Abstract
Asparagine-linked glycosylation (N-glycosylation) is necessary for the proper folding of secreted and membrane proteins, including GPCRs. Thus, many GPCRs possess the N-glycosylation motif Asn-X-Ser/Thr at their N-termini and/or extracellular loops. We found that human GPR109A (hGPR109A) has an N-glycosylation site at Asn17 in the N-terminal atypical motif, Asn17-Cys18-Cys19. Why does hGPR109A require the atypical motif, rather than the typical sequence? Here we show that Asn17-Cys18-Cys19 sequence of hGPR109A possesses 2 biologic roles. First, Asn17-X-Cys19 contributed to hGPR109A N-glycosylation by acting as an atypical motif. This modification is required for the normal surface expression of hGPR109A, as evidenced by the reduced surface expression of the nonglycosylated mutants, hGPR109A/N17A, and the finding that hGPR109A/C19S and hGPR109A/C19T, which are N-glycosylated at Asn17, exhibited expression similar to the wild-type receptor. Second, the X-Cys18-Cys19 dicysteine is indispensable for hGPR109A function. Substitution of Cys18 or Cys19 residue to Ala impaired Gi-mediated signaling via hGPR109A. We propose the disulfide bond formations of these residues with other Cys existed in the extracellular loops for the proper folding. Together, these results suggest that the atypical motif Asn17-Cys18-Cys19 is crucial for the normal surface trafficking and function of hGPR109A.—Yasuda, D., Imura, Y., Ishii, S., Shimizu, T., and Nakamura, M. The atypical N-glycosylation motif, Asn-Cys-Cys, in human GPR109A is required for normal cell surface expression and intracellular signaling.
Keywords: Nicotinic acid receptor, N-linked glycosylation, Asn-X-Cys motif, STT3B, receptor activation
Human GPR109A, also known as hydroxycarboxylic acid receptor 2 and HM74A, is a 7-transmembrane GPCR that is activated by nicotinic acid (niacin) (1–3). Nicotinic acid-induced GPR109A activation in adipocytes results in Gi-protein-mediated inhibition of adenylyl cyclase. Inhibition of cAMP synthesis in turn leads to decreased hormone-sensitive lipase activity and reduced hydrolysis of triglycerides to free fatty acids (4). In mice lacking GPR109A, the antilipolytic effects of nicotinic acid as well as decreases in plasma free fatty acid and triglyceride levels are abrogated (2).
N-Glycosylation is an essential protein modification that affects a multitude of cellular functions. The attachment of oligosaccharides chains occurs on Asn residue in proteins, within a typical Asn-X-Ser/Thr sequence, where X is any amino acid except for proline (5, 6). The Ser and Thr residues at the third position of the typical sequence are considered to be important for the determination of the motif (5, 6). However, in a few proteins, other amino acids (e.g., Cys) are found at the +2 position in this motif instead of Ser or Thr (7–17). Although the glycan structures attached to the Asn-X-Cys sequences in these proteins have been studied, the biologic requirement for this motif is still unclear. The function of N-glycosylation has been established for only a limited number of GPCRs and includes roles in receptor-agonist binding, protein folding, maturation, and stability, as well as cell surface expression and internalization (18–21). Conversely, N-glycosylation may be nonessential for GPCR function in some cases (22–24).
GPR109A has garnered great interest as a target for new drugs with therapeutic and antilipolytic benefits, and therefore, this receptor has been well described in the literature (25, 26). However, the biologic significance of N-glycosylation and its motif in GPR109A in processes such as receptor trafficking and intracellular signaling still remains unclear. In this study, we demonstrate that GPR109A has a potential atypical Asn17-X-Cys19 N-glycosylation motif in its N-terminal region. N-glycosyl modification at this site has an important role in normal cell surface expression of the receptor. Additionally, we suggest that both Cys18 and Cys19 residues in this atypical N-glycosylation motif are indispensable for nicotinic acid-induced intracellular signaling [i.e., nicotinic acid-induced cAMP synthesis reduction and intracellular (Ca2+) elevation]. Thus, our data demonstrate that, instead of the typical sequence, the atypical motif Asn17-Cys18-Cys19 is requisite for the biologic function of GPR109A.
MATERIALS AND METHODS
Materials
Nicotinic acid was purchased from Cayman Chemical (Ann Arbor, MI, USA). Anti-hemagglutinin (HA) antibody (clone 3F10), endoglycosidase H (Endo-H), and peptide N-glycosidase F (PNGase-F) were obtained from Roche Applied Science (Penzberg, Germany). Tunicamycin (TM) was purchased from Wako (Osaka, Japan), and brefeldin A (Bref-A) was from Nacalai Tesque (Kyoto, Japan).
Construction of HA-tagged GPR109A mutants and prosaposin expression plasmids
N-terminally HA-tagged human GPR109A (hGPR109A) and C-terminally HA-tagged human prosaposin (hPSAP) were generated by amplifying their entire open reading frames by PCR using cDNA as a template and KOD Plus DNA polymerase (TOYOBO, Tokyo, Japan). The primer set used to amplify HA-tagged hGPR109A was as follows: sense primer, 5′-gctcgaattccgccatgtacccctacgacgtgcccgactacgccaatcggcaccatctgcagg-3′ and antisense primer, 5′-aaaagatatcttaaggagaggttgggcccagataagaggggc-3′. The primer set used to amplify HA-tagged hPSAP was as follows: sense primer, 5′-gctcaagcttcgccatgtacgccctcttcctcctggccagcct-3′ and antisense primer, 5′-aaaactcgagctaggcgtagtcgggcacgtcgtaggggtagttccacacatggcg-3′. N-terminally HA-tagged mutant hGPR109As (hGPR109A/N2A, /N17A, /N86A, /N171A, /N175A, /N265A, /D14A, /K15A, /K16A, /C18A, /C19A, /V20A, /C19S, /C19T, /C19V, /C19Y, /C19M, /C19D, /C19E, /C100A, /C177A, /C183A, and /C266A), were generated by overlap extension PCR (27). The primer sets utilized are listed in Supplemental Table 1. The resultant fragments were subcloned into the pcDNA3 vector. The sequences of all the aforementioned constructs were confirmed using DNA sequencing.
siRNA sequences
The STT3A- and STT3B-specific small interfering RNA (siRNA) sequences were target-specific as described previously (28). The chemically custom-synthesized siRNAs and a nontargeting siRNA control were purchased from Dharmacon (Lafayette, CO, USA). The following STT3A siRNAs were used: STT3A-RNAi-113 (5′-gcgauuguccuaugagaag-3′ annealed to 5′-cuucucauaggacaaucgc-3′) and STT3A-RNAi-780 (5′-ggccguuucucucaccggc-3′ annealed to 5′-uccggugagagaaacggcc-3′). The STT3B siRNAs were as follows: STT3B-RNAi-1539 (5′-gcucuauaugcaaucagua-3′ annealed to 5′-cacugauugcauauagagc-3′) and STT3B-RNAi-2394 (5′-cagcuggauuuucguacau-3′ annealed to 5′-guguacgaaaauccagcug-3′).
Cell culture and transfection
HeLa (human epithelial carcinoma), HEK293 (human embryonic kidney), and RH7777 (rat hepatoma) cells were cultured on collagen-coated dishes (Iwaki, Tokyo, Japan), in DMEM (Sigma-Aldrich, St. Louis, MO, USA) containing 10% (v/v) fetal bovine serum at 37°C in 5% CO2. CHO-K1 (Chinese hamster ovary-K1) cells were cultured in Ham’s F-12 (Sigma-Aldrich), supplemented with 10% (v/v) fetal bovine serum at 37°C in 5% CO2. These cells were transfected with expression plasmids using Lipofectamine 2000 reagent (Invitrogen, Carlsbad, CA, USA) in OptiMEM, according to the manufacturer’s protocol. For stable expression of WT or mutant hGPR109As, transfected CHO-K1 cells were grown in the presence of Geneticin (1 mg/ml; Invitrogen) for selection, isolated, expanded, and then tested for hGPR109A expression by performing Western blot analysis. For siRNA experiments, 1 × 105 HeLa cells were seeded on 6 cm2 dishes and grown for 24 hours prior to transfection with 50 nM siRNA. Forty-eight hours after siRNA transfection, cells were subjected to receptor transfection and were assayed 16 hours later.
