Abstract
Mice lacking steroid 5α-reductase 1 and 2 were produced by gene targeting and breeding. Male mice without 5α-reductase 2 or without both enzymes had fully formed internal and external genitalia and were fertile, but had smaller prostates and seminal vesicles than controls. T accumulated to high levels in the reproductive tissues of the mutant mice. DHT administration increased seminal vesicle and coagulating gland weights in mice deficient in 5α-reductase 2 and increased the weights of the prostate, seminal vesicle, and coagulating gland in animals deficient in both enzymes. An inhibitor of both 5α-reductases (GI 208335X) decreased prostate and coagulating gland weights of control mice, but had no effect in those lacking 5α-reductase 1 and 2. Castration reduced the sizes of these tissues in animals of all genotypes. Androgen-dependent gene expression was decreased in the seminal vesicles of mice lacking one or more 5α-reductases and was restored by administration of T or DHT. Female mice missing both enzymes exhibited parturition and fecundity defects similar to those of animals without 5α-reductase 1. We conclude that T is the only androgen required for differentiation of the male urogenital tract in mice and that the synthesis of DHT serves largely as a signal amplification mechanism.
The formation of the human male phenotype requires T and DHT, which act through the AR to direct the virilization of different genital anlagen (1). T is necessary for differentiation of the Wolffian ducts into the seminal vesicles, epididymes, and ejaculatory ducts, whereas DHT is needed for the differentiation of the urogenital sinus and genital tubercle into the prostate, urethra, penis, and scrotum. The involvement of distinct androgens in these two developmental pathways is most clearly evidenced in patients with defects in the formation of T or DHT. Mutations that decrease the synthesis of T affect both developmental pathways, as T serves as a metabolic precursor of DHT (2). In contrast, a decrease in the synthesis of DHT is associated only with defects in the virilization of the urogenital sinus and genital tubercle (3, 4). Studies in male rats with pharmacological inhibitors support a two-androgen model of phenotypic sexual differentiation in this species (5–7); however, it is not clear whether the requirement for T and DHT in the formation of the male phenotype extends across the mammalian class or why this need exists.
The conversion of T to DHT is catalyzed by steroid 5α-reductase, a membrane-bound enzyme that uses NADPH as a cofactor to reduce the Δ4,5 bonds of various substrates (8). There are two 5α-reductase genes, and their encoded proteins, designated type 1 and type 2, share approximately 50% sequence identity (9). The enzymes have divergent biochemical and pharmacological properties, and they exhibit different tissue-specific and cell type-specific expression patterns that reflect their biological roles. For example, the type 2 enzyme is expressed in the mesenchyme of the urogenital sinus and genital tubercle of the rat (10, 11), a cell type that plays an instructive role in the formation of the prostate and external genitalia (12). Many species, including humans, monkeys, dogs, rats, and mice, have two 5α-reductase genes, indicating that the duplication event that gave rise to the type 1 and 2 enzymes occurred early in evolution. The pan-specific distribution and duplication of the enzyme in mammals also underscores the need for DHT in androgen action.
The contributions of individual 5α-reductase enzymes to sexual differentiation and reproductive biology are being elucidated in part through analysis of mutations in the encoding genes. Naturally occurring mutations in the human 5α-reductase 2 gene cause male pseudohermaphroditism in which the Wolffian ducts virilize normally due to the actions of T, but the prostate and external genitalia fail to form in the absence of DHT (13). An induced mutation in the mouse 5α-reductase 1 gene constructed by homologous recombination in embryonic stem (ES) cells produced male mice that were indistinguishable from wild-type counterparts, presumably due to the presence of an active type 2 enzyme. In contrast, female mice that lacked the type 1 enzyme exhibited partially penetrant defects in parturition and fecundity (14–16). These results confirmed the idea that the two 5α-reductase enzymes have different endocrine roles and suggested that the type 1 enzyme may be of greater importance in the female and the type 2 enzyme of greater importance in the male (17).
In the current study we report the construction and analysis of mice that lack 5α-reductase 2. The absence of this enzyme has no effect in the female, but causes a mild virilization defect in the male, marked by a reduction in the size of the secondary sexual glands. This phenotype is far less severe than that observed in 5α-reductase 2-deficient human males, in which the enzyme’s absence leads to a failure of these tissues to differentiate from their respective anlagen. Crossing the type 2-deficient mice with the previously constructed type 1-deficient mice produced animals that lack all known 5α-reductase enzyme activity. The phenotype of female mice missing both enzymes is no more severe than that of mice without the type 1 enzyme. The modest virilization defect observed in type 2-deficient male mice is more pronounced in animals lacking both enzymes; however, the prostate, penis, and scrotum are male in character in these mice, and the animals are fertile. These findings indicate that T is sufficient for formation of the male phenotype in mice and suggest that the conversion of T to DHT represents a signal amplification mechanism.
Materials and Methods
Mice
Animals were housed under a 12-h light cycle (lights on, 0600–1800 h) at 22 C. All mice used in these studies were of mixed strain backgrounds (C57BL/6J//129Sv/Ev). 5α-Reductase type 2-deficient mice were propagated by matings between animals homozygous for the introduced null allele. Crossing type 2-deficient mice with type 1-deficient mice (14) produced double knockout animals, which were propagated by crossing females heterozygous for the type 1 mutation and homozygous for the type 2 mutation with males homozygous for both type 1 and 2 mutations. Breeding animals of these genotypes avoided the parturition and fecundity defects in females associated with loss of the 5α-reductase 1 gene (14) and produced 50% of offspring with the desired double knockout genotype. All studies were conducted in accordance with the standards of humane animal care described in the NIH Guide for the Care and Use of Laboratory Animals using protocols approved by an institutional animal care and research advisory committee.
ES cell work
A bacteriophage P1 clone encompassing the mouse 5α-reductase type 2 gene from the 129SvJ strain was isolated from a genomic DNA library (Genome Systems, St. Louis, MO). A targeting vector derived from the pPolIIshort-neobPA-HSVTK plasmid (18) was assembled using standard methods of genetic engineering and contained a 6.8-kb BglII fragment from the type 2 gene as a long arm and a 2.3-kb XhoI fragment from the gene as a short arm (Fig. 1A). The 6.8-kb DNA fragment spanned exons 3–5, and the corresponding intervening sequences and was generated by long PCR from the bacteriophage P1 clone (19). The 2.3-kb XhoI DNA fragment spanned a portion of intervening sequence 1 and was isolated directly from the recombinant bacteriophage P1. The 2.5 kb of DNA that normally separate these two fragments in the type 2 gene were replaced by a neomycin resistance cassette (Fig. 1A).
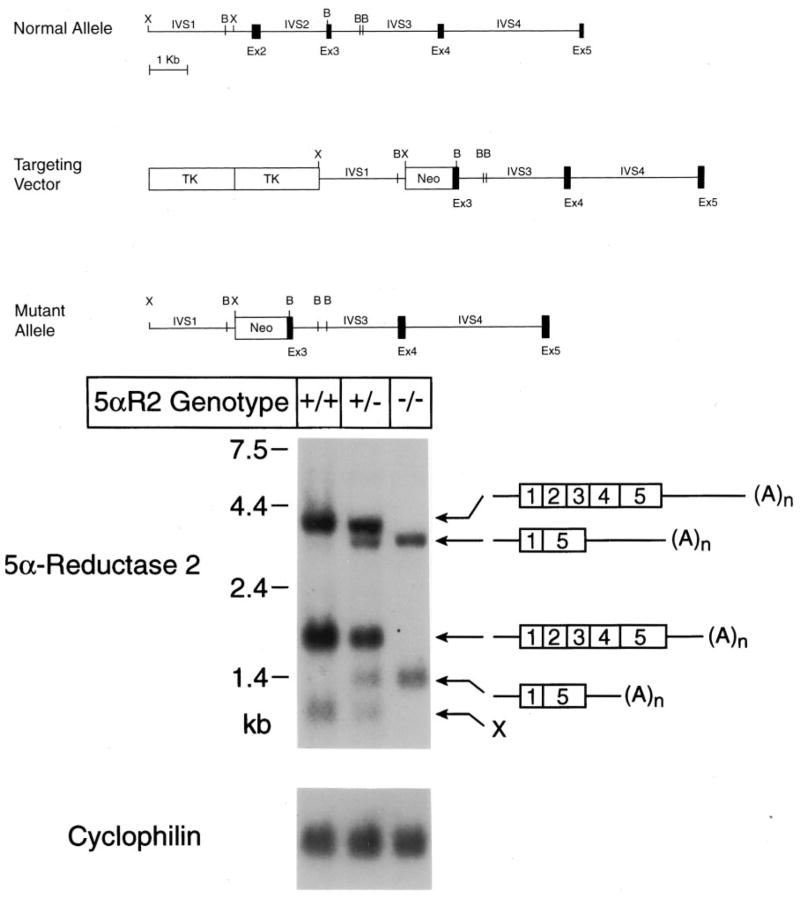
Fig. 1.
