Abstract
Chemokines are chemotactic cytokines that control the migration and positioning of immune cells in tissues and are critical for the function of the innate immune system. Chemokines control the release of innate immune cells from the bone marrow during homeostasis as well as in response to infection and inflammation. They also recruit innate immune effectors out of the circulation and into the tissue where, in collaboration with other chemoattractants, they guide these cells to the very sites of tissue injury. Chemokine function is also critical for the positioning of innate immune sentinels in peripheral tissue and then, following innate immune activation, guiding these activated cells to the draining lymph node to initiate and imprint an adaptive immune response. In this review, we will highlight recent advances in understanding how chemokine function regulates the movement and positioning of innate immune cells at homeostasis and in response to acute inflammation, and then we will review how chemokine-mediated innate immune cell trafficking plays an essential role in linking the innate and adaptive immune responses.
Chemokines regulate the movement and positioning of innate immune cells at homeostasis and in response to acute inflammation. They also coordinate interactions between the innate and adaptive immune systems.
Chemokines are chemotactic cytokines that control cell migration and cell positioning throughout development, homeostasis, and inflammation. The immune system, which is dependent on the coordinated migration of cells, is particularly dependent on chemokines for its function. Not only do chemokines guide immune effector cells to sites of infection or inflammation, but they also coordinate interactions between immune cells. By doing so, chemokines promote interactions between the innate and adaptive immune systems, thus shaping and providing the necessary context for the development of optimal adaptive immune responses. This review will aim to provide an overview of the function of the chemokine system, with emphasis placed on its role in the innate immune system.
CHEMOKINES AND CHEMOKINE RECEPTORS
The chemokine family consists of ∼50 endogenous chemokine ligands in humans and mice (Table 1). These small, 8- to 12-kDa protein ligands promote increased motility and directional migration when they bind to their corresponding cell-surface receptor. The chemokine ligands are divided into four groups based on the positioning of their initial cysteine residues: XC, CC, CXC, and CX3C. Thus, CC chemokine ligands (CCLs) have two adjoining amino-terminal cysteine residues, whereas CX3CL1 has three amino acids separating the two initial cysteine residues. Most chemokines are secreted into the extracellular space where they remain soluble or are bound to extracellular matrix components, thus forming transient or stable concentration gradients, respectively. Chemokines and their gradients are detected by binding to specific chemokine receptors.
Table 1.
Chemokines
| Chemokine | Other names | Receptor | Key/main immune function |
|---|---|---|---|
| CXCL1 | GRO-α, MGSA, mouse KC | CXCR2 | Neutrophil trafficking |
| CXCL2 | GRO-β, MIP-2α, mouse MIP2 | CXCR2 | Neutrophil trafficking |
| CXCL3 | GRO-γ, MIP-2β | CXCR2 | Neutrophil trafficking |
| CXCL4 | PF4 | ? | Procoagulant |
| CXCL5 | ENA-78, mouse LIX | CXCR2 | Neutrophil trafficking |
| CXCL6 | GCP-2 | CXCR1, CXCR2 | Neutrophil trafficking |
| CXCL7 | NAP-2 | CXCR2 | Neutrophil trafficking |
| CXCL8 | IL-8 (no mouse) | CXCR1, CXCR2 | Neutrophil trafficking |
| CXCL9 | Mig | CXCR3 | Th1 response; Th1, CD8, NK trafficking |
| CXCL10 | IP-10 | CXCR3 | Th1 response; Th1, CD8, NK trafficking |
| CXCL11 | I-TAC | CXCR3 | Th1 response; Th1, CD8, NK trafficking |
| CXCL12 | SDF-1 | CXCR4 | Bone-marrow homing |
| CXCL13 | BLC, BCA-1 | CXCR5 | B-cell and TFH-positioning LN |
| CXCL14 | BRAK | ? | Macrophage skin homing (human) |
| Cxcl15 | Lungkine (mouse only) | ? | ? |
| CXCL16 | CXCR6 | NKT and ILC migration and survival | |
| CXCL17 | ? | Macrophage and DC chemotaxis | |
| CCL1 | I-309, mouse TCA3 | CCR8 | Th2 cell and Treg trafficking |
| CCL2 | MCP-1, mouse JE | CCR2 | Inflammatory monocyte trafficking |
| CCL3 | MIP-1α | CCR1, CCR5 | Macrophage and NK-cell migration; T-cell–DC interactions |
| CCL4 | MIP-1β | CCR5 | Macrophage and NK-cell migration; T-cell–DC interactions |
| CCL5 | RANTES | CCR1, CCR3, CCR5 | Macrophage and NK-cell migration; T-cell–DC interactions |
| Ccl6 | C-10, MRP-1 (mouse only) | Unknown | ? |
| CCL7 | MCP-3, mouse Fic or MARC | CCR2, CCR3 | Monocyte mobilization |
| CCL8 | MCP-2 | CCR1, CCR2, CCR3, CCR5 (human); CCR8 (mouse) | Th2 response, skin homing mouse |
| Ccl9/10 | MIP-1γ, MRP-2 (mouse only) | Unknown | ? |
| CCL11 | Eotaxin-1 | CCR3 | Eosinophil and basophil migration |
| Ccl12 | MCP-5 (mouse only) | CCR2 | Inflammatory monocyte trafficking |
| CCL13 | MCP-4 | CCR2, CCR3, CCR5 | Th2 responses |
| CCL14 | HCC-1 | CCR1 | ? |
| CCL15 | Leukotactin-1, HCC-2, MIP-5 | CCR1, CCR3 | ? |
| CCL16 | HCC-4, NCC-4, LEC | CCR1, CCR2, CCR5 | ? |
| CCL17 | TARC | CCR4 | Th2 responses, Th2-cell migration, Treg, lung, and skin homing |
| CCL18 | PARC, DC-CK1 | CCR8 | Th2 response, marker AAM, skin homing |
| CCL19 | ELC, MIP-3β | CCR7 | T-cell and DC homing to LN |
| CCL20 | MIP-3α, LARC | CCR6 | Th17 responses, B-cell, and DC homing to gut-associated lymphoid tissue |
| CCL21 | SLC, 6CKine | CCR6, CCR7 | T-cell and DC homing to LN |
| CCL22 | MDC | CCR4 | Th2 response, Th2-cell migration, Treg migration |
| CCL23 | MPIF-1, MIP-3 | Unknown | ? |
| CCL24 | Eotaxin-2, MPIF-2 | CCR3 | Eosinophil and basophil migration |
| CCL25 | TECK | CCR9 | T-cell homing to gut, thymocyte migration |
| CCL26 | Eotaxin-3 | CCR3, CX3CR1 | Eosinophil and basophil migration |
| CCL27 | CTAK | CCR10 | T-cell homing to skin |
| CCL28 | MEC | CCR3, CCR10 | T-cell and IgA plasma–cell homing to mucosa |
| XCL1 | Lymphotactin, SCM-1α | XCR1 | Cross presentation by CD8+ DCs |
| XCL2 | SCM-1β | XCR1 | Cross presentation by CD8+ DCs |
| CX3CL1 | Fractalkine | CX3CR1 | NK, monocyte, and T-cell migration |
This table was created from data adapted from Bachelerie et al. 2013.
