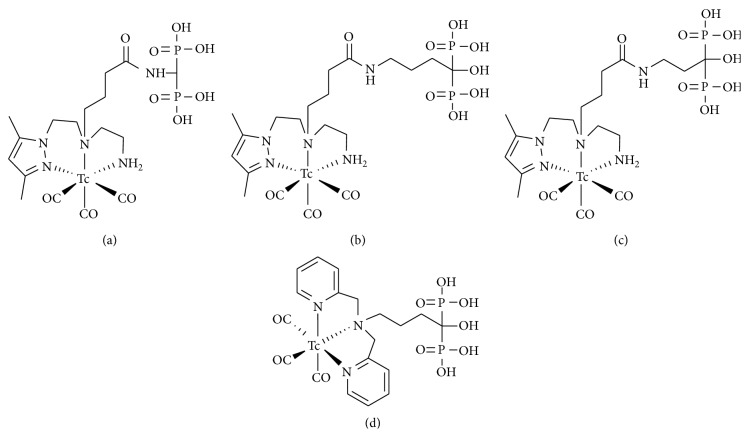
Palma et al. described 99mTc-tricarbonyl complex, which is anchored by a pyrazolyl- (Pz-) containing ligand, conjugated to bisphosphonate compounds ([99mTc(CO)3(PzNN-BP)], [99mTc(CO)3(PzNN-ALN)], and [99mTc(CO)3(PzNN-PAM)], Figures 3(a)–3(c)) [24, 25]. The structures of these technetium complexes were confirmed by reversed phase (RP) HPLC analyses. The identical retention time as the corresponding nonradioactive rhenium (Re) complexes revealed the structural analogy. Although [99mTc(CO)3(PzNN-BP)] showed moderate bone uptake, the uptake was lower than that of 99mTc-MDP. In contrast, the bone accumulation of [99mTc(CO)3(PzNN-ALN)] and [99mTc(CO)3(PzNN-PAM)] was high and comparable to that of 99mTc-MDP. The bone/blood and bone/muscle ratios of [99mTc(CO)3(PzNN-ALN)] and [99mTc(CO)3(PzNN-PAM)] were higher than that of 99mTc-MDP at 4 hours after injection because of their fast clearance. The difference in bone accumulation among 99mTc-tricarbonyl complex-conjugated bisphosphonate compounds could be derived from the introduction of a hydroxyl group at the central carbon of the bisphosphonate because bisphosphonates containing the hydroxyl group have been reported to have higher affinity for bone minerals [26–28].
Figure 3.
Chemical structures of 99mTc-tricarbonyl-complex-conjugated bisphosphonate compounds: (a) [99mTc (CO)3(PzNN-BP)], (b) [99mTc (CO)3(PzNN-ALN)], (c) [99mTc (CO)3(PzNN-PAM)], and (d) 99mTc (CO)3-DPA-alendronate.
In 2009, De Rosales et al. described 99mTc-tricarbonyl complex-conjugated bisphosphonate that has the structure similar to that of [99mTc(CO)3(PzNN-ALN)] but with dipicolylamine (DPA) was used as a chelation site (99mTc(CO)3-DPA-alendronate, Figure 3(d)) [29]. In vitro study showed that 99mTc(CO)3-DPA-alendronate had higher affinity for HA than 99mTc-MDP. In animal experiments, 99mTc(CO)3-DPA-alendronate showed high uptake in bone, comparable to 99mTc-MDP.
As mentioned above, certain 99mTc-complex-conjugated bisphosphonate compounds have shown favorable biodistribution as bone imaging agents and higher bone/blood ratios compared with those of 99mTc-MDP or 99mTc-HMDP. Consequently, these results suggest that the strategy of developing stable 99mTc-complex-conjugated bisphosphonates is promising.
3. Radiogallium-Complex-Conjugated Bisphosphonate Compounds as Bone Imaging Agents for Positron Emission Tomography (PET)
68Ga is a practical and interesting radionuclide for clinical PET because of its radiophysical properties, particularly as a 68Ge/68Ga generator-produced radionuclide has a half-life (T 1/2) of 68 minutes (Table 1) [30]. It does not require an on-site cyclotron and can be eluted on demand. Indeed, in principle, the long half-life of the parent nuclide 68Ge (T 1/2 = 270.8 days) provides a generator with a long lifespan.
The above-mentioned drug design concept, which is a stable complex-conjugated bisphosphonate, could also be applicable to gallium complexes. With the aim of developing a superior bone imaging PET tracer, some types of radiogallium complex-conjugated bisphosphonate compounds have been reported.
In 2010, Fellner et al. reported a human study of 68Ga-DOTA-conjugated bisphosphonate (68Ga-BPAMD, Figure 4(a)), containing 1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid (DOTA) as a ligand for gallium [31]. 68Ga-BPAMD showed high uptake in osteoblastic metastases lesions in a patient with prostate cancer (Figure 5). The maximal standardized uptake value (SUVmax) was 77.1 and 62.1 in the 10th thoracic and L2 vertebra for 68Ga-BPAMD compared with respective values of 39.1 and 39.2 for 18F-fluoride, which is a typical bone imaging agent for PET (Table 2), respectively. In 2012, Fellner et al. reported the findings of basic experiments on 68Ga-BPAMD using μ-PET with bone metastasis rat model [32]. 68Ga-BPAMD highly accumulated in metastatic bone lesions compared with healthy bone in the same animal (contrast factor = 3.97 ± 1.82). The same research group further reported 68Ga-DOTA-conjugated bisphosphonate derivatives, 68Ga-BPAPD and 68Ga-BPPED (Figures 4(b) and 4(c)) in 2013 [33]. The phosphinate-conjugated bisphosphonate 68Ga-BPPED showed higher accumulation in bone compared with 68Ga-BPAMD and 68Ga-BPAPD, amide-conjugated bisphosphonates. The presence of phosphinate may contribute to an additional binding to HA, leading to higher accumulation in bone.
Figure 4.
Chemical structures of precursors of 67/68Ga complex-conjugated bisphosphonate compounds: (a) BPAMD, (b) BPAPD, (c) BPPED, (d) DOTA-Bn-SCN-HBP, and (e) NOTA-BP.
Figure 5.
