SUMMARY
A major challenge in biology is determining how evolutionarily novel characters originate; however, mechanistic explanations for the origin of new characters are almost completely unknown. The evolution of pregnancy is an excellent system in which to study the origin of novelties because mammals preserve stages in the transition from egg laying to live birth. To determine the molecular bases of this transition, we characterized the pregnant/gravid uterine transcriptome from tetrapods to trace the evolutionary history of uterine gene expression. We show that thousands of genes evolved endometrial expression during the origins of mammalian pregnancy, including genes that mediate maternal-fetal communication and immunotolerance. Furthermore, thousands of cis-regulatory elements that mediate decidualization and cell-type identity in decidualized stromal cells are derived from ancient mammalian transposable elements (TEs). Our results indicate that one of the defining mammalian novelties evolved from DNA sequences derived from ancient mammalian TEs coopted into hormone-responsive regulatory elements distributed throughout the genome.
Graphical abstract
INTRODUCTION
A major challenge in biology is determining the genetic and molecular mechanisms that underlie phenotypic differences between species (Wagner and Lynch, 2010). While comparative studies in a few organisms such as plants, insects, birds, fish, and rodents have identified the molecular basis for the loss (and much more rarely the gain) of some complex traits (Hoekstra et al., 2006; Lang et al., 2012; Smith et al., 2013; Wang et al., 2011), the molecular mechanisms that underlie the divergence of morphological characters are almost entirely unknown– particularly for the origin of evolutionarily novel phenotypes (“novelties”). Among the most significant barriers to developing mechanistic explanations for the origin of evolutionary novelties is the lack of transitional forms among extant lineages and experimental systems amenable to detailed functional studies in nonmodel organisms.
Mammals are an ideal system in which toexplore the molecular mechanisms that underlie the evolution of novelties because numerous genomic and experimental resources have been developed for mammals and their slow-evolving genomes facilitate tracing the origins of the novel functional DNA sequences (Lowe and Haussler, 2012; Mikkelsen et al., 2007; Warren et al., 2008). In addition, extant mammals span several major transitions in the origins of major morphological, developmental, and physiological novelties, including mammary glands, the cochlea, placentation, and pregnancy. Monotremes such as the platypus and echidna, for example, are oviparous and lay thin, poorly mineralized eggs that hatch about 10 days after laying (Hill, 1936), but the embryo is retained in the uterus for 10–22 days, during which time it is nourished by maternal secretions delivered through a simple yolk-sac placenta (Hughes and Hall, 1998; Renfree and Shaw, 2001). Live birth (viviparity) evolved in the Therian mammals, the lineage that includes marsupial and Eutherian (“placental”) mammals, with the loss of the mineralized eggshell and yolk, and an elaboration of the placenta. Therian mammals, however, have dramatically different reproductive life histories. In marsupials, pregnancies are relatively short (mean 25 days), and in all but one lineage (macropods), gestation is completed within the span of a single estrous cycle (Renfree and Shaw, 2001). In contrast, Eutherian mammals have evolved prolonged pregnancies that can last up to 670 days (mean 131 days) and that interrupt the estrous cycle (Hughes and Hall, 1998; Renfree and Shaw, 2001).
An essential step in the establishment and maintenance of pregnancy in many Eutherian mammals is the differentiation (decidualization) of endometrial stromal fibroblasts (ESFs) into decidual stromal cells (DSCs) in response to progesterone, the second messenger cyclic AMP (cAMP), and in some species to fetal signals (Gellersen and Brosens, 2003; Gellersen et al., 2007). Decidualization induces large-scale gene regulatory, cellular, and physiological reprogramming in the endometrium, leading to dramatic gene expression changes, the influx of immunosuppressive immune cells, vascular remodeling, and secretory transformation of uterine glands (Aghajanova et al., 2011; Gellersen et al., 2007; Giudice, 2003). Decidualization evolved in the stem lineage of Eutherian mammals (Kin et al., 2014; Mess and Carter, 2006) and underlies the suite of traits that support prolonged pregnancy in Eutherians, including direct implantation of the blastocyst and trophoblast into maternal endometrium, pronounced maternal recognition of pregnancy, maternal-fetal communication, and maternal immunotolerance of the antigenically distinct fetus.
Many of the genes that underlie the origins of maternal provisioning, viviparity, decidualization, and the collection of innovations that characterize prolonged pregnancy in Eutherian mammals likely evolved to be expressed at the fetomaternal interface coincident with the origins of pregnancy. Thus, identifying these genes and determining the mechanisms by which they became expressed in the endometrium will reveal some of the crucial molecular mechanisms underlying the evolution of pregnancy. Here we use ancestral transcriptome reconstruction and functional genomics methods to delineate the evolutionary history of gene expression in the endometrium and trace the molecular origin of the regulatory landscape that orchestrates decidualization. We show that thousands of genes evolved endometrial expression coincident with the evolution of pregnancy, including the recruitment of genes that play essential roles in the establishment of fetal immunotolerance and maternal-fetal communication. Furthermore, we found that 194 distinct families of ancient mammalian transposable elements (TEs) are enriched within cis-regulatory elements active in human DSCs and that many of these TEs donated binding sites for transcription factors (TFs) that establish cell-type identity and progesterone responsiveness to DSCs. These data indicate that a defining evolutionary novelty in mammals evolved through the recruitment and loss of ancient genes into an evolutionarily novel tissue, primarily via TE-mediated origination of a new cis-regulatory landscape in endometrial stromal cells.
RESULTS
Endometrial Gene Expression Profiling
We used high-throughput Illumina sequencing (RNA-seq) to identify transcribed genes in the endometrium during pregnancy in five Eutherian mammals (dog, cow, horse, pig, and armadillo), a marsupial (short-tailed opossum), and a Monotreme (platypus) and combined these data with existing gene expression data from the decidualized Rhesus monkey endometrium (Liu et al., 2012), decidualized mouse endometrium (McConaha et al., 2011), pregnant lizard uterus (Brandley et al., 2012), chicken uterus (Chan et al., 2010), and frog uterus (Chan et al., 2009). We also used RNA-seq to identify transcribed genes in human decidualized endometrial stromal cells (DSCs) in culture and combined these data with existing gene expression data from human decidual natural killer (dNK) cells (Hanna et al., 2006), decidual macrophage cells (dMP) (Svensson et al., 2011), and decidual endothelial cells (dECs). Our complete dataset includes expression information for 19,641 protein-coding genes from 14 species, as well as all the major cell types found in the human endometrium during pregnancy; this sampling allows us to infer the lineage in which each gene evolved endometrial expression as well as the specific cell type(s) in which each gene is expressed.
Thousands of Genes Were Recruited into and Lost from Endometrial Expression in Early Mammals
We used a model-based method to transform continuous transcript abundance estimates from RNA-seq datasets into discrete character states (transcribed or not) and parsimony to reconstruct ancestral endometrial transcriptomes (Li et al., 2010; Wagner et al., 2012, 2013). We found that parsimony inferred 500 genes gained and 296 lost endometrial expression in the Mammalian stem-lineage, 1,167 genes gained and 239 lost endometrial expression in the Therian stem lineage, and 835 genes gained and 185 lost endometrial expression in the Eutherian stem lineage (Figure 1A; Table S1). We could assign the expression of 89% (2,218 of 2,480) of recruited genes to one or more of the major cell types found in the endometrium during pregnancy (Figures 1B and S1). Significantly more recruited genes were uniquely expressed in DSC than dNK (485 versus 96; p = 2.90 × 10−189, binomial test), dMP (485 versus 55; p = 4.36 × 10−269, binomial test), or dEC (485 versus 62; p = 8.13 × 10−292, binomial test), suggesting recruitment of genes into DSC expression played a particularly important role in the evolution of pregnancy.
