Key Points
Elimination of cranial radiation from therapy for childhood acute lymphoblastic leukemia has improved body-composition outcomes.
Survivors of childhood acute lymphoblastic leukemia treated without cranial radiation remain at risk for impaired fitness.
Abstract
There is limited information on body composition, energy balance, and fitness among survivors of childhood acute lymphoblastic leukemia (ALL), especially those treated without cranial radiation therapy (CRT). This analysis compares these metrics among 365 ALL survivors with a mean age of 28.6 ± 5.9 years (149 treated with and 216 without CRT) and 365 age-, sex-, and race-matched peers. We also report risk factors for outcomes among survivors treated without CRT. Male survivors not exposed to CRT had abnormal body composition when compared with peers (% body fat, 26.2 ± 8.2 vs 22.7 ± 7.1). Survivors without CRT had similar energy balance but had significantly impaired quadriceps strength (−21.9 ± 6.0 Newton-meters [Nm]/kg, 60°/s) and endurance (−11.4 ± 4.6 Nm/kg, 300°/s), exercise capacity (−2.0 ± 2.1 ml/kg per minute), low-back and hamstring flexibility (−4.7 ± 1.6 cm), and dorsiflexion range of motion (−3.1 ± 0.9°) and higher modified total neuropathy scores (+1.6 ± 1.1) than peers. Cumulative asparaginase dose ≥120 000 IU/m2 was associated with impaired flexibility, vincristine dose ≥39 mg/m2 with peripheral neuropathy, glucocorticoid (prednisone equivalent) dose ≥8000 mg/m2 with hand weakness, and intrathecal methotrexate dose ≥225 mg with dorsiflexion weakness. Physical inactivity was associated with hand weakness and decreased exercise capacity. Smoking was associated with peripheral neuropathy. Elimination of CRT from ALL therapy has improved, but not eliminated, body-composition outcomes. Survivors remain at risk for impaired fitness.
Introduction
Treatment evolution for childhood acute lymphoblastic leukemia (ALL) over the past 5 decades has resulted in 5-year survival rates exceeding 90%.1 With over 60 000 survivors of childhood ALL living in the United States,2 research has been focused on long-term medical and psychosocial outcomes and treatment-related risk factors. By the age of 50 years, it is estimated that over 40% of ALL survivors will have at least 1 severe, disabling, or life-threatening chronic condition.3 Risk for adverse energy balance, including obesity,4 poor fitness,5 suboptimal dietary intake,6,7 and physical inactivity,3 are increased in ALL survivors when compared with peers. This is likely both the result of and a contributor to poor health status. Childhood ALL survivors have the opportunity to influence their own long-term health by adopting a lifestyle that optimizes energy balance. Much of the research relating to body composition, energy balance, and fitness has focused on the impact of cranial radiation therapy (CRT) on increased risk.8-10 There is a paucity of this information among ALL survivors whose treatment did not include CRT.
The aims of this study were to (1) describe body composition, energy balance, and fitness among adult survivors of childhood ALL, contrasting those treated with and without CRT; (2) compare these metrics of ALL survivors to those of an age-, sex-, and race-matched comparison population; and (3) evaluate associations between these metrics and host-, cancer-, treatment-, and behavior-related variables among survivors not exposed to CRT.
Methods
Participants
Participants are members of the St. Jude Lifetime Cohort (SJLIFE),11 a study designed to characterize health outcomes among aging survivors of childhood cancer. Participants previously treated for childhood cancer at St. Jude Children’s Research Hospital (SJCRH), at least 10 years from diagnosis and ≥18 years of age, are invited to return to campus, where they undergo risk-based medical screening according to the Children’s Oncology Group Long Term Follow-Up Guidelines12 and a core battery of laboratory tests. For this ancillary study, eligible participants included those diagnosed with ALL between 1980 and 2003 when ≤18 years of age, without congenital neuromusculoskeletal or cardiopulmonary impairments, who were not undergoing current treatment of cancer. A comparison group, matched with survivors on age, sex, and race, was recruited from the population of friends, relatives, and family members of current patients at SJCRH (www.clinicaltrials.gov #NCT01047020). A random sample of SJCRH patients receiving active treatment or clinical follow-up (not including SJLIFE) and scheduled to be seen on campus was selected. Parents of patients were contacted to determine their interest, or interest of other adult family members or friends, in participating in the study. If interest was expressed, then a roster of interested family members/friends was obtained. Study staff then selected from the roster 1 individual who fulfilled matching criteria, who they screened for eligibility and invited to participate. Inclusion criteria were identical for the 2 groups, except the comparison group did not have a history of childhood cancer. The protocol and study documents were approved by the institutional review board. Participants provided written informed consent prior to completing study measures. All participants received a per-day financial compensation to help offset any inconveniencies resulting from their participation.
Body composition
Body composition was evaluated with standard anthropometric measures and dual x-ray absorptiometry. Height in centimeters and weight in kilograms were measured with a wall-mounted stadiometer (SECA, Hanover, MD) and an electronic scale (Scale-tronix, White Plains, NY), respectively. Waist circumference in centimeters was measured with a Gulick tape measure and divided by height to characterize waist-to-height ratio. Fat and lean mass were measured with dual x-ray absorptiometry (QDR 4500, software version 13.3:3; Hologic, Bedford, MA) in the total-body scanning mode.13-17 The scanner was calibrated weekly with known phantoms to minimize machine drift. Percent body fat and percent lean mass were calculated by dividing fat mass and fat-free mass by total body mass and multiplying the result by 100. Relative lean mass was calculated by dividing lean mass (kg) by height squared (m2).
Energy expenditure
Resting energy expenditure (REE) was evaluated with indirect calorimetry (Ultima CardiO2; MCG Diagnostics, St. Paul, MN). Participants rested in the supine position wearing a face mask attached to a low-flow pneumotach for at least 20 minutes prior to the 10-minute measurement of REE.18 REE was calculated using the modified Weir equation.19
Total daily energy expenditure (TDEE) was evaluated over 1 week with the doubly labeled water (DLW) technique.20,21 This technique evaluates the difference between elimination rates of oxygen and hydrogen from body water, representing a measure of carbon dioxide (CO2) flux, which is proportional to total energy expenditure. After baseline collection of urine samples, participants drank 2 g/kg of total body water of 10% H218O and 0.12 g/kg TBW of 2H2O, and 100 mL of tap water was used to rinse the dose container. Participants provided urine samples 1.5 and 3 hours (that were discarded) and then 4.5 and 6 hours after the dose and 2 samples in the morning 7 days later. Samples were frozen and batched shipped to the University of Pittsburgh for analysis. Isotope abundances were measured for H218O with automated peripheral devices (Finnigan GasBenchII and H/D device; Thermo Fisher Scientific GmbH, Bremen, Germany) interfaced with isotope ratio mass spectrometry (Delta PlusXP, Thermo Fisher Scientific).22 2H and 18O isotope elimination rates (kH and kO) were estimated using linear regression to include in calculations for rate of CO2 production.21,23 Energy expenditure was calculated by multiplying rCO2 by the energy equivalent of CO2 for an assumed respiratory quotient of 0.86. Activity energy expenditure was calculated by subtracting both REE and the thermal effect of food (0.10 × TDEE) from TDEE.