Glycosidase treatment and protein preparation
Twenty-four hours after transfection, cells were washed with ice-cold PBS without calcium chloride and magnesium chloride [PBS(–)] and then harvested using PBS(–) containing 2 mM EDTA. Cells were homogenized in ice-cold homogenization buffer [25 mM HEPES-NaOH (pH 7.4), 10 mM MgCl2, 0.25 M sucrose, and protease inhibitor mixture (Roche, one tablet in 50 ml)]. The lysates were centrifuged at 1000 g for 5 minutes at 4°C to remove nuclei and intact cells. The supernatants were subjected to glycosidase treatments after the protein concentration of each sample was measured by the Bradford method (29) using a Protein Assay Kit (Bio-Rad, Hercules, CA, USA). Fatty acid-free bovine serum albumin (BSA; Fraction V, Sigma-Aldrich) was used as a standard. Protein samples were treated with Endo-H (0.005 U) in 50 μl buffer [11.7 mM Na2HPO4, 168.3 mM NaH2PO4, 0.4% (w/v) SDS, 20 mM EDTA, 2% (v/v) 2-mercaptethanol] or with PNGase-F (1 U) in 50 μl buffer [139.2 mM Na2HPO4, 40.8 mM NaH2PO4, 0.4% (w/v) SDS, 20 mM EDTA, 2% (v/v) 2-mercaptethanol] for 20 hours at 4°C (30, 31). The resultant protein samples were suspended in 10% (v/v) glycerol and 0.001% (w/v) bromophenol blue and subjected to Western blot analysis.
Western blot analysis
Membrane and cytosolic protein fractions were electrophoresed on 8% and 10% SDS-polyacrylamide gels, and then transferred to nitrocellulose membranes. After blocking with 5% skim milk in TBS-T [20 mM Tris-buffered saline (pH 7.6) and 0.1% Tween 20], the blots were probed with 1 μg/ml 3F10 rat monoclonal anti-HA antibody (Roche) for 1 hour. The membranes were then washed with TBS-T and incubated with 0.4 μg/ml horseradish peroxidase-conjugated anti-rat IgG (Santa Cruz Biotechnology, CA, USA) for 1 hour. The proteins were visualized using the ECL Chemiluminescence Detection System (GE Healthcare, Little Chalfont, United Kingdom).
Flow cytometry
At 6, 12, and 24 hours after transfection, HeLa and CHO-K1 cells were incubated with 2 μg/ml of 3F10 anti-HA antibody in PBS(–) containing 2% goat serum (PBS/GS) for 30 minutes, followed by staining with 5 μg/ml phycoerythrin-conjugated anti-rat IgG (Beckman Coulter Electronics Ltd., Fullerton, CA, USA) for 30 minutes at room temperature. Cells were washed twice with PBS/goat serum and analyzed with a flow cytometer, EPICS XL (Beckman Coulter Electronics Ltd.).
Real-time reverse transcriptase PCR analysis
Total RNA was isolated from HeLa cells by using a QIAzol and RNeasy mini kit (Qiagen, Valencia, CA, USA), and cDNA was synthesized from 500 ng of total RNA by using a PrimeScript RT Reagent Kit (TaKaRa Bio., Otsu, Japan) with random hexamers. A real-time PCR assay was performed using SYBR Premix Ex Taq (TaKaRa Bio.) in a LightCycler 480 (Roche). The cycling conditions were as follows: initial denaturation at 95°C for 10 seconds, followed by 50 cycles of 95°C for 5 seconds and 60°C for 30 seconds, according to the SYBR Premix Ex Taq (Perfect Real Time) protocol. Primers were designed to span exon junctions to detect possible contaminating genomic DNA. The following primer sequences were used: β-actin forward 5′-caggatgcagaaggagatcactg-3′ and reverse 5′-tactcctgcttgctgatccacat-3′; STT3A forward 5′-tctggtaggctttgtccttctcac-3′ and reverse 5′-gcaatgatggggatgttgttct-3′; and STT3B forward 5′-gtctctgcttggggtggttatg-3′ and reverse 5′-cacttgttctgattggctggaa-3′.
cAMP measurements
Twenty-four hours after transfection, CHO-K1 cells (4 × 104 cells) were cultured on noncoated 96-well plates (Corning Costar Japan, Tokyo, Japan) for 24 hours. Cells were washed twice with buffer A [HBSS containing 25 mM HEPES-NaOH (pH 7.4) and 0.1% BSA] and preincubated in 50 μl of buffer A containing 0.5 mM 3-isobutyl-1-methylxanthine (from a 100 mM stock in DMSO stored at −30°C) for 15 minutes. Subsequently, various concentrations of nicotinic acid in buffer A (50 μl) were added, and cells were incubated for 30 minutes. The reactions were terminated by the addition of 10% Tween 20 (10 μl), followed by overnight storage at 4°C. The cAMP concentrations were measured using an AlphaScreen cAMP assay kit (PerkinElmer Life Sciences Japan, Tokyo, Japan) according to the manufacturer’s instructions. For Gi-protein inhibitory experiments, cells were pretreated with 100 ng/ml pertussis toxin (PTX) for 6 hours prior to analysis.
Intracellular [Ca2+] measurements
Stably transfected CHO-K1 cells were plated in a 96-well plate (4 × 104 cells/well) and incubated for 16 h at 37°C. The cells were then incubated with loading buffer [buffer A (1× HBSS, 2.5 mM probenecid (Sigma-Aldrich), 20 mM HEPES, 1 mM CaCl2, 1 mM MgCl2, and 0.01% BSA) containing 4 μM Fluo 3-AM (Dojindo, Kumamoto, Japan) and 0.04% pluronic acid (Molecular Probes, Eugene, OR, USA)] at 37°C for 1 hour. Cells were then washed twice with buffer A. Intracellular [Ca2+] mobilization was monitored using a scanning fluorometer (FlexStation, Molecular Devices Corp. Sunnyvale, CA, USA) by measuring emission fluorescence at 525 nm in response to excitation at 485 nm. Relative fluorescence units (maximum − minimum) are indicated.
Comprehensive search for GPCRs with atypical Asn-X-Cys sequences
Extracellular domains of 823 hGPCRs deposited in Swiss-Prot were predicted using the Transmembrane Hidden Markov Model (32). In the predicted extracellular domains of each GPCR, Asn-X-Ser/Thr and Asn-X-Cys motifs, where X is any amino acid except for proline, were searched.
Statistical analysis
Data were analyzed for statistical significance by using Prism 4 software (GraphPad Software, Inc., San Diego, CA, USA). Statistical significance was evaluated using analysis of variance and Dunnett post hoc pairwise comparisons or unpaired t tests. Differences were considered significant at P < 0.05, 0.01, and 0.001, as indicated.
