Abstract
The epidemiology of hepatitis B virus (HBV) infection is geographically diverse, with population prevalence, age and mode of acquisition, and likelihood of progression to chronic infection mutually interdependent. The burden of chronic HBV infection is increasingly being recognized, with cirrhosis and liver cancer attributable to HBV continuing to increase. The outcomes of chronic HBV infection are affected by a range of factors, including viral genotype, the presence of coinfections with other blood-borne viruses, and the impact of other causes of liver disease. The increased recognition of HBV infection as a leading cause of death globally has resulted in the development of new structures and policies at the international level; immediate attention to implementing these strategies is now required.
Infection with hepatitis B virus is a major cause of human mortality worldwide. Multiple factors (e.g., population prevalence, age, and mode of acquisition) influence its impact and strategies for its control.
Hepatitis B virus (HBV) infection results in substantial human morbidity and mortality, predominantly through the consequences of chronic infection. Recent estimates of the number of people chronically infected with HBV have ranged from 240 million (Ott et al. 2012) to 350 million (Lavanchy 2004), with more than two billion humans globally ever having been infected.
In the Global Burden of Disease Study 2010, HBV was estimated to have resulted in 786,000 deaths, the vast majority being attributable to liver cancer (341,000 deaths) and cirrhosis (312,000 deaths) (Lozano et al. 2012). As a result, HBV infection was ranked 15th among all causes of human mortality (Lozano et al. 2012).
However, the burden of HBV infection is geographically disparate, dependent on the differing modes of transmission predominant in the population and the resulting age at infection, which determines the probability of progression to chronic infection. In addition, the epidemiology of HBV infection globally is changing because of the impact of universal infant vaccination programs (Chang et al. 1997; Liang et al. 2009; Ott et al. 2012; Zoulim and Durantel 2015), and through migration between high- and low-prevalence populations (Hahne et al. 2004; Marschall et al. 2008; Kowdley et al. 2012; MacLachlan et al. 2013).
EPIDEMIOLOGY AND TRANSMISSION
HBV is transmitted through exposure to infected blood and bodily fluids (particularly semen and vaginal secretions). HBV survives for prolonged periods outside the body (Lok and McMahon 2009). Although HBV has been detected in saliva, tears, breast milk, sweat, and urine, there is minimal evidence of transmission through exposure to these fluids where no blood is present, and breastfeeding has not been shown to increase risk of infection (Zheng et al. 2011).
Most infections worldwide are acquired through perinatal transmission at birth, through horizontal transmission to/between young children, through sexual contact, and through injecting drug use (Lok and McMahon 2009). Other routes of transmission, which have declined in frequency with the implementation of control measures, include through contaminated blood or blood products and unsafe medical practices; however, health care associated infection remains a significant concern in both resource-poor (Arankalle et al. 2011) and well-resourced settings (Thompson et al. 2009).
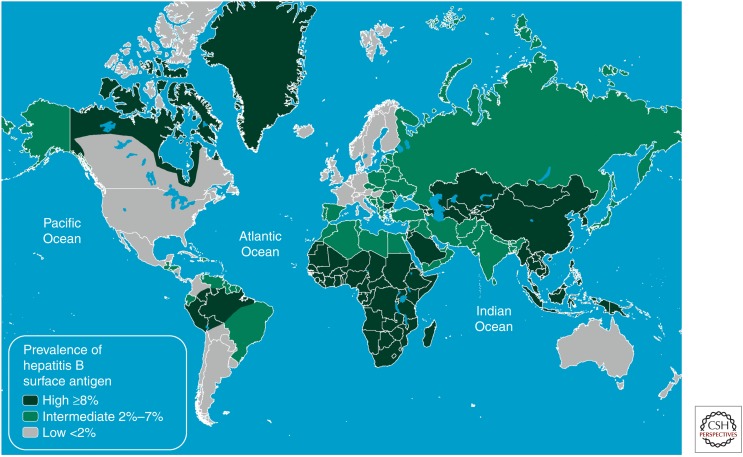
The epidemiology of hepatitis B can be described in terms of the prevalence of hepatitis B surface antigen (HBsAg) in a population, broadly classified into high- (>8% HBsAg prevalence), intermediate- (2%–7%) and low-prevalence (<2%) areas (Previsani and Lavanchy 2002). These broad categories are useful for understanding the predominant patterns of transmission and outcomes for infection, as well as the relative population burden of the consequences of chronic hepatitis B, including liver cancer (Fig. 1).
Figure 1.
Global prevalence of hepatitis B virus infection. (From the Centers for Disease Control 2012.)
High-Prevalence Populations
In countries where chronic HBV infection affects more than 8% of the population, the majority of these individuals were infected at birth or in early childhood, when the risk of progression to chronicity is high (Lavanchy 2004). High-HBV prevalence is common in much of the Asia Pacific and sub-Saharan African regions of the world. Globally, it has been estimated that 45% of the world’s population lives in an area of high prevalence (Mahoney 1999). There is evidence to suggest that vertical transmission is more common in Asia than in Africa, where a greater proportion of women are highly infectious at childbearing age, relating in part to predominant HBV genotypes that influence the likelihood of HBeAg positivity and high levels of HBV DNA during peak childbearing ages (Goldstein et al. 2005; Kramvis and Clements 2010).
The potential impact of infant vaccination against HBV (see Zoulim and Durantel 2015) is obviously greatest in high-prevalence populations. In such populations where universal infant vaccination was implemented early, not only has HBsAg dropped profoundly, but there is some evidence for significant reductions in liver cancer incidence among age cohorts eligible for free vaccine (Chang et al. 2009; Plymoth et al. 2009). In China, the prevalence of HBsAg fell from 9.7% to 1.0% in children aged less than 5 years (Liang et al. 2009), preventing an estimated 16 to 20 million cases of chronic hepatitis B. In other settings, such as the Gambia Hepatitis Intervention Study, the protective efficacy of infant vaccination in preventing chronic HBV infection was reported as ∼95% (Plymoth et al. 2009).