Dietary intake
Usual dietary intake was estimated with 3 nonconsecutive 24-hour dietary recalls (2 weekday and 1 weekend day over 1 month). The first recall was done in-person; subsequent recalls were collected via telephone by interviewers trained and certified by the University of Minnesota Nutrition Coordinating Center in the use of the Nutrition Data System for Research (NDS-R V2008-2011). Types and amounts of foods and beverages consumed during a 24-hour period (midnight to midnight) for the day preceding the interview were recorded using the standardized multiple-pass approach.24
Fitness
Fitness was evaluated by assessing flexibility, sensory integrity, balance, muscular strength, and exercise capacity. Flexibility was evaluated with the sit and reach test (Flex-tester; Novel Products, Rockton, IL)25 and by measuring passive ankle range of motion.26 Sensory integrity and balance were evaluated with the modified total neuropathy score (mTNS)27 and computerized dynamic posturography (sensory organization test, Smart Equitest; NeuroCom, Clackamas, OR),28 respectively. Muscular strength assessments included isometric sitting hand grip (kg)29 and isokinetic knee extension (Newton-meters [Nm]/kg at 60 and 300°/s) and ankle dorsiflexion (Nm/kg at 30°/s and 90°/s) measures (Biodex System III, Shirley NY).30 Resting heart and respiratory rates and blood pressure were taken after 5 minutes of quiet rest using standard procedures. Submaximal (85% of predicted heart rate) cardiopulmonary exercise testing, with continuous 12-lead electrocardiogram monitoring and breath-by-breath gas analysis (Ultima CardiO2, MCG Diagnostics), was used to evaluate peak oxygen uptake (peak volume of oxygen [VO2]).31
Moderate and vigorous physical activity
Average daily levels of moderate and vigorous physical activity (MVPA) were evaluated with actigraphy. Participants wore a triaxial accelerometer (wGT3X-BT; ActiGraph, Pensicola FL) over the right hip during waking hours for 7 days, removing it for bathing. The device collects linear accelerations in 3 planes of movement as counts per a preset epoch. Epochs were set at 60 s. ActiLife software (version 6.11.5) was used to process the accelerometer data with thresholds for MVPA set at 1952 and 5725 counts per epoch.32
Host and treatment data
Treatment data including radiation exposure and types and cumulative doses of chemotherapeutic agents were obtained from medical records by trained abstractors. Sociodemographic data and self-reported smoking status were obtained by self-administered questionnaires.
Statistics
Descriptive statistics were used to characterize ALL survivors and the comparison group. Demographic and treatment data were compared between survivor participants and nonparticipants with 2-sample t tests and χ2 statistics. Means and standard deviations (SDs) were calculated for body composition, energy balance, and fitness measures and contrasted between survivors and the comparison group, overall and by CRT exposure. For these comparisons, significance levels were set a priori at P < .002 to account for multiple testing. In models limited to survivors not exposed to CRT, multivariable linear regression was used to evaluate associations between demographic and treatment variables and the body-composition, energy-balance, and fitness outcomes. Demographic and treatment variables were entered simultaneously into the model. Independent variables with P values <.10 were retained. Those with P values <.05 were considered significant. All models were adjusted for age and sex. Analyses were completed by K.K.N., L.L., and D.K.S. in SAS version 9.3 (Cary, NC). All authors had access to the data.
Results
Participants
Among 416 potentially eligible ALL survivors, 365 (87.7%) agreed to participate and completed an evaluation; 51 either actively or passively declined participation. Among 451 potentially eligible comparison group members, 365 (80.9%) agreed to participate and completed an evaluation; 86 actively declined participation. Survivor participants did not differ from survivor nonparticipants by age, age at diagnosis, time from diagnosis, sex, race, or cranial radiation exposure. Except for a higher median dose of prednisone among survivor nonparticipants, survivor participants and nonparticipants received similar chemotherapeutic treatment exposures (Table 1). Demographic characteristics of survivors and comparison group members are shown in Table 2. By study design, survivors and comparison-group members had similar mean ages (28.6 ± 5.9 years vs 28.9 ± 7.5 years) and identical distributions by sex (47.7% male) and race (12.1% black). Survivors and comparison-group members did not differ by smoking status. However, survivors were less likely than comparison group members to report risky drinking or to have graduated from college and more likely to be unemployed. Due to availability of DLW isotopes, the numbers of survivors and comparison-group members who completed this measure were 317 and 259, respectively. Demographic characteristics did not differ among those who completed and those who did not complete the DLW measure.
Table 1.
Characteristics of ALL survivor participants and nonparticipants
| Participants (n = 365) | Nonparticipants (n = 51) | P | |
|---|---|---|---|
| Age, median (range), y | 28.5 (18.4,44.6) | 27.3 (18.0-45.9) | .09 |
| Diagnosis age, median (range), y | 5.1 (0.6-18.8) | 4.6 (2.1-18.8) | .65 |
| Survival time, median (range), y | 21.9 (11.0-30.7) | 20.3 (11.3-27.8) | .11 |
| Sex | |||
| Male, n (%) | 174 (47.7) | 25 (49.0) | .89 |
| Female, n (%) | 191 (52.3) | 26 (51.0) | |
| Race | |||
| Black, n (%) | 44 (12.1) | 5 (9.8) | .89 |
| White, n (%) | 317 (86.8) | 46 (90.2) | |
| Other, n (%) | 4 (1.1) | 0 (0.0) | |
| Cranial radiation, n (%) | 149 (40.8) | 18 (35.3) | .13 |
| Glucocorticoids | |||
| Prednisone, n (%) | 365 (100.0) | 51 (100.0) | |
| Median (range), mg/m2 | 2240 (200-23 600) | 9650 (1120-11 800) | <.001 |
| Dexamethasone, n (%) | 75 (20.5) | 6 (11.7) | |
| Median (range), mg/m2 | 1568 (72-12 880) | 1568 (1400-1624) | .83 |
| IT hydrocortisone, n (%) | 291 (79.7) | 47 (92.2) | |
| Median (range) | 391 (18-105) | 369 (104 762) | .38 |
| Antimetabolites | |||
| IV methotrexate, n (%) | 320 (87.7) | 46 (90.2) | |
| Median (range), mg/m2 | 14 757 (449-40 571) | 13 424 (4753-25 683) | .89 |
| IT methotrexate (N,%) | 365 (100.0) | 51 (100.0) | |
| Median (range), mg | 156 (12-458) | 162 (80-336) | .62 |
| 6-Mercaptopurine, n (%) | 357 (97.8) | 51 (100.0) | |
| Median (range) mg/m2 | 47 250 (5994-130 900) | 47 250 (6185-74 900) | .48 |
| IT cytarabine, n (%) | 298 (81.6) | 48 (94.1) | |
| Median (range), mg/m2 | 576 (73-11 516) | 565 (108-1142) | .38 |
| Vincristine, n (%) | 365 (100.0) | 51 (100.0) | |
| Median (range), mg/m2 | 47 (3-105) | 54 (5-118) | .06 |
| Anthracyclines | |||
| Daunorubicin, n (%) | 242 (66.3) | 40 (78.4) | |
| Median (range) mg/m2 | 75 (24-451) | 87 (48-158) | .84 |
| Doxorubicin, n (%) | 40 (11.0) | 3 (5.9) | |
| Median (range), mg/m2 | 179 (25-324) | 169 (151-187) | 1.00 |
| Epipodophyllotoxins | |||
| Etoposide, n (%) | 223 (61.1) | 36 (70.6) | |
| Median (range), mg/m2 | 9462 (400-23 630) | 9978 (883-16 215) | .54 |
| Teniposide, n (%) | 220 (60.3) | 28 (54.9) | |
| Median (range), mg/m2 | 3241 (150-10 339) | 3478 (597-7983) | .84 |
| Asparaginase | |||
| L-asparaginase, n (%) | 361 (98.9) | 51 (100.0) | |
| Median (range), IU/m2 | 70 179 (4000-99 246) | 92 051 (40 308-347 783) | .01 |
| Erwinia, n (%) | 46 (12.9) | 5 (9.8) | |
| Median (range), IU/m2 | 54 980 (10 000-118 507) | 113 398 (20 000-247 083) | .42 |
IT, intrathecal.
Table 2.