AAM, alternatively activated macrophage; ILC, innate lymphoid cell; LN, lymph node; NK, natural killer; NKT, natural killer T; TFH, follicular helper T cell; Treg, regulatory T cell.
Chemokine receptors are a group of ∼20 rhodopsin-like seven-transmembrane-spanning receptors in humans and mice (Table 2). These receptors are G-protein-coupled and signal via pertussis toxin-sensitive Gi-type G proteins. The chemokine receptors show varying levels of binding specificity and promiscuity. For example, CXCR4 binds only CXCL12, whereas CCR1 can bind to six different chemokine ligands. Despite this promiscuity, chemokine receptors do not bind different groups of chemokines (e.g., CCL and CXCL chemokines), and they are named based on the group that they bind; CCR chemokine receptors bind CCL chemokines, whereas CXCR receptors bind CXCL chemokines. In addition to the signaling receptors, chemokine receptors also include a group of four atypical receptors. These are similar in structure to the signaling receptors, but lack an intracellular motif required for signaling through Gi-type G proteins. These nonsignaling, atypical receptors appear to play primary roles in shaping chemokine gradients by scavenging and promoting transcytosis of chemokine ligands.
Table 2.
Chemokine receptors
| Receptor | Immune cell expression | Key immune function |
|---|---|---|
| G-protein-coupled chemokine receptors | ||
| CXCR1 | N>Mo, NK, MC, Ba, CD8 TEFF | Neutrophil trafficking |
| CXCR2 | N>Mo, NK, MC, Ba, CD8 T | B-cell lymphopoiesis Neutrophil egress from bone marrow Neutrophil trafficking |
| CXCR3 | Th1, CD8 TCM and TEM, NK, NKT, pDC, B, Treg, TFH | Th1-type adaptive immunity |
| CXCR4 | Most (if not all) leukocytes | Hematopoiesis Organogenesis Bone marrow homing |
| CXCR5 | B, TFH, TFR, CD8 TEM | B- and T-cell trafficking in lymphoid tissue to B-cell zone/follicles |
| CXCR6 | Th1, Th17, γδ T, iLC, NKT, NK, PC | Innate lymphoid cell function Adaptive immunity |
| CCR1 | Mo, MΦ, N, Th1, Ba, DC | Innate immunity Adaptive immunity |
| CCR2 | Mo, MΦ, Th1, iDC, Ba, NK | Monocyte trafficking Th1-type adaptive immunity |
| CCR3 | Eo>Ba, MC | Th2-type adaptive immunity Eosinophil distribution and trafficking |
| CCR4 | Th2, skin- and lung-homing T, Treg>Th17, CD8 T, Mo, B, iDC | Homing of T cells to skin and lung Th2-type immune response |
| CCR5 | Mo, MΦ, Th1, NK, Treg, CD8 T, DC, N | Type-1 adaptive immunity |
| CCR6 | Th17>iDC, γδ T, NKT, NK, Treg, TFH | iDC trafficking, GALT development Th17 adaptive immune responses |
| CCR7 | TN, TCM, TRCM, mDC, B | mDC, and B- and T-cell trafficking in lymphoid tissue to T-cell zone Egress of DC and T cells from tissue |
| CCR8 | Th2, Treg, skin TRM, γδ T, Mo, MΦ | Immune surveillance in skin type-2 adaptive immunity, thymopoiesis |
| CCR9 | Gut homing T, thymocytes, B, DC, pDC | Homing of T cells to gut GALT development and function, thymopoiesis |
| CCR10 | Skin-homing T, IgA plasmablasts | Humoral immunity at mucosal sites Immune surveillance in skin |
| XCR1 | Cross-presenting CD8+ DC, thymic DC | Ag cross-presentation by CD8+ DCs |
| CX3CR1 | Resident Mo, MΦ, MG, Th1, CD8 TEM, NK, γδ T, DC | Patrolling monocytes in innate immunity Microglial-cell and NK-cell migration type-1 adaptive immunity |
| Atypical chemokine receptors (non-G-protein-coupled signaling) | ||
| ACKR1 (DARC; Duffy) | RBC, LEC | Chemokine transcytosis Chemokine scavenging |
| ACKR2 (D6) | LEC, DC, B | Chemokine scavenging |
| ACKR3 (CXCR7) | Stromal cells, B | Shaping chemokine gradients for CXCR4 |
| ACKR4 (CCRL1; CCX-CKR) | Thymic epithelium | Chemokine scavenging |
Data in table is modified from Bachelerie et al. 2013.
B, B cell; Ba, basophil; Eo, eosinophils; GALT, gut-associated lymphoid tissue; iDC, immature DC; LEC, lymphatic endothelium; MΦ, macrophage; MG, microglia; Mo, monocyte; N, neutrophil; pDC, plasmacytoid DC; PC, plasma cell; RBC, red blood cell; TCM, central memory T cell; TEFF, effector T cell; TFH, follicular helper T cell; TFR, follicular regulatory T cell; TN, naïve T cell; TRCM, recirculating memory T cell.
EVOLUTION OF CHEMOKINES AND CHEMOKINE RECEPTORS
Chemokines are evolutionarily ancient, having first appeared 700 million years ago in a common ancestor of the vertebrate lineage. Although chemotaxis has been described in invertebrate cells, an analog of the chemokine system does not appear to exist in the invertebrates (DeVries et al. 2006). After their initial appearance, chemokines have undergone extensive and rapid evolution, owing to large gene duplication events and subsequent selection. Human chemokine ligand genes are predominantly found on chromosomes 4 and 17 in regions with significant numbers of psueodogenes, which correlates with a distant gene duplication event with subsequent diversification, selection, and loss (Zlotnik et al. 2006; Nomiyama et al. 2010). However, there is significant variation in chemokine ligands between different evolutionary lineages. The zebrafish genome contains more than 100 discrete chemokine genes (Zlotnik et al. 2006; Nomiyama et al. 2010). Whether this is the result of selection events attributable to different pathogen exposure or the result of random genetic duplication events remains to be determined.
Similar to chemokine ligands, chemokine receptors show evidence of rapid expansion and evolution after their appearance in a common vertebrate ancestor. The sea lamprey, a jawless fish, contains six known chemokine receptors. Concurrent with their ligands, further expansion of chemokine receptors occurred with species diversification. The elephant shark, a cartilaginous fish, contains 13 putative chemokine receptors, whereas the zebrafish contains at least 32 different chemokine receptors (Zlotnik et al. 2006). Mammals contain less, indicating that this expansion in chemokine receptors occurred following the split of the teleost lineage with the mammalian lineage. Humans and mice contain 17 signaling chemokine receptors, with the majority present on human chromosome 3 (Zlotnik et al. 2006).
