Abstract
Adverse pregnancy outcomes significantly contribute to morbidity and mortality for mother and child, with lifelong health consequences for both. The innate and adaptive immune system must be regulated to insure survival of the feta allograft, and the complement system is no exception. An intact complement system optimizes placental development and function and is essential to maintain host defense and fetal survival. Complement regulation is apparent at the placental interface from early pregnancy with some degree of complement activation occurring normally throughout gestation. However, a number of pregnancy complications including early pregnancy loss, fetal growth restriction, hypertensive disorders of pregnancy and preterm birth are associated with excessive or misdirected complement activation, and are more frequent in women with inherited or acquired complement system disorders or complement gene mutations. Clinical studies employing complement biomarkers in plasma and urine implicate dysregulated complement activation in components of each of the adverse pregnancy outcomes. In addition, mechanistic studies in rat and mouse models of adverse pregnancy outcomes address the complement pathways or activation products of importance and allow critical analysis of the pathophysiology. Targeted complement therapeutics are already in use to control adverse pregnancy outcomes in select situations. A clearer understanding of the role of the complement system in both normal pregnancy and complicated or failed pregnancy will allow a rational approach to future therapeutic strategies for manipulating complement with the goal of mitigating adverse pregnancy outcomes, preserving host defense, and improving long term outcomes for both mother and child.
Keywords: pregnancy, preeclampsia, preterm birth, complement system, miscarriage, fetal growth restriction, placenta
1. Introduction
Pregnancy is a complex process with incredible changes occurring in the cardiovascular and immune system of the mother to insure a successful birth. More than 4 million births occur in the United States alone each year with the majority of them being uneventful (but no less miraculous). Despite modern medicine and the advancements that have been made in prenatal care, adverse pregnancy outcomes still occur at a disturbing rate, including spontaneous abortion and early pregnancy loss, preterm labor, preterm birth, fetal growth restriction and gestational hypertension including preeclampsia and HELLP syndrome (hemolysis, elevated liver enzymes, low platelet count). Preterm birth rates have increased during the last 20 years, with up to 12% of births in the United States occurring before 37 weeks of a normal 40 week gestation. Preeclampsia is characterized by new onset high blood pressure and often protein in the urine after 20 weeks of gestation (ACOG, 2013) and affects 3–6% of all pregnancies (Ananth et al., 2013). In the 1990s, preeclampsia accounted for 15% of maternal deaths in pregnancy (NHLBI, 2000). The maternal mortality has improved, with 9% mortality attributable to hypertensive disorders of pregnancy in 2010 (Creanga et al., 2015), yet the only clear treatment for preeclampsia is delivery of the placenta. Adverse pregnancy outcomes take a severe toll on quality of life with long-term adverse cardiovascular and respiratory sequelea in both mother and child. Survivors of fetal growth restriction and preterm birth have increased risk of cardiovascular disease as adults (Lewandowski et al., 2014; Lewandowski and Leeson, 2014) as well as metabolic and respiratory complications and developmental delays (Saigal and Doyle, 2008). An increased lifetime risk for cardiovascular disease is also associated with mothers who experienced hypertension in pregnancy (Bellamy et al., 2007; Veerbeek et al., 2015).
Significant adaptations of the immune system occur in pregnancy, both in the innate and adaptive arms, to insure survival of the fetal allograft and maintenance of an immune system to defend the mother and fetus from invaders. The complement system is no exception with marked changes apparent to protect the fetus from complement system attack. In addition, newer evidence indicates the importance of the complement system in orchestrating normal development (Kolev et al., 2014), not just protecting from infection. We will first review the changes in the complement system and its regulators that occur to insure a successful normal pregnancy and then discuss studies demonstrating dysregulation of the complement system in adverse pregnancy outcomes, consequences of that dysregulation, and potential therapeutic strategies.
2. Complement System and Normal Pregnancy
2.1 Complement System
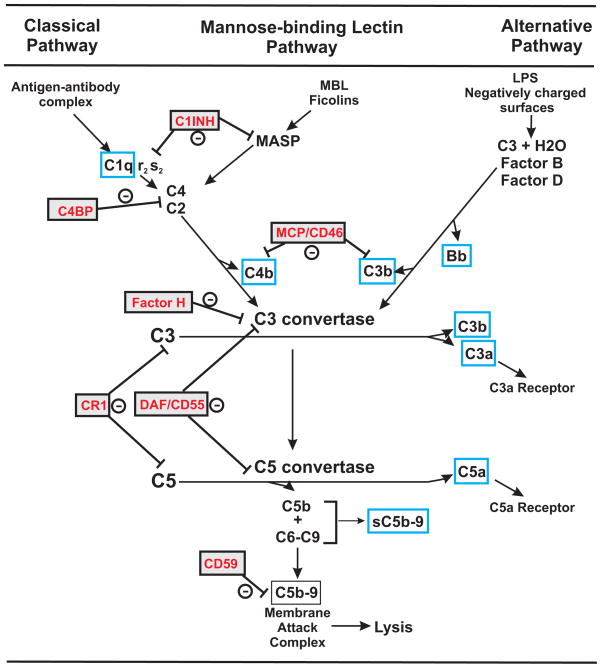
The complement system is comprised of more than 50 proteins and normally operates at a low steady state level of activation. However, the complement cascade may be amplified by one or more of 3 activation pathways; classical, lectin or alternative (Figure 1). Activation through C3 can result in covalent binding of the C3b fragment to invaders or self i.e. C3 deposition. Activation through C9 can lead to lysis of target cells and is a prominent host defense mechanism for many microorganisms. C3a and C5a are fluid phase activation products that interact with G protein coupled receptors on numerous cell types to elicit inflammation and immune cell activation. C5b-9 forms a membrane pore resulting in cell lysis. Sublytic concentrations of the Membrane Attack Complex C5b-9 can stimulate cells and upregulate adhesion molecules. Also, a cytolytically inactive C5b-9 complex that is soluble (sC5b-9) and is detectable in plasma or serum can also be formed and initiate cytokine synthesis and vascular leakage in endothelial cells (Tedesco et al., 1997). Complement activation is controlled by soluble and membrane bound inhibitors, primarily at C3 and C5b-9 (Figure 1). C1-INH inhibits both C1r and C1s of the classical pathway and the mannose associated serine proteases (MASP) of the MBL pathway. Factor H and C4 binding protein (C4BP) are soluble plasma regulators that target C3 and C4, respectively, to limit activation. Complement receptor 1 (CR1), CD55 (DAF; Decay accelerating factor), CD46 (MCP, Membrane cofactor protein) and CD59 are membrane-associated regulators that limit activation on self. In contrast to widespread expression in humans, in mice and rats the CD46 molecule is exclusively expressed in testis. Mice and rats also have complement receptor 1-related gene protein y (Crry) that regulates C3 activation and has CD46 and CD55 like activity (Naik et al., 2013).
Figure 1. Complement System.
Three activation pathways of complement are depicted with regulators of complement activation in red text. Blue boxes highlight products of complement activation of interest in normal pregnancy or associated with adverse pregnancy outcomes. The C3 convertase is a molecular complex generated from the classical or lectin pathway (C4bC2a) and the alternative pathway (C3bBb) that cleaves C3 generating the C5 convertase (C4bC2aC3b or C3bBbC3b). Once activated, C3b and C4b can be further degraded to smaller fragments C3d and C4d that are still covalently bound to target and maintain many biological activities but do not participate as components of the C3 and C5 convertase to propagate activation of the pathway. The rodent specific regulator of complement activation Crry (CR-1 related gene y) is not included but has CD46/CD55 like activities. C1-INH, C1 inhibitor; MCP, membrane cofactor protein; C4BP, C4 binding protein; CR1, complement receptor 1; DAF, decay accelerating factor; MASP, mannose associated serine protease; MBL, mannose binding lectin
2.2 Placental development
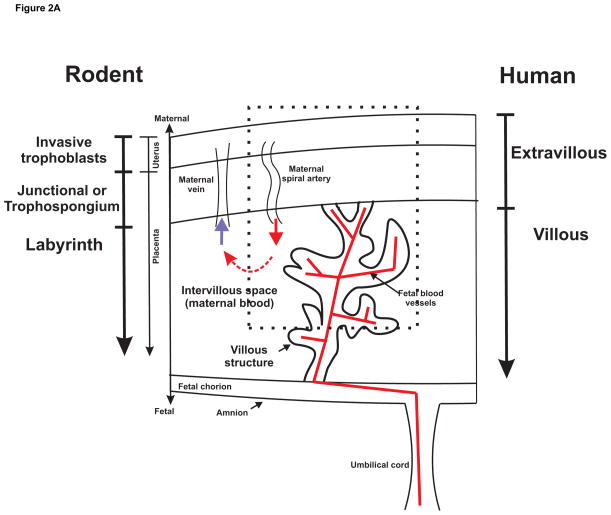
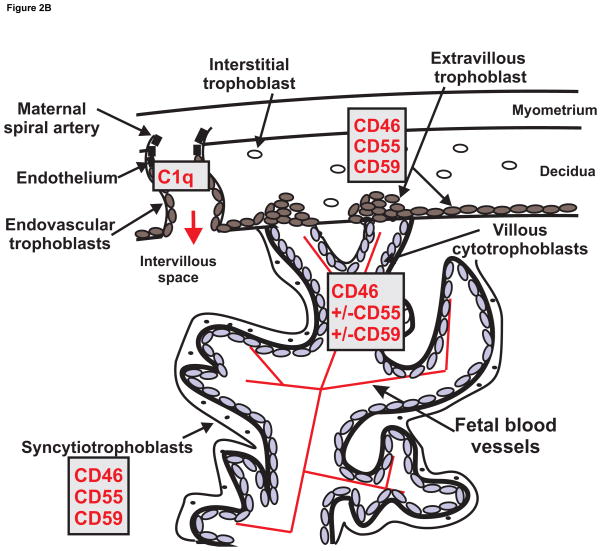
The fetus and the placenta express paternal antigens and thus present as foreign to the maternal immune system. This semi-allogeneic graft requires special protection from the maternal immune system and the exact mechanism of protected status of the fetus is under continuing investigation. The human placenta (as well as that of rabbit, guinea pig, mouse and rat) is a hemichorial structure meaning that maternal blood is in direct contact with the fetal chorion. The placental villi are fetal structures that bridge the uterus and the fetal chorion and carry the fetal vasculature (Figure 2A). The maternal blood pours into the intervillous space bathing placental villi to provide nutrients and oxygen for active transport or diffusion into the fetal blood vessels. Maternal IgG antibodies are transported by endocytosis to reach the fetal circulation. In the human, the regions are often designated as extravillous and villous, whereas in the rodent, the placental layers are designated as the labyrinth and trophospongium or junctional layer. The trophospongium layer is both fetal and maternal with fetal trophoblasts invading to remodel maternal spiral arteries. The villous or labyrinth space is of fetal origin with villous structures carrying fetal blood vessels into the intervillous space for exchange of oxygen and nutrients. The placental villi and the intervillous space are lined by fetal trophoblasts, the outermost epithelial like cell layer of the developing fetus. The trophoblasts differentiate with the task of invading the maternal uterine decidua to remodel spiral arteries and establish a high flow low resistance source of maternal blood that enters the intervillous space and bathes the placental villi. The different trophoblast cell types are depicted in Figure 2B. The interstitial and endovascular trophoblasts invade maternal decidua to direct remodeling of maternal spiral arteries to a low resistance blood vessel that can provide adequate maternal blood to the intervillous space. The villous cytotrophoblasts differentiate into the outer syncytiotrophoblast layer on the placental villi in contact with the intervillous space with maternal blood and complement. The fetal trophoblast on the outermost layer of the embryonic placental villi is the syncytiotrophoblast that establishes the interface between maternal blood and the fetus. It is formed by fusion of the underlying layer of villous cytotrophoblasts and lacks gaps for immune cell penetration. The fetal derived trophoblast is the only cell in contact with the maternal blood so this is an important site for control of complement activation by endogenous complement regulators. Complement system activation at the syncytiotrophoblast must be carefully controlled so the fetus is not harmed by the mothers innate immune complement system. In addition, a functioning intact complement system is of critical importance in maintaining host defense to protect the fetus and mother from infection.
Figure 2.
Figure 2A. Schematic of a portion of a hemochorial placenta depicting comparable zones in human and rodent. Portion enclosed by dashed line is expanded in Figure 2B to illustrate in more detail the villous structure and maternal spiral artery with subtypes of trophoblasts and complement components/regulators of most interest.
Figure 2B. Schematic of hemochorial placenta illustrating subtypes of trophoblasts and associated selective complement components/regulators of interest that participate in normal placental development and protection of the trophoblast subpopulations from maternal complement attack. Intervillous space and maternal spiral artery is illustrated with maternal blood and plasma complement proteins bathing the villous structure for exchange of nutrients and oxygen with the fetal blood supply within the villous structure.
2.3 Complement and placental development
Complement is very important in a normal pregnancy for development of placenta and consequently for normal development of the fetus. At initial stages of pregnancy, the uterine wall undergoes changes and transforms into decidual tissue that is important for implantation. Inflammation accompanies the trophoblast invasion of the decidual tissue and successful embryo implantation. Numerous changes in the adaptive immune response are also important in success of this process, but we will concentrate primarily on the role of the complement system in successful trophoblast invasion and fetal development. Activated C3 participates in normal phagocytic activity of the mouse trophoblast in vitro suggesting that C3 may assist in trophoblast invasion of the decidua and endometrial blood vessels (Albieri et al., 1999). Some trophoblasts invade the uterine decidua as endovascular trophoblasts and migrate up the uterine spiral artery to replace the endothelial cells and result in vascular remodeling and a high flow, low resistance vessel. Normally endothelial cells do not synthesize complement components. However, Bulla et al (Bulla et al., 2008) demonstrated that endothelial cells in decidual tissue secrete C1q during pregnancy and C1q is seen at contact sites between endovascular trophoblasts invading the spiral arteries and decidual endothelial cells. No C4 is detected co-localized with C1q suggesting that C1q does not initiate complement activation at this location. In vitro studies suggest that C1q is likely a bridge allowing adherence of decidual endothelial cells and the endovascular trophoblast. MBL is known to inhibit this interaction (Agostinis et al., 2012). Moreover, MBL is increased in normal pregnancy compared to non-pregnant women (van de Geijn et al., 2007) suggesting it may play a role in limiting endovascular trophoblast invasion of spiral arteries.
The importance of local production of C1q is evident from the localization of C1q in areas of invading trophoblasts in human placentas (Agostinis et al., 2010; Bulla et al., 2012) as well as by abnormal placentation in C1q deficient mice. Compared to wild type mice, C1q deficient mice have reduced placental labyrinth development and spiral artery remodeling with the end result being reduced litter size and fetal weight. C1q deficient animals demonstrated a preeclamptic like phenotype with increased blood pressure and proteinuria indicating the critical importance of C1q for a normal uneventful pregnancy. Also, this suggests the possibility that lack of C1q, either acquired or genetic, in women may lead to abnormal placentation and pregnancy complications (Singh et al., 2011). This is consistent with the increased incidence of pregnancy complications in women with acquired or genetic deficiency of classical pathway components such as in systemic lupus erythematosus (SLE), malaria, or antiphospholipid antibody syndrome (APLAS) (discussed in section 3.2).