Mutation of mouse 5α-reductase 2 gene. A, Structures of the normal 5α-reductase type 2 gene, the targeting vector used to remove a portion of the gene extending from intervening sequence 1 through exon 3 and the mutant allele. The positions at which the restriction enzymes XhoI (X) and BamHI (B) cleave the DNAs are indicated. Exons (Ex) are indicated by black boxes, and intervening sequences (IVS) by connecting lines. Two viral thymidine kinase (TK) genes and a gene encoding neomycin resistance (Neo) are indicated by boxes, which are not drawn to scale. B, RNA blotting in wild-type and 5α-reductase type 2 knockout mice. Poly(A)+ RNA was isolated from the epididymes of animals (n = 2) of the indicated type 2 genotype. Aliquots (5 μg) of RNA were size-fractionated by agarose gel electrophoresis in the presence of glyoxal, transferred to a nylon membrane, and subjected to blot hybridization using a radiolabeled probe derived from exon 1 of the mouse type 2 gene. After washing, the filter was exposed to x-ray film for 18 h. The locations to which standards of known size migrated to in the gel are indicated on the left of the autoradiogram (upper panel). The exon structures of the 5α-reductase type 2 mRNAs deduced in RT-PCR experiments are shown on the right of the autoradiogram. The structure of the mRNA marked X was not elucidated. The filter was stripped of bound radioactivity by washing at high temperature and then subjected to a second round of hybridization using a radiolabeled probe made from a cyclophilin cDNA. As indicated by the autoradiogram shown in the lower panel, equal amounts of mRNA were analyzed in each lane.
Fifty micrograms of the targeting vector were linearized and electroporated into the I1C line of ES cells derived from 129Sv/Ev mice and grown under standard conditions (20). The transfected ES cells were subjected to positive selection with the antibiotic geneticin (G418; 190 μg/ml) and to negative selection with gancyclovir (2 μM). Clones bearing homologous recombination events were identified by Southern blotting (21). Six positive clones were identified of 600 screened, and 4 of these were injected into blastocysts derived from C57BL/6J mice. High percentage (>50%) male chimeras from 3 ES cell clones were crossed with female C57BL/6J mice to generate 2 independent lines of animals carrying the disrupted 5α-reductase 2 allele. The presence of the mutation in the offspring of chimeric males was detected by a PCR-based assay.
RNA blotting
Total RNA was prepared from tissues using RNA Stat 60 (Tel-Test B, Inc., Friendswood, TX). Polyadenylated [poly(A)+] RNA was purified by oligo(deoxythymidine)-cellulose chromatography using a kit (Pharmacia LKB, Piscataway, NJ). The purified RNA was size-fractionated by electrophoresis through 1.4% (wt/vol) agarose gels, transferred to nylon filters (Biotrans, ICN Biomedicals, Inc., Cleveland, OH) by capillary blotting, and subjected to blot hybridization using standard methods (19). A probe corresponding to exon 1 of the encoding gene was used to detect the 5α-reductase type 2 mRNA. cDNAs purchased from Incyte Genomics (St. Louis, MO) were used as hybridization probes in the experiments presented in Fig. 7.
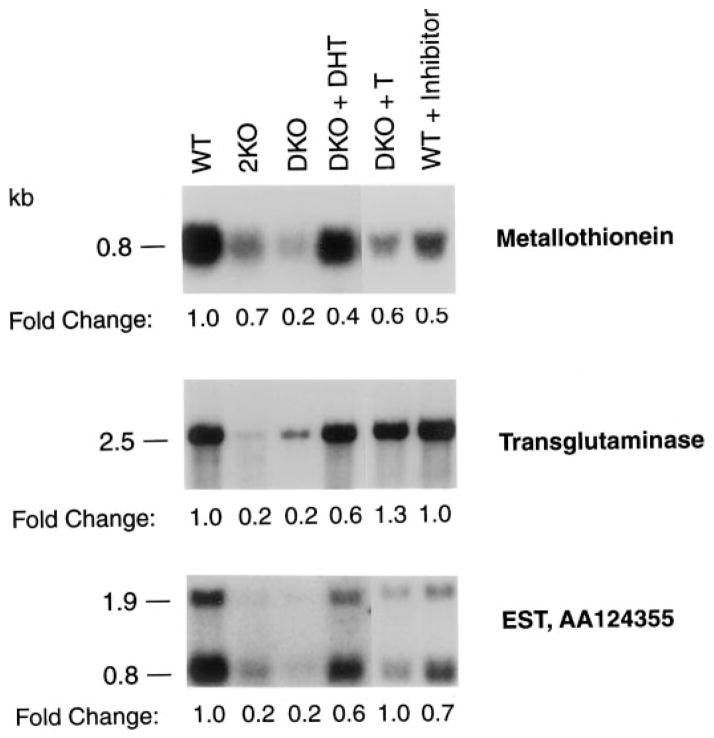
Fig. 7.
Gene expression in mice lacking 5α-reductase. Poly(A)+-enriched RNA was purified from the seminal vesicles/coagulating glands of 3-month-old male mice of the indicated 5α-reductase genotypes (WT, wild-type; 2KO, type 2 knockout; DKO, type 1 and 2 double knockout) and treatment groups (DKO + DHT, double knockout mice treated for 20 d with pellets containing DHT; DKO + T, double knockout mice treated for 20 d with pellets containing T; WT + inhibitor, wild-type mice treated for 9 d with the 5α-reductase inhibitor GI 208335X). Aliquots (5 μg) were separated by electrophoresis through agarose gels and then subjected to blot hybridization with probes derived from cDNAs encoding metallothionein 1 (top panel), transglutaminase (middle panel), and EST AA124355 (bottom panel). Exposure times for autoradiography were 6, 18, and 96 h, respectively, for the top, middle, and bottom panels. The sizes of the mRNAs detected with each probe are indicated on the left of the autoradiograms. The fold changes in mRNA levels between the various animal groups are indicated below the autoradiograms and were determined by scanning densitometry using signals from a cyclophilin cDNA probe as a loading control.
Serum hormone measurements
T, DHT, androstenedione, E2, 5α-androstan-3α,17β-diol glucuronide, and LH were measured in the sera of 3-month-old male mice by RIA (22). Blood was drawn from the inferior vena cava, cells were removed by centrifugation, and the resulting sera were stored at −20 C until analyzed. T, androstenedione, and E2 were extracted into ether, separated by chromatography on Sepharose LH-20, and subjected to RIA at the Oregon Regional Primate Research Center (Beaverton, OR). 5α-Androstan-3α,17β-diol glucuronide and DHT levels were measured using kits from Diagnostics Systems Laboratories, Inc. (Webster, TX). Dr. Terry Nett (Animal Reproduction and Biotechnology Laboratory, Colorado State University, Ft. Collins, CO) measured LH concentrations.
Tissue hormone measurements
Androstenedione and T levels were measured in the prostates and seminal vesicles of 3-month-old male mice. Prostates (5–15 mg) and seminal vesicles (50–150 mg) were dissected and stored at −80 C until analyzed. For extraction, the tissues were thawed, weighed on a microbalance, and homogenized in 1 ml PBS at 4 C using a Polytron (Brinkmann Instruments, Inc., Westbury, NY). Steroids were extracted into ether from the homogenates and subsequently measured as described above and previously (22).
Steroid 5α-reductase enzyme activity
Tissues were dissected from 3-month-old wild-type and knockout animals and homogenized in 10 mM potassium phosphate (pH 7.0), 150 mM potassium chloride, 0.3 M sucrose, and 1 mM EDTA. Protein concentrations were determined using a kit (Bio-Rad Laboratories, Inc., Hercules, CA). 5α-Reductase type 1 enzyme activity was assayed by incubating tissue homogenates (150 μg protein) in 0.1 M Tris-citrate buffer (pH 7.0) containing 5 μM [14C]T (NEN Life Science Products, Boston, MA) and 5 mM NADPH (Sigma, St. Louis, MO) in a total volume of 0.5 ml for 1 h at 37 C. 5α-Reductase type 2 enzyme activity was measured similarly, except that the pH of the 0.1 M Tris-citrate buffer was 5.0. In both assays, steroids were extracted into 5 ml methylene chloride at the end of the incubation period and taken to dryness under a stream of nitrogen. The resulting pellets were dissolved in 20 μl chloroform-methanol (2:1, vol/vol), spotted onto Silica Gel 150 TLC plates (catalogue no. 4855-82, Whatman, Clifton, NJ), and resolved by development in chloroform-ethylacetate (3:1, vol/vol). Radioactive steroids were detected by autoradiography using Kodak XAR-5 film (Eastman Kodak Co., Rochester, NY). Exposure times were 12–16 h. The sensitivity of the assay under these conditions was approximately 1 pmol/min·mg protein.
Animal studies
Castration was performed at 10–12 wk of age. Animals were anesthetized, the abdomen/scrotal area was cleaned, and a single incision was made. The testis, epididymes, and epididymal fat pad were removed after ligation of the spermatic vein. The incision was closed with sutures and staples. The animals were killed 9–10 d later, and tissue weights were determined after dissection.
Steroid hormones were administered by sc implantation of pellets containing T or DHT (5 mg, 21-d release; Innovative Research of America, Sarasota, FL). Pellets were inserted on the backs of 10-wk-old animals as previously described (14). Twenty days after pellet insertion, the animals were killed, and blood and tissues were harvested.
Inhibitor studies were performed using GI 208335X (N-[1-(4-trifluoromethylphenyl)-cycopentenyl]3-oxo-4-aza-5α-androst-1-ene-17β-carboxamide, empirical formula C31H39F3N2O2, Mr = 528), a compound that inhibits both 5α-reductase type 1 and 2 (obtained from Dr. Stephen Frye, GlaxoSmithKline, Research Triangle Park, NC). Adult male mice, 10–12 wk old, were injected sc with 10 mg/kg BW GI 208335X for 9 d. On d 10 of the experiment, the animals were killed, and blood and tissues were collected.
