Abstract
Background
Survival of patients with congenital heart disease has improved such that there are now more adults than children living with these conditions. Complex single ventricle congenital heart disease requiring Fontan palliation is associated with multiple risk factors for impaired bone accrual. Bone density and structure have not been characterized in these patients.
Methods
Tibia peripheral quantitative computed tomography (pQCT) was used to assess trabecular and cortical volumetric bone mineral density (vBMD), cortical dimensions, and calf muscle area in 43 Fontan participants (5–33 years old), a median of 10 years following Fontan palliation. pQCT outcomes were converted to sex- and race-specific Z-scores relative to age based on >700 healthy reference participants. Cortical dimensions and muscle area were further adjusted for tibia length.
Results
Height Z-scores were lower in Fontan compared to reference participants (mean±SD: −0.291.00 vs. 0.250.93, p<0.001); BMI Z-scores were similar (0.160.88 vs. 0.351.02, p=0.1). Fontan participants had lower trabecular vBMD Z-scores (−0.850.96 vs. 0.011.02, p<0.001); cortical vBMD Z-scores were similar (−0.170.98 vs. 0.001.00, p=0.27). Cortical dimensions were reduced with lower cortical area (−0.590.84 vs. 0.000.88, p<0.001) and periosteal circumference (−0.500.82 vs. 0.000.84, p<0.001) Z-scores, compared to reference participants. Calf muscle area Z-scores were lower in the Fontan participants (−0.450.98 vs. 0.000.96, p=0.003) and lower calf muscle area Z-scores were associated with smaller periosteal circumference Z-scores (R=0.62, p<0.001). Musculoskeletal deficits were not associated with age, Fontan characteristics, parathyroid hormone or vitamin D levels.
Conclusions
Children and young adults demonstrate low trabecular vBMD, cortical structure and muscle area following Fontan. Muscle deficits were associated with smaller periosteal dimensions. Future studies should determine the fracture implications of these deficits and identify interventions to promote musculoskeletal development.
Keywords: Congenital heart disease, Fontan, bone mineral density, peripheral quantitative computed tomography, muscle
1. INTRODUCTIONa
Over the four decades since its initial description, the Fontan operation has evolved as the final palliative procedure for various types of single ventricle heart disease. Through this total cavopulmonary connection, systemic venous blood flows passively to the lungs for oxygenation while the single ventricular mass supplies cardiac output to the body. Over the last forty years, outcomes following the Fontan have improved dramatically. Surgical mortality is now less than 5% [1–4], and transplant-free survival for the earliest Fontan patients is greater than 80% over a 15 to 20 year period [5]. As survival improves, focus has shifted toward the long-term consequences of this abnormal physiology characterized by elevated central venous pressure and often diminished cardiac output. These consequences are particularly important as there are now more adults than children living with congenital heart disease in the United States [6].
Fontan patients have multiple risk factors for poor growth, abnormal bone accrual, and muscle deficits, including hepatic dysfunction, vitamin D and other nutritional deficiencies, decreased weight-bearing activity, and treatment with medications including diuretics and anti-coagulants [7, 8]. Protein losing enteropathy (PLE) imposes additional risk factors; a recent report of 12 Fontan patients with PLE demonstrated a significant reduction in dual energy x-ray absorptiometry (DXA) areal bone mineral density (BMD) relative to age in association with glucocorticoid therapy and impaired growth [9].
While poor linear growth is well-documented in Fontan patients [10–13], bone studies are otherwise limited to a case series of six Fontan patients, demonstrating significant deficits in distal radius volumetric BMD (vBMD) [14]. We recently demonstrated substantial growth failure and deficits in appendicular lean mass, as measured by whole body DXA, in 46 children and young adults following Fontan palliation [15]. As muscles increase during growth, bones adapt by increasing cortical dimensions, with lifelong implications for bone strength. Consistent with this functional muscle-bone unit, we have demonstrated that greater muscle mass, muscle strength, and physical activity during childhood and adolescence are independently associated with cortical bone acquisition as a consequence of greater periosteal expansion in healthy children and adolescents [16, 17]. The relationship between muscle deficits and cortical bone structure has not been addressed in the high-risk Fontan population.
Tibia peripheral quantitative computed tomography (pQCT) provides three-dimensional measures of trabecular and cortical vBMD (g/cm3) and cortical bone structure that are highly correlated with fracture load [18]. Additionally, pQCT provides measures of calf muscle and fat cross-sectional area (CSA). This technique represents an important advance over DXA measures that are subject to the confounding effects of poor growth on areal BMD (g/cm2) [19] and fail to distinguish between trabecular and cortical bone density and structure. The objectives of this pQCT study were to characterize vBMD, cortical structure, and the muscle-bone unit in children, adolescents and young adults after Fontan palliation, and to identify risk factors for musculoskeletal abnormalities.