Figure 1. Evolution of the Endometrial Transcriptome in Tetrapods.
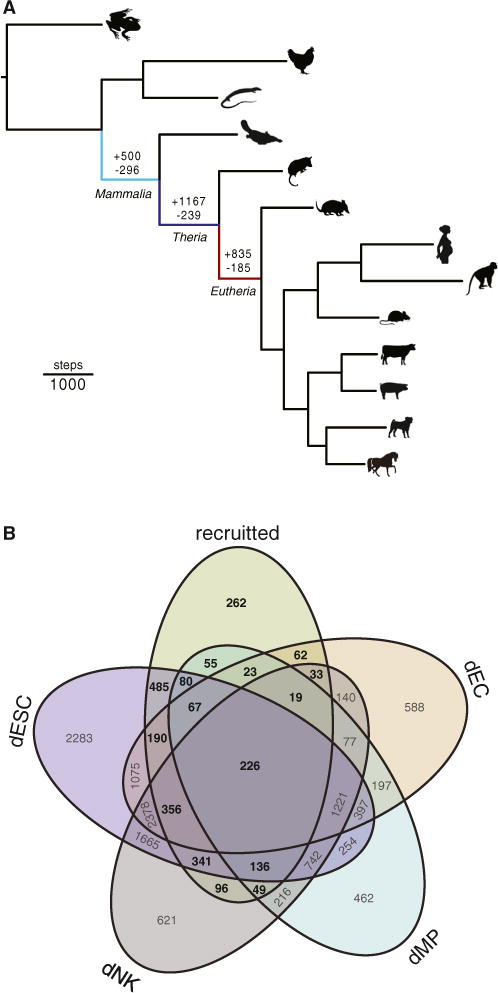
(A) Parsimony reconstruction of gene expression gain and loss events in the tetrapod endometrium. The numbers above branches indicate the number of genes recruited into (+) or lost (−) from endometrial expression in the stem lineages of Mammalia (light blue), Theria (blue), and Eutheria (red). Branch lengths are proportional to gene expression recruitment and loss events inferred by Wagner parsimony (see inset scale bar).
(B) Expression of recruited genes in the major endometrial cell types. Recruited, total number of genes recruited into endometrial expression in the stem lineages of Mammalia, Theria, and Eutheria (n = 2,502).
Recruited Genes Are Enriched in Immune, Signaling, and Reproductive Functions
We annotated each gene that evolved endometrial expression in the Mammalian, Therian, and Eutherian stem lineage by its expression domains in mouse to infer from which organ and tissue systems the expression of these genes were enriched and therefore likely recruited. We found that the expression of recruited genes was enriched in 33 distinct anatomical systems (false discovery rate [FDR] = 1%; Table S2) and that most genes were likely recruited from neural systems such as the brain (1083 genes), from digestive systems such as the gut (717 genes), and from the hemolymphoid system (661 genes; Figure 2A).
Figure 2. Recruited Genes Are Enriched in Immune, Signaling, and Reproductive Functions.
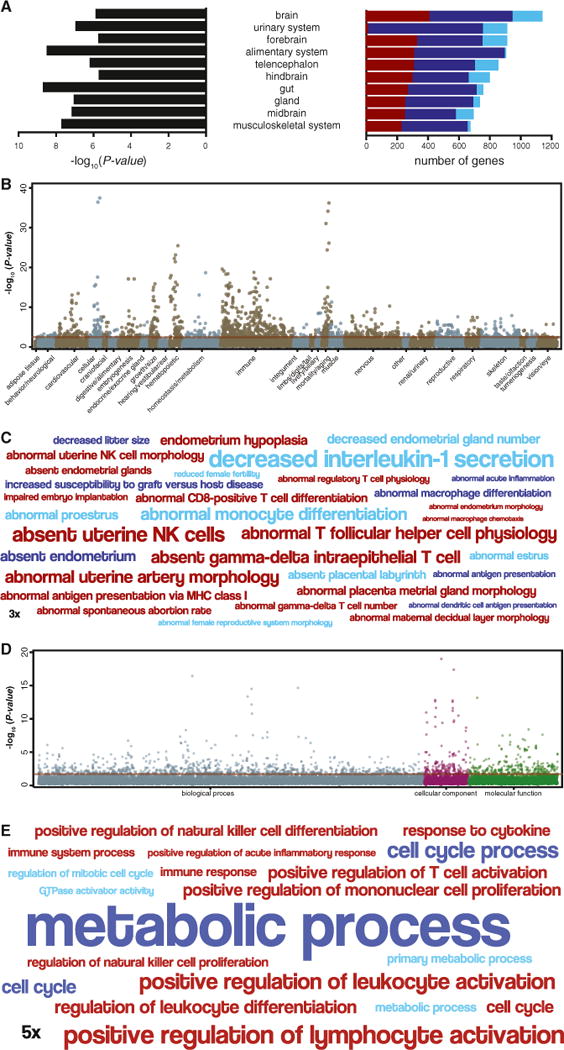
(A) Top 10 anatomical systems in which the expression of recruited genes is enriched. Anatomical system, center; −log10 p value of enrichment (hypergeometric), left. Stacked bar chart shows the number of genes recruited into endometrial expression in the Mammalian (light blue), Therian (blue), and Eutherian (red) stem lineage that are expressed in each anatomical system.
(B) Manhattan plot of −log10 p values (hypergeometric test) for mouse knockout phenotypes enriched among recruited genes. Phenotypes are grouped by anatomical system. Horizontal red line indicates the dataset-wide false discovery rate (FDR q value = 0.1).
(C) Word cloud of mouse knockout phenotypes enriched among genes recruited into endometrial expression in the Mammalian (light blue), Therian (blue), and Eutherian (red) stem-lineage. Abnormal phenotypes are scaled to the log2 enrichment of that term (see inset scale).
(D) Manhattan plot of −log10 p values (hypergeometric test) for GO terms enriched among recruited genes. Go terms are grouped into biological process, cellular component, and molecular function. Horizontal red line indicates the dataset-wide false discovery rate (FDR q value = 0.1).
(E) Word cloud of GO terms enriched among genes recruited into endometrial expression in the Mammalian (light blue), Therian (blue), and Eutherian (red) stem lineage. GO terms are scaled to the log2 enrichment of that term (see inset scale).
We also annotated genes that were recruited into endometrial expression by their Gene Ontology (GO) terms and mouse knockout phenotypes to infer the functional consequences of their recruitment. We found that Mammalian, Therian, and Eutherian recruited genes were enriched (FDR = 1%) in 87, 480, and 288 knockout phenotypes (Figures 2B and 2C; Table S3) and in 71, 93, and 28 GO terms (Figures 2D and 2E; Table S4), respectively, most of which are related to metabolic processes, immune responses, cell-cell signaling, cell-cell communication, and uterine defects. Among the most significantly enriched mouse knockout phenotypes, for example, are “absent uterine NK cells” (55.50-fold, hypergeometric p = 2.33 × 10−7, FDR q = 1.23 × 10−5), “abnormal professional antigen presenting cell physiology” (2.84-fold, hypergeometric p = 6.11 × 10−16, FDR q = 1.94 × 10−13), “abnormal female reproductive system morphology” (1.98-fold, hypergeometric p = 9.96 × 10−5, FDR q = 1.79 × 10−3), and “decreased interleukin-1 secretion” (101-fold, hypergeometric p = 1.46 × 10−4, FDR q = 6.83 × 10−3). The most enriched GO terms included “positive regulation of leukocyte activation” (2.85-fold, hypergeometric p = 8.30 × 10−7, FDR q = 1.91 × 10−3), “GTPase activator activity” (2.58-fold, hypergeometric p = 1.79 × 10−3, FDR q = 4.92 × 10−2), and “response to cytokine” (1.90-fold, hypergeometric p = 3.32 × 10−4, FDR q = 4.31 × 10−2). These data indicate that many of the pathways essential for implantation, such as regulation of GTPase activity (Grewal et al., 2010), and the establishment of maternal-fetal immunotolerance, such as the recruitment of uterine NK into the endometrium during pregnancy (Hanna et al., 2006), and maternal-fetal signaling through cytokines evolved in the stem-lineage of Therian and Eutherian mammals.
