Abstract
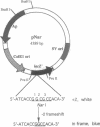
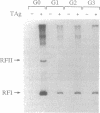
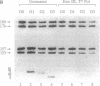
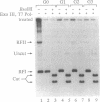
We have analyzed the effects of site-specific N-2-acetylaminofluorene (AAF) adducts on the efficiency and frameshift fidelity of bidirectional replication of double-stranded DNA in a human cell extract. Plasmid vectors were constructed containing the simian virus 40 origin of replication and single AAF adducts at one of three guanines in the Nar I sequence GGCGCC in a lacZ reporter gene. The presence of an AAF adduct diminishes replication efficiency in HeLa cell extracts by 70-80%. Replication product analyses reveal unique termination sites with each damaged vector, suggesting that when the replication fork encounters an AAF adduct, it often stops before incorporation opposite the adduct. We also observed a higher proportion of products representing replication of the undamaged strand compared to the damaged strand. This suggests that the undamaged strand is replicated more readily, either by uncoupling the first fork to encounter the lesion or by replication using the fork arriving from the other direction. Also included among replication products are covalently closed monomer-length molecules resistant to cleavage at the AAF-modified Nar I site. This resistance is characteristic of substrates containing the AAF adduct, suggesting that translesion bypass had occurred. Transformation of Escherichia coli cells with the replicated damaged DNA yielded lacZ alpha revertant frequencies significantly above values obtained with undamaged DNA or with damaged DNA not replicated in vitro. This increase was only seen with the substrate modified at the third guanine position. Analysis of mutant DNA demonstrated the loss of a GC dinucleotide at the Nar I sequence. Generation of this position-dependent AAF-induced frameshift error in a human replication system is consistent with previous observations in E. coli suggesting that, after incorporation of dCMP opposite modified guanine in the third position, realignment of the template-primer occurs to form an intermediate with two unpaired nucleotides in the template strand.
Full text
PDF
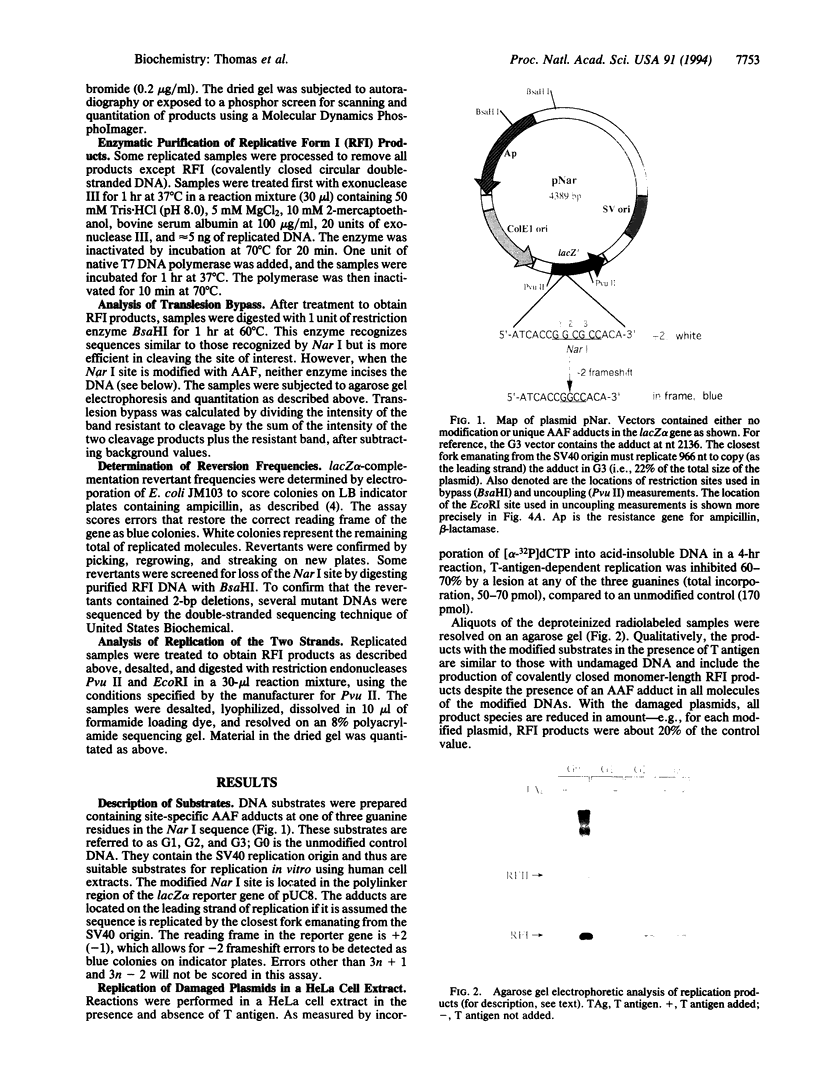

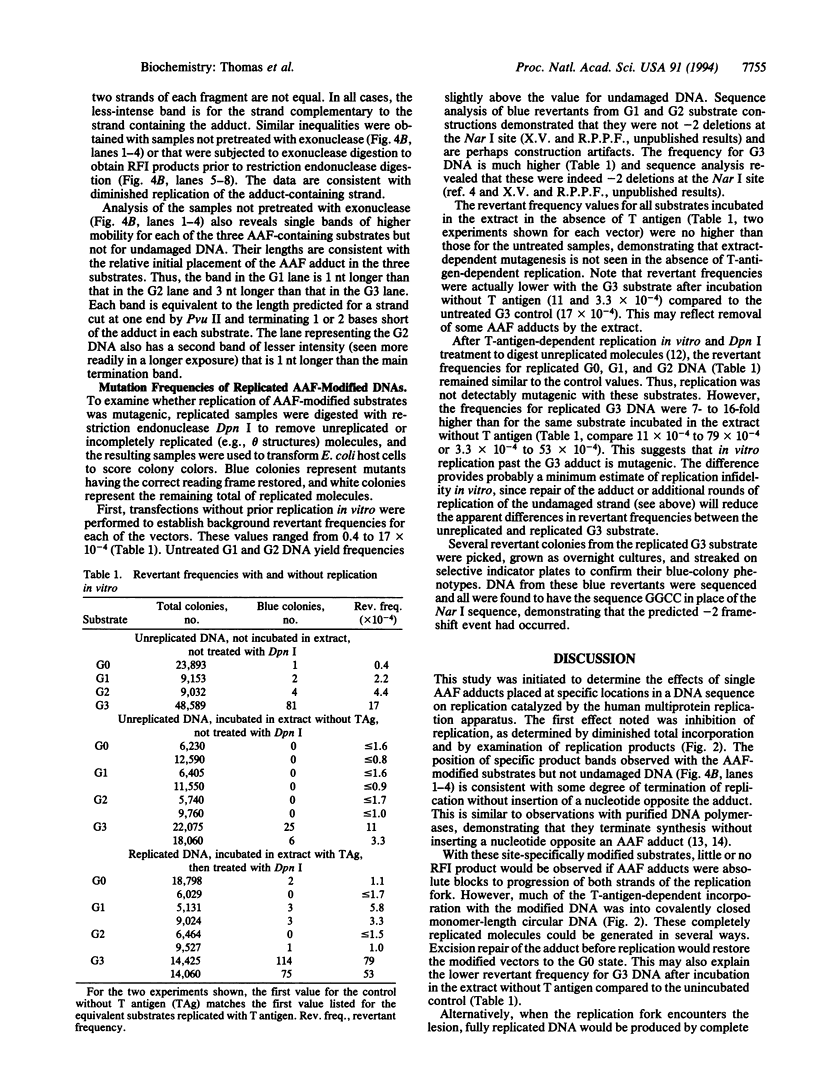