2. MATERIALS AND METHODS
2.1 Study Subjects
Fontan participants > 5 years of age were prospectively enrolled in this cross-sectional study of growth, nutrition, body composition, and bone health from July 2011 through October 2013, as previously described [15]. Subjects were eligible if they had single ventricle physiology and had undergone Fontan palliation. Exclusion criteria included: pregnancy, Fontan baffle obstruction, single lung physiology, moderate to severe chronic kidney disease (estimated glomerular filtration rate < 60 mL/min/1.73 m2), moderate to severe hepatic impairment (transaminases > 2 times the upper limit of normal), and inability to complete the study procedures due to significant developmental delay. As Fontan patients affected by PLE may be at increased risk of bone disease due to protein malabsorption, hypocalcemia, inflammation, and the medications used to treat disease symptoms, the data presented here excludes three participants with PLE. A subset of participants was recruited from an existing exercise cardiac magnetic resonance (CMR) protocol that was limited to Fontan participants ≥ 12 years of age. Fontan participants were compared with a cohort of 730 healthy reference participants (ages 5 to 30 years) from the greater Philadelphia area [17, 20–24]. The study protocol was approved by the Institutional Review Board at The Children’s Hospital of Philadelphia. Informed consent was obtained from participants >18 years of age, and assent as well as parental consent in those <18 years.
2.2 Demographic and Fontan Characteristics
Demographic characteristics and pertinent Fontan variables including cardiac anatomy, ventricular morphology, presence of heterotaxy syndrome or a genetic syndrome, date and type of Fontan, presence of a fenestration, medications, and supplements were obtained from the medical record and confirmed in patient interviews.
2.3 Anthropometry and Pubertal Development
Weight was measured using a digital scale (Scaltronix, White Plains, NY). Height was measured using a stadiometer (Holtain Ltd, Croswell, Crymych, United Kingdom) and tibia length using sliding calipers (Segmometer, Rosscraft Innovations, Vancouver, BC). Pubertal status (Tanner stage) was assessed with a validated self-assessment questionnaire [25].
2.4 Tibia pQCT
Bone and muscle measures in the left tibia were obtained by a Stratec XCT2000 pQCT device (Orthometrix, White Plains, NY) with a 12-detector unit, voxel size of 0.4 mm, slice thickness of 2.3 mm, and scan speed of 25 mm/seconds. Scans were analyzed with Stratec software version 5.50. A scout view was obtained to place the reference line at the proximal medial border of the distal tibia growth plate in participants with open growth plates and at the medial distal endplate in participants with fused growth plates, as previously described [17]. Measurements were obtained at 3%, 38%, and 66% of tibia length proximal to the reference line. Trabecular vBMD (mg/cm3) was measured at the 3% (metaphysis) site. Cortical vBMD (mg/cm3), cortical CSA (mm2), cortical thickness (mm), periosteal circumference (mm), and endosteal circumference (mm) were measured at the 38% (diaphysis) site. Muscle and fat CSA (mm2) were evaluated at the 66% site. The manufacturer's (Orthometrix) hydroxyapatite phantom was scanned daily for quality assurance. Our laboratory’s coefficients of variation ranged from 0.5% to 1.6% for pQCT outcomes.
2.5 Laboratory Studies
Serum albumin, ionized calcium, and intact parathyroid hormone (PTH) were measured in the clinical laboratory using standard techniques. Brain natriuretic peptide (BNP) was measured using a chemiluminescent microparticle immunoassay. Quantification of circulating 25 (OH) vitamin D was performed by tandem mass-spectrometry, as previously described [26, 27]. Insulin-like growth factor-1 (IGF-1) was measured by Esoterix Laboratory Services (Calabasas Hills, CA) using a radioimmunoassay. IGF-1 levels were converted to sex-, age-, and Tanner-specific Z-scores using Esoterix laboratory’s reference data; adult participants were considered to be 18 years old.
2.6 Non-invasive Cardiac Imaging
Echocardiograms and CMRs were reviewed when available. Echocardiograms were performed on a Phillips IE33 machine (Phillips, Andover, MA) according to our standard imaging protocol. Five or 8 MHz transducers were used according to patient’s size and acoustic windows. Qualitative ventricular function and the degree of atrioventricular valve regurgitation were obtained from the echocardiogram report. CMRs were performed on a 1.5T Avanto Magnetic Resonance Imaging scanner (Siemens Medical Solutions, Erlangen, Germany) with a 6-channel phased-array body coil using our standard imaging protocol. Phase contrast velocity mapping was used to determine the summed caval flow and indexed to body surface area.
