Abstract
Alzheimer's disease (AD), the leading cause of dementia, has reached epidemic proportions, with major social, medical and economical burdens. With no currently available curative treatments, both the World Health Organization and the G8 Dementia Summit recently identified dementia and AD prevention as a major public health priority. Dementia and AD have a wide range of risk factors (genetic, vascular/metabolic and lifestyle-related), which often co-occur and thus interact with each other. Previous intervention efforts aimed at preventing dementia and AD focused on the management of single risk factors, with relatively modest findings. Also, the effect of risk factors depends on age at exposure, indicating that the timing of preventive interventions needs to be carefully considered. In view of the complex multifactorial nature of AD, as well as its long pre-clinical (asymptomatic) phase, interventions simultaneously targeting multiple risk factors and disease mechanisms at an early stage of the disease are most likely to be effective. Three large European multidomain prevention trials have been launched with the goal of preventing cognitive decline, dementia and AD in older adults with different risk profiles. Pharmacological trials are also shifting towards prevention of Alzheimer dementia, by targeting at-risk individuals prior to the onset of cognitive symptoms. The current review will summarize and discuss the evidence on risk and protective factors from observational studies, ongoing lifestyle-related and pharmacological randomized controlled trials (RCTs), as well as future directions for dementia and AD prevention.
Introduction
As the worldwide population ages, dementia has reached epidemic proportions, with major social, medical and economical burdens. Dementia is a syndrome associated with progressive declines in cognitive capacities and impairments that interfere with daily functioning. These conditions are the primary cause of dependency, disability and institutionalization among older populations [1-3]. Cerebrovascular and neurodegenerative diseases account for the majority of dementia cases, while a small percentage is due to potentially treatable illnesses, for example, normal pressure hydrocephalus, human immunodeficiency virus (HIV) infection, and thyroid disorders. AD, a neurodegenerative disorder, is the leading cause of dementia, accounting for 60–70% of cases [2,3], although increasing evidence shows that mixed brain pathologies (AD and vascular) account for most dementia cases, especially in very advanced age (age 85+) [4,5]. It has been estimated that 44 million people worldwide were living with dementia in 2013 and this number is predicted to double every 20 years, reaching 76 million by 2030, and 136 million by 2050 [3,6]. Annually, there are 7.7 million new cases of dementia globally [2]. The estimated worldwide annual socioeconomic cost of dementia was US$604 billion in 2010, which was higher than the costs of cancer and cardiovascular disease combined [1]. Currently, there is no cure for dementia and AD, and both the World Health Organization and the G8 Dementia Summit recently identified dementia and AD prevention as a major public health priority [7,8].
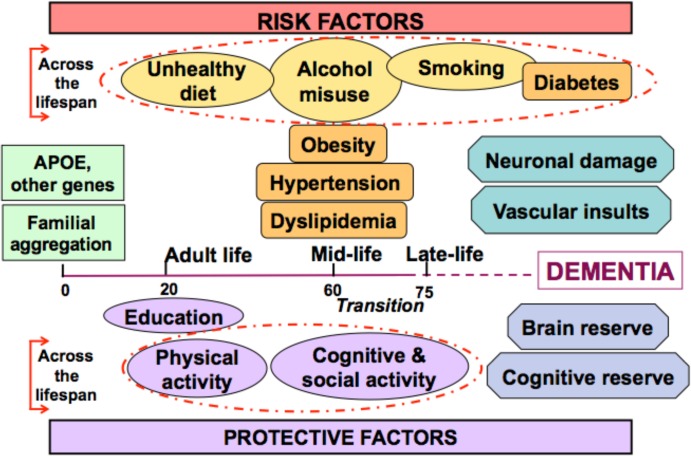
Dementia and AD are multifactorial disorders, and evidence from the last decades has revealed that genetic, vascular and lifestyle-related risk factors often co-occur and interact across the lifespan to determine the risk of developing dementia and AD later in life (Figure 1). The frequent co-occurrence of AD and cerebrovascular disease is consistent with the evidence that both disorders share several risk and protective factors, supporting the validity of dementia syndrome as a target for prevention. As many epidemiological findings discussed in this review apply to both dementia and AD, these terms are used interchangeably (dementia/AD) whenever applicable.
Figure 1. Risk factors for dementia and Alzheimer's disease across the lifespan (Figure modified from [51]).
While the precise underlying etiology of AD is unknown, extensive knowledge is available regarding its clinical and pathological features. The neuropathological hallmarks of AD involve the formation of extracellular beta amyloid (Aβ) plaques and intraneuronal neurofibrillary tangles, which are both associated with synaptic and neuronal loss. Various mechanisms have been described to explain the link between genetic and environmental factors and AD pathophysiology, including oxidative stress, inflammatory and vascular-related pathways [9,10].
To date, preventive interventions to manage vascular and lifestyle risk factors are focusing on late-onset dementia/AD (age ≥65 years), while pharmacological preventive interventions are mainly targeting early-onset dementia due to AD (age <65 years). The current review will summarize evidence on dementia risk factors from observational studies, ongoing lifestyle-related and/or pharmacological RCTs, as well as future directions for dementia/AD prevention.
Observational studies
Evidence from observational studies has revealed that while some dementia/AD risk factors are non-modifiable, many risk factors are modifiable through lifestyle alterations and/or pharmacological treatment. The most important non-modifiable risk factor is age, and dementia/AD incidence substantially increases with advanced age [2,5]. Other non-modifiable risk factors for late-life AD are familial aggregation and the apolipoprotein E (APOE) ε4 allele; the main genetic risk factor for sporadic AD. The APOE ε4 allele increases the risk for AD in a dose-response manner, where those with homozygous ε4 allele (two ε4 copies) have a higher risk than individuals with heterozygous ε4 allele (a combination of one ε4 copy, and an alternative copy: ε2 or ε3 allele), who in turn have a higher risk than individuals with no ε4 copies [11-14]. Moreover, the APOE ε4 allele reduces the age of AD onset, amplifies the negative effects of lifestyle-related factors and modulates responsivity to interventions [15-20]. It has been estimated that 15 to 20% of dementia and AD are attributable to the APOE ε4 allele [14,21].
Other genes have been proposed as risk factors for AD (a full meta-analysis can be found on the Alzgene website, http://www.alzgene.org ), and it has been estimated that about 70% of AD risk can be attributed to genetics [22]. Still, the risk conferred by single genes is small and combinations of risk alleles need to be identified [22]. Genetic causes of AD include autosomal-dominant mutations in three genes (Presenilin 1 [PSEN1], Presenilin 2 [PSEN2] and the amyloid precursor protein [APP]), which are implicated in early-onset AD and account for approximately 1% of all cases [5,23,24].
Modifiable risk factors for dementia/AD fall into several categories. First, adequate evidence is present for vascular risk factors, including midlife hypertension, diabetes mellitus, smoking, midlife obesity, stroke and cardiovascular disease (Table 1) [25]. Some of these factors increase dementia risk when present in midlife, emphasizing the importance of applying a life-course perspective when examining risk factors and implementing preventive interventions [26-30]. Second, protective nutritional components include omega-3 fatty acids and unsaturated fats, antioxidants, vitamins and moderate alcohol consumption [31-35]. The importance of dietary patterns (e.g. Mediterranean diet) is also recognized, as nutritional components interact to produce synergistic effects (for a review see [36]). Third, lifestyle and psychosocial factors can also modify dementia/AD risk. For example, while living alone, having feelings of loneliness, depression, social isolation and psychosocial stress can increase dementia/AD risk, higher levels of education, engaging in exercise, and cognitively and socially stimulating activities are protective [25,37-39].