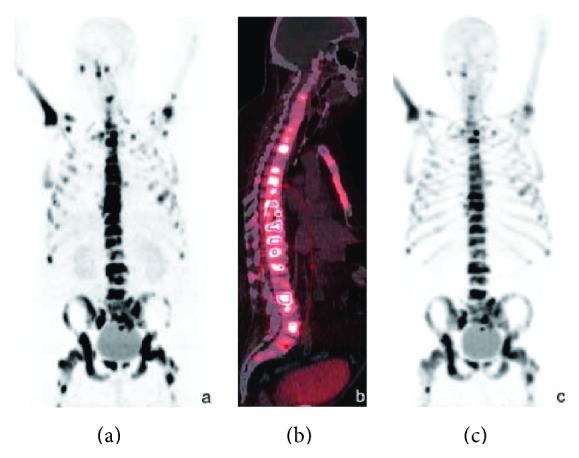
68Ga-BPAMD was injected intravenously into a patient with extensive bone metastases of prostate cancer. 68Ga-BPAMD [maximum intensity projection (MIP) 50 min after injection (p.i.), 462 MBq] revealed intense accumulation in multiple osteoblastic lesions in the central skeleton, ribs, and proximal extremities: (a) = coronal PET, (b) = sagittal PET/CT. For comparison, (c) shows 18F-fluoride PET (sagittal, MIP 90 min p.i., 270 MBq). With kind permission from Springer Science+Business Media: Eur J Nucl Med Mol Imaging, PET/CT imaging of osteoblastic bone metastases with 68Ga-bisphosphonates: first human study, 37, 2010, 834, Fellner et al.
In 2011, we also reported 67Ga-DOTA complex-conjugated bisphosphonate (67Ga-DOTA-Bn-SCN-HBP, Figure 4(d)) [34]. Although the aim was to develop a superior 68Ga-labeled bone imaging agent for PET, in the initial basic studies 67Ga was used because of its longer half-life. In biodistribution experiments in normal mice, 67Ga-DOTA-Bn-SCN-HBP rapidly and highly accumulated in bone but was rarely observed in tissues other than bone. As a result, the bone/blood ratio of 67Ga-DOTA-Bn-SCN-HBP was comparable to that of 99mTc-HMDP, which is a gold standard for a bone scintigraphy agent.
Furthermore, in 2011, Suzuki et al. reported 68Ga-NOTA-conjugated bisphosphonate, containing 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA) as a ligand for gallium (68Ga-NOTA-BP, Figure 4(e)) [35]. In biodistribution experiments using Wistar rats, 68Ga-NOTA-BP showed faster clearance and a higher bone/blood ratio than 99mTc-MDP and 18F-fluoride. Moreover, in PET study using a mouse model of bone metastasis, 68Ga-NOTA-BP showed high accumulation of radioactivity in osteolytic lesions in the tibia.
These results suggest that the drug design concept of radio gallium complex-conjugated bisphosphonate could be useful for the development of 68Ga PET imaging agents for bone disorders such as bone metastases.
4. Re-Complex-Conjugated Bisphosphonate for Palliation of Bone Metastases
Gamma ray emitter radionuclide and positron emitter radionuclide-labeled bone-seeking agents are used for the diagnosis of bone metastases. A prominent symptom caused by bone metastases is pain, which has a significant impact on the patients' quality of life. Bone-seeking agents labeled with high-energy beta particle emitter radionuclides and alpha particle emitter radionuclides are used for palliation of pain caused by bone metastases. Rhenium, which has similar chemical properties to technetium, because they are members of family VIIA of the periodic table, has two useful radionuclides, 186Re and 188Re, which are useful for radionuclide therapy [36]. Both rhenium radionuclides emit not only beta particles for therapy but also gamma rays, which are suitable for diagnoses: 186Re (T 1/2 = 3.78 days, β max − = 1.07 MeV, γ = 137 keV) and 188Re (T 1/2 = 16.98 hours, β max − = 2.12 MeV, γ = 155 keV) (Table 1). In addition, 188Re has a further advantage for clinical use because it is obtained from an in-house alumina-based 188W/188Re generator, similar to a 99Mo/99mTc generator [37].
When considering the use of rhenium in bone-seeking agents for palliation, similar to 99mTc-MDP and 99mTc-HMDP, it is known that rhenium coordinates with some bisphosphonate derivatives. 186/188Re-1-hydroxyethylidene-1,1-diphosphonate (186/188Re-HEDP, Figure 1(d)), which has high affinity for bone, has been prepared and used for clinical research [9, 38, 39]. Although the chemical properties of rhenium are similar to those of technetium as mentioned above, rhenium is more easily oxidized than technetium [40], and it has been reported that 186Re-HEDP is not as stable as 99mTc-bisphosphonate complexes [41]. Some studies reported that 186Re-HEDP showed unexpected gastric uptake in patients with bone metastases [42, 43]. This may be derived from the accumulation of free rhenium (perrhenate: ReO4 −) in the stomach due to the instability of 186Re-HEDP [40, 44]. Moreover, as with 99mTc-MDP and 99mTc-HMDP, the phosphonate groups in 186/188Re-HEDP are used as both ligands for coordination and as carrier to HA in bone, which may decrease the inherent affinity of HEDP for bone.