Intermediate-Prevalence Populations
Regions of the world in which HBV prevalence is classified as intermediate (2%–7%) include North Africa and the Middle East, parts of Eastern and Southern Europe, parts of Latin America, and South Asia. These represent a similar proportion of the global population to high-prevalence areas (slightly more than 40%) (Trépo et al. 2014). In these regions, transmission occurs either perinatally or horizontally (Lavanchy 2004). Although the predominant mode of transmission varies according to country, perinatal acquisition is thought to be less common in intermediate compared with high-prevalence countries, owing to a lower prevalence of high infectivity among women of childbearing age (Mahoney 1999).
As discussed above, the categorization of prevalence is subject to change with the impact of immunization and other prevention programs, and a number of countries previously categorized as high prevalence are now estimated to have a population seroprevalence below 8% (Chang et al. 2009; Liang et al. 2009). This decrease in prevalence through the impact of vaccination has also been shown in intermediate-prevalence countries in Europe (Salleras et al. 2005; Sagnelli et al. 2014).
Low-Prevalence Populations
People living in low-HBV-prevalence countries make up the minority of the global population (∼12%), and include Australia, Asia, Northern and Western Europe, Japan, North America, and some countries in South America.
In low-prevalence areas, the incidence of vertical and horizontal transmission in childhood is low, with most incident infections occurring in adolescence and adulthood through sexual contact, injecting drug use, and other blood-related exposures, including historically in healthcare settings.
A recent systematic review suggested that worldwide, 1.2 million people who inject drugs (PWID) are living with chronic hepatitis B (range 0.3–2.7 million), and 6.4 million have previously been exposed (Nelson et al. 2011). Regions with a low overall prevalence of chronic hepatitis B (CHB) but a relatively high burden of PWID living with CHB include Eastern Europe (280,000; 22.8% of the global total), and North America (272,500; 22.2% of the global total). Other people at increased risk of acquiring HBV in adulthood include those who are or who have been incarcerated, men who have sex with men, sex workers, and homeless people (Kowdley et al. 2012).
Global migration from higher prevalence to lower-prevalence countries is also an important determinant of the burden of chronic hepatitis B in many countries, where the prevalence in migrants generally reflects that of their country of origin. In many otherwise low-prevalence countries, the majority of people living with chronic HBV were born overseas in endemic areas (Hahne et al. 2004; Marschall et al. 2008; Kowdley et al. 2012; MacLachlan et al. 2013).
Indigenous Peoples
In many areas, indigenous peoples experience a higher prevalence of CHB and also an increased burden of associated liver disease. This was first documented among Aboriginal and Torres Strait Islander people in Australia, leading to HBsAg initially being named the “Australia antigen” (Blumberg et al. 1965). However, many other First Peoples—including Maori, Indigenous Taiwanese, Indigenous peoples of the Amazon, Native North Americans, and Inuit peoples of the circumpolar regions—have subsequently been noted to have a higher prevalence of CHB (Mahoney 1999; Harpaz et al. 2000; Robinson et al. 2005; Viana et al. 2005; Weinbaum et al. 2008; Graham et al. 2013). Other specific ethnic groups have been shown to have a higher prevalence of CHB in countries, such as India (Batham et al. 2007) and China (Liang et al. 2009).
The reason for higher prevalence among indigenous peoples is not completely understood, but is likely to be multifactorial. Possible contributions include earlier age of pregnancy (relating to greater likelihood of high-maternal viral load among women with CHB), greater residential density, specific HBV genotypes (Davies et al. 2013), practices eliciting blood-to-blood contact including ritual body modification or scarification, and inadequate access to timely vaccination and other elements of effective primary health care, exacerbated in some circumstances by residence in remote locations.
Global Estimates of Hepatitis B Prevalence
Recent estimates of global CHB infection prevalence were developed following a systematic literature review and subsequent age-specific estimation of global HBsAg prevalence for the years 1990 and 2005, published in 2012 (Ott et al. 2012). This collaboration between the World Health Organization and the U.S. Centers for Disease Control emphasized the regional differences in prevalence discussed above, and also highlighted reducing population hepatitis B prevalence, predominantly attributable to the influence of infant hepatitis B vaccination.
The highest prevalence of up to 12% among adults was estimated to occur in Western sub-Saharan Africa, followed by East and Southeast Asia and the remaining parts of sub-Saharan Africa with estimates of ∼5%–7% HBsAg prevalence among adults. The lowest-prevalence areas remain Northern and Western Europe and North America. At a global level, it was estimated that the prevalence of CHB reduced from 4.2% in 1990 to 3.7% in 2005, resulting in an estimate of 240 million people living with chronic hepatitis B in 2005 (Ott et al. 2012).
Global Estimates of Mortality Attributable to Hepatitis B
The Global Burden of Disease Study 2010 (GBD 2010) shows an increasing burden of mortality attributable to liver disease, including hepatitis B (Lozano et al. 2012). Earlier GBD studies had not categorically assigned deaths from cirrhosis and liver cancer to their ultimate causes, arguably contributing to the underestimated impact of viral hepatitis on human health (Cowie et al. 2013). This is reflected in the historical lack of public health priority for viral hepatitis globally (Lemoine et al. 2013; see www.who.int/csr/disease/hepatitis/GHP_framework.pdf), particularly given that many of these deaths are preventable.
In GBD 2010, the total number of deaths attributable to hepatitis B was 786,000, of which 132,200 (17%) were estimated to be caused by acute hepatitis B, 341,400 (43%) were caused by liver cancer, and 312,400 (40%) were caused by cirrhosis (Lozano et al. 2012). This represents a substantial change over the preceding two decades (Table 1).
Table 1.
Global Burden of Disease Study estimates of the attributable mortality of hepatitis B, 1990–2010
| 1990 | 2010 | |
|---|---|---|
| Total hepatitis B attributable deaths | 520,400 | 786,000 |
| Acute hepatitis B | 68,600 (46,700–84,400) | 132,200 (91,100–169,700) |
| Liver cancer secondary to hepatitis B | 210,200 (176,900–239,400) | 341,400 (290,100–402,600) |
| Cirrhosis secondary to hepatitis B | 241,700 (198,500–270,500) | 312,400 (270,800–378,300) |
Data in parentheses have 95% uncertainty intervals. (Adapted from data in Lozano et al. 2012.)