Characteristics of the study participants
| Survivors | Comparison group (n = 365) | P† | |||
|---|---|---|---|---|---|
| Overall (n = 365) | No CRT (n = 216) | CRT (n = 149)* | |||
| Mean age (SD), y | 28.6 (5.9) | 26.9 (5.4) | 31.2 (5.7) | 28.9 (7.5) | .48 |
| Age | |||||
| 18-29 y | 212 (58.0) | 152 (70.4) | 60 (40.3) | 216 (59.2) | .73 |
| 30-39 y | 132 (36.2) | 57 (26.4) | 75 (50.3) | 124 (33.9) | |
| 40-45 y | 21 (5.8) | 7 (3.2) | 14 (9.4) | 25 (6.8) | |
| Sex | |||||
| Male | 191 (47.7) | 107 (49.5) | 84 (56.4) | 191 (47.7) | 1.00 |
| Female | 174 (52.3) | 109 (50.5) | 65 (43.6) | 174 (52.3) | |
| Race | |||||
| Black | 44 (12.1) | 20 (9.3) | 24 (16.1) | 44 (12.1) | 1.00 |
| White | 317 (86.8) | 195 (90.3) | 122 (81.9) | 317 (86.8) | |
| Other | 4 (1.1) | 1 (0.5) | 3 (2.0) | 4 (1.1) | |
| Ethnicity | |||||
| Hispanic | 16 (4.4) | 10 (4.6) | 6 (4.0) | 16 (4.4) | 1.00 |
| Non-Hispanic | 349 (95.6) | 206 (95.4) | 143 (96.0) | 349 (95.6) | |
| Smoking | |||||
| Never | 236 (64.7) | 128 (59.3) | 108 (72.5) | 229 (62.7) | .67 |
| Ever | 38 (10.4) | 26 (12.0) | 12 (8.1) | 37 (10.4) | |
| Current | 91 (24.9) | 62 (28.7) | 29 (19.5) | 99 (28.3) | |
| Risky drinking‡ | 157 (43.0) | 105 (48.6) | 52 (34.8) | 179 (49.0) | .02 |
| College degree | 118 (35.2) | 64 (29.6) | 54 (36.2) | 150 (43.4) | .01 |
| Unemployed | 86 (23.6) | 48 (22.2) | 38 (25.5) | 60 (16.4) | .01 |
Data are presented as n (%) unless otherwise indicated.
A total of 41 received ≥24 Gy; 108 received <24 Gy CRT.
Survivors overall vs comparison group.
More than 4 drinks in 1 day or >14 drinks in 1 week for males and >3 drinks in 1 day or >7 drinks in 1 week for women.
Body composition
Means and SDs for body composition, energy balance, and dietary intake among survivors (by CRT exposure) and in comparison group members are shown in Table 3. On average, ALL survivors treated with CRT were shorter but had similar weight and, thus, had higher body mass index (BMI) values than comparison group members. Waist circumference, waist to height ratio, and total and percent fat mass were also higher among male survivors and among CRT-exposed female survivors than in comparison-group members. Lean mass and lean mass relative to height were lower among male survivors and CRT-exposed female survivors than in comparison-group members. Female survivors not exposed to CRT did not differ from the comparison group for mean values of body-composition variables. Survivors exposed to CRT had higher BMI and percent body fat values than survivors not exposed to CRT.
Table 3.
Mean body composition and energy balance among ALL survivors (by CRT exposure) and comparison group members
| No CRT | CRT | Comparison group | P* | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | A vs C | B vs C | A vs B | |
| Females† | |||||||||
| Body composition | |||||||||
| Height (cm) | 163.9 | 6.3 | 158.0 | 7.5 | 164.2 | 5.4 | .82 | <.001 | <.001 |
| Weight (kg) | 71.1 | 18.3 | 81.2 | 24.3 | 74.9 | 22.2 | .28 | .07 | .01 |
| BMI (kg/m2) | 26.4 | 6.4 | 32.3 | 8.5 | 27.8 | 8.0 | .24 | <.001 | <.001 |
| Waist circumference (cm) | 81.6 | 14.0 | 93.6 | 18.1 | 82.8 | 16.9 | .87 | <.001 | <.001 |
| Waist to height ratio | 0.5 | 0.1 | 0.6 | 0.1 | 0.5 | 0.1 | .81 | <.001 | <.001 |
| Fat mass (kg) | 25.2 | 11.3 | 34.6 | 14.7 | 27.6 | 14.5 | .26 | <.001 | <.001 |
| Percent body fat | 34.0 | 7.5 | 41.3 | 6.2 | 34.9 | 8.5 | .53 | <.001 | <.001 |
| Fat-free mass (kg) | 45.9 | 8.1 | 46.6 | 10.8 | 47.3 | 8.7 | .39 | .47 | .99 |
| Percent fat-free mass | 66.0 | 7.5 | 58.7 | 6.2 | 65.1 | 8.5 | .53 | <.001 | <.001 |
| Relative lean mass (g/m2) | 17.2 | 2.9 | 18.4 | 3.0 | 17.5 | 3.7 | .36 | .03 | .006 |
| Energy expenditure (kcal) | |||||||||
| Resting (adjusted for lean mass) | 1339 | 271 | 1297 | 266 | 1330 | 263 | .69 | .26 | .22 |
| Total daily (adjusted for weight)‡ | 2840 | 438 | 2432 | 419 | 2598 | 475 | <.001 | .009 | <.001 |
| Activity energy expenditure (adjusted for weight)‡ | 1232 | 469 | 949 | 443 | 1032 | 488 | <.001 | .21 | <.001 |
| Daily average minutes of MVPA | 14 | 136 | 11 | 10 | 17 | 14 | .1 | .005 | .27 |
| Energy intake | |||||||||
| Kcal total | 1822 | 595 | 1737 | 570 | 1815 | 594 | .92 | .46 | .35 |
| Kcal fat | 621 | 261 | 620 | 255 | 619 | 264 | .97 | .98 | .99 |
| Kcal protein | 279 | 94 | 282 | 94 | 275 | 92 | .68 | .61 | .88 |
| Kcal carbohydrate | 911 | 324 | 823 | 321 | 893 | 330 | .65 | .14 | .08 |
| Sodium (mg) | 3018 | 1106 | 3101 | 1091 | 3049 | 1121 | .82 | .74 | .63 |
| Fiber (g) | 13 | 10 | 12 | 6 | 14 | 6 | .47 | .02 | .09 |
| Estimated % dietary intake underreporting (TDEE − total/TDEE) | 30.4 | 29.5 | 29.5 | 27.7 | 28.2 | 31.4 | .55 | .76 | .83 |
| Males§ | |||||||||
| Body composition | |||||||||
| Height (cm) | 176.1 | 8.3 | 172.9 | 7.9 | 178.9 | 7.0 | .004 | <.001 | .003 |
| Weight (kg) | 87.7 | 24.7 | 87.8 | 18.6 | 87.3 | 19.1 | .24 | .39 | .09 |
| BMI (kg/m2) | 28.8 | 7.2 | 28.4 | 5.1 | 27.2 | 5.6 | .02 | .12 | .62 |
| Waist circumference (cm) | 91.1 | 16.9 | 95.3 | 12.3 | 89.0 | 13.5 | .007 | .04 | .74 |
| Waist to height ratio | 0.5 | 0.1 | 0.6 | 0.1 | 0.5 | 0.1 | <.001 | <.001 | .52 |
| Fat mass (kg) | 25.1 | 14.2 | 23.6 | 9.6 | 20.9 | 11.0 | .002 | .07 | .41 |
| Percent body fat | 26.2 | 8.2 | 27.2 | 6.0 | 22.7 | 7.1 | <.001 | <.001 | .34 |
| Fat-free mass (kg) | 64.9 | 12.5 | 61.1 | 11.1 | 66.4 | 9.9 | .28 | <.001 | .02 |
| Percent fat free mass | 74.5 | 8.2 | 71.9 | 6.0 | 77.3 | 7.1 | <.001 | <.001 | .34 |
| Relative lean mass (g/m2) | 20.8 | 2.8 | 20.4 | 2.8 | 20.7 | 2.8 | .75 | .43 | .35 |
| Energy expenditure (kcal) | |||||||||
| Resting (adjusted for lean mass) | 1636 | 393 | 1600 | 403 | 1666 | 401 | .53 | .21 | .54 |
| Total daily (adjusted for weight)|| | 3506 | 631 | 3290 | 576 | 3423 | 691 | .29 | .11 | .02 |
| Activity energy expenditure (adjusted for weight)|| | 1533 | 693 | 1406 | 651 | 1452 | 760 | .35 | .61 | .2 |
| Daily average minutes of MVPA | 21 | 14 | 18 | 14.7 | 25.2 | 19.7 | .07 | .004 | .3 |
| Energy intake | |||||||||
| Kcal total | 2409 | 776 | 2400 | 788 | 2425 | 788 | .87 | .8 | .93 |
| Kcal fat | 841 | 300 | 829 | 312 | 838 | 401 | .93 | .82 | .78 |
| Kcal protein | 388 | 134 | 372 | 135 | 400 | 138 | .48 | .13 | .42 |
| Kcal carbohydrate | 1129 | 419 | 1180 | 422 | 1120 | 428 | .86 | .28 | .4 |
| Sodium (mg) | 4193 | 1386 | 3863 | 1402 | 4114 | 1451 | .64 | .18 | .11 |
| Fiber (g) | 14 | 7 | 16 | 8 | 18 | 8 | .004 | .29 | .14 |
| Estimated % dietary intake underreporting (TDEE − total/TDEE) | 27.7 | 28.7 | 24.6 | 27.4 | 27.5 | 31.5 | .97 | .44 | .45 |
A, no-CRT group; B, CRT group; C, comparison group; kcal, kilocalories.
