Abstract
Amyloid beta (Aβ) peptides, 36-43 amino acids in length, are produced from β- and γ-secretase cleavage of the amyloid precursor protein (AβPP), and are one of the causative agents of Alzheimer disease (AD). Here we show that an ELISA can detect total rodent Aβ without interference from physiological concentrations of human Aβ. In cultured dissociated rat cortical neurons and rat and mouse hippocampal organotypic slices, we apply the assay to measure the production of Aβ in response to treatment with hydrogen peroxide, a known stimulator of Aβ secretion, or human Aβ dimer/trimer (Aβd/t), fractionated from the culture medium of 7PA2 cells. Peroxide increases Aβ secretion by about 2 fold, similar to results from previous reports that used a different assay. Of greater significance is that physiologically relevant concentrations (~250 pM) of human Aβd/t increase rodent Aβ secretion from cultured rat cortical neurons by >3 fold over 4 days. Surprisingly, neither treatment with peroxide nor human Aβd/t leads to accumulation of intracellular Aβ. Human Aβd/t increased >2 fold the Aβ secreted by organotypic hippocampal slices from tau knock-out mice whether or not they expressed a human tau transgene, suggesting tau plays no role in enhanced Aβ secretion. Together, these results support an Aβ-mediated feed-forward mechanism in AD progression.
Keywords: rodent Aβ ELISA, Aβ dimer/trimer, hippocampal neurons, cortical neurons, organotypic hippocampal slice culture, tau knockout mice
Introduction
Alzheimer disease (AD) is the major form of dementia that affects the aged. It has about a 50% probability of occurrence in every person living to age 85 and beyond [1]. The pathological hallmarks of the disease are extracellular amyloid plaques, composed primarily of the amyloid beta (Aβ) peptide, and striated neuropil threads and neurofibrillary tangles formed from hyperphosphorylated tau [2]. Familial AD, representing 1% or less of AD cases, arises from mutations in genes affecting the production or clearance in the brain of the amyloid beta (Aβ) peptides [3], which are excised from the transmembrane amyloid precursor protein (AβPP) through the actions of β- and γ-secretases [4–7] and range from 36 to 43 amino acids. However, Aβ peptides also accumulate in the other 99% of AD cases, called sporadic AD, although mechanisms driving their production are unclear [3,6].
Different isoforms and different conformations or aggregation states of the Aβ peptides deliver different signals to neurons and have remarkably different neuro- and synapto-toxicities. The Aβ1-42 peptides are more amyloidogenic than the Aβ1-40 peptides and correlate better with AD and its progression [8,9]. Fibrillar forms of the Aβ species are less toxic than the soluble oligomeric forms [10]. An oligomeric fraction, called Aβ-derived diffusible ligands (ADDLs), affects synapses at submicromolar concentrations [10]. However, an even more active form of Aβ, with maximal activity at subnanomolar concentrations, is secreted from a cultured Chinese hamster ovary cell line (7PA2 cells) expressing a mutated form of human AβPP [11]. This material contains SDS-stable human Aβ (HAβ) dimers and trimers (HAβd/t), which can be isolated by gel filtration; the isolated HAβd/t has a marked effect on synaptic function, both in cultured slices and when injected into rodent brain [12–15]. An SDS-stable HAβ dimer, the major soluble species extracted from postmortem AD brain, is also active at subnanomolar concentrations [16]. In fact, the presence of this SDS-stable HAβ dimer strongly correlates with AD type dementia [17].
Excessive production of HAβ from AβPP occurs in familial AD due to mutations in AβPP and its processing enzymes or in proteins that normally clear the excess HAβ, but the factors causing excess HAβ production in sporadic AD are less well understood. Within 60 min of treatment, synthetic HAβ oligomers at 2 μM inhibit axonal transport of mitochondria and vesicles containing neurotrophin receptors in mouse hippocampal neurons [18]. Transport inhibition is dependent on the presence of the microtubule-binding and stabilizing protein tau. It has been proposed that stalled vesicles containing AβPP might be the sites for enhanced production of HAβ [19], since up to 70% of the Aβ secreted from cells arises from β- and γ-secretase cleavage of AβPP within the lipid environment of endosomes [20–23].
Enzyme-linked immunosorbant assays (ELISAs) are the standard means for quantifying either Aβ1-40 or Aβ1-42 from rodents or humans [24–26]. Here we characterize and apply an ELISA for total rodent Aβ (RAβ) that can be used in the presence of physiologically relevant amounts of HAβd/t to show that both peroxide and HAβd/t increase RAβ secretion but not internal Aβ pools in cultured neurons.
Materials and Methods
Reagents
Unless otherwise noted, all chemicals are reagent grade and were obtained from Sigma-Aldrich Co. (St. Louis, MO), and all tissue culture reagents were obtained from Life Technologies (Carlsbad, CA). Synthetic human amyloid beta (HAβ1-42) was obtained from AnaSpec, Inc. (San Jose, CA), and synthetic rodent amyloid beta (RAβ1-42 and RAβ1-40) were gifts from Covance (Princeton, NJ). As previously described [27], the HAβ monomer (HAβm) and HAβd/t fractions (Supplementary Figure 1) were isolated by size-exclusion chromatography from conditioned culture medium of Chinese hamster ovary (CHO) cells, clone 7PA2 (a gift from Dennis Selkoe, Harvard Medical School), which express a mutant human AβPP [11]. Unless otherwise noted, these were used at the equivalent of 1x concentration (for HAβm this value is ~800 pM and for HAβd/t ~250 pM as determined by dot blots). Medium from wild type CHO cells was fractionated identically by size-exclusion, and fractions eluting at the equivalent position of HAβd/t were used as one control.
Dot-blot assay for quantifying Aβ in 7PA2 cell medium
HAβ was quantified in 7PA2 cell culture medium using dot blots with synthetic HAβ1-42 as a standard [28]. Briefly, samples were applied to nitrocellulose (0.1 μm), the membrane was boiled 10 min in phosphate buffered saline (PBS) [29], and HAβ was detected after overnight incubation at 4°C with 6E10 antibody (Covance, Emoryville, CA) followed by a goat-anti-mouse antibody conjugated to DyLight 680 (1:15,000 for 45 min; Thermo Scientific, Rockford, IL). Spots were imaged with a LI-COR Odyssey IR Imaging System, and intensities quantified using TotalLab software (Nonlinear Dynamics, Newcastle Upon Tyne, UK).
Sandwich ELISA
Synthetic RAβ1-40 and RAβ1-42 were solubilized to 1 mg/mL in 0.1% ammonium hydroxide. Aliquots (10 μL) were dried in a speed-vac and pellets were stored at −80°C. Costar 96-well white solid plates were coated with 100 μL of 2, 5, or 10 μg/mL of capture antibody, a rabbit polyclonal raised against RAβ (Covance, SIG-39153). The detection antibody is horseradish peroxidase-conjugated mouse monoclonal, (4G8; Covance) that is reactive to both HAβ and RAβ. Other details of the ELISA are in Supplementary Information.
Aliquots of culture medium from dissociated primary neurons, N2a cells or organotypic hippocampal slice cultures were diluted appropriately with PBS containing Tween and BSA (PBSTB; Covance), and 100 μL was used per well in the RAβ ELISA. The intracellular pools of RAβ were measured in peroxide or HAβd/t stressed cells and unstressed control cells after the medium was removed and the cells were washed in PBS. Cells were lysed in PBSTB containing 0.1% NP-40 for 5 min at room temperature, and the lysate was used directly in the RAβ ELISA.