As a result, GBD 2010 estimates hepatitis B to be the 15th ranked cause of human death (Lozano et al. 2012). When considered together with deaths attributable to hepatitis C, these infections resulted in 1.29 million deaths in 2010, ranking ninth as a cause of human mortality (Cowie et al. 2013). For comparison, in recently published GBD data for 2013, HIV/AIDS was estimated to have caused 1.34 million deaths, tuberculosis for 1.29 million deaths, and malaria for 854,000 deaths (Murray et al. 2014).
The increasing recognition of the burden of viral hepatitis globally has led to increased international attention, represented by the passing of resolutions in the World Health Assembly and creation of a Global Hepatitis Programme by the World Health Organization (see below).
NATURAL HISTORY
Infection and Immunity
The natural history of CHB can be complex, and varies significantly under the influence of a variety of host and viral factors (Table 2). Host factors include sex, age at infection, and the presence of comorbidities, coinfections, and exposure to alcohol, tobacco, and dietary aflatoxin (Fattovich et al. 2008; El-Serag 2012; Trépo et al. 2014). Viral factors that affect natural history include genotype and, possibly, the presence of specific mutations associated with progressive liver disease (Kramvis and Kew 2005; Fattovich et al. 2008).
Table 2.
Factors associated with progression to cirrhosis and hepatocellular carcinoma (HCC) in people living with chronic hepatitis B
| Viral factors | High levels of HBV replication |
| Coinfection with hepatitis C, hepatitis D, or HIV | |
| Genotype (C > B) | |
| Host factors | Age >40 years and/or longer duration of infection |
| Male sex | |
| Presence of cirrhosis (for development of HCC) | |
| Race (African, Asian) | |
| Family history of HCC | |
| Other factors | Heavy alcohol consumption |
| Aflatoxin exposure | |
| Tobacco |
Adapted from data in Fattovich et al. (2008), Lok and McMahon (2009), El-Serag (2012), and Trépo et al. (2014).
As discussed by Tan et al. 2015, the likelihood of progression to chronicity, following infection with HBV, is predominantly determined by age at infection. Infants infected at birth develop chronic infection in 90% of cases, whereas for children aged between 1 and 5 years, this falls to 25%–30%, and, for immunocompetent adults, the likelihood of progression to chronicity is ∼5% (Edmunds et al. 1993). This highlights the importance of primary prevention through vaccination in infancy to prevent long-term adverse outcomes of HBV, particularly in high-HBV-prevalence populations (Zoulim and Durantel 2015).
For individuals with CHB acquired early in life, the course of infection often follows a series of phases determined by the interplay between viral replication and the host’s immune response. These phases, described in more detail in Tan et al. (2015), are:
immune tolerance—high infectivity, little ongoing liver damage;
immune clearance—variable infectivity, active inflammation of the liver;
immune control—low infectivity, little ongoing liver damage; and
immune escape—variable infectivity, recurrent liver inflammation.
In early life, CHB generally involves asymptomatic infection and does not result in significant liver damage (Fattovich et al. 2008). However, HBV still accounts for a considerable number of hepatocellular carcinoma (HCC) cases in children living in endemic areas and incidence of HCC in children has decreased since the implementation of universal vaccination in some countries, such as Taiwan (Chang et al. 2009).
Elevated baseline serum HBV DNA has been shown to be associated with subsequent development of cirrhosis (Iloeje et al. 2006) and HCC (Chen et al. 2006); more recent evidence involving repeated measurement during follow-up has further shown that the long-term persistence of high-HBV DNA levels predicts HCC risk (Chen et al. 2011), and that the association increases along a gradient of viral load. A similar relationship was shown with alanine aminotransferase (ALT) level (Chen et al. 2011). Further study is needed to determine whether these associations are replicated in other populations, as these studies have most commonly been conducted in individuals of Asian background, and largely those with hepatitis B genotype B or C.
Genotype
There is a strong relationship between HBV genotype (see Lin and Kao 2015) and geography worldwide, and genotype has been shown to influence the natural history and, in turn, transmission patterns of hepatitis B infection. The distribution of genotypes worldwide is shown in Table 3.
Table 3.
Global distribution of hepatitis B virus genotypes
| Genotype | Prevalent regions |
|---|---|
| A | Sub-Saharan Africa (A1); Northern Europe (A2); North America (A2); Northern and Western Europe |
| B | Eastern and Southeast Asia; Oceania; North America |
| C | North, East, and Southeast Asia; Oceania; North America |
| D | Southern and Eastern Europe; South-Central Asia; Western Asia; North Africa; Oceania |
| E | West Africa |
| F | North America; Latin America; the Caribbean |
| G | Western Europe; North America |
| H | Latin America; the Caribbean |
Regions based on the United Nations Population Dictionary (United Nations Statistics Division 2013). (Adapted from data in Kramvis et al. 2005, McMahon 2009, and Trépo et al. 2014.)
Genotype also influences the progression of viral infection through phases (see above), which, in turn, determines infectivity and age at transmission or infection. In Asia, where genotypes B and C predominate, transmission is most commonly vertical at the time of birth; where genotypes A, D, and E are most common, such as Africa, Eastern Europe, and the Middle East, transmission is more commonly horizontal in early childhood (McMahon 2009).
HBV genotypes and subgenotypes may also have relevance to hepatitis B control efforts through vaccination. Mismatch between the strain used to derive hepatitis B vaccine (serotype adw) and that which is prevalent in a given population may result in increased vaccine escape and reduced efficacy at a population level (Viviani et al. 2008; Davies et al. 2013).
COINFECTION
Overlapping patterns of endemicity are observed between HBV and other blood-borne viruses because of shared modes of transmission. This can result in HBV coinfection with hepatitis C virus (HCV), hepatitis D virus (HDV), and HIV, and can include multiple coinfections. Coinfection with HBV and one or more of the other blood-borne viruses is associated with modification of the natural history of liver disease and typically poorer outcomes than are observed in HBV mono-infection. Given this increased risk of complications, prevention of coinfection in those living with HBV (through harm reduction) or with HCV or HIV (through HBV vaccination) is of high priority.