2.4 The complement system in normal pregnancy
2.4.1 Complement in the circulation
Changes in circulating complement could provide readily accessible biomarkers for predicting adverse pregnancy outcomes if we understand what is ‘normal’ for pregnancy. Older literature demonstrates increased C3 in serum of the mother at term compared to non-pregnant women (Millar and Mills, 1972). Both C3 and total hemolytic complement were investigated over the continuum of pregnancy with an initial decrease in serum levels of C3 in first trimester and an increase in second and third trimester. After delivery, C3 returned to early pregnancy values (Baines et al., 1974). Earlier studies are contradictory with no change in complement noted over the course of pregnancy (Prall and Kantor, 1966). Total hemolytic complement is a relatively insensitive measure and significant complement activation can occur with little change in this measure. A more recent study by Derzsy et al (Derzsy et al., 2010) using more contemporary techniques did a thorough examination of healthy non-pregnant and pregnant women to measure circulating levels of complement components and activation products C4d, C3a, sC5b-9 along with the regulator Factor H to determine if increased inflammation in the form of complement activation is associated with normal pregnancy compared to non-pregnant women. In normal pregnancy at 36–37 weeks of gestation, concentrations of C4d, C3a, sC5b-9, C3, C9, and Factor H were greater than non-pregnant women whereas C1-INH was lower. Increases in the C4d/C4 ratio, the C3a/C3 ratio and sC5b-9 indicated increased complement activation via the classical or lectin pathway in normal pregnancy with no significant change in the alternative pathway fragment Bb. This study was consistent with an earlier study by Richani demonstrating increased C3a and C5a during pregnancy (Richani et al., 2005). In contrast, in an African green monkey model of influenza during pregnancy, investigators found that C3, C3a and C4 were actually decreased late in gestation compared to non pregnant animals, correlating with decreased viral neutralization and influenza susceptibility late in pregnancy (Mayer and Parks, 2014). Clearly when considering complement and its activation in adverse pregnancy outcomes, a gestationally matched control group of pregnant women with no adverse outcomes must be used rather than simply using non-pregnant controls.
2.4.2 Complement in placenta
Complement components are primarily synthesized by liver, but can also be synthesized locally in numerous tissues including placenta. It is particularly important to know what is happening locally in the placenta during pregnancy – where maternal blood meets the semiallogeneic fetal syncytiotrophoblast. Most studies have examined term placenta available after normal birth or early placenta obtained at abortion. Lokki et al (Lokki et al., 2014) probed for plasma regulators of complement activation in term placenta and demonstrated C4BP evident in syncytial bodies or areas of damaged syncytiotrophoblasts and Factor H in tissue stroma. Buurma (Buurma et al., 2012) clearly documented message for CD46, CD55 and CD59 in placenta during normal pregnancy. Older studies of others examined the distribution of complement regulators CD46, CD55 and CD59 on syncytiotrophoblasts, extravillous trophoblasts and villous cytotrophoblasts using immunohistochemistry. On the syncytiotrophoblast, the differentiated fused trophoblast that lines the intervillous space, all three membrane bound complement regulators are consistently noted in first, second and third trimester placenta i.e. CD46, CD55 and CD59 (Figure 2B) (Holmes et al., 1992; Holmes et al., 1990; Hsi et al., 1991; Lokki et al., 2014; Nishikori et al., 1993). Using trophoblasts isolated from first through third trimester, Holmes (Holmes et al., 1992; Holmes et al., 1990) noted increased presence of all three regulators in second and third trimester. Extravillous trophoblasts, whether in trophoblastic columns invading the decidua or as interstitial or endovascular trophoblasts, also consistently express all 3 regulators, CD46, CD55 and CD59 (Holmes et al., 1992; Holmes et al., 1990; Hsi et al., 1991; Nishikori et al., 1993). The underlying layer of villous cytotrophoblasts is apparent early in pregnancy and there is general agreement in the literature that villous cytotrophoblasts have CD46 (Holmes et al., 1992; Holmes et al., 1990; Hsi et al., 1991; Nishikori et al., 1993). However, the presence of CD55 in villous cytotrophoblasts of first term placenta has been reported by some (Hsi et al., 1991; Nishikori et al., 1993) and less consistently by others (Holmes et al., 1992; Holmes et al., 1990). CD59 was also inconsistently reported in villous cytotrophoblasts, being rarely evident in the study of Nishikori (Nishikori et al., 1993) but positive in the study by Holmes (Holmes et al., 1992). It is conceivable that cells most in contact with maternal tissue, the syncytio- and extravillous trophoblasts would need most protection from the maternal complement activation, whereas the villous cytotrophoblast is largely confined to the fetal compartment shielded from the maternal immune system by the syncytiotrophoblast. Certainly, significant variability can occur when dealing with placental samples from women of varying gestational ages and using different antibodies and techniques.
Despite the presence of complement regulators in placenta, some degree of complement activation still occurs in placenta during normal pregnancy. Bulla et al (Bulla et al., 2009) demonstrated very nicely that both human trophoblast cell lines, as well as freshly isolated human first trimester trophoblasts secrete C3 and C4 protein and have mRNA for the later components C6-9, with particularly high mRNA for C7. Presumably these complement components provide a local source of innate immune protection from infection. An early study by Faulk et al, (Faulk et al., 1980; Faulk and Johnson, 1977) demonstrated C3, particularly C3d, in trophoblast basement membranes of full term placenta along with C9 but not C1q, C4 or C2. The most consistent staining of trophoblast basement membranes was demonstrated with anti C3 with C1q occasionally found in walls of large blood vessels. Tedesco (Tedesco et al., 1990) documented the terminal complement complex C5b-9 in normal term placenta in the decidua and stroma of chorionic villi. In normal uteroplacental spiral arteries (4–40 wk gestational age), C1q, C3d, C4, C6 and C9 were all evident (Wells et al., 1987). More recent studies by Lokki et al (Lokki et al., 2014) and Buurma (Buurma et al., 2012) demonstrated clear C1q and C9 staining along with C3d, C4BP and Factor H in normal placenta. As in the circulation, assessing complement activation in placenta associated with adverse pregnancy outcomes needs to be carefully compared to the same parameters in a normal pregnancy at the same time point.
2.5 Complement and embryo/fetal development
Besides playing critical roles in host defense and pattern recognition, recent studies are defining an important role for the complement system in embryonic and fetal development. Embryotrophic factors largely derived from the uterus promote embryo growth prior to development of placenta. In the human, embryotrophic factor 3 (ETF-3) is the most abundant embryotrophic factor and contains C3 along with C3b and its degradation product iC3b. iC3b is the most potent embryotrophic factor in mice preimplantation in the oviduct (Lee et al., 2009). In the rat, C3 on the visceral yolk sac is important for embryonic development in early post implantation embryos. In explant rat embryo culture, adding intact C3 significantly favors development, without a requirement for C3 activation (Usami et al., 2010). Thus, C3 appears to be an important embryotrophic factor prior to formation of the placenta.
Whether complement plays an unrecognized role in long term health outcomes associated with preterm and low birth weight pregnancies by altering developmental trajectories of fetal organs and/or the pool of resident progenitor cells in those organs remains largely unexplored. Complement receptors are expressed on hematopoietic stem-like progenitor cells (HSPCs) and studies by Reca et al and others report that activation of complement receptors modulates cellular functions such as homing response and regeneration (Ratajczak et al., 2004; Reca et al., 2003). Likewise, previous studies report interactions between platelet derived microparticles and complement factors that may play important roles in HSPC function (Baj-Krzyworzeka et al., 2002). In preeclampsia, endothelial progenitor cells are decreased in cord blood compared to normal pregnancy (Beasley et al., 2014; Munoz-Hernandez et al., 2014). Further research is needed to determine if complement activation in adverse pregnancy outcomes alters levels of HSPCs and directly contributes to long term health concerns in offspring.
3 Complement Deficiencies and Pregnancy
3.1 Complement deficiencies and mutations and pregnancy complications
Inherited complement deficiencies are rare with population prevalence estimated at 0.03% (Figueroa and Densen, 1991; Grumach and Kirschfink, 2014). Homozygous C2 deficiency is one of the most commonly inherited complement defects, occurring in up to 0.01% of the population (Alper, 1987). In general, severe deficiency of complement components predisposes to infection with encapsulated bacteria, such as Neisseria meningitidis or Streptococcus pneumonia. Deficiencies in C1, C2, and C4 may predispose to collagen vascular disease and systemic lupus erythematosus (SLE) (Aggarwal et al., 2010; Jonsson et al., 2007; Meyer et al., 1985). Descriptions in pregnancy are limited to case reports or familial clusters of heterozygous carriers (Dantant et al., 1978; Dixit et al., 1985; Sullivan and Winkelstein, 1994). In light of the critical position of C3 in the complement cascade, inherited deficiency of this component results in overwhelming infections at an early age and no cases of human pregnancy have been reported in the literature among women with inherited C3 deficiency.
Although true complement deficiencies are rare, there is increasing evidence that inherited gene mutations in complement regulatory proteins predispose to adverse pregnancy outcomes (Mohlin et al., 2013; Salmon et al., 2011). Most commonly, these are loss of function gene mutations in complement regulators resulting in increased complement activation. Gain of function mutations in complement effectors have not yet been linked to adverse pregnancy outcomes, but they have been identified in individuals with atypical hemolytic uremic syndrome (Loirat and Fremeaux-Bacchi, 2011). In a study among women with recurrent pregnancy loss (defined as ≥3 consecutive embryonic losses before 10 weeks gestation or ≥2 fetal losses after 10 weeks gestation), specific mutations in C4b binding protein (C4BP) and CD46 were identified (Mohlin et al., 2013). C4BP polymorphism R120H was detected in two subjects with recurrent pregnancy loss and was associated with decreased C3b degradation capability. Two polymorphisms in CD46 included P324L, which caused significant decreased cell surface expression of CD46 and N213I, which was associated with decreased degradation of both C4b and C3b. In a large prospective investigation among women with SLE or with aPL antibodies (PROMISSE Study) (Salmon et al., 2011), 18% of those with preeclampsia had detectable mutations in genes encoding complement regulatory proteins MCP (CD46), factor I, or factor H. Three women with preeclampsia were heterozygous for the MCP gene variant A304V, shown to be deficient in cell-surface alternative pathway activation (Fang et al., 2008a). The same variant was detected in 7% of non-autoimmune patients with severe preeclampsia or HELLP syndrome, and has been detected in patients with aHUS (Caprioli et al., 2006).
Studies in animal models with genetic deficiencies have also demonstrated the importance of an intact complement system in a successful pregnancy. As previously noted, mice deficient in C1q experience abnormal placentation and fetal loss (Singh et al., 2011). Crry knockout mice experience complete fetal loss during embryonic development (Xu et al., 2000) due to excessive alternative pathway complement activation through C3. Experiments demonstrated that animals deficient in C3, Factor B and properdin (Kimura et al., 2010) were protected from fetal loss in Crry knockout mice, whereas C4 or C5 deficient animals were not. More detailed study of Crry knockout mice indicated that a normal placental vasculature does not develop (Mao et al., 2003). C3 deficient mice have a higher fetal resorption rate with reduced placental development in the labyrinth and spongiotrophoblast layer than control mice (Chow et al., 2009). In addition, fetal and placental weight decreased suggesting fetal growth restriction as a result of the C3 deficiency.
3.2 Complement Deficiencies and Pregnancy complications
True complement deficiencies are associated with severe, recurrent infections and are uncommon in pregnancy. However, there is increasing recognition of acquired complement deficiency in pregnancy, which may be triggered by underlying complement gene mutations or complement-associated disorders such as SLE or paroxysmal nocturnal hemoglobinuria (PNH). In pregnancy, deficiencies in specific complement regulators (e.g., PNH or complement gene mutations) may predispose to excess complement activation or alternatively, complement deficiency may arise as the end result of excess complement activation (e.g., SLE). Depending on the underlying trigger and the gestational age of the pregnancy, complement biomarkers and clinical manifestations of disease will vary. We will review pregnancy complications in select situations where deficiencies or mutations of complement, whether genetic or acquired, are associated with pregnancy complications.
3.2.1 Systemic lupus erythematosus (SLE) and antiphospholipid antibody syndrome (APLAS)
Immune complex formation triggers complement activation and predisposes to adverse pregnancy outcomes in women with SLE and APLAS. Recurrent embryonic loss (<10 weeks), unexplained fetal death (>10 weeks), and severe preeclampsia or growth restriction <34 weeks are heightened in both disorders, but these adverse pregnancy outcomes specifically define the diagnosis of APLAS. SLE is defined clinically, but is associated with a number of auto-antibodies including anti-nuclear, anti-Smith and anti-double stranded DNA antibodies. Anti-C1q antibodies have also been described in lupus nephritis (Marto et al., 2005). Anti-phospholipid (aPL) antibodies (e.g., lupus anticoagulant, anti-cardiolipin antibody, anti-β2 glycoprotein I) are diagnostic for APLAS.
In light of immune complex formation and complement deposition in the kidney, SLE flares are often associated with low C3 and C4 levels (Birmingham et al., 2010). Normalization of C3 and C4, irrespective of therapy, is associated with a reduction in severe flares (Stohl et al., 2012). Lupus flares in pregnancy are often characterized by severe hypertension and proteinuria, and distinction from severe preeclampsia is difficult. While preeclampsia is not characterized by low C3 or C4 levels as seen with lupus in pregnancy (Buyon et al., 1986), the ratio of complement split products (i.e., C3a/C3 and C4d/C4) are elevated in preeclampsia suggesting excess complement activation with relative depletion of C3 and C4 (Derzsy et al., 2010). On the other hand, both severe preeclampsia and lupus have been associated with increased terminal complement activation as measured by plasma and urine biomarkers C5a and sC5b-9 (Burwick et al., 2013; Chiu et al., 1998; Gou et al., 2013). In preeclampsia, urinary excretion of sC5b-9 correlates most closely with kidney injury molecule 1 (KIM-1), a marker of proximal tubule injury (Burwick et al., 2014b). Extrinsic C5 activation by serine proteases in the coagulation cascade may also contribute to increased levels of C5a and sC5b-9 in severe preeclampsia (Amara et al., 2010; Burwick et al., 2014a; Huber-Lang et al., 2006).
