Abstract
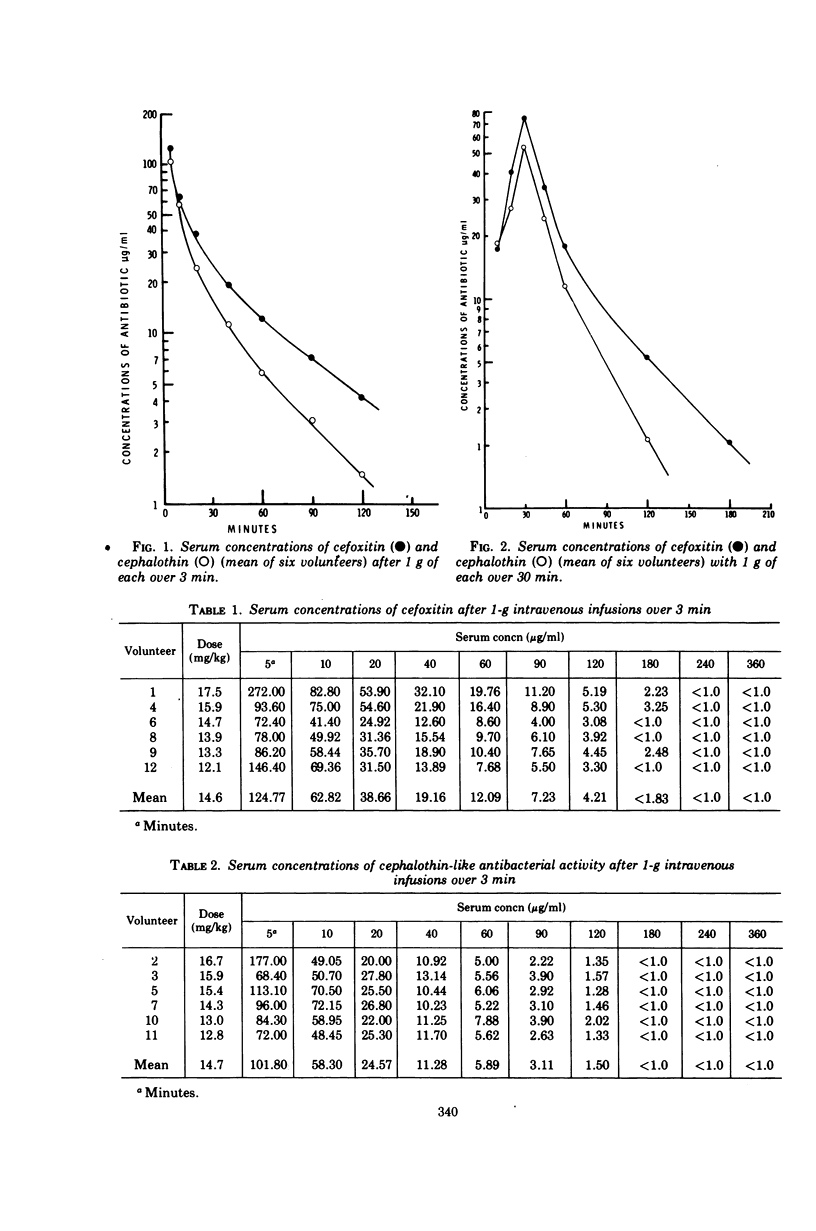
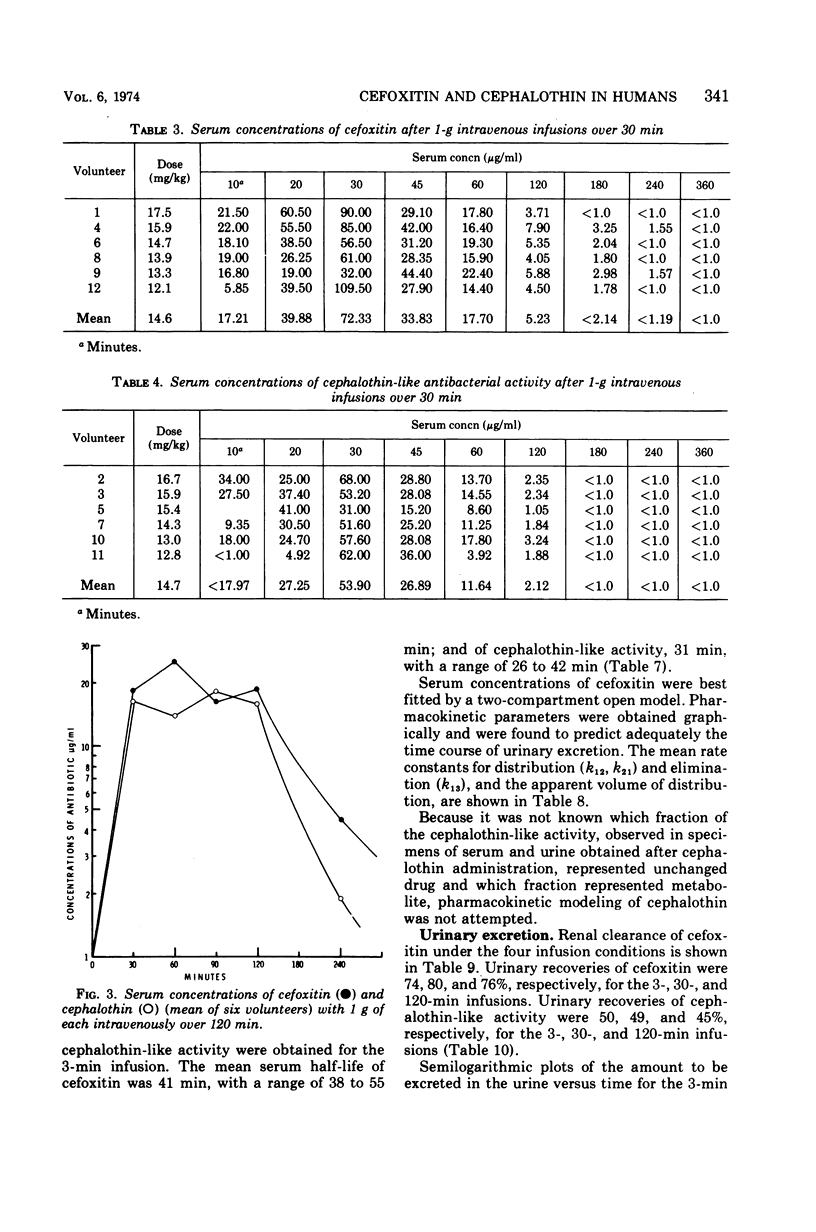
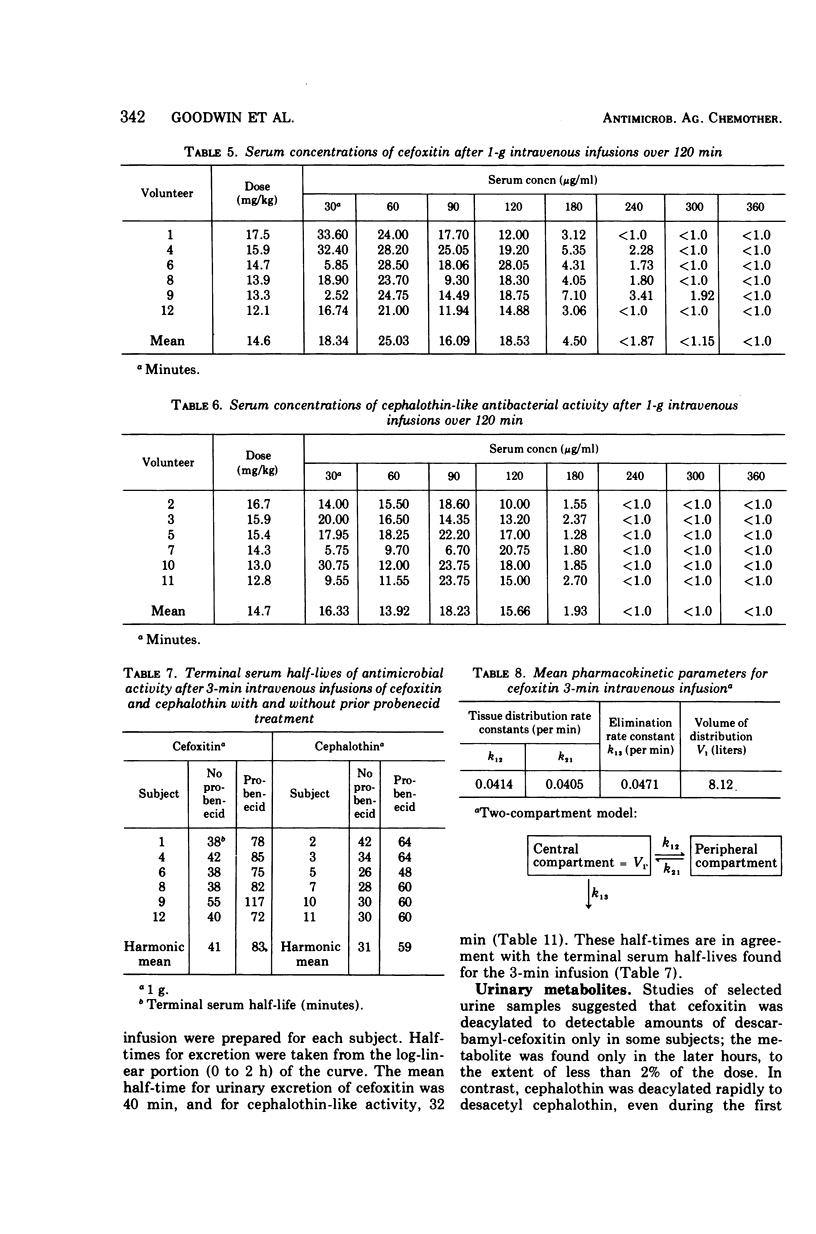
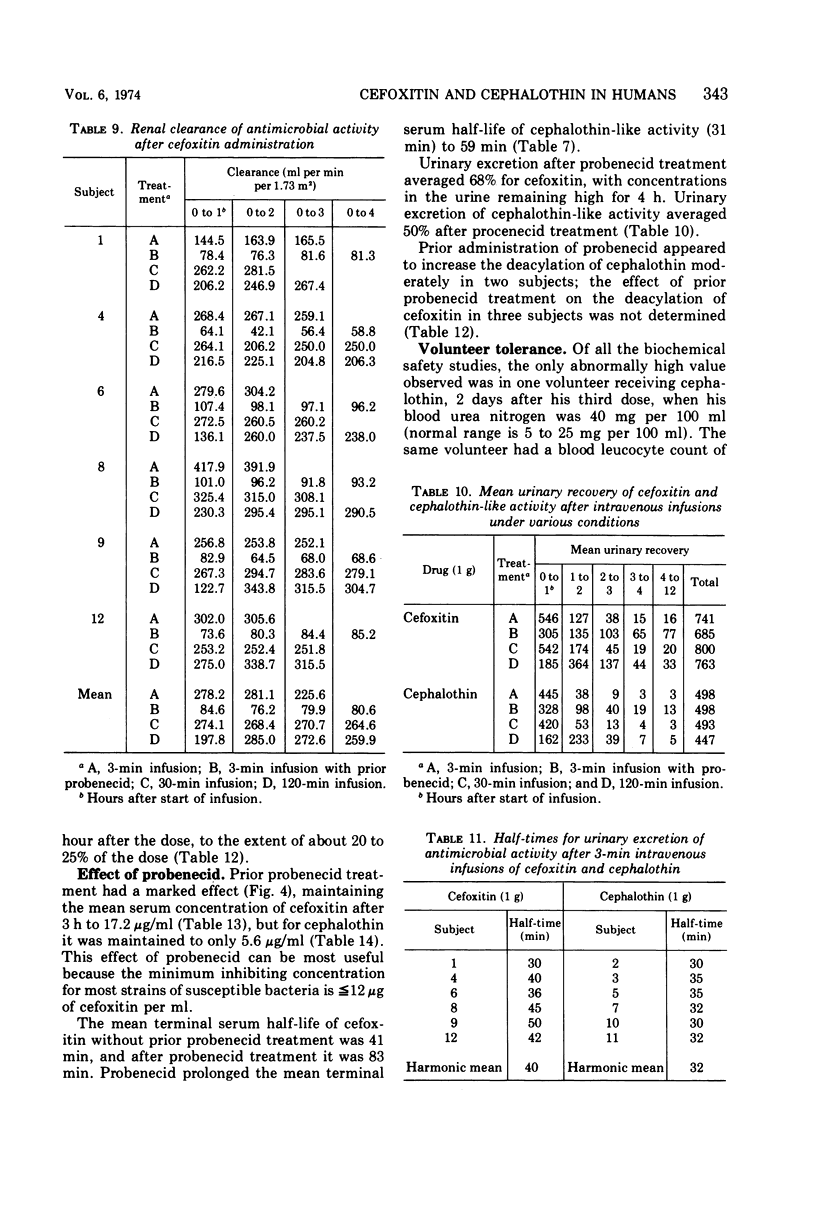
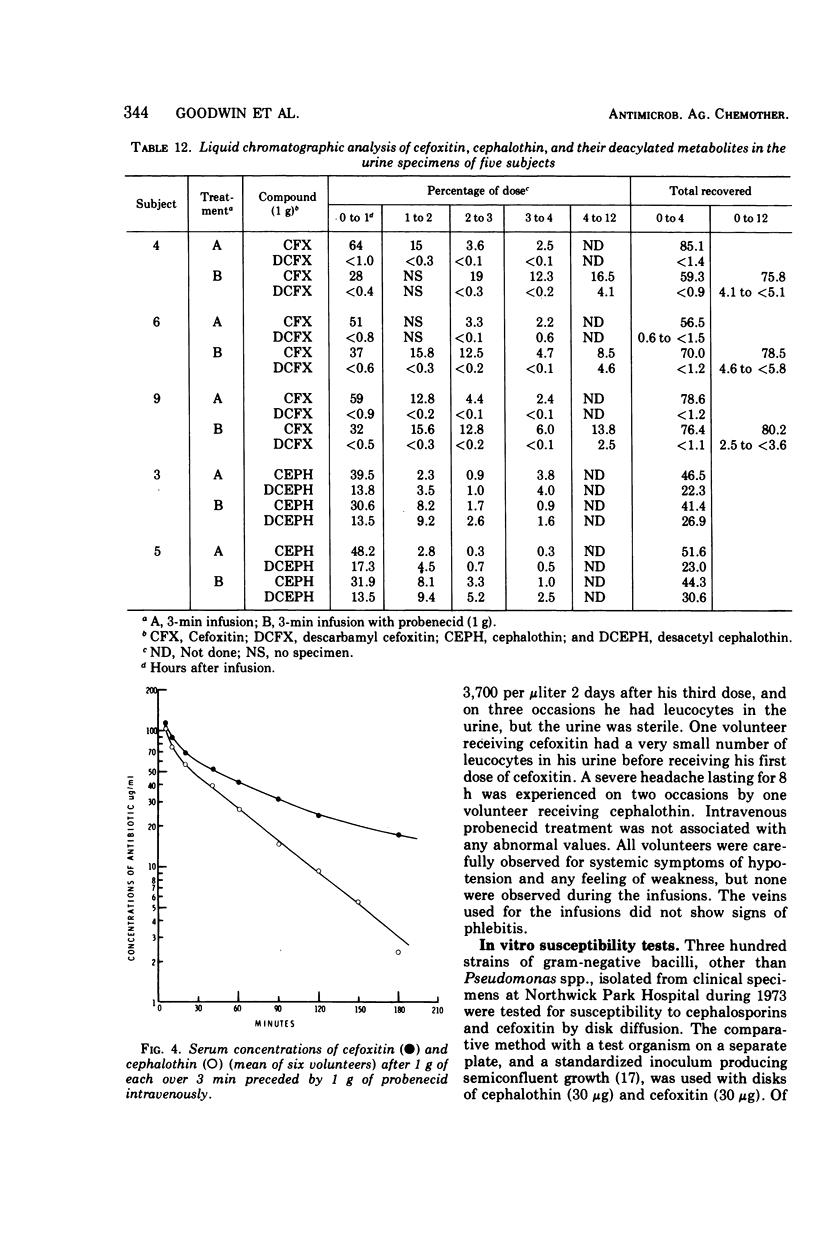
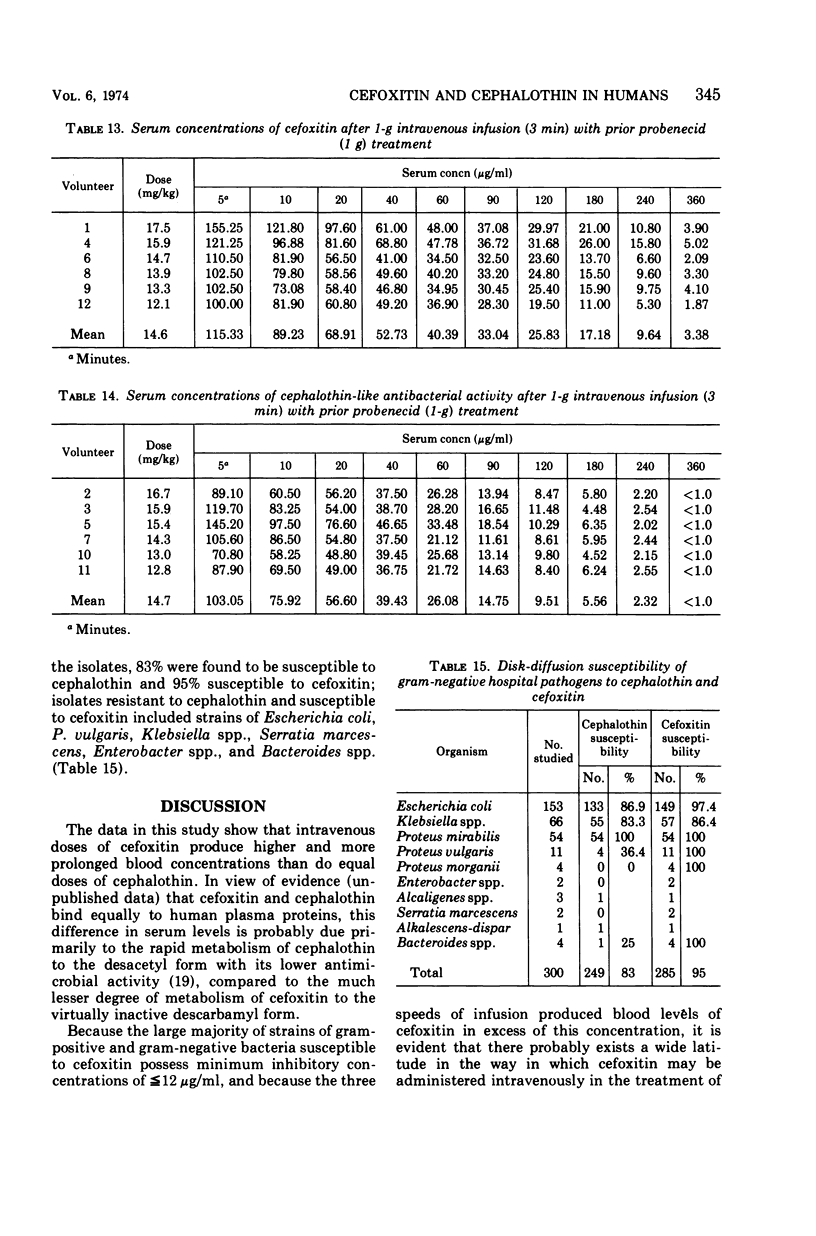
Using a randomized crossover design, 1-g intravenous doses of cephalothin and cefoxitin, a cephalosporinase-resistant cephamycin, were infused into 12 normal adult males over periods of 120, 30, and 3 min, the last with and without prior intravenous infusions of probenecid (1 g). Mean peak serum concentrations of antibiotic activity after cephalothin infusions were 23, 56, 103, and 102 μg/ml, respectively, and after cefoxitin infusions they were 27, 74, 115, and 125 μg/ml, respectively. Probenecid treatment prolonged the terminal serum half-life of cephalothin-like activity from 0.52 to 1.0 h, and of cefoxitin