HIV
Of the estimated 29.2 million individuals living with HIV globally (Murray et al. 2014), between 5% and 10% are thought to be living with HBV coinfection (Puoti et al. 2008; Thio 2009; Kourtis et al. 2012), representing ∼1.7–3.5 million people. As a proportion of the estimated 240–350 million people living with CHB worldwide, this represents a prevalence of HIV coinfection of ∼0.5%–1.5%. The frequency of coinfection varies considerably according to population group and region, and is related to both CHB and HIV endemicity and transmission patterns.
In low-HBV-prevalence countries, HBV and HIV transmission largely occur in adulthood through sexual contact and injecting drug use; and although overall prevalence of coinfection is low, it can be significant in some groups, such as men who have sex with men and people who inject drugs (Kourtis et al. 2012). In some populations, up to 25% of people living with HIV are also infected with HBV (Thio 2009).
In addition to adverse impact on the natural history of CHB, antiretroviral treatment in the setting of coinfection can be more complex owing to the potential of selecting for resistance mutations in HBV, the possibility of immune reconstitution flares in hepatitis B on initiation of antiretroviral therapy, and poor outcomes when HBV-active regimens are inadvertently ceased (Thio 2009). In people with more advanced immunodeficiency, the efficacy of hepatitis B vaccination is also reduced (Landrum et al. 2011).
In the era of increased access to antiretroviral therapy, liver disease is a major cause of non-AIDS-related mortality in individuals living with HIV (Puoti et al. 2008; Sulkowski 2008). In contrast, the scaling up of access to antiretroviral regimens in low-resource settings, which are also endemic for CHB, can lead to a situation in which the only people able to access treatment for hepatitis B are those who are coinfected with HIV (Cowie et al. 2013; Lemoine et al. 2013). Although this increased access for those at greater risk for progressing to advanced liver disease is a very positive development, the lack of access to those living with HBV mono-infection in these countries remains a substantial concern.
HCV
Like HBV infection, HCV is one of the most common chronic infectious diseases worldwide, affecting an estimated 150 to 170 million people globally (Mohd Hanafiah et al. 2013; see www.who.int/csr/disease/hepatitis/GHP_framework.pdf). Between 7 and 20 million are thought to be coinfected with HBV, or ∼2%–8% of the total number living with chronic HBV (Wu and Liu 2012). Reflecting the epidemiology of HCV in general, risk factors for HCV coinfection in people living with CHB include injecting drug use, a history of blood transfusion, and other parenteral exposures. Regional differences in prevalence are observed within some countries (Gaeta et al. 2003; Li et al. 2013).
There is evidence that HCV coinfection in people living with CHB increases the risk of progression to cirrhosis and HCC (Donato et al. 1998; Shi et al. 2005; Amin et al. 2006; Fattovich et al. 2008; Lok and McMahon 2009; Oh et al. 2012). The effect of coinfection on risk has been shown to vary between HBV-endemic, HCV-endemic, and low-prevalence areas (Cho et al. 2011).
HDV
HDV is a satellite RNA virus, which only infects individuals also infected with HBV (Rizzetto 2009). Globally, HDV infection occurs in ∼5% of those living with HBV; however, the prevalence of coinfection varies widely and, in many areas, is not known (Hughes et al. 2011). HDV is transmissible through blood-borne, sexual, percutaneous, permucosal, and perinatal means, although perinatal transmission is less common than for HBV. The predominant modes of transmission are thought to be through intrafamilial horizontal transmission (such as between young children), sexual, injecting drug use, and other parenteral exposures, such as unsafe medical procedures (Hughes et al. 2011).
Although the pattern of HDV endemicity is related to HBV, many areas that have high-HBV prevalence do not have significant rates of HDV. HDV endemic areas have traditionally included Central Africa, the Horn of Africa, the Amazon Basin, Eastern and Mediterranean Europe, the Middle East, and parts of Asia (Rizzetto et al. 1990; Hughes et al. 2011).
However, the prevalence of HDV has been shown to be declining in a number of regions, such as Southern Europe (Gaeta et al. 2000), thought to be associated with the improvement of primary HBV prevention through vaccination, harm reduction in people who inject drugs, and general improvement in socioeconomic conditions. Conversely, prevalence has shown an increase in some previously low-prevalence areas, predominantly caused by increasing migration from endemic areas (Wedemeyer et al. 2007; Shadur et al. 2013).
Coinfection with HDV is associated with a higher likelihood of progressions to cirrhosis and related mortality; however, the impact of HDV on HCC risk specifically remains uncertain (Wedemeyer 2010; Hughes et al. 2011).
EPIDEMIOLOGY OF HCC AND HBV
HCC is a major adverse outcome of HBV infection, and an important cause of mortality in a global context. In 2012, 782,000 people were diagnosed with liver cancer and it caused 746,000 deaths (Ferlay 2012), with HCC making up the vast majority of all liver cancers (Jemal et al. 2011). As a cause of cancer morbidity and mortality, this makes liver cancer the second most common cause of cancer death worldwide, responsible for one in ten cancer deaths.
