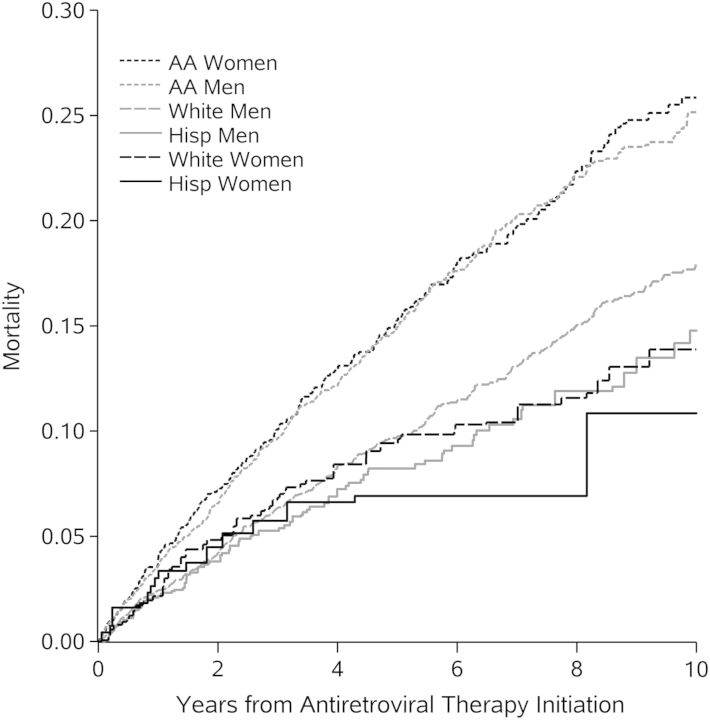
Among antiretroviral therapy initiators in the Centers for AIDS Research Network of Integrated Clinical Systems, 10-year all-cause mortality was significantly higher among black patients than among white men. White women and Hispanic patients had lower 10-year mortality than white men.
Keywords: HIV, health status disparities, cohort studies, survival analysis, antiretroviral therapy
Abstract
Background. Ensuring equal access to antiretroviral therapy (henceforth therapy) should alleviate disparities in health outcomes among persons infected with human immunodeficiency virus (HIV). However, evidence supporting the persistence of disparities in survival following therapy initiation is mixed.
Methods. Patients initiating therapy in eight academic medical centers in the Centers for AIDS Research Network of Integrated Clinical Systems between 1 January 1998 and 30 December 2011. Patients (n = 10 017) were followed from therapy initiation until death from any cause, administrative censoring at 10 years after therapy initiation or the end of follow-up on 31 December 2011. The 10-year risk of all-cause mortality was calculated from standardized Kaplan–Meier survival curves.
Results. Patients were followed for a median of 4.7 years (interquartile range: 2.2, 8.2). During 51 121 person-years of follow-up, 1224 of the 10 017 patients died. The overall 10-year mortality risk was 20.2% (95% confidence interval [CI], 19.2%, 21.3%). Black men and women experienced standardized 10-year all-cause mortality risks that were 7.2% (95% CI, 4.3%, 10.1%) and 7.9% (95% CI, 3.9%, 12.0%) larger (absolute difference) than white men. White women, Hispanic men, and Hispanic women all had lower 10-year mortality than white men.
Conclusions. These data serve as a call to action to identify modifiable mechanisms leading to these observed mortality disparities among HIV-infected black patients. Effective interventions are needed to ensure that the goal of the National HIV/AIDS Strategy to overcome health disparities becomes a reality.
Documented differences in mortality by race, ethnicity, and sex among people infected with human immunodeficiency virus (HIV) [1] may be attributable to differences in the rates of diagnosis, linkage to care following diagnosis, or initiation of effective combination antiretroviral therapy (henceforth therapy). Once HIV-infected persons initiate therapy, mortality rates are dramatically reduced [2, 3], and we would expect disparities in mortality to be attenuated [1, 4, 5]. However, evidence supporting the existence of disparities in survival following therapy initiation is mixed [6, 7]. In this paper, we describe 10-year all-cause mortality after therapy initiation in the Centers for AIDS Research Network of Integrated Clinical Systems (CNICS) between 1998 and 2011 by race, ethnicity, and sex among a sample of adult patients in the United States. We standardized results to control for differences in pretreatment health status.
METHODS
Study Population
CNICS is a clinical cohort study that includes detailed information on demographics, laboratory measures, medication use, and mortality for HIV-infected patients 18 years of age and older who initiated primary care at any of 8 CNICS sites after 1 January 1995 (or the site-specific CNICS inception date) [8]. Institutional review boards at each site approved participation in CNICS, and this analysis of deidentified data was determined not to be human subjects research by the institutional review board of the University of North Carolina at Chapel Hill.
The study population included patients who initiated a first combination therapy regimen, defined as 3 or more different antiretroviral drugs, between 1 January 1998 and 30 December 2011. Race and ethnicity were patient-reported and categorized independently in the original data according to the Health Resource and Service Administration federal standards [9]. We combined race and ethnicity into one variable for analysis and classified patients as Hispanic if Hispanic ethnicity was indicated regardless of race; <2% of Hispanic patients reported their race as black and 16% did not indicate any race. Non-Hispanic patients were classified as black, white, or other race.
