Abstract
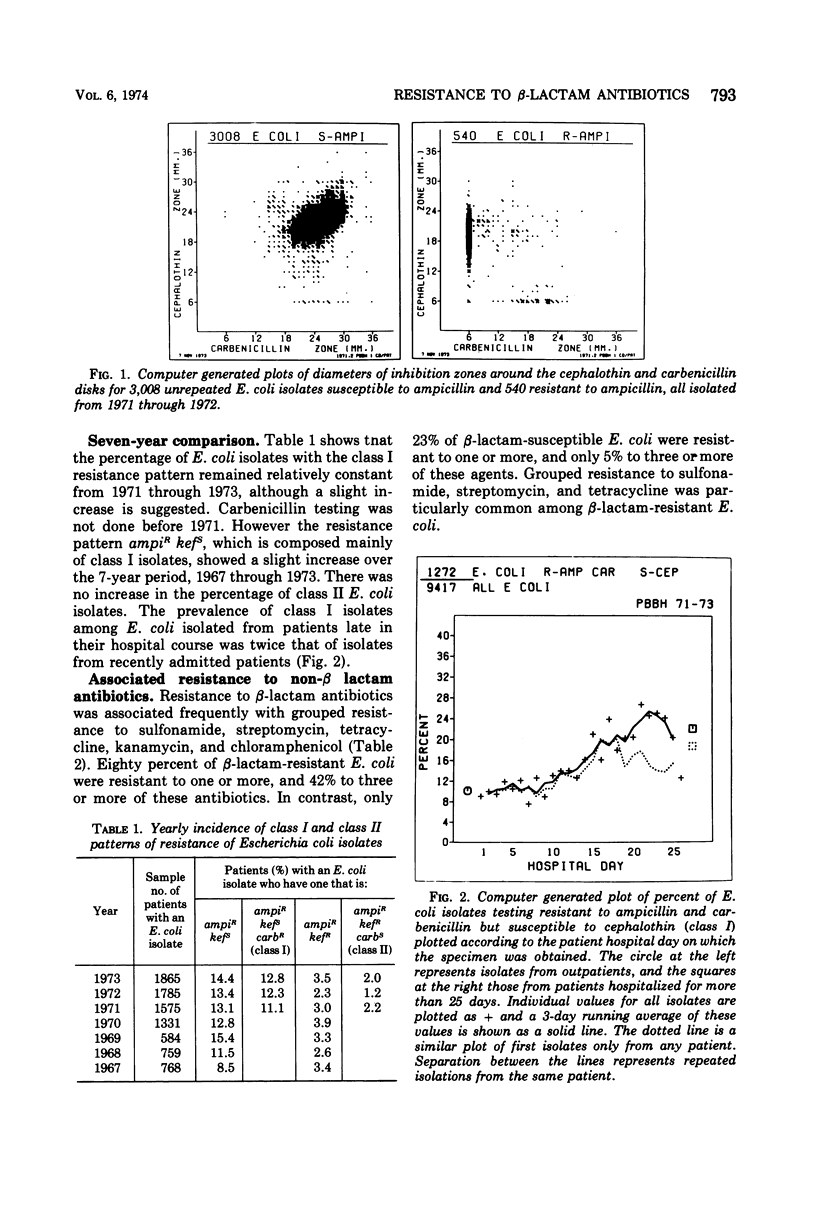
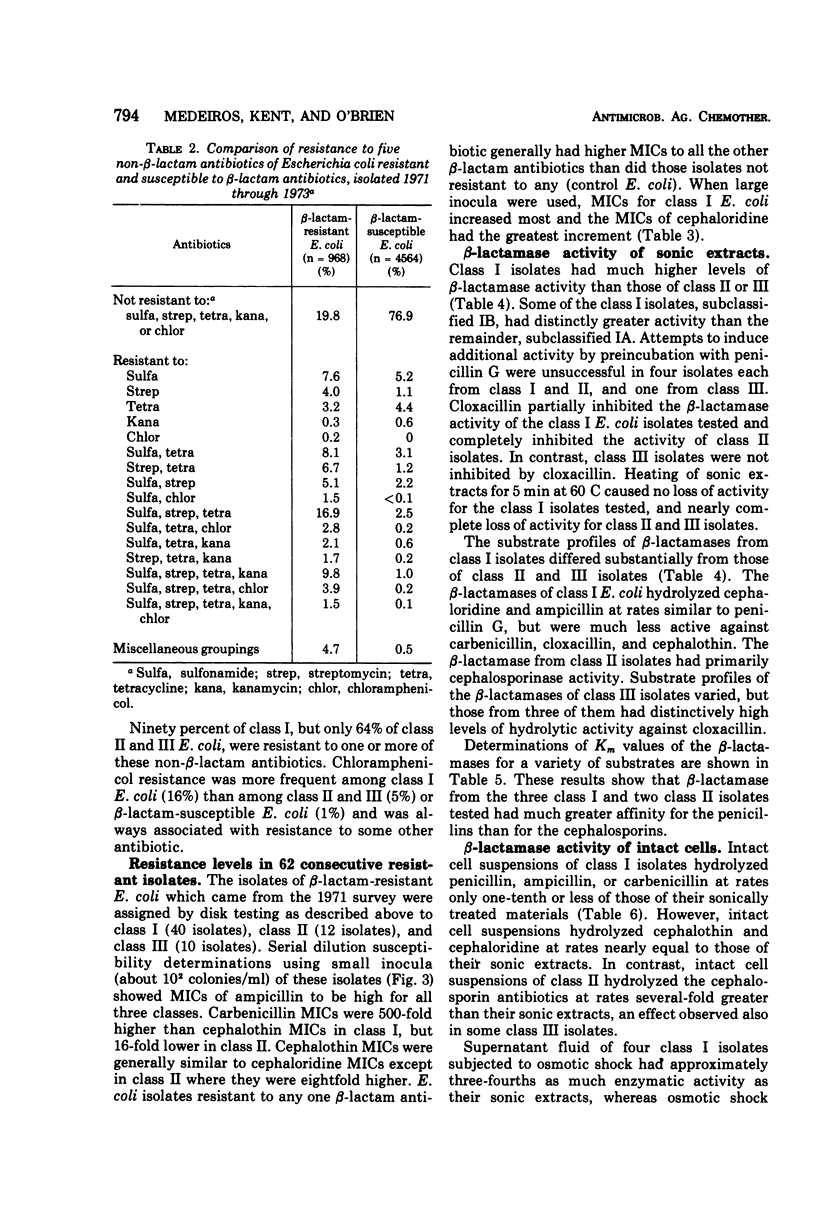
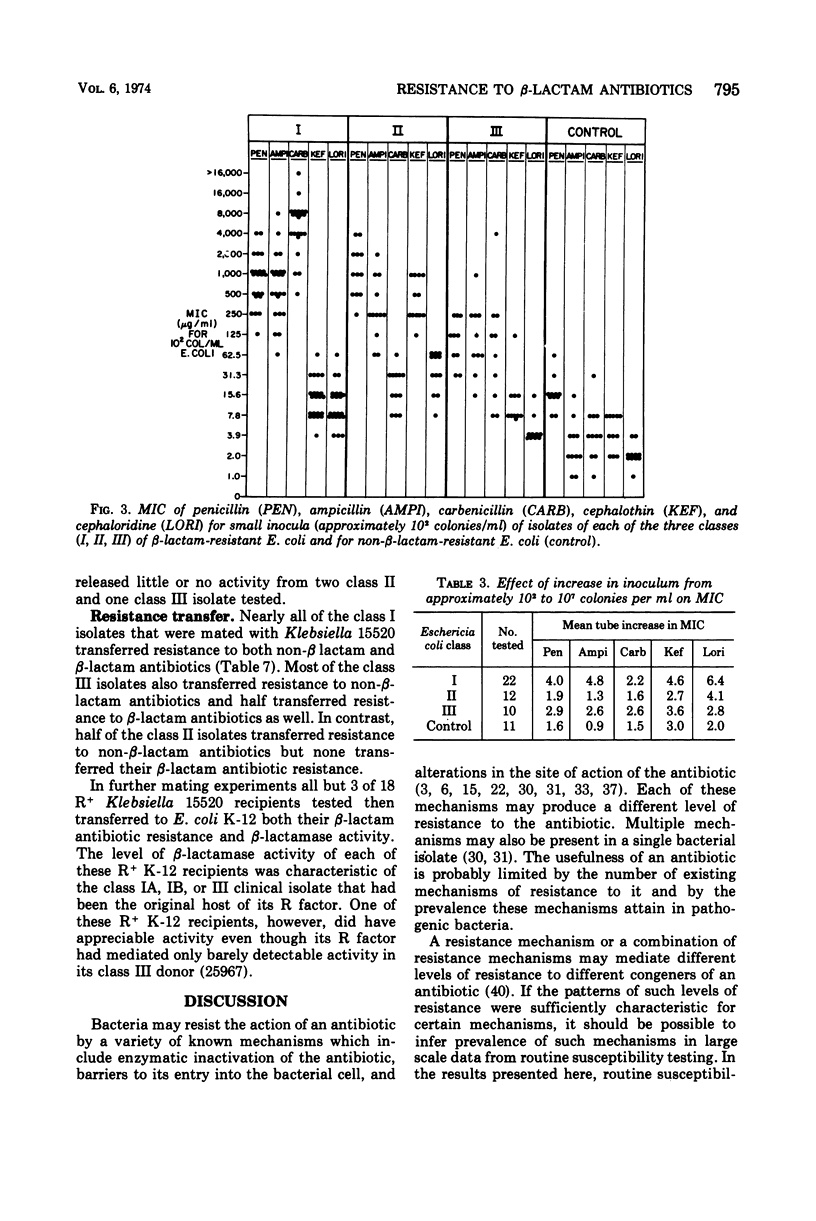
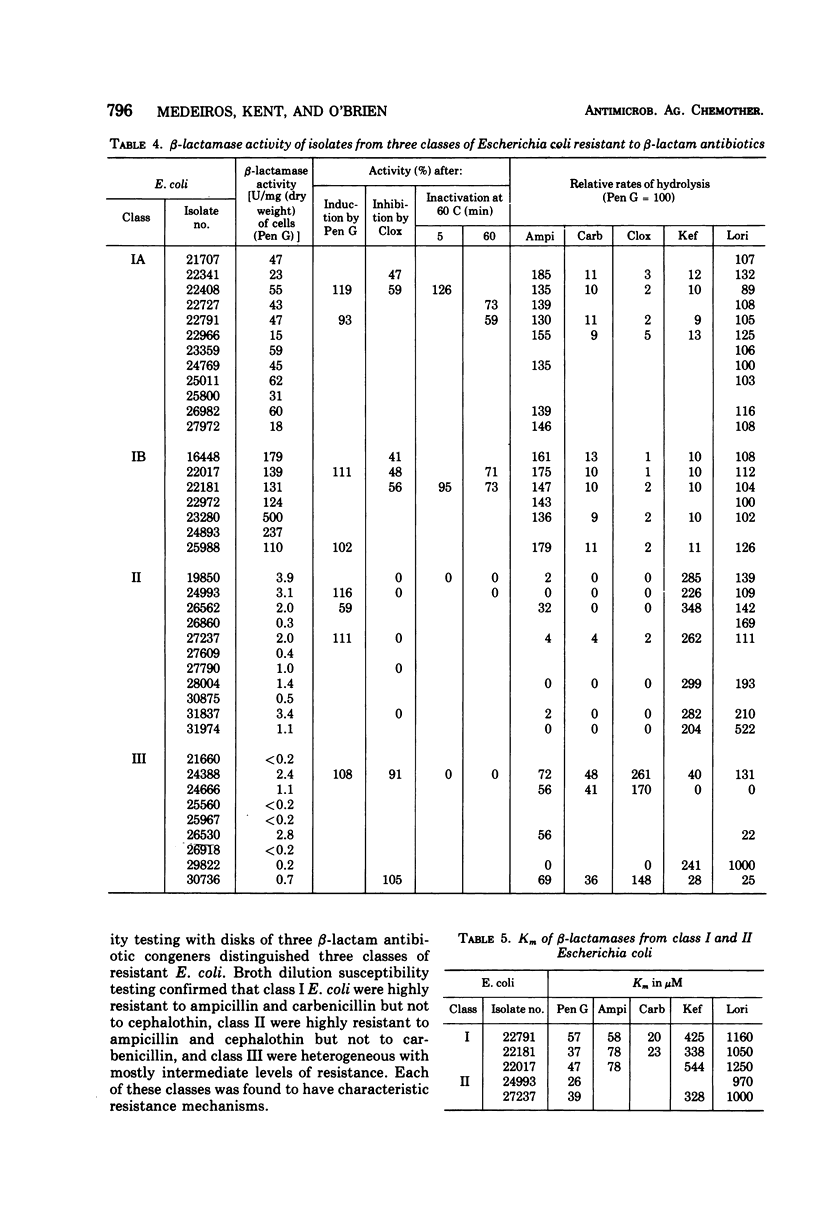
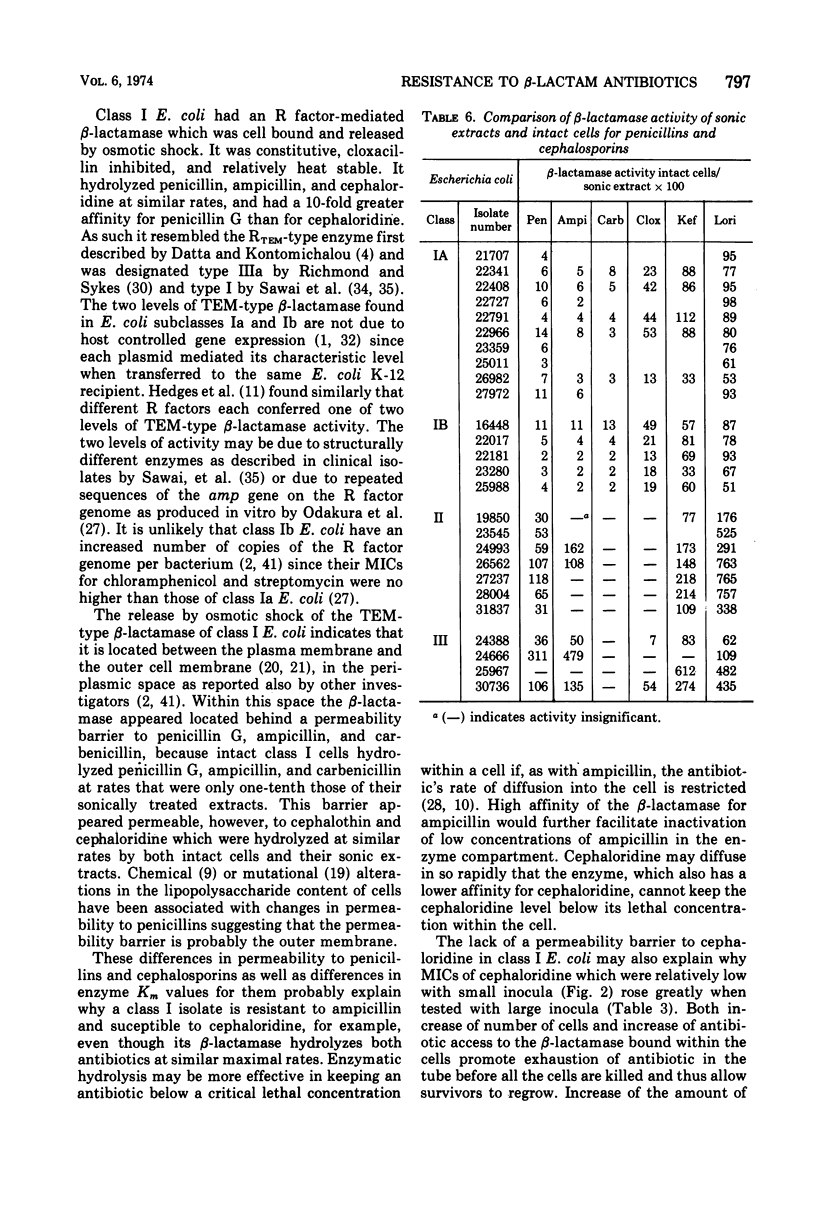
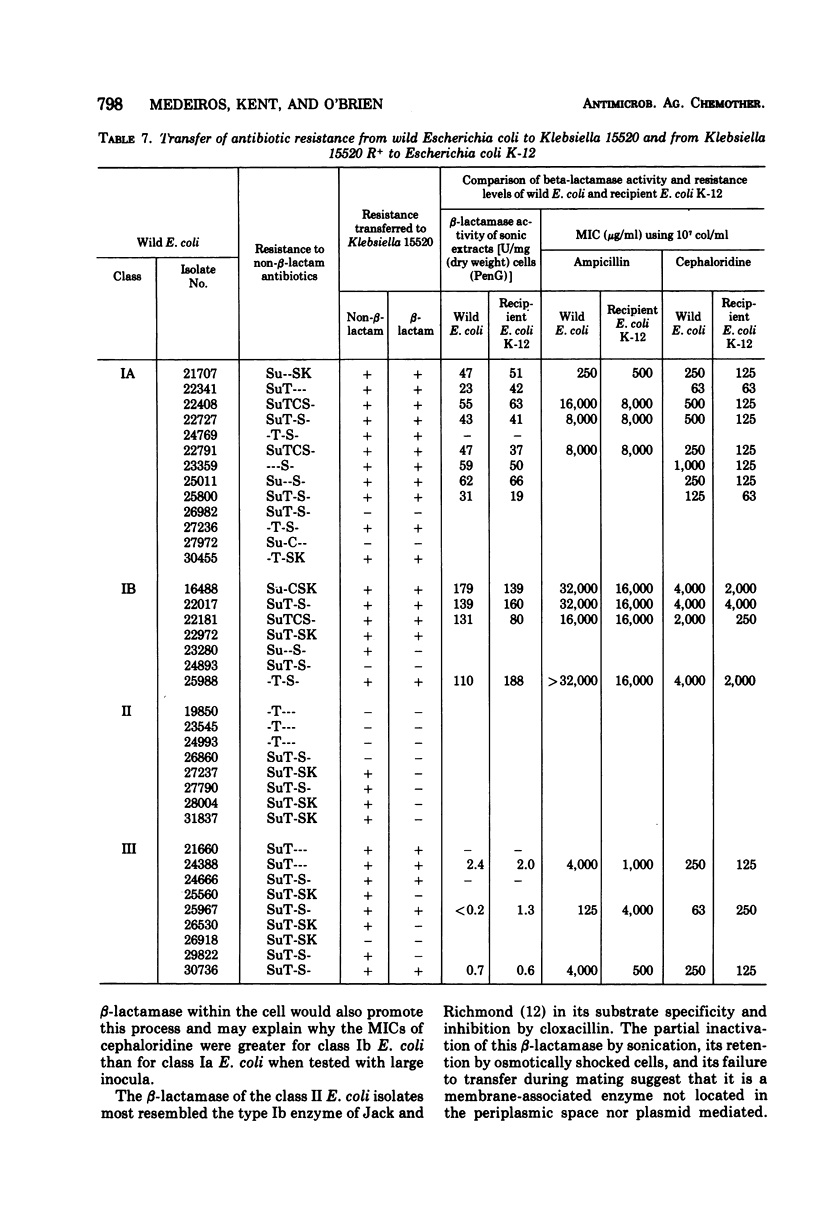
A survey of clinical isolates from a hospital laboratory showed that Escherichia coli could be grouped into three classes of beta-lactam-antibiotic resistance by results of routine susceptibility testing to ampicillin, cephalothin, and carbenicillin. E. coli highly resistant to ampicillin and carbenicillin but not to cephalothin (class I) were found to have one of two levels of R factor-mediated, periplasmic-β-lactamase which resembled RTEM and was located behind a permeability barrier to penicillins but not to cephalosporins. This permeability barrier appeared to act synergistically with the β-lactamase in producing high levels of resistance to penicillins. E. coli highly resistant to ampicillin and cephalothin but not carbenicillin (class II) were found to have a β-lactamase with predominantly cephalosporinase activity which was neither transferable nor releasable by osmotic shock. E. coli moderately resistant to one or to all three of these antibiotics (class III) were found to have low levels of different β-lactamases including a transferable β-lactamase which resembled R1818. Thus, different