Abstract
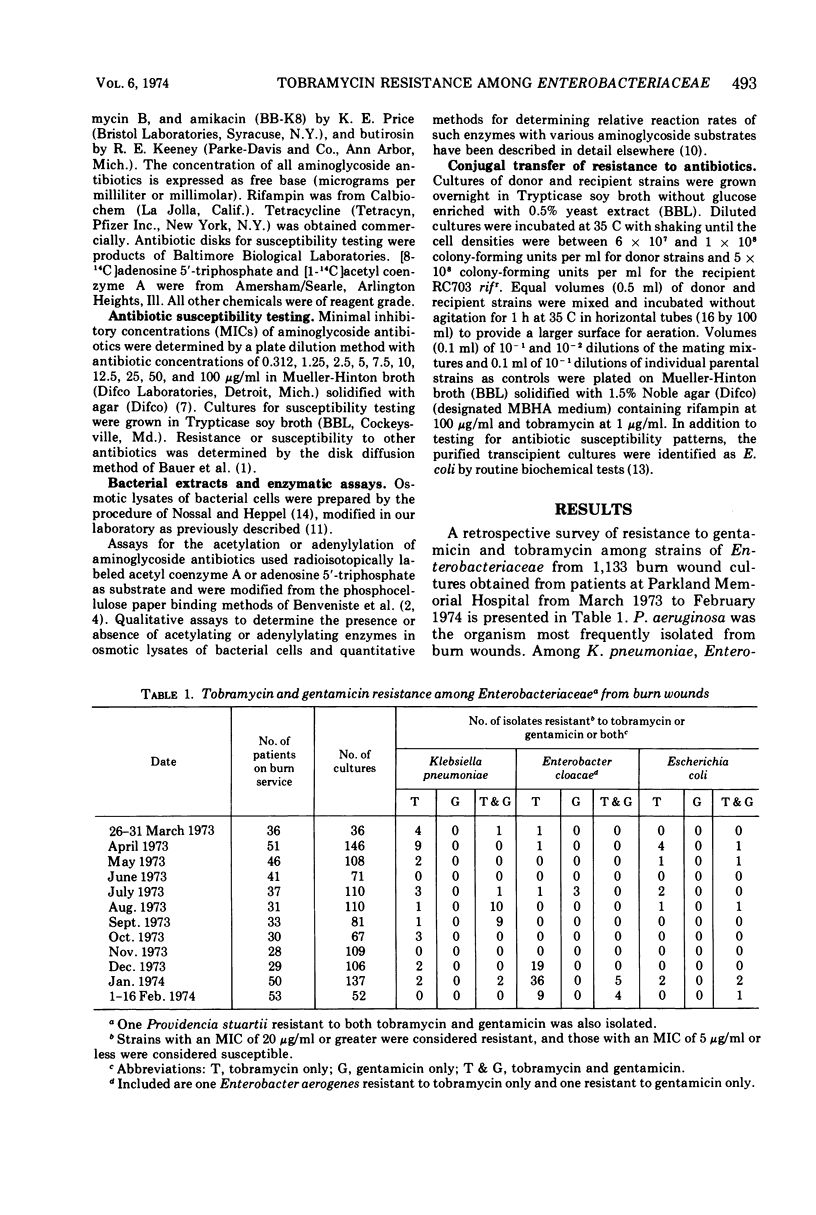
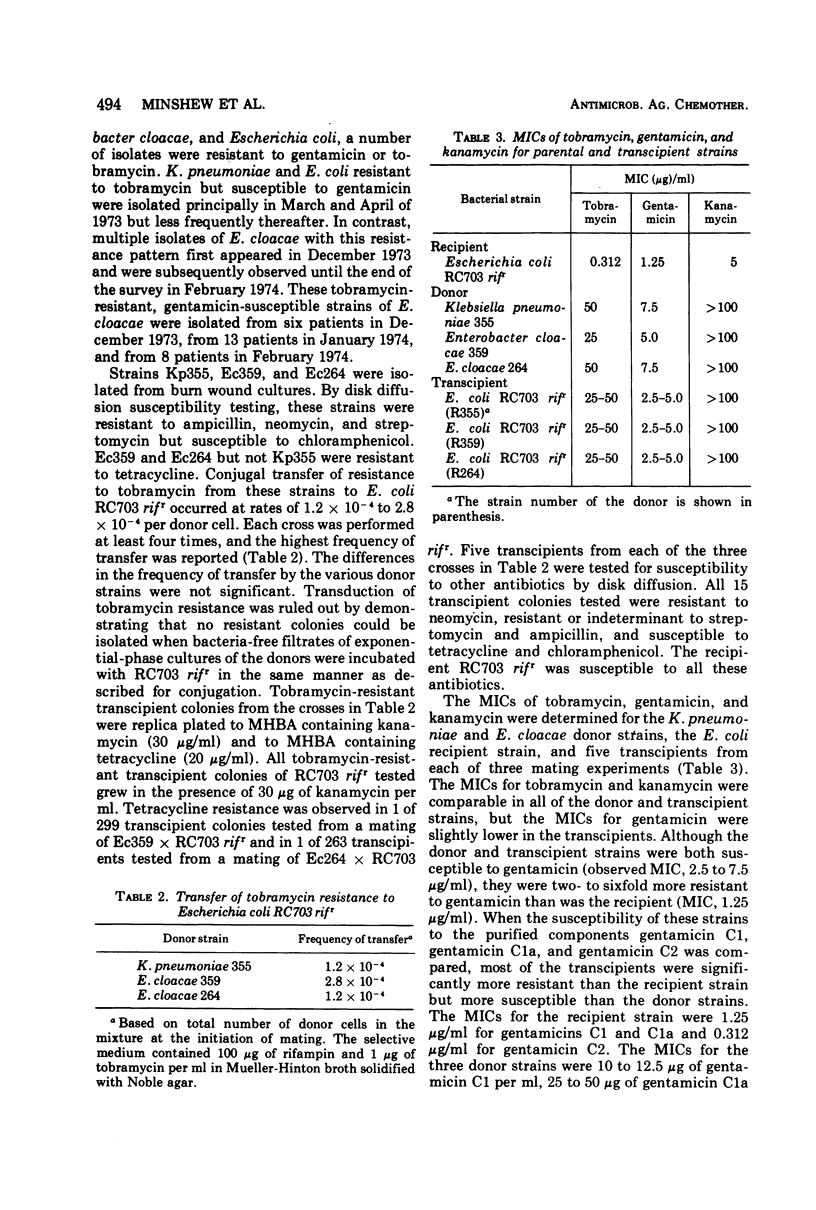
Among gram-negative bacilli isolated from burn wound cultures, some strains of Enterobacteriaceae were resistant to tobramycin (minimal inhibitory concentration [MIC]≥ 20 μg/ml) but susceptible to gentamicin (MIC ≤ 5 μg/ml). One Klebsiella pneumoniae and two Enterobacter cloacae strains were selected for studies on their mechanisms of resistance to aminoglycoside antibiotics. Resistance to high concentrations of tobramycin (MICs of 25 to 50 μg/ml) was conjugally transferred to a susceptible Escherichia coli strain at rates of 1.2 × 10−4 to 2.8 to 10−4 per donor cell, suggesting that resistance is controlled by R factors. Resistances to tobramycin, kanamycin, and neomycin were cotransferred. Enzymatic activities were present that acetylated tobramycin, gentamicin, and kanamycin in osmotic lysates from the donor and transcipient strains. Enzymatic adenylylation of these aminoglycosides was not observed. The aminoglycoside-acetylating activities from K. pneumoniae and E. cloacae resembled kanamycin acetyltransferase (KAT) in their specificity for aminoglycoside substrates. Not all isolates of bacteria that produce KAT are resistant to tobramycin, but the factors that determine susceptibility or resistance to tobramycin in KAT-producing bacteria have not yet been established.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bauer A. W., Kirby W. M., Sherris J. C., Turck M. Antibiotic susceptibility testing by a standardized single disk method. Am J Clin Pathol. 1966 Apr;45(4):493–496. [PubMed] [Google Scholar]
- Benveniste R., Davies J. Enzymatic acetylation of aminoglycoside antibiotics by Escherichia coli carrying an R factor. Biochemistry. 1971 May 11;10(10):1787–1796. doi: 10.1021/bi00786a009. [DOI] [PubMed] [Google Scholar]
- Benveniste R., Davies J. Mechanisms of antibiotic resistance in bacteria. Annu Rev Biochem. 1973;42:471–506. doi: 10.1146/annurev.bi.42.070173.002351. [DOI] [PubMed] [Google Scholar]