During the study period, 11 463 men and women initiated therapy in CNICS. We excluded 518 with reported race/ethnicity other than white, black or Hispanic, or sex other than male or female (ie, intersex). We excluded 928 patients (8%) for missing baseline data for race/ethnicity (n = 97, <1%), injection drug use (n = 219, 2%), and CD4 cell count or HIV-1 RNA viral load proximate to therapy initiation (n = 612, 6%). The final study population included 10 017 patients.
Mortality Ascertainment
The outcome was death from any cause. Mortality information is obtained from clinic sources, death certificates, and the US Social Security Death Index, which is queried regularly by contributing sites.
Statistical Analysis
We measured survival time in days from therapy initiation to death, administratively censoring patients on 31 December 2011 (patients from one site were administratively censored on 15 September 2010) or at 10 years of follow-up to maintain adequately sized risk sets (ie, >20 persons).
We estimated mortality risk using the complement of the Kaplan–Meier survival function [10]. To isolate disparities arising after therapy initiation, we standardized our results to the study sample according to the distribution of risk factors for mortality measured at or just prior to therapy initiation using stabilized inverse probability weights [11, 12]. We estimated the denominator of the weights for race/ethnicity and sex categories using polytomous logistic regression, conditional on calendar date of therapy initiation, age at therapy initiation, CD4 cell count (cells/mm3), and HIV-1 RNA plasma concentration (viral load) (log10 copies/mL) most proximate to therapy initiation measured between 6 months prior to 14 days after therapy initiation, ART naivety (ie, no evidence of prior exposure to mono or dual therapy), prior AIDS diagnosis, history of injection drug use, history of hepatitis C virus infection, and CNICS site. We used restricted quadratic splines (with knots at the 5th, 35th, 65th, and 95th percentiles) to flexibly model all continuous covariates [13]. We stabilized the weights using the site-specific distribution of race/ethnicity and sex. The estimated weights had a mean of 1.00 (range: 0.14, 4.44).
We compared the crude and standardized absolute and relative difference in 10-year mortality risk by race/ethnicity and sex categories. We also calculated hazard ratios using a Cox regression model [14] using Efron approximation for tied death times [15]. We assessed the proportional hazards assumptions by visual inspection of log cumulative hazard functions by time (Supplementary Figure 1), as well as by statistical test of the product terms for race/ethnicity and sex categories with time. We saw no important violations of the proportional hazards assumption. We provide site-stratified hazard ratios as supplementary analysis.
We calculated 95% confidence intervals (CIs) for the standardized mortality risk differences and risk ratios using a standard error estimated from 200 nonparametric bootstrap random samples drawn with replacement [16]. For the standardized hazard ratios, we calculated CIs using the robust standard error [17].
To assess whether the magnitude of disparities in survival have changed over time, we stratified follow-up time into early and late calendar periods, varying the cut point from 2002 to 2008 by increments of 2 years, and then tested the significance of interactions between time period and race/ethnicity and sex in a Cox model. To assess how disparities observed in the CNICS compared with survival disparities seen in the US general population, we present age- and calendar time-standardized mortality rates for the general population, which we calculated from vital statistics data downloaded from CDC WONDER [18]. To assess possible hypotheses about mechanisms for disparities in survival, we calculated the proportion of patients retained in care (no gaps in laboratory monitoring >1 year within 2 years of therapy initiation) and the proportion of patients who achieved and maintained viral suppression (<400 copies/mL) at 1 year after therapy initiation, stratified by race/ethnicity and sex. Patients who died within the first 2 years of follow-up without experiencing a gap in laboratory monitoring were considered retained in care. Patients who died before achieving viral suppression (1%) were considered not virally suppressed.
All analyses were conducted using SAS version 9.3 (SAS Institute, Cary, North Carolina).
RESULTS
The median age in the study sample was 40 years (interquartile range [IQR]: 33, 46) and the median year of therapy initiation was 2006 (IQR: 2003, 2009). The median baseline CD4 count and viral load were 238 cells/mm3 (IQR: 85, 385) and 4.7 log10 copies/mL (IQR: 3.9, 5.3), respectively (Table 1). Most patients (88%) were treatment-naive, 27% had a prior AIDS-defining condition, 18% had a history of injection drug use, and 16% had a history of hepatitis C virus infection. The median time spent in the CNICS prior to initiation of therapy was 68 days (IQR: 21–371 days). The prevalence of a prior AIDS-defining condition at baseline was 22% among white men, compared with 28%–34% among other race/ethnicity and sex groups. Additionally, 30% of white women had a history of injection drug use, compared to only 12%–19% in other groups, with a correspondingly high risk of HCV infection.
Table 1.