RESULTS
N-glycan modification of hGPR109A
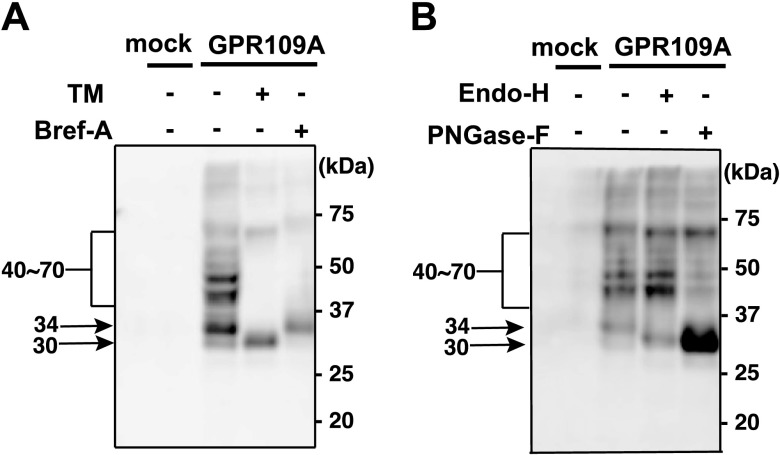
To examine whether hGPR109A N-glycosyl modified, we first generated N-terminally HA-tagged hGPR109A, expressed the receptor proteins in HeLa cells, and performed Western blotting by using anti-HA antibody. Twenty-four hours after transfection, the proteins of several different molecular masses (broad bands at 40–70 kDa, and narrow bands at 34 kDa and 30 kDa) were detected when probing for hGPR109A (Fig. 1A). The broad bands (40–70 kDa) were drastically reduced after treatment with TM, an inhibitor of protein N-glycosylation, or Bref-A, an inhibitor of the early secretory pathway. The 30-kDa band was detected after treatment with TM, and the 34-kDa band remained after Bref-A treatment (Fig. 1A). The broad bands were reduced by PNGase-F treatment, which cleaves all N-glycan types but not by Endo-H treatment, which specifically digests high-mannose glycans (Fig. 1B). In contrast, the 34-kDa band shifted to 30 kDa in response to PNGase-F and Endo-H treatments (Fig. 1B). These data suggested that the 34-kDa and 30-kDa bands corresponded to core glyco-chain-conjugated and nonglycosylated forms, respectively. As was observed in our previous studies (33, 34), the detected proteins migrated through the gel faster than their calculated molecular masses (∼42 kDa for nonglycosylated hGPR109A). Similar results were obtained in RH7777 and HEK293 cells (Supplemental Fig. 1). Taken together, these results suggest that hGPR109A is subject to N-glycosyl modification(s).
Figure 1.
Human GPR109A is an N-glycosylated receptor. A) Four hours after the HeLa cells were transfected with empty vector (mock) or HA-tagged hGPR109A, they were pretreated with 3.0 μg/ml TM (+) or 1.0 μg/ml Bref-A (+), or were left untreated (−). After 20 h, the protein concentrations of cell homogenate supernatants were measured and separated using SDS-PAGE. After staining with anti-HA and horseradish peroxidase-conjugated anti-rat IgG as primary and secondary antibodies, respectively, the molecular masses of the expressed HA-tagged hGPR109A products were determined using Western blot analysis. B) Twenty-four hours after transfection with empty vector (mock) or HA-tagged hGPR109A, HeLa cells were homogenized and treated with Endo-H (+) or PNGase-F (+), or were left untreated (−). The molecular mass of hGPR109A was determined using SDS-PAGE and Western blot analyses, as described in the Materials and Methods. The positions of molecular markers are indicated on the right, and the approximate molecular sizes of hGPR109A are shown on the left. Data are representative of 3 independent experiments that yielded similar results.
Role of the Asn17-X-Cys19 motif in hGPR109A N-glycosylation
hGPR109A does not have a typical Asn-X-Ser/Thr motif for N-glycosylation in its extracellular domains; however, 6 Asn residues reside in these regions (Fig. 2A). To determine the N-glycosylation sites in hGPR109A, we generated an Ala substitution mutant receptor for each of these 6 Asn residues. Western blot analyses clearly showed that the hGPR109A mutant in which Asn17 was replaced by Ala (N17A) appeared as a single 30-kDa band, whereas the other mutants generated signals similar to WT, suggesting that N-glycosyl modification occurred on hGPR109A Asn17 (Fig. 2B). To determine which residues were important for this modification, we constructed mutant receptors that were Ala-substituted in proximal Asn17 amino acids. As shown in Fig. 2C, a 30 kDa band, which was similar to that of hGPR109A/N17A, was obtained for hGPR109A/C19A, demonstrating the significance of this residue for the N-glycosylation. Taken together, these data suggest that the N-terminal atypical motif Asn17-X-Cys19 is involved in the hGPR109A N-glycosylation.
Figure 2.
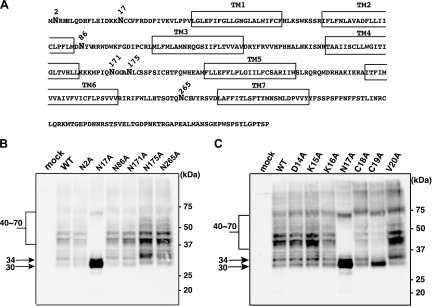
N-glycans occupy the atypical motif Asn17-X-Cys19 of hGPR109A. A) The amino acid sequence of hGPR109A was obtained from the UniprotKB/Swiss-Prot database (ID: Q8TDS4). Putative transmembrane domains are boxed and 6 Asn residues within the extracellular regions are shown in bold. B) hGPR109A/WT and Ala substitution mutations (1 of 6 extracellular Asn residues) were each transiently expressed in HeLa cells, and the molecular mass of each product was determined using SDS-PAGE and Western blot analyses. C) hGPR109A/WT and Ala substitution mutants (amino acids proximal to Asn17) were each transiently expressed in HeLa cells and the molecular mass of each product was determined using SDS-PAGE and Western blot analyses, as described in the Materials and Methods. The positions of molecular markers are indicated on the right, and the approximate molecular sizes are shown on the left. Data are representative of 3 independent experiments that yielded similar results.
Importance of N-glycosylation for hGPR109A cell surface expression
Next, we examined the importance of the N-glycosylation in hGPR109A function. As shown in Fig. 3A, B, the TM treatment significantly impaired the surface expression of hGPR109A. Furthermore, the hGPR109A mutant lacking N-glycans, hGPR109A/N17A, had significantly reduced the cell surface expression compared with that of the WT receptor (Fig. 3C, D). These results demonstrate that N-glycosylation is important for normal surface expression. N-glycosyl modification at Asn17 was observed even if the Cys19 residue was replaced with Ser or Thr, effectively generating a typical motif at the position of the atypical motif (Fig. 3E). Because hGPR109A/C19S and hGPR109A/C19T mutants showed similar surface expression to that of the WT receptor (Fig. 3F), N-glycosylation is important for normal surface trafficking of hGPR109A.
Figure 3.
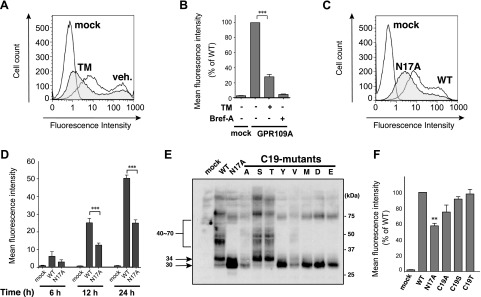
hGPR109A N-glycosylation is important for its normal cell surface expression. A) Surface expression levels of hGPR109A in HeLa cells were determined using flow cytometric analysis 24 hours after transfection with the empty vector (mock) or hGPR109A/WT. hGPR109A/WT-expressing cells were treated with 3.0 μg/ml TM or vehicle (veh.) for 20 h. After staining with anti-HA and phycoerythrin-conjugated anti-rat IgG as primary and secondary antibodies, respectively, the fluorescence intensity of each live cell was measured using flow cytometry. B) Four hours after transfection with hGPR109A/WT, HeLa cells were pretreated with 3.0 μg/ml TM (+) or 1.0 μg/ml Bref-A (+), or were left untreated (−) for 20 hours. Mean fluorescence intensities of the transfected cells were measured using flow cytometry. C) Twenty-four hours after transfection with mock plasmids, or those encoding WT or an N-glycosylation-defective hGPR109A mutant (N17A), hGPR109A surface expression levels in the transfected HeLa cells were determined using flow cytometric analysis. Representative results from the flow cytometric analyses are shown. D) HeLa cells were transfected with mock, WT, or N17A plasmids, and subjected to flow cytometric analyses at 6 hours, 12 hours, and 24 hours after transfection. Surface expression levels of the expressed receptors are represented as mean fluorescence intensities. E) hGPR109A/WT and the Cys19 substitution mutant, hGPR109A/C19A, /C19S, /C19T, /C19Y, /C19V, /C19M, /C19D, and /C19E, were each transiently expressed in HeLa cells, and the molecular mass of each product was determined using SDS-PAGE and Western blot analyses, as described in the Materials and Methods. The positions of molecular markers are indicated on the right, and the approximate molecular sizes of the products are shown on the left. The data are representative of 3 independent experiments that yielded similar results. F) HeLa cells were transfected with mock, hGPR109A/WT, /N17A, /C19A, /C19S, or /C19T plasmids, and subjected to the flow cytometric analyses at 6 hours, 12 hours, and 24 hours after transfection. Surface expression levels of each receptor are represented as mean fluorescence intensities. All data are represented as means ± se of 3 independent experiments. **P < 0.01, ***P < 0.001 vs. hGPR109A/WT; ANOVA with Tukey post hoc pairwise comparisons.