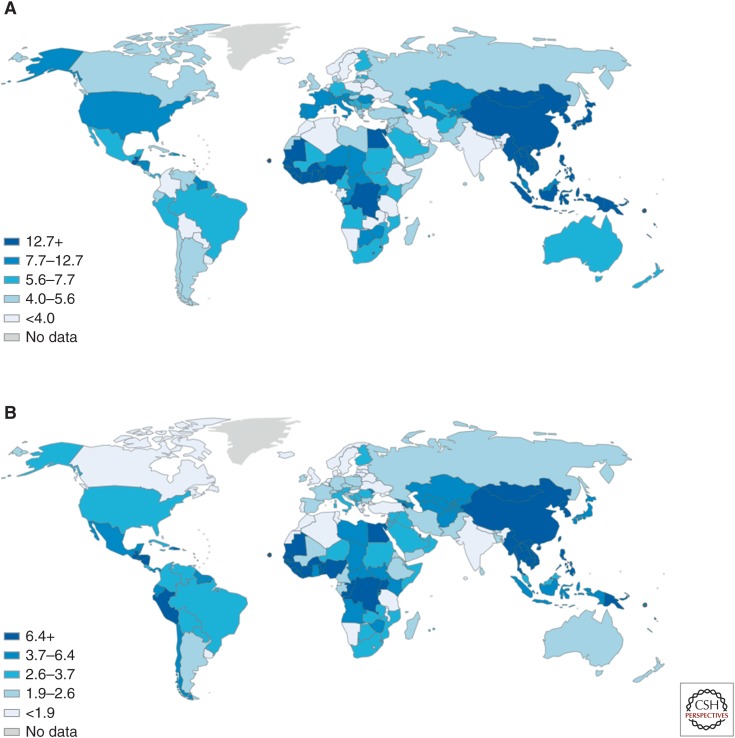
The burden of liver cancer is geographically disparate, and, in 2012, nearly two-thirds of all liver cancer cases were in the Western Pacific world region. This disproportionate impact is largely driven by high incidence in China, where half of worldwide liver cancer cases occurred. Other regions of high burden include the Africa region, where, although the number of cases is much lower, the relative burden is high, with liver cancer ranking as the fourth most common cancer, and the Eastern Mediterranean region, where it ranks as fifth most common. This geographic distribution is largely driven by HBV, which is estimated to be responsible for most cases of HCC globally (Jemal et al. 2011; Lozano et al. 2012).
Given the high variation in HBV prevalence globally and the importance of HBV as a cause of HCC, there is a clear geographic relationship between HBV prevalence and the burden of HCC (see Figs. 1 and 2). The attribution of HBV as a cause of HCC is dependent on HBV prevalence, and the proportion of cancers attributable to HBV varies considerably according to region. HBV is estimated to cause around a quarter of liver cancer cases in developed countries but up to 60% in developing countries (Jemal et al. 2011). In China, up to 90% of liver cancer is attributable to HBV (Gust 1996). This also influences the overall etiology of liver disease according to country, with those of high-HBV prevalence shown to have a relatively higher ratio of HCC compared with cirrhosis-related liver deaths.
Figure 2.
Global incidence of liver cancer, age standardized rates per 100,000 population, 2012 in (A) males, and (B) females. (Data from Ferlay 2012.)
The disproportionately high incidence of HCC in some regions is also influenced by other factors, such as the presence of aflatoxin B1 contamination, which has a synergistic relationship with HBV in promoting HCC development. HBV genotype is another factor that is geographically determined and related to HCC incidence, with studies demonstrating that in the Asia-Pacific region, genotype C is associated with more rapid progression to HCC than genotype B. Genotype D has also been shown to lead to higher incidence of HCC than genotype A in those areas in which it is prevalent, such as North America and Western Europe (El-Serag 2012).
Survival following liver cancer diagnosis remains poor even in well-resourced health systems with multiple therapeutic options, including liver transplantation (Nguyen et al. 2009). For example, despite the substantial improvements in cancer mortality overall, liver cancer mortality continues to increase in these settings, with liver cancer the fastest increasing cause of cancer death in the United States and Australia (MacLachlan and Cowie 2012; El-Serag and Kanwal 2014). This emphasizes the need for increased attention to diagnosis, management, and treatment of hepatitis B (along with hepatitis C and other causes of chronic liver disease), with increasing evidence that antiviral therapy can substantially reduce the incidence of liver cancer (Papatheodoridis et al. 2010).
THE GLOBAL HEPATITIS PROGRAMME OF THE WORLD HEALTH ORGANIZATION
The World Health Assembly adopted resolution WHA63.18 in May 2010 in recognition of the increasing burden of viral hepatitis on global health (see apps.who.int/gb/ebwha/pdf_files/WHA63/A63_R18-en.pdf). In response, the World Health Organization (WHO) established the Global Hepatitis Programme in Geneva, with focal points in each WHO regional office, to coordinate global efforts to address viral hepatitis and to support member states in developing the necessary capacity and policies to reduce the burden of viral hepatitis globally.
An early undertaking of the WHO Global Hepatitis Programme was to publish, in December 2012, the “Prevention and Control of Viral Hepatitis Infection: Framework for Global Action” (see www.who.int/csr/disease/hepatitis/GHP_framework.pdf). The goal of the WHO viral hepatitis strategy is to reduce transmission, morbidity, mortality, and socioeconomic impact of viral hepatitis globally, using a health systems approach. The framework presented in this publication consists of four axes:
Axis 1. Raising awareness, promoting partnerships and mobilizing resources;
Axis 2. Evidence-based policy and data for action;
Axis 3. Prevention of transmission; and
Axis 4. Screening, care, and treatment.
From the perspective of enhanced epidemiological information, proposed activities under Axis 2 include updating global prevalence estimates for viral hepatitis, developing standards for communicable disease surveillance relating to viral hepatitis, and provision of guidance for serological surveys to monitor trends and evaluate the impact of prevention measures.
Appropriate implementation of this framework will require substantial political commitment and mobilization of funding at national, regional, and global levels. However, with increasing recognition that viral hepatitis is a major cause of human deaths—on a comparable scale to HIV/AIDS, tuberculosis, and malaria (Lozano et al. 2012; Cowie et al. 2013)—it is essential that the opportunity to reduce the burden of viral hepatitis, including hepatitis B, is realized.
CONCLUDING REMARKS
The burden of adverse outcomes related to hepatitis B on individuals and communities, particularly in high-prevalence populations, is increasingly recognized. Although ensuring high coverage of infant vaccination will have a profound impact on this burden in coming decades, attention must be given to comprehensive policy responses now. Understanding the epidemiology of HBV infection will enable evidence-based and cost-effective public health and clinical interventions within countries and at the global level.
Footnotes
Editors: Christoph Seeger and Stephen Locarnini
Additional Perspectives on Hepatitis B and Delta Viruses available at www.perspectivesinmedicine.org
REFERENCES
*Reference is also in this collection.