Comparisons are adjusted for age.
No CRT , n = 109; CRT, n = 65; comparison group, n = 174.
Among females, 155 survivors and 131 comparison-group members completed the DLW measure. Energy expenditure was estimated with analysis of variance using fat-free mass or body weight as covariates.
No CRT , n = 107; CRT, n = 84; comparison group, n = 191.
Among males, 162 survivors and 128 comparison-group members completed the DLW measure. Energy expenditure was estimated with analysis of variance using fat-free mass or body weight as covariates.
Energy expenditure
After adjusting for lean mass, average REE was lower among survivors exposed to CRT than among comparison-group members (Table 3). Weight-adjusted TDEE differed between all groups and was lowest among survivors exposed to CRT. Activity energy expenditure, adjusted for body weight, was also lowest among survivors exposed to CRT. Daily minutes of MVPA were lower among ALL survivors treated with or without CRT than among comparison group members.
Dietary intake
Mean total and nutrient-specific daily dietary intake from 3 24-hour recalls did not differ between groups. Daily dietary intake was uniformly underreported by ∼30% across groups compared with TDEE.
Fitness
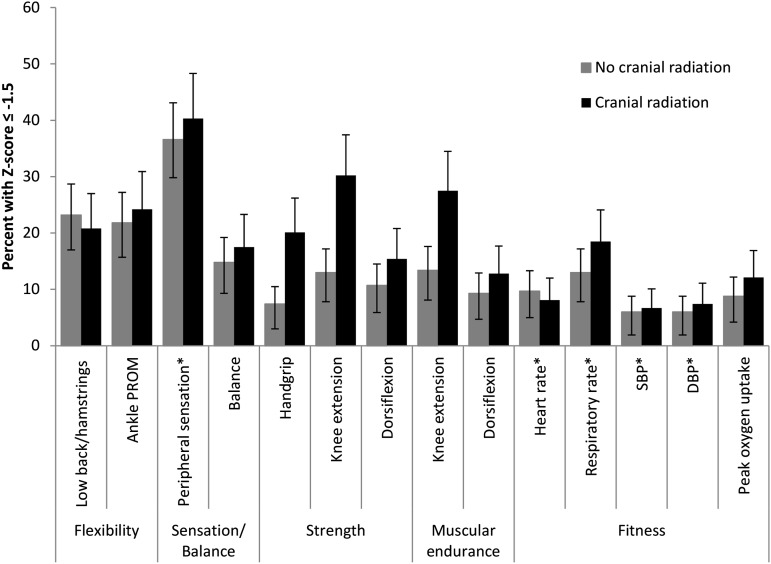
Fitness measures for ALL survivors and the percentage of survivors who scored ≤−1.5 SD based on comparison-group–based age- and sex-specific z scores are presented in Tables 4 and 5 and Figure 1. Sex-specific comparisons are shown in supplemental Tables 1 and 2 (available on the Blood Web site). On average, flexibility, peripheral sensory integrity, proximal muscle strength, and cardiopulmonary-fitness values were lower in ALL survivors than in comparison-group members. ALL survivors with CRT exposure had the lowest mean values for each of these outcomes. However, survivors not exposed to CRT also had significant fitness deficits with most effect sizes ≥0.5.
Table 4.
Means and SDs for fitness measures for ALL survivors by CRT exposures and comparison group members
| No CRT (n = 216) | CRT (n = 149) | Comparison group (n = 365) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A vs C | B vs C | A vs B | ||||||||||
| Mean | SD | Mean | SD | Mean | SD | ES | P | ES | P | ES | P | |
| Flexibility | ||||||||||||
| Sit and reach test (cm) | 22.4 | 10.8 | 20.9 | 10.4 | 27.1 | 9.2 | 1.5 | <.001 | 2.0 | <.001 | 0.5 | .98 |
| Passive ankle dorsiflexion range of motion (degrees) | 12.1 | 7.0 | 11.2 | 6.6 | 15.2 | 6.1 | 1.2 | <.001 | 1.6 | <.001 | 0.4 | .3 |
| Modified total neuropathy score (0-24) | 2.6 | 2.5 | 3.5 | 2.3 | 1.0 | 1.4 | 1.1 | <.001 | 1.9 | <.001 | 0.6 | <.001 |
| Sensory organization test (%) | 79.8 | 8.1 | 77.4 | 10.2 | 80.0 | 7.6 | 0.1 | .67 | 0.9 | .002 | 0.8 | .02 |
| Muscle strength | ||||||||||||
| Hand grip (kg) | 38.2 | 12.3 | 38.4 | 13.9 | 40.0 | 12.3 | 0.5 | .22 | 0.4 | <.001 | 0.1 | .02 |
| Quadriceps 60°/s (Nm/kg) | 182.5 | 57.1 | 158.3 | 54.7 | 204.4 | 63.1 | 2.8 | <.001 | 6.0 | <.001 | 3.2 | <.001 |
| Dorsiflexion 60°/s (Nm/kg) | 24.8 | 10.6 | 25.5 | 9.4 | 25.8 | 9.3 | 0.3 | .19 | 0.1 | .81 | 0.2 | .42 |
| Muscular endurance | ||||||||||||
| Quadriceps 300°/s (Nm/kg) | 81.7 | 30.5 | 69.4 | 24.2 | 93.1 | 35.1 | 2.0 | <.001 | 4.4 | <.001 | 2.3 | .002 |
| Dorsiflexion 300°/s (Nm/kg) | 22.2 | 8.1 | 22.0 | 7.7 | 22.6 | 7.8 | 0.1 | .33 | 0.2 | .48 | 0.1 | .90 |
| Cardiopulmonary fitness | ||||||||||||
| Resting heart rate | 72.5 | 10.9 | 74.5 | 10.6 | 70.8 | 11.4 | 0.5 | .10 | 1.1 | <.001 | 0.6 | .05 |
| Resting respiratory rate | 17.9 | 1.7 | 18.8 | 1.8 | 17.5 | 1.8 | 0.3 | <.001 | 1.1 | 0.001 | 0.6 | <.001 |
| Systolic blood pressure | 120.0 | 12.6 | 120.6 | 12.1 | 121.7 | 11.3 | 0.5 | .21 | 0.3 | .12 | 0.2 | .69 |
| Diastolic blood pressure | 72.7 | 8.4 | 75.3 | 8.5 | 72.9 | 8.3 | 0.1 | .73 | 0.8 | .02 | 0.9 | .06 |
| Peak VO2 (mL/kg per min) | 25.5 | 6.2 | 22.1 | 5.5 | 27.5 | 8.3 | 0.7 | <.001 | 2.1 | <.001 | 1.4 | <.001 |
A, no-CRT group; B, CRT group; C, comparison group; ES, effect size.
Table 5.
Percentages of ALL survivors whose performance on fitness measures is 1.5 SD lower (or higher) than age- and sex-specific comparison-group values
| No CRT (n = 216) | CRT (n = 149) | |||
|---|---|---|---|---|
| % | 95% CI | % | 95% CI | |
| Flexibility | ||||
| Sit and reach test | 23.2 | (17.7, 29.4) | 20.8 | (14.6, 28.2) |
| Passive ankle dorsiflexion range of motion | 21.8 | (16.4, 27.9) | 24.2 | (17.5, 31.8) |
| Modified total neuropathy score | 36.6 | (30.1, 43.4) | 40.3 | (32.3, 48.6) |
| Sensory organization test* | 14.8 | (10.4, 20.3) | 17.5 | (11.7, 24.5) |
| Muscle strength | ||||
| Hand grip | 7.4 | (4.3, 11.8) | 20.1 | (14.0, 27.5) |
| Quadriceps 60°/s | 13.0 | (8.8, 18.2) | 30.2 | (23.0, 38.3) |
| Dorsiflexion 60°/s | 10.7 | (6.9, 15.5) | 15.4 | (10.0, 22.3) |
| Muscular endurance | ||||
| Quadriceps 300°/s | 13.4 | (9.2, 18.7) | 27.5 | (20.5, 35.4) |
| Dorsiflexion 90°/s | 9.3 | (5.7, 13.9) | 12.8 | (7.9, 19.2) |
| Cardiopulmonary fitness | ||||
| Resting heart rate* | 9.7 | (6.1, 14.4) | 8.1 | (4.2, 13.6) |
| Resting respiratory rate* | 13.0 | (8.8, 18.2) | 18.5 | (12.9, 26.0) |
| Systolic blood pressure* | 6.0 | (3.2,10.1) | 6.7 | (3.3, 12.0) |
| Diastolic blood pressure* | 6.0 | (3.2, 10.1) | 7.4 | (3.7, 12.8) |
| Peak VO2 | 8.8 | (5.4, 13.4) | 12.1 | (7.3, 18.4) |
The expected percentage in the population is 6.7%.