STT3B-mediated N-glycan conjugation on Asn17
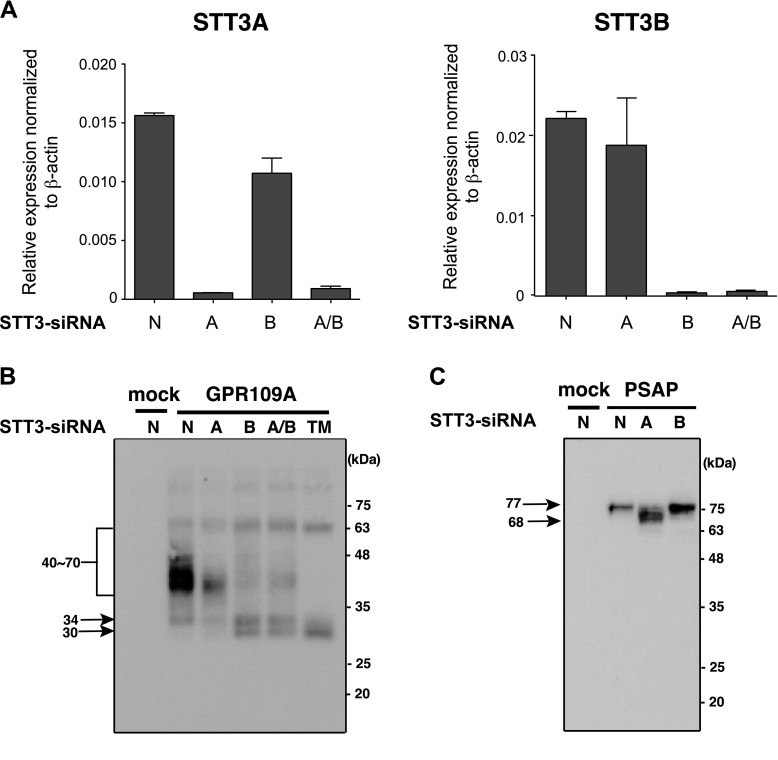
Oligosaccharyltransferase (OST) isoforms with distinct catalytic subunits, STT3A and STT3B, act sequentially to perform protein N-glycosylation (28). To gain a better understanding of how the hGPR109A atypical motif becomes N-glycosylated, we investigated the involvement of both STT3 isoforms in this process by using STT3A- and STT3B-specific siRNAs. We evaluated the inhibitory effects of STT3A- and STT3B-specific siRNAs on the expression of each mRNA in HeLa cells. The quantitative reverse transcriptase PCR analysis revealed that STT3A mRNA was specifically decreased by STT3A-siRNA treatment such that the levels in these cells were lower than those in cells treated by control-siRNA (Fig. 4A, left). In contrast, STT3B-siRNA treatment led to a marked reduction in STT3B mRNA (Fig. 4A, right), indicating that the STT3 isoforms are specifically depleted by their respective siRNAs. As shown in Fig. 4B, STT3B mRNA depletion decreased the hGPR109A broad bands and increased the 30 kDa band. In contrast, STT3A knockdown failed to reduce the N-glycosylated forms of hGPR109A, although this treatment effectively impaired PSAP N-glycosylation, which is mediated by STT3A (Fig. 4C) (28). Thus, these data suggest that the STT3B isoform preferentially contributed to the hGPR109A N-glycosylation.
Figure 4.
STT3B is required for efficient hGPR109A N-glycosylation. A) Endogenously expressed STT3A and STT3B mRNAs were knocked down in HeLa cells by using 50 nM isoform-specific STT3 siRNAs (STT3A-siRNA, STT3B-siRNA, or mixture of STT3A and STT3B-siRNAs). Relative STT3A and STT3B mRNA expression levels were determined using real-time reverse transcriptase PCR analysis. Values were normalized to β-actin mRNA levels. B, C) HeLa cells were cultured for 24 hours prior to transfection with 50 nM siRNA (STT3A-siRNA, STT3B-siRNA, mixture of STT3A and STT3B-siRNAs, or nontargeting siRNA). Forty-eight hours after siRNA transfection, the cells were transfected with hGPR109A/WT and hPSAP plasmids, and were harvested 16 hours later. The molecular masses of hGPR109A and hPSAP were determined using SDS-PAGE and Western blot analyses, as described in the Materials and Methods. A, STT3A-siRNA; B, STT3B-siRNA; A/B, mixture of STT3A and STT3B-siRNAs; N, nontargeting siRNA; TM: tunicamycin-treated.
Requirement of atypical motif dicysteine Cys18-Cys19 for hGPR109A function
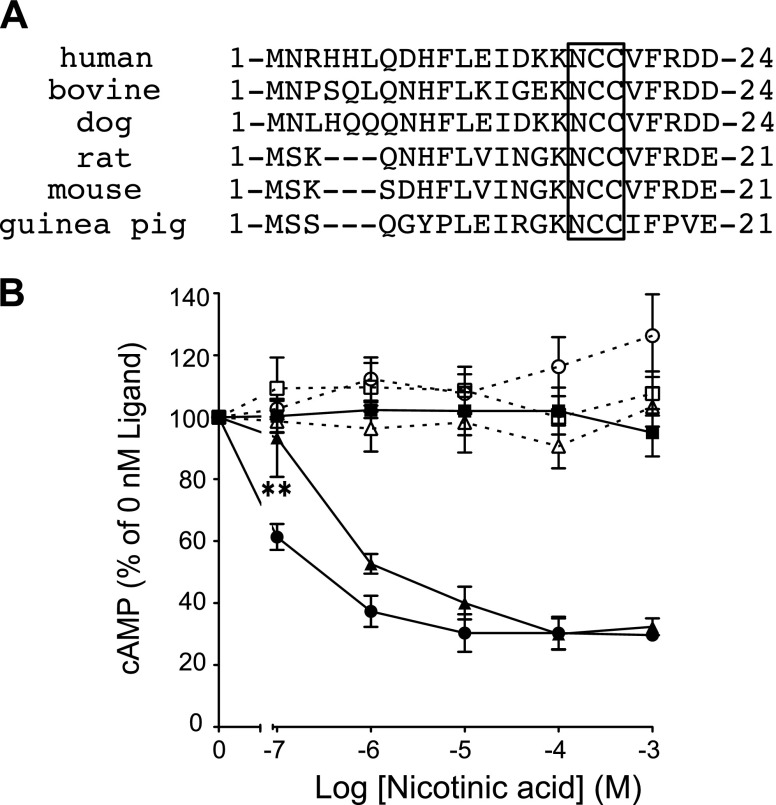
The hGPR109A atypical motif is conserved in several species (Fig. 5A), implying the importance of this motif in the function of GPR109A. Thus, we next sought to determine why hGPR109A requires the atypical N-glycosylation sequence, rather than the typical motif. First, to test whether N-glycosylation is required for hGPR109A function, we tested the ligand-dependent changes in cAMP production in response to hGPR109A/N17A expression in CHO-K1 cells. As previously reported, hGPR109A transduced intracellular signaling through Gi-type proteins, specifically the nicotinic acid-dependent reduction of cAMP production, an effect that was attenuated by PTX treatment (Fig. 5B). In hGPR109A/N17A-expressing cells, we observed Gi-dependent signaling, as indicated by dose-dependent inhibition of cAMP accumulation, although the efficacy (EC50) of the mutant was slightly lower than that of WT (WT, 0.30 ± 0.07 nM; N17A-mutant, 1.27 ± 0.46 nM; mean ± se, n = 4). These data suggest that this modification somehow contributes to Gi-type protein activation via hGPR109A (Fig. 5B). Next, to examine whether the amino acid residues flanking Asn17 are critical for hGPR109A activity, we constructed the Ala-substituted mutants, hGPR109A/D14A, /K15A, /K16A, /C18A, /C19A, and /V20A, and evaluated the effect of each mutation on forskolin-induced cAMP accumulation. Two mutants, hGPR109A/C18A and /C19A, markedly reduced the nicotinic acid-evoked inhibitory effect observed with expression of the WT receptor (Fig. 6A, B). To better understand the importance of the Cys19 residue, we evaluated the activities of 8 mutant receptors with Cys19 substituted for several other amino acids. All substitution mutants, including hGPR109A/C19S and /C19T that had N-glycan at Asn17, markedly attenuated the inhibition of forskolin-induced cAMP accumulation (Fig. 6C, D). The importance of Cys18 and Cys19 residues for hGPR109A activation was further confirmed in experiments in which we observed the agonist-induced increase in intracellular [Ca2+] (Fig. 6E). Thus, Cys18 and Cys19 residues in the atypical hGPR109A N-glycosylation motif are crucial for nicotinic acid-induced hGPR109A activation.