Characteristics at Antiretroviral Therapy Initiation, 10 017 HIV-Infected Adults, 1998–2011a
| Overall n = 10 017 | Black Men n = 2679 | Black Women n = 1,232 | White Men n = 4228 | White Women n = 457 | Hispanic Men n = 1252 | Hispanic Women n = 169 | |
|---|---|---|---|---|---|---|---|
| Age, years | 40 (33, 46) | 40 (33, 47) | 40 (33, 47) | 40 (34, 46) | 41 (32, 46) | 37 (31, 43) | 39 (30, 47) |
| Calendar year | 2006 (2003, 2009) | 2006 (2002, 2009) | 2005 (2002, 2008) | 2007 (2003, 2009) | 2005 (2003, 2009) | 2007 (2004, 2010) | 2007 (2004, 2009) |
| CD4 count, cells/mm3 | 238 (85, 385) | 196 (44, 343) | 210 (64, 348) | 276 (126, 428) | 256 (114, 407) | 226 (77, 364) | 217 (91, 324) |
| Viral load, log10 copies/mL | 4.7 (3.9, 5.3) | 4.7 (4.1, 5.3) | 4.7 (3.9, 5.3) | 4.7 (3.9, 5.3) | 4.7 (3.8, 5.3) | 4.7 (3.9, 5.3) | 4.5 (3.4, 5.0) |
| Therapy naive, n (%) | 8,787 (88%) | 2,364 (88%) | 1,030 (84%) | 3,724 (88%) | 397 (87%) | 1,122 (90%) | 151 (89%) |
| AIDS, n (%) | 2,735 (27%) | 866 (32%) | 415 (34%) | 912 (22%) | 127 (28%) | 364 (29%) | 51 (30%) |
| Injection drug use, n (%) | 1,758 (18%) | 505 (19%) | 224 (18%) | 715 (17%) | 139 (30%) | 144 (12%) | 31 (18%) |
| Hepatitis C viral infection, n (%) | 1,572 (16%) | 516 (19%) | 222 (18%) | 528 (12%) | 133 (29%) | 137 (11%) | 36 (21%) |
Abbreviation: HIV, human immunodeficiency virus.
a Median (quartiles) unless noted otherwise.
Patients were followed for a median of 4.7 years (IQR: 2.2, 8.2). During 51 121 person-years of follow-up, 1224 of the 10 017 patients died. The overall crude 10-year mortality risk was 20.2% (95% CI, 19.2%, 21.3%), and the overall crude mortality rate was 2.39 deaths per 100 person-years (95% CI, 2.26, 2.53). The crude 10-year mortality risk was 27.0% (95% CI, 24.6%, 29.3%) among black men and 25.0% (95% CI, 21.6%, 28.4%) among black women. In contrast, the crude 10-year mortality risk was only 16.8% (95% CI, 15.1%, 18.5%) among white men and 17.5% (95% CI, 12.9%, 22.1%) among white women. Among Hispanic men and women, the crude 10-year mortality risk was 12.5% (95% CI, 9.5%, 15.5%) and 12.0% (95% CI, 4.5%, 19.4%), respectively.
Black men and women experienced a standardized 10-year mortality risk that was 7.2% (95% CI, 4.3%, 10.1%) and 7.9% (95% CI, 3.9%, 12.0%) larger (absolute difference) than white men. The standardized 10-year risk of mortality was 4.0% (95% CI, −8.5%, 0.4%) less among white women compared to white men. Among Hispanic men and women, the standardized 10-year risk of mortality was 3.2% (95% CI, −7.1%, 0.8%) and 7.1% (95% CI, −16.1%, 1.9%) less, respectively than the mortality risk among white men (Figure 1). Standardized risk ratios and hazard ratios exhibited a similar pattern (Table 2). Stratifying on CNICS site did not substantively change the results, but it slightly attenuated the estimated relative hazard of all-cause mortality for black men and women and shifted the estimated relative hazard for white women and Hispanic patients downward, away from the null (Supplementary Table 2).
Figure 1.
Cumulative all-cause mortality standardizeda to total study sample by years from therapy initiation, race/ethnicity and sex, 10 017 human immunodeficiency virus-infected adults, 1998–2011. aStandardized by baseline covariates: age at therapy initiation, CD4 count and viral load, all modeled with restricted quadratic splines (with 4 knots located at the 5th, 35th, 65th and 95th percentiles), antiretroviral therapy naivety, prior diagnosis of any AIDS-defining condition at therapy initiation, injection drug use, and history of hepatitis C virus infection. Abbreviation: AA, African-American.
Table 2.