Table 1. Risk and protective factors for late-onset dementia and Alzheimer's disease (Table adapted from [5]).
| Risk factors | Protective factors |
|---|---|
|
Age Genetic Familial aggregation APOE ε4 Different genes (e.g., CR1, PICALM, CLU, TREM2, TOMM40) have been proposed (www.alzgene.org) Vascular and metabolic Cerebrovascular lesions Cardiovascular diseases Diabetes mellitus and pre-diabetes Midlife positive association but late-life negative association Hypertension High BMI (overweight and obesity) High serum cholesterol Lifestyle Smoking High alcohol intake Diet Saturated fats Homocysteine Others Depression Traumatic brain injury Occupational exposure (extremely low-frequency electromagnetic field, heavy metals) Infective agents (Herpes Simplex Virus Type I, Clamydophila pneumonia, Spirochetes) |
Genetic Different genes (e.g. APP, APOE ε2) have been proposed (www.alzgene.org) Psychosocial factors High education and socioeconomic status High work complexity Rich social network and social engagement Mentally stimulating activity Lifestyle Physical activity Moderate alcohol intake Diet Mediterranean diet Polyunsaturated fatty acids and fish-related fats Vitamin B6, B12, folate Antioxidant vitamins (A, C, E) Vitamin D Drugs Antihypertensive drugs Statins HRT NSAIDs |
APP, amyloid precursor protein; APOE, apolipoprotein E; BMI, body mass index; CLU, clusterin; CR1, complement component receptor 1; HRT, hormone-replacement therapy; NSAID, non-steroidal anti-inflammatory drug; PICALM, phosphatidylinositol binding clathrin assembly protein; TOMM40, translocase of outer mitochondrial membrane 40 homolog; TREM2, triggering receptor expressed on myeloid cells 2.
A large number of risk and protective factors for dementia and Alzheimer's disease have been investigated, and there are varying degrees of evidence to support these factors.
Cumulative exposure to risk factors needs to be accounted for, as it impacts the overall risk of late-life dementia/AD. Risk scores have been developed to assess dementia risk and target interventions for at-risk individuals using motivating and educational tools. The Cardiovascular Risk Factors, Aging and Dementia (CAIDE) Dementia Risk Score was the first tool developed using midlife risk profiles to predict dementia risk in later life (20 years later) [40]. This validated risk score combines low education with vascular factors (hypertension, obesity, hypercholesterolemia, and physical inactivity) [41]. A mobile application was recently developed for rapid and accessible usage of the CAIDE Dementia Risk score [42,43]. Other risk scores were subsequently developed (for a review see [44]).
A recent estimate took into account the prevalence and co-occurrence of the main modifiable risk factors and investigated combined effects of reducing their occurrence. It showed that up to a third of worldwide AD cases are attributable to seven risk factors (diabetes mellitus, midlife hypertension, midlife obesity, physical inactivity, depression, smoking, low education), and a 10–20% reduction of all these risk factors might decrease AD prevalence by 8–15% in 2050 [45]. These findings further highlight the potential of multimodal preventive interventions—simultaneously addressing multiple risk factors—as preventive measures for dementia and AD. In line with these estimates, recent studies reported that improved lifestyle, medical care, and socioeconomic factors might have contributed to the reduced age-specific incidence of dementia observed during the past two decades [46-50]. However, while the age-specific incidence of dementia has been decreasing in some developed countries, the global occurrence of dementia is continuing to rise, especially considering the worldwide aging population [51].
Collectively, observational evidence provides strong grounds to target vascular and lifestyle-related risk factors that increase dementia/AD risk. Even modest reductions in these factors can considerably mitigate total risk and delay the onset age of dementia/AD [52].
Intervention studies
Based on evidence from observational studies, several intervention studies were designed to prevent or delay dementia/AD onset, including trials testing medications, food supplements, and lifestyle interventions. When collectively evaluated by the State-of-the-Science Conference held by the National Institutes of Health (NIH), it was concluded that the evidence for preventive interventions of dementia/AD was inadequate, with an exception of adequate evidence for anti-hypertensive medications [53-55]. It is plausible that observed associations from epidemiological studies do not necessarily imply that risk factors can be modified to alter dementia risk. However, inconsistent findings from intervention studies may have also resulted from several methodological issues: a) short intervention durations/follow-up periods may have prevented the observation of time-lagged intervention effects on cognition; b) late intervention timing (after the “window of opportunity” during which interventions have the highest potential for effectiveness); c) small sample sizes (insufficient power); d) considerable diversity in dosages/compounds of nutritional supplements used; e) use of unimodal interventions (e.g. diet or exercise) that are based on unimodal hypotheses, which are unlikely to adequately target the multiple interacting risk factors for multifactorial conditions; and f) the definition of outcome variables, which differs between studies—some studies measured dementia or AD incidence, others measured cognitive performance or decline, rendering comparisons difficult [56-58]. These aforementioned methodological shortcomings may explain the discrepancy in findings and guide in improving future intervention trials.
Several steps have been recently taken in this direction, with expert groups and regulatory authorities, such as the US Food and Drug Administration (FDA) and the European Medicines Agency (EMA), providing a new framework for how drugs can be studied in RCTs on early AD. This includes: the development of new diagnostic criteria, which facilitates the identification of the early stages of AD and more homogeneous groups of patients [59-61]; the use of biomarkers, either as enrichment strategies or supportive of a disease-modifying effect [62-64]; and the definition of new trial designs, robust analytical methods, and reliable indicators of clinical efficacy (for a review see [65]). These innovative approaches to RCT design are expected to strengthen the quality of future RCT findings.
Ongoing multidomain prevention trials
Using the cumulative evidence from epidemiological studies, a few research groups have recently taken the aforementioned limitations into account and shifted to conducting multidomain lifestyle RCTs that simultaneously target several modifiable risk factors in order to prevent cognitive impairment and dementia/AD [56]. Currently, there are three ongoing large multidomain RCTs in Europe: the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER), the Multidomain Alzheimer Prevention Trial (MAPT), and the Prevention of Dementia by Intensive Vascular Care (PreDIVA) study (Table 2) [66-69].
Table 2. Characteristics of selected RCTs for prevention of cognitive impairment, dementia and Alzheimer's disease based on multidomain interventions (Table adapted from [5]).
| RCT | FINGER | MAPT | PreDIVA | ||
|---|---|---|---|---|---|
| Sample size | 1260 community dwellers, from previous population-based observational cohorts | 1680 community dwellers | 3533 community dwellers | ||
| Main inclusion criteria | CAIDE Dementia Risk Score >6 and cognition at mean level/slightly lower than expected for age (CERAD test battery) | Frail elderly people, subjective memory complaint, slow walking speed, limitation in IADL (MMSE≥24) | All elderly within GP practices, non demented (MMSE >23) | ||
| Age at enrolment | 60–77 yrs | ≥70 yrs | 70–78 yrs | ||
| Study design | Multi-center, randomized, parallel-group controlled trial | Multi-center, randomized, controlled trial | Multi-center, cluster-randomized, parallel group controlled trial | ||
| Intervention | Multi-domain: nutritional guidance, physical activity, cognitive training, increased social activity and intensive monitoring and management of metabolic and vascular risk factors | Multi-domain: vascular care, nutritional advice, exercise advice, cognitive training, and/or DHA 800 mg/day | Multi-domain: nurse-led vascular care including medical treatment of risk factors, nutritional advice, exercise advice | ||
| Duration | 2 yrs + 5 yrs extended follow-up | 3 yrs + 2 yrs extended follow-up | 6 yrs | ||
| Outcomes | Primary: change in cognitive function (neuropsychological test battery, Trail Making, Stroop), Secondary: dementia, cardiovascular events, depression, disability, quality of life, health resources utilisation, AD biomarkers change |
Primary: change in cognitive function (Grober and Buschke memory test) Secondary: cognition (MMSE, CDR), functional status, depression, health resources utilisation, AD biomarkers change |
Primary: dementia, disability Secondary: cognitive decline (MMSE, VAT), depression, cardiovascular events |
||
| Status | Intervention was completed in March 2014 | Intervention was completed in March 2014 | Ongoing, will be completed in 2015 |
CDR, clinical dementia rating scale; DHA, docosahexaenoic acid; FINGER, Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability; GP, general practitioner; IADL, instrumental activities of daily living; MAPT, Multidomain Alzheimer Prevention Study; PreDIVA, Prevention of Dementia by Intensive Vascular Care; VAT, visual association test.