CI, confidence interval.
Value is 1.5 SD higher than age- and sex-specific comparison-group values.
Figure 1.
Percentages (and 95% confidence intervals) of ALL survivors whose performance on fitness measures is 1.5 SD lower (or higher*) than age and sex-specific comparison-group values.# Low-back and hamstring flexibility was measured with the sit and reach test. Peripheral sensation was measured with the mTNS. Strength in the knee extensors and dorsiflexors was evaluated with isokinetic dynamometry at 60°/s. Muscular endurance was evaluated with isokinetic dynamometry at 300°/s in the quadriceps and 90°/s in the dorsiflexors. DBP, diastolic blood pressure; SBP, systolic blood pressure. #The expected percentage in the population is 6.7%.
Associations between treatment and fitness
Results of multivariable linear regression analyses evaluating associations between host and treatment factors and fitness measures among survivors not exposed to CRT are shown in Table 6. After adjusting for age and sex, those with cumulative doses of asparaginase ≥120 000 IU/m2 had lower average performance on the sit and reach test of 4.8 (1.5) cm and less ankle dorsiflexion range of motion of 2.4 (1.1) degrees compared with those exposed to cumulative doses of <120 000 IU/m2. Cumulative doses of vincristine ≥39 mg/m2 were associated with a 1.1 (0.4) point higher score on the mTNS, cumulative doses of intrathecal methotrexate ≥225 mg were associated with a 4.1 (1.9) lower percent on the sensory organization test, and with 5.2 (2.4) Nm/kg lower dorsiflexion strength at 60°/s and 3.9 (1.9) Nm/kg lower dorsiflexion strength at 90°/s. Cumulative doses of glucocorticoids in prednisone equivalents ≥8000 mg/m2 were associated with 2.2 (1.0) kg lower grip strength when compared with those who had cumulative doses of <8000 mg/m2. Smoking was associated with an increased score on the mTNS. Participating in ≥30 minutes per day of moderate or vigorous physical activity was associated with higher grip strength and peak VO2. There were no significant treatment-related risk factors identified for quadriceps weakness.
Table 6.
Multivariable linear regression showing associations among host, treatment, and lifestyle factors among ALL survivors treated without CRT (n = 216)
| Sit and reach test (cm) | Ankle dorsiflexion ROM (°) | Modified total neuropathy score* | Sensory organization test (%) | Grip strength (kg) | Quadriceps strength 60°/s (Nm/kg) | Quadriceps strength 300°/s (Nm/kg) | Dorsiflexion strength 60°/s (Nm/kg) | Dorsiflexion strength 90°/s (Nm/kg) | Peak VO2 (mL/kg per min) | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| β | SE | P | β | SE | P | β | SE | P | β | SE | P | β | SE | P | β | SE | P | β | SE | P | β | SE | P | β | SE | P | β | SE | P | |
| Female sex | 9.51 | 1.32 | <.001 | 1.42 | 0.95 | .14 | 0.20 | 0.34 | .57 | −0.25 | 1.14 | .83 | −20.04 | 1.00 | <.001 | −50.96 | 6.79 | <.001 | −19.68 | 3.83 | <.001 | −4.92 | 1.40 | <.001 | −2.45 | 1.10 | .03 | −3.91 | 0.80 | <.001 |
| Age (y) | −0.39 | 0.13 | .003 | 0.04 | 0.09 | .65 | 0.07 | 0.03 | .10 | −0.12 | 0.11 | .27 | −0.21 | 0.10 | .03 | −1.98 | 0.63 | .002 | −1.28 | 0.36 | <.001 | −0.19 | 0.14 | .18 | −0.16 | 0.11 | .14 | −0.21 | 0.08 | .009 |
| Asparaginase ≥120 000 IU/m2 | −4.81 | 1.49 | .001 | −2.40 | 1.07 | .03 | NS | NS | NS | NS | NS | NS | NS | NS | ||||||||||||||||
| Glucocorticoids ≥8000 mg/m2† | NS | NS | NS | NS | −2.18 | 1.03 | .04 | NS | NS | NS | NS | NS | ||||||||||||||||||
| IT methotrexate ≥225 mg2 | NS | NS | NS | −4.11 | 1.93 | .03 | NS | NS | NS | −5.17 | 2.41 | .03 | −3.89 | 1.88 | 0.04 | NS | ||||||||||||||
| IV methotrexate ≥16 000 mg/m2 | NS | NS | NS | NS | NS | NS | NS | −2.87 | 1.53 | .06 | −1.84 | 1.20 | 0.12 | NS | ||||||||||||||||
| Vincristine ≥39 mg/m2 | NS | NS | 1.10 | 0.36 | .003 | NS | NS | NS | NS | NS | NS | NS | ||||||||||||||||||
| <30 min/day MVPA | NS | NS | NS | NS | −2.83 | 1.17 | .02 | NS | NS | NS | NS | −3.50 | 0.92 | <.001 | ||||||||||||||||
| Current smoker | NS | NS | 0.75 | 0.38 | .05 | NS | NS | NS | NS | NS | NS | NS | ||||||||||||||||||
Variables with P > .10 in univariate analyses were not included in multiple-variable models (anthracyclines, epipodophyllotoxins, cytarabine, 6-mercaptopurine); variables with P < .10 in multivariable models were retained.
NS, nonsignificant; ROM, range of motion; SE, standard error; IT, intrathecal.
Higher is worse.
Prednisone equivalent dose.
Discussion
In this large cohort of ALL survivors treated on contemporary therapeutic protocols, female survivors not exposed to CRT appear to have body-composition values similar to their peers. However, as with patients exposed to CRT, they remain at risk for fitness abnormalities, including problems with proximal strength, exercise capacity, flexibility, and peripheral sensorimotor integrity. We found associations between asparaginase exposure and muscle flexibility and between cumulative glucocorticoid exposure and hand strength, findings that have not been previously documented among long-term survivors of childhood ALL. Our results confirm previously documented associations between vincristine and peripheral sensorimotor impairments and intrathecal methotrexate exposure and balance problems, and we provide results of the most extensive clinical assessment of long-term ALL survivors evaluated decades following treatment. Our study also observed previously unreported associations with smoking and impaired peripheral sensorimotor integrity and that daily MVPA of ≥30 minutes was associated with increased grip strength and with exercise capacity.
