Abstract
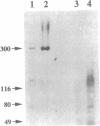
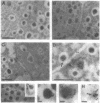
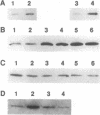
Calcium ions play a central role in stimulus-secretion coupling in pancreatic beta cells, and an elevation of cytosolic Ca2+ levels is necessary for insulin secretion. Inositol 1,4,5-trisphosphate mobilizes intracellular Ca2+ stores in the beta cell by binding to specific receptors that are ligand-activated Ca2+ channels. The inositol trisphosphate receptors comprise a family of structurally related proteins with distinct but overlapping tissue distributions. Previous studies indicated that the predominant inositol trisphosphate receptor subtype expressed in rat pancreatic islets was the protein designated IP3R-3. We have confirmed the expression of IP3R-3 in pancreatic islets by immunohistocytochemistry and localized this protein to the secretory granules of insulin-secreting beta cells and somatostatin-secreting delta cells by immunogold electron microscopy. Secretory granules contain high levels of Ca2+, and the presence of IP3R-3 in the granule provides a mechanism for mobilizing granule Ca2+ stores in response to glucose and/or hormones. The release of Ca2+ from granule stores would increase the Ca2+ concentration in the surrounding cytoplasm and promote rapid exocytosis of granules, especially those granules in close proximity to the plasma membrane. The levels of IP3R-3 were increased in pancreatic islets of diabetic rats and rats that had been refed after a period of fasting. They were also increased in rat insulinoma RINm5F cells cultured in 25 mM glucose compared with cells cultured in 5 mM glucose. The localization of IP3R-3 to secretory granules of insulin-secreting beta cells and somatostatin-secreting delta cells suggests that granule Ca2+ stores actively participate in the secretory process and that their release is regulated by inositol 1,4,5-trisphosphate. The regulation of IP3R-3 levels by glucose, diabetes, and refeeding may allow the beta cell to adjust the insulin secretory response to changing physiological conditions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrahamsson H., Gylfe E., Hellman B. Influence of external calcium ions on labelled calcium efflux from pancreatic beta-cells and insulin granules in mice. J Physiol. 1981 Feb;311:541–550. doi: 10.1113/jphysiol.1981.sp013603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ammälä C., Larsson O., Berggren P. O., Bokvist K., Juntti-Berggren L., Kindmark H., Rorsman P. Inositol trisphosphate-dependent periodic activation of a Ca(2+)-activated K+ conductance in glucose-stimulated pancreatic beta-cells. Nature. 1991 Oct 31;353(6347):849–852. doi: 10.1038/353849a0. [DOI] [PubMed] [Google Scholar]
- Aunis D., Bader M. F. The cytoskeleton as a barrier to exocytosis in secretory cells. J Exp Biol. 1988 Sep;139:253–266. doi: 10.1242/jeb.139.1.253. [DOI] [PubMed] [Google Scholar]
- Bani Sacchi T., Bani D. New views on the identification of the various cell types in the pancreatic islets of the rat. An ultrastructural and morphometrical study. Acta Anat (Basel) 1985;122(1):1–17. doi: 10.1159/000145977. [DOI] [PubMed] [Google Scholar]
- Berridge M. J. Inositol trisphosphate and calcium signalling. Nature. 1993 Jan 28;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- Blondel O., Takeda J., Janssen H., Seino S., Bell G. I. Sequence and functional characterization of a third inositol trisphosphate receptor subtype, IP3R-3, expressed in pancreatic islets, kidney, gastrointestinal tract, and other tissues. J Biol Chem. 1993 May 25;268(15):11356–11363. [PubMed] [Google Scholar]
- Burgoyne R. D., Cheek T. R. Reorganisation of peripheral actin filaments as a prelude to exocytosis. Biosci Rep. 1987 Apr;7(4):281–288. doi: 10.1007/BF01121449. [DOI] [PubMed] [Google Scholar]
- Burgoyne R. D., Morgan A. Regulated exocytosis. Biochem J. 1993 Jul 15;293(Pt 2):305–316. doi: 10.1042/bj2930305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham A. M., Ryugo D. K., Sharp A. H., Reed R. R., Snyder S. H., Ronnett G. V. Neuronal inositol 1,4,5-trisphosphate receptor localized to the plasma membrane of olfactory cilia. Neuroscience. 1993 Nov;57(2):339–352. doi: 10.1016/0306-4522(93)90067-p. [DOI] [PubMed] [Google Scholar]
- Ferris C. D., Snyder S. H. Inositol 1,4,5-trisphosphate-activated calcium channels. Annu Rev Physiol. 1992;54:469–488. doi: 10.1146/annurev.ph.54.030192.002345. [DOI] [PubMed] [Google Scholar]