FINGER (http://ClinicalTrials.gov NCT01041989) is a 2-year multicenter RCT aiming to prevent cognitive impairment, dementia and disability in a sample of 1260 community dwelling at-risk older adults (aged 60–77 years), selected according to the CAIDE Dementia Risk Score and the CERAD (Consortium to Establish a Registry for Alzheimer's Disease) neuropsychological test battery [40,70]. Participants were randomly selected from population-based, non-intervention surveys, which provide extensive retrospective information [71]. The multidomain intervention involved nutritional guidance, exercise, cognitive training, social activity, and intensive monitoring/management of metabolic/vascular risk factors (impaired glucose tolerance, obesity, hypertension, and hypercholesterolemia). Participants in the control group received regular health advice. The primary outcome was cognitive performance measured by a comprehensive Neuropsychological Test Battery (NTB) after 2 years [66]. The 2-year intervention was recently finalized (spring 2014). Participants reported positive intervention experiences, showed high adherence rates and a low dropout rate (11%), demonstrating the feasibility of this type of intervention. After the 2-year program, a significant beneficial intervention effect was seen on global cognition: improvement in NTB total score (pre-specified primary outcome) during 24 months was 25% higher in the intervention than the control group, indicating the possibility of improving/maintaining cognitive functions in older individuals at risk of dementia [72]. Furthermore, the risk for cognitive impairment was 31% higher in the control group compared to the intervention group. To determine the intervention effect on dementia and AD incidence, a 7-year extended follow-up of the study participants started in 2015.
MAPT (http://ClinicalTrials.gov NCT00672685) is a French, multicenter 3-year RCT, in which 1680 older adults (age ≥70 years) were recruited using a frailty definition that includes three components: memory complaints, limitation in one instrumental activity of daily living, and slow walking speed. Enrolled participants were randomized to four groups: omega-3 supplementation; multidomain intervention; omega-3 plus multidomain intervention; or placebo. The multidomain intervention involved group sessions (cognitive training, nutrition and exercise advice) and personalized consultations to identify and manage risk factors for dementia and frailty. The study aims to investigate the intervention effects on cognitive changes after 3 years using the Grobe and Buschke Test (memory recall) [68,69]. The 3-year intervention was completed in March 2014, and 5-year extended follow-up will be completed in 2016.
PreDIVA (http://Controlled-Trials.com ISRCTN29711771) is a cluster-randomized RCT among 3533 Dutch older adults (aged 70–78 years). Participants were recruited from primary care settings, with a follow-up duration of 6 years. The study aims to investigate the effects of nurse-led intensive vascular care on dementia incidence and disability. Intensive vascular care includes consultations with a study nurse and tailored interventions for medical risk factors (diabetes, hypertension, hypercholesterolemia) and lifestyle-related risk factors (diet, body weight, exercise, smoking habits). The control group receives standard care according to Dutch general practice [67].
Considering the similarities between the three aforementioned trials (FINGER, MAPT, and PreDIVA), the trial leaders recently established the European Dementia Prevention Initiative (EDPI) network to promote international collaborations and share/harmonize data analyses [73]. Collectively, the joint analyses within EDPI will demonstrate the extent to which dementia/AD can be prevented by multidomain interventions and the most effective strategies. The EDPI recently launched a collaborative multidomain RCT Healthy Aging Through Internet Counseling in the Elderly (HATICE) to prevent dementia and cardiovascular diseases among 4250 at-risk older adults, recruited in three European countries (Finland, France, and the Netherlands) [74]. Through a user-friendly Internet platform, HATICE participants consult with a coach who offers comprehensive tailored advice to manage vascular and lifestyle risk factors. More recently, EDPI members and other researchers launched the Multimodal Preventive Trials for AD (MIND-AD) project, funded by the Joint Programme - Neurodegenerative Disease Research (JPND) initiative. The MIND-AD project aims to develop effective AD/dementia prevention strategies while harmonizing and optimizing methodologies for multinational and multimodal prevention RCTs across the entire spectrum of AD (that is, from asymptomatic at-risk subjects to early-symptomatic stages of Alzheimer dementia) [75]. This project will build upon experiences and data from all the above-mentioned prevention studies, and it will also investigate for the first time whether a multidomain prevention trial is effective for the prodromal (early stages) of AD through a proof-of-concept multinational pilot trial. If successful, the multimodal intervention strategy can be easily adapted for different healthcare systems and can thus facilitate the implementation of preventive measures.
Ongoing trials with potential disease-modifying drugs
Since Aβ is believed to have a key role in AD pathogenesis, many RCTs have tested drugs targeting this protein in subjects with dementia due to AD, with negative findings thus far [65,76,77]. As previously suggested, this may have resulted from starting treatments too late in the disease process among subjects with overt dementia, while a beneficial effect might be achieved if treatments are administered in earlier, asymptomatic (or preclinical) stages of AD [58,78,79]. In order to test this hypothesis, recent prevention trials are focusing on asymptomatic individuals who are at risk of developing dementia/AD, either due to genetic causal factors (carriers of autosomal-dominant mutations: PSEN1, PSEN2, or APP) or brain Aβ accumulation. Currently, there are three ongoing prevention trials that test the safety and efficacy of potential disease-modifying anti-amyloid drugs: the Alzheimer's Prevention Initiative - Autosomal Dominant Alzheimer's Disease treatment trial (API-ADAD), the Dominantly Inherited Alzheimer's Network (DIAN) and the Anti-Amyloid Treatment in Asymptomatic Alzheimer's Disease (ADCS-A4) (Table 3). All trials include extensive cognitive assessments, neuroimaging and biomarker measures to track AD onset/progression. These ongoing initiatives are separate, but also interconnected. The Collaboration for Alzheimer's Prevention (CAP) is a consortium including, among others, researchers leading these RCTs, and aims to optimize methodologies, harmonize data collection, and plan future trials based on these aforementioned studies [80]. CAP also provides feedback to regulatory bodies (FDA and EMA) that is used to refine rules supporting preventive RCTs in AD [81]
Table 3. Characteristics of selected pharmacological RCTs for prevention of Alzheimer's disease using compounds targeting beta-amyloid (Table adapted from [5]).
| Prevention trials based on a genetic risk or an amyloid-beta biomarker risk | |||||
|---|---|---|---|---|---|
| RCT | API | DIAN | ADCS-A4 | ||
| Sample size | 300 members of Colombian families (Antioquia) with early-onset AD. Subjects with no cognitive impairment. A small number of individuals from USA (collaboration with the DIAN network) will also be included |
210 members of families with early-onset AD. Subjects can be asymptomatic or have very mild memory and thinking problems including mild dementia | 1150 older adults with no cognitive impairment | ||
| Main inclusion criteria | Carriers of a mutated PSEN1 gene. Non-carriers will also be included, to ensure double-blinding about the genetic status | Carriers (n=105) of mutation in PSEN1, or PSEN2, or APP. Non-carriers (n=105) will also be included, to ensure double-blinding about the genetic status | Evidence of brain amyloid accumulation (PET). Subjects with no evidence of amyloid burden will also be included | ||
| Age at enrolment | 30-60 yrs | 18-80 yrs | 65-85 yrs | ||
| Study design | Phase II randomized, double blind, placebo controlled trial | Phase II/III randomized, double blind, placebo controlled trial | Phase III randomized, double blind, placebo controlled trial | ||
| Intervention | Anti-amyloid monoclonal antibody: Crenezumab (Genentech) | Two anti-amyloid therapies: the anti-amyloid monoclonal antibodies Gantenerumab (La Roche) and Solanezumab (Eli Lilly) | Anti-amyloid monoclonal antibody: Solanezumab (Eli Lilly) | ||
| Duration | 5 yrs (interim analysis at 2 yrs) | 2 yrs + 3 yrs extension | 3 yrs + 2 yrs extension | ||
| Outcomes | Primary: cognitive function Secondary: AD biomarkers change, including brain amyloid load and brain atrophy |
Initial phase (2 yrs): AD biomarkers change, including brain and CSF amyloid, to identify the most promising drug candidate Follow-up phase (3 yrs): cognitive function |
Primary: cognitive function Secondary: AD biomarkers change |
||
| Status | Started in 2013 | Started in 2012 | Started in 2014 | ||
ADCS-A4, Anti-Amyloid Treatment of Asymptomatic Alzheimer's disease; API, Alzheimer's Prevention Initiative; APOE, apolipoprotein E; APP, amyloid precursor protein; DIAN, Dominantly Inherited Alzheimer Network; PET, positron emission tomography; PSEN1, presenilin 1; PSEN2, presenilin 1.