Pervasive Loss of Ion Transporters from Endometrial Expression in Early Mammals
Next we annotated genes that lost endometrial expression by their mouse knockout phenotypes and GO terms to infer the functional consequences of their loss. Genes that lost endometrial expression in the Mammalian, Therian, and Eutherian stem lineages were significantly enriched (FDR = 1%) in 133, 11, and 15 knockout phenotypes (Figures S2A and S2B) and in 115, 16, and 46 GO terms (Figures S2C and S2D), respectively, most of which were related to ion transport and behavioral defects associated with impaired ion transport in the nervous system. Unexpectedly, the most significantly enriched mouse knockout phenotypes are related to abnormal behavior, for example, “abnormal anxiety-related response” (5.04-fold, hypergeometric p = 7.53 × 10−9, FDR q = 2.13 × 10−5), and “abnormal chemical nociception” (13.19-fold, hypergeometric p = 1.02 × 10−6, FDR q = 2.55 × 10−4), whereas the most enriched GO terms included “transporter activity” (2.48-fold, hypergeometric p = 4.00 × 10−8, FDR q = 3.13 × 10−5), “positive regulation of secretion” (3.73-fold, hypergeometric p = 9.58 × 10−5, FDR q = 0.037), and “transporter activity” (2.50-fold, hypergeometric p = 8.46 × 10−6, FDR q = 0.004). These data suggest that the enrichment of abnormal behavioral phenotypes in the mouse knockout data is related to the loss of ion channels with pleiotropic functions in behavioral regulation in the nervous and reproductive systems.
Consistent with the mouse knockout phenotype data and GO analyses, we found that numerous ion transporters and ligand-gated and voltage-gated ion channels lost endometrial expression during the evolution of mammalian pregnancy (Figure S2E). Among the ion transporters and ion channels that lost endometrial expression are several that have previously been shown to be important for mineralization of avian eggshells, including ATP2B2, SLC12A5, SLC12A8, SLC26A9, and TRPV5 (Brionne et al., 2014; Jonchère et al., 2010, 2012). These data suggest the loss of ion channels from endometrial expression in early mammals is associated with the reduced mineralization of egg-shells in the mammalian stem lineage (Hill, 1936) and the complete loss of the eggshell in the Therian stem-lineage (Renfree and Shaw, 2001).
Recruited Genes Are More Dynamically Expressed than Ancestrally Expressed Genes
Next, we asked whether recruited genes had different expression dynamics compared with ancestrally expressed genes using several existing endometrial gene expression datasets (Talbi et al., 2006; Hess et al., 2007; Altmäe et al., 2010). We found a progressive increase in the tissue specificity of recruited genes, such that more recently recruited genes were significantly more tissue specific as well as more specifically expressed in the uterus than more anciently recruited and ancestrally expressed genes (Figure 3A). Transcripts of more recently recruited genes were also expressed at significantly lower levels, but had a greater range of expression, than more anciently recruited and ancestrally expressed genes (Figure 3B). To determine whether ancestral and recruited genes are differentially regulated during the reproductive cycle, we compared their expression levels in proliferative, early secretory, middle secretory, and late secretory phase human endometria. We found that Therian and Eutherian recruited genes had significantly greater variance in expression levels compared with ancestrally expressed genes throughout the menstrual cycle but that Mammalian recruited genes had similar expression dynamics as ancestrally expressed genes (Figure 3C). These data indicate that Therian and Eutherian recruited genes are more strongly differentially regulated during the menstrual cycle than mammalian recruited and ancestrally expressed genes. Similarly, recruited genes had significantly greater variance in expression levels in decidualized human endometrial cells 3 and 12 hr after treatment with trophoblast-conditioned media than ancestrally expressed genes(Figure 3D), indicating that they are more responsive to fetal signals than ancestrally expressed genes. Finally, recruited genes were more misregulated than ancestrally expressed genes during the window of implantation in the endometria of women with unexplained infertility compared with fertile controls (Figure 3E), consistent with an important role for recruited genes in the establishment of pregnancy.
Figure 3. Expression Dynamics of Recruited Genes.
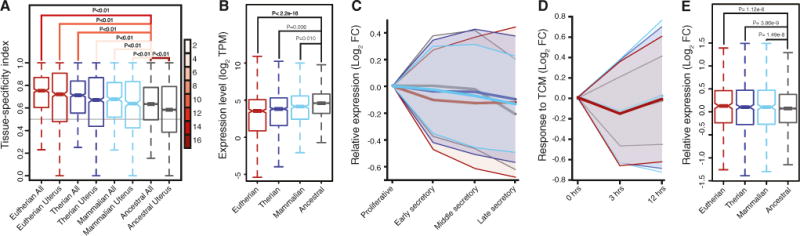
(A) Tissue specificity of recruited and ancestrally expressed genes across 27 tissues (all) and how specific the expression those genes are in the uterus. 0, not tissue specific; 1, tissue specific; p values shown for paired t tests, lines connecting comparisons are colored by the t value of the comparison (see scale at right).
(B) Expression level (log2 transcripts per million [TPM]) of recruited and ancestrally expressed genes; p values shown for paired t tests.
(C) Relative expression (log2 fold change [FC] of secretory samples relative to proliferative samples) of recruited and ancestrally expressed genes throughout the human menstrual cycle. Mean ± variance.
(D) Response of recruited and ancestrally expressed genes expressed in human endometrial stromal cells to trophoblast conditioned media (TCM); log2 fold change (FC) in gene expression 3 and 12 hr after treatment relative to control conditioned media (0 hr). Mean ± variance.
(E) Relative expression (log2 fold change [FC]) of recruited and ancestrally expressed genes in the endometria of women with unexplained infertility compared to fertile controls; p values shown for F test for variance.
Endometrial Regulatory Elements Are Enriched in Ancient Mammalian TEs
Previous studies have shown that TEs can act as hormone responsive regulatory elements in DSCs (Gerlo et al., 2006; Lynch et al., 2011; Emera and Wagner, 2012), suggesting that Mammalian-, Therian-, and Eutherian-specific TEs (hereafter “ancient mammalian” TEs) may have played a role in the recruitment of genes in endometrial expression in early mammals. To identify regulatory elements that may be derived from ancient mammalian TEs (AncMam-TEs) we mapped enhancers (H3K27ac chromatin immunoprecipitation sequencing [ChIP-seq]), promoters (H3K4me3 ChIP-seq), and regions of open chromatin (FAIRE-seq and DNaseI-seq) in cAMP/progesterone-treated human DSCs. We then intersected the location of these regulatory elements with the location of AncMam-TEs across the human genome (hg19).