Standardizeda 10-year Mortality Risk Differences and Hazard Ratios and by Race/Ethnicity and sex, 10 017 HIV-Infected Adults, 1998–2011
| Observed |
Standardizeda |
||||||
|---|---|---|---|---|---|---|---|
| No. Deaths | No. Person-years | 10-year Mortality Risk, % | 10-year Mortality Risk, % | Risk Difference, % (95% CI) | Risk Ratio (95% CI) | Hazard Ratio (95% CI) | |
| Overall | 1224 | 51,121.1 | 20.2 | 20.2 | … | … | … |
| Race/ethnicity/sex | |||||||
| Black women | 207 | 6,730.0 | 25.0 | 25.8 | 7.9 (3.9, 12.0) | 1.44 (1.21, 1.72) | 1.57 (1.32, 1.87) |
| Black men | 458 | 13,338.4 | 27.0 | 25.2 | 7.2 (4.3, 10.1) | 1.40 (1.23, 1.61) | 1.53 (1.33, 1.76) |
| White men | 405 | 21,792.3 | 16.8 | 18.0 | 0 | 1 | 1 |
| Hispanic men | 86 | 5,924.6 | 12.5 | 14.8 | −3.2 (−7.1, .8) | 0.82 (.63, 1.08) | 0.82 (.64, 1.05) |
| White women | 53 | 2,482.0 | 17.5 | 13.9 | −4.0 (−8.5, .4) | 0.77 (.57, 1.05) | 0.85 (.61, 1.19) |
| Hispanic women | 15 | 853.9 | 12.0 | 10.8 | −7.1 (−16.1, 1.9) | 0.61 (.26, 1.42) | 0.68 (.37, 1.23) |
| Race/ethnicity | |||||||
| White | 458 | 24,274.3 | 16.9 | 17.2 | 0 | 1 | 1 |
| Black | 665 | 20,068.4 | 26.3 | 25.4 | 8.2 (5.6, 10.8) | 1.48 (1.30, 1.67) | 1.29 (1.12, 1.49) |
| Hispanic | 101 | 6,778.5 | 12.5 | 14.5 | −2.7 (−6.6, 1.1) | 0.84 (.65, 1.09) | 0.75 (.59, .96) |
| Sex | |||||||
| Men | 949 | 41,055.3 | 19.7 | 19.8 | 0 | 1 | 1 |
| Women | 275 | 10,065.8 | 22.2 | 22.0 | 2.2 (−0.7, 5.2) | 1.11 (.97, 1.28) | 0.95 (.82, 1.10) |
Abbreviations: CI, confidence interval; HIV, human immunodeficiency virus; No., number.
a Standardized for calendar date of therapy initiation, age at therapy initiation, CD4 cell count (cells/mm3) and HIV-1 RNA plasma concentration (viral load) (log10 copies/mL) most proximate to therapy initiation measured between 6 months prior to 14 days after therapy initiation, antiretroviral therapy naivety (ie, no evidence of prior exposure to mono or dual therapy), prior AIDS diagnosis, history of injection drug use, and history of hepatitis C virus infection; additionally, race/ethnicity risk was standardized for sex and sex risk was standardized for race/ethnicity.
Disparities in survival did not change significantly over the study period (P-values for interaction between time period and race/ethnicity and sex were .14 when the time period was divided into pre- and post-2002, .66 for 2004, .75 for 2006, and .43 for 2008).
The mortality rate ratio comparing black men with white men in the CNICS was higher than the mortality rate ratio comparing black men with white men in the US general population. The mortality rate ratio comparing black women with white men vastly exceeds the mortality rate ratio comparing black women with white men in the US general population. The relative rate of mortality for Hispanic men and women compared with white men in the CNICS was higher and closer to the null than the relative rate of mortality for Hispanic men and women with white men in the US general population (Table 3).
Table 3.
Crude Mortality Rate Ratios for 10 017 HIV-Infected Adults in the CNICS, 1998–2011, and age- and Calendar Time-standardized Mortality Rates in the US General Population Based on Vital Statistics Data, 1999–2011
| Race/Ethnicity/Sex | CNICS, 1998–2011 |
US Population, 1999–2011 [18] |
||||
|---|---|---|---|---|---|---|
| No. Deaths | No. Person-years | Mortality Rate per 10 000 Person-years | Rate Ratio | Standardized Mortality Ratea | Rate Ratio | |
| Black women | 207 | 6,730.0 | 307.6 | 1.66 | 45.7 | 1.05 |
| Black men | 458 | 13,338.4 | 343.4 | 1.85 | 73.9 | 1.70 |
| White men | 405 | 21,792.3 | 185.8 | 1.00 | 43.6 | 1.00 |
| Hispanic men | 86 | 5,924.6 | 145.2 | 0.78 | 27.0 | 0.62 |
| White women | 53 | 2,482.0 | 213.5 | 1.15 | 25.9 | 0.59 |
| Hispanic women | 15 | 853.9 | 175.7 | 0.95 | 18.5 | 0.42 |
Abbreviations: CNICS, Centers for AIDS Research Network of Integrated Clinical Systems; HIV, human immunodeficiency virus.
aDirectly standardized to match the age group and calendar time distribution of the CNICS data.
Overall, 66.6% (95% CI, 65.7%, 67.5%) of patients were virally suppressed around 1 year after ART initiation and 75.9% (95% CI, 75.1%, 76.8%) of patients were retained in care at 2 years after ART initiation. There were substantial differences in viral suppression at 1 year post-ART initiation. Only 59.5% (95% CI, 58.0%, 61.1%) of black patients were virally suppressed 1 year after ART initiation, compared to 70.9% (95% CI, 69.6%, 72.2%) of white patients and 72.0% (95% CI, 69.7%, 74.3%) of Hispanic patients. Patterns were similar in men and women. There were only minor, nonsignificant differences in retention in care at 2 years post-ART initiation by race/ethnicity and sex. Weighting the data to standardize on baseline covariates had little impact on the results (Table 4).