APLAS complicates disease in a subset of patients with SLE, but it also occurs in the absence of clinical lupus. For women with pregnancies complicated by severe, early-onset preeclampsia or growth restriction (<34 weeks gestation), the persistence of circulating aPL antibodies is sufficient to diagnose APLAS. This emphasizes the pathophysiologic link between autoantibodies, complement activation, and placentally driven adverse pregnancy outcomes. For example, aPL antibodies may fix complement to platelets, contributing to platelet activation and venous thrombosis (Lood et al., 2014). This may occur within the placental vasculature or systemic circulation. In mice, targeted C5 inhibition is sufficient to mitigate aPL associated thrombosis (Romay-Penabad et al., 2014) and pregnancy loss (Girardi et al., 2006). In human pregnancy, low molecular weight heparin reduces adverse pregnancy outcomes in women with APLAS (Bates et al., 2008) although it is unclear if this is due specifically to an anti-complement or anti-thrombotic effect or both.
3.2.2 Paroxysmal nocturnal hemoglobinuria (PNH)
Paroxysmal nocturnal hemoglobinuria (PNH) results from an acquired defect in glycosylphosphatidylinositol (GPI) anchoring of complement regulators CD55 and CD59 in blood cells, predisposing to complement-mediated hemolysis, thrombocytopenia and thrombosis. Complement hemolytic activity (e.g., CH50 assay) may be used to assess disease activity and response to treatment in PNH, and CH50 activity >10% of normal values correlates with the need for blood transfusion (Peffault de Latour et al., 2014). Nearly half of pregnancies in women with PNH result in either spontaneous miscarriages or early termination, and among those carrying the pregnancy beyond the second trimester more than 50% are delivered prematurely (Bais et al., 1994; Fieni et al., 2006; Ray et al., 2000). However, the anti C5 antibody eculizumab was FDA approved for treatment of PNH in 2007 and its use has successfully allowed management of pregnancy in women with PNH (Kelly et al., 2010).
3.2.3 Atypical hemolytic uremic syndrome (aHUS)
Atypical hemolytic uremic syndrome is a life-threatening disorder characterized by microangiopathic hemolytic anemia, thrombocytopenia and renal failure. Uncontrolled complement activation is a common feature of disease and complement gene mutations have been found in more than 50% of patients with aHUS, including women with pregnancy associated HUS (Noris and Remuzzi, 2010). Interestingly, similar complement gene mutations are found in women with preeclampsia and HELLP syndrome (Fang et al., 2008a; Salmon et al., 2011). Serum C5a and sC5b-9 levels are also elevated in all three conditions (Burwick et al., 2013; Cataland et al., 2014; Haeger et al., 1990). Eculizumab is now FDA approved for treatment of aHUS and it appears to be effective in mitigating complement-mediated hemolysis, platelet destruction and kidney injury (Legendre et al., 2013).
HELLP syndrome and aHUS have been compared because they are both thrombotic microangiopathies associated with hemolytic anemia and thrombocytopenia (Fang et al., 2008b). HELLP syndrome is most often present in conjunction with preeclampsia, but it can occur independently in 10–15% of cases. Complicating matters, aHUS can occur in pregnancy or postpartum. When HELLP presents in the absence of hypertension the distinction from pregnancy associated aHUS is less clear, but liver dysfunction suggests the former and acute renal failure the latter. In women with prior aHUS, pregnancy may trigger recurrent disease in up to 20%, and most recurrences are among those with gene mutations in complement regulatory proteins (e.g., Factor H, Factor I, MCP) (Fakhouri et al., 2010). Outcomes for these women are particularly poor, with over 80% requiring hemodialysis and over 60% reaching end stage renal disease within one month of the episode. Rates of pregnancy loss and preeclampsia are also elevated.
Haeger et al. reported increased complement activation in 10 women with HELLP syndrome as assessed by plasma levels of C3a, C5a, and Cb5b-9 (Haeger et al., 1990). Over the next few decades, advancements in the understanding of complement disorders led investigators to consider that complement gene mutations might predispose to HELLP syndrome, as seen with aHUS. Fang et al. described a mutation in MCP (A304V) in a subject with HELLP syndrome that was also seen in subjects with aHUS, shiga toxin associated HUS, and glomerulonephritis with C3 deposits (Fang et al., 2008a). It was determined that the A304V mutation led to deficient control of the alternative pathway of complement activation on a cell surface. This mutation was subsequently reported by Salmon et al. in a subject with HELLP syndrome and neonatal demise at 22 weeks (Salmon et al., 2011) and by Fakhouri et al. in a subject with HELLP syndrome and renal involvement (Fakhouri et al., 2008). Fakhouri et al. also identified other complement gene mutations in HELLP syndrome subjects, including Factor H (1 subject) and Factor I (2 subjects). More recently, Crovetto et al. (Crovetto et al., 2012) evaluated 33 women with HELLP syndrome and detected complement gene mutations in three subjects (9.1%), including Factor I (2 subjects) and MCP (1 subject). The overall prevalence of complement gene mutations in women with HELLP syndrome remains unclear due to small sample size, but it is likely to be lower than the prevalence in individuals with aHUS (>50%). Regardless, complement gene mutation testing may ultimately be useful for providers managing high-risk pregnancies such as those with a prior history of HUS or HELLP syndrome.
3.2.4 Placental malaria
In placental malaria, parasitized RBCs have access to the intervillous space (Conroy et al., 2011). Infection with the malarial plasmodium results in excessive complement activation, and when the individual is pregnant, this increased activation occurs in the intervillous space as well, exposing the fetus and syncytiotrophoblasts and developing placenta to excessive complement activation products. Placental malaria predisposes to hypertension, which is not typical of malaria infection outside of pregnancy (Muehlenbachs et al., 2006; Ndao et al., 2009). Some very elegant studies by Conroy et al demonstrated an important role for complement activation and C5a in the placental insufficiency and low birth weight associated with placental malaria (Biryukov and Stoute, 2014; Conroy et al., 2013). Besides hypertension in the mother with placental malaria, fetal growth restriction results in long term consequences for the child in terms of poor health outcomes.
4. Complement and early pregnancy loss
Miscarriage, affecting approximately 15% of pregnancies, is spontaneous loss of pregnancy where the fetus is not viable. In general, miscarriage is categorized as antibody dependent and antibody independent miscarriage or spontaneous abortion. Cunningham et al (Cunningham and Tichenor, 1995) examined trophoblasts from recurrent spontaneous abortions associated with hypocomplementemia and noted a significant reduction in DAF/CD55 expression in the trophoblasts, with a loss of protection from complement activation resulting in an unsuccessful pregnancy. They estimated that up to 20% of cases of first trimester loss are associated with hypocomplementemia (Tichenor et al., 1995). Indeed, in the human placenta after spontaneous abortion a significant decrease in complement regulators CD46 and CD55 with a subsequent increase in complement activation has been described (Banadakoppa et al., 2014), reaffirming the importance of limiting placental complement activation to ensure a successful pregnancy.
4.1 Antibody dependent miscarriage models
As discussed in section 3.2.1 women with APLAS are plagued with thrombosis and increased risk of pregnancy complications and early pregnancy loss. To examine the mechanism of this fetal loss aPL antibodies were administered to a mouse and resulted in fetal loss and fetal growth restriction in survivors with significant neutrophil accumulation at sites of fetal loss (Girardi, 2008). Using the power of mouse genetics, as well as neutrophil depletion, they demonstrated the importance of neutrophils and the classical and alternative pathway through C5 in the fetal loss. In addition, using C5aR deficient animals as well as the C5aR antagonist PMX-53 [AcPhe(L-ornithine-Pro-D-cyclohexylalanine-Trp-Arg)] C5a was demonstrated to be critical for the pathology. C5a was also essential for increased tissue factor expression (Redecha et al., 2007) thus connecting the thrombosis and complement activation in this animal model of APLAS. Placental inflammation and fetal death are also associated with another inflammatory disease neuromyelitis optica with antibodies against aquaporin. Injection of these antibodies into mice results in activated complement, miscarriage and damaged fetoplacental unit (Saadoun et al., 2013).
4.2 Antibody independent miscarriage models
An antibody independent mouse model of pregnancy loss that has also been extensively used is an abortion prone mating, the CBA/J female X DBA/2 male, that results in increased fetal resorption and growth restriction. In this model, the semiallogeneic placenta is likely being rejected by effector cells from the mother. The control mating is the MHC identical CBA/J X BALB/c mating combination that has disparate minor loci (Chaouat et al., 2009). C5a is the key complement activation product resulting in fetal rejection and growth restriction in the CBA/J X DBA/2 mating (Girardi et al., 2006). Normal placental development is affected. There is a large volume of literature regarding the importance of angiogenesis in development of normal vasculature for the placenta with the proper balance of angiogenic [e.g. vascular endothelial growth factor (VEGF), Placental growth factor (PlGF)] and anti-angiogenic factors [soluble fms-like tyrosine kinase-1 (sFlt-1)] being important. In the CBA/J female X DBA/2 male abortion prone mating, C5a triggers release of sFlt-1 (a soluble VEGF receptor) from monocytes, thus sequestering VEGF and affecting angiogenic balance and normal placental development leading to pregnancy loss and fetal growth restriction (Girardi et al., 2006). Inhibition of complement activation in this model using the C3 inhibitor CR2-Crry on gestation day 5 blocked placental complement activation and prevented placental dysfunction (Qing et al., 2011). However, in this model, placental dysfunction was not associated with hypertension, but with oxidative stress and increased sFlt-1, an occurrence that is consistent with reports from the clinical literature investigating idiopathic fetal growth restriction (Crispi et al., 2006; Espinoza et al., 2007; Shibata et al., 2005; Wathen et al., 2006).
C57Bl/6 mice provide another example of potential complement involvement in antibody independent pregnancy loss. About 10% of fetuses from C57Bl/6 mice are aborted at day 14 of gestation. Immunohistochemical examination of abortion sites compared to normal sites revealed increased C3 in cytoplasm of invasive trophoblast giant cells. High levels of adipsin, a mouse homolog of alternative pathway human Factor D, was also present (Kusakabe et al., 2008) suggesting complement involvement in the pregnancy loss. However, further studies in this model are necessary to determine if the complement activation is causative rather than a result of the spontaneous abortion.
5. Complement and Fetal growth restriction
A growth restricted neonate is 3 times more likely to die as a neonate than a normal weight child (Garite et al., 2004). Adverse long-term consequences of fetal growth restriction can include increased risk for respiratory (e.g. BPD), cardiovascular, or metabolic diseases as well as neurodevelopmental outcomes that may result in a variety of mental health issues. Thus, understanding the underlying causes of fetal growth restriction and its potential prevention are of considerable interest for improving public health.
Unilateral uterine ischemia/reperfusion has been used as a model of fetal growth restriction. In this mouse model, partial flow restriction for 30 min (fetal growth restriction) or total flow restriction for 5 min (fetal loss) was accomplished mechanically clamping the uterine and ovarian arteries of the right horn on gestation day 13.5. The left horn was not manipulated. When assessed on gestation day 18.5, unilateral ischemia reperfusion resulted in bilateral fetal loss and fetal growth restriction. Blood pressure was not measured. In the absence of C5, the mouse was protected from ischemia reperfusion induced fetal growth restriction and fetal loss. Uterine myeloperoxidase indicative of neutrophil infiltration was increased following ischemia reperfusion, but not in C5 deficient mice (Qu et al., 2012).
The reduced uterine perfusion pressure (RUPP) model of placental ischemia in the rat leads to fetal growth restriction and hypertension (See section 6.2). Interestingly our studies in the RUPP model indicate that inhibition of complement activation reduces blood pressure but does not improve fetal growth or viability (Lillegard et al., 2013; Lillegard et al., 2014), whereas in unilateral uterine ischemia reperfusion in the mouse, C5 deficiency protected from fetal growth restriction (Qu et al., 2012). Also, a case report by Burwick and Feinberg showed that anti C5 antibody treatment improved outcomes in preeclampsia and HELLP syndrome including lengthening gestation by 17 days and allowing fetal growth and maturation (Burwick and Feinberg, 2013). Although exciting, this observation highlights a limitation of many current animal models for studying pregnancy disorders. In particular, rodent models cannot easily incorporate the pre-term delivery aspect of preeclampsia and fetal growth restriction. Taken together, the exact role for complement factors in fetal growth restriction remains unclear. While a certain amount of complement activation is required during early pregnancy for normal embryonic and fetal development, excessive complement activation in early or late pregnancy can be deleterious. Hence, continued studies in the basic sciences as well as the clinic are required to identify further therapeutic targets to improve maternal and fetal well-being.
6. Complement and Hypertensive Disorders of Pregnancy
Hypertensive disorders of pregnancy are classified into 4 categories: 1) Preeclampsia –eclampsia, 2) chronic hypertension of any cause (pre-dates pregnancy or evident in early pregnancy before 20 weeks gestation), 3) chronic hypertension with superimposed preeclampsia, 4) gestational hypertension. The diagnosis of preeclampsia has required the presence of proteinuria since at least the 1930’s (Irving, 1939) and continuously through 2013. Prior to 2013, the American College of Obstetricians and Gynecologists (ACOG) required ≥300mg protein in a 24 hour urine collection to make a formal diagnosis of preeclampsia (2002). However, due to the heterogeneous and systemic nature of disease, ACOG has broadened the diagnosis of preeclampsia (ACOG, 2013). The diagnosis of preeclampsia and gestational hypertension remain dependent on new onset blood pressure ≥140 mmHg systolic or ≥90 mmHg diastolic after 20 weeks of gestation. However, proteinuria (≥300mg protein in a 24 hour urine collection or urine spot protein/creatinine ratio ≥0.3) is sufficient but not necessary for the diagnosis of preeclampsia. In the absence of proteinuria, preeclampsia is now confirmed by new onset hypertension with signs or symptoms of systemic disease including thrombocytopenia, impaired liver function, renal insufficiency, or cerebral or visual disturbances. Gestational hypertension is distinguished from preeclampsia by absence of proteinuria or systemic signs or symptoms. Hypertensive disorders of pregnancy are also characterized by fetal growth restriction and are a leading cause of medically indicated preterm birth. Thus, the discussion here will concentrate primarily on the role of the complement system in mediating the hypertension or proteinuria associated with preeclampsia with full knowledge that the complement system may also be involved in preterm birth (Section 7) and fetal growth restriction (Section 5) due to hypertensive disorders of pregnancy. It should also be noted that the studies cited are generally based on the ‘old’ classification of preeclampsia with the requirement for proteinuria. Preeclampsia is also distinguished as early onset vs late onset preeclampsia (Moore and Redman, 1983; Paruk and Moodley, 2000) based on evidence that the two entities have distinct pathophysiologic underpinnings (Nelson et al., 2014; Pinheiro et al., 2014) Investigators have most often classified early-onset and late-onset disease as preeclampsia that prompts delivery <34 weeks or ≥34 weeks gestation, respectively.
It is generally considered that preeclampsia and related hypertensive disorders of pregnancy arise from early placental aberrations that impair blood flow and oxygenation to the placenta. A leading hypothesis is that spiral artery remodeling is impaired in placental development leading to reduced perfusion of the intervillous space and relative placental ischemia. Two questions arise regarding pathophysiology: what causes the impaired placental development, and once placental ischemia has occurred, what leads to the increased blood pressure and fetal growth restriction. As appropriate, we will consider complement involvement in both stages of development of preeclampsia.