Morphometry
Semiquantitative morphometric analyses to determine the numbers of epithelial and stromal cells in androgen-dependent tissues were carried out as previously described (23). Briefly, tissues were dissected from wild-type, 5α-reductase 1 and 2 double knockout, and castrated wild-type mice; fixed in Bouin’s solution; sectioned at 5 μm; stained with hematoxylin and eosin; and examined by light microscopy (Eclipse 1000, Nikon, Melville, NY). The numbers of stromal cells, epithelial cells, and glandular lumens present in 20 grids from each tissue section were counted with the aid of the NIH Image version I.58 software program (http://rsb.info.nih.gov/nih-image/). These data were expressed as ratios of cell types to determine the effects of gene and organ loss on tissue architecture.
Assessment of reproductive function
Reproduction in knockout animals was assessed by harem mating experiments in which individual males of different 5α-reductase genotypes were housed with three wild-type C57BL/6J females from 0800–1300 h daily. At 1300 h females were examined for the presence of a copulatory plug in the vagina (Exp 1) or for the presence of sperm in the vagina by lavage (Exp 2). In Exp 1, wild-type (n = 3), 5α-reductase type 2 knockout (n = 3), and 5α-reductase type 1 and 2 double knockout (n = 3) males were used, and the experiment was continued over a 40-d period. The efficiencies of plugging were 47.5%, 19.2%, and 17.5% for wild-type, type 2-deficient, and type 1- and 2-deficient male animals, respectively. Females with plugs were removed from the cages on the day of insemination and replaced with fresh females over the course of the experiment. The frequency of pregnancies carried to term in the plugged females was 60%, 64%, and 51% in matings with wild-type, type 2-deficient or type 1- and 2-deficient males, respectively. The average litter sizes resulting from these pregnancies were 6.4, 7.5, and 6.6 pups, respectively.
In the second experiment wild-type (n = 3) and 5α-reductase type 1-and 2-deficient males (n = 3) were mated with three C57BL/6J females over a 23-d period. Daily lavages were performed to determine the presence of sperm in the vagina as an indicator of mating. In matings involving wild-type and double knockout males, the mating frequencies were 32% and 44%, the pregnancy rates were 96% and 60%, and the average litter sizes were eight and eight pups, respectively.
Sperm counts were measured by dissection of the vas and cauda epididymes into 1 ml PBS. Three small incisions were made in each fragment, and the tissues were gently massaged to release the sperm. The tissue and sperm were placed in a 5% CO2 incubator for 1 h to facilitate dispersal. Sperm counts were determined by light microscopy using a hemocytometer.
Microarray analysis
Total RNA was prepared from seminal vesicles/coagulating glands of wild-type and mutant mice by extraction with TRIzol (Life Technologies, Inc./BRL, Gaithersburg, MD) and checked for integrity by agarose gel electrophoresis. The RNA was shipped to Incyte Genomics (St. Louis, MO) and used to prepare poly(A)+ RNA, which was converted into fluorescent dye-labeled cDNA for use as hybridization probes with the mouse GEM1 microarray containing approximately 8000 cDNA clones. Differentially expressed cDNAs in the tissues of the wild-type and double knockout mice were identified, purchased as plasmid DNAs, and used in the RNA blotting experiments of Fig. 7.
Results
Mice deficient in 5α-reductase 2 were produced by homologous recombination in ES cells. Schematics of the normal allele, the targeting vector used for recombination, and the expected structure of the mutant allele are shown in Fig. 1A. The targeting vector was designed to inactivate the gene by replacing a DNA segment encompassing a portion of intron 1 through a portion of exon 3 with a bacterial gene encoding neomycin resistance. ES cells with the desired recombination event were detected at a frequency of 1% after electroporation and plus-minus selection, and of these, four were injected into blastocysts to produce three lines of chimeric male mice. Two lines transmitted the mutation to their offspring, as judged by PCR- and Southern blotting-based genotyping assays. Mice from these lines exhibited the same phenotype and were used interchangeably in the studies described below.
Blotting experiments were performed to assess the effects of the introduced mutation on 5α-reductase 2 mRNA levels. As indicated by the data in Fig. 1B, three mRNAs of 4.2, 2.0, and 1.2 kb were detected in the epididymis of wild-type mice. In animals heterozygous for the deletion allele, these same three mRNAs were present together with two additional species of 4.0 and 1.4 kb, whereas animals homozygous for the mutation had only the latter two mRNAs. RT-PCR and DNA-sequencing experiments showed that the aberrant mRNAs associated with the deletion allele contained exon 1 spliced to exon 5. The size variation in mRNAs containing this arrangement of exons arose from the use of different polyadenylation sequences located within the 3′-flanking region of the gene (see schematics of Fig. 1B). The translational reading frame of exon 1 is not in phase with that of exon 5; thus, the mRNAs transcribed from the deletion allele were predicted to encode a truncated and inactive 5α-reductase 2 enzyme of 116 amino acids. To ensure that the induced mutation eliminated enzyme activity, a truncated cDNA encoding the first 116 amino acids of the protein was generated and introduced into cultured human embryonic kidney 293 cells by transfection. Assay of the transfected cells using [14C]T substrate revealed that the truncated protein had no measurable 5α-reductase enzyme activity (data not shown).
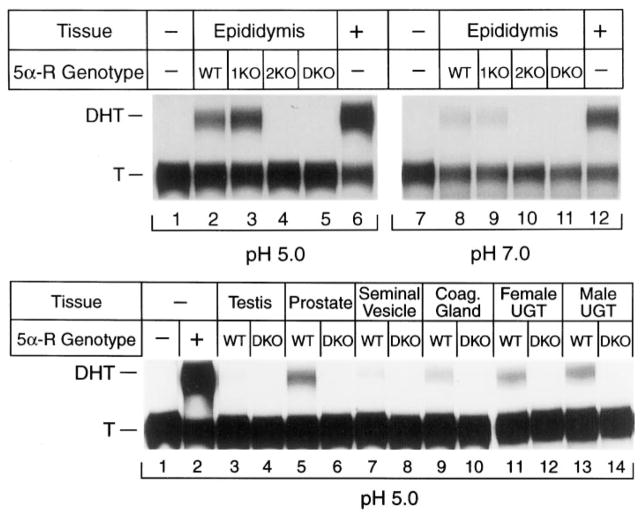
To confirm that the introduced mutation led to loss of enzyme activity in vivo, extracts were prepared from the epididymes of adult male mice with different 5α-reductase genotypes and assayed for activity (Fig. 2). This tissue is a particularly rich source of 5α-reductase 2 in the mouse (14), and the contributions of each enzyme to total activity can be partially distinguished by carrying out assays in buffers of different pH (24, 25). Extracts from wild-type mice converted T into DHT at both an acidic pH (type 2 enzyme activity; Fig. 2A, lane 2) and a neutral pH (type 1 enzyme activity; Fig. 2A, lane 8). In contrast, extracts from mice with a mutation in the type 1 gene exhibited normal or slightly elevated levels of 5α-reductase activity at pH 5.0 (lane 3) and reduced activity at pH 7.0 (lane 9). Mice with a mutation in the type 2 gene had a marked reduction in activity at pH 5.0 (lane 4) and a decrease at pH 7.0 (lane 10). The latter result indicated that approximately 50% of the activity detected at pH 7.0 was due to the type 2 enzyme. Extracts from mice lacking both 5α-reductase 1 and 2 exhibited no 5α-reductase enzyme activity at either pH (lanes 5 and 11). In a similar series of experiments, no 5α-reductase enzyme activity was detected at acidic (Fig. 2B) or neutral (data not shown) pH in extracts from double knockout mice prepared from adult testis, prostate, seminal vesicle, or coagulating gland or male and female embryonic d 18 urogenital tracts.
Fig. 2.
5α-Reductase enzyme activity in wild-type and knockout mice. A and B, Extracts were prepared from different adult (3 months of age) or fetal (embryonic d 18) tissues of the indicated 5α-reductase genotypes (WT, wild-type; 1KO, type 1 knockout; 2KO, type 2 knockout; DKO, type 1 and 2 double knockout). Aliquots containing 150 μg protein were incubated for 60–120 min at 37 C in the presence of 5.0 μM [14C]T and 5 mM NADPH in buffers of the indicated pH. 5α-Reductase type 1 activity predominates at pH 7.0, whereas type 2 activity predominates at pH 5.0 (24, 25). Steroids were isolated by extraction with organic solvents, separated by TLC, and detected by autoradiography. The positions to which T substrate and DHT product migrated to on the plates are indicated on the left. Lanes are numbered below the autoradiograms. UGT, Urogenital tract.
Type 2-deficient and double knockout male mice born to mothers of varying 5α-reductase genotypes were grossly normal. The anogenital distances of newborn animals harboring the introduced mutations were not different from those of controls, they did not exhibit nipple formation, and the animals appeared to undergo puberty within the same time period as normal males based on the onset of mating at 6–8 wk of age.