- Fujimoto T., Nakade S., Miyawaki A., Mikoshiba K., Ogawa K. Localization of inositol 1,4,5-trisphosphate receptor-like protein in plasmalemmal caveolae. J Cell Biol. 1992 Dec;119(6):1507–1513. doi: 10.1083/jcb.119.6.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutton J. C., Penn E. J., Peshavaria M. Low-molecular-weight constituents of isolated insulin-secretory granules. Bivalent cations, adenine nucleotides and inorganic phosphate. Biochem J. 1983 Feb 15;210(2):297–305. doi: 10.1042/bj2100297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. A., Steiner J. P., Klein M. G., Schneider M. F., Snyder S. H. IP3 receptor: localization to plasma membrane of T cells and cocapping with the T cell receptor. Science. 1992 Aug 7;257(5071):815–818. doi: 10.1126/science.1323146. [DOI] [PubMed] [Google Scholar]
- Lacy P. E., Kostianovsky M. Method for the isolation of intact islets of Langerhans from the rat pancreas. Diabetes. 1967 Jan;16(1):35–39. doi: 10.2337/diab.16.1.35. [DOI] [PubMed] [Google Scholar]
- Lange K., Brandt U. The IP3-sensitive calcium store of HIT cells is located in a surface-derived vesicle fraction. FEBS Lett. 1993 Apr 12;320(3):183–188. doi: 10.1016/0014-5793(93)80582-f. [DOI] [PubMed] [Google Scholar]
- Malviya A. N., Rogue P., Vincendon G. Stereospecific inositol 1,4,5-[32P]trisphosphate binding to isolated rat liver nuclei: evidence for inositol trisphosphate receptor-mediated calcium release from the nucleus. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9270–9274. doi: 10.1073/pnas.87.23.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A. Calcium release and internal calcium regulation in acinar cells of exocrine glands. J Membr Biol. 1991 Dec;124(3):189–197. doi: 10.1007/BF01994353. [DOI] [PubMed] [Google Scholar]
- Orci L., Gabbay K. H., Malaisse W. J. Pancreatic beta-cell web: its possible role in insulin secretion. Science. 1972 Mar 10;175(4026):1128–1130. doi: 10.1126/science.175.4026.1128. [DOI] [PubMed] [Google Scholar]
- Peng Y. W., Sharp A. H., Snyder S. H., Yau K. W. Localization of the inositol 1,4,5-trisphosphate receptor in synaptic terminals in the vertebrate retina. Neuron. 1991 Apr;6(4):525–531. doi: 10.1016/0896-6273(91)90055-5. [DOI] [PubMed] [Google Scholar]
- Prentki M., Matschinsky F. M. Ca2+, cAMP, and phospholipid-derived messengers in coupling mechanisms of insulin secretion. Physiol Rev. 1987 Oct;67(4):1185–1248. doi: 10.1152/physrev.1987.67.4.1185. [DOI] [PubMed] [Google Scholar]
- Ravazzola M., Malaisse-Lagae F., Amherdt M., Perrelet A., Malaisse W. J., Orci L. Patterns of calcium localization in pancreatic endocrine cells. J Cell Sci. 1976 Jun;21(1):107–117. doi: 10.1242/jcs.21.1.107. [DOI] [PubMed] [Google Scholar]
- Roe M. W., Lancaster M. E., Mertz R. J., Worley J. F., 3rd, Dukes I. D. Voltage-dependent intracellular calcium release from mouse islets stimulated by glucose. J Biol Chem. 1993 May 15;268(14):9953–9956. [PubMed] [Google Scholar]
- Ross C. A., Danoff S. K., Schell M. J., Snyder S. H., Ullrich A. Three additional inositol 1,4,5-trisphosphate receptors: molecular cloning and differential localization in brain and peripheral tissues. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4265–4269. doi: 10.1073/pnas.89.10.4265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross C. A., Meldolesi J., Milner T. A., Satoh T., Supattapone S., Snyder S. H. Inositol 1,4,5-trisphosphate receptor localized to endoplasmic reticulum in cerebellar Purkinje neurons. Nature. 1989 Jun 8;339(6224):468–470. doi: 10.1038/339468a0. [DOI] [PubMed] [Google Scholar]
- Thorn P. Spatial aspects of Ca2+ signalling in pancreatic acinar cells. J Exp Biol. 1993 Nov;184:129–144. doi: 10.1242/jeb.184.1.129. [DOI] [PubMed] [Google Scholar]
- Wolters G. H., Pasma A., Konijnendijk W., Bouman P. R. Effects of calcium manipulation and glucose stimulation on a histochemically detectable mobile calcium fraction in isolated rat pancreatic islets. Histochemistry. 1980;66(2):125–135. doi: 10.1007/BF00494640. [DOI] [PubMed] [Google Scholar]
- Wolters G. H., Pasma A., Wiegman J. B., Konijnendijk W. Glucose-induced changes in histochemically determined Ca2+ in B-cell granules, 45Ca uptake, and total Ca2+ of rat pancreatic islets. Diabetes. 1984 May;33(5):409–414. doi: 10.2337/diab.33.5.409. [DOI] [PubMed] [Google Scholar]
- Yoo S. H., Albanesi J. P. Inositol 1,4,5-trisphosphate-triggered Ca2+ release from bovine adrenal medullary secretory vesicles. J Biol Chem. 1990 Aug 15;265(23):13446–13448. [PubMed] [Google Scholar]
- Zawalich W. S., Rasmussen H. Control of insulin secretion: a model involving Ca2+, cAMP and diacylglycerol. Mol Cell Endocrinol. 1990 Apr 17;70(2):119–137. doi: 10.1016/0303-7207(90)90152-x. [DOI] [PubMed] [Google Scholar]