6.1 Human studies
Early studies using CH50 measurements of total hemolytic complement activity revealed no difference in plasma from normal pregnancies vs. preeclamptic pregnancies (Kitzmiller et al., 1973). Advances in measurement of complement activation products in clinical studies have clearly demonstrated that complement activation is even greater in preeclamptic pregnancies compared to normal pregnancies. Derzsy et al. (Derzsy et al., 2010) reported ↑C3a/C3 ratio and ↑sC5b-9 in preeclamptic pregnancies compared to normal pregnancies, as well as a significant decrease in C3. These measures all support the conclusion that excessive complement activation had occurred in the preeclamptic pregnancy leading to a depletion of C3 in the plasma – C3 synthesis was outpaced by C3 activation. They also found that increased terminal lytic pathway activation (sC5b-9) was associated with fetal growth restriction. Soto et al. (Soto et al., 2010) compared complement activation products C3a, C4a and C5a in preeclamptic pregnancies vs pregnancies with small for gestational age fetuses and noted increased C5a was associated with preeclampsia but not small for gestational age fetuses. Both of these studies evaluated complement activation products in the last half of pregnancy when preeclamptic symptoms were evident.
To determine if complement activation products early in pregnancy were predictive and/or potentially causal in preeclampsia, Lynch and colleagues (Lynch et al., 2008) measured complement activation products and followed patients for development of preeclampsia and other pregnancy complications. They reported increased Factor Bb suggesting excessive alternative pathway activation early in pregnancy, and this increase was associated with preeclampsia development later in pregnancy. Predictive differences in C3a or sC5b-9 were not detected. In continuing studies, outcomes were expanded to include hypertensive disease of pregnancy, preterm birth (<37 wk), premature rupture of the membranes, intrauterine fetal loss, and growth restriction. In this analysis women in the highest quartile of C3a were 3 times more likely to have an adverse pregnancy outcome. (Lynch et al., 2011). Hypertension, preterm birth and premature rupture of membranes had the strongest association with high C3a levels early in pregnancy.
In patients with severe preeclampsia, increased complement activation products C5a and sC5b-9 have also been detected in plasma and urine indicating activation of the terminal complement components (Burwick et al., 2013). Urinary excretion of sC5b-9 was markedly increased in severe preeclampsia but minimal or absent in gravidas with chronic hypertension or healthy controls. Furthermore, urinary detection of sC5b-9 correlated well with the anti-angiogenic condition, characterized by increased sFlt-1 and decreased PlGF and VEGF (Guseh et al., 2014). Overall, clinical data clearly suggest excessive complement activation is associated with adverse outcomes of pregnancy, including high blood pressure and renal insufficiency. The excessive complement activation precedes the symptoms and may be predictive, suggesting a causal role in the process. However, excessive complement activation also occurs during preeclampsia, indicating that increased complement activation perpetuates pathology during later pregnancy when preeclamptic symptoms are evident.
More recent studies have examined changes in complement regulators to determine if decreased regulation in placenta is responsible in part for increased complement activation in preeclampsia compared to normal pregnancy. Mutations in complement regulatory proteins have been detected in 8–18% of women with preeclampsia (Salmon et al., 2011). The involvement of early complement components in preeclampsia is suggested by the fact that deficiency in C4A or C4B is twice as prevalent in early onset preeclampsia patients compared to normal pregnant controls (Lokki et al., 2014). C4 is produced by 2 loci with the normal situation involving 2 C4A and 2 C4B genes. In control pregnancies, higher C4 deposition and reduced presence of C4BP is contrasted with low C4 deposition and high C4BP in early and late onset preeclampsia (Lokki et al., 2014). C4BP was intense in apoptotic fragments and syncytial bodies. In contrast, Buurma observed increased C4d on syncytiotrophoblasts in preeclamptics compared to controls (Buurma et al., 2012). Differences in CD46, CD55 and CD59 by immunohistochemistry were not evident in normal pregnancies vs early and late onset preeclampsia (Lokki et al., 2014). However, mRNA for CD55 (DAF, decay accelerating factor) and CD59, but not CD46 were significantly upregulated in placenta from preeclamptic pregnancies (Buurma et al., 2012; Lokki et al., 2014). Overall these studies point to dysregulation of complement activation in preeclamptic placenta in response to placental insufficiency and damage, with upregulation of endogenous regulators in an attempt to keep complement activation under control.
6.2 Animal studies
Animal studies of hypertensive disorders of pregnancy are important to critically test hypotheses regarding pathophysiology and putative treatments that are not possible in humans, as well as to inform critical studies in humans. Many studies have utilized mice and rats that share hemichorial placentation with humans. Both humans and rats exhibit deep trophoblast invasion in placental development, in contrast to mouse where invasion is shallow and placentation is more superficial (Soares et al., 2012). Genetic manipulation of the mouse has been very valuable, and tools for genetic manipulation of the rat are becoming increasingly available. Studies have primarily examined pathophysiology of preeclampsia once placental ischemia has been realized with a few studies investigating the cause of the placental insufficiency. As previously noted, C1q deficient mice result in an abnormal placenta being formed and increased blood pressure developing over pregnancy with increased protein in the urine (Singh et al., 2011). Clearly, C1q is necessary for normal placental development and in the absence of a normal placenta, the mice develop defective decidual invasion and symptoms of preeclampsia.
Other models of preeclampsia are focused on events in the later part of pregnancy to investigate the consequences of placental ischemia once it has developed. In these models, preeclampsia symptoms are initiated either by surgical intervention to cause placental ischemia or by infusion of mediators resulting from placental ischemia. Placental ischemia in rat and mouse results in elaboration of many factors including but not limited to agonistic autoantibodies to the angiotensin II type 1 receptor (AT1-AA), TNF, endothelin, increased sFlt-1, decreased VEGF and increased reactive oxygen species (LaMarca et al., 2013). Studies by Lillegard et al (Lillegard et al., 2013; Lillegard et al., 2014) using the reduced uterine perfusion pressure (RUPP) model of placental ischemia in the rat were the first to mechanistically link complement activation, particularly C3a and C5a, with the hypertension caused by placental ischemia. Chronic placental ischemia in the RUPP model (mechanically reducing blood flow to the uteroplacental unit to ~40%) results in increased blood pressure in the mother, fetal growth restriction, and complement activation. Inhibiting complement activation using a soluble version of endogenous CR1 (sCR1) significantly attenuated hypertension. Continued studies using the C5a receptor antagonist PMX 53 and the C3a receptor antagonist SB290157 demonstrated that C3a and C5a were the important products of complement activation responsible for the hypertension. In addition, PMX 53 prevented endothelial dysfunction in small mesenteric blood vessels and changes in heart rate that occur following placental ischemia suggesting that they both contribute to hypertension. The conclusion of these studies regarding C3a involvement relies on specificity of SB290157 at the dosage used (5 mg/kg). Certainly SB290157 has been criticized for its partial agonist activity as well as its effect on neutrophils so the involvement of C3a in placental ischemia induced hypertension should be considered cautiously (Proctor et al., 2004). What is unclear at this point is whether complement activation and C3a/C5a generation are linked to other mediator systems with a known role in placental ischemia induced hypertension i.e. endothelin, reactive oxygen species, AT1-AA, VEGF, sFlt-1, or whether complement activation acts in parallel with these other systems to result in hypertension.
Infusion of sFlt-1, AT1-AA or TNF can also mimic symptoms of preeclampsia in rodent models, and manipulating these mediator systems attenuates placental ischemia-induced hypertension (LaMarca et al., 2013). Wang et al hypothesized that interaction of the AT1-AA with the angiotensin receptor would be responsible for increased complement activation and the preeclamptic symptoms in a mouse model. Infusion of the human AT1-AA mimicked symptoms of preeclampsia with increased blood pressure, albuminuria, fetal growth restriction and significant complement deposition in placenta and kidney (Wang et al., 2012). SB290157 (30 mg/kg) attenuated the hypertension, albuminuria, intrauterine growth restriction and sFlt-1 changes suggesting that the C3a receptor was an important mediator of these events and contributed to the angiogenic imbalance in preeclampsia. Again studies with SB290157 need to be viewed with caution given its documented off target effects.
Evidence for complement involvement in pathophysiology of preeclampsia has also been reported using another rat model of preeclampsia developed by crossing rats transgenic for the human angiotensinogen gene and the human renin gene to produce a female rat that develops hypertension during pregnancy with excessive stimulation of angiotensin receptor (Dechend et al., 2005). Increased AT1-AA are also produced by this rat and react with a defined epitope on the second extracellular loop of the receptor resulting in activation that can be prevented by the epitope or the angiotensin receptor antagonist losartan. Complement activation was noted in the kidney of these animals as evidenced by glomerular C3 deposition. In addition, increased C3 was apparent in the placental blood vessels of these rats (Hering et al., 2008) co-localized with actin indicating it was associated with vascular smooth muscle. In contrast to C1q deficient mice where placental development is reduced and hypertension ensues, this rat model results in significantly deeper trophoblast invasion and placental development associated with complement activation and increased C3 in placental blood vessels with hypertension.
7. Complement and Preterm Birth
7.1 Preterm birth
Preterm birth constitutes delivery before 37 weeks of gestation and approximately 12% of births in United States are preterm. Approximately a third of those births are medically indicated (Goldenberg et al., 2008) to protect the health of the mother and/or child. The remaining 70% of the preterm births are spontaneous – occurring after spontaneous onset of labor. Studies suggest spontaneous and medically indicated preterm births are clinical subtypes with possible overlapping mechanisms (Ananth, 2014; Ananth and Vintzileos, 2006a; Iams, 2014; Roberts, 2014). Ischemic placental disease includes preeclampsia, intrauterine growth restriction, and placental abruption (Ananth and Vintzileos, 2006b; Friedman and Cleary, 2014; Roberts, 2014) and accounts for half of medically indicated preterm births in United States or about 2% of all births (Friedman and Cleary, 2014). Spontaneous preterm labor can occur for many different reasons including infection, cervical disease, disruption of maternal fetal tolerance, and vascular disease (Romero et al., 2014). Labor involves uterine contractility, cervical dilatation and rupture of the chorioamniotic membranes. With spontaneous preterm labor one or more of these events is prematurely activated by the disease process. Strategies to prevent preterm birth are urgently needed (Newnham et al., 2014).
7.2 Complement and preterm birth in humans
Increased levels of Factor Bb early in pregnancy are associated with preterm birth (Lynch et al., 2008; Vaisbuch et al., 2010) and occurred whether or not intra-amniotic infection or inflammation was present. Increased C5a also was evident in the circulation of women delivering preterm, but only associated with intra-amniotic infection or inflammation (Soto et al., 2005) indicating increased Bb has greater potential as a predictor of preterm birth of multiple causes. Spontaneous idiopathic preterm birth is associated with coding variants in CR1 (McElroy et al., 2013) reaffirming that control of complement activation helps to ensure a successful pregnancy.
Excessive inflammation has been posited as a mechanism that leads to preterm birth. Complement regulator CD55 normally increases during pregnancy on circulating white blood cells and is even further elevated in subjects with preterm labor (Nowicki et al., 2009) presumably due to activation of the inflammatory response in the maternal peripheral circulation. Greater increases in CD55 were seen in women who experienced preterm labor yet carried their pregnancies past 34 weeks suggesting that the increase in CD55 was an attempt to limit inflammation and extend the pregnancy (Pacheco et al., 2011). CD55 in peripheral white blood cells may be a useful predictor of preterm labor (Nowicki et al., 2009), either alone or in combination with TLR4 (Pratap et al., 2013), consistent with the extensive literature on crosstalk with TLR4 and complement activation (Song, 2012).
7.3 Complement and preterm birth – animal models
Preterm birth in humans can have numerous causes including infection and inflammation. Girardi et al developed a mouse model of inflammation induced preterm delivery using LPS administered intravaginally on gestation day 15 in the mouse. In this model, more than 90% of the females delivered within 24–36 hours after LPS and there was no maternal morbidity or mortality observed. LPS is both a TLR4 ligand as well as an activator of the alternative complement pathway. Complement activation via a C5a/C5aR interaction resulted in macrophage recruitment and matrix metalloprotease (MMP) production resulting in cervical ripening and preterm birth (Gonzalez et al., 2011b). Now this mechanism is different from the mechanism that leads to cervical remodeling at term where cervical fibroblasts and columnar epithelial cells are the source of MMPs (Gonzalez et al., 2013) leading to cervical ripening without complement recruitment of macrophages. These data suggest that monitoring complement activation may serve as a biomarker for preterm birth (Gonzalez et al., 2011a) but the clinical translation of this information has not yet been realized.
A clear complication of preterm delivery is inadequate development of critical organ systems including the brain. In their model of preterm birth, Girardi et al also found that complement inhibition using a C5aR deficient mouse prevented fetal brain cortical abnormalities associated with inflammation induced preterm birth (Pedroni et al., 2014). Using ultrasmall superparamagnetic iron oxides conjugated to anti C3 and magnetic resonance imaging, they were able to detect C3 deposition in placenta and brain in animals undergoing LPS induced preterm birth or APLAS preterm birth (Girardi et al., 2014). This noninvasive in utero method can determine the presence of C3 deposition in the placenta and fetal brain and may serve as a very useful experimental method to further probe complement involvement in preterm birth.
8. Therapeutic manipulation of the complement system in pregnancy
The association between complement activation and adverse pregnancy outcomes suggests that targeted complement regulation has the potential to reduce maternal and neonatal harms. Targeted therapeutics could potentially act by preventing complement activation, mitigating the effects of activated complement components, or by depleting complement. Sensibly, there has been hesitancy to introduce complement blockade in pregnancy in light of the potential risk for infection in a vulnerable host. Terminal complement deficiency has been associated with increased risk of infections with encapsulated organisms, particularly Neisseria species (Hellerud et al., 2010; Petersen et al., 1979). While blockade of the complement cascade at the level of C5 could theoretically pose a lower risk of infection than does blocking complement at the level of C3, all pregnant women with complement deficiency (including C5 blockade) are advised to receive the meningococcal vaccine (Cohn et al., 2013).
Use of eculizumab for treatment of PNH has provided evidence that complement blockade is well tolerated and beneficial in pregnancy (Hallstensen et al., 2014; Kelly et al., 2010). As noted previously, PNH carries high morbidity in pregnancy and historically, more than 50% of pregnancies end in fetal loss or premature delivery (Bais et al., 1994; Fieni et al., 2006; Ray et al., 2000). Due to these maternal and neonatal risks, eculizumab is often initiated or maintained for pregnant women with PNH and a few cases have been described (Hallstensen et al., 2014; Kelly et al., 2010). Eculizumab levels in cord blood have been minimal or undetectable and importantly, the low cord levels of eculizumab that have been found do not alter classical or alternative complement signaling pathways in the neonate (Hallstensen et al., 2014).