Figure 5.
hGPR109A N-glycosylation contributes to nicotinic acid-induced intracellular signaling. A) Amino acid sequences of mammalian GPR109As were obtained from the National Center for Biotechnology Information (NCBI) database (human ID: EU012026, bovine predicted sequence ID: XM002694482, dog predicted sequence ID: XM543378, rat ID: NM181476, mouse ID: NM030701, guinea pig ID: EF185820). N-terminal Asn-Cys-Cys sequences in each GPR109A are boxed. B) Stimulatory effects of nicotinic acid on forskolin-induced cAMP accumulation in CHO-K1 cells transiently expressing hGPR109A/WT (full circles) or hGPR109A/N17A (full triangles), or in empty vector-transfected cells (mock, full squares). These cells, hGPR109A/WT (open circles), hGPR109A/N17A (open triangles), and mock (open squares) cells, were pretreated with 100 ng/ml PTX for 6 h. Data are represented as the mean ± se (n = 3). Statistical analysis was carried out for hGPR109A/N17A (nontreated, full triangles). Data are representative of 3 independent experiments that yielded similar results. **P < 0.01 vs. hGPR109A/WT; ANOVA with Tukey post hoc pairwise comparisons.
Figure 6.
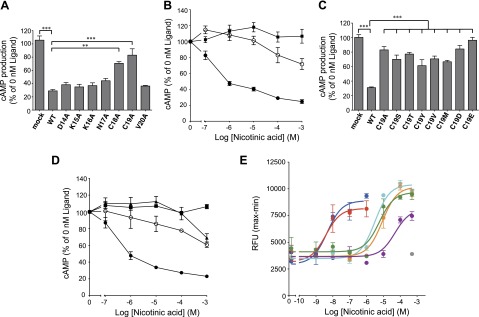
Cys18 and Cys19 residues in the hGPR109A atypical N-glycosylation motif are essential for nicotinic acid-induced intracellular signaling. A) Effects of 100 μM nicotinic acid on forskolin-induced cAMP accumulation in CHO-K1 cells transiently expressing hGPR109A/WT or Ala substitution mutants (amino acids proximal to Asn17), or empty vector-transfected cells (mock) are shown. **P < 0.01, ***P < 0.001 vs. hGPR109A/WT; ANOVA with Tukey post hoc pairwise comparisons. B) Effects of nicotinic acid on forskolin-induced cAMP accumulation in CHO-K1 cells transiently expressing hGPR109A/WT (full circles), /C19A (open circles), or those transfected with empty vector (mock, full squares). C) Stimulatory effects of 100 μM nicotinic acid on the forskolin-induced cAMP accumulation in CHO-K1 cells transiently expressing hGPR109A/WT or Cys19 substitution hGPR109A mutants, or those transfected with empty vector (mock). ***P < 0.001 vs. hGPR109A/WT; ANOVA with Tukey post hoc pairwise comparisons. D) Effects of nicotinic acid on forskolin-induced cAMP accumulation in CHO-K1 cells transiently expressing WT (full circles), hGPR109A/C19S (full triangles), or /C19T (open circles), or those transfected with empty vector (mock, full squares). E) Effects of nicotinic acid on intracellular [Ca2+] levels in CHO-K1 cells stably expressing hGPR109A/WT (blue), /N17A (red), /C18A (aqua), /C19A (green), /C19S (purple), or /C19T (orange), or those transfected with empty vector (mock, gray). All data are represented as means ± se (n = 3). Data are representative of 3 independent experiments yielding similar results. Average EC50 values for hGPR109A/WT, /N17A, /C18A, /C19A, /C19S, and /C19T in 3 independent experiments were 2.5 nM (WT), 3.5 nM (N17A), 1.9 μM (C18A), 4.6 μM (C19A), 71.3 μM (C19S), and 4.1 μM (C19T).
Significance of extracellular domain cysteine residues for hGPR109A activation
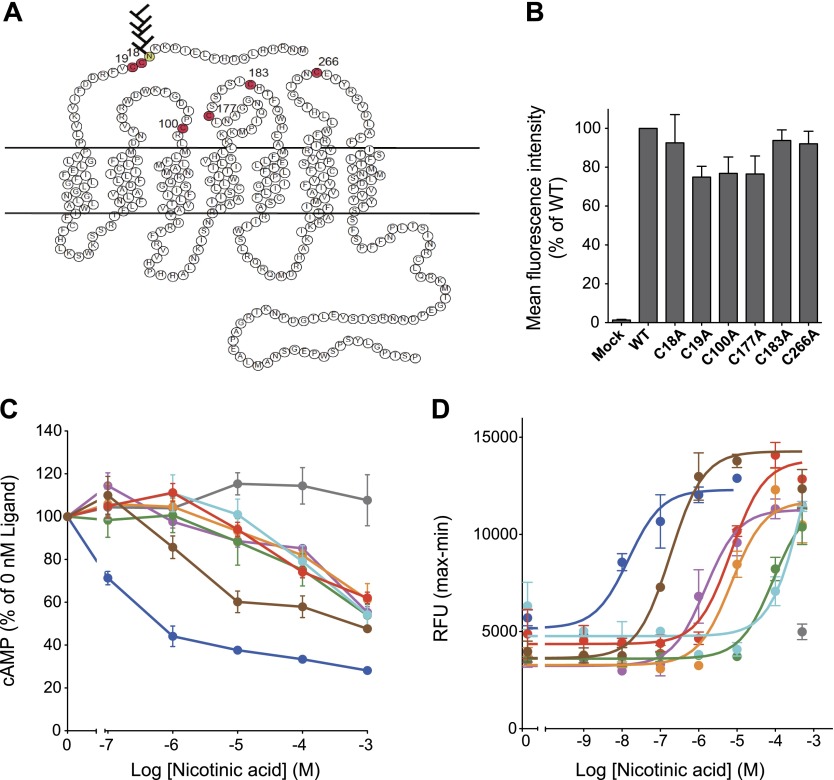
In addition to Cys18 and Cys19, 4 other cysteine residues are found in the extracellular regions (Cys100, Cys177, Cys183, and Cys266) that presumably form disulfide bonds (Fig. 7A). We next examined the significance of all 6 cysteine residues in hGPR109A activity. Disulfide bonds formed in the extracellular regions are known to be required to allow GPCRs pass through the quality check in the endoplasmic reticulum (ER). As expected, hGPR109A mutants lacking Cys19, Cys100, or Cys177 residues tended to reduce the hGPR109A surface trafficking compared with the WT receptor (Fig. 7B). We examined the nicotinic acid-stimulated cAMP and intracellular [Ca2+] responses in cells expressing WT or Cys-mutant receptors. As shown in Fig. 7C, D, all tested Cys-mutants drastically impaired nicotinic acid-induced responses compared to the WT receptor. Taken together, these data suggest that not only Cys18 and Cys19 residues in the N-terminal region, but also the Cys100, Cys177, Cys183, and Cys266 residues, located in the extracellular loops, are important for hGPR109A activity. We speculate that the disulfide bonds, which are indispensable for nicotinic acid-induced hGPR109A activation, form between these residues.