Human Aβ interference in rodent Aβ ELISA
Synthetic HAβ1-42 (AnaSpec) was solubilized with 1,1,1,3,3,3-Hexafluoro-2-propanol to 1 mg/mL, air dried, and stored at −80°C. Pellets were solubilized with 10 μL dimethylsulfoxide (DMSO) and diluted with Neurobasal medium containing B27 supplement (Life Technologies, Carlsbad, CA) to their desired concentration. In one set of experiments, RAβ1-42 was maintained at 150 pg/mL and the amount of HAβ1-42 was varied from 10 pg/mL to 1 μg/mL. In a second set of experiments, HAβ1-42 was maintained at 1.9 ng/mL (equal to the highest concentration of HAβd/t used in cell treatments), and RAβ was varied. Tubes containing mixed H/RAβ1-42 were incubated at 37°C for 72 hrs, the time in which rodent cells are exposed to HAβ, to determine if possible co-oligomerization between HAβ and RAβ might affect the ELISA results. Detection antibody at a final concentration of 1 μg/mL was added to the samples, and after incubation for 30 min, samples were added to sandwich ELISA plates and processed as described for Sandwich ELISA.
DNA assay
Calf thymus DNA was solubilized with 100 mM Tris, 10 mM EDTA, pH 8.0 (TE buffer). Its final concentration determined spectrophotmetrically using an extinction coefficient of 0.02 μg/mL−1·cm−1 at 260 nm [30]. Cells grown in Lab-Tek 8 well chamber slides were lysed with 200 μL of DNA lysis buffer (25 mM NaOH, 10 mM EDTA, pH 12.0) and wells were washed 2x with 300 μL of TE buffer, which was added to the lysate. Standards and samples were diluted appropriately with TE buffer containing 1:10,000 SybrGreen I (Life Technologies). Lysates from the dissociated neuronal cell cultures were diluted 1:50 in TE buffer containing SybrGreen I. Organotypic mouse hippocampal slices were removed from the coverslip, lysed in 200 μL of DNA lysis buffer for 30 min at room temperature, and 600 μL of TE buffer was added. Slice lysates were further diluted 1:200 in TE buffer containing SybrGreen I. Samples and standards were added at 100 μL per well onto 96-well white solid plates (Costar, Corning Inc.). Fluorescence was quantified for 0.1 s per well on a Perkin-Elmer Victor V multi-mode microplate reader equipped with fluorescein filters. Complete cell lysis was confirmed by fluorescence microscopy of wells stained with SyberGreen I.
Neuronal cell culture
All studies with tissue and cells from mice and rats were performed according to the National Research Council’s guide for the care and use of laboratory animals by following protocols approved by the Institutional Animal Care and Use Committee. E18 rat cortical and hippocampal neurons were obtained from timed-pregnant dams purchased from Harlan (Indianapolis, IN) and were prepared as previously described [31]. After counting, 300,000 cells were plated per well onto poly-D-lysine coated, 8-well chamber slides (Lab-Tek, Thermo Scientific, Portsmouth, NH). Neurons were cultured in 400 μL of Neurobasal medium supplemented with 1x B27, 2 mM GlutaMAX, and 100 μg/mL penicillin/streptomycin (Pen/Strep; Life Technologies).
Mouse N2a neuroblastoma cells were obtained from ATCC and cultured in Dulbecco’s Modified Eagle Medium (D-MEM) with 4.5 g/L D-glucose, 2 mM L-glutamine, 110 mg/L sodium pyruvate, and 10% fetal bovine serum. Cells were plated at 5,000 cells per well onto 8-well chamber slides. One day after plating, cells were stressed with varying concentrations of hydrogen peroxide.
Organotypic hippocampal slice cultures
Hippocampal slices (400 μm thick) were prepared from P6 Sprague Dawley rat pups [32] and cultured on membranes in 6-well dishes as previously described [33]. Using a procedure [34] with modifications described in detail in Supplementary Information, hippocampal slices (300 μm thick) on glass coverslips were prepared from P7 mouse pups (TAU−/− (B6.Cg-Mapttm1(EGFP)Klt); Jackson Labs, Bar Harbor, ME) and TAU−/− mice carrying human tau transgene (B6.Cg- Mapttm1(EGFP)Klt Tg(MAPT)8cPdav/J). The original slice medium (per 205 mL: 50 mL horse serum, 50 mL Hanks BSS, 4 mL 25% glucose, 100 mL minimum essential medium containing GlutaMAX (250 μL/100 mL), HEPES (4.76g/L) and Pen/Strep (1 mL)) was replaced on day 2 with Neurobasal A medium containing (per 50 mL: 48 mL Neurobasal A, 180 μL 25% glucose, 625 μL GlutaMAX, 1 mL B27 supplements and 250 μL Pen/Strep). This medium was changed every 2–3 days thereafter. After dissection, slices were allowed to recover for at least 1 week before treatment.
Statistics
All experiments were performed multiple times (number (n) listed in figure legends) with at least triplicate samples for every point. However, because data sets were nearly identical, in many figures data are shown from only one experiment. Error bars on each plot are standard deviations, and p values were determined from paired two-tailed t tests.
Results
Determining the maximum level of HAβ that does not interfere in RAβ ELISA
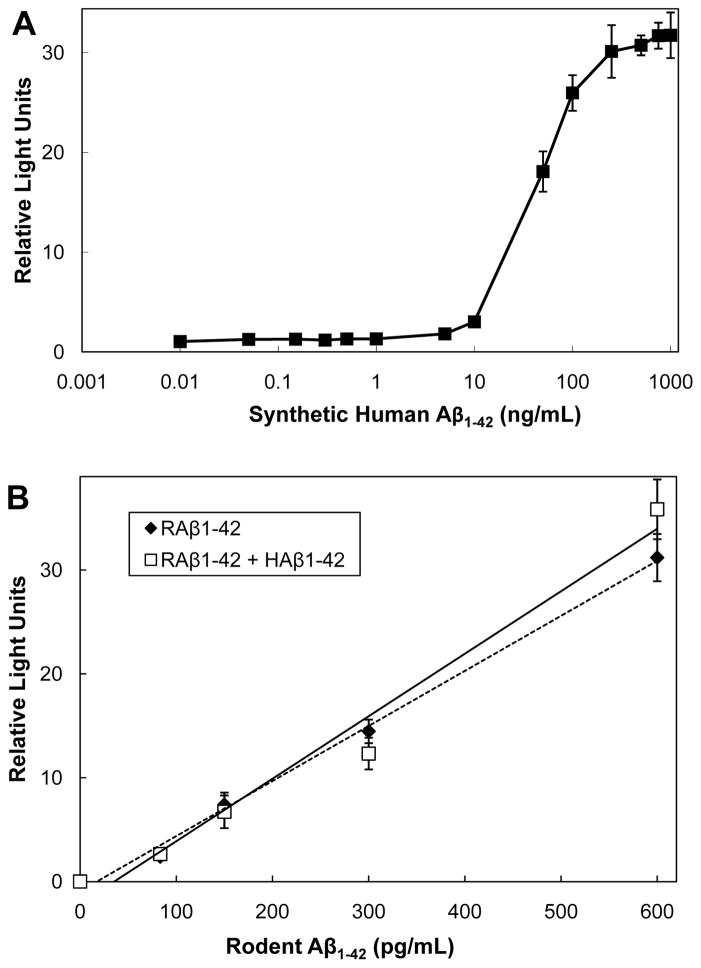
RAβ and HAβ peptide sequences differ in only three residues: Arg5, Tyr10, and His13 in human Aβ are Gly5, Phe10, and Arg13 in rodent Aβ. All are located within the epitope recognized by the RAβ capture antibody. By inference, the epitope recognized by the detection antibody is subject to interference by Aβ from non-rodent species. To utilize the ELISA to detect RAβ in the presence of HAβ, the maximum level of HAβ that would not interfere in the assay needed to be determined. A constant level of RAβ1-42 (150 pg/mL) was maintained in the assay while human Aβ1-42 was added from 10 pg/mL to 1 μg/mL (Figure 1A). Human Aβ1-42 at <5 ng/mL had no effect on the ability of the ELISA to quantify correctly the RAβ. The increased signal obtained at concentrations of HAβ >10 ng/mL could arise either from a weak affinity of capture antibody for HAβ, or from co-oligomerization between RAβ and HAβ that might occur at higher Aβ concentrations [35,36].