API-ADAD (http://ClinicalTrials.gov NCT01998841) is a potentially label-enabling trial testing the anti-amyloid compound crenezumab [Genentech] in 300 asymptomatic members of an extended large family in Colombia with the PSEN1 E280A mutation, for which the median onset age of mild cognitive impairment (MCI) is 44 years and the onset of dementia is 49 years [82,83]. The goal of the study is to assess whether administration of crenezumab in the preclinical stage can delay or prevent the onset of symptomatic AD. Using a randomized, double-blind, placebo-controlled, parallel group design, participants who are mutation carriers will be randomized to receive crenezumab or placebo. For comparisons with non-carriers of PSEN1, the trial will include a cohort of non-carriers who will only receive placebo. The API trial is also due to launch another preventive study in 2015 (API APOE trial) in which two anti-amyloid drugs (a vaccine and a beta-secretase1 inhibitor) will be tested in cognitively normal subjects who are homozygous APOE ε4 carriers and aged 60 to 75 years [84].
DIAN (http://ClinicalTrials.gov NCT01760005) is an observational study funded by the American National Institute on Aging to develop an international multicenter registry of symptomatic and asymptomatic individuals with genetic mutations for autosomal-dominant AD (PSEN1, PSEN2, APP) [85,86]. Their non-carrier family members are also recruited as controls with similar genetics. The target sample is 400 individuals, and 13 international research institutes are currently involved in the study. The DIAN Trials Unit (DIAN-TU) was recently established to test two anti-amyloid drugs (Solanezumab [Eli Lilly and Company] and Gantenerumab [Hoffmann-La Roche]) among mutation carriers at risk of AD, including 210 asymptomatic or mild symptomatic subjects [87]. Mutation non-carriers are also recruited (placebo arms). The primary outcomes in the DIAN-TU RCT are changes in biomarkers after 2 years. After this period, the agent(s) showing beneficial biomarker effects will be further tested in larger trials with cognitive outcomes.
The ADCS-A4 study (http://ClinicalTrials.gov NCT02008357) [88] is a placebo-controlled, potential label-enabling RCT conducted in 1150 asymptomatic individuals (aged 65–85 years) who show brain Aβ accumulation (on positron emission tomography [PET] imaging scans) and are thus considered at risk of AD [80]. The goal is to assess whether administration of anti-amyloid therapy prior to symptoms onset prevents dementia. In this first A4 trial, participants will be randomized to receive solanezumab or placebo, and alternative drugs may be used in future A4 trials. The study will also investigate the impact of disclosing to participants their amyloid status by including psychological assessments and measures of adverse reactions to amyloid status disclosure. Participants without high Aβ levels at initial screening will serve as a comparison group by participating in an observational arm of the A4 study.
Other RCTs among asymptomatic individuals include: the PREVENT Alzheimer Program launched by the Centre for Studies on Prevention of AD (StoP-AD) at the Douglas Mental Health University Institute (Montreal, Canada), which will test preventive interventions (naproxen, nasal insulin or others) in older adults with a family history of AD [89]; and the TOMORROW trial (http://ClinicalTrials.gov NCT01931566), a multicenter label-enabling trial that tests whether a potential disease-modifying drug (Pioglitazone [Takeda]) can postpone the onset of MCI due to AD, and whether a genetic-based (using APOE4 and TOMM40) biomarker risk algorithm predicts risk of MCI due to AD. Collectively, the trials will demonstrate whether preventive treatments at the early asymptomatic (or mild symptomatic) stage are effective, and whether alternative drug targets are required.
Future directions
Due to the worldwide aging population, dementia/AD occurrence will continue to rise. Prevention has been highlighted as one of the key elements in managing the dementia epidemic, in a similar manner to what has been proposed for other major non-communicable diseases. Merely delaying the onset of AD/dementia will considerably alleviate social and economic burdens. Lifestyle-related interventions provide hope to prevent or postpone dementia/AD in the general population. Many experts agree that sufficient evidence is already available to justify immediate action, including prevention/management of vascular and lifestyle-related risk factors (smoking, hypertension, obesity, diabetes mellitus and physical inactivity) [90]. This is particularly important considering the trend towards increased prevalence of diabetes mellitus and obesity, which will impact future dementia/AD prevalence, while reinforcing the need to prevent these risk factors [91-94]. Similar to earlier discoveries that lifestyle-related interventions can protect against cardiovascular diseases, recent evidence confirms the adage “what is good for the heart, is good for the brain”. However, more RCTs are required to refine the knowledge on specific factors that optimize various prevention domains, such as nutrition or exercise. There is also a knowledge gap regarding the window of opportunity for some pharmacological interventions that so far have not been effective in terms of preventing dementia (e.g. statins and hormone replacement therapy) [95,96]. Further evidence is also needed on the underlying mechanisms that reduce dementia risk in prevention trials.
Evidence from the FINGER multimodal prevention trial supports the feasibility and effectiveness of this intervention approach, and the other EDPI trials will provide further insight. These trials will also identify whether barriers to adherence need to be addressed, whether subgroups are most responsive to interventions, and whether windows of opportunity exist for the different intervention types. Such acquired findings will further improve the methods of future trials. Multimodal lifestyle-related preventive strategies have additional benefits as they are not specific to reducing dementia risk; they also prevent chronic conditions including cardiovascular diseases, diabetes and cancer [97]. Translation of these findings will lead to the implementation of cost-effective interventions at a population level, while tailoring interventions for various sub-populations.
Pharmacological trials were previously conducted in advanced AD stages, showing relatively poor results. The ongoing trials in at-risk subjects will determine whether administering potentially disease-modifying treatments prior to symptoms onset is effective. If findings from the trials are positive, the next task will be to assess whether a similar approach can be adapted for patients at risk for Alzheimer dementia in the general population. It is currently unclear whether interventions and findings from preclinical studies on early-onset AD are generalizable to preclinical stages of late-onset AD, which are more heterogeneous. The two groups of aforementioned trials represent two extremes of the prevention continuum; lifestyle-related prevention trials target large-scale populations with common risk factors, while drug trials focus on highly specific groups with rare genetic mutations or brain Aβ accumulation. Both prevention approaches have benefits for various target populations, however, it will be important for future trials to narrow this gap by combining both approaches and adapting them for various target groups.
It was recently suggested that alternative targeted and adaptive trial designs (which have been used for cancer) might be more effective for complex and heterogeneous diseases such as AD [98]. More optimal designs would test the effects of treatments in heterogeneous samples while simultaneously improving and tailoring the trials for sub-populations as novel biomarkers are identified and validated [98]. Future trials will also benefit from including more enrichment cohorts as well as multitarget therapies, beyond anti-amyloid treatments, with increased collaborations among pharmaceutical companies (“multicompany trials”), as carried out by the DIAN-TU trial [58,99]. It will also be important to establish academic-industrial partnerships such as those developed within the Innovative Medicine Initiative - European Prevention of AD Consortium (IMI-EPAD), the largest public-private partnership in health sciences in Europe [100].
Future successful prevention efforts will require large-scale multinational collaborations. Similar to EDPI and CAP, international umbrella organizations can serve as a model to maximize power and harmonize data collection and analyses among trials. Such initiatives will optimize the use of existing infrastructure, prevent redundant investigations and errors while rapidly improving methodologies and translating findings to clinical practice and the general population in order to successfully prevent dementia/AD.
Acknowledgments
Miia Kivipelto receives research support from the Academy of Finland, the Swedish Research Council, American Alzheimer Association, AXA Research Fund and EU 7th framework large collaborative project grant (HATICE) and the Sheika Salama Bint Hamdan Alahyan Foundation. Shireen Sindi receives postdoctoral funding from the Fonds de la recherche en santé du Québec (FRSQ). Francesca Mangialasche is supported by the Ragnhild och Einar Lundströms-Minne-Lindhés Foundation, Gun and Bertil Stohnes and Gamla-Tjänarinnor.