2.7 Statistical Analysis
Height and body mass index (BMI, kg/m2) were converted to sex-specific Z-scores (standard deviation scores) relative to age using the 2000 National Center for Health Statistics (NCHS) growth data [28]. Height Z-scores in participants ≥ 20 years of age (the upper bound of the NCHS reference data) were generated relative to a 20 year old, and BMI Z-scores were limited to participants < 20 years of age. pQCT outcomes were converted to race- and sex-specific Z-scores using the LMS method (Chartmaker Program version 2.3) based on the reference participants [29]. This method accounts for the non-linearity, heteroscedasticity and skew of bone data with age in children, adolescents, and young adults. The trabecular and cortical vBMD outcomes were assessed relative to age. The cortical dimensions and muscle and fat CSA were highly correlated with tibia length (all p <0.001); therefore, Z-scores for these parameters were generated relative to age and further adjusted for tibia length for age-Z-score, as previously described [22, 30]. This adjustment addresses the significant linear growth failure observed in childhood chronic diseases. pQCT Z-scores were generated relative to age 30 in the two participants that were 32 and 33 years old. The mean Z-scores for all pQCT outcomes were 0.00 to 0.01 in the reference participants, with standard deviations ranging from 0.84 to 1.02.
Continuous variables were expressed as means ± standard deviation or median (range). As none of the growth or pQCT variables were skewed, group differences between Fontan and reference participants were assessed using Student’s t-test. The associations between pQCT Z-scores and Fontan characteristics were examined using Pearson or Spearman rank correlations (if not normally distributed) for continuous variables and comparisons of Z-scores according to categorical variables such as the presence of a systemic right ventricle or vitamin D deficiency [defined as 25(OH) vitamin D < 20 ng/mL consistent with the Institute of Medicine definition] [31]. The associations between pQCT calf muscle CSA and cortical dimension Z-scores were examined by linear regression. Analyses were performed using Stata 12.0 (Stata Corp., College Station, TX, USA). A p value < 0.05 was considered significant and two-sided tests were used throughout
3. RESULTS
3.1 Subject Characteristics
Table 1 summarizes the demographic and anthropometric characteristics of the 43 Fontan participants. Fontan participants had lower mean height Z-scores compared to reference participants (−0.291.00 vs. 0.250.93, p<0.001) and exhibited significantly delayed puberty, as previously described [15]. Fontan participants were older than reference participants within Tanner stages 2, 3, and 4, adjusted for sex and race (p<0.001). BMI Z-scores did not differ in Fontan participants compared with reference participants (0.160.88 vs. 0.351.02, p=0.1) among the 35 participants < 20 years of age.
Table 1.
Demographic and Anthropometric Characteristics of the Fontan Participants
| Variable | Fontan participants |
|---|---|
| N | 43 |
| Age, median (range) year | 12.8 (5.1–33.5) |
| Interval from Fontan, median (range) year | 9.8 (2–26.7) |
| Female, n (%) | 22 (51) |
| Race, n (%) | |
| White | 31 (72) |
| Black | 6 (14) |
| Other | 6 (14) |
| Tanner stage 1–2, n(%) | 21 (49) |
| Height Z-score, mean ± SD | −0.29±1.00 |
| Range | −4.30 to 0.95 |
| Body mass index Z-score, mean ± SDa | 0.16±0.88 |
| Range | −2.49 to 2.24 |
SD, standard deviation
Limited to participants less than 20 years of age.
The disease-specific characteristics of the Fontan participants are summarized in Table 2. Single right ventricular physiology was most common (42%), and the most common cardiac diagnosis was hypoplastic left heart syndrome (33%). Four patients (9%) had heterotaxy syndrome. One patient had Turner syndrome; another had VACTERL association. There were no patients on systemic steroids at the time of the study. The median IGF-1 Z-score was 0.06 (−2.72 to 3.00). Eighteen patients (42%) had PTH levels > 53 pg/mL, the upper limit of normal for our clinical laboratory. Eleven patients (26%) were vitamin D deficient with levels < 20 ng/mL. Hyperparathyroidism was related to vitamin D deficiency. Black participants were more commonly vitamin D deficient compared with non-black participants (67% vs. 19%, p=0.01).
Table 2.