Figure 1. Effects of human Aβ on the rodent Aβ ELISA.
(A) Effects of variable amounts of human Aβ1-42 on the ELISA detection of a fixed amount (150 pg/ml) of rodent Aβ1-42. Sample mixtures were incubated at 37°C to allow possible co-oligomerization to occur before the addition of detection antibody. Points are averages of triplicate samples; the standard deviation shown by the error bars is less than symbol size for concentrations below 10 ng/mL. (B) Effects of a fixed amount of human Aβ1-42 (1.9 ng/ml) on the rodent Aβ ELISA standard curve. Samples of each concentration were incubated at 37°C for 3 days before assay to mimic the incubation conditions for secreted RAβ in the presence of the HAβd/t. Points are averages of triplicate samples. n=2
We also performed the RAβ ELISA using constant HAβ (1.9 ng/mL, about the maximum concentration in the HAβd/t fraction) with increasing concentrations of RAβ (from 10 pg/mL to 1 μg/mL). At concentrations of RAβ <600 pg/mL, the presence of HAβ had no effect on the standard curve, demonstrating that within its linear region (10–600 pg/mL, Supplementary Figure 2) the ELISA is specific for RAβ (Figure 1B).
Measurement of secreted Aβ from a rodent cell line
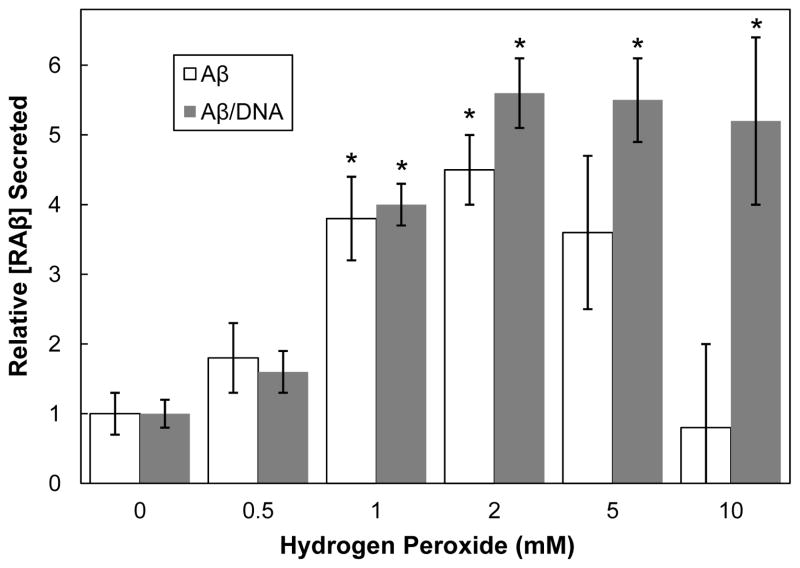
A human neuroblastoma cell line was used previously to demonstrate that peroxide induces Aβ secretion through JNK-dependent activation of γ-secretase [37]. Therefore, we first utilized the ELISA with a mouse neuroblastoma cell line (N2a) to detect RAβ in culture medium of cells stressed for 3 days with varying levels of hydrogen peroxide (0.5 mM to 10 mM). A significant increase (p≤0.05) in secreted RAβ was measured for peroxide concentrations above 0.5 mM with a maximum of ~5 fold for cells treated with 2 mM peroxide (Figure 2). However, microscopic observation of the wells showed significant cell loss at peroxide concentrations above 2 mM.
Figure 2. Normalizing the amounts of secreted RAβ to cell numbers allows better comparisons between samples.
N2a cells were treated with different concentrations of hydrogen peroxide and the total RAβ secreted was determined after 3 days and normalized to levels secreted from untreated cells. An apparent decline in secreted RAβ occurred at peroxide concentrations above 2 mM. However, after correcting for cell loss by normalizing secreted RAβ to DNA in each well, the production of RAβ reached a plateau at or above 2 mM peroxide. * values of p ≤ 0.05 compared to untreated samples. n=3
To normalize Aβ secretion to cell number, the DNA level in each well was quantified using SybrGreen I (Supplementary Figure 3), a specific DNA-binding fluorescent dye [38]. After medium was removed from the wells to analyze for RAβ, the remaining cells were lysed, DNA was quantified, and the RAβ secreted per ng DNA was calculated. Secretion of RAβ per ng DNA increased with increasing peroxide up to a plateau at 2 mM (Figure 2).
Application of the rodent Aβ ELISA to primary neurons
Primary E18 rat cortical neurons, cultured in 8-well chamber slides for 3 days, were left untreated or treated with varying concentrations of peroxide on day 3. After three days of treatment culture medium was removed and assayed for RAβ, and cells were lysed to quantify either DNA or the internal pool of Aβ. Untreated neurons secreted 63 ± 5 pg (n=3) of Aβ per well (0.4 mL), or 157.0 pg/mL (35 pM) over the course of three days. The DNA content of each well (1.72 ± .09 μg) can be used to calculate that there are 0.036 pg Aβ/ng of DNA. To calculate the number of molecules of Aβ secreted by each cell, one can assume that there are 6.5 pg of DNA/cell [39]; therefore, over a three day period, each cell secretes 220 ag of Aβ (or ~29,000 molecules), which equals about 6–7 Aβ peptides secreted per min per neuron under non-stress growth conditions.