- Benveniste R., Yamada T., Davies J. Enzymatic Adenylylation of Streptomycin and Spectinomycin by R-Factor-Resistant Escherichia coli. Infect Immun. 1970 Jan;1(1):109–119. doi: 10.1128/iai.1.1.109-119.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodey G. P., Stewart D. In vitro studies of tobramycin. Antimicrob Agents Chemother. 1972 Sep;2(3):109–113. doi: 10.1128/aac.2.3.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusch J. L., Barza M., Bergeron M. G., Weinstein L. Cross-resistance of Pseudomonas to gentamicin and tobramycin. Antimicrob Agents Chemother. 1972 Mar;1(3):280–281. doi: 10.1128/aac.1.3.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger L. M., Sanford J. P., Zweighaft T. Tobramycin: bacteriological evaluation. Am J Med Sci. 1973 Feb;265(2):135–142. [PubMed] [Google Scholar]
- Crowe C. C., Sanders E. Is there complete cross-resistance of gram-negative bacilli to gentamicin and tobramycin? Antimicrob Agents Chemother. 1972 Nov;2(5):415–416. doi: 10.1128/aac.2.5.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Bene V. E., Farrar W. E., Jr Tobramycin: in vitro activity and comparison with kanamycin and gentamicin. Antimicrob Agents Chemother. 1972 Apr;1(4):340–342. doi: 10.1128/aac.1.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. K., Minshew B. H., Gould I. K., Sanford J. P. Resistance of Pseudomonas aeruginosa to gentamicin and related aminoglycoside antibiotics. Antimicrob Agents Chemother. 1974 Sep;6(3):253–262. doi: 10.1128/aac.6.3.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes R. K., Sanford J. P. Enzymatic assay for gentamicin and related aminoglycoside antibiotics. J Infect Dis. 1974 May;129(5):519–527. doi: 10.1093/infdis/129.5.519. [DOI] [PubMed] [Google Scholar]
- Lundbäck A. K., Nordström K. Mutations in Escherichia coli K-12 decreasing the rate of streptomycin uptake: synergism with R-factor-mediated capacity to inactivate streptomycin. Antimicrob Agents Chemother. 1974 May;5(5):500–507. doi: 10.1128/aac.5.5.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Price K. E., Pursiano T. A., DeFuria M. D. Activity of BB-K8 (amikacin) against clinical isolates resistant to one or more aminoglycoside antibiotics. Antimicrob Agents Chemother. 1974 Feb;5(2):143–152. doi: 10.1128/aac.5.2.143. [DOI] [PMC free article] [PubMed] [Google Scholar]