Fontan Clinical Characteristics
| Variable | Value |
|---|---|
| Anatomy, n (%) | |
| HLHS | 14 (33) |
| Tricuspid atresia | 7 (16) |
| Unbalanced AV canal defect | 7 (16) |
| Other single ventricle variants | 7 (16) |
| DORV | 6 (14) |
| PA/IVS | 2 (5) |
| Heterotaxy syndrome, n (%) | 4 (9) |
| Ventricular morphology, n (%) | |
| RV | 18 (42) |
| LV | 15 (35) |
| Mixed | 10 (23) |
| Type of Fontan, n (%) | |
| Extracardiac conduit | 25 (58) |
| Lateral tunnel | 18 (42) |
| Fenestration, n (%)a | 35 (81) |
| Medications, n (%) | |
| Aspirin | 40 (93) |
| ACEI | 19 (44) |
| Sildenafil | 3 (7) |
| Supplements, n (%) | |
| Calcium | 9 (21) |
| Vitamin D | 20 (47) |
| Laboratory values | |
| Albumin (g/dL) | 4.7 (3.4–5.3) |
| Ionized calcium | 1.22 (1.08–1.31) |
| Intact PTH (pg/mL) | 47.8 (12.3–140) |
| 25 (OH) Vitamin D (ng/mL) | 29.9 (4.5–64.2) |
| BNP (pg/mL) | 18.1 (10–246.8) |
| IGF-1 Z-score | 0.06 (−2.72 to 3.00) |
| Echocardiographic assessment, n (%)b | |
| Normal or low normal ventricular function | 29 (78) |
| Absent or mild AV valve regurgitation | 32 (86) |
| Indexed systemic flow on CMR, (L/min/m2)c | 2.3 (1.9–3.3) |
Results expressed as median (range) or n (%).
at time of initial operation;
N=37;
N=19
HLHS, hypoplastic left heart syndrome; AV, atrioventricular valve; DORV, double outlet right ventricle; PA/IVS, pulmonary atresia/intact ventricular septum; RV, right ventricle; LV, left ventricle; ACEI, angiotensin-converting enzyme inhibitor; PTH, parathyroid hormone; BNP, brain type natriuretic peptide; IGF-1, insulin-like growth factor-1; CMR, cardiac magnetic resonance; L/min/m2, liters per minute per meters squared
3.2 pQCT Results
Trabecular vBMD and cortical structure Z-scores were significantly lower in Fontan participants, compared with reference participants (Table 3). The smaller cortical area and thickness were due to a smaller periosteal circumference. The endosteal circumference Z-scores did not differ between the two groups.
Table 3.
pQCT Bone and muscle area Z-scores for Fontan participants
| Variable | Z-score | p value compared with reference participants |
|---|---|---|
| Trabecular vBMD | −0.85±0.96 | <0.001 |
| Cortical vBMD | −0.17±0.98 | 0.27 |
| Cortical area | −0.59±0.84 | <0.001 |
| Cortical thickness | −0.49±0.87 | 0.002 |
| Periosteal circumference | −0.50±0.82 | <0.001 |
| Endosteal circumference | −0.21±1.02 | 0.14 |
| Calf muscle area | −0.45±0.98 | 0.003 |
| Calf fat area | −0.28±1.17 | 0.07 |
Results expressed as mean ± SD
vBMD, volumetric bone mineral density
Calf muscle CSA Z-scores were significantly lower in Fontan participants compared with reference participants (Table 3); calf fat CSA Z-scores did not differ between the two groups. In the Fontan cohort, lower calf muscle area Z-scores were associated with smaller cortical periosteal circumference Z-scores (R=0.62, p<0.001) (Figure) and smaller cortical area Z-scores (R=0.55, p <0.001). Adjustment for calf muscle area Z-scores attenuated the cortical deficits in Fontan patients, however periosteal circumference Z-scores (β = −0.27, p=0.009) and cortical area Z-scores (β = −0.41, p<0.001) remained significantly lower compared with reference participants in the adjusted models.
Figure.
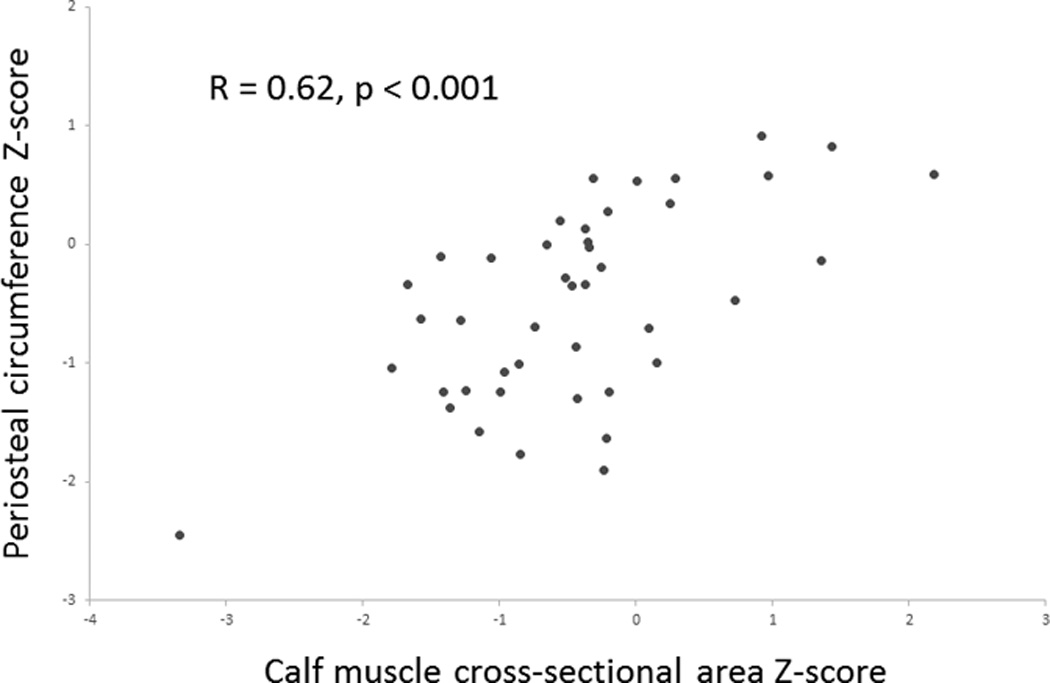
In the Fontan participants, lower calf muscle area Z-scores were associated with smaller cortical periosteal circumference Z-scores (R=0.62, p<0.001).