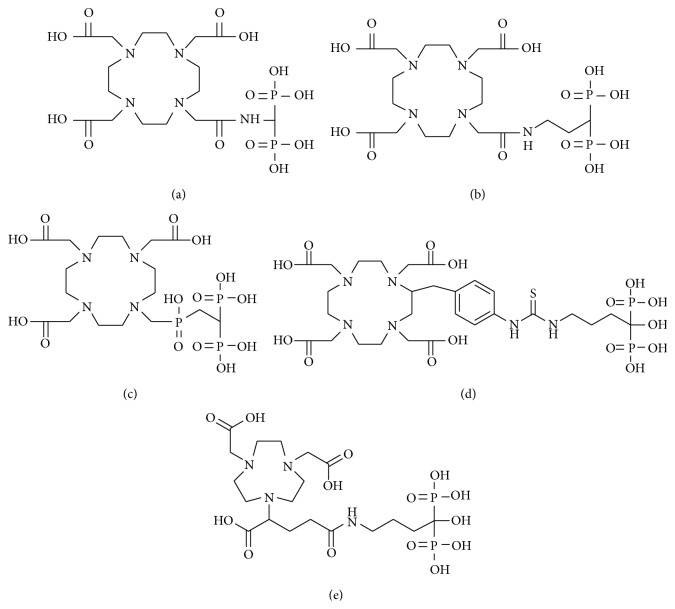
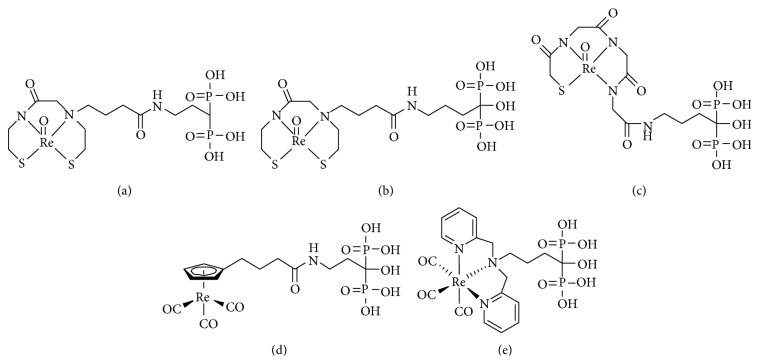
To overcome these problems, designing a stable 186/188Re-complex-conjugated bisphosphonate would be useful. Therefore, we studied 186Re-monoaminemonoamidedithiol- (MAMA-) and 186Re-mercaptoacetylglycylglycylglycine- (MAG3-) conjugated bisphosphonate compounds (186Re-MAMA-BP, 186Re-MAMA-HBP, and 186Re-MAG3-HBP: Figures 6(a)–6(c)) and reported their findings in 2004, 2006, and 2005, respectively [28, 45, 46]. In an in vitro stability experiment in buffer solution, the Re-complex-conjugated bisphosphonate compounds, 186Re-MAMA-HBP, 186Re-MAMA-BP, and 186Re-MAG3-HBP, were more stable than 186Re-HEDP. In the biodistribution experiments performed in normal mice, 186Re-MAMA-HBP, 186Re-MAMA-BP, and 186Re-MAG3-HBP showed lower accumulation of radioactivity in stomach compared with 186Re-HEDP. This result indicates that the drug design of Re-complex-conjugated bisphosphonates enabled better stability in vitro and in vivo. Of 186Re-complex-conjugated bisphosphonate compounds, 186Re-MAG3-HBP showed the most favorable biodistribution characteristics as bone-seeking radiopharmaceuticals such as high and selective bone accumulation, based on high hydrophilicity (log P value: −2.68 ± 0.01) and the introduction of a hydroxyl group to the central carbon of the bisphosphonate structure. Previous studies suggested that the hydroxyl group affects affinity for bone minerals [26, 27]. We evaluated the therapeutic potential of 186Re-MAG3-HBP for the palliation of metastatic bone pain using an animal model of bone metastasis [47]. The planar image of 186Re-MAG3-HBP showed high accumulation of radioactivity in bone metastasis lesion. 186Re-MAG3-HBP was more effective for palliation and was compared with 186Re-HEDP using the hind paw withdrawal response to stimulation with von Frey filaments. Moreover, although 186Re-HEDP did not affect tumor growth, 186Re-MAG3-HBP significantly inhibited tumor growth.
Figure 6.
Chemical structures of 186/188Re-complex-conjugated bisphosphonate compounds: (a) 186Re-MAMA-BP, (b) 186Re-MAMA-HBP, (c) 186Re-MAG3-HBP, (d) [186Re]CpTR-Gly-APD, and (e) 188Re(CO)3-DPA-alendronate.
In 2007, Uehara et al. reported [186Re]CpTR-Gly-APD (Figure 6(d)), which is a tricarbonyl [186Re][(cyclopentadienylcarbonyl amino)-acetic acid] rhenium complex ([186Re]CpTR-Gly)-conjugated bisphosphonate [48]. [186Re]CpTR-Gly-APD showed characteristics superior to those of 186Re-HEDP, such as higher stability in plasma, a higher binding rate for HA, higher bone accumulation, and lower plasma protein binding. When [186Re]CpTR-Gly-APD with HEDP (9.0 mg/kg) was administered to mice, the accumulation of radioactivity in bone significantly decreased and the blood clearance was delayed. Therefore, the authors concluded that the specific activity of 186Re-labeled bisphosphonate compounds is very important to bone accumulation and blood clearance.
In the 99mTc-complex-conjugated bisphosphonate session, 99mTc(CO)3-DPA-alendronate has been discussed above. Using the same ligand, in 2010, De Rosales et al. reported 188Re(CO)3-DPA-alendronate (Figure 6(e)) [49]. 188Re(CO)3-DPA-alendronate can easily be synthesized with high specific activities and high yields (≥96%). 188Re(CO)3-DPA-alendronate showed higher stability in vitro compared with 188Re-HEDP, which oxidized to 188ReO4 − (up to 75%) when placed in PBS for 48 hours at 37°C. In in vivo imaging, 188Re(CO)3-DPA-alendronate showed superior biodistribution of radioactivity than 188Re-HEDP; that is, 188Re(CO)3-DPA-alendronate highly accumulated in metabolically active bone, such as the joints with low soft-tissue uptake.
These results indicate that the concept of the stable 186Re-complex-conjugated bisphosphonates could be more useful and that novel 186Re-complex-conjugated bisphosphonate complexes could be attractive candidates as palliative agents in metastatic bone pain.
5. Aspartic Acid Peptides as Carriers of Radionuclides to Bone
Several major noncollagenous bone proteins, such as osteopontin and bone sialoprotein, have repeating sequences of acidic amino acids (Asp or Glu) in their structures, offering potential HA binding sites [50–52]. It has been reported that polyglutamic and polyaspartic acids have a high affinity for HA and could be used as carriers for drug delivery to bone [53–55].