Figure 7.
Extracellular cysteine residues in hGPR109A are important for nicotinic acid-induced intracellular signaling. A) Secondary structure of hGPR109A. Extracellular, transmembrane, and cytoplasmic regions are based on the structure of rhodopsin. Amino acid symbols in red indicate cysteine residues in the extracellular regions of hGPR109A. B) Twenty-four hours after transfection with mock, hGPR109A/WT, /C18A, /C19A, /C100A, /C177A, /C183A, or /C266A plasmids, surface expression levels in CHO-K1 cells were determined using flow cytometric analysis. C) Stimulatory effects of nicotinic acid on forskolin-induced cAMP accumulation in CHO-K1 cells transiently expressing hGPR109A/WT (blue), /C18A (purple), /C19A (orange), /C100A (green), /C177A (aqua), /C183A (red), /C266A (brown), or those transfected with empty vector (mock, gray). D) Stimulatory effects of nicotinic acid on the intracellular [Ca2+] levels in CHO-K1 cells stably expressing hGPR109A/WT (blue), /C18A (purple), /C19A (orange), /C100A (green), /C177A (aqua), /C183A (red), or /C266A (brown), or those transfected with empty vector (mock, gray). Data are represented as means ± se (n = 3) and are representative of 3 independent experiments that yielded similar results. Average EC50 values for hGPR109A/WT, /C18A, /C19A, /C100A, /C177A, /C183A, and /C266A in 3 independent experiments were 7.9 nM (WT), 1.8 μM (C18A), 7.4 μM (C19A), 77.4 μM (C100A), 300 μM (C177A), 9.5 μM (C183A), and 541 nM (C266A).
DISCUSSION
N-glycosylation is a common post-translational modification of GPCRs and is known to play an important role in receptor folding, trafficking, and intracellular signaling of various members of this family (35–41). However, the functional requirements of N-glycosylation of GPR109A and its modification motif remained unknown. In this study, we clearly showed that hGPR109A is N-glycosylated at Asn17 of the atypical motif Asn17-Cys18-Cys19. To date, N-glycosylation of the atypical Asn-X-Cys sequence has been reported for several proteins: human α1T-glycoprotein (8), human von Willebrand factor (9), human CD69 (10), human α-lactalbumin (11), human and murine fetal antigen 1 (12, 13), human and bovine protein C (14, 15), recombinant human epidermal growth factor receptor (16), and plant B subunit of Shiga toxin (17). In addition, Zielinska et al. recently identified 65 Asn-X-Cys motifs including 5 Asn-Cys-Cys sequences, that are N-glycosylated, by a glycoproteomics analysis of mouse tissues (42), indicating that the atypical motif is not as rare as initially we believed. In these reports, N-glycosylation occupancy at the atypical sequence and N-glycan structures have been elucidated; however, little is known about the biologic requirements of this modification and motif. The importance of N-glycosylation at the typical motif for cell surface expression varies depending on the GPCR. For example, disruption of N-glycosylation sites strongly affects cell surface expression of the calcium receptor (43), vasointestinal peptide receptor (44), and the β2-adrenergic receptor (20) but does not alter the expression of the V2 vasopressin receptor (45) and parathyroid hormone receptor (46). In this study, flow cytometric analyses revealed that the normal cell surface expression of hGPR109A was reduced by TM treatment or N-glycosylation site mutations. N-glycans are known to mediate the interaction of nascent proteins with ER quality control chaperones such as calnexin and calreticulin to prevent their misfolding (47). Thus, the reduced cell surface expression of hGPR109A could be due to the decrease in the receptor export from the ER. Indeed, we performed immunohistochemical experiments to see the intracellular localizations of WT, N17A, and C19A mutants. Although the surface localizations of these receptors were confirmed, small amount of N17A was accumulated in the ER (data not shown). These results suggest the importance of N-glycosylation for normal surface expression of this receptor.
Ruiz-Canada et al (28) recently showed that distinct catalytic subunits within the OST enzyme complex, STT3A and STT3B, act sequentially and complementarily to optimize protein N-glycosylation. STT3A is primarily responsible for cotranslational modification of typical motifs when the nascent polypeptide enters the ER lumen. Conversely, the STT3B is less competent to generate cotranslational glycoproteins, but has the capacity to mediate post-translational modification of skipped glycosylation sites in unfolded proteins (28). In this study, STT3B-siRNA treatment markedly impaired hGPR109A N-glycosylation, suggesting that STT3B containing OSTs contribute to this modification. The STT3B-mediated N-glycosylation might depend on the location of its recognition motif, especially in N terminus, because N-glycan does not attach to Asn175-Leu176-Cys177 sequence located in the second extracellular loop of hGPR109A (Fig 2B).
To examine the requirements of Cys18 and Cys19 residues for nicotinic acid-induced signaling, we established CHO-K1 cell lines that stably expressed hGPR109A with mutations in these residues. Interestingly, hGPR109A/C18A, /C19A, /C19S, and /C19T markedly attenuated the inhibitory effects of forskolin-induced cAMP accumulation and intracellular [Ca2+] mobilization, suggesting that hGPR109A Cys18 and Cys19 residues are essential for the nicotinic acid-induced Gi-protein activation. Previous studies reported that hGPR109A Cys18 and Cys19 residues are important for radioligand and guanosine 5′-O-(3-thiotriphosphate) binding in COS-7 cells stably expressing hGPR109A mutations lacking these residues, when transiently coexpressed with the human Go2-protein (48). These data further support our finding that extracellular Cys residues, including Cys18 and Cys19, are indispensable for GPR109A function. We attribute the functional deficiencies of hGPR109A/C19S and /C19T to aberrant nicotinic acid binding.
Elimination of disulfide bonding due to mutagenesis leads to severely disrupted structures, followed by impaired intracellular trafficking and signaling (49, 50). Previously, the structural requirements of hGPR109A for the binding of nicotinic acid were characterized by site-directed mutagenesis of putative ligand-binding residues (51). In that study, the disulfide bond between Cys100 and Cys177 was proposed because C100A and C177A mutants had a similar impact on the cell surface expression. Moreover, it was reported that both Cys183 and Cys266 are important for the intracellular [Ca2+] signaling, suggesting that these Cys residues additionally form disulfide bonds, possibly with Cys18 and Cys19 (51). In this study, we demonstrated that not only Cys18 and Cys19 but also Cys100, Cys177, Cys183, and Cys266 residues are important for nicotinic acid-stimulated cAMP reduction and intracellular [Ca2+] response, although the bonding pattern of the other 2 disulfides is less clear. Taken together, these results suggest that the functional requirement of hGPR109A Cys18 and Cys19 residues is to form disulfide bonds that stabilize the conformation of the receptor.
We identified 44 GPCRs that have the Asn-X-Cys sequence in their N-termini, by performing in silico analysis of 823 human GPCRs (Supplemental Table 2). Notably, 11 of these GPCRs, including hGPR109A, hGPR109B, and hCX3C chemokine receptor 1, lack the Asn-X-Ser/Thr motif in their extracellular domains. Because they are similar to that of hGPR109A, these atypical motifs possibly have dual pivotal functions in N-glycosyl modification and disulfide bond formation.