Our finding that female survivors without CRT exposure are not at increased risk for abnormal body composition is consistent with reports from the Childhood Cancer Survivor Study (CCSS), where self-reported height and weight, represented as BMI, did not differ among adult survivors of childhood ALL treated without CRT (N = 333; age at follow-up, 32.3 ± 4.8 years) compared with siblings (N = 2167) at either their baseline or a follow-up questionnaire 7.8 years later. BMI values in the CCSS cohort (survivors 25.3 [SD, 5.1], siblings 26.0 [SD, 5.3] kg/m2) were somewhat lower than in our study (survivors 27.3 ± 6.9, comparison group 27.5 ± 6.9 kg/m2), potentially because CCSS participants self-reported height and weight.4 This should be good news for female ALL survivors not exposed to CRT. However, both our study and data from CCSS indicate that half of ALL survivors not exposed to CRT are overweight or obese, increasing their risk for other chronic health conditions, such as diabetes,33 heart disease,34 and some types of cancer.35 This problem is evident early in survivorship. Steinberger et al reported 10.4% higher fat mass among adolescent leukemia survivors when compared with a healthy comparison group.36 Clinicians who care for these survivors should provide counseling and, where appropriate, referral for intervention to help achieve a healthy weight.37
Our study clearly documents that fitness deficits remain a problem for long-term survivors of childhood ALL, even when they are not exposed to CRT. In our cohort, proximal muscle strength and exercise capacity were impaired in ALL survivors, even among survivors who were not exposed to CRT. Jarvela et al5 reported deficits in muscle performance and a 14% deficit in peak VO2 when comparing 21 ALL survivors 16 to 30 years of age to age- and sex-matched controls, and Hartman et al37 reported that mean performance on the 6-minute walk test was 2 SD below predicted, 5 years after therapy among 34 ALL survivors aged 9 to 18 years. In contrast, Taskinen et al38 reported no differences in performance on 6 timed measures of physical fitness when comparing 45 ALL survivors aged 9 to 20 years to established reference values.38 In our multivariable analysis among those not exposed to CRT, we found no specific chemotherapy agents or doses associated with proximal muscle weakness or exercise capacity. This suggests that exposure to cellular toxic therapies may confer nonspecific general damage to underlying cellular structures that persists years after exposure.
Limitations in ankle dorsiflexion range of motion have been reported by others,39-41 particularly in children during or soon after curative therapy. However, limitations in flexibility in the low back/hamstrings have not been described previously. This finding is in contrast to a study in a small group (N = 45) of younger (median age, 13.3; range, 9.2-20.1 years) ALL survivors treated without CRT, in which deficits in flexibility were not detected.38 In our cohort, impaired dorsiflexion and low-back/hamstring flexibility were associated with higher cumulative dose of asparaginase. Hovi et al previously described a similar association, but between l-asparaginase exposure and muscular endurance (performance on sit-up and push-up tests), in a group of 43 female ALL survivors 14 to 30 (mean, 19) years of age.42 The mechanisms by which exposure to asparaginase results in long-term muscular damage are not clear. However, asparaginase can increase systemic exposure to glucocorticoid, and both drugs are often given concomitantly.43,44 Moreover, asparaginase metabolizes both asparagine and glutamine, limiting extracellular supply of these amino acids and interfering with DNA, RNA, and protein synthesis. Acute effects on normal tissue include hepatotoxicity, hyperglycemia, and hemorrhage or thrombosis.45 Muscle cells may also be vulnerable. Muscular stiffness suggests potential depletion of muscle satellite cell pools, necessary for muscular repair and prevention of injury-related muscle fibrosis throughout life.46 Preclinical work that specifically examines muscle and associated satellite-cell response to asparaginase may provide insight into a potential mechanism of injury.
Our analysis confirmed previously reported associations between the cumulative dose of vincristine and peripheral sensorimotor impairment, and between the cumulative dose of intrathecal methotrexate and poor balance, among ALL survivors.47 Vincristine causes death of rapidly dividing leukemic cells by inhibiting assembly of microtubulin structures, thereby arresting mitosis in metaphase. Microtubule cytoskeletons of axons are also affected, with accumulation of neurofilaments in spinal ganglion cells suggesting problems with axonal transport.48 Methotrexate acts on leukemic cells and also likely impacts neurons by competitively inhibiting dihydrofolate reductase, depleting folic acid, and interfering with synthesis of DNA, RNA, thymidylates, and proteins.49-52
We also found that higher cumulative doses of glucocorticoids, which exert catabolic effects on muscle by inhibiting protein synthesis and by activating proteolysis, were associated with lower hand grip strength, but not with quadriceps strength. This discrepancy may be due to differential anatomical distribution of muscle fiber type. Glucocorticoid induced atrophy is characterized by decreased fiber cross-sectional area and reduced myofibrillar protein content in fast-twitch glycolytic muscles.53 Upper extremity muscles have a greater proportion of fast-twitch muscle fibers than the quadriceps.54 Because sensorimotor function and balance are necessary for negotiating daily environments55,56 and adequate hand strength is required for performance of fine motor tasks,57 these findings are important. All 3 of these agents are included in the backbone of current treatment protocols for children with ALL.58
Our data also suggested that lifestyle may play a role in fitness impairments observed among adult survivors of childhood ALL. Smoking was associated with reduced peripheral sensorimotor integrity and is a risk factor for peripheral vascular disease in other populations59; symptoms of peripheral vascular disease include both numbness and weakness. Because adult survivors of childhood ALL are potentially at increased risk for peripheral vascular disease,60 they should be counseled not to smoke. Daily MVPA was associated with exercise capacity, which is not unexpected.61 Because these outcomes were measured in a cross-sectional fashion, it is difficult to ascertain whether impaired exercise capacity limits ability to participate in MVPA or if low levels of MVPA limit exercise capacity. Nevertheless, adult survivors of childhood ALL may benefit from interventions designed to improve exercise capacity and promote regular physical activity.
Limitations
The results of our study should be considered in the context of several limitations. First, even though participation rates were excellent and our sample was representative of ALL survivors treated at our institution from 1980 to 2003, not all of our potentially eligible population agreed to participate. If survivors who were healthier chose not to participate, our estimates may be biased away from the null. Conversely, if survivors who were less healthy chose not to participate, our estimates may be biased toward the null. Second, our comparison population was composed of friends and family members of patients treated at our institution. They were, on average, overweight and less fit than expected based on published population norms.62 Thus, it is possible that results underestimate the magnitude of energy balance and fitness deficits experienced by ALL survivors. Third, several of our lifestyle measures (eg, smoking and diet) were based on self-report and may not be completely accurate. Although we used 24-hour dietary recalls to minimize recall bias, our dietary measure substantially underestimated daily intake for both survivors and the comparison group. Finally, our single-institution sample may not be completely representative of ALL survivors treated from 1980 to 2003.
Conclusions
Elimination of CRT for treatment of childhood ALL has improved, but not completely eliminated, deficits in body composition for this population. In addition, regardless of previous exposure to CRT, significant proportions of adult survivors of ALL are at risk for impaired flexibility, peripheral sensorimotor deficits, proximal muscle weakness, and poor exercise tolerance, which in the general population are deficits associated with increased risk of morbidity and mortality.63,64 Research is needed to determine if rehabilitation services during and immediately following therapy can prevent long-term impairments. Clinicians who treat these survivors should monitor them for these outcomes and make appropriate referrals for lifestyle interventions when appropriate.
Acknowledgments
This work was supported by National Institutes of Health National Cancer Institute grants CA132901 (K.K.N.) and CA21765 and by the American Lebanese Syrian Associated Charities.
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: K.K.N. designed the study, supervised data collection, interpreted and analyzed data, and jointly wrote the first manuscript draft; J.P.D. designed the study, supervised DLW specimen analysis, interpreted data, and jointly wrote the first manuscript draft; S.C.K. supervised collection and analysis of body composition data, interpreted data, and revised the manuscript; D.A.M., C.-H.P., and W.C. interpreted data and jointly wrote the first manuscript draft; R.E.K., C.R.H., and J.Q.L. coordinated data collection, interpreted data, and revised the manuscript; L.L. and D.K.S. analyzed and interpreted data and revised the manuscript; and L.L.R. and M.M.H. designed the study, interpreted data, and revised the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kirsten K. Ness, 262 Danny Thomas Pl, Mail Stop 735, Memphis, TN 38105; e-mail: kiri.ness@stjude.org.
References
- 1.Pui CH, Campana D, Pei D, et al. Treating childhood acute lymphoblastic leukemia without cranial irradiation. N Engl J Med. 2009;360(26):2730–2741. doi: 10.1056/NEJMoa0900386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review (CSR) 1975-2011. http://seer.cancer.gov/csr/1975_2011/. Accessed April 30, 2014.