mechanisms producing resistance to β-lactam antibiotics could be deduced from the patterns of resistance to ampicillin, cephalothin, and carbenicillin found on routine susceptibility testing. E. coli of class I were much more prevalent than the other classes and the proportion of E. coli that were class I increased with duration of patient hospitalization. The incidence of class I E. coli rose only slightly over the past 7 years and that of class II E. coli remained constant despite increased usage of both cephalothin and ampicillin. These observations emphasize that the properties of the apparently limited number of individual resistance mechanisms that exist in a bacterial flora, such as their genetic mobility and linkages and the spectrum of their antibiotic inactivating enzymes and permeability barriers, may govern the effect that usage of an antibiotic has upon the prevalence of resistance to it and to other antibiotics.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Curtis N. A., Richmond M. H., Stanisich V. R-factor mediated resistance to penicillins which does not involve a beta-lactamase. J Gen Microbiol. 1973 Nov;79(1):163–166. doi: 10.1099/00221287-79-1-163. [DOI] [PubMed] [Google Scholar]
- Curtis N. A., Richmond M. H., Sykes R. B. "Periplasmic" location of a -lactamase specified either by a plasmid or a chromosomal gene in Escherichia coli. J Bacteriol. 1972 Dec;112(3):1433–1434. doi: 10.1128/jb.112.3.1433-1434.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Faiers M. C., Reeves D. S., Brumfitt W., Orskov F., Orskov I. R Factors in Escherichia coli in faeces after oral chemotherapy in general practice. Lancet. 1971 Feb 13;1(7694):312–315. doi: 10.1016/s0140-6736(71)91042-7. [DOI] [PubMed] [Google Scholar]
- Datta N., Kontomichalou P. Penicillinase synthesis controlled by infectious R factors in Enterobacteriaceae. Nature. 1965 Oct 16;208(5007):239–241. doi: 10.1038/208239a0. [DOI] [PubMed] [Google Scholar]
- Davies J. E., Rownd R. Transmissible multiple drug resistance in Enterobacteriaceae. Science. 1972 May 19;176(4036):758–768. doi: 10.1126/science.176.4036.758. [DOI] [PubMed] [Google Scholar]
- Hamilton-Miller J. M. Effect of EDTA upon bacterial permeability to benzylpenicillin. Biochem Biophys Res Commun. 1965 Sep 22;20(6):688–691. doi: 10.1016/0006-291x(65)90070-7. [DOI] [PubMed] [Google Scholar]
- Hamilton-miller J. M. Use of Michaelis-Menten kinetics in the analysis of synergism between beta-lactam antibiotics. J Theor Biol. 1971 May;31(2):171–176. doi: 10.1016/0022-5193(71)90181-0. [DOI] [PubMed] [Google Scholar]