Table 4.
Crude and Standardizeda Probabilities of Retention in Careb at 2 Years post-ART Initiation and Viral Suppression at 1 Year post-ART Initiation, by Race/Ethnicity and Sex, 10 017 HIV-Infected Adults, 1998–2011
| Crude |
Standardized |
|||
|---|---|---|---|---|
| Retention in Care 2 Years After ART Initiation, % (95% CI) | Viral Suppression at Approximately 1 Year After ART Initiation, % (95% CI) | Retention in Care 2 Years After ART Initiation, % (95% CI) | Viral Suppression at Approximately 1 Year After ART Initiation, % (95% CI) | |
| Overall | 75.9 (75.1, 76.8) | 66.6 (65.7, 67.5) | ||
| Race/ethnicity/sex | ||||
| Black women | 76.2 (73.8, 78.6) | 57.8 (55.0, 60.6) | 76.0 (73.6, 78.4) | 57.7 (54.9, 60.5) |
| Black men | 75.1 (73.5, 76.7) | 60.3 (58.4, 62.2) | 75.3 (73.6, 76.9) | 60.5 (58.7, 62.4) |
| White men | 76.4 (75.1, 77.7) | 72.0 (70.6, 73.3) | 76.2 (74.9, 77.5) | 71.7 (70.3, 73.1) |
| Hispanic men | 76.2 (73.8, 78.6) | 73.1 (70.6, 75.5) | 77.0 (74.7, 79.4) | 72.3 (69.9, 74.8) |
| White women | 74.0 (69.9, 78.0) | 61.3 (56.8, 65.7) | 75.2 (71.3, 79.2) | 64.3 (59.9, 68.9) |
| Hispanic women | 78.1 (71.9, 84.3) | 63.9 (56.2, 71.1) | 76.9 (70.5, 83.4) | 65.3 (58.0, 72.5) |
| Race/ethnicity | ||||
| White | 76.2 (75.0, 77.4) | 70.9 (69.6, 72.2) | 75.8 (74.6, 77.1) | 70.2 (68.9, 71.5) |
| Black | 75.5 (74.1, 76.8) | 59.5 (58.0, 61.1) | 75.3 (74.0, 76.7) | 59.8 (58.2, 61.3) |
| Hispanic | 76.4 (74.2, 78.6) | 72.0 (69.7, 74.3) | 76.9 (74.7, 79.1) | 71.5 (69.2, 73.8) |
| Sex | ||||
| Men | 76.0 (75.0, 76.9) | 68.3 (67.3, 69.3) | 76.1 (75.1, 77.0) | 68.1 (67.1, 69.1) |
| Women | 75.8 (73.9, 77.8) | 59.2 (56.9, 61.5) | 75.2 (73.2, 77.2) | 60.0 (57.8, 62.2) |
Data can be referenced [18].
Abbreviations: ART, antiretroviral therapy; CI, confidence interval; HIV, human immunodeficiency virus.
a Standardized for calendar date of therapy initiation, age at therapy initiation, CD4 cell count (cells/mm3) and HIV-1 RNA plasma concentration (viral load) (log10 copies/mL) most proximate to therapy initiation measured between 6 months prior to 14 days after therapy initiation, ART naivety (ie, no evidence of prior exposure to mono or dual therapy), prior AIDS diagnosis, history of injection drug use, and history of hepatitis C virus infection; additionally, race/ethnicity risk was standardized for sex and sex risk was standardized for race/ethnicity.
bRetention in care defined as no gaps in laboratory monitoring of ≥1 year.
DISCUSSION
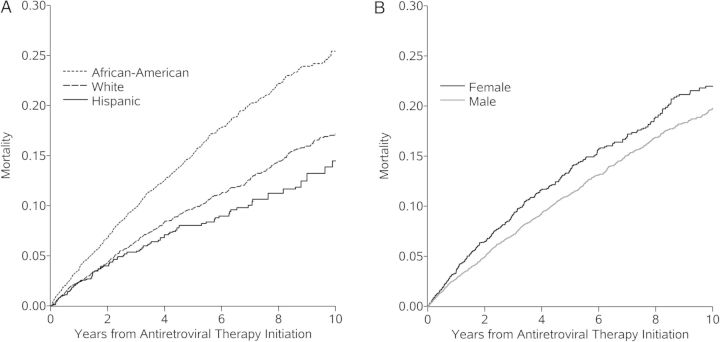
We observed markedly higher risk of death from any cause among black men and women compared to other race/ethnicity groups in the CNICS cohort following ART initiation (Figure 2A). Survival by race/ethnicity and sex varies substantially in the general US population due to differences in the prevalence of non-HIV-related conditions like diabetes, hypertension, kidney disease and violence, for example [19]. As a result, by estimating the risk of all-cause mortality, rather than focusing specifically on AIDS-defining illness-associated mortality, we have likely captured differences in the risk of non-AIDS-related causes of death. However, the disparities seen in the CNICS comparing mortality among black persons with white men exceeds the disparities in the US general population comparing black person with white men, especially for black women [18]. As we have standardized estimates to control for disparities in HIV-related health status prior to therapy initiation [4–6, 20], the overall lower survival among HIV-infected black persons is only compounded by disparities after therapy initiation as is evident in contrasts of crude mortality risks. Although a thorough mediation analysis is beyond the scope of this study, we observed lower viral suppression at 1-year after therapy initiation among black patients. Other studies have documented greater loss-to-follow-up, more missed visits, and poorer therapy adherence among black patients [21, 22], which may explain some of the survival disparities observed in this cohort.