Abbreviations
- AD
Alzheimer's disease
- ADCS-A4
Anti-Amyloid Treatment in Asymptomatic Alzheimer's Disease
- API
Alzheimer's Prevention Initiative
- APOE
apolipoprotein E
- APP
amyloid precursor protein
- Aβ
beta amyloid
- CAIDE
Cardiovascular Risk Factors, Aging and Dementia study
- CAP
Collaboration for Alzheimer's Prevention
- DIAN
Dominantly Inherited Alzheimer's Network
- DIAN-TU
Dominantly Inherited Alzheimer's Network - Trials Unit
- EDPI
European Dementia Prevention Initiative
- EMA
European Medicines Agency
- FDA
US Food and Drug Administration
- FINGER
Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability
- HATICE
Healthy Aging Through Internet Counseling in the Elderly
- HIV
human immunodeficiency virus
- MAPT
Multidomain Alzheimer Preventive Trial
- MCI
mild cognitive impairment
- MIND-AD
Multimodal Preventive Trials for Alzheimer's Disease
- NTB
neuropsychological test battery
- PreDIVA
Prevention of Dementia by Intensive Vascular Care
- PSEN1
presenilin 1
- PSEN2
presenilin 2
- RCT
randomized controlled trial
The electronic version of this article is the complete one and can be found at: http://f1000.com/prime/reports/m/7/50
Disclosures
Miia Kivipelto has served on scientific advisory boards for Pfizer Inc., Elan Corporation, Alzheon and Nutricia and received speaker honoraria from Janssen, Novartis, Pfizer Inc., and Merz.
References
- 1.Wimo A, Jönsson L, Bond J, Prince M, Winblad B. The worldwide economic impact of dementia 2010. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2013;9:1–11. doi: 10.1016/j.jalz.2012.11.006. e3. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization (WHO) Dementia: a public health priority. Geneva: World Health Organization—Alzheimer's Disease International. 2012 [Google Scholar]
- 3.Alzheimer's Disease International The Global Impact of Dementia 2013-2050: Policy Brief for Heads of Government. 2013 [Google Scholar]
- 4.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 5.Solomon A, Mangialasche F, Richard E, Andrieu S, Bennett DA, Breteler M, Fratiglioni L, Hooshmand B, Khachaturian AS, Schneider LS, Skoog I, Kivipelto M. Advances in the prevention of Alzheimer's disease and dementia. Journal of internal medicine. 2014;275:229–50. doi: 10.1111/joim.12178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brookmeyer R, Johnson E, Ziegler-Graham K, Arrighi HM. Forecasting the global burden of Alzheimer's disease. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2007;3:186–91. doi: 10.1016/j.jalz.2007.04.381. [DOI] [PubMed] [Google Scholar]
- 7.Mental Health . World Health Organisation; 2012. Dementia: a public health priority. [ http://www.who.int/mental_health/publications/dementia_report_2012/en/] [Google Scholar]
- 8.Global Action Against Dementia G8 Dementia Summit Declaration. 2013 [ https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/265869/2901668_G8_DementiaSummitDeclaration_acc.pdf]
- 9.Wyss-Coray T, Rogers J. Inflammation in Alzheimer disease-a brief review of the basic science and clinical literature. Cold Spring Harbor perspectives in medicine. 2012;2:a006346. doi: 10.1101/cshperspect.a006346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Orsucci D, Mancuso M, Ienco EC, Simoncini C, Siciliano G, Bonuccelli U. Vascular factors and mitochondrial dysfunction: a central role in the pathogenesis of Alzheimer's disease. Current neurovascular research. 2013;10:76–80. doi: 10.2174/156720213804805972. [DOI] [PubMed] [Google Scholar]
- 11.Qiu C, Kivipelto M, Agüero-Torres H, Winblad B, Fratiglioni L. Risk and protective effects of the APOE gene towards Alzheimer's disease in the Kungsholmen project: variation by age and sex. Journal of neurology, neurosurgery, and psychiatry. 2004;75:828–33. doi: 10.1136/jnnp.2003.021493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Corder EH, Saunders AM, Strittmatter WJ, Schmechel DE, Gaskell PC, Small GW, Roses AD, Haines JL, Pericak-Vance MA. Gene dose of apolipoprotein E type 4 allele and the risk of Alzheimer's disease in late onset families. Science (New York, N.Y.) 1993;261:921–3. doi: 10.1126/science.8346443. [DOI] [PubMed] [Google Scholar]
- 13.Poirier J, Davignon J, Bouthillier D, Kogan S, Bertrand P, Gauthier S. Apolipoprotein E polymorphism and Alzheimer's disease. Lancet. 1993;342:697–9. doi: 10.1016/0140-6736(93)91705-Q. [DOI] [PubMed] [Google Scholar]
- 14.Slooter AJ, Cruts M, Kalmijn S, Hofman A, Breteler MM, van Broeckhoven C, van Duijn CM. Risk estimates of dementia by apolipoprotein E genotypes from a population-based incidence study: the Rotterdam Study. Archives of neurology. 1998;55:964–8. doi: 10.1001/archneur.55.7.964. [DOI] [PubMed] [Google Scholar]
- 15.Seripa D, Panza F, Franceschi M, D‘Onofrio G, Solfrizzi V, Dallapiccola B, Pilotto A. Non-apolipoprotein, E, and apolipoprotein E genetics of sporadic Alzheimer's disease. Ageing research reviews. 2009;8:214–36. doi: 10.1016/j.arr.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 16.Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, Myers RH, Pericak-Vance MA, Risch N, van Duijn CM. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–56. doi: 10.1001/jama.1997.03550160069041. [DOI] [PubMed] [Google Scholar]
- 17.Reiman EM, Chen K, Liu X, Bandy D, Yu M, Lee W, Ayutyanont N, Keppler J, Reeder SA, Langbaum Jessica BS, Alexander GE, Klunk WE, Mathis CA, Price JC, Aizenstein HJ, DeKosky ST, Caselli RJ. Fibrillar amyloid-beta burden in cognitively normal people at 3 levels of genetic risk for Alzheimer's disease. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:6820–5. doi: 10.1073/pnas.0900345106. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/1160510
- 18.Nagy Z, Esiri MM, Jobst KA, Johnston C, Litchfield S, Sim E, Smith AD. Influence of the apolipoprotein E genotype on amyloid deposition and neurofibrillary tangle formation in Alzheimer's disease. Neuroscience. 1995;69:757–61. doi: 10.1016/0306-4522(95)00331-C. [DOI] [PubMed] [Google Scholar]
- 19.Kivipelto M, Rovio S, Ngandu T, Kåreholt I, Eskelinen M, Winblad B, Hachinski V, Cedazo-Minguez A, Soininen H, Tuomilehto J, Nissinen A. Apolipoprotein E epsilon4 magnifies lifestyle risks for dementia: a population-based study. Journal of cellular and molecular medicine. 2008;12:2762–71. doi: 10.1111/j.1582-4934.2008.00296.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salloway S, Sperling R, Gilman S, Fox NC, Blennow K, Raskind M, Sabbagh M, Honig LS, Doody R, van Dyck CH, Mulnard R, Barakos J, Gregg KM, Liu E, Lieberburg I, Schenk D, Black R, Grundman M. A phase 2 multiple ascending dose trial of bapineuzumab in mild to moderate Alzheimer disease. Neurology. 2009;73:2061–70. doi: 10.1212/WNL.0b013e3181c67808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Frikke-Schmidt R, Nordestgaard BG, Thudium D, Moes Grønholdt ML. APOE genotype predicts AD and other dementia but not ischemic cerebrovascular disease. Neurology. 2001;56:194–200. doi: 10.1212/WNL.56.2.194. [DOI] [PubMed] [Google Scholar]
- 22.Ballard C, Gauthier S, Corbett A, Brayne C, Aarsland D, Jones E. Alzheimer's disease. Lancet. 2011;377:1019–31. doi: 10.1016/S0140-6736(10)61349-9. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718074541
- 23.Wu L, Rosa-Neto P, Hsiung GR, Sadovnick AD, Masellis M, Black SE, Jia J, Gauthier S. Early-onset familial Alzheimer's disease (EOFAD) The Canadian journal of neurological sciences. Le journal canadien des sciences neurologiques. 2012;39:436–45. doi: 10.1017/S0317167100013949. [DOI] [PubMed] [Google Scholar]