THE CHEMOKINE SYSTEM IN INNATE IMMUNE CELL HOMEOSTASIS
Developing Immune Cells
Maintenance of hematopoietic stem cells and developing innate immune cells takes place largely in the bone marrow (BM) and is dependent on CXCL12/CXCR4 interactions. The retention of hematopoietic stem cells within BM niches is dependent on CXCL12, which is produced by CXCL12-abundant reticular cells, and binds to CXCR4 on hematopoietic stem cells (Ara et al. 2003). As immune cell development progresses past the hematopoietic stem cell, CXCL12/CXCR4 interactions remain essential for BM retention and normal development of multiple immune lineages, including B cells, monocytes, macrophages, neutrophils, natural killer (NK) cells, and plasmacytoid dendritic cells (Mercier et al. 2012). Developing neutrophils are retained in the BM by CXCR4 signals, and their maturation coincides with down-regulation of CXCR4, permitting mature neutrophils to enter the peripheral blood (Suratt et al. 2004; Broxmeyer et al. 2005). This process is defective in patients with warts, hypogammaglobulinemia, infections, and myelokathexis (WHIM) syndrome, which is caused by mutations in CXCR4 that enhance responsiveness to CXCL12 (Hernandez et al. 2003; Gulino et al. 2004). Neutrophils in the WHIM syndrome are unable to normally decrease responsiveness to CXCL12 and are therefore trapped within the BM, resulting in peripheral neutropenia.
CXCR4-mediated signaling plays a major role in promoting BM retention of many immune cells. However, exit from the BM may not be entirely passive. In studies examining monocyte development and release from the BM, blockade of CXCR4 induces only a small increase in the number of peripheral blood monocytes (Wang et al. 2009). However, blockade of CCR2, which is uniformly expressed by early monocytes, leads to decreases in circulating monocytes with concomitant increases in BM monocytes (Serbina and Pamer 2006; Wang et al. 2009). However, the source of CCR2 ligands under homeostatic conditions is unclear.
Neutrophils
After release from the BM, neutrophils enter the bloodstream where they await inflammatory stimuli that would promote their migration into peripheral tissue. The peripheral bloodstream is thought to be the major compartment for neutrophils under homeostatic conditions; however, recent reports indicate that CXCR4 signaling may promote the formation of a marginated pool of neutrophils in the lung vasculature that can be rapidly mobilized (Devi et al. 2013). CXCR4 may also play an important role in neutrophil elimination; senescent neutrophils up-regulate expression of CXCR4 and this may promote reentry into the BM where they undergo apoptotic cell death (Martin et al. 2003).
Monocytes
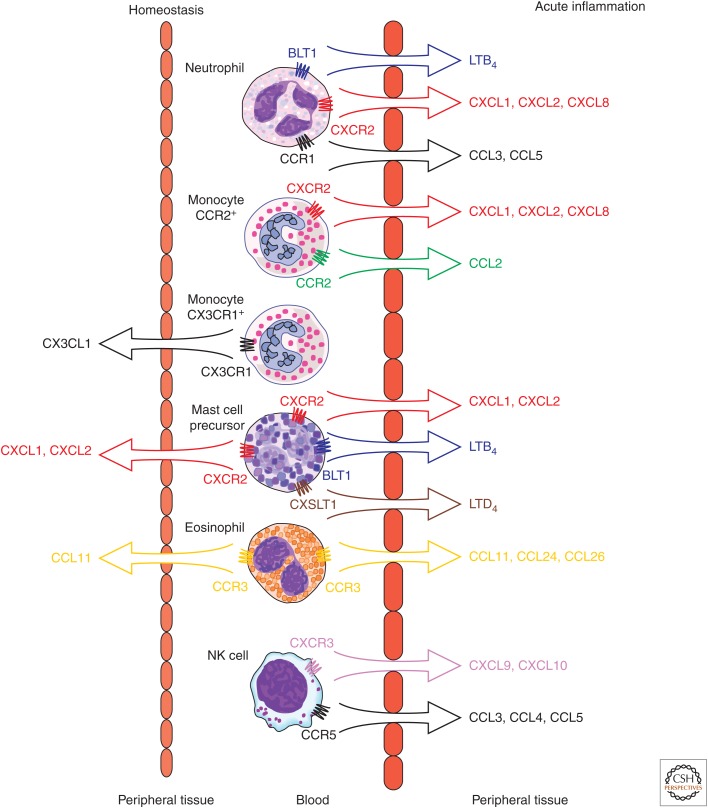
Exit of monocytes from the BM appears to be dependent on CXCR4 and possibly CCR2-mediated signaling under homeostatic conditions. Upon exiting, monocytes differentiate into proinflammatory and anti-inflammatory subsets based on their expression of CCR2 and CX3CR1, respectively. The proinflammatory (CCR2+) monocyte population remains within the peripheral blood and spleen, whereas the anti-inflammatory (CX3CR1+) population is distributed in the peripheral blood and nonlymphoid organs (Fig. 1) (Geissmann et al. 2003). This is likely because of differential homeostatic expression of CCR2 and CX3CR1 ligands; in the absence of inflammation, there is little expression of CCR2 ligands in the periphery, whereas CX3CL1 is homeostatically expressed by endothelium in various tissues (Foussat et al. 2000). However, CX3CR1+ monocytes may follow additional chemokine signals as they enter the periphery and develop into tissue macrophages under homeostatic conditions. CXCL14 is homeostatically produced by human fibroblasts in the skin and lamina propria, and tissue macrophages can be found in close association with CXCL14-producing cells (Kurth et al. 2001). However, the full role of CXCL14 and its receptor, which remains to be identified, is not fully appreciated.
Figure 1.
Chemokine control of innate immune cell migration in homeostasis and inflammation. Under homeostatic conditions, CX3CR1+ monocytes presumably migrate into the periphery following CX3CL1 gradients, mast cell precursors migrate into the gastrointestinal tract following CXCL1 and CXCL2 gradients, and eosinophils migrate into tissue following CCL11 gradients. In acute inflammation, neutrophils may leave the bloodstream and migrate into the periphery following gradients of LTB4, CXCL1, CXCL2, CXCL8, CCL3, and CCL5. CCR2+ monocytes will migrate following CXCL1, CXCL2, CXCL8, and CCL2 gradients. Mast cell precursors also migrate in response to leukotriene B4 (LTB4) and leukotrine D4 (LTD4) via activation of their respective G-protein-coupled receptors BLT1 and CYSLT1. Eosinophils will migrate into inflammatory sites in response to CCL11, CCL24, and CCL26 gradients. NK cells will migrate following CXCL9, CXCL10, CCL3, CCL4, and CCL5 gradients.
Eosinophils and Basophils
Under homeostatic conditions, eosinophils are found in the blood and the peripheral tissues, with the gastrointestinal tract making up the main reservoir of eosinophils. This baseline migration into the periphery is largely dependent on the production of CCL11 (eotaxin-1)/CCR3 interactions that promote eosinophil release from the BM and entry into peripheral tissues (Fig. 1) (Palframan et al. 1998; Mishra et al. 1999). Basophils express CXCR4 and so it is possible that they are released into the peripheral blood by CXCR4-mediated mechanisms, where they remain in the absence of inflammatory stimuli. Human basophils constitutively express CXCR1, CXCR4, CCR1, CCR2, and a majority express CCR3 (Uguccioni et al. 1997; Marone et al. 2005).
Mast Cells
Mast cells (MCs) are found throughout all vascularized tissues, where they act as innate immune sentinels and effector cells. MC precursors express CXCR4, as well as CXCR2, CCR3, and CCR5, and likely exit the BM following similar pathways to other innate immune cells (Ochi et al. 1999). They then migrate into the periphery via unknown mechanisms, although migration in response to epithelial- and fibroblast-derived stem cell factor (SCF) has been postulated (Nilsson et al. 1996). Migration signals may be tissue dependent; CXCR2 deficiency in MC precursors leads to a specific defect in intestinal homing of MC precursors and loss of intestinal MCs (Fig. 1) (Abonia et al. 2005). Once in the peripheral tissues, MCs further differentiate and alter their chemokine receptor expression; whether this is because of constitutive signaling necessary for tissue retention is unknown (Halova et al. 2012).