There is scant human data on the use of complement blockers specifically for the prevention of adverse pregnancy outcomes. Studies in a mouse model of antiphospholipid antibody mediated fetal loss have demonstrated that controlling complement activation using a neutralizing antibody to C5 (Agostinis et al., 2014; Girardi et al., 2003) or using a CH2 deleted recombinant antibody to β2-Glycoprotein 1 (Agostinis et al., 2014) can prevent fetal loss. In humans, a single case report describes successful use of eculizumab for the treatment of preeclampsia and HELLP syndrome (Burwick and Feinberg, 2013). Treatment with eculizumab was commenced, after meningococcal vaccination, at a dose of 1200mg IV. The patient tolerated the treatment without adverse effect and the pregnancy was prolonged 17 days with transient resolution of HELLP syndrome and a neonate born at 292/7 weeks gestation with no major complications. The cord blood level was low (15ug/ml) and likely insufficient to block complement, as others have reported. In a subsequent report, the authors report that sC5b-9, but not C5a, levels in maternal blood and urine decreased after treatment, concordant with disease resolution (Burwick et al., 2014a). This unique case description supports the belief that complement activation is an important mediator of disease in preeclampsia and HELLP syndrome and that reduction in terminal complement activation may mitigate features of disease. The ability of complement blockers to mitigate other adverse pregnancy outcomes, such as growth restriction or preterm birth, has yet to be reported in human investigations.
There are many other potential therapeutic agents that target complement and they include, but are not limited to: Compstatin derivatives (e.g., Cp40, C3 inhibitor) (Risitano et al., 2014); soluble CR1 (CDX-1135, Celldex Therapeutics, C3 convertase inhibitor) (Lillegard et al., 2013; Weisman et al., 1990); Pexelizumab (C5 inhibitor) (Armstrong et al., 2007; Martel et al., 2012); C5a antagonists (e.g., PMX53) (Finch et al., 1999; Lillegard et al., 2014); and recombinant human cobra venom factor (Vogel et al., 2014). These agents have been tested in a variety of pre-clinical and clinical studies (Ricklin and Lambris, 2013a; Ricklin and Lambris, 2013b) but none have been specifically utilized in pregnancy. While it is a daunting task to investigate novel agents in pregnancy, and there are legitimate safety considerations for each, the building literature on complement activation and adverse pregnancy outcomes urges investigators and the pharmaceutical industry to pursue more trials in the field. In the interim, important information may be gleaned from complement inhibitors already in use (e.g., eculizumab) or existing agents with anti-complement effects such as heparin (Garlatti et al., 2010; Girardi et al., 2004; Oberkersch et al., 2010). Heparin, unfractionated or low molecular weight, appears to decrease the recurrence of placentally-driven adverse pregnancy outcomes (Ghidini, 2014; Kupferminc et al., 2011) and may improve live birth rate in APLAS (Girardi et al., 2004; Rai et al., 1997). Until additional complement therapeutics become readily available, it may be helpful to investigate the anti-complement effects of heparin in pregnancy to better understand the role of complement blockade in reducing adverse pregnancy outcomes. While novel and existing agents will need continued safety testing for pregnancy, we ultimately believe that the complement cascade can be specifically targeted to improve pregnancy outcomes. The optimal complement therapeutic for a given pregnancy condition should have an acceptable safety profile while offering the hope of a healthier pregnancy outcome for both mother and child.
9. Conclusions and Future Directions
An intact complement system is important for a successful normal pregnancy, both for host defense and to ensure proper placental and fetal development. Complement regulation in the placenta is extremely important to prevent the mother’s innate immune system from harming the fetus. Although some degree of complement activation compared to the non-pregnant state is requisite for a successful pregnancy, recent studies have made it clear that too little or too much complement at the wrong time in gestation can have devastating consequences for the mother and the baby. Indeed, adverse pregnancy outcomes can result from excess activation leading to placental damage or fetal demise, or be the result of inherited or acquired complement deficiencies that do not facilitate adequate placental and fetal development, or the normal regulation of complement activation. Hypertensive disorders of pregnancy with compromised placental perfusion can result from excessive activation of the system or can result from deficiencies in the system not allowing the proper development and perfusion of the uteroplacental unit. Because of the variety of outcomes associated with deviations [in either direction] from the normal level of complement activation there may be a need to develop reference values across the gestational time span.
One of the many challenges is to develop procedures for using excessive complement activation, or complement mutations or dysregulation, as biomarkers to predict the varied adverse outcomes i.e. early pregnancy loss, hypertension, preterm birth, that might occur throughout gestation. Future investigations should also consider the role of inherited fetal complement gene mutations (maternal or paternal) in adverse pregnancy outcomes. Throughout this review we have alluded to maternal mutations but it is possible that fetal inheritance of complement gene mutations is more closely linked to altered complement regulation at the placental interface. Considering the role of the paternal genes on placental development this may be especially important.
Another challenge facing the complement field in general is how to therapeutically impact adverse complement activation without jeopardizing the complement activation necessary for host defense. Many lessons are being learned from use of the anti-C5 antibody eculizumab, including the management of preeclampsia and HELLP syndromes. Clearly therapeutic manipulation of complement should be directed at preventing abnormal placental or fetal development so that fetal demise, growth restriction, or hypertension does not occur. However, as evidenced in our review, striking an acceptable balance of complement activation may not be a straightforward endeavor.
HIGHLIGHTS.
Complement is essential for normal placental and fetal development
Increased complement activation occurs in adverse pregnancy outcomes
Mutations or deficiency in complement are associated with significant pregnancy loss
Clinical and animal studies expose the critical nature of complement dysregulation
Therapeutic complement regulation is an option to mitigate adverse pregnancy outcomes
Acknowledgments
This work was supported in part by NIH R15 HL109843 (JFR, JSG), R01HD079547-01 (sub-contract to JSG), Mission Support Award Grant #64553600, Department of Obstetrics and Gynecology Oregon Health and Science University (RMB).
Abbreviations
- aHUS
atypical hemolytic uremic syndrome
- aPL
antiphospholipid
- APLAS
antiphospholipid antibody syndrome
- AT1-AA
agonistic autoantibodies to the angiotensin II type 1 receptor
- CH50
complement hemolytic activity
- C1-INH
C1 esterase inhibitor
- CR1
Complement receptor 1
- Crry
Complement receptor 1-related gene protein y
- C4BP
C4 binding protein
- DAF
decay accelerating factor, CD55
- HELLP
hemolysis, elevated liver enzymes, low platelet count
- HSPC
hematopoietic stem-like progenitor cells
- MASP
Mannose associated serine protease
- MCP
membrane cofactor protein, CD46
- MBL
mannose binding lectin
- MMP
matrix metalloprotease
- PlGF
placental growth factor
- PNH
paroxysmal nocturnal hemoglobinuria
- RUPP
reduced utero-placental perfusion pressure
- sFlt-1
soluble fms-like tyrosine kinase-1
- SLE
systemic lupus erythematosus
- VEGF
vascular endothelial growth factor
Footnotes
Disclosures
The authors have no conflicts to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jean F. Regal, Email: jregal@d.umn.edu.
Jeffrey S. Gilbert, Email: jgilbert@d.umn.edu.
Richard M. Burwick, Email: burwick@ohsu.edu.
References
- ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33. Obstet Gynecol. 2002 Jan;99:159–67. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- ACOG. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122:1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- Aggarwal R, Sestak AL, D’Sousa A, Dillon SP, Namjou B, Scofield RH. Complete complement deficiency in a large cohort of familial systemic lupus erythematosus. Lupus. 2010;19:52–7. doi: 10.1177/0961203309346508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostinis C, Bossi F, Masat E, Radillo O, Tonon M, De Seta F, Tedesco F, Bulla R. MBL interferes with endovascular trophoblast invasion in pre-eclampsia. Clinical & developmental immunology. 2012;2012:484321. doi: 10.1155/2012/484321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostinis C, Bulla R, Tripodo C, Gismondi A, Stabile H, Bossi F, Guarnotta C, Garlanda C, De Seta F, Spessotto P, Santoni A, Ghebrehiwet B, Girardi G, Tedesco F. An alternative role of C1q in cell migration and tissue remodeling: contribution to trophoblast invasion and placental development. J Immunol. 2010;185:4420–9. doi: 10.4049/jimmunol.0903215. [DOI] [PubMed] [Google Scholar]
- Agostinis C, Durigutto P, Sblattero D, Borghi MO, Grossi C, Guida F, Bulla R, Macor P, Pregnolato F, Meroni PL, Tedesco F. A non-complement-fixing antibody to beta2 glycoprotein I as a novel therapy for antiphospholipid syndrome. Blood. 2014;123:3478–87. doi: 10.1182/blood-2013-11-537704. [DOI] [PubMed] [Google Scholar]
- Albieri A, Kipnis T, Bevilacqua E. A possible role for activated complement component 3 in phagocytic activity exhibited by the mouse trophoblast. Am J Reprod Immunol. 1999;41:343–52. doi: 10.1111/j.1600-0897.1999.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Alper CA. Inherited deficiencies of complement components in man. Immunol Lett. 1987;14:175–81. doi: 10.1016/0165-2478(87)90098-8. [DOI] [PubMed] [Google Scholar]
- Amara U, Flierl MA, Rittirsch D, Klos A, Chen H, Acker B, Bruckner UB, Nilsson B, Gebhard F, Lambris JD, Huber-Lang M. Molecular intercommunication between the complement and coagulation systems. J Immunol. 2010;185:5628–36. doi: 10.4049/jimmunol.0903678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananth CV. Ischemic placental disease: a unifying concept for preeclampsia, intrauterine growth restriction, and placental abruption. Seminars in perinatology. 2014;38:131–2. doi: 10.1053/j.semperi.2014.03.001. [DOI] [PubMed] [Google Scholar]
- Ananth CV, Keyes KM, Wapner RJ. Pre-eclampsia rates in the United States, 1980–2010: age-period-cohort analysis. BMJ. 2013;347:f6564. doi: 10.1136/bmj.f6564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ananth CV, Vintzileos AM. Epidemiology of preterm birth and its clinical subtypes. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2006a;19:773–82. doi: 10.1080/14767050600965882. [DOI] [PubMed] [Google Scholar]
- Ananth CV, Vintzileos AM. Maternal-fetal conditions necessitating a medical intervention resulting in preterm birth. Am J Obstet Gynecol. 2006b;195:1557–63. doi: 10.1016/j.ajog.2006.05.021. [DOI] [PubMed] [Google Scholar]
- Armstrong PW, Granger CB, Adams PX, Hamm C, Holmes D, Jr, O’Neill WW, Todaro TG, Vahanian A, Van de Werf F. Pexelizumab for acute ST-elevation myocardial infarction in patients undergoing primary percutaneous coronary intervention: a randomized controlled trial. JAMA. 2007;297:43–51. doi: 10.1001/jama.297.1.43. [DOI] [PubMed] [Google Scholar]
- Baines MG, Millar KG, Mills P. Studies of complement levels in normal human pregnancy. Obstet Gynecol. 1974;43:806–10. [PubMed] [Google Scholar]
- Bais J, Pel M, von dem Borne A, van der Lelie H. Pregnancy and paroxysmal nocturnal hemoglobinuria. Eur J Obstet Gynecol Reprod Biol. 1994;53:211–4. doi: 10.1016/0028-2243(94)90121-x. [DOI] [PubMed] [Google Scholar]
- Baj-Krzyworzeka M, Majka M, Pratico D, Ratajczak J, Vilaire G, Kijowski J, Reca R, Janowska-Wieczorek A, Ratajczak MZ. Platelet-derived microparticles stimulate proliferation, survival, adhesion, and chemotaxis of hematopoietic cells. Exp hematology. 2002;30:450–459. doi: 10.1016/s0301-472x(02)00791-9. [DOI] [PubMed] [Google Scholar]
- Banadakoppa M, Chauhan MS, Havemann D, Balakrishnan M, Dominic JS, Yallampalli C. Spontaneous abortion is associated with elevated systemic C5a and reduced mRNA of complement inhibitory proteins in placenta. Clinical and experimental immunology. 2014;177:743–9. doi: 10.1111/cei.12371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SM, Greer IA, Pabinger I, Sofaer S, Hirsh J. Venous thromboembolism, thrombophilia, antithrombotic therapy, and pregnancy: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition) Chest. 2008;133:844S–886S. doi: 10.1378/chest.08-0761. [DOI] [PubMed] [Google Scholar]
- Beasley KM, Lovering AT, Gilbert JS. Decreased endothelial progenitor cells in preeclampsia and consequences for developmental programming. Hypertension. 2014;64:23–25. doi: 10.1161/HYPERTENSIONAHA.114.03200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ. 2007;335:974. doi: 10.1136/bmj.39335.385301.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birmingham DJ, Irshaid F, Nagaraja HN, Zou X, Tsao BP, Wu H, Yu CY, Hebert LA, Rovin BH. The complex nature of serum C3 and C4 as biomarkers of lupus renal flare. Lupus. 2010;19:1272–80. doi: 10.1177/0961203310371154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biryukov S, Stoute JA. Complement activation in malaria: friend or foe? Trends in molecular medicine. 2014;20:293–301. doi: 10.1016/j.molmed.2014.01.001. [DOI] [PubMed] [Google Scholar]