from 0.68 to 1.4 h. In contrast to cephalothin, which was found to be metabolized about 25% to the less active desacetyl form, cefoxitin was metabolized less than 2% to the virtually inactive descarbamyl form, as judged from urinary recoveries. Neither antibiotic displayed detectable organ toxicity. Of 300 recent clinical isolates of gram-negative bacilli other than Pseudomonas spp., 83% were susceptible to cephalothin but 95% were susceptible to cefoxitin. Organisms resistant to cephalothin but susceptible to cefoxitin included strains of Escherichia coli, Proteus vulgaris, Klebsiella spp., Serratia marcescens, Enterobacter spp., and Bacteroides spp.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAYTON P. G., YU T. F., CHEN W., BERGER L., WEST L. A., GUTMAN A. B. The physiological disposition of probenecid, including renal clearance, in man, studied by an improved method for its estimation in biological material. J Pharmacol Exp Ther. 1963 Jun;140:278–286. [PubMed] [Google Scholar]
- Daoust D. R., Onishi H. R., Wallick H., Hendlin D., Stapley E. O. Cephamycins, a new family of beta-lactam antibiotics: antibacterial activity and resistance to beta-lactamase degradation. Antimicrob Agents Chemother. 1973 Feb;3(2):254–261. doi: 10.1128/aac.3.2.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bene V. E., Farrar W. E., Jr Cephalosporinase activity in Bacteroides fragilis. Antimicrob Agents Chemother. 1973 Mar;3(3):369–372. doi: 10.1128/aac.3.3.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith R. S., Black H. R. Blood, urine and tissue concentrations of the cephalosporin antibiotics in normal subjects. Postgrad Med J. 1971 Feb;47(Suppl):32–40. [PubMed] [Google Scholar]