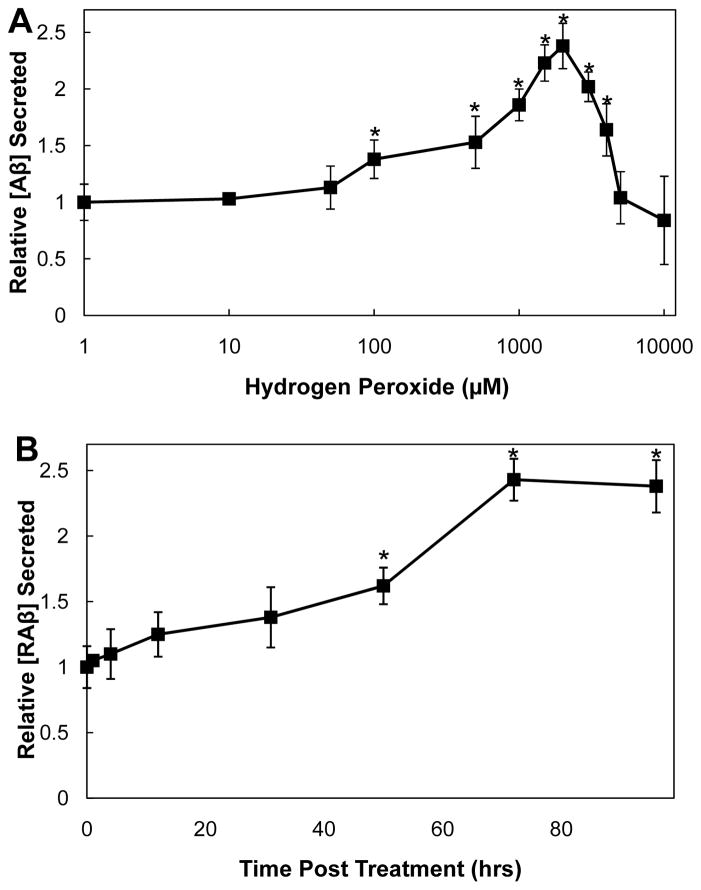
Cortical neurons stressed for three days with various concentrations of hydrogen peroxide showed a significant (p≤0.05) increase in Aβ secreted per cell only at or above peroxide concentrations of 100 μM. The maximal secretion was observed at 2 mM peroxide, resulting in a 2.4 ± 0.2 fold increase over untreated neurons (Figure 3A). Concentrations of peroxide over 2 mM resulted in increasing cell death, manifested by a DNA decline (data not shown). Our measured increase in Aβ secreted is similar to the increase (2.4 ± 0.6) observed in chick tectal neurons exposed to only 10–20 μM peroxide for 20 h [40]. It is important to note that we maintained the B27 supplements in our neuronal culture medium because the supplements contain factors in addition to antioxidants that help keep the cells viable over the 3 day culture period. Our use of B27 likely explains why much higher concentrations of peroxide were required to induce the same levels of secretion as in experiments with chick tectal neurons.
Figure 3. Hydrogen peroxide enhances secretion of RAβ from primary cortical neurons.
(A) Dose-response of hydrogen peroxide-induced RAβ secretion after 3 days in rat E18 cortical neurons, normalized for DNA content. Values are normalized to untreated samples. Points are averages of triplicate samples. * values with p ≤0.05 compared to untreated. RAβ secretion reached a peak with the addition of 2 mM peroxide. n=5 (B) Time course of RAβ secretion from rat E18 cortical neurons in response to 2 mM hydrogen peroxide and normalized for DNA content. Values shown are relative to the zero time sample. Points are averages of triplicate samples. * values with p ≤0.05 compared to 0 time. n=5
An increase in the internal pool of Aβ has been reported in neurons responding to certain types of stress [41] and has been associated with synaptic dysfunction in mouse models of AD [42,43]. Thus, we quantified the internal pool of Aβ in lysed neurons untreated or treated with peroxide. Untreated neurons contained 4.3 ± 0.6 pg of Aβ per ng DNA and 2 mM peroxide-treated neurons contained 4.9 ± 0.8 pg of Aβ per ng DNA. These values do not differ significantly.
We next determined the time course of Aβ production in cortical neurons stressed with 2 mM peroxide. Although increased secretion was observed as early as 12 h after treatment, the increase was not statistically significant (p ≤ 0.05) until 50 h, with maximal secretion obtained at 72 h (Figure 3B).
Because dissociated neurons might behave differently from neurons maintained in an environment closer to in situ, we also determined how peroxide affected RAβ secretion from rat hippocampal organotypic slices, an easily manipulated ex vivo model. Each untreated slice secretes 85 ± 8 pg (n=3) of Aβ over the course of 3 days. The secretion is increased 2.1± 0.3 fold by 2 mM peroxide treatment. This is a 1.5 ± 0.3 fold increase when normalized for DNA, still significant but less than the 2.3 to 2.4 fold increase that was obtained for dissociated cortical neurons (Figures 3A and 4). The reduced value in slices might arise from the DNA contribution of non-neuronal cells or from less exposure of the neurons within the slice from the peroxide added to the underlying medium.
Figure 4. Relative effects of hydrogen peroxide (2 mM), HAβm, and HAβd/t on RAβ secretion from dissociated cortical neurons and organotypic hippocampal slices.
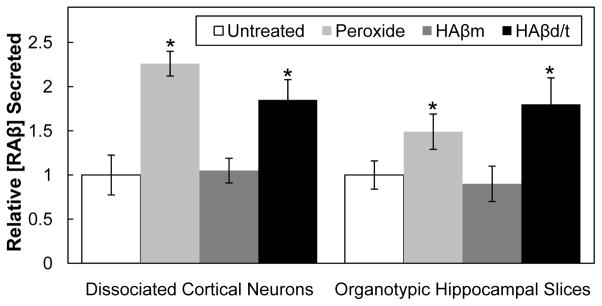
Values are triplicate samples normalized for DNA content and are expressed relative to untreated samples. * values with p ≤ 0.05 compared to their untreated control. n=3
HAβ dimer/trimer induces secretion of RAβ
If traditionally prepared synthetic HAβ oligomers are used, >45 ng/mL (10 nM) are needed to obtain physiological or morphological effects on neurons [19]. These concentrations are far above the levels that interfere in the RAβ ELISA. However, the secreted form of HAβ, containing SDS-stable dimers and trimers (HAβd/t) [11,] and HAβ dimers extracted from postmortem AD brain [16], are effective in a far lower range, that of pM [15], which does not interfere with the ELISA. Thus, we can for the first time directly assay the effects of physiologically relevant amounts of HAβ on RAβ secretion.
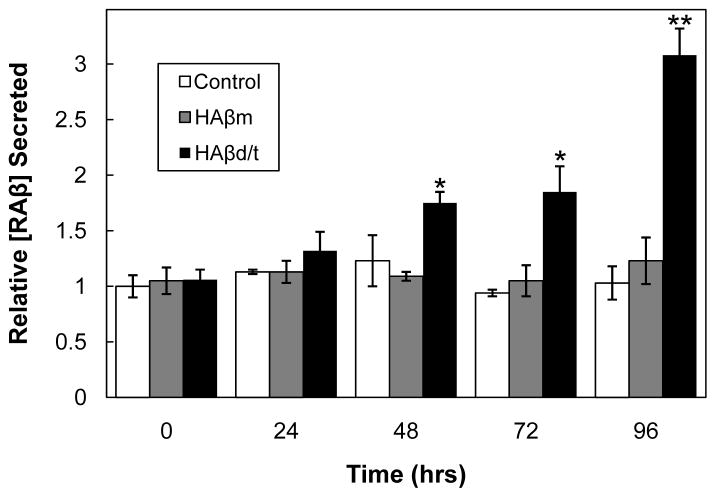
The amounts (in monomer equivalents) of HAβm or HAβd/t were quantified using a dot blot assay because the membrane could be boiled. Boiling is essential for exposing epitopes of the HAβd/t for detection. This step is essential because oligomers are inefficiently measured by ELISA [44]. Because RAβ does not oligomerize at its secreted concentrations, heating samples of culture medium or cell extracts prior to the RAβ ELISA is not required. HAβm at 3.6 ng/mL (0.8 nM) and HAβd/t at 1.1 ng/mL (about 0.25 nM), as well as their respective controls (equivalent fractions from gel filtration of medium from wild type CHO cells), were added to 3 day old cultures of rat cortical neurons. Secreted RAβ per ng DNA was quantified at 0, 24, 48, 72 and 96 h (Figure 5). The presence of HAβ did not interfere in the RAβ ELISA, as shown in Figure 1B for synthetic peptide and it was confirmed here for the secreted peptides: untreated and HAβ-treated groups at time zero are identical (Figure 5). Treatment of neurons for 4 days with HAβd/t, but not HAβm, induced 3-fold greater secretion of RAβ than did fractions from control CHO cell medium or untreated cells. Because of potential co-oligomerization between HAβd/t and RAβ that could have altered the ELISA results, we boiled some of the samples before performing the RAβ ELISA, but detected no differences between boiled and unboiled samples (data not shown). By 96 h the amount of RAβ secreted (>600 pg/mL) was over half the amount of added HAβd/t, so if co-oligomerization had occurred an altered signal after boiling should have been detected.