- Botto M, Kirschfink M, Macor P, Pickering MC, Wurzner R, Tedesco F. Complement in human diseases: Lessons from complement deficiencies. Mol Immunol. 2009;46:2774–83. doi: 10.1016/j.molimm.2009.04.029. [DOI] [PubMed] [Google Scholar]
- Bulla R, Agostinis C, Bossi F, Rizzi L, Debeus A, Tripodo C, Radillo O, De Seta F, Ghebrehiwet B, Tedesco F. Decidual endothelial cells express surface-bound C1q as a molecular bridge between endovascular trophoblast and decidual endothelium. Mol Immunol. 2008;45:2629–40. doi: 10.1016/j.molimm.2007.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulla R, Bossi F, Agostinis C, Radillo O, Colombo F, De Seta F, Tedesco F. Complement production by trophoblast cells at the feto-maternal interface. Journal of reproductive immunology. 2009;82:119–25. doi: 10.1016/j.jri.2009.06.124. [DOI] [PubMed] [Google Scholar]
- Bulla R, Bossi F, Tedesco F. The complement system at the embryo implantation site: friend or foe? Frontiers in immunology. 2012;3:55. doi: 10.3389/fimmu.2012.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burwick RM, Burwick NR, Feinberg BB. Eculizumab fails to inhibit generation of C5a in vivo. Blood. 2014a;124:3502–3. doi: 10.1182/blood-2014-07-589366. [DOI] [PubMed] [Google Scholar]
- Burwick RM, Easter SR, Dawood HY, Yamamoto HS, Fichorova RN, Feinberg BB. Complement activation and kidney injury molecule-1-associated proximal tubule injury in severe preeclampsia. Hypertension. 2014b;64:833–8. doi: 10.1161/HYPERTENSIONAHA.114.03456. [DOI] [PubMed] [Google Scholar]
- Burwick RM, Feinberg BB. Eculizumab for the treatment of preeclampsia/HELLP syndrome. Placenta. 2013;34:201–3. doi: 10.1016/j.placenta.2012.11.014. [DOI] [PubMed] [Google Scholar]
- Burwick RM, Fichorova RN, Dawood HY, Yamamoto HS, Feinberg BB. Urinary excretion of c5b-9 in severe preeclampsia: tipping the balance of complement activation in pregnancy. Hypertension. 2013;62:1040–5. doi: 10.1161/HYPERTENSIONAHA.113.01420. [DOI] [PubMed] [Google Scholar]
- Buurma A, Cohen D, Veraar K, Schonkeren D, Claas FH, Bruijn JA, Bloemenkamp KW, Baelde HJ. Preeclampsia is characterized by placental complement dysregulation. Hypertension. 2012;60:1332–7. doi: 10.1161/HYPERTENSIONAHA.112.194324. [DOI] [PubMed] [Google Scholar]
- Buyon JP, Cronstein BN, Morris M, Tanner M, Weissmann G. Serum complement values (C3 and C4) to differentiate between systemic lupus activity and pre-eclampsia. The American journal of medicine. 1986;81:194–200. doi: 10.1016/0002-9343(86)90251-2. [DOI] [PubMed] [Google Scholar]
- Caprioli J, Noris M, Brioschi S, Pianetti G, Castelletti F, Bettinaglio P, Mele C, Bresin E, Cassis L, Gamba S, Porrati F, Bucchioni S, Monteferrante G, Fang CJ, Liszewski MK, Kavanagh D, Atkinson JP, Remuzzi G. Genetics of HUS: the impact of MCP, CFH, and IF mutations on clinical presentation, response to treatment, and outcome. Blood. 2006;108:1267–79. doi: 10.1182/blood-2005-10-007252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataland SR, Holers VM, Geyer S, Yang S, Wu HM. Biomarkers of terminal complement activation confirm the diagnosis of aHUS and differentiate aHUS from TTP. Blood. 2014;123:3733–8. doi: 10.1182/blood-2013-12-547067. [DOI] [PubMed] [Google Scholar]
- Chaouat G, Petitbarat M, Bulla R, Dubanchet S, Valdivia K, Ledee N, Steffen T, Jensenius JC, Tedesco F. Early regulators in abortion and implications for a preeclampsia model. Journal of reproductive immunology. 2009;82:131–40. doi: 10.1016/j.jri.2009.08.004. [DOI] [PubMed] [Google Scholar]
- Chiu YY, Nisihara RM, Wurzner R, Kirschfink M, de Messias-Reason IJ. SC5b-9 is the most sensitive marker in assessing disease activity in Brazilian SLE patients. Journal of investigational allergology & clinical immunology. 1998;8:239–44. [PubMed] [Google Scholar]
- Chow WN, Lee YL, Wong PC, Chung MK, Lee KF, Yeung WS. Complement 3 deficiency impairs early pregnancy in mice. Molecular reproduction and development. 2009;76:647–55. doi: 10.1002/mrd.21013. [DOI] [PubMed] [Google Scholar]
- Cohn AC, MacNeil JR, Clark TA, Ortega-Sanchez IR, Briere EZ, Meissner HC, Baker CJ, Messonnier NE. Prevention and control of meningococcal disease: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. Recommendations and reports: Morbidity and mortality weekly report. Recommendations and reports/Centers for Disease Control. 2013;62:1–28. [PubMed] [Google Scholar]
- Conroy AL, McDonald CR, Silver KL, Liles WC, Kain KC. Complement activation: a critical mediator of adverse fetal outcomes in placental malaria? Trends in parasitology. 2011;27:294–9. doi: 10.1016/j.pt.2011.02.005. [DOI] [PubMed] [Google Scholar]
- Conroy AL, Silver KL, Zhong K, Rennie M, Ward P, Sarma JV, Molyneux ME, Sled J, Fletcher JF, Rogerson S, Kain KC. Complement activation and the resulting placental vascular insufficiency drives fetal growth restriction associated with placental malaria. Cell host & microbe. 2013;13:215–26. doi: 10.1016/j.chom.2013.01.010. [DOI] [PubMed] [Google Scholar]
- Creanga AA, Berg CJ, Syverson C, Seed K, Bruce FC, Callaghan WM. Pregnancy-related mortality in the United States, 2006–2010. Obstet Gynecol. 2015;125:5–12. doi: 10.1097/AOG.0000000000000564. [DOI] [PubMed] [Google Scholar]
- Crispi F, Dominguez C, Llurba E, Martin-Gallan P, Cabero L, Gratacos E. Placental angiogenic growth factors and uterine artery Doppler findings for characterization of different subsets in preeclampsia and in isolated intrauterine growth restriction. Am J Obstet Gynecol. 2006;195:201–7. doi: 10.1016/j.ajog.2006.01.014. [DOI] [PubMed] [Google Scholar]
- Crovetto F, Borsa N, Acaia B, Nishimura C, Frees K, Smith RJ, Peyvandi F, Palla R, Cugno M, Tedeschi S, Castorina P, Somigliana E, Ardissino G, Fedele L. The genetics of the alternative pathway of complement in the pathogenesis of HELLP syndrome. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2012;25:2322–5. doi: 10.3109/14767058.2012.694923. [DOI] [PubMed] [Google Scholar]
- Cunningham DS, Tichenor JR., Jr Decay-accelerating factor protects human trophoblast from complement-mediated attack. Clinical immunology and immunopathology. 1995;74:156–61. doi: 10.1006/clin.1995.1023. [DOI] [PubMed] [Google Scholar]
- Dantant M, Rivat C, Gilbert D, Fontaine M, Cavelier B, Godin M, Fillastre JP. Selective deficiencies in complement component: a family with hereditary C2 deficiency. Biomedicine/[publiee pour l’A.A.I.C.I.G] 1978;28:185–90. [PubMed] [Google Scholar]
- Dechend R, Gratze P, Wallukat G, Shagdarsuren E, Plehm R, Brasen JH, Fiebeler A, Schneider W, Caluwaerts S, Vercruysse L, Pijnenborg R, Luft FC, Muller DN. Agonistic autoantibodies to the AT1 receptor in a transgenic rat model of preeclampsia. Hypertension. 2005;45:742–6. doi: 10.1161/01.HYP.0000154785.50570.63. [DOI] [PubMed] [Google Scholar]
- Derzsy Z, Prohaszka Z, Rigo J, Jr, Fust G, Molvarec A. Activation of the complement system in normal pregnancy and preeclampsia. Mol Immunol. 2010;47:1500–6. doi: 10.1016/j.molimm.2010.01.021. [DOI] [PubMed] [Google Scholar]
- Dixit R, Krieg AM, Atkinson JP. Thrombotic thrombocytopenic purpura developing during pregnancy in a C2-deficient patient with a history of systemic lupus erythematosus. Arthritis Rheum. 1985;28:341–4. doi: 10.1002/art.1780280316. [DOI] [PubMed] [Google Scholar]
- Espinoza J, Romero R, Nien JK, Gomez R, Kusanovic JP, Goncalves LF, Medina L, Edwin S, Hassan S, Carstens M, Gonzalez R. Identification of patients at risk for early onset and/or severe preeclampsia with the use of uterine artery Doppler velocimetry and placental growth factor. Am J Obstet Gynecol. 2007;196:326.e1–13. doi: 10.1016/j.ajog.2006.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fakhouri F, Jablonski M, Lepercq J, Blouin J, Benachi A, Hourmant M, Pirson Y, Durrbach A, Grunfeld JP, Knebelmann B, Fremeaux-Bacchi V. Factor H, membrane cofactor protein, and factor I mutations in patients with hemolysis, elevated liver enzymes, and low platelet count syndrome. Blood. 2008;112:4542–5. doi: 10.1182/blood-2008-03-144691. [DOI] [PubMed] [Google Scholar]
- Fakhouri F, Roumenina L, Provot F, Sallee M, Caillard S, Couzi L, Essig M, Ribes D, Dragon-Durey MA, Bridoux F, Rondeau E, Fremeaux-Bacchi V. Pregnancy-associated hemolytic uremic syndrome revisited in the era of complement gene mutations. J Am Soc Nephrol. 2010;21:859–67. doi: 10.1681/ASN.2009070706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang CJ, Fremeaux-Bacchi V, Liszewski MK, Pianetti G, Noris M, Goodship TH, Atkinson JP. Membrane cofactor protein mutations in atypical hemolytic uremic syndrome (aHUS), fatal Stx-HUS, C3 glomerulonephritis, and the HELLP syndrome. Blood. 2008a;111:624–32. doi: 10.1182/blood-2007-04-084533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang CJ, Richards A, Liszewski MK, Kavanagh D, Atkinson JP. Advances in understanding of pathogenesis of aHUS and HELLP. British journal of haematology. 2008b;143:336–48. doi: 10.1111/j.1365-2141.2008.07324.x. [DOI] [PubMed] [Google Scholar]
- Faulk WP, Jarret R, Keane M, Johnson PM, Boackle RJ. Immunological studies of human placentae: complement components in immature and mature chorionic villi. Clinical and experimental immunology. 1980;40:299–305. [PMC free article] [PubMed] [Google Scholar]
- Faulk WP, Johnson PM. Immunological studies of human placentae: identification and distribution of proteins in mature chorionic villi. Clinical and experimental immunology. 1977;27:365–75. [PMC free article] [PubMed] [Google Scholar]
- Fieni S, Bonfanti L, Gramellini D, Benassi L, Delsignore R. Clinical management of paroxysmal nocturnal hemoglobinuria in pregnancy: a case report and updated review. Obstetrical & gynecological survey. 2006;61:593–601. doi: 10.1097/01.ogx.0000234794.27485.59. [DOI] [PubMed] [Google Scholar]
- Figueroa JE, Densen P. Infectious diseases associated with complement deficiencies. Clinical microbiology reviews. 1991;4:359–95. doi: 10.1128/cmr.4.3.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch AM, Wong AK, Paczkowski NJ, Wadi SK, Craik DJ, Fairlie DP, Taylor SM. Low-molecular-weight peptidic and cyclic antagonists of the receptor for the complement factor C5a. Journal of medicinal chemistry. 1999;42:1965–74. doi: 10.1021/jm9806594. [DOI] [PubMed] [Google Scholar]
- Friedman AM, Cleary KL. Prediction and prevention of ischemic placental disease. Seminars in perinatology. 2014;38:177–82. doi: 10.1053/j.semperi.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Garite TJ, Clark R, Thorp JA. Intrauterine growth restriction increases morbidity and mortality among premature neonates. Am J Obstet Gynecol. 2004;191:481–7. doi: 10.1016/j.ajog.2004.01.036. [DOI] [PubMed] [Google Scholar]
- Garlatti V, Chouquet A, Lunardi T, Vives R, Paidassi H, Lortat-Jacob H, Thielens NM, Arlaud GJ, Gaboriaud C. Cutting edge: C1q binds deoxyribose and heparan sulfate through neighboring sites of its recognition domain. J Immunol. 2010;185:808–12. doi: 10.4049/jimmunol.1000184. [DOI] [PubMed] [Google Scholar]
- Ghidini A. Overview of low molecular weight heparin for preventative treatment of adverse obstetric outcomes related to abnormal placentation. Prenatal diagnosis. 2014;34:649–54. doi: 10.1002/pd.4385. [DOI] [PubMed] [Google Scholar]
- Girardi G. Complement inhibition keeps mothers calm and avoids fetal rejection. Immunological investigations. 2008;37:645–59. doi: 10.1080/08820130802191615. [DOI] [PubMed] [Google Scholar]
- Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, Hollmann TJ, Casali P, Caroll MC, Wetsel RA, Lambris JD, Holers VM, Salmon JE. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest. 2003;112:1644–54. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi G, Fraser J, Lennen R, Vontell R, Jansen M, Hutchison G. Imaging of activated complement using ultrasmall superparamagnetic iron oxide particles (USPIO) - conjugated vectors: an in vivo in utero non-invasive method to predict placental insufficiency and abnormal fetal brain development. Molecular psychiatry. 2014 doi: 10.1038/mp.2014.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nature medicine. 2004;10:1222–6. doi: 10.1038/nm1121. [DOI] [PubMed] [Google Scholar]
- Girardi G, Yarilin D, Thurman JM, Holers VM, Salmon JE. Complement activation induces dysregulation of angiogenic factors and causes fetal rejection and growth restriction. J Exp Med. 2006;203:2165–75. doi: 10.1084/jem.20061022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldenberg RL, Culhane JF, Iams JD, Romero R. Epidemiology and causes of preterm birth. Lancet. 2008;371:75–84. doi: 10.1016/S0140-6736(08)60074-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JM, Dong Z, Romero R, Girardi G. Cervical remodeling/ripening at term and preterm delivery: the same mechanism initiated by different mediators and different effector cells. PloS one. 2011a;6:e26877. doi: 10.1371/journal.pone.0026877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JM, Franzke CW, Yang F, Romero R, Girardi G. Complement activation triggers metalloproteinases release inducing cervical remodeling and preterm birth in mice. Am J Pathol. 2011b;179:838–49. doi: 10.1016/j.ajpath.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez JM, Romero R, Girardi G. Comparison of the mechanisms responsible for cervical remodeling in preterm and term labor. Journal of reproductive immunology. 2013;97:112–9. doi: 10.1016/j.jri.2012.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gou SJ, Yuan J, Wang C, Zhao MH, Chen M. Alternative complement pathway activation products in urine and kidneys of patients with ANCA-associated GN. Clinical journal of the American Society of Nephrology: CJASN. 2013;8:1884–91. doi: 10.2215/CJN.02790313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grumach AS, Kirschfink M. Are complement deficiencies really rare? Overview on prevalence, clinical importance and modern diagnostic approach. Mol Immunol. 2014;61:110–7. doi: 10.1016/j.molimm.2014.06.030. [DOI] [PubMed] [Google Scholar]