We found that 59.9% of DNaseI-seq peaks, 30.0% of FAIRE-seq peaks, 57.7% of H3K27ac peaks, and 31.5% of H3K4me3 peaks overlapped AncMam-TE (Figure 4A), most of which were Mammalian- or Eutherian-specific (Figure 4B; Table S5). We also identified 194 AncMam-TEs families that were significantly enriched within cis-regulatory elements compared to their genomic abundances (Figure 4C; Table S6), 89.2% (173 of 194) of which are Eutherian specific (Figure 4D). Among the enriched AncMam-TEs are 930 copies of MER20 (1.98-fold, p = 2.79 × 10−8) a Eutherian-specific hAT-Charlie DNA transposon we have previously shown to function frequently as cAMP/progesterone-responsive cis-regulatory element in hDSCs (Lynch et al., 2011) and 377 individual “exapted” TEs previously shown to have evolved under strong purifying selection near developmental genes (Lowe et al., 2007). Among the exapted repeats are 25 copies of MER121 (5.42-fold, p = 0), one of the most constrained TE families in mammalian genomes (Kamal et al., 2006). These data indicate that AncMam-TEs, particularly Eutherian-specific TEs, played an important role in the genesis of cis-regulatory elements in DSCs coincident with the origins of decidualization.
Figure 4. Ancient Mammalian TEs Are Enriched in Regulatory Elements Active in Decidualized Human Endometrial Stromal Cells.
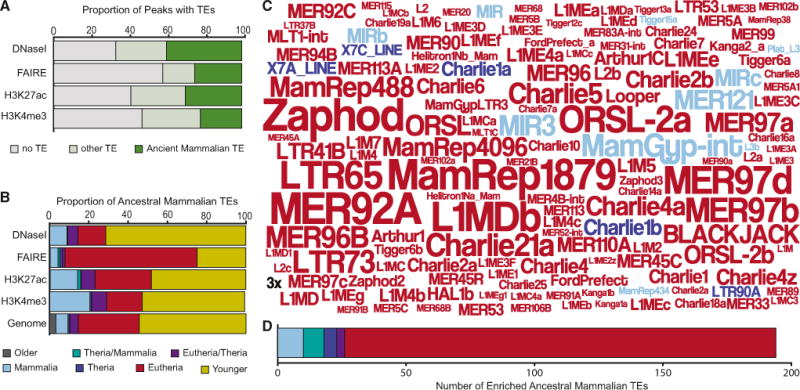
(A) Proportion of DNaseI-seq, FAIRE-seq, H3K27ac-seq, and H3K4me3-seq peaks that do not overlap annotated TEs (gray), overlap “ancient mammalian” TEs, i.e., those that integrated into the genome in the stem lineage of Mammalia, Thera, or Eutheria (green), and other TEs (light green).
(B) Proportion of TEs in DNaseI-, FAIRE-, H3K27ac-, and H3K4me3-seq peaks and the human genome (hg18) by age class.
(C) Word cloud of the 150 most enriched TEs among ancient mammalian elements, in DNaseI-, FAIRE-, H3K27ac-, and H3K4me3-seq peaks. The size of the transposon names corresponds to its enrichment (see inset 3-fold scale). Colors indicate lineage in which the TE originated: Mammalian-specific (light blue), Therian or Mammalian (green), Therian specific (blue), Eutherian or Therian (purple), Eutherian specific (red).
(D) Number of enriched TEs among ancient mammalian elements in DNaseI-, FAIRE-, H3K27ac-, and H3K4me3-seq peaks by age class.
Ancient Mammalian TEs Are Associated with Progesterone-Responsive Genes
Our observation that some AncMam-TE families are enriched within active regulatory elements in DSCs suggests they may play a role in orchestrating the transcriptional response of ESFs cells to cAMP and progesterone. To test this hypothesis, we used RNA-seq (Figure 5A) to compare the expression levels of genes associated with AncMam-TE containing cis-regulatory elements and genes without such association between undifferentiated ESFs and decidualized DSCs (Figure 5B). We found that genes associated with AncMam-TE containing regulatory elements were no more strongly differentially regulated upon cAMP/progesterone-induced decidualization than genes without such associations when we compared all genes expressed in DSCs (Figure 5C). However, we found that unambiguously recruited genes associated with AncMam-TE containing regulatory elements were more strongly differentially regulated upon decidualization than recruited genes without AncMam-TE containing regulatory elements (Figure 5C). Recruited genes associated with AncMam-TE containing regulatory elements were also more strongly differentially regulated upon decidualization than ancestrally expressed genes associated with AncMam-TE containing regulatory elements. These results suggest that AncMam-TEs play a role in orchestrating the transcriptional response of DSCs to cAMP/progesterone, particularly for those genes that evolved endometrial expression during the origins of pregnancy and decidualization.
Figure 5. Genes Associated with Ancient Mammalian TE-Derived Regulatory Elements Are More Strongly Differentially Regulated during Decidualization than Genes without TE-Derived Regulatory Elements.
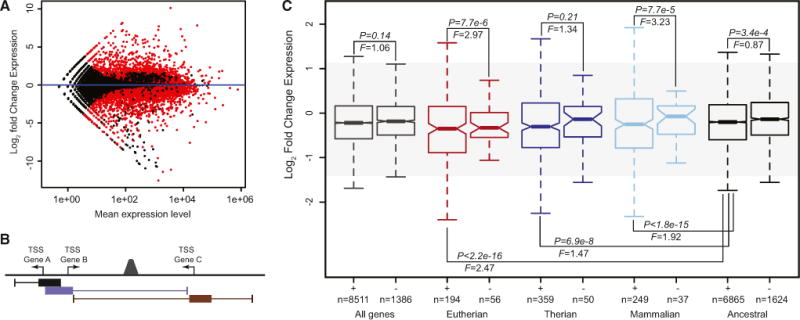
(A) MA-plot showing changes in gene expression 48hrs after treatment of human endometrial stromal cells with cAMP/Progesterone. Red dots indicate significantly differentially expressed genes (n = 7,337, N = 16,551).
(B) Cartoon of the GREAT association rule used to associate regulatory elements with nearby genes: regulatory element peaks located +5,000 to −1,000 bp of transcription start sites (TSSs) were defined as the basal regulatory domain (filled boxes represented below each TSS) for each gene (regardless of other nearby genes). Basal regulatory domains were extended in both directions to the nearest gene’s basal domain (thin lines) but no more than 100 kb. Under this association rule, for example, the regulatory element peak located between Gene B and Gene C would be associated with these genes but not Gene A.
(C) Recruited genes associated with ancient mammalian TE-derived regulatory elements (+) are more strongly differentially regulated upon cAMP/progesterone treatment (decidualization) than genes without TE-derived regulatory elements (−). Genes are grouped into all genes expressed in DSCs (gray), Eutherian recruited genes (red), Therian recruited genes (blue), Mammalian recruited genes (light blue), and ancestrally expressed genes (black). F is the ratio of variances from a two-sample F test, and p is the significance of the two-sample F test.
Ancient Mammalian TEs Mediate Decidualization and Endometrial Cell-type Identity
Next, we generated ChIP-seq data for the progesterone receptor (PGR), the principle transcriptional effector of progesterone signaling and decidualization, from cAMP/Progesterone treated DSCs and integrated this dataset with previously published ChIP-seq data for 132 TFs to identify TF binding sites (TFBSs) enriched in AncMam-TEs compared with non-TE derived regions of the genome (Eijkelenboom et al., 2013; Lo and Matthews, 2012; Tewari et al., 2012; Wang etal., 2012). We identified 50 TFBSs that were significantly enriched (FDR = 5%) in the AncMam-TE-derived portions of regulatory elements (Figure 6A; Table S7). Among the TFs with binding sites enriched in AncMam-TEs are those that mediate responses to hormones such as PGR (10-fold enrichment, p < 1.0 × 10−5) (Conneely et al., 2001; Lydon et al., 1995), NR4A1 (2.6-fold, p = 6.6 × 10−4) (Jiang et al., 2011), and ERRA (3-fold, p = 1.0 × 10−10) (Bombail et al., 2010), TFs essential for the successful establishment of pregnancy such as c-MYC (1.3-fold, p = 5.6 × 10−21) (Davis et al., 1993), AHR (1.5-fold, p = 4.0 × 10−4) (Hernández-Ochoa et al., 2009), and STAT1 (1.2-fold, p = 7.5 × 10−9) (Christian et al., 2001), as well as general TFs and numerous chromatin remodeling factors (Figure 6B). De novo motif discovery also identified many enriched motifs in the AncMam-TE-derived regions of regulatory element peaks compared with TE-free regulatory elements, many of which match consensus binding sites for TFs that function as master regulators of DSC cell-type identity including FOX, COUP-TF, HOX, and AHR/ARNT (Figure 6C). These data indicate that TEs contributed binding sites for TFs that directly mediate the response of stromal cells to progesterone (PGR) and that establish cell-type identity in endometrial cells (HOX and FOX) during the origins of decidualization.