In 2013, Yanagi et al. reported 99mTc-complex-conjugated aspartic acid (Asp) peptides [56]. They selected EC as a ligand to prepare stable technetium complexes, conjugated with one or two penta d-Asp peptides [99mTc-EC-(d-Asp)5 or 99mTc-EC-[(d-Asp)5]2, Figures 7(a) and 7(b)]. The HA binding of 99mTc-EC-[(d-Asp)5]2 was higher than that of 99mTc-EC-(d-Asp)5. 99mTc-EC-[(d-Asp)5]2 showed significantly lower accumulation in normal bone of mice compared with 99mTc-MDP. In contrast, when compared with 99mTc-MDP, 99mTc-EC-[(d-Asp)5]2 showed the same degree of accumulation in a osteogenic lesion of tumor-bearing rat models. Thus, the uptake ratio of osteogenic lesion to normal bone (osteogenic lesion/normal bone) of 99mTc-EC-[(d-Asp)5]2 after injection was higher than that of 99mTc-MDP. The authors supposed that the higher osteogenic lesion/normal bone ratio derived forms the higher molecular size, which was determined by permeability through a membrane filter (10 kDa), of 99mTc-EC-[(d-Asp)5]2 compared with that of 99mTc-MDP and 99mTc-EC-(d-Asp)5.
Figure 7.
Chemical structures of radiometal complex-conjugated aspartic acid peptide compounds: (a) 99mTc-EC-(d-Asp)5, (b) 99mTc-EC-[(d-Asp)5]2, and (c) 67Ga-DOTA-(Asp)n.
In 2013, we reported 67Ga-DOTA-conjugated l-Asp peptides (67Ga-DOTA-(Asp)n, Figure 7(c)), which had varying peptide lengths (n = 2, 5, 8, 11, or 14) [57]. Binding affinity to HA of 67Ga-DOTA-(Asp)n increased with an increase in the length of the aspartate peptide. The HA binding of 67Ga-DOTA-conjugated bisphosphonate, 67Ga-DOTA-Bn-SCN-HBP, was inhibited by lower concentrations of alendronate, one of bisphosphonate compounds, compared with 67Ga-DOTA-(Asp)14. In biodistribution experiments of normal mice, 67Ga-DOTA-(Asp)8, 67Ga-DOTA-(Asp)11, and 67Ga-DOTA-(Asp)14 selectively and highly accumulated in bone (10.5 ± 1.5, 15.1 ± 2.6, and 12.8 ± 1.7% ID/g, resp.). Although the bone accumulation of 67Ga-DOTA-(Asp)n was lower than that of 67Ga-DOTA-Bn-SCN-HBP, the blood clearance of 67Ga-DOTA-(Asp)n was more rapid. Accordingly, the bone/blood ratios of 67Ga-DOTA-(Asp)11 and 67Ga-DOTA-(Asp)14 were comparable to that of 67Ga-DOTA-Bn-SCN-HBP. Moreover, the inhibition of radioactive bone accumulation by alendronate was greater after injection of 67Ga-DOTA-Bn-SCN-HBP than that of 67Ga-DOTA-(Asp)14.
These results indicate that not only bisphosphonate molecules but also acidic amino acid peptide sequences could be useful as carriers of radionuclides to bone metastases lesions. Moreover, radiometal complex-conjugated acidic amino acid peptides may provide slightly different information from radiometal complex-conjugated bisphosphonates.
6. Carbon-11 Labeled Cathepsin K Inhibitors
Cathepsin K is a member of the papain family of cysteine peptidases with a primary physiological function of cleavage of type I and type II collagen [58]. The enzyme is highly expressed in activated osteoclasts, and a change in the number of the osteoclast is related to bone diseases such as osteoporosis [59]. Therefore, it could be useful to determine the changes in osteoclast numbers in such disease states by imaging cathepsin K. Because many inhibitors of cathepsin K have been synthesized and evaluated both in vitro and in vivo, their derivatives may be candidates as mother compounds for cathepsin K imaging agents. The possibility of targeting cathepsin K in in vivo imaging, using a near-infrared reporter probe, was confirmed in a previous report [60].
Rodnick et al. reported carbon-11-labeled cathepsin K inhibitors with high affinity as cathepsin K imaging agents, 2-cyano-4-(cyclohexylamino)-N-(4-[11C]methoxyphenethyl)-pyrimidine-5-carboxamide ([11C]1, Figure 8(a)) and 2-cyano-N-(4-[11C]methoxyphenethyl)-4-(neopentylamino) pyrimidine-5-carboxamide ([11C]2, Figure 8(b)) in 2014 [61]. Nonradioactive counterparts of [11C]1 and [11C]2 were reported in 2007 [62]. In that study, because the pyrimidine core structure docked into the cathepsin K active site, many types of derivatives based on a pyrimidine scaffold were synthesized and evaluated as cathepsin K inhibitors. Among them, the nonradioactive counterparts showed greater affinity and selectivity for cathepsin K. For inhibition of cathepsin K, cathepsin L, and cathepsin S, IC50 values of compound 1 were 0.022, 0.17, and 0.7, and those of compound 2 were <0.003, 1.2, and 0.9, respectively. [11C]1 and [11C]2 were radiosynthesized by standard reaction conditions used for alkylation reactions with [11C]methyl iodide. In vivoμ-PET imaging experiments showed that [11C]1 and [11C]2 inhibitors have a higher uptake in actively growing bone regions, such as distal ulnar, carpal, distal and proximal humeral, distal femur, and proximal tibia, than in nontarget regions such as muscle. The uptake in specific bone regions was based on specific binding to cathepsin K because the uptake was inhibited by pre- or coinjection of an excess amount of ligands. These results indicated that radiolabeled cathepsin K inhibitors could have potential as in vivo imaging agents to determine a change in the number of osteoclasts.
Figure 8.
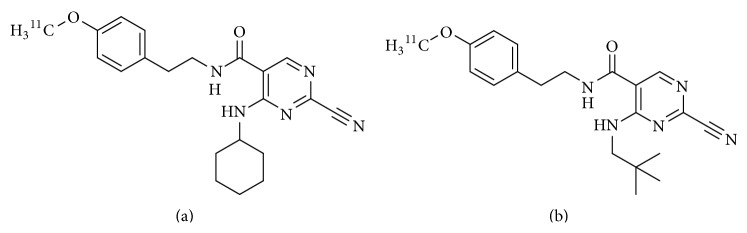
Chemical structures of carbon-11 labeled cathepsin K inhibitors: (a) 2-cyano-4-(cyclohexylamino)-N-(4-[11C]methoxyphenethyl)-pyrimidine-5-carboxamide and (b) 2-cyano-N-(4-[11C]methoxyphenethyl)-4-(neopentylamino) pyrimidine-5-carboxamide.