Figure 2.
Cumulative all-cause mortality standardizeda to the total study sample by years from therapy initiation and (A) race/ethnicity or (B) sex, 10 017 human immunodeficiency virus-infected adults, 1998–2011. aStandardized by baseline covariates: sex (A) or race (B), age at therapy initiation, CD4 count and viral load, all modeled with restricted quadratic splines (with 4 knots located at the 5th, 35th, 65th, and 95th percentiles), antiretroviral therapy naivety, prior diagnosis of any AIDS-defining condition at therapy initiation, injection drug use, and history of hepatitis C virus infection.
The 10-year mortality risk in the CNICS cohort was slightly lower for Hispanic patients compared to non-Hispanic white patients (Figure 2A). Although Hispanic men and women in the CNICS tend to experience lower mortality rates than white men, the magnitude of rate ratio is less than the rate ratio comparing Hispanic men and women to white men in the general population [18]. The survival advantage that Hispanic persons in the general population is still incompletely understood [23]. As such, it is difficult to interpret the relative risk of mortality for Hispanics in the CNICS as a survival advantage (since they do better than their white male counterparts) or as a disadvantage (since they do not do as much better as their HIV-uninfected counterparts). This study is one of a few with sufficient size to estimate survival among Hispanic ART initiators. In some settings, Hispanic patients appear to have poorer retention in care [24] and lower probability achieving viral suppression [25] as compared to white patients. However, other studies have found no evidence of ethnic disparities for similar outcomes [26], or even a lower hazard of AIDS incidence or death for Hispanic patients with equal access to care [7]. The discrepant results may be due to many factors including: exposure misclassification associated with self-reported ethnicity; geographical differences in structural discrimination and access to care experienced by Hispanics; or the heterogeneity of the Hispanic community, owing to different countries of origin, residency statuses and generations since immigration to the United States, among other things [23, 27, 28].
Mortality risk observed in the CNICS was similar or slightly higher among women than among men after accounting for differences in baseline covariates (including race/ethnicity) (Figure 2B). In other cohorts, mortality rates were higher among men than among women [29] or there were no differences in survival between men and women [30]. Although women have been reported to be more likely than men to be retained in care [26, 31], we observed similar prevalence of gaps in care by two years after therapy initiation. Notably, we found that while black women had poorer survival than black males, white and Hispanic women generally had better survival than white and Hispanic men, respectively. Few previous studies that have examined survival by sex have simultaneously stratified by race/ethnicity, despite evidence of heterogeneity of HIV death rates within strata of both race/ethnicity and sex [32, 33].
We estimated all-cause mortality following therapy initiation by race/ethnicity, and sex, standardized to the total cohort at therapy initiation to control for baseline differences in health status. We view race, ethnicity, and sex as markers for unmeasured factors, such as environment, income, social status, social capital, discrimination, structural violence and other phenomena, which may partially or completely explain the demographic disparities we observed [34]. We did not standardize for these factors because our purpose was not to explain demographic disparities but rather to document their presence and estimate their magnitude.
Measurement bias is unlikely to explain the strong survival disparities observed in this study. Sex, race, ethnicity, and death are likely measured with negligible error. Race and ethnicity are collected differently across sites but are typically based on self-report and are classified in CNICS using Health Resources and Services Administration standards. Patients were classified as having a single race, which may have oversimplified race in multiracial patients, although the proportion of the US population that is multiracial is relatively low (2.9%, according to the 2010 Census) [35]. Selection bias is also unlikely to explain our observed results, as the primary outcome, mortality, was ascertained via a national database; patients were not lost to follow-up because administrative censoring was unnecessary (eg, after prolonged gap in labs or visits). Additionally, nearly all members of the eligible cohort were included in these analyses (only 8% of otherwise eligible subjects were excluded due to missing data).
Our findings may or may not generalize to the US population [36]. The CNICS cohort has proportionately more white patients, fewer young adults, more men, and more injection drug users than are HIV-diagnosed and living in the United States. Furthermore, CNICS clinics are all associated with academic medical centers, which may not reflect the HIV care provided in nonacademic settings. However, the geographic distribution of study sites more closely resembles the US population than studies conducted in any single clinic.
A subsequent investigation into the causes of death would be invaluable to tease out the relative contributions of AIDS-related and non-AIDS-related mortality to the disparity described in this study. At this time, however, cause of death data is not available for all CNICS patients, and the data that are available are generally from the underlying cause of death on the death certificate, which has been shown to have poor specificity [37, 38].