Genetic deficiencies in 5α-reductase did alter steroid hormone levels in the sera of 3-month-old male mice (Table 1). T levels increased in both the type 2-deficient and double knockout animals; however, the observed differences did not reach statistical significance. DHT levels decreased in double knockout mice to below the detectable range of the RIA (Table 1). Androstenedione levels were increased in the type 2-deficient mice, but not to statistical significance, and were significantly increased in double knockout mice (P = 0.03; Table 1). E2 and LH levels were unchanged between wild-type and knockout mice, and in agreement with the latter data, no changes were detected in pituitary levels of LH α-or β-subunit mRNAs or proteins (data not shown). Finally, the levels of 5α-androstan-3α,17β-diol glucuronide, a DHT metabolite, were significantly reduced in both type 2-deficient and double knockout mice (P = 0.001; Table 1).
TABLE 1.
Steroid hormone levels in sera of 3-month-old male mice
| 5α-Reductase genotypea | T (ng/ml) | DHT (pg/ml)b | Androstenedione (ng/ml) | E2 (pg/ml) | Adiol (ng/ml)c | LH (ng/ml) |
|---|---|---|---|---|---|---|
| Wild type | 2.3 ± 0.6 (38)d | 78.3 ± 15.4 (11) | 0.090 ± 0.020 (38) | 6 ± 0.6 × 10−3 (16) | 0.6 ± 0.06 (11) | 2.6 ± 0.8 (11) |
| 2KO | 3.9 ± 1.1 (37) | Not determined | 0.144 ± 0.024 (37) | 6 ± 1.2 × 10−3 (12) | 0.1 ± 0.02e (10) | 4.4 ± 1.2 (13) |
| P | 0.001 | |||||
| DKO | 3.6 ± 0.8 (36) | 18.4 ± 4.4e (13) | 0.165 ± 0.027e (36) | 6 ± 1.0 × 10−3 (12) | 0.1 ± 0.03e | 2.7 ± 0.9 (14) |
| P | 0.003 | 0.03 | 0.001 |
Steroid hormone levels were measured by RIA in the sera of individual animals, and the mean and SEM were calculated for each genotype.
2KO, 5α-Reductase type 2 knockout mice; DKO, 5α-reductase types 1 and 2 double knockout mice.
The limit of detection in this assay was 25 ng/ml or more.
Adiol, 5α-Androstan-3α,17β-diol-glucuronide. The limit of detection in this assay was 0.14 ng/ml.
The number of animals assayed is in parentheses.
Significantly different from wild type, with P values as indicated.
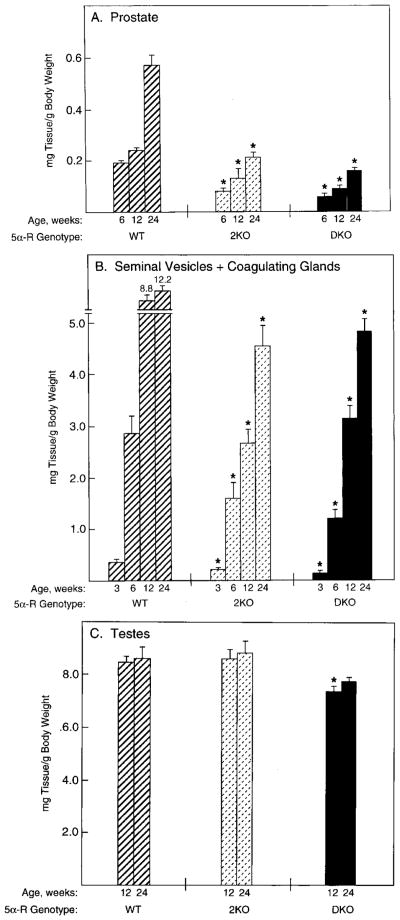
Mutation of the 5α-reductase type 2 gene or of both the type 1 and type 2 genes caused a reduction in the weights of androgen-dependent tissues. The prostates of wild-type mice increased in weight from about 0.2 to 0.6 mg/g BW between postnatal wk 6 and 24 (Fig. 3A). This trend was also evident in mice lacking the type 2 gene and in the double knockout animals; however, in animals of these genotypes the tissues were less than 50% of the weights of age-matched wild-type controls (Fig. 3A). Similar decreases in the weights of the seminal vesicles and coagulating glands were observed in the knockout vs. wild-type animals during postnatal wk 3–24 (Fig. 3B). The weights of the testes were not different between wild-type and type 2-deficient mice, whereas those of the double knockout mice were slightly smaller (Fig. 3C). Semiquantitative morphometric analyses (23) of the prostates of the type 2-deficient and double knockout mice revealed that the decrease in weight was due to cellular hypotrophy rather than hypoplasia. The stromal to epithelial cell ratio in wild-type mice (n = 3) was 1.1, and those in the type 2-deficient (n = 4) and double knockout mice (n = 4) were 0.73 and 0.75, respectively. In a control experiment the ratio of stromal to epithelial cells increased to 2.3 in the prostates of wild-type mice castrated 14 d before tissue harvest.
Fig. 3.
Androgen-responsive tissue weights in wild-type and knockout mice. Tissues were dissected from male mice of the indicated ages and 5α-reductase genotypes (WT, wild-type; 2KO, type 2 knockout; DKO, type 1 and 2 double knockout) and weighed. Plotted values are expressed as milligrams of tissue per g BW and represent the mean ± SEM of values obtained from 7–12 animals for each time point and genotype. Asterisks above the histogram bars indicate organ weights that were significantly different (P ≤ 0.05) from those of wild-type mice. A, Prostate; B, seminal vesicles plus coagulating glands; C, testes.
The androgen contents of several reproductive tissues were assessed by RIA. The prostates of wild-type mice contained low levels of androstenedione and T (Table 2). In contrast, these steroids accumulated to markedly high levels in the prostates of type 2-deficient and double knockout mice. Large increases in androstenedione and T also were detected in the seminal vesicles and coagulating glands of the mutant mice (Table 2). These data and those presented in Fig. 3 indicated that mice deficient in 5α-reductase type 2 or both type 1 and type 2 had smaller prostates, seminal vesicles, and coagulating glands despite prominent elevations in the tissue content of T.
TABLE 2.
Steroid hormone levels in tissues of 3-month-old male mice
| Tissue | na | 5α-Reductase genotypeb | Androstenedione (ng/g) | T (ng/g) |
|---|---|---|---|---|
| Prostate | 12 | Wild-type | 0.8 ± 0.1 | 0.2 ± 0.1 |
| 9 | 2KO | 6.6 ± 1.2c | 47.4 ± 12d | |
| 10 | DKO | 5.7 ± 1.0c | 47.7 ± 13.7e | |
| Seminal vesicle | 12 | Wild-type | 0.2 ± 0.04 | 0.1 ± 0.03 |
| 9 | 2KO | 0.5 ± 0.2 | 5.6 ± 1.7f | |
| 10 | DKO | 0.8 ± 0.2c | 12.3 ± 1.9g | |
| Coagulating gland | 12 | Wild-type | 0.2 ± 0.03 | 0.5 ± 0.2 |
| 9 | 2KO | 1.5 ± 0.4h | 9.9 ± 3.2i | |
| 10 | DKO | 2.8 ± 0.7e | 20.4 ± 3.8j |
Tissues were dissected, and steroids were purified by extraction with organic solvents. Steroid hormone measurements were made in individual tissues by RIA, and the mean and SEM were calculated.
Number of animals in which measurements were made.
2KO, 5α-Reductase type 2 knockout mice; DKO, 5α-reductase type 1 and 2 double knockout mice.
P = 0.001 vs. wild-type.
P = 0.004 vs. wild-type.
P = 0.007 vs. wild-type.
P = 0.011 vs. wild-type.
P = 0.0001 vs. wild-type.
P = 0.012 vs. wild-type.
P = 0.017 vs. wild-type.
P = 0.005 vs. wild-type.
Loss of 5α-reductase type 2 or of both the type 1 and 2 enzymes had effects on male fertility and fecundity (26, 27). For example, in a harem mating experiment in which formation of a vaginal plug was monitored as an end point, double knockout males plugged 18% of wild-type females compared with a 47.5% efficiency measured for wild-type males. When the presence of sperm in the vagina was monitored in a second experiment as an indication of mating, double knockout males impregnated 44% of wild-type females. The increase in apparent mating frequency detected in the latter assay was probably a consequence of the smaller secondary sexual organs in the knockout mice. These would be expected to secrete less seminal fluid upon ejaculation, which, in turn, would decrease the formation of copulatory plugs.
In agreement with the mating frequency data, sperm counts in double knockout mice (17.9 ± 8.8 × 106; n = 11) were not statistically different from those in wild-type controls (25 ± 8.5 × 106; n = 8). The data in Table 3 indicated that the litter sizes fathered by type 2-deficient or type 1- and 2-deficient male mice were no different from those of wild-type males. Loss of the type 1 enzyme in females caused a marked decrease in litter size, as expected from previous studies (15); however, loss of the type 2 enzyme did not affect this parameter, and the fecundity of double knockout females was not lower than that of type 1-deficient animals (Table 3).
TABLE 3.