Figure 6. Ancient Mammalian TEs Are Enriched in Binding Sites for TFs that Mediate Hormone Responsiveness and Endometrial Cell Identity.

(A) Distribution of enriched (red) and depleted (blue) TFBSs in ancient TE-derived segments of FAIRE-, DNaseI-, H3K27ac-, and H3K4me3-seq peaks compared with TF ChIP-seq peaks with no TE overlap. Fifty and 75 TFBSs were significantly enriched and depleted, respectively, at FDR = 5%.
(B) Word cloud of TFBSs enriched in ancient mammalian TE segments of ChIP-seq peaks compared to peaks with no TE overlap. Colors indicate TFs that mediate hormone responses (purple), remodel chromatin (light purple), have known functions in endometrial cells or that mediate immune responses (green), or with general regulatory functions (light green). Data shown for ≥ 1.2-fold enriched TFs at FDR = 5%.
(C) Word cloud of TF motifs enriched in ancient mammalian TE segments of ChIP-seq peaks compared with peaks with no TE overlap. Colors indicate motifs enriched in FAIRE- (red), DNaseI- (tan), H3K27ac- (dark blue), and H3K4me3-seq peaks (light blue). Data shown for ≥ 3-fold enriched motifs at FDR = 1 × 10−4 and E1 × 10−4.
Ancient Mammalian TEs Donated Functional PGR Binding Sites to the Genome
Our observations that AncMam-TEs are enriched in PGR binding sites and are associated with genes that are strongly differentially expressed in DSCs upon decidualization prompted us to explore AncMam-TE derived PGR binding sites in greater detail. We found that nearly all (1,721 of 1,777) of the AncMam-TE-derived PGR binding sites were found within Mammalian- or Eutherian-specific TEs (Figure 7A; Table S8) and that 53.7% of the AncMam-TEs that are enriched in PGR binding sites were Eutherian-specific (Figure 7B; Table S8). We also found that all genes associated with AncMam-TE-derived PGR binding sites were more strongly upregulated and more strongly differentially regulated upon decidualization than genes not associated with AncMam-TE-derived PGR binding sites (Figure 7C). Similarly, recruited and ancestrally expressed genes associated with AncMam-TE-derived PGR binding sites were more strongly up-regulated and more strongly differentially regulated than recruited and ancestral genes not associated with AncMam-TE-derived PGR binding sites or than recruited and ancestral genes without TE-derived regulatory elements (Figure 7C).
Figure 7. Ancient Mammalian TEs Globally Remodeled PGR Binding Site Architecture across the Genome.
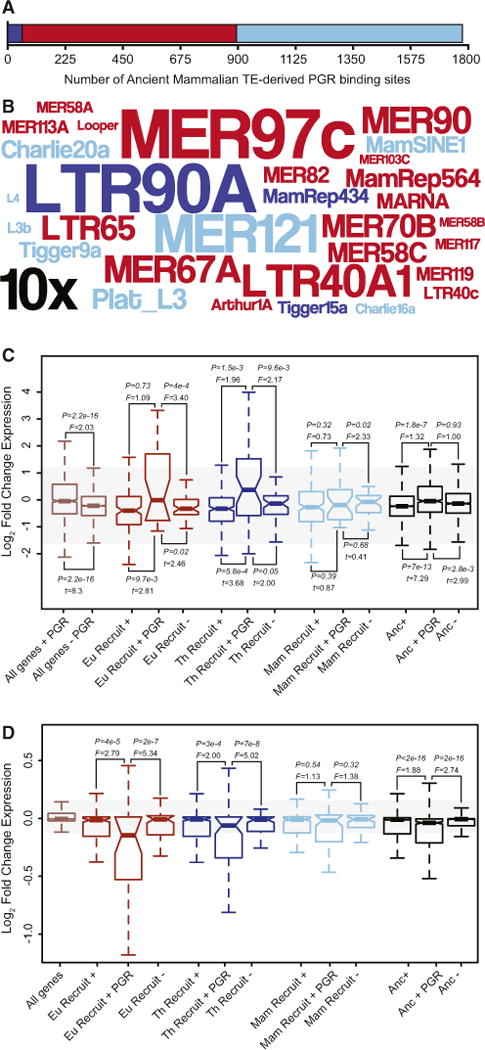
(A) The number of PGR ChIP-seq peaks that contain Mammalian-specific (light blue), Therian-specific (blue), and Eutherian-specific (red) TEs in primary cultures of hESCs treated with 10 nM 17β-estradiol, 100 nM medroxyprogesterone acetate, and 1 mM 8-bromo-cAMP.
(B) Word cloud of ancient mammalian TEs enriched within PGR ChIP-seq peaks. Colors follow (A). Inset scale (10×) shows 10-fold enrichment. Only elements with R2-fold enrichment are shown.
(C) Genes associated with ancient mammalian TE-derived PGR binding sites (+ PGR) are more strongly differentially regulated in hESCs upon decidualization than genes associated with ancient mammalian TE as a whole (+) and genes without TE-derived regulatory elements (−). Eu Recruit, Eutherian recruited gene; Th Recruit, Therian recruited gene; Mam Recruit, Mammalian recruited gene; Anc, ancestrally expressed gene. F is the ratio of variances from a two-sample F test.
(D) Genes associated with ancient mammalian TE-derived PGR biding sites (+ PGR) are more strongly dysregulated by PGR knockdown in DSCs than either genes associated with ancient mammalian TE as a whole (+) and genes without TE-derived regulatory elements (−). F is the ratio of variances from a two-sample F test.
These data suggest that AncMam-TE derived PGR binding sites orchestrate most of the transcriptional response of DSCs to progesterone. To test further this hypothesis, we used previously published data to compare the effects of PGR knockdown in cAMP/progesterone-treated DSCs (Pabona et al., 2012) on the expression of genes associated with AncMam-TE derived regulatory elements and PGR binding sites to genes without such associations. We found that ancestrally expressed and recruited genes associated with AncMam-TE-derived PGR binding sites were more strongly misregulated by PGR knockdown than genes without AncMam-TE-derived PGR binding sites (Figure 7D). These data are consistent with an important role for TE-derived PGR binding sites in the transcriptional response of ESFs to cAMP/progesterone and therefore decidualization into DSCs.
DISCUSSION
A major challenge in developmental evolution is determining the genetic changes that underlie morphological differences between species, particularly the changes that have generated evolutionary novelties such as mammalian pregnancy (Wagner and Lynch, 2010). While it is clear that the evolution of gene regulatory networks is an essential part of developmental evolution, the mechanisms that underlie regulatory network divergence are debated (Carroll, 2005; 2008; Lynch and Wagner, 2008; Prud’homme et al., 2007; Wagner and Lynch, 2010). Comparative studies in a few well-studied organisms suggest that gain and loss of cis-regulatory elements provides the molecular basis for the divergence of morphological traits between species (Hoekstra et al., 2006; Lang et al., 2012; Smith et al., 2013; Wang et al., 2011), but whether the gradual gene-by-gene gain and loss of individual regulatory elements is sufficient to explain the origin of new gene regulatory networks and therefore the origin of evolutionary novelties is not clear.