Dendritic Cells
Under homeostatic conditions, dendritic cells (DCs) develop in situ from DC precursor cells that first populate the periphery. No common chemokine for DC precursor positioning has been described and the chemokines that guide this transit remain largely unknown, although CCL2, CXCL14, and CCL20 have been implicated. CCR2 deficiency leads to decreases in the skin resident langerin-positive DCs, but not in other DC populations (Bogunovic et al. 2006). CCL20/CCR6 interactions are essential for normal DC migration to the subepithelial dome of the Peyer’s patches, but no other DC populations are affected (Cook et al. 2000; Varona et al. 2001). Finally, CXCL14 has been postulated to play a role in DC precursor positioning based on its ability to induce chemotaxis in human DC precursors (Schaerli et al. 2005). However, CXCL14-deficient mice show no defect in DC positioning, casting doubt on the universal role of CXCL14 in DC positioning (Meuter et al. 2007).
DC precursors differentiate into immature resident DCs that survey the periphery for signs of infection or inflammation. Immature, resident DCs express various chemokine receptors (e.g., CCR1, CCR2, CCR5, CCR6, CXCR1, CXCR2, and CXCR4) that allow DCs to migrate to sites of inflammation, but they may also actively promote their maintenance in the periphery (Randolph et al. 2008). However, even in the absence of inflammatory stimuli, DCs can become “semi-mature,” a state defined by increased surface expression of major histocompatibility complex class II (MHCII) and CCR7. The up-regulation of CCR7 allows these DCs to sense CCL21, which is produced by the lymphatic vessels in peripheral tissues, thus permitting semi-mature DCs to migrate into the lymphatics (Forster et al. 1999; Weber et al. 2013). CCR7 signaling is a common pathway for homeostatic DC migration from diverse peripheral tissues; CCR7 has been shown to be essential for migration of DCs from the skin, lung, and gastrointestinal tract (Ohl et al. 2004; Worbs et al. 2006). However, there is redundancy in CCR7 ligands; within the peripheral tissues, CCL21 promotes migration to the lymphatic vessels, but once inside the vessel, CCL19 and CCL21 act additively to promote migration to the lymph node (LN).
Because soluble or particulate antigens may have direct access to the secondary lymphoid organs via the lymphatics or bloodstream, proper homeostatic positioning of DCs within the secondary lymphoid organs is essential for immune defense. Within the LN, the fibroblastic reticular cells homeostatically produce CCL19 and CCL21, which promote DC localization to the T-cell area. In the spleen, CD4+ DCs express high levels of the G-protein-coupled receptor EBI2, the receptor for 7α, 25-dihydroxycholesterol, which is necessary for the normal positioning of CD4+ DCs in the bridging channels of the spleen and the subsequent immune response to particulate antigens (Gatto et al. 2013). Finally, although sphingosine-1-phosphate receptor 1 (S1PR1) blockade has no effect on DC localization in the LN, it does lead to the redistribution of immature CD4+ DCs from the bridging channels to the splenic marginal zone (Czeloth et al. 2007). This is specific to DC localization under homeostatic conditions; activated or mature DC positioning is not affected. Thus, although DC migration is a universal pathway governed by CCR7 signaling, positioning in the periphery and secondary lymphoid organs may be specific for different DC subsets.
Innate Lymphoid Cells
Innate lymphoid cells (ILCs) are a broad group of innate lymphocyte-like cells that are notable for not undergoing recombination of antigen receptors and clonal selection. They have been organized into three groups based on cytokine production. Group 1 ILCs includes ILC1 and classical NK cells. Human NK cells can be split into CD56dim and CD56bright populations. Under homeostatic conditions, CD56dim NK cells comprise the majority of blood resident NK cells, whereas CD56bright are preferentially localized to secondary lymphoid organs. As expected, this differential localization is accompanied by differences in chemokine receptor expression. Although all NK cells express CXCR1, CXCR3, and CXCR4, CD56high NK cells also express CCR7, allowing for homeostatic migration to the LNs (Maghazachi 2010). Group 2 ILCs include ILC2, also referred to as natural helper cells or nuocytes. ILC2 express CXCR4 and CCR9, which promote their homeostatic distribution (Walker et al. 2013). Using a CXCR6 reporter mouse, ∼50% of ILC2 was also shown to express the CXCR6 reporter (Roediger et al. 2013). However, ILC2 may not actually express CXCR6 and expression of the reporter may simply reflect prior expression by an ILC2 precursor cell (Possot et al. 2011; Constantinides et al. 2014). Finally, group 3 ILCs include ILC3 and lymphoid tissue- inducer (LTi) cells. LTi cells differentiate from a CXCR6high LTi precursor cell and the mature LTi cells express CXCR5 and CCR6 (Constantinides et al. 2014). This CCR6 expression allows LTi cells to migrate into the intestinal epithelium in response to epithelial derived CCL20 and β-defensins, which are produced in response to commensal bacteria (Sawa et al. 2010).
THE CHEMOKINE SYSTEM IN ACUTE INFLAMMATION
Acute inflammation is a complex process that must coordinate the dual goals of providing initial immune protection as well as to initiate the adaptive immune response. This coordination starts with the homeostatic prepositioning of innate immune cells throughout the periphery, where they act as local sensors of infection and inflammation through the activation of pattern recognition receptors (PRRs), the inflammasome, and/or RNA and DNA sensors. Examples of these prepositioned cellular sensors of infection or inflammation include MCs, macrophages, and DCs. Upon activation, these local innate immune cells release inflammatory cytokines and chemokines that promote the entry of additional, often blood resident, innate effector cells such as neutrophils and monocytes. These cells follow chemokine gradients to the site of inflammation, but can themselves be activated to produce additional inflammatory cytokines and chemokines that promote further inflammatory cell entry. This feed-forward mechanism not only allows for rapid amplification of the effector response, but also allows the innate immune system to shape the inflammatory response. At the same time, activated DCs change their responsiveness to chemokine gradients, allowing for rapid migration to secondary lymphoid organs and initiation of the adaptive immune response. We will discuss the role of the chemokine system in shaping the acute inflammatory response as well as its role in shaping the activation of the adaptive immune response.
Induction of Acute Inflammation by Resident Immune Cells
With the exception of neutrophils, monocytes, and basophils, almost all innate immune cells are present to some extent in the periphery under homeostatic conditions. There they lie in wait as sensors of pathogen invasion, via PRRs, or tissue damage, via the interleukin (IL)-33 pathway as one example. MCs and macrophages are classically described as essential immune sensors, based on their expression of a wide variety of PRRs and their broad localization throughout all vascularized tissues. MCs are uniquely capable of responding immediately to infectious or inflammatory stimuli. Lipopolysaccharide (LPS) stimulation of murine peritoneal MCs leads to immediate release of CXCL1 and CXCL2-containing granules, but not histamine-containing granules, as well as transcriptional activation of CXCL1 and CXCL2. This promotes early (within 2 h of stimulus) neutrophil recruitment that is abolished in MC-depleted mice, but not in macrophage-depleted mice (De Filippo et al. 2013). Additionally, in mouse models of airway hyperreactivity, MCs release preformed CCL1, promoting early migration of CCR8 expressing Th2 effector cells (Gonzalo et al. 2007). However, release of preformed mediators acts only temporarily to promote inflammatory cell entry; sustained recruitment requires transcriptional activation and secretion of additional chemokines.