Figure 5. Time course of RAβ secretion from rat cortical neurons, untreated or treated with HAβm or HAβd/t.
Rat cortical neurons grown 3 days before treatment were left untreated (controls) or were treated with fractions containing HAβm (3.6 ng/mL), HAβd/t (1.1 ng/mL) or equivalent volumes of the same fractions from wild type CHO cell culture medium. All controls using fractionated medium from wild type CHO cells were not significantly different from the untreated controls of the same time point so only untreated controls are shown. Values shown are from triplicate samples and are relative to the zero time control. * values with p ≤ 0.05, or ** p ≤ 0.05, compared to their untreated control. n=3
The effects of HAβm and HAβd/t on the secretion of Aβ from rat hippocampal organotypic slices was also determined (Figure 4). When compared to untreated slices on a per slice basis, HAβm treatment caused no increase (0.9 ± 0.2 fold) in RAβ secretion, whereas HAβd/t treatment significantly (p≤0.05) increased secretion (1.8 ± 0.2 fold). This increase is similar to that observed in dissociated cortical neuronal cultures.
There were no significant differences in the amount of RAβ in the intracellular pools measured in lysates of control cells and cells treated with HAβd/t. Untreated neurons contained 5.1 ± 0.4, cells treated with HAβm contained 4.8 ± 0.3, and cells treated with HAβd/t contained 5.4 ± 0.5 pg RAβ/ng DNA. These values are similar to the intracellular RAβ amounts reported above (4.3 to 4.9 ± 0.8 pg/ng DNA) for control and peroxide treated neurons.
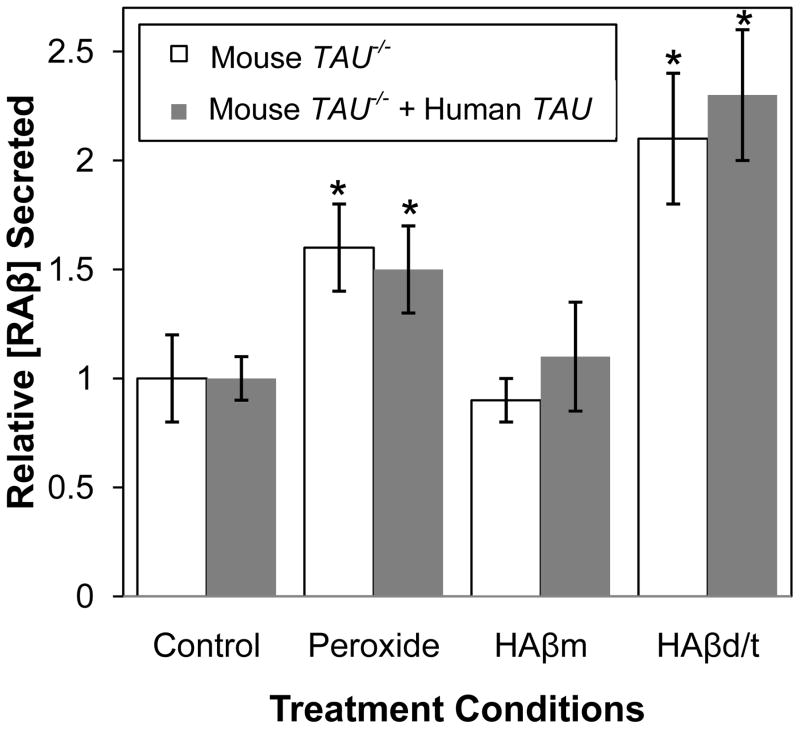
To determine if the effects of HAβd/t on RAβ secretion are dependent on the microtubule protein tau, we treated organotypic hippocampal slices from transgenic mice that are either tau null (TAU−/−) or TAU−/− carrying a human tau transgene. Stabilized slices were stressed with 2 mM peroxide, HAβm (3.6 ng/ml), or HAβd/t (1.1 ng/ml). After 3 days of treatment RAβ levels per ng DNA were quantified (Figure 6). Untreated TAU−/− slices secreted 46±6 pg/slice/3 days, which is about half the 85±8 pg/slice/3 days we obtained from the rat hippocampal slices. However, this value is quite comparable considering their difference in size. The mouse slices are 25% thinner to allow better aeration, which is needed when grown on coverslips instead of membranes. There was no difference in Aβ secretion between untreated slices not expressing or expressing the human tau transgene. Tau null slices and those expressing the transgenic human tau showed a nearly identical increase over unstressed slices in levels of secreted RAβ when stressed with peroxide (1.6 ± 0.2 and 1.5 ± 0.2 fold respectively). Slices treated with HAβm showed no change in secreted RAβ levels compared to untreated slices. RAβ secreted from slices treated with HAβd/t increased 2.1 ± 0.3 fold (tau null) and 2.3 ± 0.3 fold (transgenic human tau). Taken together, these results suggest that RAβ secretion is not dependent on tau and that rat and mouse hippocampal slices secrete similar levels of RAβ.
Figure 6. Tau has no effect on RAβ secretion induced by either peroxide or HAβd/t.
Triplicate samples each containing two organotypic hippocampal slices from mice that are either TAU−/− or TAU−/− but expressing a human tau transgene were cultured for 10 or more days and treated with 2 mM peroxide, HAβm (3.6 ng/mL) or Hβd/t (1.1 ng/mL). After 3 days, secreted RAβ was quantified and normalized to DNA per sample. Values shown are averages from the triplicate samples relative to untreated (control) slices from the same genotype, but there was no significant difference in the control amounts secreted for each genotype (Mouse TAU−/− 46±6 pg/slice/3 days, Mouse TAU−/− + Human TAU 48±8 pg/slice/3 days). * values with p 0.05 compared with mouse TAU−/− control. n=2
Discussion
We have developed an ELISA that allows us to test directly the effects of various HAβ species on Aβ production. With it we have discovered that the HAβd/t drives a feed-forward cycle of Aβ secretion. Recent studies show the dimer is the major soluble form of SDS-stable Aβ oligomer extracted from human AD brain and is the minimal synaptotoxic species [11,12,15,16]. Our results add to the importance of the Aβ dimer by showing that it is the minimal species able to stimulate the feed-forward response in Aβ production. The dimer is found in AD brain, but not in brains of stroke patients or patients diagnosed with diseases unrelated to Aβ overproduction [16], and its presence is correlated with severity of AD dementia [17]. It is of interest to note that HAβm does not show any detrimental effects on synaptic function. In fact it has a role in neuroprotection [45], perhaps acting as a scavenger for metal induced oxidative stress [46]. Here we show that HAβm does not cause an increase in RAβ secretion, providing additional support that HAβm is not a pathogenic species in AD.