- Amin J, Law MG, Bartlett M, Kaldor JM, Dore GJ. 2006. Causes of death after diagnosis of hepatitis B or hepatitis C infection: A large community-based linkage study. Lancet 368: 938–945. [DOI] [PubMed] [Google Scholar]
- Arankalle VA, Gandhi S, Lole KS, Chadha MS, Gupte GM, Lokhande MU. 2011. An outbreak of hepatitis B with high mortality in India: Association with precore, basal core promoter mutants and improperly sterilized syringes. J Viral Hepat 18: e20–e28. [DOI] [PubMed] [Google Scholar]
- Batham A, Narula D, Toteja T, Sreenivas V, Puliyel JM. 2007. Sytematic review and meta-analysis of prevalence of hepatitis B in India. Indian Pediatr 44: 663–674. [PubMed] [Google Scholar]
- Blumberg BS, Alter HJ, Visnich S. 1965. A “new” antigen in leukemia sera. JAMA 191: 541–546. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control. 2012. Hepatitis B—2012 Yellow Book. wwwnc.cdc.gov/travel/yellowbook/2014/chapter-3-infectious-diseases-related-to-travel/hepatitis-b. [Google Scholar]
- Chang MH, Chen CJ, Lai MS, Hsu HM, Wu TC, Kong MS, Liang DC, Shau WY, Chen DS. 1997. Universal hepatitis B vaccination in Taiwan and the incidence of hepatocellular carcinoma in children. Taiwan Childhood Hepatoma Study Group. N Engl J Med 336: 1855–1859. [DOI] [PubMed] [Google Scholar]
- Chang MH, You SL, Chen CJ, Liu CJ, Lee CM, Lin SM, Chu HC, Wu TC, Yang SS, Kuo HS, et al. 2009. Decreased incidence of hepatocellular carcinoma in hepatitis B vaccinees: A 20-year follow-up study. J Natl Cancer Inst 101: 1348–1355. [DOI] [PubMed] [Google Scholar]
- Chen CJ, Yang HI, Su J, Jen CL, You SL, Lu SN, Huang GT, Iloeje UH. 2006. Risk of hepatocellular carcinoma across a biological gradient of serum hepatitis B virus DNA level. JAMA 295: 65–73. [DOI] [PubMed] [Google Scholar]
- Chen CF, Lee WC, Yang HI, Chang HC, Jen CL, Iloeje UH, Su J, Hsiao CK, Wang LY, You SL, et al. 2011. Changes in serum levels of HBV DNA and alanine aminotransferase determine risk for hepatocellular carcinoma. Gastroenterology 141: e1242. [DOI] [PubMed] [Google Scholar]
- Cho LY, Yang JJ, Ko KP, Park B, Shin A, Lim MK, Oh JK, Park S, Kim YJ, Shin HR, et al. 2011. Coinfection of hepatitis B and C viruses and risk of hepatocellular carcinoma: Systematic review and meta-analysis. Int J Cancer 128: 176–184. [DOI] [PubMed] [Google Scholar]
- Cowie BC, Carville KS, MacLachlan JH. 2013. Mortality due to viral hepatitis in the Global Burden of Disease Study 2010: New evidence of an urgent global public health priority demanding action. Antivir Ther 18: 953–954. [DOI] [PubMed] [Google Scholar]
- Davies J, Littlejohn M, Locarnini SA, Whiting S, Hajkowicz K, Cowie BC, Bowden DS, Tong SY, Davis JS. 2013. The molecular epidemiology of hepatitis B in the indigenous people of Northern Australia. J Gastroenterol Hepatol 28: 1234–1241. [DOI] [PubMed] [Google Scholar]
- Donato F, Boffetta P, Puoti M. 1998. A meta-analysis of epidemiological studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma. Int J Cancer 75: 347–354. [DOI] [PubMed] [Google Scholar]
- Edmunds WJ, Medley GF, Nokes DJ, Hall AJ, Whittle HC. 1993. The influence of age on the development of the hepatitis B carrier state. Proc Biol Sci 253: 197–201. [DOI] [PubMed] [Google Scholar]
- El-Serag HB. 2012. Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142: e1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag HB, Kanwal F. 2014. Epidemiology of hepatocellular carcinoma in the United States: Where are we? Where do we go? Hepatology 60: 1767–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattovich G, Bortolotti F, Donato F. 2008. Natural history of chronic hepatitis B: Special emphasis on disease progression and prognostic factors. J Hepatol 48: 335–352. [DOI] [PubMed] [Google Scholar]
- Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. 2012. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11, International Agency for Research on Cancer, Lyon, France. [Google Scholar]
- Gaeta GB, Stroffolini T, Chiaramonte M, Ascione T, Stornaiuolo G, Lobello S, Sagnelli E, Brunetto MR, Rizzetto M. 2000. Chronic hepatitis D: A vanishing disease? A multicenter Italian study. Hepatology 32: 824–827. [DOI] [PubMed] [Google Scholar]
- Gaeta GB, Stornaiuolo G, Precone DF, Lobello S, Chiaramonte M, Stroffolini T, Colucci G, Rizzetto M. 2003. Epidemiological and clinical burden of chronic hepatitis B virus/hepatitis C virus infection. A multicenter Italian study. J Hepatol 39: 1036–1041. [DOI] [PubMed] [Google Scholar]
- Goldstein ST, Zhou F, Hadler SC, Bell BP, Mast EE, Margolis HS. 2005. A mathematical model to estimate global hepatitis B disease burden and vaccination impact. Int J Epidemiol 34: 1329–1339. [DOI] [PubMed] [Google Scholar]
- Graham S, Guy RJ, Cowie B, Wand HC, Donovan B, Akre SP, Ward JS. 2013. Chronic hepatitis B prevalence among Aboriginal and Torres Strait Islander Australians since universal vaccination: A systematic review and meta-analysis. BMC Infect Dis 13: 403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gust ID. 1996. Epidemiology of hepatitis B infection in the Western Pacific and South East Asia. Gut 38: S18–S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahne S, Ramsay M, Balogun K, Edmunds WJ, Mortimer P. 2004. Incidence and routes of transmission of hepatitis B virus in England and Wales, 1995–2000: Implications for immunisation policy. J Clin Virol 29: 211–220. [DOI] [PubMed] [Google Scholar]