3.3 Associations between pQCT Outcomes and Fontan Characteristics
Bone and muscle deficits were not associated with age, delayed puberty, time from Fontan, cardiac anatomy, Fontan characteristics, presence of heterotaxy syndrome, PTH or 25 (OH) vitamin D levels, IGF-1 Z-scores, BNP level, medications or supplements, qualitative ventricular function or atrioventricular valve regurgitation on echocardiogram, or indexed systemic flow on CMR. The Z-score distributions for the bone and muscle outcomes did not differ according to Tanner stage.
4. DISCUSSION
This study demonstrates substantial deficits in trabecular vBMD, cortical structure, and muscle area in a population of children, adolescents, and young adults with Fontan physiology, compared to reference participants. The deficits in cortical dimensions were highly correlated with muscle deficits, consistent with our observations in multiple chronic diseases [24, 32–34], and remained significant after adjustment for these muscle deficits. This is the largest comprehensive description of musculoskeletal outcomes in Fontan patients, and is strengthened by the inclusion of a robust reference sample, facilitating adjustment for age, sex, race and growth failure.
A prior study in 12 Fontan patients with PLE noted marked reductions in height and DXA BMD Z-scores [9]. However, DXA is a two-dimensional technique that summarizes total bone mineral content within the projected bone area to generate areal BMD (g/cm2). Areal BMD systematically underestimates volumetric BMD (g/cm3) in children with poor growth [19]. Furthermore, trabecular and cortical bone mineral content are superimposed in the DXA scan, potentially concealing discrete disease effects on trabecular and cortical vBMD and cortical structure.
We used pQCT, a 3-dimensional technology which provides measures of vBMD as well as measures of bone structure that correlate with fracture failure load [35]. Importantly, the measures of cortical dimensions were adjusted for tibia length for age-Z-scores, eliminating the confounding effect of poor growth. A previous study used radial pQCT to assess musculoskeletal outcomes in 29 adolescent and young adult patients with various forms of congenital heart disease, compared with a healthy reference sample [14]. That study included six Fontan patients and demonstrated significantly worse deficits in trabecular vBMD compared with patients with other forms of congenital heart disease. Cortical and muscle deficits were not observed in the larger cohort overall and results in the six Fontan participants were not provided.
The deficits in trabecular vBMD, cortical structure and muscle in Fontan patients are similar to, if not worse than, those previously described by our group in other pediatric chronic diseases know to impact bone health. These include Crohn’s disease, acute lymphoblastic leukemia, chronic kidney disease, hematopoetic stem cell transplantation and renal transplantation [22–24, 32, 33]. These bone deficits expose a vulnerability of Fontan patients during childhood and adolescence. Failure to achieve normal peak bone mass likely has important implications for life-long bone health. The deficits in cortical structure are particularly concerning as bone structure is critical to bone strength and failure to accrue periosteal dimensions is likely irreversible. In studies of fracture biomechanics, cortical area explains 77% of the variability of bone failure load and correlates with fracture rate [35]. The fracture implications of these structural deficits in the Fontan population are unknown.