Although preformed MC chemokines are necessary for immediate neutrophil recruitment in an LPS intraperitoneal injection model, neutrophil migration is normal in MC-deficient mice within 4 h after LPS injection (De Filippo et al. 2013). This is attributable to chemokine production by macrophages, which produce a wide range of chemokines including, but not limited to, CXCL1, CXCL2, CXCL8, CCL2, CCL3, CCL4, and CCL5. Similarly, MCs have been shown to produce CCL2, CCL3, CCL4, CCL5, CCL11, CCL20, CXCL1, CXCL2, CXCL8, CXCL9, CXCL10, and CXCL11 (Marshall 2004). Finally, viral exposure of DCs leads to the production of many of the same chemokines as well as CXCL16 (Piqueras et al. 2006). Group 2 ILCs appear to play an important role in acute inflammation in response to epithelial damage. IL-33 produced in response to epithelial damage by the protease allergen papain activates ILC2, leading to their production of CCL17 and CCL22 (Halim et al. 2014). Depletion of any of these cell types results in impaired inflammatory cell migration, although the degree of impairment is dependent on the model used.
In addition to producing chemokines, resident innate immune cells also produce inflammatory cytokines, such as tumor necrosis factor (TNF) and IL-1. These cytokines can alter the chemokine environment by inducing further production of chemokines, altering the presentation of chemokines by endothelium, or by altering the response to chemokine gradients. Cytokine-activated epithelium can produce a host of chemokines, including CCL2, CCL3, CCL4, CCL5, CXCL1, CXCL2, CXCL3, CXCL5, and CXCL8 (Kagnoff and Eckmann 1997). Likewise, cytokines can alter chemokine presentation by endothelial cells. In a model of experimental autoimmune encephalitis, IL-17 stimulation of brain endothelial cells leads to abluminal expression of CXCR7, which acts to remove CXCL12. In the absence of endothelial CXCL12, leukocytes enter the brain and induce disease pathogenesis (Cruz-Orengo et al. 2011). Whether this occurs in other disease settings and in other organs remains to be seen. Finally, cytokines can also impact the responsiveness of immune cells to available chemokines. For instance, activated ILC2 releases IL-13, which promotes increased DC responsiveness to CCR7 ligands and thus induces DC migration into the draining LNs (Halim et al. 2014).
ENTRY OF BLOOD-BORNE CELLS INTO SITES OF ACUTE INFLAMMATION
Neutrophils
Although resident innate immune cells are the initial responders to inflammatory cues, circulating innate cells such as neutrophils, monocytes, and eosinophils quickly become the major immune cells during acute inflammation. Neutrophils are stereotypical cells of acute inflammation; they express many chemokine receptors, including CXCR2 and CCR1 in mice and CXCR1 and CXCR2 in humans, which respond to early chemokines released by MCs and macrophages (Fig. 1). Once activated in tissue, neutrophils up-regulate other chemokine receptors, such as CCR5, which has been shown to act as a chemokine scavenger (Ariel et al. 2006). Neutrophils also express chemotactic receptors for complement, lipid mediators, such as leukotriene B4 (LTB4), and bacterial products, such as formylated peptides, including formyl-methionyl-leucyl-phenylalanine (fMLP). But how do neutrophils respond to distant chemokine gradients that exist beyond the endothelium? As previously discussed, endothelial cells can be activated by inflammatory cytokines produced by innate immune cells. Additionally, the pericyte, a structural support cell of the endothelium, has been shown to be a “hot spot” for neutrophil migration and to up-regulate adhesion molecules and chemokines after activation via PRRs (Proebstl et al. 2012; Stark et al. 2013). This activation induces the endothelial expression of P-selectin, E-selectin, and integrins, which bind to neutrophils, slowing their movement and causing them to roll along the endothelium. Rolling neutrophils are then able to bind to chemokines, such as CXCL1, CXCL2, or CXCL8, which are presented on the luminal surface of the endothelium. There are two established sources for these luminal chemokines: direct production by the activated endothelium or endothelial presentation of distantly produced chemokines. In the second case, the atypical chemokine receptor, known as the Duffy antigen receptor for chemokines (DARC or ACKR1), binds free chemokines on the abluminal surface of the endothelium. The DARC/chemokine complex is then transcytosed across the endothelial cell and ultimately presented on the luminal surface, where the bound chemokine is able to activate and promote immune cell transmigration (Proudfoot et al. 2003; Pruenster et al. 2009). Given the indiscriminate tissue damage seen in acute inflammation, this process is under tight control with rapid endocytosis and destruction of immobilized chemokines (Hillyer and Male 2005).
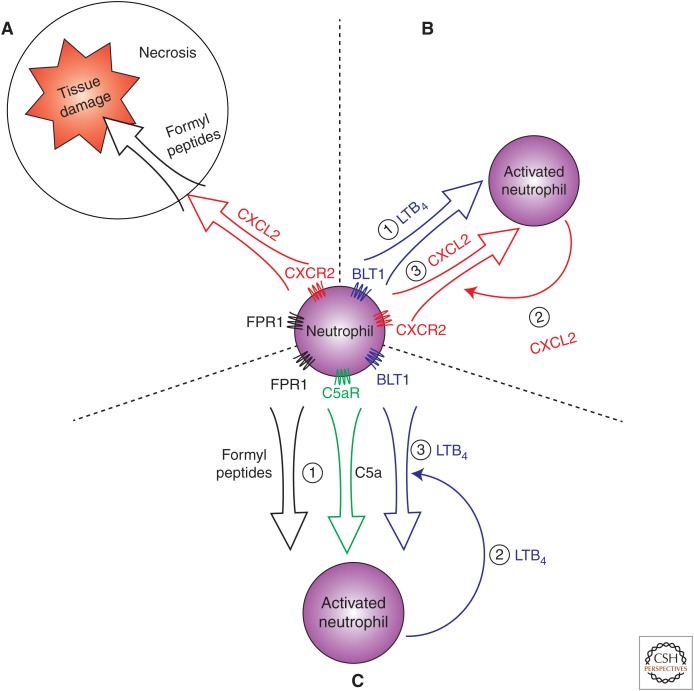
Once they have transcytosed, neutrophils follow chemoattractant gradients as they migrate through the interstitium to sites of acute inflammation. Along the way, activated neutrophils produce CCL3, CCL4, CCL5, CCL20, CXCL1, CXCL8, CXCL9, and CXCL10. Thus, they can further amplify the initial acute inflammatory response by inducing additional leukocyte entry (Bennouna et al. 2003). Chemokines make up one class of chemoattractants; however, neutrophils can respond to a wide variety of chemoattractant molecules that include lipid mediators, bacterial products, and complement fragments. These different classes of chemoattractants can act simultaneously or sequentially. One example of sequential activity of chemoattractants is in a model of sterile thermal tissue injury. In this model, CXCL2 gradients form around the area of tissue injury and promote neutrophil chemotaxis toward the injury site (McDonald et al. 2010). However, because chemokine production is dependent on live cells, the central site of necrosis lacked a supportive CXCL2 gradient. Instead, endogenous formyl peptides, which are produced by mitochondria and released upon cellular damage, promoted neutrophil chemotaxis to the site of cellular injury (McDonald et al. 2010; Zhang et al. 2010). Thus, CXCL2 gradients promoted initial migration to the area of necrosis, followed by FPR1 signaling that promoted neutrophil entry into the necrotic center (Fig. 2A).