- Harpaz R, McMahon BJ, Margolis HS, Shapiro CN, Havron D, Carpenter G, Bulkow LR, Wainwright RB. 2000. Elimination of new chronic hepatitis B virus infections: Results of the Alaska immunization program. J Infect Dis 181: 413–418. [DOI] [PubMed] [Google Scholar]
- Hughes SA, Wedemeyer H, Harrison PM. 2011. Hepatitis delta virus. Lancet 378: 73–85. [DOI] [PubMed] [Google Scholar]
- Iloeje UH, Yang HI, Su J, Jen CL, You SL, Chen CJ. 2006. Predicting cirrhosis risk based on the level of circulating hepatitis B viral load. Gastroenterology 130: 678–686. [DOI] [PubMed] [Google Scholar]
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. 2011. Global cancer statistics. CA Cancer J Clin 61: 69–90. [DOI] [PubMed] [Google Scholar]
- Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. 2012. HIV–HBV coinfection—A global challenge. N Engl J Med 366: 1749–1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowdley KV, Wang CC, Welch S, Roberts H, Brosgart CL. 2012. Prevalence of chronic hepatitis B among foreign-born persons living in the United States by country of origin. Hepatology 56: 422–433. [DOI] [PubMed] [Google Scholar]
- Kramvis A, Clements CJ. 2010. Implementing a birth dose of hepatitis B vaccine for home deliveries in Africa—Too soon? Vaccine 28: 6408–6410. [DOI] [PubMed] [Google Scholar]
- Kramvis A, Kew MC. 2005. Relationship of genotypes of hepatitis B virus to mutations, disease progression and response to antiviral therapy. J Viral Hepat 12: 456–464. [DOI] [PubMed] [Google Scholar]
- Kramvis A, Kew M, Francois G. 2005. Hepatitis B virus genotypes. Vaccine 23: 2409–2423. [DOI] [PubMed] [Google Scholar]
- Landrum ML, Hullsiek KH, Chun HM, Crum-Cianflone NF, Ganesan A, Weintrob AC, Barthel RV, O’Connell RJ, Agan BK. 2011. The timing of hepatitis B virus (HBV) immunization relative to human immunodeficiency virus (HIV) diagnosis and the risk of HBV infection following HIV diagnosis. Am J Epidemiol 173: 84–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavanchy D. 2004. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat 11: 97–107. [DOI] [PubMed] [Google Scholar]
- Lemoine M, Nayagam S, Thursz M. 2013. Viral hepatitis in resource-limited countries and access to antiviral therapies: current and future challenges. Future Virol 8: 371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Long Y, Wang T, Xiao D, Zhang J, Guo Z, Wang B, Yan Y. 2013. Epidemiology of hepatitis C virus infection in highly endemic HBV areas in China. PLoS ONE 8: e54815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Bi S, Yang W, Wang L, Cui G, Cui F, Zhang Y, Liu J, Gong X, Chen Y, et al. 2009. Epidemiological serosurvey of hepatitis B in China—Declining HBV prevalence due to hepatitis B vaccination. Vaccine 27: 6550–6557. [DOI] [PubMed] [Google Scholar]
- *.Lin CL, Kao JH. 2015. Hepatitis B virus genotypes and variants. Cold Spring Harb Perspect Med 5: a021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lok AS, McMahon BJ. 2009. Chronic hepatitis B: Update 2009. Hepatology 50: 661–662. [DOI] [PubMed] [Google Scholar]
- Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, et al. 2012. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: A systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLachlan JH, Cowie BC. 2012. Liver cancer is the fastest increasing cause of cancer death in Australians. Med J Aust 197: 492–493. [DOI] [PubMed] [Google Scholar]
- MacLachlan JH, Allard N, Towell V, Cowie BC. 2013. The burden of chronic hepatitis B virus infection in Australia, 2011. Aust NZ J Public Health 37: 416–422. [DOI] [PubMed] [Google Scholar]
- Mahoney FJ. 1999. Update on diagnosis, management, and prevention of hepatitis B virus infection. Clin Microbiol Rev 12: 351–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marschall T, Kretzschmar M, Mangen MJ, Schalm S. 2008. High impact of migration on the prevalence of chronic hepatitis B in the Netherlands. Eur J Gastroenterol Hepatol 20: 1214–1225. [DOI] [PubMed] [Google Scholar]
- McMahon BJ. 2009. The influence of hepatitis B virus genotype and subgenotype on the natural history of chronic hepatitis B. Hepatol Int 3: 334–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohd Hanafiah K, Groeger J, Flaxman AD, Wiersma ST. 2013. Global epidemiology of hepatitis C virus infection: New estimates of age-specific antibody to HCV seroprevalence. Hepatology 57: 1333–1342. [DOI] [PubMed] [Google Scholar]
- Murray CJ, Ortblad KF, Guinovart C, Lim SS, Wolock TM, Roberts DA, Dansereau EA, Graetz N, Barber RM, Brown JC, et al. 2014. Global, regional, and national incidence and mortality for HIV, tuberculosis, and malaria during 1990-2013: A systematic analysis for the Global Burden of Disease Study 2013. Lancet 384: 1005–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PK, Mathers BM, Cowie B, Hagan H, Des Jarlais D, Horyniak D, Degenhardt L. 2011. Global epidemiology of hepatitis B and hepatitis C in people who inject drugs: Results of systematic reviews. Lancet 378: 571–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen VT, Law MG, Dore GJ. 2009. Hepatitis B-related hepatocellular carcinoma: Epidemiological characteristics and disease burden. J Viral Hepat 16: 453–463. [DOI] [PubMed] [Google Scholar]