7. Dual-Modality Single Photon Emission Computed Tomography/Near-Infrared (SPECT/NIR) Fluorescent Probe
Recently, multimodality molecular imaging combining several imaging techniques has attracted much attention in basic scientific and clinical research. Nuclear medical imaging can detect deep tissues in the body with high sensitivity, but there are some problems such as relatively poor spatial resolution [63].
Optical imaging is a relatively new imaging modality that offers real-time and nonradioactive and high-resolution imaging of fluorophores in lesion sites, but it is difficult to detect a deep tissue using this technique [64]. Fluorescence imaging with near-infrared (NIR, 700–900 nm wavelength) light reveals relatively low tissue absorption. IRDye78 is a heptamethine indocyanine-type NIR fluorophore with peak absorption at 771 nm and peak excitation emission at 806 nm. Pam78 (Figure 9(a)), a IRDye78-conjugated pamidronate (one of the bisphosphonate derivatives), has been reported as a NIR fluorescence imaging probe targeted to HA [65]. HA is considered to be a good marker for some diseases because calcification (HA deposition) occurs during the processes of cancer and atherosclerosis.
Figure 9.
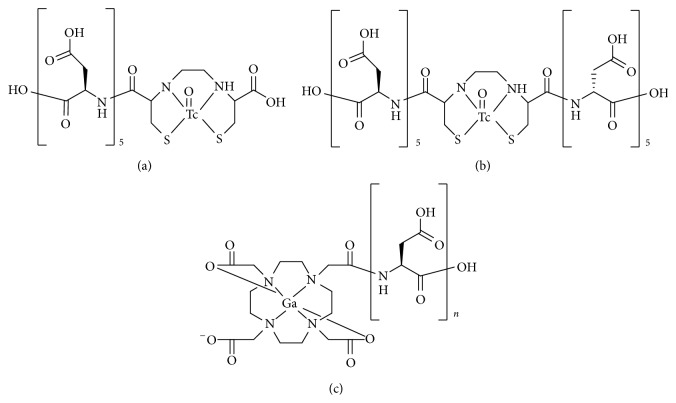
Chemical structures of a heptamethine indocyanine-type NIR fluorophore conjugated bisphosphonate: (a) Pam78 and a trifunctional diagnostic agent and (b) Pam-Tc/Re-800.
Bhushan et al. reported the trifunctional diagnostic agent Pam-Tc/Re-800 (Figure 9(b)) in 2008 [66]. Pam-Tc/Re-800 possesses a radiometal complex as a nuclear imaging probe, a fluorescent site as a fluorescence imaging probe, and bisphosphonate having high affinity for HA as a carrier to bone in a molecule. In an in vitro experiment, Pam-Tc/Re-800 showed specific and selective binding to HA. In the fluorescence imaging of microcalcified breast cancer rat model, Pam-Re-800 detected breast cancer microcalcifications. In SPECT/CT imaging, Pam-Tc-800 showed not only accumulation in normal bone but also highly sensitive detection of breast cancer microcalcifications. In biodistribution experiments, the total body clearance of Pam-Tc-800 at 4 hours after injection was comparable to that of 99mTc-MDP. Moreover, Pam-Tc-800 showed a higher uptake in bone and tumor than 99mTc-MDP. These results indicated that the novel trifunctional agent could provide simultaneous imaging by SPECT and NIR fluorescence. Dual-modality imaging may compensate for the drawbacks of the other modalities.
8. Summary
In this paper, several well-designed bone-seeking compounds were reviewed. They are chemically well-characterized and different from 99mTc-MDP and 99mTc-HMDP. Some demonstrated superior biodistribution characteristics. The mechanism by which all the compounds (except the carbon-11 labeled cathepsin K inhibitors) accumulate in bone is derived due to a high affinity for HA. We estimate that 68Ga-DOTA-conjugated bisphosphonate compounds, such as 68Ga-BPAMD and 68Ga-DOTA-Bn-SCN-HBP, are the most promising diagnostic agents for bone metastases because they show superior biodistribution characteristic and 68Ga is a useful PET radionuclide in clinical. Moreover, as DOTA ligand could form a complex with not only 67/68Ga but also 177Lu and 90Y, the palliation therapy is applicable using the same ligand. Namely, this system is “theranostics”, which is a combination of diagnosis and therapy.
Thus, the information from imaging data and the type of bone metastasis susceptible to treatment should be similar to those for existing bone-seeking radiopharmaceuticals. We hope that novel bone-seeking compounds that possess a different accumulation mechanism will be developed in the near future.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
References
- 1.Subramanian G., McAfee J. G., Blair R. J., Kallfelz F. A., Thomas F. D. Technetium 99m methylene diphosphonate: a superior agent for skeletal imaging: comparison with other technetium complexes. Journal of Nuclear Medicine. 1975;16(8):744–755. [PubMed] [Google Scholar]
- 2.Domstad P. A., Coupal J. J., Kim E. E. 99mTc-hydroxymethane diphosphonate: a new bone imaging agent with a low tin content. Radiology. 1980;136(1):209–211. doi: 10.1148/radiology.136.1.6446106. [DOI] [PubMed] [Google Scholar]
- 3.Love C., Din A. S., Tomas M. B., Kalapparambath T. P., Palestro C. J. Radionuclide bone imaging: an illustrative review. Radiographics. 2003;23(2):341–358. doi: 10.1148/rg.232025103. [DOI] [PubMed] [Google Scholar]
- 4.Mari C., Catafau A., Carrio I. Bone scintigraphy and metabolic disorders. Quarterly Journal of Nuclear Medicine. 1999;43(3):259–267. [PubMed] [Google Scholar]
- 5.Davis J., Cook N. D., Pither R. J. Biologic mechanisms of 89SrCl2 incorporation into type I collagen during bone mineralization. Journal of Nuclear Medicine. 2000;41(1):183–188. [PubMed] [Google Scholar]
- 6.Paes F. M., Serafini A. N. Systemic metabolic radiopharmaceutical therapy in the treatment of metastatic bone pain. Seminars in Nuclear Medicine. 2010;40(2):89–104. doi: 10.1053/j.semnuclmed.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Paes F. M., Ernani V., Hosein P., Serafini A. N. Radiopharmaceuticals: when and how to use them to treat metastatic bone pain. Journal of Supportive Oncology. 2011;9(6):197–205. doi: 10.1016/j.suponc.2011.06.004. [DOI] [PubMed] [Google Scholar]