- Guseh SH, Feinberg BB, Dawood HY, Yamamoto HS, Fichorova RN, Burwick RM. Urinary Excretion of C5b-9 is Associated With the Anti-Angiogenic State in Severe Preeclampsia. Am J Reprod Immunol. 2014 doi: 10.1111/aji.12349. [DOI] [PubMed] [Google Scholar]
- Haeger M, Unander M, Bengtsson A. Enhanced anaphylatoxin and terminal C5b-9 complement complex formation in patients with the syndrome of hemolysis, elevated liver enzymes, and low platelet count. Obstet Gynecol. 1990;76:698–702. [PubMed] [Google Scholar]
- Hallstensen RF, Bergseth G, Foss S, Jaeger S, Gedde-Dahl T, Holt J, Christiansen D, Lau C, Brekke OL, Armstrong E, Stefanovic V, Andersen JT, Sandlie I, Mollnes TE. Eculizumab treatment during pregnancy does not affect the complement system activity of the newborn. Immunobiology. 2014 doi: 10.1016/j.imbio.2014.11.003. [DOI] [PubMed] [Google Scholar]
- Hellerud BC, Aase A, Herstad TK, Naess LM, Kristiansen LH, Troseid AM, Harboe M, Lappegard KT, Brandtzaeg P, Hoiby EA, Mollnes TE. Critical roles of complement and antibodies in host defense mechanisms against Neisseria meningitidis as revealed by human complement genetic deficiencies. Infect Immun. 2010;78:802–9. doi: 10.1128/IAI.01044-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hering L, Herse F, Verlohren S, Park JK, Wellner M, Qadri F, Pijnenborg R, Staff AC, Huppertz B, Muller DN, Luft FC, Dechend R. Trophoblasts reduce the vascular smooth muscle cell proatherogenic response. Hypertension. 2008;51:554–9. doi: 10.1161/HYPERTENSIONAHA.107.102905. [DOI] [PubMed] [Google Scholar]
- Holmes CH, Simpson KL, Okada H, Okada N, Wainwright SD, Purcell DF, Houlihan JM. Complement regulatory proteins at the feto-maternal interface during human placental development: distribution of CD59 by comparison with membrane cofactor protein (CD46) and decay accelerating factor (CD55) Eur J Immunol. 1992;22:1579–85. doi: 10.1002/eji.1830220635. [DOI] [PubMed] [Google Scholar]
- Holmes CH, Simpson KL, Wainwright SD, Tate CG, Houlihan JM, Sawyer IH, Rogers IP, Spring FA, Anstee DJ, Tanner MJ. Preferential expression of the complement regulatory protein decay accelerating factor at the fetomaternal interface during human pregnancy. J Immunol. 1990;144:3099–105. [PubMed] [Google Scholar]
- Hsi BL, Hunt JS, Atkinson JP. Differential expression of complement regulatory proteins on subpopulations of human trophoblast cells. Journal of reproductive immunology. 1991;19:209–23. doi: 10.1016/0165-0378(91)90036-p. [DOI] [PubMed] [Google Scholar]
- Huber-Lang M, Sarma JV, Zetoune FS, Rittirsch D, Neff TA, McGuire SR, Lambris JD, Warner RL, Flierl MA, Hoesel LM, Gebhard F, Younger JG, Drouin SM, Wetsel RA, Ward PA. Generation of C5a in the absence of C3: a new complement activation pathway. Nature medicine. 2006;12:682–7. doi: 10.1038/nm1419. [DOI] [PubMed] [Google Scholar]
- Iams JD. Preterm birth categories-labels with consequences. Am J Obstet Gynecol. 2014;210:97–8. doi: 10.1016/j.ajog.2013.10.013. [DOI] [PubMed] [Google Scholar]
- Irving FC. A Study of Five Hundred Consecutive Cases of Pre-Eclampsia. Canadian Medical Association journal. 1939;40:137–40. [PMC free article] [PubMed] [Google Scholar]
- Jonsson G, Sjoholm AG, Truedsson L, Bengtsson AA, Braconier JH, Sturfelt G. Rheumatological manifestations, organ damage and autoimmunity in hereditary C2 deficiency. Rheumatology (Oxford) 2007;46:1133–9. doi: 10.1093/rheumatology/kem023. [DOI] [PubMed] [Google Scholar]
- Kelly R, Arnold L, Richards S, Hill A, Bomken C, Hanley J, Loughney A, Beauchamp J, Khursigara G, Rother RP, Chalmers E, Fyfe A, Fitzsimons E, Nakamura R, Gaya A, Risitano AM, Schubert J, Norfolk D, Simpson N, Hillmen P. The management of pregnancy in paroxysmal nocturnal haemoglobinuria on long term eculizumab. British journal of haematology. 2010;149:446–50. doi: 10.1111/j.1365-2141.2010.08099.x. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Zhou L, Miwa T, Song WC. Genetic and therapeutic targeting of properdin in mice prevents complement-mediated tissue injury. J Clin Invest. 2010;120:3545–54. doi: 10.1172/JCI41782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitzmiller JL, Stoneburner L, Yelenosky PF, Lucas WE. Serum complement in normal pregnancy and pre-eclampsia. Am J Obstet Gynecol. 1973;117:312–5. doi: 10.1016/0002-9378(73)90031-8. [DOI] [PubMed] [Google Scholar]
- Kolev M, Le Friec G, Kemper C. Complement - tapping into new sites and effector systems. Nat Rev Immunol. 2014;14:811–20. doi: 10.1038/nri3761. [DOI] [PubMed] [Google Scholar]
- Kupferminc M, Rimon E, Many A, Maslovitz S, Lessing JB, Gamzu R. Low molecular weight heparin versus no treatment in women with previous severe pregnancy complications and placental findings without thrombophilia. Blood coagulation & fibrinolysis: an international journal in haemostasis and thrombosis. 2011;22:123–6. doi: 10.1097/MBC.0b013e328343315c. [DOI] [PubMed] [Google Scholar]
- Kusakabe K, Naka M, Ito Y, Eid N, Otsuki Y. Regulation of natural-killer cell cytotoxicity and enhancement of complement factors in the spontaneously aborted mouse placenta. Fertility and sterility. 2008;90:1451–9. doi: 10.1016/j.fertnstert.2007.07.1331. [DOI] [PubMed] [Google Scholar]
- LaMarca B, Cornelius D, Wallace K. Elucidating immune mechanisms causing hypertension during pregnancy. Physiology (Bethesda) 2013;28:225–33. doi: 10.1152/physiol.00006.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee YL, Cheong AW, Chow WN, Lee KF, Yeung WS. Regulation of complement-3 protein expression in human and mouse oviducts. Molecular reproduction and development. 2009;76:301–8. doi: 10.1002/mrd.20955. [DOI] [PubMed] [Google Scholar]
- Legendre CM, Licht C, Muus P, Greenbaum LA, Babu S, Bedrosian C, Bingham C, Cohen DJ, Delmas Y, Douglas K, Eitner F, Feldkamp T, Fouque D, Furman RR, Gaber O, Herthelius M, Hourmant M, Karpman D, Lebranchu Y, Mariat C, Menne J, Moulin B, Nurnberger J, Ogawa M, Remuzzi G, Richard T, Sberro-Soussan R, Severino B, Sheerin NS, Trivelli A, Zimmerhackl LB, Goodship T, Loirat C. Terminal complement inhibitor eculizumab in atypical hemolytic-uremic syndrome. N Engl J Med. 2013;368:2169–81. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- Lewandowski AJ, Davis EF, Yu G, Digby JE, Boardman H, Whitworth P, Singhal A, Lucas A, McCormick K, Shore AC, Leeson P. Elevated Blood Pressure in Preterm-Born Offspring Associates With a Distinct Antiangiogenic State and Microvascular Abnormalities in Adult Life. Hypertension. 2014 doi: 10.1161/HYPERTENSIONAHA.114.04662. [DOI] [PubMed] [Google Scholar]
- Lewandowski AJ, Leeson P. Preeclampsia, prematurity and cardiovascular health in adult life. Early human development. 2014;90:725–729. doi: 10.1016/j.earlhumdev.2014.08.012. [DOI] [PubMed] [Google Scholar]
- Lillegard KE, Johnson AC, Lojovich SJ, Bauer AJ, Marsh HC, Gilbert JS, Regal JF. Complement activation is critical for placental ischemia-induced hypertension in the rat. Mol Immunol. 2013;56:91–7. doi: 10.1016/j.molimm.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillegard KE, Loeks-Johnson AC, Opacich JW, Peterson JM, Bauer AJ, Elmquist BJ, Regal RR, Gilbert JS, Regal JF. Differential effects of complement activation products c3a and c5a on cardiovascular function in hypertensive pregnant rats. J Pharmacol Exp Ther. 2014;351:344–51. doi: 10.1124/jpet.114.218123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loirat C, Fremeaux-Bacchi V. Atypical hemolytic uremic syndrome. Orphanet journal of rare diseases. 2011;6:60. doi: 10.1186/1750-1172-6-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lokki AI, Heikkinen-Eloranta J, Jarva H, Saisto T, Lokki ML, Laivuori H, Meri S. Complement activation and regulation in preeclamptic placenta. Frontiers in immunology. 2014;5:Article 312. doi: 10.3389/fimmu.2014.00312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lood C, Tyden H, Gullstrand B, Sturfelt G, Jonsen A, Truedsson L, Bengtsson AA. Platelet activation and anti-phospholipid antibodies collaborate in the activation of the complement system on platelets in systemic lupus erythematosus. PloS one. 2014;9:e99386. doi: 10.1371/journal.pone.0099386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch AM, Gibbs RS, Murphy JR, Byers T, Neville MC, Giclas PC, Salmon JE, Van Hecke TM, Holers VM. Complement activation fragment Bb in early pregnancy and spontaneous preterm birth. Am J Obstet Gynecol. 2008;199:354.e1–8. doi: 10.1016/j.ajog.2008.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch AM, Gibbs RS, Murphy JR, Giclas PC, Salmon JE, Holers VM. Early elevations of the complement activation fragment C3a and adverse pregnancy outcomes. Obstet Gynecol. 2011;117:75–83. doi: 10.1097/AOG.0b013e3181fc3afa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao D, Wu X, Deppong C, Friend LD, Dolecki G, Nelson DM, Molina H. Negligible role of antibodies and C5 in pregnancy loss associated exclusively with C3-dependent mechanisms through complement alternative pathway. Immunity. 2003;19:813–22. doi: 10.1016/s1074-7613(03)00321-2. [DOI] [PubMed] [Google Scholar]
- Martel C, Granger CB, Ghitescu M, Stebbins A, Fortier A, Armstrong PW, Bonnefoy A, Theroux P. Pexelizumab fails to inhibit assembly of the terminal complement complex in patients with ST-elevation myocardial infarction undergoing primary percutaneous coronary intervention. Insight from a substudy of the Assessment of Pexelizumab in Acute Myocardial Infarction (APEX-AMI) trial. American heart journal. 2012;164:43–51. doi: 10.1016/j.ahj.2012.04.007. [DOI] [PubMed] [Google Scholar]
- Marto N, Bertolaccini ML, Calabuig E, Hughes GR, Khamashta MA. Anti-C1q antibodies in nephritis: correlation between titres and renal disease activity and positive predictive value in systemic lupus erythematosus. Annals of the rheumatic diseases. 2005;64:444–8. doi: 10.1136/ard.2004.024943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer AE, Parks GD. An AGM model for changes in complement during pregnancy: neutralization of influenza virus by serum is diminished in late third trimester. PloS one. 2014;9:e112749. doi: 10.1371/journal.pone.0112749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy JJ, Gutman CE, Shaffer CM, Busch TD, Puttonen H, Teramo K, Murray JC, Hallman M, Muglia LJ. Maternal coding variants in complement receptor 1 and spontaneous idiopathic preterm birth. Human genetics. 2013;132:935–42. doi: 10.1007/s00439-013-1304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer O, Hauptmann G, Tappeiner G, Ochs HD, Mascart-Lemone F. Genetic deficiency of C4, C2 or C1q and lupus syndromes. Association with anti-Ro (SS-A) antibodies. Clinical and experimental immunology. 1985;62:678–84. [PMC free article] [PubMed] [Google Scholar]
- Millar KG, Mills P. C 1 3 and IgG levels in mothers and babies at delivery. Obstet Gynecol. 1972;39:527–32. [PubMed] [Google Scholar]
- Mohlin FC, Mercier E, Fremeaux-Bacchi V, Liszewski MK, Atkinson JP, Gris JC, Blom AM. Analysis of genes coding for CD46, CD55, and C4b-binding protein in patients with idiopathic, recurrent, spontaneous pregnancy loss. Eur J Immunol. 2013;43:1617–29. doi: 10.1002/eji.201243196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore MP, Redman CW. Case-control study of severe pre-eclampsia of early onset. Br Med J (Clin Res Ed) 1983;287:580–3. doi: 10.1136/bmj.287.6392.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muehlenbachs A, Mutabingwa TK, Edmonds S, Fried M, Duffy PE. Hypertension and maternal-fetal conflict during placental malaria. PLoS medicine. 2006;3:e446. doi: 10.1371/journal.pmed.0030446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Hernandez R, Miranda ML, Stiefel P, Lin RZ, Praena-Fernandez JM, Dominguez-Simeon MJ, Villar J, Moreno-Luna R, Melero-Martin JM. Decreased level of cord blood circulating endothelial colony-forming cells in preeclampsia. Hypertension. 2014;64:165–171. doi: 10.1161/HYPERTENSIONAHA.113.03058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik A, Sharma S, Quigg RJ. Complement regulation in renal disease models. Seminars in nephrology. 2013;33:575–85. doi: 10.1016/j.semnephrol.2013.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndao CT, Dumont A, Fievet N, Doucoure S, Gaye A, Lehesran JY. Placental malarial infection as a risk factor for hypertensive disorders during pregnancy in Africa: a case-control study in an urban area of Senegal, West Africa. Am J Epidemiol. 2009;170:847–53. doi: 10.1093/aje/kwp207. [DOI] [PubMed] [Google Scholar]
- Nelson DB, Ziadie MS, McIntire DD, Rogers BB, Leveno KJ. Placental pathology suggesting that preeclampsia is more than one disease. Am J Obstet Gynecol. 2014;210:66.e1–7. doi: 10.1016/j.ajog.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Newnham JP, Dickinson JE, Hart RJ, Pennell CE, Arrese CA, Keelan JA. Strategies to prevent preterm birth. Frontiers in immunology. 2014;5:584. doi: 10.3389/fimmu.2014.00584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHLBI. Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000;183:S1–S22. [PubMed] [Google Scholar]
- Nishikori K, Noma J, Hirakawa S, Amano T, Kudo T. The change of membrane complement regulatory protein in chorion of early pregnancy. Clinical immunology and immunopathology. 1993;69:167–74. doi: 10.1006/clin.1993.1166. [DOI] [PubMed] [Google Scholar]
- Noris M, Remuzzi G. Genetics and genetic testing in hemolytic uremic syndrome/thrombotic thrombocytopenic purpura. Seminars in nephrology. 2010;30:395–408. doi: 10.1016/j.semnephrol.2010.06.006. [DOI] [PubMed] [Google Scholar]
- Nowicki S, Izban MG, Pawelczyk E, Agboto VK, Pratap S, Olson G, Nowicki B. Preterm labor: CD55 in maternal blood leukocytes. Am J Reprod Immunol. 2009;61:360–7. doi: 10.1111/j.1600-0897.2009.00702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberkersch R, Attorresi AI, Calabrese GC. Low-molecular-weight heparin inhibition in classical complement activation pathway during pregnancy. Thrombosis research. 2010;125:e240–5. doi: 10.1016/j.thromres.2009.11.030. [DOI] [PubMed] [Google Scholar]
