Abstract
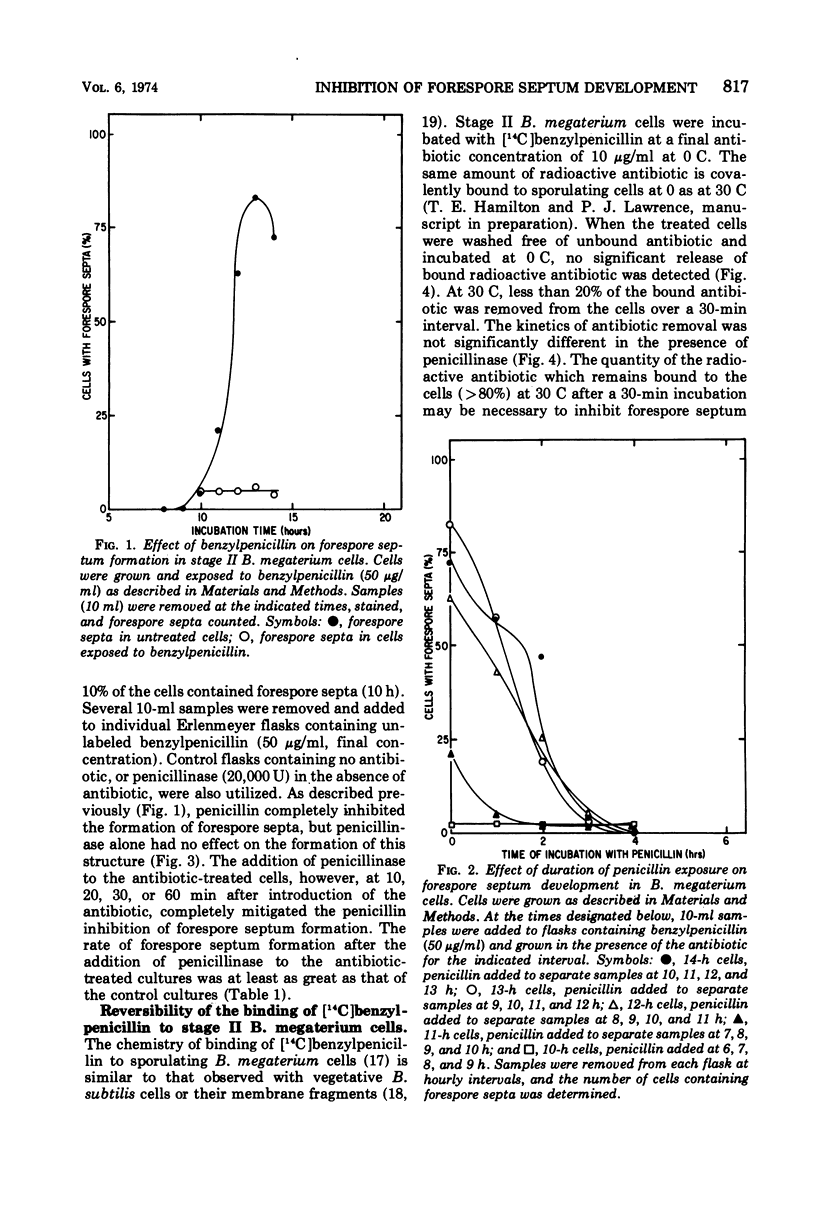
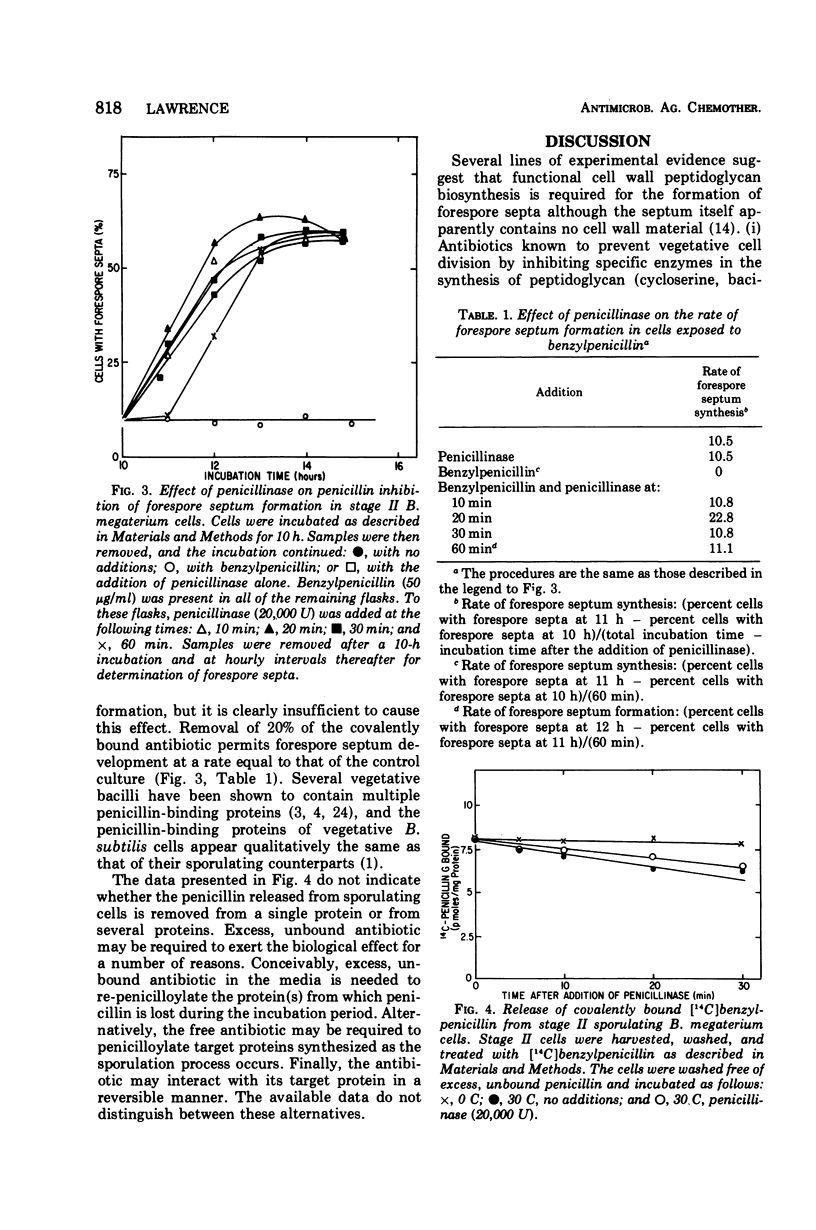
Benzylpenicillin inhibits the development of the forespore septum in sporulating Bacillus megaterium cells. The inhibitory effect is a function of the duration of exposure to the antibiotic and is completely reversible by penicillinase. Under the incubation conditions employed, less than 20% of the covalently bound antibiotic is released from the cells. The penicillin which remains bound to the cells after treatment with penicillinase may be necessary but is not sufficient for the effect; unbound antibiotic in the sporulation medium is also required.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anwar R. A., Blumberg P. M., Strominger J. L. Penicillin binding components in Bacillus subtilis during sporulation. J Bacteriol. 1974 Feb;117(2):924–925. doi: 10.1128/jb.117.2.924-925.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Five penicillin-binding components occur in Bacillus subtilis membranes. J Biol Chem. 1972 Dec 25;247(24):8107–8113. [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Inactivation of D-alanine carboxypeptidase by penicillins and cephalosporins is not lethal in Bacillus subtilis. Proc Natl Acad Sci U S A. 1971 Nov;68(11):2814–2817. doi: 10.1073/pnas.68.11.2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg P. M., Strominger J. L. Isolation by covalent affinity chromatography of the penicillin-binding components from membranes of Bacillus subtilis. Proc Natl Acad Sci U S A. 1972 Dec;69(12):3751–3755. doi: 10.1073/pnas.69.12.3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COOPER P. D. Site of action of radiopenicillin. Bacteriol Rev. 1956 Mar;20(1):28–48. doi: 10.1128/br.20.1.28-48.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow C. T., Takahashi I. Acid-soluble nucleotides in an asporogenous mutant of Bacillus subtilis. J Bacteriol. 