- 8.Liepe K., Kotzerke J. Internal radiotherapy of painful bone metastases. Methods. 2011;55(3):258–270. doi: 10.1016/j.ymeth.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Ogawa K., Washiyama K. Bone target radiotracers for palliative therapy of bone metastases. Current Medicinal Chemistry. 2012;19(20):3290–3300. doi: 10.2174/092986712801215865. [DOI] [PubMed] [Google Scholar]
- 10.Eary J. F., Collins C., Stabin M., et al. Samarium-153-EDTMP biodistribution and dosimetry estimation. Journal of Nuclear Medicine. 1993;34(7):1031–1036. [PubMed] [Google Scholar]
- 11.Bianchi L., Baroli A., Marzoli L., Verusio C., Chiesa C., Pozzi L. Prospective dosimetry with 99mTc-MDP in metabolic radiotherapy of bone metastases with 153Sm-EDTMP. European Journal of Nuclear Medicine and Molecular Imaging. 2009;36(1):122–129. doi: 10.1007/s00259-008-0926-7. [DOI] [PubMed] [Google Scholar]
- 12.Dickie G. J., Macfarlane D. Strontium and samarium therapy for bone metastases from prostate carcinoma. Australasian Radiology. 1999;43(4):476–479. doi: 10.1046/j.1440-1673.1999.00716.x. [DOI] [PubMed] [Google Scholar]
- 13.Lam M. G. E. H., Dahmane A., Stevens W. H. M., Van Rijk P. P., De Klerk J. M. H., Zonnenberg B. A. Combined use of zoledronic acid and 153Sm-EDTMP in hormone-refractory prostate cancer patients with bone metastases. European Journal of Nuclear Medicine and Molecular Imaging. 2008;35(4):756–765. doi: 10.1007/s00259-007-0659-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parker C., Nilsson D Heinrich S., Helle S. I., et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. The New England Journal of Medicine. 2013;369(3):213–223. doi: 10.1056/NEJMoa1213755. [DOI] [PubMed] [Google Scholar]
- 15.Sartor O., Coleman R., Nilsson S., et al. Effect of radium-223 dichloride on symptomatic skeletal events in patients with castration-resistant prostate cancer and bone metastases: results from a phase 3, double-blind, randomised trial. The Lancet Oncology. 2014;15(7):738–746. doi: 10.1016/S1470-2045(14)70183-4. [DOI] [PubMed] [Google Scholar]
- 16.Tanabe Zodda S. J. P., Deutsch E., Heineman W. R. Effect of pH on the formation of Tc(NaBH4)-MDP radiopharmaceutical analogues. International Journal of Applied Radiation and Isotopes. 1983;34(12):1577–1584. doi: 10.1016/0020-708X(83)90002-9. [DOI] [PubMed] [Google Scholar]
- 17.Libson K., Deutsch E., Barnett B. L. Structural characterization of a 99Tc-diphosphonate complex. Implications for the chemistry of 99mTc skeletal imaging agents. Journal of the American Chemical Society. 1980;102(7):2476–2478. doi: 10.1021/ja00527a066. [DOI] [Google Scholar]
- 18.Verbeke K., Rozenski J., Cleynhens B., et al. Development of a conjugate of 99mTc-EC with aminomethylenediphosphonate in the search for a bone tracer with fast clearance from soft tissue. Bioconjugate Chemistry. 2002;13(1):16–22. doi: 10.1021/bc0001600. [DOI] [PubMed] [Google Scholar]
- 19.Ogawa K., Mukai T., Inoue Y., Ono M., Saji H. Development of a novel 99mTc-chelate-conjugated bisphosphonate with high affinity for bone as a bone scintigraphic agent. Journal of Nuclear Medicine. 2006;47(12):2042–2047. [PubMed] [Google Scholar]
- 20.Liu L., Zhong G., Wei Y., Zhang M., Wang X. Synthesis and biological evaluation of a novel 99mTc complex of HYNIC-conjugated aminomethylenediphosphonate as a potential bone imaging agent. Journal of Radioanalytical and Nuclear Chemistry. 2011;288(2):467–473. doi: 10.1007/s10967-010-0942-5. [DOI] [Google Scholar]
- 21.Liu S., Edwards D. S., Looby R. J., et al. Labeling a hydrazino nicotinamide-modified cyclic IIb/IIIa receptor antagonist with 99mTc using aminocarboxylates as coligands. Bioconjugate Chemistry. 1996;7(1):63–71. doi: 10.1021/bc950069+. [DOI] [PubMed] [Google Scholar]
- 22.Ono M., Arano Y., Uehara T., et al. Intracellular metabolic fate of radioactivity after injection of technetium-99m-labeled hydrazino nicotinamide derivatized proteins. Bioconjugate Chemistry. 1999;10(3):386–394. doi: 10.1021/bc980105f. [DOI] [PubMed] [Google Scholar]
- 23.Ono M., Arano Y., Mukai T., et al. Plasma protein binding of 99mTc-labeled hydrazino nicotinamide derivatized polypeptides and peptides. Nuclear Medicine and Biology. 2001;28(2):155–164. doi: 10.1016/S0969-8051(00)00200-6. [DOI] [PubMed] [Google Scholar]
- 24.Palma E., Oliveira B. L., Correia J. D. G., Gano L., Maria L., Santos I. C. A new bisphosphonate-containing 99mTc(I) tricarbonyl complex potentially useful as bone-seeking agent: synthesis and biological evaluation. Journal of Biological Inorganic Chemistry. 2007;12(5):667–679. doi: 10.1007/s00775-007-0215-0. [DOI] [PubMed] [Google Scholar]
- 25.Palma E., Correia J. D. G., Oliveira B. L., Gano L., Santos I. C. 99mTc(CO)3-labeled pamidronate and alendronate for bone imaging. Dalton Transactions. 2011;40(12):2787–2796. doi: 10.1039/c0dt01396j. [DOI] [PubMed] [Google Scholar]
- 26.Van Beek E., Hoekstra M., van de Ruit M., Löwik C., Papapoulos S. Structural requirements for bisphosphonate actions in vitro. Journal of Bone and Mineral Research. 1994;9(12):1875–1882. doi: 10.1002/jbmr.5650091206. [DOI] [PubMed] [Google Scholar]
- 27.Meyer J. L., Nancollas G. H. The influence of multidentate organic phosphonates on the crystal growth of hydroxyapatite. Calcified Tissue Research. 1973;13(1):295–303. doi: 10.1007/BF02015419. [DOI] [PubMed] [Google Scholar]