- Kirby W. M., De Maine J. B., Serrill W. S. Pharmacokinetics of the cephalosporins in healthy volunteers and uremic patients. Postgrad Med J. 1971 Feb;47(Suppl):41–46. [PubMed] [Google Scholar]
- Lacey R. W., Bruten D. M., Gillespie W. A., Lewis E. L. Trimethoprim-resistant coliforms. Lancet. 1972 Feb 19;1(7747):409–410. doi: 10.1016/s0140-6736(72)90857-4. [DOI] [PubMed] [Google Scholar]
- Lakke J. P., Korf J., Van Praag H. M., Schut T. [Predictive value of the probenecid test for the effect of L-DOPA therapy in Parkinson's disease]. Nat New Biol. 1972 Apr 19;236(68):208–209. doi: 10.1038/newbio236208a0. [DOI] [PubMed] [Google Scholar]
- May J. R., Davies J. Resistance of Haemophilus influenzae to trimethoprim. Br Med J. 1972 Aug 12;3(5823):376–377. doi: 10.1136/bmj.3.5823.376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. K., Celozzi E., Kong Y., Pelak B. A., Kropp H., Stapley E. O., Hendlin D. Cephamycins, a new family of beta-lactam antibiotics. IV. In vivo studies. Antimicrob Agents Chemother. 