- Pacheco LD, Hankins GD, Costantine MM, Anderson GD, Pawelczyk E, Nowicki S, Nowicki BJ. The role of human decay-accelerating factor in the pathogenesis of preterm labor. American journal of perinatology. 2011;28:565–70. doi: 10.1055/s-0031-1274510. [DOI] [PubMed] [Google Scholar]
- Paruk F, Moodley J. Maternal and neonatal outcome in early- and late-onset pre-eclampsia. Seminars in neonatology: SN. 2000;5:197–207. doi: 10.1053/siny.2000.0023. [DOI] [PubMed] [Google Scholar]
- Pedroni SM, Gonzalez JM, Wade J, Jansen MA, Serio A, Marshall I, Lennen RJ, Girardi G. Complement inhibition and statins prevent fetal brain cortical abnormalities in a mouse model of preterm birth. Biochimica et biophysica acta. 2014;1842:107–15. doi: 10.1016/j.bbadis.2013.10.011. [DOI] [PubMed] [Google Scholar]
- Peffault de Latour R, Fremeaux-Bacchi V, Porcher R, Xhaard A, Rosain J, Cadena Castaneda D, Vieira-Martins P, Roncelin S, Rodriguez-Otero P, Plessier A, Sicre de Fontbrune F, Abbes S, Robin M, Socie G. Assessing complement blockade in patients with paroxysmal nocturnal hemoglobinuria receiving eculizumab. Blood. 2014 doi: 10.1182/blood-2014-03-560540. [DOI] [PubMed] [Google Scholar]
- Petersen BH, Lee TJ, Snyderman R, Brooks GF. Neisseria meningitidis and Neisseria gonorrhoeae bacteremia associated with C6, C7, or C8 deficiency. Annals of internal medicine. 1979;90:917–20. doi: 10.7326/0003-4819-90-6-917. [DOI] [PubMed] [Google Scholar]
- Pinheiro CC, Rayol P, Gozzani L, Reis LM, Zampieri G, Dias CB, Woronik V. The relationship of angiogenic factors to maternal and neonatal manifestations of early-onset and late-onset preeclampsia. Prenatal diagnosis. 2014;34:1084–92. doi: 10.1002/pd.4432. [DOI] [PubMed] [Google Scholar]
- Prall RH, Kantor FS. Serum complement in eclamptogenic toxemia. Am J Obstet Gynecol. 1966;95:530–3. doi: 10.1016/0002-9378(66)90145-1. [DOI] [PubMed] [Google Scholar]
- Pratap S, Brown LE, Izban MG, Nowicki S, Nowicki BJ. Accurate preterm labor diagnosis using a CD55-TLR4 combination biomarker model. Journal of biomedical science and engineering. 2013;6:253–257. doi: 10.4236/jbise.2013.63031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proctor LM, Arumugam TV, Shiels I, Reid RC, Fairlie DP, Taylor SM. Comparative anti-inflammatory activities of antagonists to C3a and C5a receptors in a rat model of intestinal ischaemia/reperfusion injury. Br J Pharmacol. 2004;142:756–64. doi: 10.1038/sj.bjp.0705819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qing X, Redecha PB, Burmeister MA, Tomlinson S, D’Agati VD, Davisson RL, Salmon JE. Targeted inhibition of complement activation prevents features of preeclampsia in mice. Kidney Int. 2011;79:331–9. doi: 10.1038/ki.2010.393. [DOI] [PubMed] [Google Scholar]
- Qu XW, Jilling T, Neerhof MG, Luo K, Hirsch E, Thaete LG. Unilateral uterine ischemia/reperfusion-induced bilateral fetal loss and fetal growth restriction in a murine model require intact complement component 5. Journal of reproductive immunology. 2012;95:27–35. doi: 10.1016/j.jri.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rai R, Cohen H, Dave M, Regan L. Randomised controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibodies) BMJ. 1997;314:253–7. doi: 10.1136/bmj.314.7076.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak J, Reca R, Kucia M, Majka M, Allendorf DJ, Baran JT, Janowska-Wieczorek A, Wetsel RA, Ross GD, Ratajczak MZ. Mobilization studies in mice deficient in either C3 or C3a receptor (C3aR) reveal a novel role for complement in retention of hematopoietic stem/progenitor cells in bone marrow. Blood. 2004;103:2071–2078. doi: 10.1182/blood-2003-06-2099. [DOI] [PubMed] [Google Scholar]
- Ray JG, Burows RF, Ginsberg JS, Burrows EA. Paroxysmal nocturnal hemoglobinuria and the risk of venous thrombosis: review and recommendations for management of the pregnant and nonpregnant patient. Haemostasis. 2000;30:103–17. doi: 10.1159/000022532. [DOI] [PubMed] [Google Scholar]
- Reca R, Mastellos D, Majka M, Marquez L, Ratajczak J, Franchini S, Glodek A, Honczarenko M, Spruce LA, Janowska-Wieczorek A, Lambris JD, Ratajczak MZ. Functional receptor for C3a anaphylatoxin is expressed by normal hematopoietic stem/progenitor cells, and C3a enhances their homing-related responses to SDF-1. Blood. 2003;101:3784–3793. doi: 10.1182/blood-2002-10-3233. [DOI] [PubMed] [Google Scholar]
- Redecha P, Tilley R, Tencati M, Salmon JE, Kirchhofer D, Mackman N, Girardi G. Tissue factor: a link between C5a and neutrophil activation in antiphospholipid antibody induced fetal injury. Blood. 2007;110:2423–31. doi: 10.1182/blood-2007-01-070631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richani K, Soto E, Romero R, Espinoza J, Chaiworapongsa T, Nien JK, Edwin S, Kim YM, Hong JS, Mazor M. Normal pregnancy is characterized by systemic activation of the complement system. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2005;17:239–45. doi: 10.1080/14767050500072722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Lambris JD. Complement in immune and inflammatory disorders: therapeutic interventions. J Immunol. 2013a;190:3839–47. doi: 10.4049/jimmunol.1203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricklin D, Lambris JD. Progress and Trends in Complement Therapeutics. Adv Exp Med Biol. 2013b;735:1–22. doi: 10.1007/978-1-4614-4118-2_1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risitano AM, Ricklin D, Huang Y, Reis ES, Chen H, Ricci P, Lin Z, Pascariello C, Raia M, Sica M, Del Vecchio L, Pane F, Lupu F, Notaro R, Resuello RR, DeAngelis RA, Lambris JD. Peptide inhibitors of C3 activation as a novel strategy of complement inhibition for the treatment of paroxysmal nocturnal hemoglobinuria. Blood. 2014;123:2094–101. doi: 10.1182/blood-2013-11-536573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JM. Pathophysiology of ischemic placental disease. Seminars in perinatology. 2014;38:139–145. doi: 10.1053/j.semperi.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romay-Penabad Z, Carrera Marin AL, Willis R, Weston-Davies W, Machin S, Cohen H, Brasier A, Gonzalez EB. Complement C5-inhibitor rEV576 (coversin) ameliorates in-vivo effects of antiphospholipid antibodies. Lupus. 2014;23:1324–6. doi: 10.1177/0961203314546022. [DOI] [PubMed] [Google Scholar]
- Romero R, Dey SK, Fisher SJ. Preterm labor: one syndrome, many causes. Science. 2014;345:760–5. doi: 10.1126/science.1251816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saadoun S, Waters P, Leite MI, Bennett JL, Vincent A, Papadopoulos MC. Neuromyelitis optica IgG causes placental inflammation and fetal death. J Immunol. 2013;191:2999–3005. doi: 10.4049/jimmunol.1301483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saigal S, Doyle LW. An overview of mortality and sequelae of preterm birth from infancy to adulthood. Lancet. 2008;371:261–9. doi: 10.1016/S0140-6736(08)60136-1. [DOI] [PubMed] [Google Scholar]
- Salmon JE, Heuser C, Triebwasser M, Liszewski MK, Kavanagh D, Roumenina L, Branch DW, Goodship T, Fremeaux-Bacchi V, Atkinson JP. Mutations in complement regulatory proteins predispose to preeclampsia: a genetic analysis of the PROMISSE cohort. PLoS medicine. 2011;8:e1001013. doi: 10.1371/journal.pmed.1001013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata E, Rajakumar A, Powers RW, Larkin RW, Gilmour C, Bodnar LM, Crombleholme WR, Ness RB, Roberts JM, Hubel CA. Soluble fms-like tyrosine kinase 1 is increased in preeclampsia but not in normotensive pregnancies with small-for-gestational-age neonates: relationship to circulating placental growth factor. The Journal of clinical endocrinology and metabolism. 2005;90:4895–903. doi: 10.1210/jc.2004-1955. [DOI] [PubMed] [Google Scholar]
- Singh J, Ahmed A, Girardi G. Role of complement component C1q in the onset of preeclampsia in mice. Hypertension. 2011;58:716–24. doi: 10.1161/HYPERTENSIONAHA.111.175919. [DOI] [PubMed] [Google Scholar]
- Soares MJ, Chakraborty D, Karim Rumi MA, Konno T, Renaud SJ. Rat placentation: an experimental model for investigating the hemochorial maternal-fetal interface. Placenta. 2012;33:233–43. doi: 10.1016/j.placenta.2011.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song WC. Crosstalk between complement and toll-like receptors. Toxicol Pathol. 2012;40:174–82. doi: 10.1177/0192623311428478. [DOI] [PubMed] [Google Scholar]
- Soto E, Romero R, Richani K, Espinoza J, Chaiworapongsa T, Nien JK, Edwin SS, Kim YM, Hong JS, Goncalves LF, Yeo L, Mazor M, Hassan SS, Kusanovic JP. Preeclampsia and pregnancies with small-for-gestational age neonates have different profiles of complement split products. The journal of maternal-fetal & neonatal medicine: the official journal of the European Association of Perinatal Medicine, the Federation of Asia and Oceania Perinatal Societies, the International Society of Perinatal Obstet. 2010;23:646–57. doi: 10.3109/14767050903301009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto E, Romero R, Richani K, Espinoza J, Nien JK, Chaiworapongsa T, Santolaya-Forgas J, Edwin SS, Mazor M. Anaphylatoxins in preterm and term labor. Journal of perinatal medicine. 2005;33:306–13. doi: 10.1515/JPM.2005.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stohl W, Hiepe F, Latinis KM, Thomas M, Scheinberg MA, Clarke A, Aranow C, Wellborne FR, Abud-Mendoza C, Hough DR, Pineda L, Migone TS, Zhong ZJ, Freimuth WW, Chatham WW. Belimumab reduces autoantibodies, normalizes low complement levels, and reduces select B cell populations in patients with systemic lupus erythematosus. Arthritis Rheum. 2012;64:2328–37. doi: 10.1002/art.34400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KE, Winkelstein JA. Prenatal diagnosis of heterozygous deficiency of the second component of complement. Clinical and diagnostic laboratory immunology. 1994;1:606–7. doi: 10.1128/cdli.1.5.606-607.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco F, Pausa M, Nardon E, Introna M, Mantovani A, Dobrina A. The cytolytically inactive terminal complement complex activates endothelial cells to express adhesion molecules and tissue factor procoagulant activity. J Exp Med. 1997;185:1619–27. doi: 10.1084/jem.185.9.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedesco F, Radillo O, Candussi G, Nazzaro A, Mollnes TE, Pecorari D. Immunohistochemical detection of terminal complement complex and S protein in normal and pre-eclamptic placentae. Clinical and experimental immunology. 1990;80:236–40. doi: 10.1111/j.1365-2249.1990.tb05240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tichenor JR, Bledsoe LB, Opsahl MS, Cunningham DS. Activation of complement in humans with a first-trimester pregnancy loss. Gynecologic and obstetric investigation. 1995;39:79–82. doi: 10.1159/000292384. [DOI] [PubMed] [Google Scholar]
- Usami M, Mitsunaga K, Miyajima A, Sunouchi M, Doi O. Complement component C3 functions as an embryotrophic factor in early postimplantation rat embryos. The International journal of developmental biology. 2010;54:1277–85. doi: 10.1387/ijdb.092993mu. [DOI] [PubMed] [Google Scholar]
- Vaisbuch E, Romero R, Erez O, Mazaki-Tovi S, Kusanovic JP, Soto E, Dong Z, Chaiworapongsa T, Kim SK, Ogge G, Pacora P, Yeo L, Hassan SS. Activation of the alternative pathway of complement is a feature of pre-term parturition but not of spontaneous labor at term. Am J Reprod Immunol. 2010;63:318–30. doi: 10.1111/j.1600-0897.2009.00800.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van de Geijn FE, Roos A, de Man YA, Laman JD, de Groot CJ, Daha MR, Hazes JM, Dolhain RJ. Mannose-binding lectin levels during pregnancy: a longitudinal study. Hum Reprod. 2007;22:362–71. doi: 10.1093/humrep/del392. [DOI] [PubMed] [Google Scholar]
- Veerbeek JH, Hermes W, Breimer AY, van Rijn BB, Koenen SV, Mol BW, Franx A, de Groot CJ, Koster MP. Cardiovascular Disease Risk Factors After Early-Onset Preeclampsia, Late-Onset Preeclampsia, and Pregnancy-Induced Hypertension. Hypertension. 2015 doi: 10.1161/HYPERTENSIONAHA.114.04850. [DOI] [PubMed] [Google Scholar]
- Vogel CW, Finnegan PW, Fritzinger DC. Humanized cobra venom factor: structure, activity, and therapeutic efficacy in preclinical disease models. Mol Immunol. 2014;61:191–203. doi: 10.1016/j.molimm.2014.06.035. [DOI] [PubMed] [Google Scholar]
- Wang W, Irani RA, Zhang Y, Ramin SM, Blackwell SC, Tao L, Kellems RE, Xia Y. Autoantibody-mediated complement C3a receptor activation contributes to the pathogenesis of preeclampsia. Hypertension. 2012;60:712–21. doi: 10.1161/HYPERTENSIONAHA.112.191817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wathen KA, Tuutti E, Stenman UH, Alfthan H, Halmesmaki E, Finne P, Ylikorkala O, Vuorela P. Maternal serum-soluble vascular endothelial growth factor receptor-1 in early pregnancy ending in preeclampsia or intrauterine growth retardation. The Journal of clinical endocrinology and metabolism. 2006;91:180–4. doi: 10.1210/jc.2005-1076. [DOI] [PubMed] [Google Scholar]
- Weisman HF, Bartow T, Leppo MK, Boyle MP, Marsh HC, Jr, Carson GR, Roux KH, Weisfeldt ML, Fearon DT. Recombinant soluble CR1 suppressed complement activation, inflammation, and necrosis associated with reperfusion of ischemic myocardium. Trans Assoc Am Physicians. 1990;103:64–72. [PubMed] [Google Scholar]
- Wells M, Bennett J, Bulmer JN, Jackson P, Holgate CS. Complement component deposition in uteroplacental (spiral) arteries in normal human pregnancy. Journal of reproductive immunology. 1987;12:125–35. doi: 10.1016/0165-0378(87)90040-4. [DOI] [PubMed] [Google Scholar]
- Xu C, Mao D, Holers VM, Palanca B, Cheng AM, Molina H. A critical role for murine complement regulator crry in fetomaternal tolerance. Science. 2000;287:498–501. doi: 10.1126/science.287.5452.498. [DOI] [PubMed] [Google Scholar]