- Oh JK, Shin HR, Lim MK, Cho H, Kim DI, Jee Y, Yun H, Yoo KY. 2012. Multiplicative synergistic risk of hepatocellular carcinoma development among hepatitis B and C co-infected subjects in HBV endemic area: A community-based cohort study. BMC Cancer 12: 452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ott JJ, Stevens GA, Groeger J, Wiersma ST. 2012. Global epidemiology of hepatitis B infection: New estimates of age-specific HBsAg seroprevalence and endemicity. Vaccine 30: 2212–2219. [DOI] [PubMed] [Google Scholar]
- Papatheodoridis GV, Lampertico P, Manolakopoulos S, Lok A. 2010. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: A systematic review. J Hepatol 53: 348–356. [DOI] [PubMed] [Google Scholar]
- Plymoth A, Viviani S, Hainaut P. 2009. Control of hepatocellular carcinoma through hepatitis B vaccination in areas of high endemicity: Perspectives for global liver cancer prevention. Cancer Lett 286: 15–21. [DOI] [PubMed] [Google Scholar]
- Previsani N, Lavanchy D. 2002. Hepatitis B. Department of Communicable Diseases Surveillance and Response, World Health Organisation, Geneva. [Google Scholar]
- Puoti M, Manno D, Nasta P, Carosi G. 2008. Hepatitis B virus and HIV coinfection in low-income countries: Unmet needs. Clin Infect Dis 46: 367–369. [DOI] [PubMed] [Google Scholar]
- Rizzetto M. 2009. Hepatitis D: Thirty years after. J Hepatol 50: 1043–1050. [DOI] [PubMed] [Google Scholar]
- Rizzetto M, Ponzetto A, Forzani I. 1990. Hepatitis delta virus as a global health problem. Vaccine 8: S10–S14; discussion S21–S23. [DOI] [PubMed] [Google Scholar]
- Robinson T, Bullen C, Humphries W, Hornell J, Moyes C. 2005. The New Zealand Hepatitis B Screening Programme: Screening coverage and prevalence of chronic hepatitis B infection. NZ Med J 118: U1345. [PubMed] [Google Scholar]
- Sagnelli E, Sagnelli C, Pisaturo M, Macera M, Coppola N. 2014. Epidemiology of acute and chronic hepatitis B and delta over the last 5 decades in Italy. World J Gastroenterol 20: 7635–7643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salleras L, Dominguez A, Bruguera M, Cardenosa N, Batalla J, Carmona G, Navas E, Taberner JL. 2005. Dramatic decline in acute hepatitis B infection and disease incidence rates among adolescents and young people after 12 years of a mass hepatitis B vaccination programme of pre-adolescents in the schools of Catalonia (Spain). Vaccine 23: 2181–2184. [DOI] [PubMed] [Google Scholar]
- Shadur B, Maclachlan J, Cowie B. 2013. Hepatitis D virus in Victoria 2000–2009. Intern Med J 43: 1081–1087. [DOI] [PubMed] [Google Scholar]
- Shi J, Zhu L, Liu S, Xie WF. 2005. A meta-analysis of case-control studies on the combined effect of hepatitis B and C virus infections in causing hepatocellular carcinoma in China. Br J Cancer 92: 607–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulkowski MS. 2008. Management of hepatic complications in HIV-infected persons. J Infect Dis 197: S279–S293. [DOI] [PubMed] [Google Scholar]
- *.Tan A, Koh S, Bertoletti A. 2015. Immune response in HBV infection. Cold Spring Harb Perspect Med 10.1101/cshperspect.a021428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thio CL. 2009. Hepatitis B and human immunodeficiency virus coinfection. Hepatology 49: S138–S145. [DOI] [PubMed] [Google Scholar]
- Thompson ND, Perz JF, Moorman AC, Holmberg SD. 2009. Nonhospital health care-associated hepatitis B and C virus transmission: United States, 1998–2008. Ann Intern Med 150: 33–39. [DOI] [PubMed] [Google Scholar]
- Trépo C, Chan HL, Lok A. 2014. Hepatitis B virus infection. Lancet 10.1016/S0140-6736(14)60220-8. [DOI] [PubMed] [Google Scholar]
- United Nations Statistics Division. 2013. Composition of macro geographical (continental) regions, geographical sub-regions, and selected economic and other groupings. United Nations Statistics Division, unstats.un.org/unsd/default.htm. [Google Scholar]
- Viana S, Parana R, Moreira RC, Compri AP, Macedo V. 2005. High prevalence of hepatitis B virus and hepatitis D virus in the Western Brazilian Amazon. Am J Trop Med Hyg 73: 808–814. [PubMed] [Google Scholar]
- Viviani S, Carrieri P, Bah E, Hall AJ, Kirk GD, Mendy M, Montesano R, Plymoth A, Sam O, Van der Sande M, et al. 2008. 20 years into the Gambia Hepatitis Intervention Study: Assessment of initial hypotheses and prospects for evaluation of protective effectiveness against liver cancer. Cancer Epidemiol Biomarkers Prev 17: 3216–3223. [DOI] [PubMed] [Google Scholar]
- Wedemeyer H. 2010. Re-emerging interest in hepatitis delta: New insights into the dynamic interplay between HBV and HDV. J Hepatol 52: 627–629. [DOI] [PubMed] [Google Scholar]
- Wedemeyer H, Heidrich B, Manns MP. 2007. Hepatitis D virus infection—Not a vanishing disease in Europe! Hepatology 45: 1331–1332; author reply 1332–1333. [DOI] [PubMed] [Google Scholar]
- Weinbaum CM, Williams I, Mast EE, Wang SA, Finelli L, Wasley A, Neitzel SM, Ward JW. 2008. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep 57: 1–20. [PubMed] [Google Scholar]
- Wu Q, Liu Q. 2012. Do hepatitis B virus and hepatitis C virus co-infections increase hepatocellular carcinoma occurrence through synergistically modulating lipogenic gene expression? Hepatol Res 42: 733–740. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Lu Y, Ye Q, Xia Y, Zhou Y, Yao Q, Wei S. 2011. Should chronic hepatitis B mothers breastfeed? A meta analysis. BMC Public Health 11: 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Zoulim F, Durantel D. 2015. Antiviral therapies and prospects for a cure of chronic hepatitis B. Cold Spring Harb Perspect Med 10.1101/cshperspect.a021501. [DOI] [PMC free article] [PubMed] [Google Scholar]