TE-mediated rewiring of gene regulatory networks is an attractive alternative to the gene-by-gene origination of cis-regulatory elements because it provides a mechanism to rapidly distribute nearly identical copies of regulatory elements across the genome that are capable of responding to the same input signals (Britten and Davidson, 1969; Davidson and Britten, 1979; McClintock, 1984). Thus far, however, there is little evidence that TEs contribute to the genesis of novel regulatory networks rather than contributing to the turnover of regulatory elements within an existing gene regulatory network. Our results indicate that a major mechanism in the origin of pregnancy was the recruitment of genes that were ancestrally expressed in other organ and tissue systems into endometrial expression, which imparted the endometrium with new functions such as immunoregulation and maternal-fetal signaling. Our data also suggest that TEs that amplified prior to the divergence of Eutherian mammals played a central role in recruiting these genes into endometrial expression and thereby in the origin of decidualization because they apparently deposited binding sites for master transcriptional regulators of endometrial stromal cell-type identity and progesterone responsiveness to numerous genes across the genome.
Taken together, our data suggest that novel gene regulatory networks and cell-type identities can evolve through large-scale genome-wide changes rather than gradual gene-by-gene changes (Goldschmidt, 1940). TEs may play a particularly important role in this process because they provide a mechanism to coordinately regulate the expression of numerous genes to the same stimuli upon their integration into multiple locations in the genome, alleviating the need for the de novo evolution of cis-regulatory elements capable of directing stereotyped responses to the same stimuli one gene at a time across the genome (Britten and Davidson, 1969; Davidson and Britten, 1979; Feschotte, 2008; McClintock, 1984).
EXPERIMENTAL PROCEDURES
Transcriptome Sequencing
Total RNA from endometrial samples from midstage pregnant platypus, opossum, armadillo, and dog, and day 14–18 postimplantation cow, horse, and pig were dissected was extracted using the QIAGEN RNA-Easy Midi RNA-extraction kit followed by on-column DNase treatment (QIAGEN). RNA samples were sequenced using the Illumina Genome Analyzer II platform (75 bp reads), following the protocol suggested by Illumina for sequencing of cDNA samples. mRNA-seq reads were quality controlled following standard methods and species specific reads mapped to the human (GRCh37), macaque (MMUL_1), mouse (NCBIM37), dog (BROADD2), cow (UMD3.1), horse (EquCab2), pig (Sscrofa9), armadillo (dasNov2), opossum (monDom5), platypus (OANA5), and chicken (WASHUC2) gene builds at Ensembl using Bowtie with default parameters.
Parsimony Reconstruction of Gene Expression Gain/Loss
We used parsimony to reconstruct gene expression gains and losses using the method implemented in the pars program from PhyML (v.2.4.4). We used Mesquite (v.2.75) to identify the number of genes that most parsimoniously gained or lost endometrial expression; expression was classified as most parsimoniously a gain if a gene was inferred as not expressed the ancestral node (state 0) but inferred likely expressed in a descendent node (state 1/[0/1]) and vice versa for the classification of a loss from endometrial expression.
ChIP-Seq, DNaseI-Seq, and FAIRE-Seq Data Generation
See the Supplemental Experimental Procedures.
Identification of TE-Containing Regulatory Elements
To identify regulatory elements derived from TEs, we first intersected regulatory element peaks from the analyses described above with human TE annotation of the hg18 assembly (files from the U.C.S.C genome browser, with Repeat Masker v.3.2.7 and repbase libraries released on 20050112) using BEDTools. The ratio between counts (in-set divided by in-genome) is used to estimate whether a given TE is enriched or depleted. Significance of enrichment was inferred on TE counts with three standard statistical tests (binomial, hypergeometric, and Poisson models), but figures are built on length since it is more representative of a given TE contribution.
Region-Gene Associations, Motif, and TFBS Finding
We used the Genomic Regions Enrichment of Annotations Tool (GREAT) (McLean et al., 2010) to associated regulatory elements with nearby genes using the default association rules. To identify ancestral and recruited genes that were expressed in human endometrial stromal cells (hESCs), we intersected ancestral and recruited genes sets with the set of genes expressed in hESCs. To identify sequence motifs that were enriched in “TE-derived” regulatory elements, we used a de novo motif discovery approach. First we used CisFinder webserver (http://lgsun.grc.nia.nih.gov/CisFinder/) to identify overrepresented short DNA motifs in TE-derived regulatory elements, the top 10 scoring motifs from CisFinder were annotated using the STAMP webserver (http://www.benoslab.pitt.edu/stamp/) with TRANSFAC (families labeled) motifs.
TE and Regulatory Element Correlations
See the Supplemental Experimental Procedures.
Supplementary Material
Highlights.
Thousands of genes gained uterine expression during the origins of pregnancy
Genes that mediate maternal-fetal immunotolerance evolved expression in Eutherians
Ancient transposable elements donated cis-regulatory elements to recruited genes
Ancient transposable elements coordinate the uterine progesterone response
Acknowledgments
This work was funded by a University of Chicago new lab startup package and a Burroughs Wellcome Preterm Birth Initiative grant (V.J.L.) and a grant from the John Templeton Foundation, number 12793 Genetics and the Origin of Organismal Complexity (G.P.W). The generation of mRNA-seq data from cow, pig, and horse was supported by a German Ministry for Education and Research grant (BMBF, FUGATO-plus, COMPENDIUM) to S.B. We thank R.W. Truman (National Hansen’s Disease Program/U.S. National Institutes of Allergy and Infectious Diseases IAA-2646) and K. Smith for the generous gifts of pregnant armadillo and opossum uterus and R. Bjornson and N. Carriero for assistance with RNA-seq read mapping. This work was also supported by the NIH (grant number R01GM077582 to C.F). We also thank C. Ober for comments on an earlier version of this manuscript and A. Smit for assistance for TE age classifications.
Footnotes
ACCESSION NUMBERS
The mRNA-seq data reported in this article have been deposited in NCBI GEO under accession numbers GSE57714, GSE30708, GSE29553, GSE21046, GSE43667, and GSE48862. The ChIP-seq and DNaseI-seq data have been deposited under accession number GSE61793, and the FAIRE-seq data have been deposited under accession number GSM1011119.
Supplemental Information includes Supplemental Experimental Procedures, two figures, eight tables, and one data file and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2014.12.052.