- 28.Ogawa K., Mukai T., Arano Y., et al. Rhemium-186-monoaminemonoamidedithiol-conjugated bisphosphonate derivatives for bone pain palliation. Nuclear Medicine and Biology. 2006;33(4):513–520. doi: 10.1016/j.nucmedbio.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 29.De Rosales R. T. M., Finucane C., Mather S. J., Blower P. J. Bifunctional bisphosphonate complexes for the diagnosis and therapy of bone metastases. Chemical Communications. 2009;(32):4847–4849. doi: 10.1039/b908652h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhernosekov K. P., Filosofov D. V., Baum R. P., et al. Processing of generator-produced 68Ga for medical application. Journal of Nuclear Medicine. 2007;48(10):1741–1748. doi: 10.2967/jnumed.107.040378. [DOI] [PubMed] [Google Scholar]
- 31.Fellner M., Baum R. P., Kubíček V., et al. PET/CT imaging of osteoblastic bone metastases with 68Ga-bisphosphonates: first human study. European Journal of Nuclear Medicine and Molecular Imaging. 2010;37(4, article 834) doi: 10.1007/s00259-009-1355-y. [DOI] [PubMed] [Google Scholar]
- 32.Fellner M., Biesalski B., Bausbacher N., et al. 68Ga-BPAMD: PET-imaging of bone metastases with a generator based positron emitter. Nuclear Medicine and Biology. 2012;39(7):993–999. doi: 10.1016/j.nucmedbio.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 33.Bruland Ø. S., Nilsson S., Fisher D. R., Larsen R. H. High-linear energy transfer irradiation targeted to skeletal metastases by the α-emitter 223Ra: adjuvant or alternative to conventional modalities? Clinical Cancer Research. 2006;12(20, part 2):6250s–6257s. doi: 10.1158/1078-0432.CCR-06-0841. [DOI] [PubMed] [Google Scholar]
- 34.Ogawa K., Takai K., Kanbara H., et al. Preparation and evaluation of a radiogallium complex-conjugated bisphosphonate as a bone scintigraphy agent. Nuclear Medicine and Biology. 2011;38(5):631–636. doi: 10.1016/j.nucmedbio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki K., Satake M., Suwada J., et al. Synthesis and evaluation of a novel 68Ga-chelate-conjugated bisphosphonate as a bone-seeking agent for PET imaging. Nuclear Medicine and Biology. 2011;38(7):1011–1018. doi: 10.1016/j.nucmedbio.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 36.Ferro-Flores G., Arteaga de Murphy C. Pharmacokinetics and dosimetry of 188Re-pharmaceuticals. Advanced Drug Delivery Reviews. 2008;60(12):1389–1401. doi: 10.1016/j.addr.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 37.Knapp F. F., Jr., Beets A. L., Guhlke S., et al. Availability of rhenium-188 from the alumina-based tungsten-188/Rhenium-188 generator for preparation of rhenium-188-labeled radiopharmaceuticals for cancer treatment. Anticancer Research. 1997;17(3):1783–1795. [PubMed] [Google Scholar]
- 38.Liepe K., Runge R., Kotzerke J. The benefit of bone-seeking radiopharmaceuticals in the treatment of metastatic bone pain. Journal of Cancer Research and Clinical Oncology. 2005;131(1):60–66. doi: 10.1007/s00432-004-0625-0. [DOI] [PubMed] [Google Scholar]
- 39.Finlay I. G., Mason M. D., Shelley M. Radioisotopes for the palliation of metastatic bone cancer: a systematic review. The Lancet Oncology. 2005;6(6):392–400. doi: 10.1016/S1470-2045(05)70206-0. [DOI] [PubMed] [Google Scholar]
- 40.Deutsch E., Libson K., Vanderheyden J. L., Ketring A. R., Maxon H. R. The chemistry of rhenium and technetium as related to the use of isotopes of these elements in therapeutic and diagnostic nuclear medicine. Nuclear Medicine and Biology. 1986;13(4):465–477. doi: 10.1016/0883-2897(86)90027-9. [DOI] [PubMed] [Google Scholar]
- 41.Arano Y., Ono M., Wakisaka K., et al. Synthesis and biodistribution studies of 186Re complex of 1-hydroxyethylidene-1,1-diphosphonate for treatment of painful osseous metastases. Radioisotopes. 1995;44(8):514–522. [Google Scholar]
- 42.De Winter F., Brans B., Van De Wiele C., Dierckx R. A. Visualization of the stomach on rhenium-186 HEDP imaging after therapy for metastasized prostate carcinoma. Clinical Nuclear Medicine. 1999;24(11):898–899. doi: 10.1097/00003072-199911000-00022. [DOI] [PubMed] [Google Scholar]
- 43.Limouris G. S., Shukla S. K., Condi-Paphiti A., et al. Palliative therapy using rhenium-186-HEDP in painful breast osseous metastases. Anticancer Research. 1997;17(3B):1767–1772. [PubMed] [Google Scholar]
- 44.Lin W.-Y., Hsieh J.-F., Tsai S.-C., Yen T.-C., Wang S. J., Knapp F. F., Jr. A comprehensive study on the blockage of thyroid and gastric uptakes of 188Re-perrhenate in endovascular irradiation using liquid-filled balloon to prevent restenosis. Nuclear Medicine and Biology. 2000;27(1):83–87. doi: 10.1016/S0969-8051(99)00079-7. [DOI] [PubMed] [Google Scholar]
- 45.Ogawa K., Mukai T., Arano Y., et al. Design of a radiopharmaceutical for the palliation of painful bone metastases: rhenium-186-labeled bisphosphonate derivative. Journal of Labelled Compounds and Radiopharmaceuticals. 2004;47(11):753–761. doi: 10.1002/jlcr.864. [DOI] [Google Scholar]
- 46.Ogawa K., Mukai T., Arano Y., et al. Development of a rhenium-186-labeled MAG3-conjugated bisphosphonate for the palliation of metastatic bone pain based on the concept of bifunctional radiopharmaceuticals. Bioconjugate Chemistry. 2005;16(4):751–757. doi: 10.1021/bc040249w. [DOI] [PubMed] [Google Scholar]