Figure 2.
Mechanisms of neutrophil migration in the inflamed periphery. (A) Neutrophils migrate to sites of tissue damage by following sequential chemoattractant gradients. Neutrophils first follow CXCL2 gradients, and then, in areas of tissue necrosis, follow gradients of endogenous formyl peptides via FPR1 signaling. (B) Neutrophils can amplify their own recruitment through the production of chemokines. Neutrophils migrate to inflammatory sites by following LTB4 gradients (1) where they are activated by inflammatory stimuli and produce additional chemokines, such as CXCL2 (2) that promote further neutrophil entry by establishing an additional chemokine gradient (3). (C) Neutrophils can be activated by chemotactic agents, such as formyl peptides or C5a, leading to LTB4 production, which establishes a relay system for neutrophil swarming. Formyl-peptide or C5a-activated neutrophils (1) produce LTB4 (2), which creates a chemoattactant gradient that induces more neutrophils to enter and swarm to the site of inflammation (3).
Beyond providing linear directional information, different chemoattractant classes may play important roles in permitting neutrophils to amplify their own migration. Such interactions between chemoattractant classes have been shown in the case of LTB4 and chemokines. Using a mouse model of autoimmune arthritis, we and other investigators have shown that LTB4 acting through the G-protein-coupled receptor BLT1 on neutrophils is necessary for initial neutrophil recruitment into the joint (Chen et al. 2006; Kim et al. 2006). Once inside the joint, these intial “scout” neutrophils are activated by synovial-immune complexes to produce neutrophil-active chemokines, such as CXCL2, as well as the proinflammatory cytokine IL-1β. This inflammatory cytokine then activates local synoviocytes as well as macrophages and endothelial cells to produce additional acute inflammatory chemokines, such as CCL3, CCL4, CCL5, CXCL1, and CXCL2. These chemokines then rapidly amplify neutrophil entry into the joint by binding to the CCR1 and CXCR2 receptors on neutrophils (Chou et al. 2010). Thus, LTB4 induces a necessary initial neutrophil migration that then amplifies inflammatory cell influx characteristic of acute inflammation (Fig. 2B).
In addition to creating sequential waves of neutrophil recruitment, different chemoattractant classes may enhance the response to each other or act to amplify the chemotactic gradient. Stimulation of the G-protein-coupled formyl peptide receptor FPR1 on neutrophils leads to neutrophil LTB4 production, which then feeds back in an autocrine and paracrine fashion and promotes further neutrophil migration toward formyl peptides (Afonso et al. 2012). Likewise, in the murine model of immune-complex-induced arthritis discussed above, activation of the G-protein-coupled complement receptor C5aR was required for the generation of LTB4 and the induction of arthritis (Sadik et al. 2012). Indeed, production of LTB4 by neutrophils appears to be an essential mechanism for extending the range and enhancing the stability of chemotactic signals. Activated neutrophils produce LTB4, which then acts as a neutrophil chemoattractant. Thus, LTB4 production by activated neutrophils can feed-forward and extend the range of initial chemotactic signals. Additionally, this LTB4 can activate neutrophils, promoting further LTB4 production, which acts to further amplify the initial inflammatory stimulus (Afonso et al. 2012). LTB4-mediated amplification of neutrophil activation and chemotaxis induces the coordinated chemotaxis and clustering (i.e., swarming) of neutrophils at sites of tissue damage in vivo (Fig. 2C) (Lammermann et al. 2013).
Monocytes
Along with neutrophils, blood-borne monocytes are recruited early in the setting of acute inflammation by activated endothelium. As discussed previously, monocytes are divided into CCR2+ and CX3CR1+ groups. CCR2+ monocytes are largely resident in the peripheral blood during homeostasis, but rapidly migrate to areas of acute inflammation. Although they are defined by their expression of CCR2, they also express CXCR2, which may play an important role in initial activation and transmigration. In an atheroma model, CXCL8/CXCR2 interactions were necessary for firm adhesion of monocytes to vascular endothelium (Gerszten et al. 1999). CCR2 signaling then promotes proinflammatory monocyte migration into peripheral tissues in response to stable extracellular matrix–associated CCL2 gradients (Fig. 1) (Kuziel et al. 1997; Proudfoot et al. 2003). Interestingly, although CXCL2 does not appear to promote monocyte migration in response to CXCL8, it does promote migration in response to its atypical ligand macrophage migration inhibitory factor (MIF). MIF binds to CD74 and CXCR2 on monocytes and macrophages, leading to CXCR2 signaling and integrin-dependent chemotaxis of monocytes, which is necessary for maintenance of atherosclerotic plaques (Bernhagen et al. 2007). Additionally, CCR1 and CCR5 have been shown to promote chemotaxis of CCR2+ monocytes in in vitro experiments, but in vivo data are lacking (Weber et al. 2001; Le Borgne et al. 2006; Shi and Pamer 2011).
In addition to promoting CCR2+ monocyte recruitment to sites of acute inflammation, CCL2 may also have long-range effects in draining LNs and BM. Soluble CCL2 has been detected in the afferent lymph, where it drains to the LN, binds to HEVs, and induces CCR2+ monocyte entry into the draining LN (Palframan et al. 2001). Additionally, CCL2 plays an important role in mobilizing inflammatory monocytes from the BM (Fig. 1). The source of this CCL2 is unclear. It is possible that locally produced CCL2 enters the systemic circulation, but given the mechanisms in place to remove circulating chemokines, there may not be sufficient concentrations of CCL2 that reach the BM. Alternatively, small amounts of pathogen-associated molecular patterns may enter the systemic circulation during infection, activate PRRs on BM niche cells, and induce local CCL2 production by BM cells (Shi et al. 2011). Given the heterogeneity of acute inflammatory stimuli that induce monocyte egress, it is likely that multiple mechanisms contribute to the chemokine-dependent mobilization of inflammatory monocytes.
The chemotactic properties of CX3CR1+ monocytes are less understood. Anti-inflammatory monocytes are characterized by their high-level expression of CX3CR1 and lack of CCR2, although CCR2+ monocytes also express intermediate levels of CX3CR1, and CX3CR1 deficiency leads to minimal defects in migration of monocytes. CX3CR1 was shown to play a role in the early migration of inflammatory monocytes into the spleen after Listeria infection, but this was a temporary defect (Auffray et al. 2007). Instead, CX3CR1 may fulfill nonchemotactic roles. CX3CR1 has been shown to play an essential role in promoting integrin-mediated adhesion within the vessel, which allows for the patrolling phenotype of CX3CR1+ monocytes (Auffray et al. 2007). Additionally, CX3CR1 may provide a prosurvival signal to CX3CR1+ monocytes, which may underlie the proatherogenic role of CX3CL1/CX3CR1 (Moatti et al. 2001; Landsman et al. 2009).