In summary, hGPR109A is N-glycosylated at Asn17 at the atypical Asn-X-Cys motif, and this modification is important for the normal surface expression of the receptor. Interestingly, we found that the OST catalytic subunit STT3B contributes to hGPR109A N-glycosylation. This information may provide insights into the roles of STT3B-mediated N-glycosylation at the atypical motif. Furthermore, Cys18 and Cys19 residues in hGPR109A are essential for nicotinic acid-induced intracellular signaling, suggesting that these residues may play important roles in disulfide bond formation, contributing to structural stabilization of the receptor.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Y. Kita (University of Tokyo), Dr. H. Shindou (National Center for Global Health and Medicine), and Drs. N. Akahoshi and T. Ohto (Akita University), and to all members of their laboratory for technical advice and useful discussions. The authors also thank Mrs. C. Kanokoda and Mrs. Y. Sugimoto-Aruga for their technical support. This work was supported in part by a Grant-in-Aid from the Ministry of Education, Culture, Sports, Science and Technology of Japan (to D.Y., M.N., and T.S.), and grants from the Japan Society for the Promotion of Science (Global COE program) and the Center for NanoBio Integration at the University of Tokyo.
Glossary
- Bref-A
brefeldin A
- BSA
bovine serum albumin
- Endo-H
endoglycosidase H
- ER
endoplasmic reticulum
- h
human
- HA
hemagglutinin
- N-glycosylation
asparagine-linked glycosylation
- OST
oligosaccharyltransferase
- PNGase-F
peptide N-glycosidase F
- PSAP
prosaposin
- PTX
pertussis toxin
- siRNA
small interfering RNA
- TBS-T
20 mM Tris-buffered saline and 0.1% Tween 20
- TM
tunicamycin
- WT
wild-type
Footnotes
This article includes supplemental data. Please visit http://www.fasebj.org to obtain this information.
REFERENCES
- 1.Soga T., Kamohara M., Takasaki J., Matsumoto S., Saito T., Ohishi T., Hiyama H., Matsuo A., Matsushime H., Furuichi K. (2003) Molecular identification of nicotinic acid receptor. Biochem. Biophys. Res. Commun. 303, 364–369 [DOI] [PubMed] [Google Scholar]
- 2.Tunaru S., Kero J., Schaub A., Wufka C., Blaukat A., Pfeffer K., Offermanns S. (2003) PUMA-G and HM74 are receptors for nicotinic acid and mediate its anti-lipolytic effect. Nat. Med. 9, 352–355 [DOI] [PubMed] [Google Scholar]
- 3.Wise A., Foord S. M., Fraser N. J., Barnes A. A., Elshourbagy N., Eilert M., Ignar D. M., Murdock P. R., Steplewski K., Green A., Brown A. J., Dowell S. J., Szekeres P. G., Hassall D. G., Marshall F. H., Wilson S., Pike N. B. (2003) Molecular identification of high and low affinity receptors for nicotinic acid. J. Biol. Chem. 278, 9869–9874 [DOI] [PubMed] [Google Scholar]
- 4.Offermanns S. (2006) The nicotinic acid receptor GPR109A (HM74A or PUMA-G) as a new therapeutic target. Trends Pharmacol. Sci. 27, 384–390 [DOI] [PubMed] [Google Scholar]
- 5.Bause E., Hettkamp H. (1979) Primary structural requirements for N-glycosylation of peptides in rat liver. FEBS Lett. 108, 341–344 [DOI] [PubMed] [Google Scholar]
- 6.Marshall R. D. (1974) The nature and metabolism of the carbohydrate-peptide linkages of glycoproteins. Biochem. Soc. Symp. 40, 17–26 [PubMed] [Google Scholar]
- 7.Valliere-Douglass J. F., Eakin C. M., Wallace A., Ketchem R. R., Wang W., Treuheit M. J., Balland A. (2010) Glutamine-linked and non-consensus asparagine-linked oligosaccharides present in human recombinant antibodies define novel protein glycosylation motifs. J. Biol. Chem. 285, 16012–16022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Araki T., Haupt H., Hermentin P., Schwick H. G., Kimura Y., Schmid K., Torikata T. (1998) Preparation and partial structural characterization of alpha1T-glycoprotein from normal human plasma. Arch. Biochem. Biophys. 351, 250–256 [DOI] [PubMed] [Google Scholar]
- 9.Titani K., Kumar S., Takio K., Ericsson L. H., Wade R. D., Ashida K., Walsh K. A., Chopek M. W., Sadler J. E., Fujikawa K. (1986) Amino acid sequence of human von Willebrand factor. Biochemistry 25, 3171–3184 [DOI] [PubMed] [Google Scholar]
- 10.Vance B. A., Wu W., Ribaudo R. K., Segal D. M., Kearse K. P. (1997) Multiple dimeric forms of human CD69 result from differential addition of N-glycans to typical (Asn-X-Ser/Thr) and atypical (Asn-X-cys) glycosylation motifs. J. Biol. Chem. 272, 23117–23122 [DOI] [PubMed] [Google Scholar]
- 11.Giuffrida M. G., Cavaletto M., Giunta C., Neuteboom B., Cantisani A., Napolitano L., Calderone V., Godovac-Zimmermann J., Conti A. (1997) The unusual amino acid triplet Asn-Ile-Cys is a glycosylation consensus site in human alpha-lactalbumin. J. Protein Chem. 16, 747–753 [DOI] [PubMed] [Google Scholar]
- 12.Jensen C. H., Krogh T. N., Højrup P., Clausen P. P., Skjødt K., Larsson L. I., Enghild J. J., Teisner B. (1994) Protein structure of fetal antigen 1 (FA1). A novel circulating human epidermal-growth-factor-like protein expressed in neuroendocrine tumors and its relation to the gene products of dlk and pG2. Eur. J. Biochem. 225, 83–92 [DOI] [PubMed] [Google Scholar]
- 13.Krogh T. N., Bachmann E., Teisner B., Skjødt K., Højrup P. (1997) Glycosylation analysis and protein structure determination of murine fetal antigen 1 (mFA1)—the circulating gene product of the delta-like protein (dlk), preadipocyte factor 1 (Pref-1) and stromal-cell-derived protein 1 (SCP-1) cDNAs. Eur. J. Biochem. 244, 334–342 [DOI] [PubMed] [Google Scholar]
- 14.Gil G. C., Velander W. H., Van Cott K. E. (2009) N-glycosylation microheterogeneity and site occupancy of an Asn-X-Cys sequon in plasma-derived and recombinant protein C. Proteomics 9, 2555–2567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stenflo J., Fernlund P. (1982) Amino acid sequence of the heavy chain of bovine protein C. J. Biol. Chem. 257, 12180–12190 [PubMed] [Google Scholar]
- 16.Sato C., Kim J. H., Abe Y., Saito K., Yokoyama S., Kohda D. (2000) Characterization of the N-oligosaccharides attached to the atypical Asn-X-Cys sequence of recombinant human epidermal growth factor receptor. J. Biochem. 127, 65–72 [DOI] [PubMed] [Google Scholar]
- 17.Matsui T., Takita E., Sato T., Kinjo S., Aizawa M., Sugiura Y., Hamabata T., Sawada K., Kato K. (2011) N-glycosylation at noncanonical Asn-X-Cys sequences in plant cells. Glycobiology 21, 994–999 [DOI] [PubMed] [Google Scholar]
- 18.Davis D., Liu X., Segaloff D. L. (1995) Identification of the sites of N-linked glycosylation on the follicle-stimulating hormone (FSH) receptor and assessment of their role in FSH receptor function. Mol. Endocrinol. 9, 159–170 [DOI] [PubMed] [Google Scholar]
- 19.Michineau S., Muller L., Pizard A., Alhenc-Gélas F., Rajerison R. M. (2004) N-linked glycosylation of the human bradykinin B2 receptor is required for optimal cell-surface expression and coupling. Biol. Chem. 385, 49–57 [DOI] [PubMed] [Google Scholar]
- 20.Rands E., Candelore M. R., Cheung A. H., Hill W. S., Strader C. D., Dixon R. A. (1990) Mutational analysis of beta-adrenergic receptor glycosylation. J. Biol. Chem. 265, 10759–10764 [PubMed] [Google Scholar]
- 21.Shukla A. K., Reinhart C., Michel H. (2006) Comparative analysis of the human angiotensin II type 1a receptor heterologously produced in insect cells and mammalian cells. Biochem. Biophys. Res. Commun. 349, 6–14 [DOI] [PubMed] [Google Scholar]