- 3.Wilson CL, Stratton K, Leisenring WL, et al. Decline in physical activity level in the Childhood Cancer Survivor Study cohort. Cancer Epidemiol Biomarkers Prev. 2014;23(8):1619–1627. doi: 10.1158/1055-9965.EPI-14-0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garmey EG, Liu Q, Sklar CA, et al. Longitudinal changes in obesity and body mass index among adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26(28):4639–4645. doi: 10.1200/JCO.2008.16.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Järvelä LS, Niinikoski H, Lähteenmäki PM, et al. Physical activity and fitness in adolescent and young adult long-term survivors of childhood acute lymphoblastic leukaemia. J Cancer Surviv. 2010;4(4):339–345. doi: 10.1007/s11764-010-0131-0. [DOI] [PubMed] [Google Scholar]
- 6.Robien K, Ness KK, Klesges LM, Baker KS, Gurney JG. Poor adherence to dietary guidelines among adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2008;30(11):815–822. doi: 10.1097/MPH.0b013e31817e4ad9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith WA, Li C, Nottage KA, et al. Lifestyle and metabolic syndrome in adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort Study. Cancer. 2014;120(17):2742–2750. doi: 10.1002/cncr.28670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gurney JG, Ness KK, Stovall M, et al. Final height and body mass index among adult survivors of childhood brain cancer: childhood cancer survivor study. J Clin Endocrinol Metab. 2003;88(10):4731–4739. doi: 10.1210/jc.2003-030784. [DOI] [PubMed] [Google Scholar]
- 9.Meacham LR, Gurney JG, Mertens AC, et al. Body mass index in long-term adult survivors of childhood cancer: a report of the Childhood Cancer Survivor Study. Cancer. 2005;103(8):1730–1739. doi: 10.1002/cncr.20960. [DOI] [PubMed] [Google Scholar]
- 10.Oeffinger KC, Mertens AC, Sklar CA, et al. Childhood Cancer Survivor Study. Obesity in adult survivors of childhood acute lymphoblastic leukemia: a report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21(7):1359–1365. doi: 10.1200/JCO.2003.06.131. [DOI] [PubMed] [Google Scholar]
- 11.Hudson MM, Ness KK, Nolan VG, et al. Prospective medical assessment of adults surviving childhood cancer: study design, cohort characteristics, and feasibility of the St. Jude Lifetime Cohort study. Pediatr Blood Cancer. 2011;56(5):825–836. doi: 10.1002/pbc.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Children's Oncology Group. Long-Term Follow-Up Guidelines for Survivors of Childhood, Adolescent and Young Adult Cancers, Version 4.0. Monrovia, CA: Children's Oncology Group; 2013. http://www.survivorshipguidelines.org.
- 13.Jensen MD, Kanaley JA, Roust LR, et al. Assessment of body composition with use of dual-energy x-ray absorptiometry: evaluation and comparison with other methods. Mayo Clin Proc. 1993;68(9):867–873. doi: 10.1016/s0025-6196(12)60695-8. [DOI] [PubMed] [Google Scholar]
- 14.Thomas SR, Kalkwarf HJ, Buckley DD, Heubi JE. Effective dose of dual-energy X-ray absorptiometry scans in children as a function of age. J Clin Densitom. 2005;8(4):415–422. doi: 10.1385/jcd:8:4:415. [DOI] [PubMed] [Google Scholar]
- 15.Njeh CF, Samat SB, Nightingale A, McNeil EA, Boivin CM. Radiation dose and in vitro precision in paediatric bone mineral density measurement using dual X-ray absorptiometry. Br J Radiol. 1997;70(835):719–727. doi: 10.1259/bjr.70.835.9245884. [DOI] [PubMed] [Google Scholar]
- 16.Kalender WA. Effective dose values in bone mineral measurements by photon absorptiometry and computed tomography. Osteoporos Int. 1992;2(2):82–87. doi: 10.1007/BF01623841. [DOI] [PubMed] [Google Scholar]
- 17.Njeh CF, Fuerst T, Hans D, Blake GM, Genant HK. Radiation exposure in bone mineral density assessment. Appl Radiat Isot. 1999;50(1):215–236. doi: 10.1016/s0969-8043(98)00026-8. [DOI] [PubMed] [Google Scholar]
- 18.Snodgrass JJ, Leonard WR, Tarskaia LA, Schoeller DA. Total energy expenditure in the Yakut (Sakha) of Siberia as measured by the doubly labeled water method. Am J Clin Nutr. 2006;84(4):798–806. doi: 10.1093/ajcn/84.4.798. [DOI] [PubMed] [Google Scholar]
- 19.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109(1-2):1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schoeller DA. Recent advances from application of doubly labeled water to measurement of human energy expenditure. J Nutr. 1999;129(10):1765–1768. doi: 10.1093/jn/129.10.1765. [DOI] [PubMed] [Google Scholar]
- 21.Schoeller DA. Measurement of energy expenditure in free-living humans by using doubly labeled water. J Nutr. 1988;118(11):1278–1289. doi: 10.1093/jn/118.11.1278. [DOI] [PubMed] [Google Scholar]
- 22.DeLany JP, Kelley DE, Hames KC, Jakicic JM, Goodpaster BH. High energy expenditure masks low physical activity in obesity. Int J Obes (Lond) 2013;37(7):1006–1011. doi: 10.1038/ijo.2012.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Racette SB, Schoeller DA, Luke AH, Shay K, Hnilicka J, Kushner RF. Relative dilution spaces of 2H- and 18O-labeled water in humans. Am J Physiol. 1994;267(4 Pt 1):E585–E590. doi: 10.1152/ajpendo.1994.267.4.E585. [DOI] [PubMed] [Google Scholar]
- 24.Feskanich D, Sielaff BH, Chong K, Buzzard IM. Computerized collection and analysis of dietary intake information. Comput Methods Programs Biomed. 1989;30(1):47–57. doi: 10.1016/0169-2607(89)90122-3. [DOI] [PubMed] [Google Scholar]
- 25.Shephard RJ, Berridge M, Montelpare W. On the generality of the “sit and reach” test: an analysis of flexibility data for an aging population. Res Q Exerc Sport. 1990;61(4):326–330. doi: 10.1080/02701367.1990.10607495. [DOI] [PubMed] [Google Scholar]
- 26.Moseley AM, Crosbie J, Adams R. Normative data for passive ankle plantarflexion—dorsiflexion flexibility. Clin Biomech (Bristol, Avon) 2001;16(6):514–521. doi: 10.1016/s0268-0033(01)00030-4. [DOI] [PubMed] [Google Scholar]
- 27.Wampler MA, Miaskowski C, Hamel K, Byl N, Rugo H, Topp KS. The modified total neuropathy score: a clinically feasible and valid measure of taxane-induced peripheral neuropathy in women with breast cancer. J Support Oncol. 2006;4:W9–W16. [Google Scholar]
- 28.Nashner LM, Peters JF. Dynamic posturography in the diagnosis and management of dizziness and balance disorders. Neurol Clin. 1990;8(2):331–349. [PubMed] [Google Scholar]
- 29.Mathiowetz V, Kashman N, Volland G, Weber K, Dowe M, Rogers S. Grip and pinch strength: normative data for adults. Arch Phys Med Rehabil. 1985;66(2):69–74. [PubMed] [Google Scholar]
- 30.Harbo T, Brincks J, Andersen H. Maximal isokinetic and isometric muscle strength of major muscle groups related to age, body mass, height, and sex in 178 healthy subjects. Eur J Appl Physiol. 2012;112(1):267–275. doi: 10.1007/s00421-011-1975-3. [DOI] [PubMed] [Google Scholar]
- 31.Wang CY, Haskell WL, Farrell SW, et al. Cardiorespiratory fitness levels among US adults 20-49 years of age: findings from the 1999-2004 National Health and Nutrition Examination Survey. Am J Epidemiol. 2010;171(4):426–435. doi: 10.1093/aje/kwp412. [DOI] [PubMed] [Google Scholar]
- 32.Freedson PS, Melanson E, Sirard J. Calibration of the Computer Science and Applications, Inc. accelerometer. Med Sci Sports Exerc. 1998;30(5):777–781. doi: 10.1097/00005768-199805000-00021. [DOI] [PubMed] [Google Scholar]
- 33.Bizzarri C, Bottaro G, Pinto RM, Cappa M. Metabolic syndrome and diabetes mellitus in childhood cancer survivors. Pediatr Endocrinol Rev. 2014;11(4):365–373. [PubMed] [Google Scholar]