Eosinophils
Eosinophils express the chemokine receptors CCR1 and CCR3, allowing them to respond to a wide variety of inflammatory stimuli. However, eosinophils are best characterized by their role in allergic and parasitic responses, during which they migrate in response to the eotaxins (CCL11, CCL24, and CCL26). Eotaxins can be released directly by stimulation of innate immune cells, but experiments in murine asthma models have illustrated that IL-4 and IL-13 exposure are necessary for optimal production of CCL11, CCL24, and CCL26 (Menzies-Gow and Robinson 2001). In murine models of airway inflammation, CCL11 and CCL24 play additive roles in promoting eosinophil migration into the lung (Humbles et al. 2002; Mattes et al. 2002; Pope et al. 2005). The role of CCL26 is less understood, but it likely plays an important role in human eosinophil migration as it is the predominant chemokine that drives eosinophil migration across IL-4-activated endothelial and epithelial monolayers (Fig. 1) (Cuvelier and Patel 2001; Yuan et al. 2006). CCL11 activity is regulated by CD26, a cell-surface protease that cleaves CCL11 into a partial antagonist: a protein that cannot induce chemotaxis, but can still bind to CCR3 and desensitize its response to CCL11. This leads to the inhibition of CCR3-mediated chemotaxis, and ultimately to the suppression of eosinophilic inflammation (Struyf et al. 1999; Yan et al. 2012). Finally, analogous to the inflammation-amplification mechanisms used by other acute inflammatory cells, thymic stromal lymphopoietin (TSLP)–stimulated eosinophils also produce CCL2, CXCL1, and CXCL8 promoting further influx of inflammatory monocytes and neutrophils (Wong et al. 2010).
Dendritic Cells
Once activated by inflammatory cytokines or PRR ligation, DCs undergo a maturation process and down-regulate expression of chemokine receptors expressed on immature DCs and up-regulate CCR7 expression (Sallusto et al. 1998). Thus, instead of altering the expression of chemokine ligands, DC migration occurs in response to alterations in chemokine receptor expression. Mature, CCR7high, DCs follow stable gradients of CCL21, which increase the mobility and directional migration of mature DCs toward the lymphatic endothelium (Tal et al. 2011). There, DCs enter the lumen of the lymphatic vessel via gaps in the basement membrane beneath the lymphatic endothelial cells (Pflicke and Sixt 2009). Once in the lumen, they crawl along following the direction of lymphatic flow until they reach the collecting lymphatics, at which point they freely flow with the lymph to the draining LN and then transmigrate through the floor of the subcapsular sinus and into the T-cell zone in a CCR7-dependent process (Braun et al. 2011).
Innate Lymphoid Cells
As discussed above, innate lymphocytes are thought to primarily be tissue resident in order to provide a first line of defense, especially at mucosal sites. However, parabiosis studies have revealed that there are both tissue-resident and recirculating populations of NK and natural killer T (NKT) cells (Thomas et al. 2011; Sojka et al. 2014). Innate lymphocytes constitutively express inflammatory chemokine receptors, which allow them to rapidly migrate and respond to inflammatory stimuli. NK cells use CCR5 signaling to migrate to sites of acute inflammation in murine-infection models, whereas CXCR3 signaling is used in models of cardiac transplant and hepatitis (Hancock et al. 2001; Hokeness et al. 2005; Khan et al. 2006; Wald et al. 2006). The infiltration of activated, IFN-γ-producing NK cells into peripheral tissues then promotes further cell migration via production of the IFN-γ-inducible CXCR3 ligands, CXCL9 and CXCL10 (Fig. 1). NKT cells have similarly been shown to initially migrate into the acutely inflamed liver via a CXCR6-dependent process where they then promote local accumulation of monocytes and macrophages (Wehr et al. 2013). Chemokine-mediated trafficking of innate lymphoid cells also serves to link innate and adaptive immune responses. For example, CXCR3-mediated NK cell migration guides IFN-γ-secreting NK cells into the LN where they help prime the LN for a Th1 response (Martin-Fontecha et al. 2004). Similarly, in the periphery, a blockade of NK cell CX3CR1-mediated migration into the brain in a mouse model of experimental autoimmune encephalitis led to enhanced disease caused by unabated Th17 formation in the absence of NK-cell-derived IFN-γ (Hao et al. 2010).
THE CHEMOKINE SYSTEM IN INNATE CONTROL OF ADAPTIVE IMMUNITY
Once in the draining LN or other secondary lymphoid organ, mature DCs migrate to specific regions and secrete their own chemokines to enhance their interactions with adaptive immune cells. The combination of specific localization and chemoattraction of adaptive immune cells appears to be necessary for optimal activation and instruction of the developing adaptive immune response.
CD4+ Th1 Priming
Th1 cells have been shown to preferentially express the chemokine receptors CCR5, CXCR3, and CXCR6, but CXCR3 may play a specific role in CD4+ Th1 priming (Bromley et al. 2008; Groom et al. 2012). In response to cutaneous immunization with antigen-pulsed mature DCs or immunization with antigen and adjuvant, CXCL9 and CXCL10 were produced in the interfollicular and medullary regions, respectively, in the draining LN. This expression promoted migration of CD4+ T cells to these DC-rich regions, while, at the same time, activated DCs produced CXCL10. Together, this led to clustering and the formation of stable contacts between CD4+ T cells and DCs, which were necessary for optimal Th1 differentiation (Groom et al. 2012). In addition to promoting direct interactions between CD4+ T cells and DCs, this localization may also place developing Th1 cells close to innate sources of IFN-γ, such as CXCR3 expressing NK, NKT, γδ T, and innate-like CD8+ T cells (Bajenoff et al. 2006; Groom and Luster 2011; Kastenmuller et al. 2012; Oghumu et al. 2013).
CD4+ Th2 and Tfh Priming
Like CD4+ Th1 cells, optimal induction of Th2 cell differentiation appears to be dependent on chemokine guidance. Using a helminth infection model, Th2 and T follicular helper (Tfh) cell induction was shown to be dependent on CXCL13/CXCR5 interactions (Leon et al. 2012). CXCR5-mediated signaling in both DCs and CD4+ T cells led to migration to the perifollicular regions of the LN in response to B-cell-derived CXCL13. A blockade of CXCR5 signaling prevented optimal Th2 and Tfh differentiation (Leon et al. 2012). However, it remains unclear why localization to this region is necessary for Th2 differentiation and whether an innate immune cell involved in cytokine production or Th2 skewing cell is similarly localized to this region.
CD8+ T-Cell Priming
Optimal priming of CD8+ T cells requires interactions with DCs that have been “licensed” by CD4+ T cells. This requires the coordinated migration of DCs, CD4+ T cells and CD8+ T cells, which appears to be caused by CCR5- and CCR4-mediated signaling. In response to CD4+ T-cell and DC interactions, both cells secrete CCR5 ligands, which bind to a subset of CD8+ T cells within the LN (Castellino et al. 2006). This promotes sustained contacts between CD8+ T cells and the CD4+ licensed DCs, which is necessary for optimal CD8+ T-cell memory responses. However, the precise role for CCR5 remains unclear as viral infection models using different vaccinia virus strains have shown contradictory results for the role of CCR5 in CD8+ T-cell priming (Hickman et al. 2011; Kastenmuller et al. 2013). Finally, in a model of CD8+ T-cell priming using injection of α-galactosylceramide, CCR4 signaling promoted stable contacts between CD8+ T cells and DCs (Semmling et al. 2010). However, whether this pathway is relevant in the setting of more physiologic immune stimuli remains unknown.