- 24.Bateman RJ, Aisen PS, Strooper B de, Fox NC, Lemere CA, Ringman JM, Salloway S, Sperling RA, Windisch M, Xiong C. Autosomal-dominant Alzheimer's disease: a review and proposal for the prevention of Alzheimer's disease. Alzheimer's research & therapy. 2011;3:1. doi: 10.1186/alzrt59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mangialasche F, Weili X, Kivipelto M. Prevention of Alzheimer's Disease: Intervention Studies. In: Zerr I, editor. Understanding Alzheimer's Disease. InTech; 2013. [DOI] [Google Scholar]
- 26.Qiu C, Winblad B, Fratiglioni L. The age-dependent relation of blood pressure to cognitive function and dementia. The Lancet. Neurology. 2005;4:487–99. doi: 10.1016/S1474-4422(05)70141-1. [DOI] [PubMed] [Google Scholar]
- 27.Mielke MM, Zandi PP, Sjögren M, Gustafson D, Ostling S, Steen B, Skoog I. High total cholesterol levels in late life associated with a reduced risk of dementia. Neurology. 2005;64:1689–95. doi: 10.1212/01.WNL.0000161870.78572.A5. [DOI] [PubMed] [Google Scholar]
- 28.Kivipelto M, Helkala E, Laakso MP, Hänninen T, Hallikainen M, Alhainen K, Iivonen S, Mannermaa A, Tuomilehto J, Nissinen A, Soininen H. Apolipoprotein E epsilon4 allele, elevated midlife total cholesterol level, and high midlife systolic blood pressure are independent risk factors for late-life Alzheimer disease. Annals of internal medicine. 2002;137:149–55. doi: 10.7326/0003-4819-137-3-200208060-00006. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1008693
- 29.Kivipelto M, Ngandu T, Fratiglioni L, Viitanen M, Kåreholt I, Winblad B, Helkala E, Tuomilehto J, Soininen H, Nissinen A. Obesity and vascular risk factors at midlife and the risk of dementia and Alzheimer disease. Archives of neurology. 2005;62:1556–60. doi: 10.1001/archneur.62.10.1556. [DOI] [PubMed] [Google Scholar]
- 30.Atti AR, Palmer K, Volpato S, Winblad B, Ronchi D de, Fratiglioni L. Late-life body mass index and dementia incidence: nine-year follow-up data from the Kungsholmen Project. Journal of the American Geriatrics Society. 2008;56:111–6. doi: 10.1111/j.1532-5415.2007.01458.x. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/720519008
- 31.von Arnim Christine AF, Gola U, Biesalski HK. More than the sum of its parts? Nutrition in Alzheimer's disease. Nutrition. 2010;26:694–700. doi: 10.1016/j.nut.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 32.Eskelinen MH, Ngandu T, Tuomilehto J, Soininen H, Kivipelto M. Midlife coffee and tea drinking and the risk of late-life dementia: a population-based CAIDE study. Journal of Alzheimer's disease: JAD. 2009;16:85–91. doi: 10.3233/JAD-2009-0920. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/1147566
- 33.Jicha GA, Markesbery WR. Omega-3 fatty acids: potential role in the management of early Alzheimer's disease. Clinical interventions in aging. 2010;5:45–61. doi: 10.2147/CIA.S5231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anttila T, Helkala E, Viitanen M, Kåreholt I, Fratiglioni L, Winblad B, Soininen H, Tuomilehto J, Nissinen A, Kivipelto M. Alcohol drinking in middle age and subsequent risk of mild cognitive impairment and dementia in old age: a prospective population based study. BMJ (Clinical research ed.) 2004;329:539. doi: 10.1136/bmj.38181.418958.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panza F, Frisardi V, Seripa D, Logroscino G, Santamato A, Imbimbo BP, Scafato E, Pilotto A, Solfrizzi V. Alcohol consumption in mild cognitive impairment and dementia: harmful or neuroprotective? International journal of geriatric psychiatry. 2012;27:1218–38. doi: 10.1002/gps.3772. [DOI] [PubMed] [Google Scholar]
- 36.Mi W, van Wijk N, Cansev M, Sijben John WC, Kamphuis Patrick JGH. Nutritional approaches in the risk reduction and management of Alzheimer's disease. Nutrition (Burbank, Los Angeles County, Calif.) 2013;29:1080–9. doi: 10.1016/j.nut.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 37.Maguire NC, Drozd K, Luff RD, Kantor RR. Immunohistochemical localization of glutathione S-transferase in preneoplastic and neoplastic lesions of the human uterine cervix. Acta cytologica. 1991;35:94–9. [PubMed] [Google Scholar]
- 38.Johansson L, Guo X, Hällström T, Norton MC, Waern M, Ostling S, Bengtsson C, Skoog I. Common psychosocial stressors in middle-aged women related to longstanding distress and increased risk of Alzheimer's disease: a 38-year longitudinal population study. BMJ open. 2013;3:e003142. doi: 10.1136/bmjopen-2013-003142. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718129107
- 39.Ahlskog JE, Geda YE, Graff-Radford NR, Petersen RC. Physical exercise as a preventive or disease-modifying treatment of dementia and brain aging. Mayo Clinic proceedings. 2011;86:876–84. doi: 10.4065/mcp.2011.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kivipelto M, Ngandu T, Laatikainen T, Winblad B, Soininen H, Tuomilehto J. Risk score for the prediction of dementia risk in 20 years among middle aged people: a longitudinal, population-based study. The Lancet. Neurology. 2006;5:735–41. doi: 10.1016/S1474-4422(06)70537-3. [DOI] [PubMed] [Google Scholar]
- 41.Exalto LG, Quesenberry CP, Barnes D, Kivipelto M, Biessels GJ, Whitmer RA. Midlife risk score for the prediction of dementia four decades later. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2014;10:562–70. doi: 10.1016/j.jalz.2013.05.1772. [DOI] [PubMed] [Google Scholar]
- 42.Sindi SCE, Fokkens J, Ngandu T, Soininen H, Tuomilehto J, Kivipelto M. The CAIDE Dementia Risk Score App: The development of an evidence-based mobile application to predict dementia. 13th International Geneva/Springfield Symposium on Advances in Alzheimer Therapy; 2014; Geneva, Switzerland. [Google Scholar]
- 43.CAIDE Dementia Risk App [ http://www.memantine.com/en/patients_and_caregivers/everyday_life_management/caide_risk_factor_app/caide_risk_factor_app.php]
- 44.Imtiaz B, Tolppanen A, Kivipelto M, Soininen H. Future directions in Alzheimer's disease from risk factors to prevention. Biochemical pharmacology. 2014;88:661–70. doi: 10.1016/j.bcp.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 45.Norton S, Matthews FE, Barnes DE, Yaffe K, Brayne C. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. The Lancet. Neurology. 2014;13:788–94. doi: 10.1016/S1474-4422(14)70136-X. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718500180
- 46.Langa KM, Larson EB, Karlawish JH, Cutler DM, Kabeto MU, Kim SY, Rosen AB. Trends in the prevalence and mortality of cognitive impairment in the United States: is there evidence of a compression of cognitive morbidity? Alzheimer's & dementia: the journal of the Alzheimer's Association. 2008;4:134–44. doi: 10.1016/j.jalz.2008.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/721401658
- 47.Manton KC, Gu XL, Ukraintseva SV. Declining prevalence of dementia in the U.S. elderly population. Advances in gerontology = Uspekhi gerontologii / Rossiĭskaȋȃ akademȋȋȃ nauk, Gerontologicheskoe obshchestvo. 2005;16:30–7. [PubMed] [Google Scholar]
- 48.Matthews FE, Arthur A, Barnes LE, Bond J, Jagger C, Robinson L, Brayne C. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study, I and II. Lancet. 2013;382:1405–12. doi: 10.1016/S0140-6736(13)61570-6. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718043512
- 49.Qiu C, Strauss E von, Bäckman L, Winblad B, Fratiglioni L. Twenty-year changes in dementia occurrence suggest decreasing incidence in central Stockholm, Sweden. Neurology. 2013;80:1888–94. doi: 10.1212/WNL.0b013e318292a2f9. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718001656