Effect of 5α-reductase mutations on fecundity
| Male 5α-reductase genotype
|
Female 5α-reductase genotype
|
No. of matings | Parturition frequency (%) | Litter size | ||
|---|---|---|---|---|---|---|
| Type 1 | Type 2 | Type 1 | Type 2 | |||
| +/+ | +/+ | +/+ | +/+ | 30 | 100 | 7.3 |
| +/+ | −/− | +/+ | +/− | 7 | 100 | 7.1 |
| +/+ | +/− | +/+ | −/− | 7 | 100 | 7.6 |
| +/+ | −/− | +/+ | −/− | 47 | 100 | 6.8 |
| −/− | −/− | +/− | −/− | 24 | 100 | 6.7 |
| +/+ | +/+ | −/− | −/− | 18 | 50 | 2.9 |
| −/− | −/− | −/− | −/− | 20 | 40 | 2.3 |
Male and female animals of the indicated 5α-reductase 1 and 2 genotypes were mated, and the numbers of successful deliveries and individual litter sizes were determined. These data were used to calculate the parturition frequency, which is defined as the percentage of animals delivering on gestation d 19.5, and the mean litter size, which is defined as the number of live pups delivered per mother on gestation d 19.5.
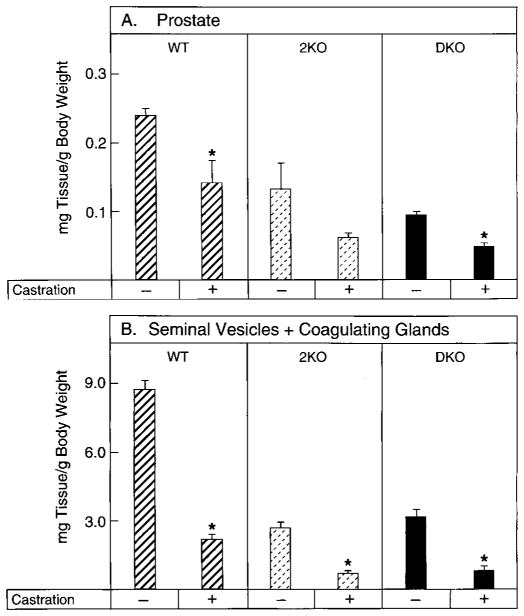
To examine the contribution of testicular androgens to the growth of the male secondary sexual organs, mice of various 5α-reductase genotypes were castrated, and the weights of the prostate and seminal vesicle/coagulating gland were measured. In wild-type mice, orchidectomy caused statistically significant declines in the weights of the prostate (Fig. 4A) and seminal vesicle/coagulating gland (Fig. 4B). In mice without the type 2 enzyme or those lacking the type 1 and 2 enzymes, removal of the testes produced a further decline in the weights of both tissues (Fig. 4, A and B). These data suggested that the internal genitalia of 5α-reductase knockout mice remained responsive to T.
Fig. 4.
Effects of castration on androgen-responsive tissue weights in wild-type and 5α-reductase knockout mice. Male mice, 2.5–3.0 months of age and of the indicated 5α-reductase genotypes (WT, wild-type; 2KO, type 2 knockout; DKO, type 1 and 2 double knockout), were subjected to sham operation (−) or castration (± ) and then maintained for 9–10 d. Tissues were thereafter dissected and weighed. Plotted values are expressed as milligrams of tissue per g BW and represent the mean ± SEM of values obtained from five or six animals for each surgical group. Asterisks above the histogram bars indicate castrate organ weights that were significantly different (P ≤ 0.05) from those of sham-operated mice. A, Prostate; B, seminal vesicles plus coagulating glands.
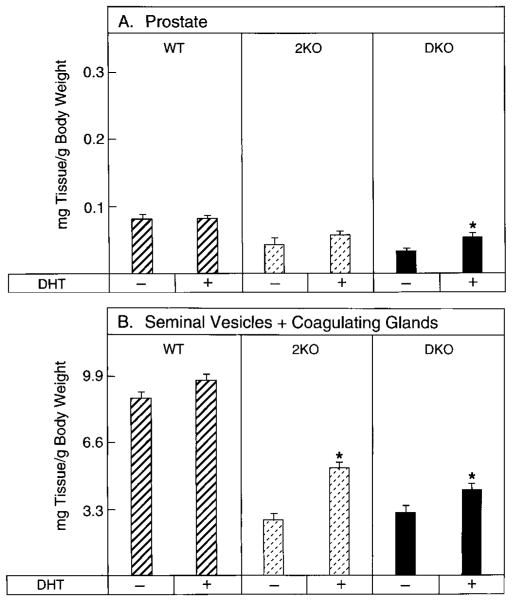
The data in Fig. 5 indicated that these tissues also retained their sensitivity to DHT in intact animals. Administration of this androgen increased prostatic weight by 22% in the type 2-deficient mice and by 41% in the double knockout animals and increased seminal vesicle/coagulating gland weights by 50% and 27%, respectively. Exogenous DHT did not increase tissue weights in wild-type mice (Fig. 5), presumably due to the presence of maximum hormone levels in these tissues.
FIG. 5.
Effects of DHT administration on androgen-responsive tissue weights in wild-type and 5α-reductase knockout mice. Time release pellets containing vehicle alone (−) or DHT (+) were implanted sc in male mice, 2.5 months of age and of the indicated 5α-reductase genotypes (WT, wild-type; 2KO, type 2 knockout; DKO, type 1 and 2 double knockout). After 20 d, tissues were dissected and weighed. Plotted values are expressed as milligrams of tissue per g BW and represent the mean ± SEM of values obtained from 9–13 animals for each treatment group. Asterisks above the histogram bars indicate organ weights that were significantly different (P ≤ 0.05) between the groups. A, Prostate; B, seminal vesicles plus coagulating glands.
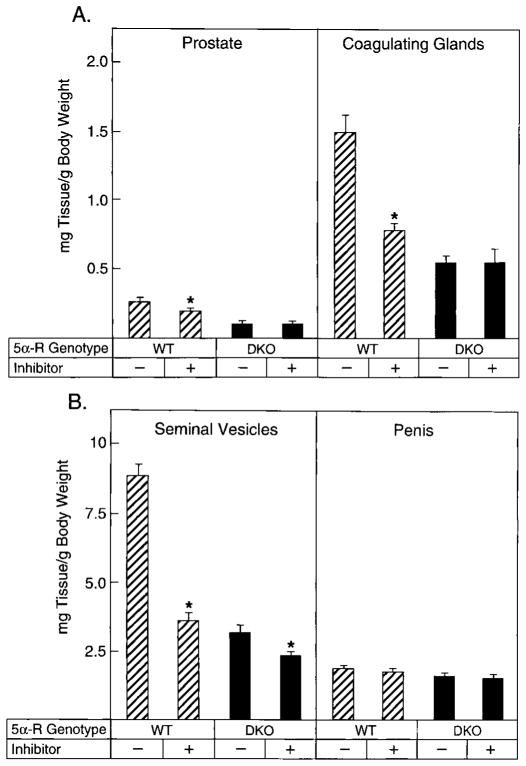
Many 5α-reductase inhibitors have been identified, and in several instances these have been used to produce a pharmacological knockout of the enzyme (28). To establish the effects of one of these inhibitors in adult wild-type and 5α-reductase deficient mice, animals (n = 7–9/genotype) were treated with GI 208335X. This compound inhibits both 5α-reductase type 1 and type 2 when delivered sc to mice (Frye, S., personal communication). As shown by the data in Fig. 6A, GI 208335X treatment of 2.5-month-old wild-type males for 9 d caused a 25% decrease in the weight of the prostate. In contrast, the drug did not affect prostatic weights in animals lacking both 5α-reductase type 1 and 2. Similar findings were obtained when the coagulating glands were examined; the drug caused a 45% decrease in the weight of this gland in wild-type mice, but had no consequences in the double knockout mice (Fig. 6A). The seminal vesicles of animals of both genotypes decreased in weight with inhibitor treatment; however, the decline observed in the wild-type animals (60%) was larger than that observed in the double knockouts (28%; Fig. 6B). Penis weights did not change in animals of either genotype (Fig. 6B).
Fig. 6.
Effect of GI 208335X administration on androgen-responsive tissue weights in wild-type and 5α-reductase knockout mice. Male animals, 2.5 months of age and of the indicated 5α-reductase genotypes (WT, wild-type; DKO, type 1 and 2 double knockout), were administered vehicle alone (−) or GI 208335X (+) as described in Materials and Methods. The GI compound inhibits both 5α-reductase enzymes. After 9 d, tissues were dissected and weighed. Plotted values are expressed as milligrams of tissue per g BW and represent the mean ± SEM of values obtained from seven to nine animals for each treatment group. Asterisks above the histogram bars indicate organ weights that were significantly different (P ≤ 0.05) between the groups. A, Prostate, coagulating glands; B, seminal vesicles, penis.
The consequences for gene expression brought on by loss of 5α-reductase were assessed in the seminal vesicle/coagulating gland by cDNA microarray technology. RNA was prepared from pooled glands dissected from wild-type (n = 2) or double knockout (n = 2) animals and converted into cDNA in the presence of a deoxynucleoside triphosphate derivatized with a fluorescent dye. Different dyes were used to label the wild-type and knockout cDNA probes, which were subsequently hybridized to a commercially available microarray containing about 8000 mouse cDNAs. Changes in gene expression were measured by fluorescence spectroscopy, and the data were organized according to rank order differences in the levels of a given mRNA. In this sample of genes, over 20 mRNAs were found to differ by more than 2-fold between mice of different genotypes. Among the differentially expressed mRNAs were those encoding metallothionein, transglutaminase, and EST AA124355, which, as indicated by the RNA blotting data shown in Fig. 7, were expressed at 5-fold higher levels in wild-type mice than in the double knockout mice. The metallothionein and transglutaminase genes are known to be regulated by androgen (29, 30). These androgen-dependent mRNAs were also present at lower levels in mice lacking just the type 2 enzyme (Fig. 7).