- Hedges R. W., Datta N., Kontomichalou P., Smith J. T. Molecular specificities of R factor-determined beta-lactamases: correlation with plasmid compatibility. J Bacteriol. 1974 Jan;117(1):56–62. doi: 10.1128/jb.117.1.56-62.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jack G. W., Richmond M. H. A comparative study of eight distinct beta-lactamases synthesized by gram-negative bacteria. J Gen Microbiol. 1970 Apr;61(1):43–61. doi: 10.1099/00221287-61-1-43. [DOI] [PubMed] [Google Scholar]
- KENNEDY R. P., PLORDE J. J., PETERSDORF R. G. STUDIES ON THE EPIDEMIOLOGY OF ESCHERICHIA COLI INFECTIONS. IV. EVIDENCE FOR A NOSOCOMIAL FLORA. J Clin Invest. 1965 Feb;44:193–201. doi: 10.1172/JCI105134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maness M. J., Sparling P. F. Multiple antibiotic resistance due to a single mutation in Neisseria gonorrhoeae. J Infect Dis. 1973 Sep;128(3):321–330. doi: 10.1093/infdis/128.3.321. [DOI] [PubMed] [Google Scholar]
- Monner D. A., Jonsson S., Boman H. G. Ampicillin-resistant mutants of Escherichia coli K-12 with lipopolysaccharide alterations affecting mating ability and susceptibility to sex-specific bacteriophages. J Bacteriol. 1971 Aug;107(2):420–432. doi: 10.1128/jb.107.2.420-432.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOVICK R. P. Micro-iodometric assay for penicillinase. Biochem J. 1962 May;83:236–240. doi: 10.1042/bj0830236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Neu H. C. The surface localization of penicillinases in Escherichia coli and Salmonella typhimurium. Biochem Biophys Res Commun. 1968 Jul 26;32(2):258–263. doi: 10.1016/0006-291x(68)90378-1. [DOI] [PubMed] [Google Scholar]