Transgenic mice expressing human mutant AβPP are commonly used for AD research. In fact, Aβ secretion in response to different effectors has been studied in cultured neurons overexpressing human AβPP [47,48]. However, quantifying the effects of HAβ on the secretion of HAβ from these neurons, as well as from chicken neurons which express identical Aβ peptides to human [49,50], would be difficult because we would be measuring small increases in the secreted HAβ pool on top of the levels of HAβ used to stimulate the cells. Further complicating the assay is the fact that the chicken and human Aβ peptides undergo oligomerization. Oligomers are not efficiently measured by a typical ELISA [44] unless samples are denatured by boiling or treated with denaturants, both of which increase the complexity and decrease the accuracy of their determination. In addition, our results suggest that the positive feedback loop through which secreted HAβd/t enhances further Aβ secretion could result in artificially high levels of secreted Aβ in response to non-Aβ effectors. High density cultures of untreated cortical neurons are secreting Aβ at about 50 pg/mL per day which amounts to >200 pg/mL over four days. HAβd/t stimulated neurons show an increase in secretion of >3 fold over controls. They would produce over 600 pg/mL, which is more than half of the 1.1 ng/mL of HAβd/t used to initiate the secretion. The 600 pg/mL, if present as Aβd/t, would have significant biological effects on the actin cytoskeleton in hippocampal neurons [28].
Thus we developed and characterized an ELISA assay for measuring total RAβ, which can be used when stressing cells with physiologically relevant concentrations of HAβd/t. The advantages of using rodent neurons from non-transgenic animals are several fold: (1) they are easier to obtain and maintain than transgenic animals [51]; (2) rodent neurons (hippocampal and cortical) are the standard model system for studying the behavioral effects of HAβ treatment, both electrophysiologically and morphologically [12,14–16, 52]; (3) the Aβ they produce does not oligomerize eliminating the need to boil or otherwise denature higher order complexes before their quantification [53,54]; (4) both rat and mouse Aβ peptides have the identical sequences and either species can be used for these assays [55]. We demonstrated the applicability of this assay to peroxide treated cortical neurons, which show a 2.3 fold increase in RAβ secretion, similar to the 2.4 fold increase reported in peroxide-treated chick neurons measured using immunoprecipitation and Western blotting [40]. In our peroxide treatments, which were performed in the presence of the B27 antioxidants, we obtained no effect on Aβ production until we exceeded 100 μM hydrogen peroxide, demonstrating the protection provided by the B27 supplement and explaining why we require higher levels of peroxide than did the previous study.
When using the ELISA to look at HAβ-induced RAβ secretion, it is necessary to use the highly active naturally secreted HAβ dimer-containing fractions (either from AD brain or 7PA2 conditioned medium). Traditionally prepared synthetic Aβ oligomers are 4000 fold less potent in biological effectiveness [28], requiring that they be used at concentrations well above those that interfere with the RAβ ELISA.
Aβ rapidly inhibits fast axonal transport in many hippocampal neurons, although in tau null neurons transport is not inhibited following Aβ treatment [18] suggesting that Aβ requires tau for its effects on vesicle transport. Between 40 and 70% of Aβ secreted by neurons is produced following endocytosis of AβPP [23]. Following endocytosis much of the AβPP and vesicular Aβ is trafficked to lysosomes and digested after endosome-lysosome fusion [56] so disruption of this transport may be one means to generate enhanced Aβ production. However, we found no significant differences in RAβ secreted in response to HAβd/t from organotypic slices from tau null mice either expressing or not expressing human tau, suggesting that tau-dependent transport inhibition per se plays no significant role in HAβd/t-induced RAβ secretion. However, transport-inhibiting cofilin-actin rods are induced in axons and dendrites of ~19% of hippocampal neurons by 1 μM synthetic Aβ oligomers [19] and ~30% of hippocampal neurons in response to 250 pM Aβd/t [28]. These Aβ-induced rods are mostly in neurons localized to the dentate gyrus and mossy fiber tract. These rods could be responsible for a non-tau-dependent transport inhibition that enhances Aβ secretion, however further work will be required to test this hypothesis.
In addition to enhanced Aβ production following endocytosis of AβPP [23], there are several Aβ-induced signaling pathways that could promote a feed-forward mechanism. One of the best characterized of these is a pathway dependent upon phosphorylated c-Jun N-terminal kinase (JNK), which can be activated by oxidative stress [37] or via many other upstream signals [57]. JNK serves as an activator for the transcription of both beta- and gamma-secretase [37,57]. Different forms of Aβ working extracellularly may signal through a number of different receptors to activate the JNK pathway [57]. Because different neuronal populations may express these receptors in different ratios, the exact signaling mechanism responsible for the enhanced Aβ production is complex and may vary in different regions of the brain. Furthermore, there is evidence for an intracellular Aβ pool that contributes to mitochondrial dysfunction and enhanced oxidative stress [58], which may also be enhanced in certain neuronal subpopulations.
In conclusion, our results show for the first time that the SDS-stable Aβd/t fraction of secreted human Aβ enhances the secretion of rodent Aβ from both dissociated rat cortical neurons and cultured rat and mouse hippocampal slices. This finding suggests the feed-forward response, generated by the HAβ species that correlates most with severity of dementia [17], could play a key role in the progression of AD.
Supplementary Material
Acknowledgments
We would like to thank Dr. Dennis Selkoe, BWH, Harvard Medical School for the gift of 7PA2 cells, Peggy Taylor of Covance for the rodent capture antibody-coated ELISA plates and the HRP-conjugated detection antibody, and Drs. Chi Pak, O’Neil Wiggan, Barbara Bernstein and Ms. Alisa Shaw for valuable discussions. This work was funded in part from NIH grant NS40371 from the National Institute of Neurological Disorders and Stroke and grant 281201 from the Alzheimer Drug Discovery Foundation.