In summary, we identified elevated and meaningful differences in mortality among black men and women following combination ART initiation in a large, demographically and geographically diverse cohort. These results serve as a call to action to identify modifiable factors that contribute to these observed differences, so that efficacious interventions may be developed and implemented so that the goal of the National HIV/AIDS Strategy [39] to overcome health disparities becomes a reality.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online (http://cid.oxfordjournals.org). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. The authors thank Marissa J. Seamans for expert advice. We also thank the patients, principal investigators, co-investigators and research staff at participating Centers for AIDS Research Network of Integrated Clinical Studies sites.
Author contributions. C. R. L. and S. R. C. had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: M. J. M., S. R. C., C. R. L. Acquisition of data: M. J. M., R. D. M., J. J. E., W. C. M., J. N. M., M. M. K. Analysis and interpretation of data: C. R. L., S. R. C. Drafting of the manuscript: C. R. L., S. R. C., M. J. M. Critical revision of the manuscript for important intellectual content: C. R. L., S. R. C., W. C. M., D. W., J. J. E., A. A. A., R. D. M., C. M., J. N. M., D. R. D., M. M. K., J. K. E., M. J. M. Obtained funding: S. R. C., J. J. E., R. D. M., C. M., J. N. M., M. M. K., M. J. M. Administrative, technical, or material support: S. R. C., W. C. M., D. W., J. J. E., A. A. A., R. D. M., W. C. M., J. N. M., D. R. D., M. M. K., M. J. M. Study supervision: S. R. C., J. J. E., R. D. M., W. C. M., J. N. M., M. M. K., M. J. M.
Role of the sponsors. The National Institutes of Health (NIH) had no role in the study design and conduct of the study, in the collection, management, analysis and interpretation of the data, or in the preparation, review, or approval of the manuscript.
Financial support. This work was supported in part by grants R24 AI067039, R0I AI100654, R01 AI103661, R01 DA11602, P30 AI094189, P30 AI027757, P30 AI027763, KL2 TR000421 and K24 DA00432 from the NIH. S. R. C. and J. J. E. are supported in part by NIH grant P30 AI50410.
Potential conflicts of interest. C. R. L. reports speaker fees from Gilead. M. J. M. reports grants and personal fees from Bristol Myers Squibb (BMS), personal fees from Gilead, personal fees from Merck Foundation, grants from Pfizer, and grants from Definicare outside of the submitted work. J. J. E. reports grants and personal fees from Merck & Co., grants and personal fees from BMS, grants and personal fees from GlaxoSmithKline/ViiV, personal fees from Gilead, personal fees from Janssen, and personal fees from Abbvie outside the submitted work. D. W. reports occasional, ad hoc consulting on epidemiological methods with NIH/NICHD; there is no overlap with the present work. A. A. A. reports consulting fees from Viiv Healthcare. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Meditz AL, MaWhinney S, Allshouse A, et al. Sex, race, and geographic region influence clinical outcomes following primary HIV-1 infection. J Infect Dis 2011; 203:442–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.HIV-CAUSAL Collaboration Ray M, Logan R, Sterne JA, et al. The effect of combined antiretroviral therapy on the overall mortality of HIV-infected individuals. AIDS 2010; 24:123–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hammer SM, Squires KE, Hughes MD, et al. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. AIDS Clinical Trials Group 320 Study Team. N Engl J Med 1997; 337:725–33. [DOI] [PubMed] [Google Scholar]
- 4.Gebo KA, Fleishman JA, Conviser R, et al. Racial and gender disparities in receipt of highly active antiretroviral therapy persist in a multistate sample of HIV patients in 2001. J Acquir Immune Defic Syndr 2005; 38:96–103. [DOI] [PubMed] [Google Scholar]
- 5.Moore RD, Keruly JC, Bartlett JG. Improvement in the health of HIV-infected persons in care: reducing disparities. Clin Infect Dis 2012; 55:1242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lemly DC, Shepherd BE, Hulgan T, et al. Race and sex differences in antiretroviral therapy use and mortality among HIV-infected persons in care. J Infect Dis 2009; 199:991–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silverberg MJ, Leyden W, Quesenberry CP, Jr, Horberg MA. Race/ethnicity and risk of AIDS and death among HIV-infected patients with access to care. J Gen Intern Med 2009; 24:1065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kitahata MM, Rodriguez B, Haubrich R, et al. Cohort profile: the Centers for AIDS Research Network of Integrated Clinical Systems. Int J Epidemiol 2008; 37:948–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Revisions to the Standards for the Classification of Federal Data on Race and Ethnicity. 1997.