1972 Mar;109(3):1175–1180. doi: 10.1128/jb.109.3.1175-1180.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H., LEVY M., FLEISCHMAN R. The binding of penicillin in relation to its cytotoxic action. IV. The amounts bound by bacteria at ineffective, growth-inhibitory, bactericidal, and maximally effective concentrations. J Bacteriol. 1955 Feb;69(2):167–172. doi: 10.1128/jb.69.2.167-172.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. The binding of penicillin in relation to its cytotoxic action. I. Correlation between the penicillin sensitivity and combining activity of intact bacteria and cell-free extracts. J Exp Med. 1954 Mar;99(3):207–226. doi: 10.1084/jem.99.3.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EAGLE H. The multiple mechanisms of penicillin resistance. J Bacteriol. 1954 Nov;68(5):610–616. doi: 10.1128/jb.68.5.610-616.1954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards J. R., Park J. T. Correlation between growth inhibition and the binding of various penicillins and cephalosporins to Staphylococcus aureus. J Bacteriol. 1969 Aug;99(2):459–462. doi: 10.1128/jb.99.2.459-462.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R. A., Murrell W. G. Simple method of detecting spore septum formation and synchrony of sporulation. J Bacteriol. 1967 Jan;93(1):495–496. doi: 10.1128/jb.93.1.495-496.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchins A. D., Slepecky R. A. Antibiotic inhibition of the septation stage in sporulation of Bacillus megaterium. J Bacteriol. 1969 Mar;97(3):1513–1515. doi: 10.1128/jb.97.3.1513-1515.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchins A. D., Slepecky R. A. Bacterial sporulation as a modified procaryotic cell division. Nature. 1969 Aug 23;223(5208):804–807. doi: 10.1038/223804a0. [DOI] [PubMed] [Google Scholar]