In our study population lower calf muscle area Z-scores were associated with smaller cortical periosteal circumference Z-scores. Similarly, a previous study demonstrated reduced forearm muscle area after heart transplantation in a population of 34 patients (5 with prior congenital heart disease) [36]. Muscle deficits were irrespective of glucocorticoid treatment and muscle area positively correlated with cortical area (R=0.43, p=0.001). These findings reflect the concept of the “muscle-bone unit” which states that as muscles increase with growth or physical activity, bones adapt by increasing dimensions and strength [16, 17, 24, 37]. Conversely, inadequate muscle loading of bone may result in secondary deficits in cortical structure. While this association is not necessarily causal in nature – inflammatory, nutritional, or endocrine factors may affect both muscles and bones – our findings highlight the potential importance of adequate muscle mass and physical activity in Fontan patients. Patients after Fontan palliation are more sedentary compared with healthy children and adolescents [8] and may not achieve daily levels of moderate to vigorous exercise [7] necessary to promote adequate bone acquisition. Regular physical activity may be important to improving bone health in Fontan patients.
This study did not address the mechanistic causes of altered bone density or structure in Fontan patients. However, as the Fontan operation creates a form of chronic heart failure characterized by mild hypoxemia, limited maximal cardiac output, and elevated central venous pressure, these physiologic derangements could adversely affect bone development. Low bone density has been described in adults with heart failure [38], while animal studies show decreased expression of markers of bone formation in hypoxic states [39].
The primary limitation of this study is the cross-sectional design. Bone density and structure reflect the cumulative effects of threats to bone health; therefore, longitudinal studies are needed to identify disease specific risk factors, and to determine the fracture implications. The cross-sectional design and limited number of participants in Tanner stages 2 through 4 limited our ability to assess the impact of delayed puberty on bone and muscle outcomes. Additionally, this study did not include an assessment of physical activity (questionnaire or otherwise) which may increase the understanding of the relationship between muscle loading and cortical bone deficits in Fontan patients. Finally, the study may have been subject to selection bias. However, the study had multiple important strengths including a large sample of Fontan participants, comprehensive laboratory studies, including vitamin D, PTH, IGF-1 and BNP levels, and a robust reference sample which allowed us to assess bone and muscle deficits, independent of age, sex, race and body size.
In conclusion, this study demonstrated deficits in bone density and bone structure in children, adolescents, and young adults with Fontan physiology. As demonstrated in other disease states, muscle deficits were associated with deficits in bone structure. Future studies including accelerometer-based measures of physical activity are needed to determine associations with musculoskeletal deficits, and to inform physical activity interventions. This evidence of trabecular and cortical deficits raises concerns about potential long-term osteopenia in this cohort of high-risk children and young adults. Longitudinal studies are necessary to determine the fracture implications of these deficits and to identify therapies to promote bone accrual.
HIGHLIGHTS.
Patients with single ventricle heart disease are at risk for impaired bone accrual.
Quantitative CT was used to assess bone in Fontan participants and controls.
Fontan participants had lower trabecular density and smaller cortical dimensions.
Calf muscle area was also significantly lower in Fontan participants.
Lower muscle area was associated with smaller periosteal circumference.
ACKNOWLEDGEMENTS
This study was funded by a Children’s Hospital of Philadelphia Cardiac Center Grant and the Robert S. and Dolores Harrington Endowment in Pediatric Cardiology at The Children's Hospital of Philadelphia. NIH support included grants T32 HL007915 (CA), K23 HL089647 (KKW), K24 DK076808 (MBL), and the Clinical and Translational Science Award UL1 RR024134 and UL1 TR000003.
Footnotes
pQCT – peripheral quantitative computed tomography; vBMD – volumetric bone mineral density; PLE – protein losing enteropathy; DXA – dual energy x-ray absorptiometry; CSA – cross sectional area; CMR – cardiac magnetic resonance; PTH – parathyroid hormone; BNP – brain natriuretic peptide; IGF-1 – insulin-like growth factor-1
REFERENCES