- 50.Schrijvers EMC, Verhaaren BFJ, Koudstaal PJ, Hofman A, Ikram MA, Breteler MMB. Is dementia incidence declining?: Trends in dementia incidence since 1990 in the Rotterdam Study. Neurology. 2012;78:1456–63. doi: 10.1212/WNL.0b013e3182553be6. [DOI] [PubMed] [Google Scholar]
- 51.Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2013;9:63–75.e2. doi: 10.1016/j.jalz.2012.11.007. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/721401309
- 52.Ritchie K, Carrière I, Ritchie CW, Berr C, Artero S, Ancelin M. Designing prevention programmes to reduce incidence of dementia: prospective cohort study of modifiable risk factors. BMJ (Clinical research ed) 2010;341:c3885. doi: 10.1136/bmj.c3885. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/725442103
- 53.Daviglus ML, Plassman BL, Pirzada A, Bell CC, Bowen PE, Burke JR, Connolly ES, Dunbar-Jacob JM, Granieri EC, McGarry K, Patel D, Trevisan M, Williams JW. Risk factors and preventive interventions for Alzheimer disease: state of the science. Archives of neurology. 2011;68:1185–90. doi: 10.1001/archneurol.2011.100. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/720365828
- 54.Peters R, Beckett N, Forette F, Tuomilehto J, Clarke R, Ritchie C, Waldman A, Walton I, Poulter R, Ma S, Comsa M, Burch L, Fletcher A, Bulpitt C. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. The Lancet. Neurology. 2008;7:683–9. doi: 10.1016/S1474-4422(08)70143-1. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718034228
- 55.Daviglus ML, Bell CC, Berrettini W, Bowen PE, Connolly ES, Cox NJ, Dunbar-Jacob JM, Granieri EC, Hunt G, McGarry K, Patel D, Potosky AL, Sanders-Bush E, Silberberg D, Trevisan M. National Institutes of Health State-of-the-Science Conference statement: preventing alzheimer disease and cognitive decline. Annals of internal medicine. 2010;153:176–81. doi: 10.7326/0003-4819-153-3-201008030-00260. [DOI] [PubMed] [Google Scholar]
- 56.Mangialasche F, Kivipelto M, Solomon A, Fratiglioni L. Dementia prevention: current epidemiological evidence and future perspective. Alzheimer's research & therapy. 2012;4:6. doi: 10.1186/alzrt104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Coley N, Andrieu S, Gardette V, Gillette-Guyonnet S, Sanz C, Vellas B, Grand A. Dementia prevention: methodological explanations for inconsistent results. Epidemiologic reviews. 2008;30:35–66. doi: 10.1093/epirev/mxn010. [DOI] [PubMed] [Google Scholar]
- 58.Carrillo MC, Brashear HR, Logovinsky V, Ryan JM, Feldman HH, Siemers ER, Abushakra S, Hartley DM, Petersen RC, Khachaturian AS, Sperling RA. Can we prevent Alzheimer's disease? Secondary “prevention” trials in Alzheimer's disease. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2013;9:123–131.e1. doi: 10.1016/j.jalz.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 59.Jack CR, Albert MS, Knopman DS, McKhann GM, Sperling RA, Carrillo MC, Thies B, Phelps CH. Introduction to the recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2011;7:257–62. doi: 10.1016/j.jalz.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718074539
- 60.Dubois B, Feldman HH, Jacova C, Hampel H, Molinuevo JL, Blennow K, DeKosky ST, Gauthier S, Selkoe D, Bateman R, Cappa S, Crutch S, Engelborghs S, Frisoni GB, Fox NC, Galasko D, Habert M, Jicha GA, Nordberg A, Pasquier F, Rabinovici G, Robert P, Rowe C, Salloway S, Sarazin M, Epelbaum S, de Souza Leonardo C, Vellas B, Visser PJ, Schneider L, et al. Advancing research diagnostic criteria for Alzheimer's disease: the IWG-2 criteria. The Lancet. Neurology. 2014;13:614–29. doi: 10.1016/S1474-4422(14)70090-0. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718426129
- 61.Dubois B, Feldman HH, Jacova C, Cummings JL, DeKosky ST, Barberger-Gateau P, Delacourte A, Frisoni G, Fox NC, Galasko D, Gauthier S, Hampel H, Jicha GA, Meguro K, O‘Brien J, Pasquier F, Robert P, Rossor M, Salloway S, Sarazin M, de Souza Leonardo C, Stern Y, Visser PJ, Scheltens P. Revising the definition of Alzheimer's disease: a new lexicon. The Lancet. Neurology. 2010;9:1118–27. doi: 10.1016/S1474-4422(10)70223-4. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/6918956
- 62.European Medicines Agency Qualification opinion of Alzheimer's disease novel methodologies/biomarkers for the use of CSF AB 1-42 and t-tau and/or PET-amyloid imaging (positive/negative) as biomarkers for enrichment, for use in regulatory clinical trials in mild and moderate Alzheimer's disease. EMA/CHMP/SAWP/893622/2011. 2012 [ http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2012/04/WC500125019.pdf]
- 63.European Medicines Agency Qualification opinion of Alzheimer's disease novel methodologies/biomarkers for PET amyloid imaging (positive/negative) as a biomarker for enrichment, for use in regulatory clinical trials in predementia Alzheimer's disease. EMA/CHMP/SAWP/892998/2011. 2012 [ http://www.ema.europa.eu/docs/en_GB/document_library/Regulatory_and_procedural_guideline/2012/04/WC500125018.pdf]
- 64.United States Food and Drug Administration Guidance for industry Alzheimer's disease: developing drugs for the treatment of early stage disease (FDA-2013-D-0077) draft. US Food and Drug Administration, Center for Drug Evaluation and Research. 2013 [Google Scholar]
- 65.Schneider LS, Mangialasche F, Andreasen N, Feldman H, Giacobini E, Jones R, Mantua V, Mecocci P, Pani L, Winblad B, Kivipelto M. Clinical trials and late-stage drug development for Alzheimer's disease: an appraisal from 1984 to 2014. Journal of internal medicine. 2014;275:251–83. doi: 10.1111/joim.12191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kivipelto M, Solomon A, Ahtiluoto S, Ngandu T, Lehtisalo J, Antikainen R, Bäckman L, Hänninen T, Jula A, Laatikainen T, Lindström J, Mangialasche F, Nissinen A, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Soininen H. The Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER): study design and progress. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2013;9:657–65. doi: 10.1016/j.jalz.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 67.Richard E, Van den Heuvel Esther, Moll van Charante Eric P, Achthoven L, Vermeulen M, Bindels PJ, Van Gool Willem A. Prevention of dementia by intensive vascular care (PreDIVA): a cluster-randomized trial in progress. Alzheimer disease and associated disorders. 2009;23:198–204. doi: 10.1097/WAD.0b013e31819783a4. [DOI] [PubMed] [Google Scholar]
- 68.Carrié I, van Kan G Abellan, Gillette-Guyonnet S, Andrieu S, Dartigues J, Touchon J, Dantoine T, Rouaud O, Bonnefoy M, Robert P, Cuffi M, Bories L, Bordes S, Gasnier Y, Desclaux F, Sudres K, Pesce A, Vellas B. Recruitment strategies for preventive trials. The MAPT study (MultiDomain Alzheimer Preventive Trial) The journal of nutrition, health & aging. 2012;16:355–9. doi: 10.1007/s12603-012-0046-8. [DOI] [PubMed] [Google Scholar]
- 69.Gillette-Guyonnet S, Andrieu S, Dantoine T, Dartigues J, Touchon J, Vellas B. Commentary on “A roadmap for the prevention of dementia II. Leon Thal Symposium 2008.” The Multidomain Alzheimer Preventive Trial (MAPT): a new approach to the prevention of Alzheimer's disease. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2009;5:114–21. doi: 10.1016/j.jalz.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 70.Morris JC, Heyman A, Mohs RC, Hughes JP, van Belle G, Fillenbaum G, Mellits ED, Clark C. The Consortium to Establish a Registry for Alzheimer's Disease (CERAD). Part I. Clinical and neuropsychological assessment of Alzheimer's disease. Neurology. 1989;39:1159–65. doi: 10.1212/WNL.39.9.1159. [DOI] [PubMed] [Google Scholar]