1972 Oct;2(4):287–290. doi: 10.1128/aac.2.4.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. K., Celozzi E., Pelak B. A., Stapley E. O., Hendlin D. Cephamycins, a new family of beta-lactam antibiotics. 3. In vitro studies. Antimicrob Agents Chemother. 1972 Oct;2(4):281–286. doi: 10.1128/aac.2.4.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi H. R., Daoust D. R., Zimmerman S. B., Hendlin D., Stapley E. O. Cefoxitin, a semisynthetic cephamycin antibiotic: resistance to beta-lactamase inactivation. Antimicrob Agents Chemother. 1974 Jan;5(1):38–48. doi: 10.1128/aac.5.1.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roe E., Lowbury E. J. Changes in antibiotic sensitivity patterns of Gram-negative bacilli in burns. J Clin Pathol. 1972 Feb;25(2):176–178. doi: 10.1136/jcp.25.2.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stapley E. O., Jackson M., Hernandez S., Zimmerman S. B., Currie S. A., Mochales S., Mata J. M., Woodruff H. B., Hendlin D. Cephamycins, a new family of beta-lactam antibiotics. I. Production by actinomycetes, including Streptomyces lactamdurans sp. n. Antimicrob Agents Chemother. 1972 Sep;2(3):122–131. doi: 10.1128/aac.2.3.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallick H., Hendlin D. Cefoxitin, a semisynthetic cephamycin antibiotic: susceptibility studies. Antimicrob Agents Chemother. 1974 Jan;5(1):25–32. doi: 10.1128/aac.5.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wick W. E. In vitro and in vivo laboratory comparison of cephalothin and desacetylcephalothin. Antimicrob Agents Chemother (Bethesda) 1965;5:870–875. [PubMed] [Google Scholar]