- 1.Rogers LS, Glatz AC, Ravishankar C, et al. 18 years of the Fontan operation at a single institution: results from 771 consecutive patients. J Am Coll Cardiol. 2012;60:1018–1025. doi: 10.1016/j.jacc.2012.05.010. [DOI] [PubMed] [Google Scholar]
- 2.O'Brien JE, Jr, Marshall JA, Young AR, Handley KM, Lofland GK. The nonfenestrated extracardiac Fontan procedure: a cohort of 145 patients. Ann Thorac Surg. 2010;89:1815–1820. doi: 10.1016/j.athoracsur.2010.02.055. [DOI] [PubMed] [Google Scholar]
- 3.Tweddell JS, Nersesian M, Mussatto KA, et al. Fontan palliation in the modern era: factors impacting mortality and morbidity. Ann Thorac Surg. 2009;88:1291–1299. doi: 10.1016/j.athoracsur.2009.05.076. [DOI] [PubMed] [Google Scholar]
- 4.Hirsch JC, Goldberg C, Bove EL, et al. Fontan operation in the current era: a 15-year single institution experience. Ann Surg. 2008;248:402–410. doi: 10.1097/SLA.0b013e3181858286. [DOI] [PubMed] [Google Scholar]
- 5.Khairy P, Fernandes SM, Mayer JE, Jr, et al. Long-term survival, modes of death, and predictors of mortality in patients with Fontan surgery . Circulation. 2008;117:85–92. doi: 10.1161/CIRCULATIONAHA.107.738559. [DOI] [PubMed] [Google Scholar]
- 6.Marelli A, Gilboa S, Devine O, et al. Estimating the congenital heart disease population in the United States in 2010 - What are the numbers? J Am Coll Cardiol. 2012;59:E787–E787. [Google Scholar]
- 7.Longmuir PE, Russell JL, Corey M, Faulkner G, McCrindle BW. Factors associated with the physical activity level of children who have the Fontan procedure. Am Heart J. 2011;161:411–417. doi: 10.1016/j.ahj.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 8.McCrindle BW, Williams RV, Mital S, et al. Physical activity levels in children and adolescents are reduced after the Fontan procedure, independent of exercise capacity, and are associated with lower perceived general health. Arch Dis Child. 2007;92:509–514. doi: 10.1136/adc.2006.105239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldberg DJ, Dodds K, Avitabile CM, et al. Children with protein-losing enteropathy after the fontan operation are at risk for abnormal bone mineral density. Pediatr Cardiol. 2012;33:1264–1268. doi: 10.1007/s00246-012-0290-z. [DOI] [PubMed] [Google Scholar]
- 10.Vogt KN, Manlhiot C, Van Arsdell G, Russell JL, Mital S, McCrindle BW. Somatic growth in children with single ventricle physiology impact of physiologic state. J Am Coll Cardiol. 2007;50:1876–1883. doi: 10.1016/j.jacc.2007.07.050. [DOI] [PubMed] [Google Scholar]
- 11.Cohen MI, Bush DM, Ferry RJ, Jr, et al. Somatic growth failure after the Fontan operation. Cardiol Young. 2000;10:447–457. doi: 10.1017/s1047951100008118. [DOI] [PubMed] [Google Scholar]
- 12.Cohen MS, Zak V, Atz AM, et al. Anthropometric measures after Fontan procedure: implications for suboptimal functional outcome. Am Heart J. 2010;160:1092–1098. doi: 10.1016/j.ahj.2010.07.039. 8 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ovroutski S, Ewert P, Alexi-Meskishvili V, et al. Comparison of somatic development and status of conduit after extracardiac Fontan operation in young and older children. Eur J Cardiothorac Surg. 2004;26:1073–1079. doi: 10.1016/j.ejcts.2004.07.021. [DOI] [PubMed] [Google Scholar]
- 14.Witzel C, Sreeram N, Coburger S, Schickendantz S, Brockmeier K, Schoenau E. Outcome of muscle and bone development in congenital heart disease. Eur J Pediatr. 2006;165:168–174. doi: 10.1007/s00431-005-0030-y. [DOI] [PubMed] [Google Scholar]
- 15.Avitabile CM, Leonard MB, Zemel BS, et al. Lean mass deficits, vitamin D status, and exercise capactiy in children and young adults after Fontan palliation. Heart. 2014 doi: 10.1136/heartjnl-2014-305723. Published Online First [27 June 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wetzsteon R, Zemel BS, Shults J, Howard KM, Kibe LW, Leonard MB. Mechanical loads and cortical bone geometry in healthy children and young adults. Bone. 2011;48:1103–1108. doi: 10.1016/j.bone.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leonard MB, Elmi A, Mostoufi-Moab S, et al. Effects of sex, race, and puberty on cortical bone and the functional muscle bone unit in children, adolescents, and young adults. J Clin Endocrinol Metab. 2010;95:1681–1689. doi: 10.1210/jc.2009-1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu D, Manske SL, Kontulainen SA, et al. Tibial geometry is associated with failure load ex vivo: a MRI, pQCT and DXA study. Osteoporos Int. 2007;18:991–997. doi: 10.1007/s00198-007-0325-0. [DOI] [PubMed] [Google Scholar]