References
- 1.Alzheimer’s Association. Alzheimer’s Disease Facts and Figures. Alzheimers Dement. 2010;6:158–194. doi: 10.1016/j.jalz.2010.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Bamburg JR, Bloom GS. Cytoskeletal pathologies of Alzheimer disease. Cell Motil Cytoskeleton. 2009;66:635–649. doi: 10.1002/cm.20388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanzi RE, Bertram L. Twenty years of the Alzheimer’s disease amyloid hypothesis: a genetic perspective. Cell. 2005;120:545–555. doi: 10.1016/j.cell.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Glenner GG, Wong CW. Alzheimer’s disease: initial report of the purification and characterization of a novel cerebrovascular amyloid protein. Biochem Biophys Res Commun. 1984;120:885–890. doi: 10.1016/s0006-291x(84)80190-4. [DOI] [PubMed] [Google Scholar]
- 5.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 6.Mattson MP. Pathways towards and away from Alzheimer’s disease. Nature. 2004;430:631–639. doi: 10.1038/nature02621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Price DL, Sisodia SS, Gandy SE. Amyloid beta amyloidosis in Alzheimer’s disease. Curr Opin Neurol. 1995;8:268–274. doi: 10.1097/00019052-199508000-00004. [DOI] [PubMed] [Google Scholar]
- 8.Finder VH, Glockshuber R. Amyloid-beta aggregation. Neurodegener Dis. 2007;4:413–427. doi: 10.1159/000100355. [DOI] [PubMed] [Google Scholar]
- 9.Portelius E, Bogdanovic N, Gustavsson MK, Volkmann I, Brinkmalm G, Zetterberg H, Winblad B, Blennow K. Mass spectrometric characterization of brain amyloid beta isoform signatures in familial and sporadic Alzheimer’s disease. Acta Neuropathol. 2010;120:185–193. doi: 10.1007/s00401-010-0690-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krafft G, Klein WL. ADDLs and the signaling web that leads to Alzheimer’s disease. Neuropharmacology. 2010;59:230–242. doi: 10.1016/j.neuropharm.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 11.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oligomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 12.Cleary JP, Walsh DM, Hofmeister JJ, Shankar GM, Kuskowski MA, Selkoe DJ, Ashe KH. Natural oligomers of the amyloid-beta protein specifically disrupt cognitive function. Nat Neurosci. 2005;8:79–84. doi: 10.1038/nn1372. [DOI] [PubMed] [Google Scholar]
- 13.Townsend M, Shankar GM, Mehta T, Walsh DM, Selkoe DJ. Effects of secreted oligomers of amyloid beta-protein on hippocampal synaptic plasticity: a potent role for trimers. J Physiol. 2006;572:477–492. doi: 10.1113/jphysiol.2005.103754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shankar GM, Bloodgood BL, Townsend M, Walsh DM, Selkoe DJ, Sabatini BL. Natural oligomers of the Alzheimer amyloid-beta protein induce reversible synapse loss by modulating an NMDA-type glutamate receptor-dependent signaling pathway. J Neurosci. 2007;27:2866–2875. doi: 10.1523/JNEUROSCI.4970-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Freir DB, Fedriani R, Scully D, Smith IM, Selkoe DJ, Walsh DM, Regan CM. Aβ oligomers inhibit synapse remodelling necessary for memory consolidation. Neurobiol Aging. 2010 Jan 22; doi: 10.1016/j.neurobiolaging.2010.01.001. 2010. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer’s brains impair synaptic plasticity and memory. Nat Med. 2008;14:837–842. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McDonald JM, Savva GM, Brayne C, Welzel AT, Forster G, Shankar GM, Selkoe DJ, Ince PG, Walsh DM. The presence of sodium dodecyl sulphate-stable Aβ dimers is strongly associated with Alzheimer-type dementia. Brain. 2010;133:1328–1341. doi: 10.1093/brain/awq065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vossel KA, Zhang K, Brodbeck J, Daub AC, Sharma P, Finkbeiner S, Cui B, Mucke L. Tau reduction prevents Aβ-induced defects in axonal transport. Science. 2010;330:398. doi: 10.1126/science.1194653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maloney MT, Minamide LS, Kinley AW, Boyle JA, Bamburg JR. Beta-secretase-cleaved amyloid precursor protein accumulates at actin inclusions induced in neurons by stress or amyloid beta: a feedforward mechanism for Alzheimer’s disease. J Neurosci. 2005;25:11313–11321. doi: 10.1523/JNEUROSCI.3711-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koo EH, Squazzo SL. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem. 1994;269:17386–17389. [PubMed] [Google Scholar]
- 21.Ehehalt R, Keller P, Haass C, Thiele C, Simons K. Amyloidogenic processing of the Alzheimer beta-amyloid precursor protein depends on lipid rafts. J Cell Biol. 2003;160:113–123. doi: 10.1083/jcb.200207113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thomas RS, Liddell JE, Murphy LS, Pache DM, Kidd EJ. An antibody to the beta-secretase cleavage site on amyloid-beta-protein precursor inhibits amyloid-beta production. J Alzheimers Dis. 2006;10:379–390. doi: 10.3233/jad-2006-10406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cirrito JR, Kang J-E, Lee J, Stewart FR, Verges DK, Silverio LM, Bu G, Mennerick S, Holtzman DM. Endocytosis is required for synaptic activity-dependent release of amyloid-beta in vivo. Neuron. 2008;58:42–51. doi: 10.1016/j.neuron.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fukumoto H, Tomita T, Matsunaga H, Ishibashi Y, Saido TC, Iwatsubo T. Primary cultures of neuronal and non-neuronal rat brain cells secrete similar proportions of amyloid beta peptides ending at Aβ40 and Aβ42. Neuroreport. 1999;10:2965–2969. doi: 10.1097/00001756-199909290-00017. [DOI] [PubMed] [Google Scholar]
- 25.Gasparini L, Rusconi L, Xu H, del Soldato P, Ongini E. Modulation of beta amyloid metabolism by non-steroidal anti-inflammatory drugs in neuronal cell cultures. J Neurochem. 2004;88:337–348. doi: 10.1111/j.1471-4159.2004.02154.x. [DOI] [PubMed] [Google Scholar]
- 26.Dasari B, Prasanthi JRP, Marwarha G, Singh BB, Ghribi O. The oxysterol 27 hydroxycholesterol increases β-amyloid and oxidative stress in retinal pigment epithelial cells. BMC Opthomol. 2010;10:22. doi: 10.1186/1471-2415-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Townsend M, Mehta T, Selkoe DJ. Soluble Aβ inhibits specific signal transduction cascades common to the insulin receptor pathway. J Biol Chem. 2007;282:33305–33312. doi: 10.1074/jbc.M610390200. [DOI] [PubMed] [Google Scholar]
- 28.Davis RC, Marsden IT, Maloney MT, Minamide LS, Podlisny M, Selkoe DJ, Bamburg JR. Amyloid beta dimers/trimers potently induce cofilin-actin rods that are inhibited by maintaining cofilin phosphorylation. Mol Neurodegen. 2011 doi: 10.1186/1750-1326-6-10. (acceptance expected within two weeks) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shankar GM, Welzel AT, McDonald JM, Selkoe DJ, Walsh DM. Isolation of Low-n amyloid β-protein oligomers from cultured cells, CSF, and Brain. Methods Mol Biol. 2011;670:33–44. doi: 10.1007/978-1-60761-744-0_3. [DOI] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch EF, Maniatis T. Molecular Cloning, a laboratory manual. 2. Vol. 3. Cold Spring Harbor Laboratory Press; 1989. p. E.5. [Google Scholar]
- 31.Minamide LS, Striegl AM, Boyle JA, Meberg PJ, Bamburg JR. Neurodegenerative stimuli induce persistent ADF/cofilin-actin rods that disrupt distal neurite function. Nat Cell Biol. 2000;2:628–636. doi: 10.1038/35023579. [DOI] [PubMed] [Google Scholar]