References
- Aghajanova L, Tatsumi K, Horcajadas JA, Zamah AM, Esteban FJ, Herndon CN, Conti M, Giudice LC. Unique transcriptome, pathways, and networks in the human endometrial fibroblast response to progesterone in endometriosis. Biol Reprod. 2011;84:801–815. doi: 10.1095/biolreprod.110.086181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altmäe S, Martínez-Conejero JA, Salumets A, Simón C, Horcajadas JA, Stavreus-Evers A. Endometrial gene expression analysis at the time of embryo implantation in women with unexplained infertility. Mol Hum Reprod. 2010;16:178–187. doi: 10.1093/molehr/gap102. [DOI] [PubMed] [Google Scholar]
- Bombail V, Gibson DA, Collins F, MacPherson S, Critchley HOD, Saunders PTK. A Role for the orphan nuclear receptor estrogen-related receptor alpha in endometrial stromal cell decidualization and expression of genes implicated in energy metabolism. J Clin Endocrinol Metab. 2010;95:E224–E228. doi: 10.1210/jc.2010-0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandley MC, Young RL, Warren DL, Thompson MB, Wagner GP. Uterine gene expression in the live-bearing lizard, Chalcides ocellatus, reveals convergence of squamate reptile and mammalian pregnancy mechanisms. Genome Biol Evol. 2012;4:394–411. doi: 10.1093/gbe/evs013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brionne A, Nys Y, Hennequet-Antier C, Gautron J. Hen uterine gene expression profiling during eggshell formation reveals putative proteins involved in the supply of minerals or in the shell mineralization process. BMC Genomics. 2014;15:220. doi: 10.1186/1471-2164-15-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britten RJ, Davidson EH. Gene regulation for higher cells: a theory. Science. 1969;165:349–357. doi: 10.1126/science.165.3891.349. [DOI] [PubMed] [Google Scholar]
- Carroll SB. Evolution at two levels. on genes and form PLoS Biol. 2005;3:e245. doi: 10.1371/journal.pbio.0030245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll SB. Evo-devo and an expanding evolutionary synthesis: a genetic theory of morphological evolution. Cell. 2008;134:25–36. doi: 10.1016/j.cell.2008.06.030. [DOI] [PubMed] [Google Scholar]
- Chan ET, Quon GT, Chua G, Babak T, Trochesset M, Zirngibl RA, Aubin J, Ratcliffe MJ, Wilde A, Brudno M, et al. Conservation of core gene expression in vertebrate tissues. J Biol. 2009;8:33. doi: 10.1186/jbiol130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YF, Marks ME, Jones FC, Villarreal G, Jr, Shapiro MD, Brady SD, Southwick AM, Absher DM, Grimwood J, Schmutz J, et al. Adaptive evolution of pelvic reduction in sticklebacks by recurrent deletion of a Pitx1 enhancer. Science. 2010;327:302–305. doi: 10.1126/science.1182213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian M, Marangos P, Mak I, McVey J, Barker F, White J, Brosens JJ. Interferon-gamma modulates prolactin and tissue factor expression in differentiating human endometrial stromal cells. Endocrinology. 2001;142:3142–3151. doi: 10.1210/endo.142.7.8231. [DOI] [PubMed] [Google Scholar]
- Conneely OM, Mulac-Jericevic B, Lydon JP, De Mayo FJ. Reproductive functions of the progesterone receptor isoforms: lessons from knock-out mice. Mol Cell Endocrinol. 2001;179:97–103. doi: 10.1016/s0303-7207(01)00465-8. [DOI] [PubMed] [Google Scholar]
- Davidson EH, Britten RJ. Regulation of gene expression: possible role of repetitive sequences. Science. 1979;204:1052–1059. doi: 10.1126/science.451548. [DOI] [PubMed] [Google Scholar]
- Davis AC, Wims M, Spotts GD, Hann SR, Bradley A. A null c-myc mutation causes lethality before 10.5 days of gestation in homozygotes and reduced fertility in heterozygous female mice. Genes Dev. 1993;7:671–682. doi: 10.1101/gad.7.4.671. [DOI] [PubMed] [Google Scholar]
- Eijkelenboom A, Mokry M, de Wit E, Smits LM, Polderman PE, van Triest MH, van Boxtel R, Schulze A, de Laat W, Cuppen E, Burgering BM. Genome-wide analysis of FOXO3 mediated transcription regulation through RNA polymerase II profiling. Mol Syst Biol. 2013;9:638. doi: 10.1038/msb.2012.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emera D, Wagner GP. Transformation of a transposon into a derived prolactin promoter with function during human pregnancy. Proc Natl Acad Sci USA. 2012;109:11246–11251. doi: 10.1073/pnas.1118566109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feschotte C. Transposable elements and the evolution of regulatory networks. Nat Rev Genet. 2008;9:397–405. doi: 10.1038/nrg2337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellersen B, Brosens J. Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. J Endocrinol. 2003;178:357–372. doi: 10.1677/joe.0.1780357. [DOI] [PubMed] [Google Scholar]
- Gellersen B, Brosens IA, Brosens JJ. Decidualization of the human endometrium: mechanisms, functions, and clinical perspectives. Semin Reprod Med. 2007;25:445–453. doi: 10.1055/s-2007-991042. [DOI] [PubMed] [Google Scholar]
- Gerlo S, Davis JRE, Mager DL, Kooijman R. Prolactin in man: a tale of two promoters. BioEssays. 2006;28:1051–1055. doi: 10.1002/bies.20468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giudice LC. Elucidating endometrial function in the post-genomic era. Hum Reprod Update. 2003;9:223–235. doi: 10.1093/humupd/dmg019. [DOI] [PubMed] [Google Scholar]
- Goldschmidt R. The Material Basis of Evolution. New Haven, CT: Yale University Press; 1940. [Google Scholar]
- Grewal S, Carver J, Ridley AJ, Mardon HJ. Human endometrial stromal cell rho GTPases have opposing roles in regulating focal adhesion turnover and embryo invasion in vitro. Biol Reprod. 2010;83:75–82. doi: 10.1095/biolreprod.109.080630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanna J, Goldman-Wohl D, Hamani Y, Avraham I, Greenfield C, Natanson-Yaron S, Prus D, Cohen-Daniel L, Arnon TI, Manaster I, et al. Decidual NK cells regulate key developmental processes at the human fetal-maternal interface. Nat Med. 2006;12:1065–1074. doi: 10.1038/nm1452. [DOI] [PubMed] [Google Scholar]
- Hernández-Ochoa I, Karman BN, Flaws JA. The role of the aryl hydrocarbon receptor in the female reproductive system. Biochem Pharmacol. 2009;77:547–559. doi: 10.1016/j.bcp.2008.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess AP, Hamilton AE, Talbi S, Dosiou C, Nyegaard M, Nayak N, Genbecev-Krtolica O, Mavrogianis P, Ferrer K, Kruessel J, et al. Decidual stromal cell responsetoparacrine signals from the trophoblast:amplification of immune and angiogenic modulators. Biol Reprod. 2007;76:102–117. doi: 10.1095/biolreprod.106.054791. [DOI] [PubMed] [Google Scholar]
- Hill JP. V. The Development of the Monotremata.–Part I. The Histology of the Oviduct during Gestation. By Catherine J. Hill. B. Sc., Ph. D. Part II. The Structure of the Egg-shell. Trans Zool Soc Lond. 1936;21:413–476. [Google Scholar]
- Hoekstra HE, Hirschmann RJ, Bundey RA, Insel PA, Crossland JP. A single amino acid mutation contributesto adaptive beach mouse color pattern. Science. 2006;313:101–104. doi: 10.1126/science.1126121. [DOI] [PubMed] [Google Scholar]
- Hughes RL, Hall LS. Early development and embryology of the platypus. Philos Trans R Soc Lond B Biol Sci. 1998;353:1101–1114. doi: 10.1098/rstb.1998.0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Hu Y, Zhao J, Zhen X, Yan G, Sun H. The orphan nuclear receptor Nur77 regulates decidual prolactin expression in human endometrial stromal cells. Biochem Biophys Res Commun. 2011;404:628–633. doi: 10.1016/j.bbrc.2010.12.027. [DOI] [PubMed] [Google Scholar]