- 19.Zemel BS, Leonard MB, Kelly A, et al. Height adjustment in assessing dual energy x-ray absorptiometry measurements of bone mass and density in children. J Clin Endocrinol Metab. 2010;95:1265–1273. doi: 10.1210/jc.2009-2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mostoufi-Moab S, Ginsberg JP, Bunin N, et al. Body composition abnormalities in long-term survivors of pediatric hematopoietic stem cell transplantation. J Pediatr. 2012;160:122–128. doi: 10.1016/j.jpeds.2011.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Baker JF, Davis M, Alexander R, et al. Associations between body composition and bone density and structure in men and women across the adult age spectrum. Bone. 2013;53:34–41. doi: 10.1016/j.bone.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mostoufi-Moab S, Brodsky J, Isaacoff EJ, et al. Longitudinal assessment of bone density and structure in childhood survivors of acute lymphoblastic leukemia without cranial radiation. J Clin Endocrinol Metab. 2012;97:3584–3592. doi: 10.1210/jc.2012-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Terpstra AM, Kalkwarf HJ, Shults J, et al. Bone density and cortical structure after pediatric renal transplantation. J Am Soc Nephrol. 2012;23:715–726. doi: 10.1681/ASN.2011050480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsampalieros A, Kalkwarf HJ, Wetzsteon RJ, et al. Changes in bone structure and the muscle-bone unit in children with chronic kidney disease. Kidney Int. 2013;83:495–502. doi: 10.1038/ki.2012.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Morris M, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J Youth Adol. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- 26.Saenger AK, Laha TJ, Bremner DE, Sadrzadeh SM. Quantification of serum 25-hydroxyvitamin D(2) and D(3) using HPLC-tandem mass spectrometry and examination of reference intervals for diagnosis of vitamin D deficiency. Am J Clin Pathol. 2006;125:914–920. doi: 10.1309/J32U-F7GT-QPWN-25AP. [DOI] [PubMed] [Google Scholar]
- 27.Ky B, Shults J, Keane MG, et al. FGF23 modifies the relationship between vitamin D and cardiac remodeling. Circ Heart Fail. 2013;6:817–824. doi: 10.1161/CIRCHEARTFAILURE.112.000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ogden CL, Kuczmarski RJ, Flegal KM, et al. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 29.Cole TJ. The LMS method for constructing normalized growth standards. Eur J Clin Nutr. 1990;44:45–60. [PubMed] [Google Scholar]
- 30.Tsampalieros A, Lam CK, Spencer JC, et al. Long-term inflammation and glucocorticoid therapy impair skeletal modeling during growth in childhood Crohn disease. J Clin Endocrinol Metab. 2013;98:3438–3445. doi: 10.1210/jc.2013-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross AC. The 2011 report on dietary reference intakes for calcium and vitamin D. Public Health Nutr. 2011;14:938–939. doi: 10.1017/S1368980011000565. [DOI] [PubMed] [Google Scholar]
- 32.Mostoufi-Moab S, Ginsberg JP, Bunin N, et al. Bone density and structure in long-term survivors of pediatric allogeneic hematopoietic stem cell transplantation. J Bone Miner Res. 2012;27:760–769. doi: 10.1002/jbmr.1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dubner SE, Shults J, Baldassano RN, et al. Longitudinal assessment of bone density and structure in an incident cohort of children with Crohn's disease. Gastroenterology. 2009;136:123–130. doi: 10.1053/j.gastro.2008.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burnham JM, Shults J, Dubner SE, Sembhi H, Zemel BS, Leonard MB. Bone density, structure, and strength in juvenile idiopathic arthritis: importance of disease severity and muscle deficits. Arthritis Rheum. 2008;58:2518–2527. doi: 10.1002/art.23683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ashe MC, Khan KM, Kontulainen SA, et al. Accuracy of pQCT for evaluating the aged human radius: an ashing, histomorphometry and failure load investigation. Osteoporos Int. 2006;17:1241–1251. doi: 10.1007/s00198-006-0110-5. [DOI] [PubMed] [Google Scholar]
- 36.Bechtold S, Putzker S, Birnbaum J, Schwarz HP, Netz H, Dalla Pozza R. Impaired bone geometry after heart and heart-lung transplantation in childhood. Transplantation. 2010;90:1006–1010. doi: 10.1097/TP.0b013e3181f6300b. [DOI] [PubMed] [Google Scholar]
- 37.Burnham JM, Shults J, Sembhi H, Zemel BS, Leonard MB. The dysfunctional muscle-bone unit in juvenile idiopathic arthritis. J Musculoskelet Neuronal Interact. 2006;6:351–352. [PubMed] [Google Scholar]
- 38.Pfister R, Michels G, Sharp SJ, Luben R, Wareham NJ, Khaw K-T. Low bone mineral density predicts incident heart failure in men and women. J Am Coll Cardiol HF. 2014;2:380–389. doi: 10.1016/j.jchf.2014.03.010. [DOI] [PubMed] [Google Scholar]
- 39.Lee AM, Morrison JL, Botting KJ, Shandala T, Xian CJ. Effects of maternal hypoxia during pregnancy on bone development in offspring: a guinea pig model. Int J Endocrinol. 2014 doi: 10.1155/2014/916918. Published Online First [14 May 2014] [DOI] [PMC free article] [PubMed] [Google Scholar]