- Nordström K., Burman L. G., Eriksson-Grennberg K. G. Resistance of Escherichia coli to penicillins. 8. Physiology of a class II ampicillin-resistant mutant. J Bacteriol. 1970 Mar;101(3):659–668. doi: 10.1128/jb.101.3.659-668.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- O'Brien T. F., Kent R. L., Medeiros A. A. Computer-generated plots of results of antimicrobial-susceptibility tests. JAMA. 1969 Oct 6;210(1):84–92. [PubMed] [Google Scholar]
- Odakura Y., Tanaka T., Hashimoto H., Mitsuhashi S. Mutation of R factors capable of specifying hypersynthesis of penicillinase. Antimicrob Agents Chemother. 1973 Mar;3(3):315–324. doi: 10.1128/aac.3.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PERCIVAL A., BRUMFITT W., DE LOUVOIS J. THE ROLE OF PENICILLINASE IN DETERMINING NATURAL AND ACQUIRED RESISTANCE OF GRAM-NEGATIVE BACTERIA TO PENICILLINS. J Gen Microbiol. 1963 Jul;32:77–89. doi: 10.1099/00221287-32-1-77. [DOI] [PubMed] [Google Scholar]
- PERRET C. J. Iodometric assay of penicillinase. Nature. 1954 Nov 27;174(4439):1012–1013. doi: 10.1038/1741012a0. [DOI] [PubMed] [Google Scholar]