- 10.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc 1958; 53:457–81. [Google Scholar]
- 11.Hernan MA, Brumback B, Robins JM. Marginal structural models to estimate the joint causal effect of nonrandomized treatments. J Am Stat Assoc 2001; 96:440–8. [Google Scholar]
- 12.Cole SR, Hernan MA. Adjusted survival curves with inverse probability weights. Comput Methods Programs Biomed 2004; 75:45–9. [DOI] [PubMed] [Google Scholar]
- 13.Howe CJ, Cole SR, Westreich DJ, Greenland S, Napravnik S, Eron JJ., Jr Splines for trend analysis and continuous confounder control. Epidemiology 2011; 22:874–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cox DR. Regression models and life tables. J R Stat Soc Series B Stat Methodol 1972; 34:187–220. [Google Scholar]
- 15.Efron B. The efficiency of Cox's likelihood function for censored data. J Am Stat Assoc 1977; 72:557–65. [Google Scholar]
- 16.Efron B, Tibshirani R. An introduction to the bootstrap. New York: Chapman & Hall, 1993. [Google Scholar]
- 17.Lin DY, Wei LJ. The robust inference for the proportional hazards model. J Am Stat Assoc 1989; 84:1074–8. [Google Scholar]
- 18.CDC WONDER, 2015. Available at: http://wonder.cdc.gov/ Accessed 22 January 2015.
- 19.Arias E. United States life tables, 2009. Natl Vital Stat Rep 2014; 62:1–63. [PubMed] [Google Scholar]
- 20.Hall HI, Frazier EL, Rhodes P, et al. Differences in human immunodeficiency virus care and treatment among subpopulations in the United States. JAMA Intern Med 2013; 173:1337–44. [DOI] [PubMed] [Google Scholar]
- 21.Whiteside YO, Cohen SM, Bradley H, et al. Progress along the continuum of HIV care among blacks with diagnosed HIV- United States, 2010. MMWR Morb Mortal Wkly Rep 2014; 63:85–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Mugavero MJ, Lin HY, Allison JJ, et al. Racial disparities in HIV virologic failure: do missed visits matter? J Acquir Immune Defic Syndr 2009; 50:100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Markides KS, Eschbach K. Aging, migration, and mortality: current status of research on the Hispanic paradox. J Gerontol B Psychol Sci Soc Sci 2005; 60(Spec No 2):68–75. [DOI] [PubMed] [Google Scholar]
- 24.Hall HI, Gray KM, Tang T, Li J, Shouse L, Mermin J. Retention in care of adults and adolescents living with HIV in 13 U.S. areas. J Acquir Immune Defic Syndr 2012; 60:77–82. [DOI] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention. Vital signs: HIV prevention through care and treatment--United States. MMWR Morb Mortal Wkly Rep 2011; 60:1618–23. [PubMed] [Google Scholar]
- 26.Yehia BR, Fleishman JA, Metlay JP, et al. Comparing different measures of retention in outpatient HIV care. AIDS 2012; 26:1131–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weinick RM, Jacobs EA, Stone LC, Ortega AN, Burstin H. Hispanic healthcare disparities: challenging the myth of a monolithic Hispanic population. Med Care 2004; 42:313–20. [DOI] [PubMed] [Google Scholar]
- 28.Franzini L, Ribble JC, Keddie AM. Understanding the Hispanic paradox. Ethn Dis 2001; 11:496–518. [PubMed] [Google Scholar]
- 29.Antiretroviral Therapy Cohort Collaboration. Life expectancy of individuals on combination antiretroviral therapy in high-income countries: a collaborative analysis of 14 cohort studies. Lancet 2008; 372:293–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet 2002; 360:119–29. [DOI] [PubMed] [Google Scholar]
- 31.Arici C, Ripamonti D, Maggiolo F, et al. Factors associated with the failure of HIV-positive persons to return for scheduled medical visits. HIV Clin Trials 2002; 3:52–7. [DOI] [PubMed] [Google Scholar]
- 32.Harrison KM, Song R, Zhang X. Life expectancy after HIV diagnosis based on national HIV surveillance data from 25 states, United States. J Acquir Immune Defic Syndr 2010; 53:124–30. [DOI] [PubMed] [Google Scholar]
- 33.Simard EP, Fransua M, Naishadham D, Jemal A. The influence of sex, race/ethnicity, and educational attainment on human immunodeficiency virus death rates among adults, 1993–2007. Arch Intern Med 2012; 172:1591–8. [DOI] [PubMed] [Google Scholar]
- 34.Kaufman JS, Cooper RS, McGee DL. Socioeconomic status and health in blacks and whites: the problem of residual confounding and the resiliency of race. Epidemiology 1997; 8:621–8. [PubMed] [Google Scholar]
- 35.Jones NA, Bullock J. The two or more races population: 2010. In: US Department of Commerce EaSA, ed. 2010 Census Briefs 2012. [Google Scholar]
- 36.HIV Surveillance Report, 2011. Centers for Disease Control and Prevention, 2013. [Google Scholar]
- 37.Hernando V, Sobrino-Vegas P, Burriel MC, et al. Differences in the causes of death of HIV-positive patients in a cohort study by data sources and coding algorithms. AIDS 2012; 26:1829–34. [DOI] [PubMed] [Google Scholar]
- 38.Weber R, Ruppik M, Rickenbach M, et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med 2013; 14:195–207. [DOI] [PubMed] [Google Scholar]
- 39.National HIV/AIDS Strategy for the United States. Office of National AIDS Policy 2010.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.