To determine whether androgens would restore the expression levels of these genes in the mutant mice to those observed in wild-type mice, double knockout animals (n = 7–9/genotype) were treated for 21 d with T or DHT, and changes in each mRNA were measured by RNA blotting. T administration increased the level of metallothionein mRNA to approximately 60% of that in wild-type mice, whereas the levels of the transglutaminase and EST AA124355 mRNAs were increased to 130% and 100%, respectively, of those found in the controls (Fig. 7). DHT also increased the amount of each mRNA, but only to levels that were 40–60% of those in wild-type mice (Fig. 7). These data confirmed the androgen responsiveness of the three genes and suggested that they were sensitive to T and DHT. This conclusion was supported by the finding that treatment of wild-type mice with the GI 208335X inhibitor decreased levels of the metallothionein and EST AA 124355 mRNAs, but did not alter transglutaminase mRNA levels (Fig. 7).
Discussion
We describe the consequences arising from loss of 5α-reductase gene expression in mice. The most surprising findings are that males lacking 5α-reductase type 2 or both 5α-reductase type 1 and type 2 have properly formed internal and external genitalia and are fertile. These outcomes are in marked contrast to the situation in the human male, in whom mutations in the 5α-reductase type 2 gene severely impede the formation of the prostate and external genitalia, and they suggest that the urogenital tract of the mouse requires only T for the differentiation of these tissues. The requirement for DHT cannot be completely dispensed with in the mouse, in that animals without 5α-reductase have smaller prostates and seminal vesicles, and the expression of androgen-responsive genes is decreased in these tissues. The hypotrophy of the secondary sexual glands and the alterations in gene expression occur despite the accumulation of T in the tissues, which further underscores the potency of DHT and the need for signal amplification.
Loss of the 5α-reductase type 2 gene in female mice did not produce adverse consequences, suggesting that this enzyme is not required for normal endocrine function in this sex. The fecundity and parturition defects observed in type 1-deficient mice (14, 15) were not worsened in the double knockout females, nor were new phenotypes evident. These findings support the hypothesis that the type 1 enzyme has evolved to play an essential role in the female, whereas the type 2 enzyme subserves in the male (17).
A simple explanation for the unexpected virilization observed in the knockout male mice would be the existence of a third 5α-reductase. A putative type 3 enzyme might be unique to the mouse, thus explaining the phenotypic differences observed between this and other species upon loss of 5α-reductase. We were, however, unable to detect 5α-reductase enzyme activity in multiple tissues from adult, fetal, and newborn double knockout mice under different assay conditions using a high specific activity T substrate. Expression cloning experiments in which 600,000 cDNA clones were screened from a liver library constructed from a 3-month-old male double knockout mouse also failed to yield a third form of the enzyme. Finally, an inhibitor of the type 1 and 2 enzymes (GI 208335X) reduced the sizes of androgen-dependent tissues in wild-type mice, but had little or no effect in double knockout animals. We thus can offer no experimental evidence in support of a third 5α-reductase enzyme.
DHT levels assessed by RIA in the sera of 5α-reductase knockout animals were reduced to below the reliable level of detection of the assay. Any residual amount of DHT may be due to cross-reaction between the antibody employed and one or more steroid metabolites that are elevated in the mutant mice. The amounts of C19 and C21 steroids with 5β stereochemistry are increased in patients with 5α-reductase 2 deficiency (4, 31–33), and it seems likely that the same is true in the knockout mice. RIA measurements of 5α-androstan-3α,17β-diol glucuronide levels revealed that this metabolite was also present at background levels in the sera of type 2-deficient and type 1- and 2-deficient mice. This steroid is produced from DHT by reductive 3α-hydroxysteroid dehydrogenases, and its presence in the plasma of humans is directly proportional to the level of DHT (34–36). Inasmuch as these findings translate to the mouse, the absence of 5α-androstan-3α,17β-diol glucuronide from the knockout animals suggests that circulating levels of DHT are, in fact, very low or absent. The steroid metabolites that accumulate in the mutant mice remain to be determined by chemical analysis.
There are two papers in the literature that report the consequences of treating mice with 5α-reductase inhibitors (37, 38). In one, the compound 6-methylene-4-pregnene-3,20-dione was administered to pregnant female mice of the ICR/JCL strain at 400 mg/kg BW from d 12–19 of gestation (37). This regimen decreased the anogenital distance in postnatal d 1 male offspring, caused hypospadiac urethras, decreased the number of prostatic buds formed along the urogenital sinus, promoted nipple formation, and decreased the epithelial volumes of the prostatic buds and coagulating glands. These abnormalities were not present in 3-month-old animals delivered from dams treated with the drug in utero, and these adults were fertile. In contrast, 129Sv/Ev/C57BL/6J mixed strain newborn male mice lacking 5α-reductase type 1 and 2 and born to double knockout mothers do not have reductions in their anogenital distances, do not have nipples, and have properly formed penile urethras. In a second published study, administration of finasteride at doses ranging from 25–250 mg/kg·d to adult CD-1 male mice caused Leydig cell hyperplasia and adenomas and increased plasma LH levels (38). Again, in contrast to these results, the double knockout mice lacking 5α-reductase studied here do not show these symptoms. We do not know whether the differences between the inhibitor-treated and knockout mice are due to the use of different mouse strains in the experiments, to possible androgenic or antiandrogenic effects of the inhibitors, or to other explanations.
In contrast to these results, extensive data are available from studies with humans and rats treated with 5α-reductase inhibitors (28), and there is a good correlation between many of these findings and those reported here for 5α-reductase knockout mice. For example, inhibition of 5α-reductase in man causes a decrease in prostatic DHT and a corresponding increase in T (39), as seen in 5α-reductase knockout mice. Treatment of male rats with 5α-reductase inhibitors causes a decrease in copulatory plug formation (26, 27), and the same phenomenon is observed in the knockout mice together with a decrease in the mRNA encoding transglutaminase, an enzyme associated with plug formation (40). These similarities suggest that the phenotypes of the knockout mice may accurately reflect the endocrine roles of 5α-reductase and DHT in this species.
With these considerations in mind, T alone appears sufficient for formation of the urogenital tract in the mouse. The involvement of a single androgen for phenotypic sexual differentiation has not been observed previously in several species, including humans, in whom mutation of the 5α-reductase type 2 gene causes male pseudohermaphroditism (13), or rats and tammar wallabies, which when given inhibitors of 5α-reductase develop a phenotype similar to that of 5α-reductase 2-deficient men (5, 7, 41, 42). Although there are differences between these species in the biosynthetic origins of DHT, the absolute requirement for the hormone is inviolate. For example, in rats, rabbits, and humans, T is synthesized by the fetal testes, secreted into the circulation, and then converted to DHT by 5α-reductase in target tissues (43, 44). In contrast, 5α-androstan-3α,17β-diol is the secreted testicular androgen in the wallaby, which is then converted back to DHT by oxidative 3α-hydroxysteroid dehydrogenases in the prostate and other target tissues (45).
Several hypotheses have been put forth to explain why two androgens are needed for formation of the male phenotype. First, different classes of androgen-responsive genes may exist, including those that respond solely to T or DHT or equally well to either androgen. Unique transcriptional responses could be mediated at the DNA level by the existence of androgen response elements of different sequences or contexts that are recognized by particular AR-ligand complexes. Alternatively, a ligand-dependent response could be mediated at the protein level by receptor-associated chaperones (46) or by the recruitment of different coactivators to a target gene by receptors activated by one or the other androgen. Despite the attractiveness of these hypotheses, there is presently little convincing evidence to support distinct gene networks or cofactors, and differential hybridization experiments in the 5α-reductase double knockout mice have to date failed to reveal genes with an absolute dependence on DHT.
A second hypothesis to explain the requirement for two androgens, and one that is endorsed by the data presented here, invokes a need for signal amplification in some target tissues. The requirement for a stronger signal may arise in a tissue due to poor vascularization and hence diminished access to hormone, to the presence of high levels of catabolic enzymes that specifically degrade one or the other androgen (47), or to the expression of receptor-binding proteins that destabilize a particular liganded complex (46). The signal amplification hypothesis is supported by biochemical studies that document a longer half-life and a higher DNA binding affinity for the human DHT-AR complex vs. the T complex (48–52). As a consequence of these properties, the effective dose of DHT required to activate an androgen-responsive marker gene by 50% is about 10-fold lower than that required to achieve the same level of induction with T. At higher hormone concentrations, however, the extents of activation achieved with both androgens are the same (49).
With respect to the virilized phenotypes of 5α-reductase knockout mice, T levels in the androgen target tissues of adult animals were 20–240 times higher than those measured in wild-type controls. If T also accumulates to this extent in fetal tissues, then this buildup would diminish or abolish the need for DHT and, hence, signal amplification. The accumulation of T to such high levels distinguishes the mouse from other species, in that dogs treated with a 5α-reductase inhibitor accumulate androstenedione, but not T, in androgen target tissues (53), and T accumulates in the prostates of inhibitor-treated humans to levels that are only 2–11 times higher than those in untreated tissues (39). T may accumulate in mouse tissues due to low levels of oxidative 17β-hydroxysteroid dehydrogenase enzyme activity (54–56), which would otherwise convert this active androgen into the far less potent steroid androstenedione, as happens in the dog (53). This interpretation supports the proposed important role of 17β-hydroxysteroid dehydrogenases in regulating tissue levels of active and inactive steroid hormones (57, 58) and, we believe, may also explain the unexpected virilization of double knockout mice.