- 71.Ngandu T, Lehtisalo J, Levälahti E, Laatikainen T, Lindström J, Peltonen M, Solomon A, Ahtiluoto S, Antikainen R, Hänninen T, Jula A, Mangialasche F, Paajanen T, Pajala S, Rauramaa R, Strandberg T, Tuomilehto J, Soininen H, Kivipelto M. Recruitment and baseline characteristics of participants in the Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER)-a randomized controlled lifestyle trial. International journal of environmental research and public health. 2014;11:9345–60. doi: 10.3390/ijerph110909345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, Bäckman L, Hanninen T, Jula A, Laatikainen T, et al. FINGER - A Multidomain Two-Year Randomised Trial to Prevent Cognitive Decline. The Lancet. 2015 doi: 10.1016/S0140-6736(15)60461-5. (In Press) [DOI] [PubMed] [Google Scholar]
- 73.European Dementia Prevention Initiative (EDPI) network. [ http://www.edpi.org/]
- 74.The Healthy Aging Through Internet Counseling in the Elderly‘ (HATICE) project. [ http://www.hatice.eu/]
- 75.Joint Programme - Neurodegenerative Disease Research (JPND) - Results of the 2013 JPND Transnational call for “European research projects for pilot studies on preventive strategies related to Neurodegenerative Diseases. [ http://www.neurodegenerationresearch.eu/initiatives/annual-calls-for-proposals/closed-calls/preventive-strategies-2013/results-of-call-for-pilot-studies-on-preventive-strategies/]
- 76.Cummings JL, Morstorf T, Zhong K. Alzheimer's disease drug-development pipeline: few candidates, frequent failures. Alzheimer's research & therapy. 2014;6:37. doi: 10.1186/alzrt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mangialasche F, Solomon A, Winblad B, Mecocci P, Kivipelto M. Alzheimer's disease: clinical trials and drug development. The Lancet. Neurology. 2010;9:702–16. doi: 10.1016/S1474-4422(10)70119-8. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/718074538
- 78.Golde TE, Schneider LS, Koo EH. Anti-aβ therapeutics in Alzheimer's disease: the need for a paradigm shift. Neuron. 2011;69:203–13. doi: 10.1016/j.neuron.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/8654956
- 79.Sperling RA, Karlawish J, Johnson KA. Preclinical Alzheimer disease-the challenges ahead. Nature reviews. Neurology. 2013;9:54–8. doi: 10.1038/nrneurol.2012.241. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/718189988
- 80.Sperling RA, Rentz DM, Johnson KA, Karlawish J, Donohue M, Salmon DP, Aisen P. The A4 study: stopping AD before symptoms begin? Science translational medicine. 2014;6:228fs13. doi: 10.1126/scitranslmed.3007941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Worley S. After disappointments, Alzheimer's researchers seek out new paths: biomarkers and combination therapies may lead to disease-modifying treatments, experts say. P T. 2014;39:365–374. [PMC free article] [PubMed] [Google Scholar]
- 82.Reiman EM, Quiroz YT, Fleisher AS, Chen K, Velez-Pardo C, Jimenez-Del-Rio M, Fagan AM, Shah AR, Alvarez S, Arbelaez A, Giraldo M, Acosta-Baena N, Sperling RA, Dickerson B, Stern CE, Tirado V, Munoz C, Reiman RA, Huentelman MJ, Alexander GE, Langbaum Jessica BS, Kosik KS, Tariot PN. Brain imaging and fluid biomarker analysis in young adults at genetic risk for autosomal dominant Alzheimer's disease in the presenilin 1 E280A kindred: a case-control study. The Lancet. Neurology. 2012;11:1048–56. doi: 10.1016/S1474-4422(12)70228-4. [DOI] [PMC free article] [PubMed] [Google Scholar]; http://f1000.com/prime/717963994
- 83.Reiman EM, Langbaum Jessica BS, Fleisher AS, Caselli RJ, Chen K, Ayutyanont N, Quiroz YT, Kosik KS, Lopera F, Tariot PN. Alzheimer's Prevention Initiative: a plan to accelerate the evaluation of presymptomatic treatments. Journal of Alzheimer's disease: JAD. 2011;26(Suppl 3):321–9. doi: 10.3233/JAD-2011-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alzheimer's Prevention Initiative. [ http://banneralz.org/research-plus-discovery/alzheimers-prevention-initiative.aspx]
- 85.Moulder KL, Snider BJ, Mills SL, Buckles VD, Santacruz AM, Bateman RJ, Morris JC. Dominantly Inherited Alzheimer Network: facilitating research and clinical trials. Alzheimer's research & therapy. 2013;5:48. doi: 10.1186/alzrt213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Morris JC, Aisen PS, Bateman RJ, Benzinger Tammie LS, Cairns NJ, Fagan AM, Ghetti B, Goate AM, Holtzman DM, Klunk WE, McDade E, Marcus DS, Martins RN, Masters CL, Mayeux R, Oliver A, Quaid K, Ringman JM, Rossor MN, Salloway S, Schofield PR, Selsor NJ, Sperling RA, Weiner MW, Xiong C, Moulder KL, Buckles VD. Developing an international network for Alzheimer research: The Dominantly Inherited Alzheimer Network. Clinical investigation. 2012;2:975–84. doi: 10.4155/cli.12.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.The Dominantly Inherited Alzheimer's Network (DIAN) [ http://www.dian-info.org/]
- 88.The A4 Study. [ http://www.adcs.org/Studies/A4.aspx]
- 89.The PREVENT Alzheimer Program. [ http://www.douglas.qc.ca/page/prevent-alzheimer-program]
- 90.Smith AD, Yaffe K. Dementia (including Alzheimer's disease) can be prevented: statement supported by international experts. Journal of Alzheimer's disease: JAD. 2014;38:699–703. doi: 10.3233/JAD-132372. [DOI] [PubMed] [Google Scholar]
- 91.International Diabetes Federation (IDF) Diabetes Atlas update 2014. [ http://www.idf.org/diabetesatlas/update-2014]
- 92.Alberti K George MM, Zimmet P. Global burden of disease--where does diabetes mellitus fit in? Nature reviews. Endocrinology. 2013;9:258–60. doi: 10.1038/nrendo.2013.54. [DOI] [PubMed] [Google Scholar]
- 93.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes research and clinical practice. 2011;94:311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 94.Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, Mullany EC, Biryukov S, Abbafati C, Abera SF, Abraham JP, Abu-Rmeileh Niveen ME, Achoki T, AlBuhairan FS, Alemu ZA, Alfonso R, Ali MK, Ali R, Guzman NA, Ammar W, Anwari P, Banerjee A, Barquera S, Basu S, Bennett DA, Bhutta Z, Blore J, Cabral N, Nonato IC, Chang J, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–81. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Henderson VW. Alzheimer's disease: review of hormone therapy trials and implications for treatment and prevention after menopause. The Journal of steroid biochemistry and molecular biology. 2014;142:99–106. doi: 10.1016/j.jsbmb.2013.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Reitz C. Dyslipidemia and the risk of Alzheimer's disease. Current atherosclerosis reports. 2013;15:307. doi: 10.1007/s11883-012-0307-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Walach H, Loef M. Towards Primary Prevention of Alzheimer's Disease. Am J Alzheimers Dis (Columbia) 2012;1:1–28. doi: 10.1016/j.jalz.2013.03.007. [DOI] [Google Scholar]
- 98.Vellas B, Carrillo MC, Sampaio C, Brashear HR, Siemers E, Hampel H, Schneider LS, Weiner M, Doody R, Khachaturian Z, Cedarbaum J, Grundman M, Broich K, Giacobini E, Dubois B, Sperling R, Wilcock GK, Fox N, Scheltens P, Touchon J, Hendrix S, Andrieu S, Aisen P. Designing drug trials for Alzheimer's disease: what we have learned from the release of the phase III antibody trials: a report from the EU/US/CTAD Task Force. Alzheimer's & dementia: the journal of the Alzheimer's Association. 2013;9:438–44. doi: 10.1016/j.jalz.2013.03.007. [DOI] [PubMed] [Google Scholar]; http://f1000.com/prime/725442104
- 99.Mullard A. Multicompany trials adapt to disciplines beyond cancer. Nature medicine. 2014;20:3. doi: 10.1038/nm0114-3. [DOI] [PubMed] [Google Scholar]
- 100.Innovative Medicine Initiative. [ http://www.imi.europa.eu]