- 34.Armstrong GT, Oeffinger KC, Chen Y, et al. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31(29):3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Berger NA. Obesity and cancer pathogenesis. Ann N Y Acad Sci. 2014;1311:57–76. doi: 10.1111/nyas.12416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Steinberger J, Sinaiko AR, Kelly AS, et al. Cardiovascular risk and insulin resistance in childhood cancer survivors. J Pediatr. 2012;160(3):494–499. doi: 10.1016/j.jpeds.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ligibel JA, Alfano CM, Courneya KS, et al. American Society of Clinical Oncology position statement on obesity and cancer. J Clin Oncol. 2014;32(31):3568–3574. doi: 10.1200/JCO.2014.58.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Taskinen MH, Kurimo M, Kanerva J, Hovi L. Physical performance of nontransplanted childhood ALL survivors is comparable to healthy controls. J Pediatr Hematol Oncol. 2013;35(4):276–280. doi: 10.1097/MPH.0b013e3182830ffa. [DOI] [PubMed] [Google Scholar]
- 39.Gocha Marchese V, Chiarello LA, Lange BJ. Strength and functional mobility in children with acute lymphoblastic leukemia. Med Pediatr Oncol. 2003;40(4):230–232. doi: 10.1002/mpo.10266. [DOI] [PubMed] [Google Scholar]
- 40.Hartman A, van den Bos C, Stijnen T, Pieters R. Decrease in peripheral muscle strength and ankle dorsiflexion as long-term side effects of treatment for childhood cancer. Pediatr Blood Cancer. 2008;50(4):833–837. doi: 10.1002/pbc.21325. [DOI] [PubMed] [Google Scholar]
- 41.Wright MJ, Halton JM, Barr RD. Limitation of ankle range of motion in survivors of acute lymphoblastic leukemia: a cross-sectional study. Med Pediatr Oncol. 1999;32(4):279–282. doi: 10.1002/(sici)1096-911x(199904)32:4<279::aid-mpo7>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 42.Hovi L, Era P, Rautonen J, Siimes MA. Impaired muscle strength in female adolescents and young adults surviving leukemia in childhood. Cancer. 1993;72(1):276–281. doi: 10.1002/1097-0142(19930701)72:1<276::aid-cncr2820720148>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 43.Kawedia JD, Liu C, Pei D, et al. Dexamethasone exposure and asparaginase antibodies affect relapse risk in acute lymphoblastic leukemia. Blood. 2012;119(7):1658–1664. doi: 10.1182/blood-2011-09-381731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang L, Panetta JC, Cai X, et al. Asparaginase may influence dexamethasone pharmacokinetics in acute lymphoblastic leukemia. J Clin Oncol. 2008;26(12):1932–1939. doi: 10.1200/JCO.2007.13.8404. [DOI] [PubMed] [Google Scholar]
- 45.Müller HJ, Boos J. Use of L-asparaginase in childhood ALL. Crit Rev Oncol Hematol. 1998;28(2):97–113. doi: 10.1016/s1040-8428(98)00015-8. [DOI] [PubMed] [Google Scholar]
- 46.Bentzinger CF, Wang YX, Dumont NA, Rudnicki MA. Cellular dynamics in the muscle satellite cell niche. EMBO Rep. 2013;14(12):1062–1072. doi: 10.1038/embor.2013.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ness KK, Hudson MM, Pui CH, et al. Neuromuscular impairments in adult survivors of childhood acute lymphoblastic leukemia: associations with physical performance and chemotherapy doses. Cancer. 2012;118(3):828–838. doi: 10.1002/cncr.26337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Topp KS, Tanner KD, Levine JD. Damage to the cytoskeleton of large diameter sensory neurons and myelinated axons in vincristine-induced painful peripheral neuropathy in the rat. J Comp Neurol. 2000;424(4):563–576. [PubMed] [Google Scholar]
- 49.Cole PD, Beckwith KA, Vijayanathan V, Roychowdhury S, Smith AK, Kamen BA. Folate homeostasis in cerebrospinal fluid during therapy for acute lymphoblastic leukemia. Pediatr Neurol. 2009;40(1):34–41. doi: 10.1016/j.pediatrneurol.2008.09.005. [DOI] [PubMed] [Google Scholar]
- 50.Kishi S, Griener J, Cheng C, et al. Homocysteine, pharmacogenetics, and neurotoxicity in children with leukemia. J Clin Oncol. 2003;21(16):3084–3091. doi: 10.1200/JCO.2003.07.056. [DOI] [PubMed] [Google Scholar]
- 51.Vezmar S, Becker A, Bode U, Jaehde U. Biochemical and clinical aspects of methotrexate neurotoxicity. Chemotherapy. 2003;49(1-2):92–104. doi: 10.1159/000069773. [DOI] [PubMed] [Google Scholar]
- 52.Vezmar S, Schüsseler P, Becker A, Bode U, Jaehde U. Methotrexate-associated alterations of the folate and methyl-transfer pathway in the CSF of ALL patients with and without symptoms of neurotoxicity. Pediatr Blood Cancer. 2009;52(1):26–32. doi: 10.1002/pbc.21827. [DOI] [PubMed] [Google Scholar]
- 53.Schakman O, Gilson H, Thissen JP. Mechanisms of glucocorticoid-induced myopathy. J Endocrinol. 2008;197(1):1–10. doi: 10.1677/JOE-07-0606. [DOI] [PubMed] [Google Scholar]
- 54.Dahmane R, Djordjevic S, Simunic B, Valencic V. Spatial fiber type distribution in normal human muscle Histochemical and tensiomyographical evaluation. J Biomech. 2005;38(12):2451–2459. doi: 10.1016/j.jbiomech.2004.10.020. [DOI] [PubMed] [Google Scholar]
- 55.Huang MH, Brown SH. Age differences in the control of postural stability during reaching tasks. Gait Posture. 2013;38(4):837–842. doi: 10.1016/j.gaitpost.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 56.Sekulic D, Spasic M, Mirkov D, Cavar M, Sattler T. Gender-specific influences of balance, speed, and power on agility performance. J Strength Cond Res. 2013;27(3):802–811. doi: 10.1519/JSC.0b013e31825c2cb0. [DOI] [PubMed] [Google Scholar]
- 57.Marmon AR, Pascoe MA, Schwartz RS, Enoka RM. Associations among strength, steadiness, and hand function across the adult life span. Med Sci Sports Exerc. 2011;43(4):560–567. doi: 10.1249/MSS.0b013e3181f3f3ab. [DOI] [PubMed] [Google Scholar]
- 58.Pui CH, Carroll WL, Meshinchi S, Arceci RJ. Biology, risk stratification, and therapy of pediatric acute leukemias: an update. J Clin Oncol. 2011;29(5):551–565. doi: 10.1200/JCO.2010.30.7405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Eraso LH, Fukaya E, Mohler ER, III, Xie D, Sha D, Berger JS. Peripheral arterial disease, prevalence and cumulative risk factor profile analysis. Eur J Prev Cardiol. 2012;21(6):704–711. doi: 10.1177/2047487312452968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dengel DR, Ness KK, Glasser SP, Williamson EB, Baker KS, Gurney JG. Endothelial function in young adult survivors of childhood acute lymphoblastic leukemia. J Pediatr Hematol Oncol. 2008;30(1):20–25. doi: 10.1097/MPH.0b013e318159a593. [DOI] [PubMed] [Google Scholar]
- 61.Kulinski JP, Khera A, Ayers CR, et al. Association between cardiorespiratory fitness and accelerometer-derived physical activity and sedentary time in the general population. Mayo Clin Proc. 2014;89(8):1063–1071. doi: 10.1016/j.mayocp.2014.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Edvardsen E, Scient C, Hansen BH, Holme IM, Dyrstad SM, Anderssen SA. Reference values for cardiorespiratory response and fitness on the treadmill in a 20- to 85-year-old population. Chest. 2013;144(1):241–248. doi: 10.1378/chest.12-1458. [DOI] [PubMed] [Google Scholar]
- 63.Kodama S, Saito K, Tanaka S, et al. Cardiorespiratory fitness as a quantitative predictor of all-cause mortality and cardiovascular events in healthy men and women: a meta-analysis. JAMA. 2009;301(19):2024–2035. doi: 10.1001/jama.2009.681. [DOI] [PubMed] [Google Scholar]
- 64.Sasaki H, Kasagi F, Yamada M, Fujita S. Grip strength predicts cause-specific mortality in middle-aged and elderly persons. Am J Med. 2007;120(4):337–342. doi: 10.1016/j.amjmed.2006.04.018. [DOI] [PubMed] [Google Scholar]