Acknowledgments
We thank Jean Wilson for advice and critical reading of the manuscript, Bob Hammer and Kathy Graves for assistance with constructing knockout mice, Dolores Vazquez and John McConnell for assistance in morphometric analyses, Kevin Anderson for maintaining the mouse colony, Terry Nett for performing gonadotropin assays, Greg Netune for RT-PCR assays, Stephen Frye for advice and 5α-reductase inhibitors, and an anonymous reviewer for suggestions concerning the measurement of steroid hormones by RIA.
This work was supported by NIH Grants HD-38127 (to D.W.R.) and RR-00163 (to D.L.H.) and the Burroughs Wellcome Fund (0203, to M.S.M.).
Abbreviations
- ES
Embryonic stem
- poly(A)+
polyadenylated
References
- 1.Wilson JD. Metabolism of testicular androgens. Handb Physiol. 1975;5:491–508. [Google Scholar]
- 2.Andersson S, Russell DW, Wilson JD. 17β-Hydroxysteroid dehydrogenase 3 deficiency. Trends Endocrinol Metab. 1996;7:121–126. doi: 10.1016/1043-2760(96)00034-3. [DOI] [PubMed] [Google Scholar]
- 3.Walsh PC, Madden JD, Harrod MJ, Goldstein JL, MacDonald PC, Wilson JD. Familial incomplete male pseudohermaphroditism, type 2. Decreased dihydrotestosterone formation in pseudovaginal perineoscrotal hypospadias. N Engl J Med. 1974;291:944–949. doi: 10.1056/NEJM197410312911806. [DOI] [PubMed] [Google Scholar]
- 4.Imperato-McGinley J, Guerrero JL, Gautier T, Peterson RE. Steroid 5α-reductase deficiency in man: an inherited form of male pseudohermaphroditism. Science. 1974;186:1213–1215. doi: 10.1126/science.186.4170.1213. [DOI] [PubMed] [Google Scholar]
- 5.Imperato-McGinley J, Binienda Z, Arthur A, Mininberg DT, Vaughan ED, Jr, Quimby FW. The development of a male pseudohermaphroditic rat using an inhibitor of the enzyme 5α-reductase. Endocrinology. 1985;16:807–812. doi: 10.1210/endo-116-2-807. [DOI] [PubMed] [Google Scholar]
- 6.George FW, Johnson L, Wilson JD. The effect of a 5α-reductase inhibitor on androgen physiology in the immature male rat. Endocrinology. 1989;125:2434–2438. doi: 10.1210/endo-125-5-2434. [DOI] [PubMed] [Google Scholar]
- 7.Anderson CA, Clark RL. External genitalia of the rat: normal development and the histogenesis of 5α-reductase inhibitor-induced abnormalities. Teratology. 1990;42:483–496. doi: 10.1002/tera.1420420505. [DOI] [PubMed] [Google Scholar]
- 8.Bruchovsky N, Wilson JD. The conversion of testosterone to 5α-androstan-17β-ol-3-one by rat prostate in vivo and in vitro. J Biol Chem. 1968;243:2012–2021. [PubMed] [Google Scholar]
- 9.Russell DW, Wilson JD. Steroid 5α-reductase: two genes/two enzymes. Annu Rev Biochem. 1994;63:25–61. doi: 10.1146/annurev.bi.63.070194.000325. [DOI] [PubMed] [Google Scholar]
- 10.Berman DM, Tian H, Russell DW. Expression and regulation of steroid 5α-reductase in the urogenital tract of the fetal rat. Mol Endocrinol. 1995;9:1561–1570. doi: 10.1210/mend.9.11.8584033. [DOI] [PubMed] [Google Scholar]
- 11.Tian H, Russell DW. Expression and regulation of steroid 5α-reductase in the genital tubercle of the fetal rat. Dev Dynamics. 1997;209:117–126. doi: 10.1002/(SICI)1097-0177(199705)209:1<117::AID-AJA11>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 12.Cunha GR, Alarid ET, Turner T, Donjacour AA, Boutin EL, Foster B. Normal and abnormal development of the male urogenital tract: role of androgens, mesenchymal-epithelial interactions, and growth factors. J Androl. 1992;13:465–475. [PubMed] [Google Scholar]
- 13.Wilson JD, Griffin JE, Russell DW. Steroid 5α-reductase 2 deficiency. Endocr Rev. 1993;14:577–593. doi: 10.1210/edrv-14-5-577. [DOI] [PubMed] [Google Scholar]
- 14.Mahendroo MS, Cala KM, Russell DW. 5α-Reduced androgens play a key role in murine parturition. Mol Endocrinol. 1996;10:380–392. doi: 10.1210/mend.10.4.8721983. [DOI] [PubMed] [Google Scholar]
- 15.Mahendroo MS, Cala KM, Landrum CP, Russell DW. Fetal death in mice lacking 5α-reductase type 1 caused by estrogen excess. Mol Endocrinol. 1997;11:917–927. doi: 10.1210/mend.11.7.9933. [DOI] [PubMed] [Google Scholar]
- 16.Mahendroo MS, Porter A, Russell DW, Word RA. The parturition defect in steroid 5α-reductase type 1 deficient mice is due to impaired cervical ripening. Mol Endocrinol. 1999;13:981–992. doi: 10.1210/mend.13.6.0307. [DOI] [PubMed] [Google Scholar]
- 17.Mahendroo MS, Russell DW. Male and female isozymes of steroid 5α-reductase. Rev Reprod. 1999;4:179–183. doi: 10.1530/ror.0.0040179. [DOI] [PubMed] [Google Scholar]
- 18.Mansour SL, Thomas KR, Capecchi MR. Disruption of the proto-oncogene int-2 in mouse embryo-derived stem cells: a general strategy for targeting mutations to non-selectable genes. Nature. 1988;336:348–352. doi: 10.1038/336348a0. [DOI] [PubMed] [Google Scholar]
- 19.Sambrook J, Russell DW. Molecular cloning: a laboratory manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 2000. pp. 1–2400. [Google Scholar]
- 20.Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo. Cold Spring Harbor: Cold Spring Harbor Laboratory Press; 1995. pp. 1–497. [Google Scholar]
- 21.Ramirez-Solis R, Rivera-Perez J, Wallace JD, Wims M, Zheng H, Bradley A. Genomic DNA microextraction: a method to screen numerous samples. Anal Biochem. 1992;201:331–335. doi: 10.1016/0003-2697(92)90347-a. [DOI] [PubMed] [Google Scholar]
- 22.Resko JA, Ellinwood WE, Pasztor LM, Buhl AE. Sex steroids in the umbilical circulation of fetal rhesus monkeys from the time of gonadal differentiation. J Clin Endocrinol Metab. 1980;50:900–905. doi: 10.1210/jcem-50-5-900. [DOI] [PubMed] [Google Scholar]
- 23.DeKlerk DP, Coffey DS. Quantitative determination of prostatic epithelial and stromal hyperplasia by a new technique: biomorphometrics. Invest Urol. 1978;16:240–245. [PubMed] [Google Scholar]
- 24.Moore RJ, Wilson JD. Steroid 5α-reductase in cultured human fibroblasts. J Biol Chem. 1976;251:5895–5900. [PubMed] [Google Scholar]
- 25.Thigpen AE, Cala KM, Russell DW. Characterization of Chinese hamster ovary cell lines expressing human steroid 5α-reductase isozymes. J Biol Chem. 1993;268:17404–17412. [PubMed] [Google Scholar]
- 26.Cukierski MA, Sina JL, Prahalada S, Wise LD, Antonello JM, MacDonald JS, Robertson RT. Decreased fertility in male rats administered the 5α-reductase inhibitor, finasteride, is due to deficits in copulatory plug formation. Reprod Toxicol. 1991;5:353–362. doi: 10.1016/0890-6238(91)90094-v. [DOI] [PubMed] [Google Scholar]
- 27.Wise LD, Minsker DH, Cukierski MA, Clark RL, Prahalada S, Antonello JM, MacDonald JS, Robertson RT. Reversible decreases of fertility in male Sprague-Dawley rats treated orally with finasteride, a 5α-reductase inhibitor. Reprod Toxicol. 1991;5:337–346. doi: 10.1016/0890-6238(91)90092-t. [DOI] [PubMed] [Google Scholar]
- 28.Harris GS, Kozarich JW. Steroid 5α-reductase inhibitors in androgen-dependent disorders. Curr Opin Chem Biol. 1997;1:254–259. doi: 10.1016/s1367-5931(97)80017-8. [DOI] [PubMed] [Google Scholar]
- 29.Ho KC, Quarmby VE, French FS, Wilson EM. Molecular cloning of rat prostate transglutaminase complementary DNA. The major androgen-regulated protein DP-1 of rat dorsal prostate and coagulating gland. J Biol Chem. 1992;267:12660–12667. [PubMed] [Google Scholar]
- 30.Tohyama C, Susuki JS, Homma S, Karasawa M, Kuroki T, Nishimura H, Nishimura N. Testosterone-dependent induction of metallothionein in genital organs of male rats. Biochem J. 1996;317:97–102. doi: 10.1042/bj3170097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterson RE, Imperato-McGinley J, Gautier T, Sturla E. Male pseudohermaphroditism due to steroid 5α-reductase deficiency. Am J Med. 1977;62:170–191. doi: 10.1016/0002-9343(77)90313-8. [DOI] [PubMed] [Google Scholar]