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bjursell G., Gussander E., Lindahl T. Long regions of single-stranded DNA in human cells. Nature. 1979 Aug 2;280(5721):420–423. doi: 10.1038/280420a0. [DOI] [PubMed] [Google Scholar]
- Burhans W. C., Vassilev L. T., Wu J., Sogo J. M., Nallaseth F. S., DePamphilis M. L. Emetine allows identification of origins of mammalian DNA replication by imbalanced DNA synthesis, not through conservative nucleosome segregation. EMBO J. 1991 Dec;10(13):4351–4360. doi: 10.1002/j.1460-2075.1991.tb05013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnouf D., Koehl P., Fuchs R. P. Single adduct mutagenesis: strong effect of the position of a single acetylaminofluorene adduct within a mutation hot spot. Proc Natl Acad Sci U S A. 1989 Jun;86(11):4147–4151. doi: 10.1073/pnas.86.11.4147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carty M. P., Hauser J., Levine A. S., Dixon K. Replication and mutagenesis of UV-damaged DNA templates in human and monkey cell extracts. Mol Cell Biol. 1993 Jan;13(1):533–542. doi: 10.1128/mcb.13.1.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs R. P., Schwartz N., Daune M. P. Hot spots of frameshift mutations induced by the ultimate carcinogen N-acetoxy-N-2-acetylaminofluorene. Nature. 1981 Dec 17;294(5842):657–659. doi: 10.1038/294657a0. [DOI] [PubMed] [Google Scholar]
- Gaudette M. F., Benbow R. M. Replication forks are underrepresented in chromosomal DNA of Xenopus laevis embryos. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5953–5957. doi: 10.1073/pnas.83.16.5953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehl P., Burnouf D., Fuchs R. P. Construction of plasmids containing a unique acetylaminofluorene adduct located within a mutation hot spot. A new probe for frameshift mutagenesis. J Mol Biol. 1989 May 20;207(2):355–364. doi: 10.1016/0022-2836(89)90259-3. [DOI] [PubMed] [Google Scholar]
- Koffel-Schwartz N., Fuchs R. P. Genetic control of AAF-induced mutagenesis at alternating GC sequences: an additional role for RecA. Mol Gen Genet. 1989 Jan;215(2):306–311. doi: 10.1007/BF00339733. [DOI] [PubMed] [Google Scholar]
- Koffel-Schwartz N., Maenhaut-Michel G., Fuchs R. P. Specific strand loss in N-2-acetylaminofluorene-modified DNA. J Mol Biol. 1987 Feb 20;193(4):651–659. doi: 10.1016/0022-2836(87)90348-2. [DOI] [PubMed] [Google Scholar]
- Kriek E., Miller J. A., Juhl U., Miller E. C. 8-(N-2-fluorenylacetamido)guanosine, an arylamidation reaction product of guanosine and the carcinogen N-acetoxy-N-2-fluorenylacetamide in neutral solution. Biochemistry. 1967 Jan;6(1):177–182. doi: 10.1021/bi00853a029. [DOI] [PubMed] [Google Scholar]