- 32.Stoppini L, Buchs PA, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 33.Davis RC, Maloney MT, Minamide LS, Flynn KC, Stonebraker MA, Bamburg JR. Mapping cofilin-actin rods in stressed hippocampal slices and the role of cdc42 in amyloid-beta-induced rods. J Alzheimers Dis. 2009;18:35–50. doi: 10.3233/JAD-2009-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gähwiler BH. Organotypic monolayer cultures of nervous tissue. J Neurosci Methods. 1981;4:329–342. doi: 10.1016/0165-0270(81)90003-0. [DOI] [PubMed] [Google Scholar]
- 35.Fung J, Frost D, Chakrabartty A, McLaurin J. Interaction of human and mouse Aβ peptides. J Neurochem. 2004;91:1398–1403. doi: 10.1111/j.1471-4159.2004.02828.x. [DOI] [PubMed] [Google Scholar]
- 36.Jankowsky JL, Younkin LH, Gonzales V, Fadale DJ, Slunt HH, Lester HA, Younkin SG, Borchelt DR. Rodent Aβ modulates the solubility and distribution of amyloid deposits in transgenic mice. J Biol Chem. 2007;282:22707–22720. doi: 10.1074/jbc.M611050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shen C, Chen Y, Liu H, Zhang K, Zhang T, Lin A, Jing N. Hydrogen peroxide promotes Abeta production through JNK-dependent activation of gamma- secretase. J Biol Chem. 2008;283:17721–17730. doi: 10.1074/jbc.M800013200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kricka LJ. Stains, labels and detection strategies for nucleic acids assays. Ann Clin Biochem. 2002;39:114–129. doi: 10.1258/0004563021901865. [DOI] [PubMed] [Google Scholar]
- 39.Ausubel FM, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K. Current Protocols in Molecular Biology. Vol. 3. John Wiley & Sons; New York: 1994. Appendix A.1- B.1. [Google Scholar]
- 40.Goldsbury C, Whiteman IT, Jeong EV, Lim YA. Oxidative stress increases levels of endogenous amyloid-beta peptides secreted from primary chick brain neurons. Aging Cell. 2008;7:771–775. doi: 10.1111/j.1474-9726.2008.00423.x. [DOI] [PubMed] [Google Scholar]
- 41.Hasegawa T, Ukai W, Jo D, Xu X, Mattson M, Nakagawa M, Araki W, Saito T, Yamada T. Homocysteic acid induces intraneuronal accumulation of neurotoxic Aβ42: Implications for the pathogenesis of Alzheimer’s disease. J Neurosci Res. 2005;80:869–876. doi: 10.1002/jnr.20514. [DOI] [PubMed] [Google Scholar]
- 42.Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular amyloid beta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/s0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 43.Casas C, Sergeant N, Itier JM, Blanchard V, Wirths O, van der Kolk N, Vingtdeux V, van de Steeg E, Ret G, Canton T, Drobecq H, Clark A, Bonici B, Delacourte A, Benavides J, Schmitz C, Tremp G, Bayer TA, Benoit P, Pradier L. Massive CA1/2 neuronal loss with intraneuronal and N-terminal truncated Abeta42 accumulation in a novel Alzheimer transgenic model. Am J Pathol. 2004;165:1289–1300. doi: 10.1016/s0002-9440(10)63388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stenh C, Englund H, Lord A, Johansson A-S, Almeida CG, Gellerfors P, Greengard P, Gouras GK, Lannfelt L, Nilsson LNG. Amyloid-beta oligomers are inefficiently measured by enzyme-linked immunosorbent assay. Ann Neurol. 2005;58:147–150. doi: 10.1002/ana.20524. [DOI] [PubMed] [Google Scholar]
- 45.Giuffrida ML, Caraci F, Pignataro B, Cataldo S, De Bona P, Bruno V, Molinaro G, Pappalardo G, Messina A, Palmigiano A, Garozzo D, Nicoletti F, Rizzarelli E, Copani A. Beta-amyloid monomers are neuroprotective. J Neurosci. 2009;29:10582–7. doi: 10.1523/JNEUROSCI.1736-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zou K, Gong JS, Yanagisawa K, Michikawa M. A novel function of monomeric amyloid beta-protein serving as an antioxidant molecule against metal-induced oxidative damage. J Neurosci. 2002;22:4822–4841. doi: 10.1523/JNEUROSCI.22-12-04833.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Busciglio J, Gabuzda DH, Matsudaira P, Yankner BA. Generation of beta-amyloid in the secretory pathway in neuronal and nonneuronal cells. Proc Natl Acad Sci USA. 1993;90:2092–2096. doi: 10.1073/pnas.90.5.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki N, Cheung T, Cai X, Odaka A, Otvos L, Eckman C, Golde T, Younkin S. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994;264:1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- 49.Esselmann H, Maler JM, Kunz N, Otto M, Paul S, Lewczuk P, Rüther E, Kornhuber J, Wiltfang J. Lithium decreases secretion of Aβ1-42 and C-truncated species Aβ1-37/38/39/40 in chicken telencephalic cultures but specifically increases intracellular Aβ1-38. Neurodegener Dis. 2004;1:236–241. doi: 10.1159/000080992. [DOI] [PubMed] [Google Scholar]
- 50.Carrodeguas JA, Rodolosse A, Garza MV, Sanz-Clemente A, Pérez-Pé R, Lacosta AM, Domínguez L, Monleón I, Sánchez-Díaz R, Sorribas V, Sarasa M. The chick embryo appears as a natural model for research in beta-amyloid precursor protein processing. Neuroscience. 2005;134:1285–1300. doi: 10.1016/j.neuroscience.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 51.Castrop H. Genetically modified mice-successes and failures of a widely used technology. Pflugers Arch. 2010;459:557–567. doi: 10.1007/s00424-009-0770-z. [DOI] [PubMed] [Google Scholar]
- 52.Wang Q, Walsh DM, Rowan MJ, Selkoe DJ, Anwyl R. Block of long-term potentiation by naturally secreted and synthetic amyloid -peptide in hippocampal slices is mediated via activation of the kinases c-Jun N-terminal activated protein kinase as well as metabotropic glutamate receptor type 5. J Neurosci. 2004;24:3370–3378. doi: 10.1523/JNEUROSCI.1633-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Atwood CS, Perry G, Zeng H, Kato Y, Jones WD, Ling K-Q, Huang X, Moir RD, Wang D, Sayre LM, Smith M, Chen SG, Bush AI. Copper mediates dityrosine cross-linking of Alzheimer’s amyloid-beta. Biochemistry. 2004;43:560–568. doi: 10.1021/bi0358824. [DOI] [PubMed] [Google Scholar]
- 54.Marksteiner J, Humpel C. Beta-amyloid expression, release and extracellular deposition in aged rat brain slices. Mol Psychiatry. 2008;13:939–952. doi: 10.1038/sj.mp.4002072. [DOI] [PubMed] [Google Scholar]
- 55.Johnstone E, Chaney M, Norris F, Pascual R, Little S. Conservation of the sequence of the Alzheimer’s disease amyloid peptide in dog, polar bear and five other mammals by cross-species polymerase chain reaction analysis. Brain Res Mol Brain Res. 1991;10:299–305. doi: 10.1016/0169-328x(91)90088-f. [DOI] [PubMed] [Google Scholar]
- 56.Lorenzen A, Samosh J, Vandewark K, Anborgh PH, Seah C, Magalhaes AC, Cregan SP, Ferguson SSG, Pasternak SH. Rapid and direct transport of cell surface APP to the lysosome defines a novel selective pathway. Mol Brain. 2010;3:11. doi: 10.1186/1756-6606-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tabaton M, Zhu X, Perry G, Smith MA, Gilberto L. Signaling effect of amyloid-β42 on the processing of AβPP. Exp Neurol. 2010;221:18–25. doi: 10.1016/j.expneurol.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Galindo MF, Ikuta I, Zhu X, Casadeus G, Jordan J. Mitochondrial biology in Alzheimer’s disease pathogenesis. J Neurochem. 2010;114:933–945. doi: 10.1111/j.1471-4159.2010.06814.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.