- Jonchère V, Réhault-Godbert S, Hennequet-Antier C, Cabau C, Sibut V, Cogburn LA, Nys Y, Gautron J. Gene expression profiling to identify eggshell proteins involved in physical defense of the chicken egg. BMC Genomics. 2010;11:57. doi: 10.1186/1471-2164-11-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonchère V, Brionne A, Gautron J, Nys Y. Identification of uterine ion transporters for mineralisation precursors of the avian eggshell. BMC Physiol. 2012;12:10. doi: 10.1186/1472-6793-12-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamal M, Xie X, Lander ES. A large family of ancient repeat elements in the human genome is under strong selection. Proc Natl Acad Sci USA. 2006;103:2740–2745. doi: 10.1073/pnas.0511238103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kin K, Maziarz J, Wagner GP. Immunohistological study of the endometrial stromal fibroblasts in the opossum, Monodelphis domestica: evidence for homology with eutherian stromal fibroblasts. Biol Reprod. 2014;90:111. doi: 10.1095/biolreprod.113.115139. [DOI] [PubMed] [Google Scholar]
- Lang M, Murat S, Clark AG, Gouppil G, Blais C, Matzkin LM, Guittard E, Yoshiyama-Yanagawa T, Kataoka H, Niwa R, et al. Mutations in the neverland gene turned Drosophila pachea into an obligate specialist species. Science. 2012;337:1658–1661. doi: 10.1126/science.1224829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Ruotti V, Stewart RM, Thomson JA, Dewey CN. RNA-Seq gene expression estimation with read mapping uncertainty. Bioinformatics. 2010;26:493–500. doi: 10.1093/bioinformatics/btp692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JL, Liang XH, Su RW, Lei W, Jia B, Feng XH, Li ZX, Yang ZM. Combined analysis of microRNome and 3′ -UTRome reveals a species-specific regulation of progesterone receptor expression in the endometrium of rhesus monkey. J Biol Chem. 2012;287:13899–13910. doi: 10.1074/jbc.M111.301275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo R, Matthews J. High-resolution genome-wide mapping of AHR and ARNT binding sites by ChIP-Seq. Toxicol Sci. 2012;130:349–361. doi: 10.1093/toxsci/kfs253. [DOI] [PubMed] [Google Scholar]
- Lowe CB, Haussler D. 29 mammalian genomes reveal novel ex-aptations of mobile elements for likely regulatory functions in the human genome. PLoS ONE. 2012;7:e43128. doi: 10.1371/journal.pone.0043128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe CB, Bejerano G, Haussler D. Thousands of human mobile element fragments undergo strong purifying selection near developmental genes. Proc Natl Acad Sci USA. 2007;104:8005–8010. doi: 10.1073/pnas.0611223104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lydon JP, DeMayo FJ, Funk CR, Mani SK, Hughes AR, Montgomery CA, Jr, Shyamala G, Conneely OM, O’Malley BW. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278. doi: 10.1101/gad.9.18.2266. [DOI] [PubMed] [Google Scholar]
- Lynch VJ, Wagner GP. Resurrecting the role of transcription factor change in developmental evolution. Evolution. 2008;62:2131–2154. doi: 10.1111/j.1558-5646.2008.00440.x. [DOI] [PubMed] [Google Scholar]
- Lynch VJ, Leclerc RD, May G, Wagner GP. Transposon-mediated rewiring of gene regulatory networks contributed to the evolution of pregnancy in mammals. Nat Genet. 2011;43:1154–1159. doi: 10.1038/ng.917. [DOI] [PubMed] [Google Scholar]
- McClintock B. The significance of responses of the genome to challenge. Science. 1984;226:792–801. doi: 10.1126/science.15739260. [DOI] [PubMed] [Google Scholar]
- McConaha ME, Eckstrum K, An J, Steinle JJ, Bany BM. Microarray assessment of the influence of the conceptus on gene expression in the mouse uterus during decidualization. Reproduction. 2011;141:511–527. doi: 10.1530/REP-10-0358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean CY, Bristor D, Hiller M, Clarke SL, Schaar BT, Lowe CB, Wenger AM, Bejerano G. GREAT improves functional interpretation of cis-regulatory regions. Nat Biotechnol. 2010;28:495–501. doi: 10.1038/nbt.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mess A, Carter AM. Evolutionary transformations of fetal membrane characters in Eutheria with special reference to Afrotheria. J Exp Zoolog B Mol Dev Evol. 2006;306:140–163. doi: 10.1002/jez.b.21079. [DOI] [PubMed] [Google Scholar]
- Mikkelsen TS, Wakefield MJ, Aken B, Amemiya CT, Chang JL, Duke S, Garber M, Gentles AJ, Goodstadt L, Heger A, et al. Genome of the marsupial Monodelphis domestica reveals innovation in non-coding sequences. Nature. 2007;447:167–177. doi: 10.1038/nature05805. [DOI] [PubMed] [Google Scholar]
- Pabona JMP, Simmen FA, Nikiforov MA, Zhuang D, Shankar K, Velarde MC, Zelenko Z, Giudice LC, Simmen RCM. Krüppel-like factor 9 and progesterone receptor coregulation of decidualizing endometrial stromal cells: implications for the pathogenesis of endometriosis. J Clin Endocrinol Metab. 2012;97:E376–E392. doi: 10.1210/jc.2011-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prud’homme B, Gompel N, Carroll SB. Emerging principles of regulatory evolution. Proc Natl Acad Sci USA. 2007;104(1):8605–8612. doi: 10.1073/pnas.0700488104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renfree M, Shaw G. Reproduction in Monotremes and Marsupials. Chichester, UK: John Wiley & Sons, Ltd; 2001. [Google Scholar]
- Smith SD, Wang S, Rausher MD. Functional evolution of an anthocyanin pathway enzyme during a flower color transition. Mol Biol Evol. 2013;30:602–612. doi: 10.1093/molbev/mss255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svensson J, Jenmalm MC, Matussek A, Geffers R, Berg G, Ernerudh J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by M-CSF and IL-10. J Immunol. 2011;187:3671–3682. doi: 10.4049/jimmunol.1100130. [DOI] [PubMed] [Google Scholar]
- Talbi S, Hamilton AE, Vo KC, Tulac S, Overgaard MT, Dosiou C, Le Shay N, Nezhat CN, Kempson R, Lessey BA, et al. Molecular phenotyping of human endometrium distinguishes menstrual cycle phases and underlying biological processes in normo-ovulatory women. Endocrinology. 2006;147:1097–1121. doi: 10.1210/en.2005-1076. [DOI] [PubMed] [Google Scholar]
- Tewari AK, Yardimci GG, Shibata Y, Sheffield NC, Song L, Taylor BS, Georgiev SG, Coetzee GA, Ohler U, Furey TS, et al. Chromatin accessibility reveals insights into androgen receptor activation and transcriptional specificity. Genome Biol. 2012;13:R88. doi: 10.1186/gb-2012-13-10-r88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner GP, Lynch VJ. Evolutionary novelties. Curr Biol. 2010;20:R48–R52. doi: 10.1016/j.cub.2009.11.010. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Kin K, Lynch VJ. Measurement of mRNA abundance using RNA-seq data: RPKM measure is inconsistent among samples. Theory Biosci. 2012;131:281–285. doi: 10.1007/s12064-012-0162-3. [DOI] [PubMed] [Google Scholar]
- Wagner GP, Kin K, Lynch VJ. A model based criterion for gene expression calls using RNA-seq data. Theory Biosci. 2013;132:159–164. doi: 10.1007/s12064-013-0178-3. [DOI] [PubMed] [Google Scholar]
- Wang Z, Young RL, Xue H, Wagner GP. Transcriptomic analysis of avian digits reveals conserved and derived digit identities in birds. Nature. 2011;477:583–586. doi: 10.1038/nature10391. [DOI] [PubMed] [Google Scholar]
- Wang J, Zhuang J, Iyer S, Lin X, Whitfield TW, Greven MC, Pierce BG, Dong X, Kundaje A, Cheng Y, et al. Sequence features and chromatin structure around the genomic regions bound by 119 human transcription factors. Genome Res. 2012;22:1798–1812. doi: 10.1101/gr.139105.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren WC, Hillier LW, Marshall Graves JA, Birney E, Ponting CP, Grützner F, Belov K, Miller W, Clarke L, Chinwalla AT, et al. Genome analysis of the platypus reveals unique signatures of evolution. Nature. 2008;453:175–183. doi: 10.1038/nature06936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