- KOLODZIEJ B. J., SLEPECKY R. A. TRACE METAL REQUIREMENTS FOR SPORULATION OF BACILLUS MEGATERIUM. J Bacteriol. 1964 Oct;88:821–830. doi: 10.1128/jb.88.4.821-830.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lawrence P. J., Rogolsky M., Hanh V. T. Binding of radioactive benzylpenicillin to sporulating Bacillus cultures: chemistry and fluctuations in specific binding capacity. J Bacteriol. 1971 Nov;108(2):662–667. doi: 10.1128/jb.108.2.662-667.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence P. J., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XV. The binding of radioactive penicillin to the particulate enzyme preparation of Bacillus subtilis and its reversal with hydroxylamine or thiols. J Biol Chem. 1970 Jul 25;245(14):3653–3659. [PubMed] [Google Scholar]
- Lawrence P. J., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. XVI. The reversible fixation of radioactive penicillin G to the D-alanine carboxypeptidase of Bacillus subtilis. J Biol Chem. 1970 Jul 25;245(14):3660–3666. [PubMed] [Google Scholar]
- Pearce S. M., Fitz-James P. C. Sporulation of a cortexless mutant of a variant of Bacillus cereus. J Bacteriol. 1971 Jan;105(1):339–348. doi: 10.1128/jb.105.1.339-348.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogolsky M., Lawrence P. J., Hanh V. T. Binding of radioactive benzylpenicillin to asporogenous mutants of Bacillus subtilis during postexponential growth. J Bacteriol. 1973 Apr;114(1):220–227. doi: 10.1128/jb.114.1.220-227.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley D., Cooper P. D., Roberts P. W. The site of action of penicillin. 1. Uptake of penicillin on bacteria. Biochem J. 1950 Feb;46(2):157–161. doi: 10.1042/bj0460157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strominger J. L., Blumberg P. M., Suginaka H., Umbreit J., Wickus G. G. How penicillin kills bacteria: progress and problems. Proc R Soc Lond B Biol Sci. 1971 Dec 31;179(1057):369–383. doi: 10.1098/rspb.1971.0103. [DOI] [PubMed] [Google Scholar]