- 47.Ogawa K., Mukai T., Asano D., et al. Therapeutic effects of a 186Re-complex-conjugated bisphosphonate for the palliation of metastatic bone pain in an animal model. Journal of Nuclear Medicine. 2007;48(1):122–127. [PubMed] [Google Scholar]
- 48.Uehara T., Jin Z. L., Ogawa K., et al. Assessment of 186Re chelate-conjugated bisphosphonate for the development of new radiopharmaceuticals for bones. Nuclear Medicine and Biology. 2007;34(1):79–87. doi: 10.1016/j.nucmedbio.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 49.de Rosales R. T. M., Finucane C., Foster J., Mather S. J., Blower P. J. 188Re(CO)3-dipicolylamine-alendronate: a new bisphosphonate conjugate for the radiotherapy of bone metastases. Bioconjugate Chemistry. 2010;21(5):811–815. doi: 10.1021/bc100071k. [DOI] [PubMed] [Google Scholar]
- 50.Butler W. T. The nature and significance of osteopontin. Connective Tissue Research. 1989;23(2-3):123–136. doi: 10.3109/03008208909002412. [DOI] [PubMed] [Google Scholar]
- 51.Oldberg A., Franzen A., Heinegard D. The primary structure of a cell-binding bone sialoprotein. The Journal of Biological Chemistry. 1988;263(36):19430–19432. [PubMed] [Google Scholar]
- 52.Oldberg A., Franzen A., Heinegard D. Cloning and sequence analysis of rat bone sialoprotein (osteopontin) cDNA reveals an Arg-Gly-Asp cell-binding sequence. Proceedings of the National Academy of Sciences of the United States of America. 1986;83(23):8819–8823. doi: 10.1073/pnas.83.23.8819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kasugai S., Fujisawa R., Waki Y., Miyamoto K.-I., Ohya K. Selective drug delivery system to bone: Small peptide (Asp)6 conjugation. Journal of Bone and Mineral Research. 2000;15(5):936–943. doi: 10.1359/jbmr.2000.15.5.936. [DOI] [PubMed] [Google Scholar]
- 54.Yokogawa K., Miya K., Sekido T., et al. Selective delivery of estradiol to bone by aspartic acid oligopeptide and its effects on ovariectomized mice. Endocrinology. 2001;142(3):1228–1233. doi: 10.1210/en.142.3.1228. [DOI] [PubMed] [Google Scholar]
- 55.Wang D., Miller S., Sima M., Kopečková P., Kopeček J. Synthesis and evaluation of water-soluble polymeric bone-targeted drug delivery systems. Bioconjugate Chemistry. 2003;14(5):853–859. doi: 10.1021/bc034090j. [DOI] [PubMed] [Google Scholar]
- 56.Yanagi M., Uehara T., Uchida Y., et al. Chemical design of 99mTc-labeled probes for targeting osteogenic bone region. Bioconjugate Chemistry. 2013;24(7):1248–1255. doi: 10.1021/bc400197f. [DOI] [PubMed] [Google Scholar]
- 57.Ogawa K., Ishizaki A., Takai K., et al. Development of novel radiogallium-labeled bone imaging agents using oligo-aspartic acid peptides as carriers. PLoS ONE. 2013;8(12) doi: 10.1371/journal.pone.0084335.e84335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Novinec M., Lenarčic B. Cathepsin K: a unique collagenolytic cysteine peptidase. Biological Chemistry. 2013;394(9):1163–1179. doi: 10.1515/hsz-2013-0134. [DOI] [PubMed] [Google Scholar]
- 59.Troeberg L., Nagase H. Proteases involved in cartilage matrix degradation in osteoarthritis. Biochimica et Biophysica Acta: Proteins and Proteomics. 2012;1824(1):133–145. doi: 10.1016/j.bbapap.2011.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kozloff K. M., Quinti L., Patntirapong S., et al. Non-invasive optical detection of cathepsin K-mediated fluorescence reveals osteoclast activity in vitro and in vivo. Bone. 2009;44(2):190–198. doi: 10.1016/j.bone.2008.10.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rodnick M. E., Shao X., Kozloff K. M., Scott P. J. H., Kilbourn M. R. Carbon-11 labeled cathepsin K inhibitors: syntheses and preliminary in vivo evaluation. Nuclear Medicine and Biology. 2014;41(5):384–389. doi: 10.1016/j.nucmedbio.2014.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Altmann E., Aichholz R., Betschart C., et al. 2-Cyano-pyrimidines: a new chemotype for inhibitors of the cysteine protease cathepsin K. Journal of Medicinal Chemistry. 2007;50(4):591–594. doi: 10.1021/jm0613525. [DOI] [PubMed] [Google Scholar]
- 63.Weissleder R., Mahmood U. Molecular imaging. Radiology. 2001;219(2):316–333. doi: 10.1148/radiology.219.2.r01ma19316. [DOI] [PubMed] [Google Scholar]
- 64.Ntziachristos V., Bremer C., Weissleder R. Fluorescence imaging with near-infrared light: new technological advances that enable in vivo molecular imaging. European Radiology. 2003;13(1):195–208. doi: 10.1007/s00330-002-1524-x. [DOI] [PubMed] [Google Scholar]
- 65.Zaheer A., Lenkinski R. E., Mahmood A., Jones A. G., Cantley L. C., Frangioni J. V. In vivo near-infrared fluorescence imaging of osteoblastic activity. Nature Biotechnology. 2001;19(12):1148–1154. doi: 10.1038/nbt1201-1148. [DOI] [PubMed] [Google Scholar]
- 66.Bhushan K. R., Misra P., Liu F., Mathur S., Lenkinski R. E., Frangioni J. V. Detection of breast cancer microcalcifications using a dual-modality SPECT/ NIR fluorescent probe. Journal of the American Chemical Society. 2008;130(52):17648–17649. doi: 10.1021/ja807099s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pandit-Taskar N., Larson S. M., Carrasquillo J. A. Bone-seeking radiopharmaceuticals for treatment of osseous metastases, part 1: alpha therapy with 223Ra-dichloride. Journal of Nuclear Medicine. 2014;55(2):268–274. doi: 10.2967/jnumed.112.112482. [DOI] [PubMed] [Google Scholar]