- Richmond M. H., Sykes R. B. The beta-lactamases of gram-negative bacteria and their possible physiological role. Adv Microb Physiol. 1973;9:31–88. doi: 10.1016/s0065-2911(08)60376-8. [DOI] [PubMed] [Google Scholar]
- Rolinson G. N. Bacterial resistance to penicillins and cephalosporins. Proc R Soc Lond B Biol Sci. 1971 Dec 31;179(1057):403–410. doi: 10.1098/rspb.1971.0105. [DOI] [PubMed] [Google Scholar]
- Rosselet A., Zimmermann W. Mutants of Pseudomonas aeruginosa with impaired -lactamase inducibility and increased sensitivity to -lactam antibiotics. J Gen Microbiol. 1973 Jun;76(2):455–457. doi: 10.1099/00221287-76-2-455. [DOI] [PubMed] [Google Scholar]
- Sawai T., Mitsuhashi S., Yamagishi S. Drug resistance of enteric bacteria. XIV. Comparison of beta-lactamases in gram-negative rod bacteria resistant to alpha-aminobenzylpenicillin. Jpn J Microbiol. 1968 Dec;12(4):423–434. doi: 10.1111/j.1348-0421.1968.tb00415.x. [DOI] [PubMed] [Google Scholar]
- Sawai T., Takahashi K., Yamagishi S., Mitsuhashi S. Variant of penicillinase mediated by an R factor in Escherichia coli. J Bacteriol. 1970 Nov;104(2):620–629. doi: 10.1128/jb.104.2.620-629.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw E. J., Datta N., Jones G., Marr F. M., Froud W. J. Effect of stay in hospital and oral chemotherapy on the antibiotic sensitivity of bowel coliforms. J Hyg (Lond) 1973 Sep;71(3):529–534. doi: 10.1017/s0022172400046519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw W. V. The enzymatic acetylation of chloramphenicol by extracts of R factor-resistant Escherichia coli. J Biol Chem. 1967 Feb 25;242(4):687–693. [PubMed] [Google Scholar]