- Lambert I. B., Napolitano R. L., Fuchs R. P. Carcinogen-induced frameshift mutagenesis in repetitive sequences. Proc Natl Acad Sci U S A. 1992 Feb 15;89(4):1310–1314. doi: 10.1073/pnas.89.4.1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J. J., Kelly T. J. Simian virus 40 DNA replication in vitro: specificity of initiation and evidence for bidirectional replication. Mol Cell Biol. 1985 Jun;5(6):1238–1246. doi: 10.1128/mcb.5.6.1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. D., Bose K. K., Rabkin S. D., Strauss B. S. Sites of termination of in vitro DNA synthesis on ultraviolet- and N-acetylaminofluorene-treated phi X174 templates by prokaryotic and eukaryotic DNA polymerases. Proc Natl Acad Sci U S A. 1981 Jan;78(1):110–114. doi: 10.1073/pnas.78.1.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaaper R. M., Koffel-Schwartz N., Fuchs R. P. N-acetoxy-N-acetyl-2-aminofluorene-induced mutagenesis in the lacI gene of Escherichia coli. Carcinogenesis. 1990 Jul;11(7):1087–1095. doi: 10.1093/carcin/11.7.1087. [DOI] [PubMed] [Google Scholar]
- Shibutani S., Grollman A. P. On the mechanism of frameshift (deletion) mutagenesis in vitro. J Biol Chem. 1993 Jun 5;268(16):11703–11710. [PubMed] [Google Scholar]
- Strauss B. S., Wang J. Role of DNA polymerase 3'----5' exonuclease activity in the bypass of aminofluorene lesions in DNA. Carcinogenesis. 1990 Dec;11(12):2103–2109. doi: 10.1093/carcin/11.12.2103. [DOI] [PubMed] [Google Scholar]
- Thomas D. C., Kunkel T. A. Replication of UV-irradiated DNA in human cell extracts: evidence for mutagenic bypass of pyrimidine dimers. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7744–7748. doi: 10.1073/pnas.90.16.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. C., Nguyen D. C., Piegorsch W. W., Kunkel T. A. Relative probability of mutagenic translesion synthesis on the leading and lagging strands during replication of UV-irradiated DNA in a human cell extract. Biochemistry. 1993 Nov 2;32(43):11476–11482. doi: 10.1021/bi00094a002. [DOI] [PubMed] [Google Scholar]
- Veaute X., Fuchs R. P. Greater susceptibility to mutations in lagging strand of DNA replication in Escherichia coli than in leading strand. Science. 1993 Jul 30;261(5121):598–600. doi: 10.1126/science.8342022. [DOI] [PubMed] [Google Scholar]