- 22.Fukushima Y., Oka Y., Saitoh T., Katagiri H., Asano T., Matsuhashi N., Takata K., van Breda E., Yazaki Y., Sugano K. (1995) Structural and functional analysis of the canine histamine H2 receptor by site-directed mutagenesis: N-glycosylation is not vital for its action. Biochem. J. 310, 553–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sawutz D. G., Lanier S. M., Warren C. D., Graham R. M. (1987) Glycosylation of the mammalian alpha 1-adrenergic receptor by complex type N-linked oligosaccharides. Mol. Pharmacol. 32, 565–571 [PubMed] [Google Scholar]
- 24.Van Koppen C. J., Nathanson N. M. (1990) Site-directed mutagenesis of the m2 muscarinic acetylcholine receptor. Analysis of the role of N-glycosylation in receptor expression and function. J. Biol. Chem. 265, 20887–20892 [PubMed] [Google Scholar]
- 25.Wanders D., Judd R. L. (2011) Future of GPR109A agonists in the treatment of dyslipidaemia. Diabetes Obes. Metab. 13, 685–691 [DOI] [PubMed] [Google Scholar]
- 26.Digby J. E., Ruparelia N., Choudhury R. P. (2012) Niacin in cardiovascular disease: recent preclinical and clinical developments. Arterioscler. Thromb. Vasc. Biol. 32, 582–588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ho S. N., Hunt H. D., Horton R. M., Pullen J. K., Pease L. R. (1989) Site-directed mutagenesis by overlap extension using the polymerase chain reaction. Gene 77, 51–59 [DOI] [PubMed] [Google Scholar]
- 28.Ruiz-Canada C., Kelleher D. J., Gilmore R. (2009) Cotranslational and posttranslational N-glycosylation of polypeptides by distinct mammalian OST isoforms. Cell 136, 272–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 30.Tan C. M., Nickols H. H., Limbird L. E. (2003) Appropriate polarization following pharmacological rescue of V2 vasopressin receptors encoded by X-linked nephrogenic diabetes insipidus alleles involves a conformation of the receptor that also attains mature glycosylation. J. Biol. Chem. 278, 35678–35686 [DOI] [PubMed] [Google Scholar]
- 31.Rutz C., Renner A., Alken M., Schulz K., Beyermann M., Wiesner B., Rosenthal W., Schülein R. (2006) The corticotropin-releasing factor receptor type 2a contains an N-terminal pseudo signal peptide. J. Biol. Chem. 281, 24910–24921 [DOI] [PubMed] [Google Scholar]
- 32.Krogh A., Larsson B., von Heijne G., Sonnhammer E. L. (2001) Predicting transmembrane protein topology with a hidden Markov model: application to complete genomes. J. Mol. Biol. 305, 567–580 [DOI] [PubMed] [Google Scholar]
- 33.Yasuda D., Okuno T., Yokomizo T., Hori T., Hirota N., Hashidate T., Miyano M., Shimizu T., Nakamura M. (2009) Helix 8 of leukotriene B4 type-2 receptor is required for the folding to pass the quality control in the endoplasmic reticulum. FASEB J. 23, 1470–1481 [DOI] [PubMed] [Google Scholar]
- 34.Hirota N., Yasuda D., Hashidate T., Yamamoto T., Yamaguchi S., Nagamune T., Nagase T., Shimizu T., Nakamura M. (2010) Amino acid residues critical for endoplasmic reticulum export and trafficking of platelet-activating factor receptor. J. Biol. Chem. 285, 5931–5940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Siffroi-Fernandez S., Giraud A., Lanet J., Franc J. L. (2002) Association of the thyrotropin receptor with calnexin, calreticulin and BiP. Effects on the maturation of the receptor. Eur. J. Biochem. 269, 4930–4937 [DOI] [PubMed] [Google Scholar]
- 36.Dong C., Filipeanu C. M., Duvernay M. T., Wu G. (2007) Regulation of G protein-coupled receptor export trafficking. Biochim. Biophys. Acta 1768, 853–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen Q., Miller L. J., Dong M. (2010) Role of N-linked glycosylation in biosynthesis, trafficking, and function of the human glucagon-like peptide 1 receptor. Am. J. Physiol. Endocrinol. Metab. 299, E62–E68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao Y. P., Morice A. H., Compton S. J., Sadofsky L. (2011) N-linked glycosylation regulates human proteinase-activated receptor-1 cell surface expression and disarming via neutrophil proteinases and thermolysin. J. Biol. Chem. 286, 22991–23002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Markkanen P. M., Petäjä-Repo U. E. (2008) N-glycan-mediated quality control in the endoplasmic reticulum is required for the expression of correctly folded delta-opioid receptors at the cell surface. J. Biol. Chem. 283, 29086–29098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whitaker G. M., Lynn F. C., McIntosh C. H., Accili E. A. (2012) Regulation of GIP and GLP1 receptor cell surface expression by N-glycosylation and receptor heteromerization. PLoS ONE 7, e32675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kohno T., Wada A., Igarashi Y. (2002) N-glycans of sphingosine 1-phosphate receptor Edg-1 regulate ligand-induced receptor internalization. FASEB J. 16, 983–992 [DOI] [PubMed] [Google Scholar]
- 42.Zielinska D. F., Gnad F., Wiśniewski J. R., Mann M. (2010) Precision mapping of an in vivo N-glycoproteome reveals rigid topological and sequence constraints. Cell 141, 897–907 [DOI] [PubMed] [Google Scholar]
- 43.Ray K., Clapp P., Goldsmith P. K., Spiegel A. M. (1998) Identification of the sites of N-linked glycosylation on the human calcium receptor and assessment of their role in cell surface expression and signal transduction. J. Biol. Chem. 273, 34558–34567 [DOI] [PubMed] [Google Scholar]
- 44.Couvineau A., Fabre C., Gaudin P., Maoret J. J., Laburthe M. (1996) Mutagenesis of N-glycosylation sites in the human vasoactive intestinal peptide 1 receptor. Evidence that asparagine 58 or 69 is crucial for correct delivery of the receptor to plasma membrane. Biochemistry 35, 1745–1752 [DOI] [PubMed] [Google Scholar]
- 45.Innamorati G., Sadeghi H., Birnbaumer M. (1996) A fully active nonglycosylated V2 vasopressin receptor. Mol. Pharmacol. 50, 467–473 [PubMed] [Google Scholar]
- 46.Bisello A., Greenberg Z., Behar V., Rosenblatt M., Suva L. J., Chorev M. (1996) Role of glycosylation in expression and function of the human parathyroid hormone/parathyroid hormone-related protein receptor. Biochemistry 35, 15890–15895 [DOI] [PubMed] [Google Scholar]
- 47.Ruddock L. W., Molinari M. (2006) N-glycan processing in ER quality control. J. Cell Sci. 119, 4373–4380 [DOI] [PubMed] [Google Scholar]
- 48.Kuei C., Yu J., Zhu J., Wu J., Zhang L., Shih A., Mirzadegan T., Lovenberg T., Liu C. (2011) Study of GPR81, the lactate receptor, from distant species identifies residues and motifs critical for GPR81 functions. Mol. Pharmacol. 80, 848–858 [DOI] [PubMed] [Google Scholar]
- 49.Savarese T. M., Wang C. D., Fraser C. M. (1992) Site-directed mutagenesis of the rat m1 muscarinic acetylcholine receptor. Role of conserved cysteines in receptor function. J. Biol. Chem. 267, 11439–11448 [PubMed] [Google Scholar]
- 50.Noda K., Saad Y., Graham R. M., Karnik S. S. (1994) The high affinity state of the beta 2-adrenergic receptor requires unique interaction between conserved and non-conserved extracellular loop cysteines. J. Biol. Chem. 269, 6743–6752 [PubMed] [Google Scholar]
- 51.Tunaru S., Lättig J., Kero J., Krause G., Offermanns S. (2005) Characterization of determinants of ligand binding to the nicotinic acid receptor GPR109A (HM74A/PUMA-G). Mol. Pharmacol. 68, 1271–1280 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.