- Slocombe B., Sutherland R. Sensitivity of Gram-negative bacilli to ampicillin after six years' clinical use. J Clin Pathol. 1969 Nov;22(6):644–648. doi: 10.1136/jcp.22.6.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slocombe B., Sutherland R. Transferable antibiotic resistance in enteropathogenic Escherichia coli between 1948 and 1968. Antimicrob Agents Chemother. 1973 Oct;4(4):459–466. doi: 10.1128/aac.4.4.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. T., Hamilton-Miller J. M., Knox R. Bacterial resistance to penicillins and cephalosporins. J Pharm Pharmacol. 1969 Jun;21(6):337–358. doi: 10.1111/j.2042-7158.1969.tb08267.x. [DOI] [PubMed] [Google Scholar]
- Smith J. T., Wyatt J. M. Relation of R factor and chromosomal beta-lactamase with the periplasmic space. J Bacteriol. 1974 Mar;117(3):931–939. doi: 10.1128/jb.117.3.931-939.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi S., O'Hara K., Sawai T., Mitsuhashi S. The purification and properties of penicillin beta-lactamases mediated by transmissible R factors in Escherichia coli. J Biochem. 1969 Jul;66(1):11–20. doi: 10.1093/oxfordjournals.jbchem.a129111. [DOI] [PubMed] [Google Scholar]