- 32.Peterson RE, Imperato-McGinley J, Gautier T, Shackleton C. Urinary steroid metabolites in subjects with male pseudohermaphroditism due to 5α-reductase deficiency. Clin Endocrinol (Oxf) 1985;23:43–53. doi: 10.1111/j.1365-2265.1985.tb00181.x. [DOI] [PubMed] [Google Scholar]
- 33.Imperato-McGinley J, Peterson RE, Gautier T, Shackleton C. Decreased urinary C19 and C21 steroid 5α-metabolites in parents of male pseudohermaphrodites with 5α-reductase deficiency: detection of carriers. J Clin Endocrinol Metab. 1985;60:553–558. doi: 10.1210/jcem-60-3-553. [DOI] [PubMed] [Google Scholar]
- 34.Kinouchi T, Horton R. 3α-Androstanediol kinetics in man. J Clin Invest. 1974;54:646–653. doi: 10.1172/JCI107802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Horton R, Hawks D, Lobo RA. 3α-,17β-Androstanediol glucoseronide in plasma: a marker of androgen action in idiopathic hirsutism. J Clin Invest. 1982;69:1203–1206. doi: 10.1172/JCI110558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ertel NH, Akgun S, Samojilik E, Kirschner MA, Imperato-McGinley J. Decreased 3α-androstanediol glucuronide levels in plasma and random urines in male pseudohermaphroditism caused by 5α-reductase deficiency. Metabolism. 1989;38:817–821. doi: 10.1016/0026-0495(89)90225-4. [DOI] [PubMed] [Google Scholar]
- 37.Iguchi T, Uesugi Y, Takasugi N, Petrow V. Quantitative analysis of the development of genital organs from the urogenital sinus of the fetal male mouse treated prenatally with a 5α-reductase inhibitor. J Endocrinol. 1991;128:395–401. doi: 10.1677/joe.0.1280395. [DOI] [PubMed] [Google Scholar]
- 38.Prahalada S, Majka JA, Soper KA, Nett TM, Bagdon WJ, Peter CP, Burek JD, MacDonald JS, van Zwieten MJ. Leydig cell hyperplasia and adenomas in mice treated with finasteride, a 5α-reductase inhibitor: a possible mechanism. Fund Applied Toxicol. 1994;22:211–219. doi: 10.1006/faat.1994.1025. [DOI] [PubMed] [Google Scholar]
- 39.McConnell JD, Wilson JD, George FW, Geller J, Pappas F, Stoner E. Finasteride, an inhibitor of 5α-reductase, suppresses prostatic dihydrotestosterone in men with benign prostatic hyperplasia. J Clin Endocrinol Metab. 1992;74:505–508. doi: 10.1210/jcem.74.3.1371291. [DOI] [PubMed] [Google Scholar]
- 40.Lundwall A, Peter A, Lovgren J, Lilja H, Malm J. Chemical characterization of the predominant proteins secreted by mouse seminal vesicles. Eur J Biochem. 1997;249:39–44. doi: 10.1111/j.1432-1033.1997.t01-2-00039.x. [DOI] [PubMed] [Google Scholar]
- 41.George FW, Peterson KG. 5α-Dihydrotestosterone formation is necessary for embryogenesis of the rat prostate. Endocrinology. 1988;122:1159–1164. doi: 10.1210/endo-122-3-1159. [DOI] [PubMed] [Google Scholar]
- 42.Ryhorchuk AR, Shaw G, Butler CM, Renfree MB. Effects of a 5α-reductase inhibitor, finasteride, on the developing prostate and testis of a marsupial. J Androl. 1997;18:123–130. [PubMed] [Google Scholar]
- 43.Wilson JD, Lasnitzki I. Dihydrotestosterone formation in fetal tissues of the rabbit and rat. Endocrinology. 1971;89:659–668. doi: 10.1210/endo-89-3-659. [DOI] [PubMed] [Google Scholar]
- 44.Siiteri PK, Wilson JD. Testosterone formation and metabolism during male sexual differentiation in the human embryo. J Clin Endocrinol Metab. 1974;38:113–125. doi: 10.1210/jcem-38-1-113. [DOI] [PubMed] [Google Scholar]
- 45.Shaw G, Renfree MB, Leihy MW, Shackleton CHL, Roitman E, Wilson JD. Prostate formation in a marsupial is mediated by the testicular androgen 5α-androstane-3α,17β-diol. Proc Natl Acad Sci USA. 2000;97:12256–12259. doi: 10.1073/pnas.220412297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freeman BC, Felts SJ, Toft DO, Yamamoto KR. The p23 molecular chaperones act at a late step in intracellular receptor action to differentially affect ligand efficacies. Genes Dev. 2000;14:422–434. [PMC free article] [PubMed] [Google Scholar]
- 47.Penning TM. Molecular endocrinology of hydroxysteroid dehydrogenases. Endocr Rev. 1997;18:281–305. doi: 10.1210/edrv.18.3.0302. [DOI] [PubMed] [Google Scholar]
- 48.Wilson EM, French FS. Binding properties of androgen receptors: evidence for identical receptors in rat testis, epididymis, and prostate. J Biol Chem. 1976;251:5620–5629. [PubMed] [Google Scholar]
- 49.Deslypere J-P, Young M, Wilson JD, McPhaul MJ. Testosterone and 5α-dihydrotestosterone interact differently with the androgen receptor to enhance transcription of the MMTV-CAT reporter gene. Mol Cell Endocrinol. 1992;88:15–22. doi: 10.1016/0303-7207(92)90004-p. [DOI] [PubMed] [Google Scholar]
- 50.Kaufman M, Pinsky L. The dissociation of testosterone- and 5α-dihydrotestosterone-receptor complexes formed within cultured human genital skin fibroblasts. J Steroid Biochem. 1983;18:121–125. doi: 10.1016/0022-4731(83)90077-8. [DOI] [PubMed] [Google Scholar]
- 51.Kovacs WJ, Griffin JE, Weaver DD, Carlson BR, Wilson JD. A mutation that causes lability of the androgen receptor under conditions that normally promote transformation to the DNA-binding state. J Clin Invest. 1984;73:1095–1104. doi: 10.1172/JCI111295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kaufman M, Pinsky L, Trifiro M, Lumbroso R, Sabbaghian N, Gottlieb B. Kinetic evidence for a unique testosterone-receptor complex in 5α-reductase sufficient genital skin fibroblasts and the effects of 5α-reductase deficiency on its formation. J Steroid Biochem. 1993;45:467–476. doi: 10.1016/0960-0760(93)90161-o. [DOI] [PubMed] [Google Scholar]
- 53.Wenderoth UK, George FW, Wilson JD. The effect of a 5α-reductase inhibitor on androgen-mediated growth of the dog prostate. Endocrinology. 1983;113:569–573. doi: 10.1210/endo-113-2-569. [DOI] [PubMed] [Google Scholar]
- 54.Wu L, Einstein M, Geissler WM, Chan HK, Elliston KO, Andersson S. Expression cloning and characterization of human 17β-hydroxysteroid dehydrogenase type 2, a microsomal enzyme possessing 20α-hydroxysteroid dehydrogenase activity. J Biol Chem. 1993;268:12964–12969. [PubMed] [Google Scholar]
- 55.Mustonen M, Poutanen M, Chotteau-Lelievre A, de Launoit Y, Isomaa V, Vaino S, Vihko R, Vihko P. Ontogeny of 17β-hydroxysteroid dehydrogenase type 2 mRNA expression in mouse placenta and fetus. Mol Cell Endocrinol. 1997;134:33–40. doi: 10.1016/s0303-7207(97)00157-3. [DOI] [PubMed] [Google Scholar]
- 56.Mustonen MV, Poutanen MH, Isomaa VV, Vihko PT, Vihko RK. Cloning of mouse 17β-hydroxysteroid dehydrogenase type 2 mRNA and analysis of the mRNAs for types 1, 2, 3, 4 and 5 in mouse embryos and adult tissues. Biochem J. 1997;325:199–205. doi: 10.1042/bj3250199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Andersson S, Geissler WM, Wu L, Davis DL, Grumbach MM, New MI, Schwarz HP, Blethen SL, Mendonca BB, Bloise W, Witchel SF, Cutler GB, Jr, Griffin JE, Wilson JD, Russell DW. Molecular genetics and pathophysiology of 17β-hydroxysteroid dehydrogenase 3 deficiency. J Clin Endocrinol Metab. 1996;81:130–136. doi: 10.1210/jcem.81.1.8550739. [DOI] [PubMed] [Google Scholar]
- 58.Labrie F, Luu-The VV, Lin S, Simard J, Labrie C. Role of 17β-hydroxysteroid dehydrogenases in sex steroid formation in peripheral intracrine tissues. Trends Endocrinol Metab. 2000;11:421–427. doi: 10.1016/s1043-2760(00)00342-8. [DOI] [PubMed] [Google Scholar]