CONCLUDING REMARKS
Chemokines are essential for the positioning of innate immune sentinels at mucosal barriers and for the recruitment of the first line of innate immune effector cells to sites of infection and inflammation. Chemokine function is also essential for maintaining an adequate pool of circulating immune cells at homeostasis and at times of stress by governing their release from the BM. Once in the tissue, chemokines collaborate with other chemoattractants, such as lipid mediators, formylated peptides, and complement components, to guide innate immune effectors to the very site of tissue damage and pathogen replication. Chemokine function is also necessary to translate an innate immune response into an adaptive immune response. Innate immune stimuli—through activation of PRRs—set in motion a genetic program that induces the expression of chemokines from resident tissue innate immune cells and also modulates the expression of chemokine receptors on DCs. The induction of chemokine and chemokine receptor expression orchestrates the movement of antigen-loaded DCs from the tissue into lymphoid tissue to activate T and B cells to initiate the adaptive immune response. Chemokines downstream from PRR activation also help guide the newly activated T cells back into the tissue where the innate immune system first sensed the foreign challenge. In addition, during secondary immune responses, chemokines induced by antigen-specific lymphocyte responses recruit innate immune cells into sites of inflammation, serving to amplify the adaptive response with innate immune effector cells. Thus, chemokines and their receptors serve a critical function in coordinating the interdependent innate and adaptive immune responses.
ACKNOWLEDGMENTS
The writers are supported by grants from the National Institutes of Health, and C.L.S. is also supported by the Asthma and Allergy Foundation of America. We thank Jason Griffith for helpful discussions in the preparation of this manuscript.
Footnotes
Editors: Ruslan M. Medzhitov
Additional Perspectives on Innate Immunity and Inflammation available at www.cshperspectives.org
REFERENCES
- Abonia JP, Austen KF, Rollins BJ, Joshi SK, Flavell RA, Kuziel WA, Koni PA, Gurish MF. 2005. Constitutive homing of mast cell progenitors to the intestine depends on autologous expression of the chemokine receptor CXCR2. Blood 105: 4308–4313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonso PV, Janka-Junttila M, Lee YJ, McCann CP, Oliver CM, Aamer KA, Losert W, Cicerone MT, Parent CA. 2012. LTB4 is a signal-relay molecule during neutrophil chemotaxis. Dev Cell 22: 1079–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ara T, Tokoyoda K, Sugiyama T, Egawa T, Kawabata K, Nagasawa T. 2003. Long-term hematopoietic stem cells require stromal cell-derived factor-1 for colonizing bone marrow during ontogeny. Immunity 19: 257–267. [DOI] [PubMed] [Google Scholar]
- Ariel A, Fredman G, Sun YP, Kantarci A, Van Dyke TE, Luster AD, Serhan CN. 2006. Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat Immunol 7: 1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auffray C, Fogg D, Garfa M, Elain G, Join-Lambert O, Kayal S, Sarnacki S, Cumano A, Lauvau G, Geissmann F. 2007. Monitoring of blood vessels and tissues by a population of monocytes with patrolling behavior. Science 317: 666–670. [DOI] [PubMed] [Google Scholar]
- Bachelerie FB-BA, Burkhardt AM, Combadiere C, Farber JM, Graham GJ, Horuk R, Sparre-Ulrich AH, Locati M, Luster AD, Mantovani A, et al. 2013. International Union of Basic and Clinical Pharmacology. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev 11: 1–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajenoff M, Breart B, Huang AY, Qi H, Cazareth J, Braud VM, Germain RN, Glaichenhaus N. 2006. Natural killer cell behavior in lymph nodes revealed by static and real-time imaging. J Exp Med 203: 619–631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennouna S, Bliss SK, Curiel TJ, Denkers EY. 2003. Cross-talk in the innate immune system: Neutrophils instruct recruitment and activation of dendritic cells during microbial infection. J Immunol 171: 6052–6058. [DOI] [PubMed] [Google Scholar]
- Bernhagen J, Krohn R, Lue H, Gregory JL, Zernecke A, Koenen RR, Dewor M, Georgiev I, Schober A, Leng L, et al. 2007. MIF is a noncognate ligand of CXC chemokine receptors in inflammatory and atherogenic cell recruitment. Nat Med 13: 587–596. [DOI] [PubMed] [Google Scholar]
- Bogunovic M, Ginhoux F, Wagers A, Loubeau M, Isola LM, Lubrano L, Najfeld V, Phelps RG, Grosskreutz C, Scigliano E, et al. 2006. Identification of a radio-resistant and cycling dermal dendritic cell population in mice and men. J Exp Med 203: 2627–2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun A, Worbs T, Moschovakis GL, Halle S, Hoffmann K, Bolter J, Munk A, Forster R. 2011. Afferent lymph-derived T cells and DCs use different chemokine receptor CCR7-dependent routes for entry into the lymph node and intranodal migration. Nat Immunol 12: 879–887. [DOI] [PubMed] [Google Scholar]
- Bromley SK, Mempel TR, Luster AD. 2008. Orchestrating the orchestrators: Chemokines in control of T cell traffic. Nat Immunol 9: 970–980. [DOI] [PubMed] [Google Scholar]
- Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, et al. 2005. Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med 201: 1307–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellino F, Huang AY, Altan-Bonnet G, Stoll S, Scheinecker C, Germain RN. 2006. Chemokines enhance immunity by guiding naive CD8+ T cells to sites of CD4+ T cell-dendritic cell interaction. Nature 440: 890–895. [DOI] [PubMed] [Google Scholar]
- Chen M, Lam BK, Kanaoka Y, Nigrovic PA, Audoly LP, Austen KF, Lee DM. 2006. Neutrophil-derived leukotriene B4 is required for inflammatory arthritis. J Exp Med 203: 837–842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou RC, Kim ND, Sadik CD, Seung E, Lan Y, Byrne MH, Haribabu B, Iwakura Y, Luster AD. 2010. Lipid-cytokine-chemokine cascade drives neutrophil recruitment in a murine model of inflammatory arthritis. Immunity 33: 266–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinides MG, McDonald BD, Verhoef PA, Bendelac A. 2014. A committed precursor to innate lymphoid cells. Nature 508: 397–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook DN, Prosser DM, Forster R, Zhang J, Kuklin NA, Abbondanzo SJ, Niu XD, Chen SC, Manfra DJ, Wiekowski MT, et al. 2000. CCR6 mediates dendritic cell localization, lymphocyte homeostasis, and immune responses in mucosal tissue. Immunity 12: 495–503. [DOI] [PubMed] [Google Scholar]
- Cruz-Orengo L, Holman DW, Dorsey D, Zhou L, Zhang P, Wright M, McCandless EE, Patel JR, Luker GD, Littman DR, et al. 2011. CXCR7 influences leukocyte entry into the CNS parenchyma by controlling abluminal CXCL12 abundance during autoimmunity. J Exp Med 208: 327–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuvelier SL, Patel KD. 2001. Shear-dependent eosinophil transmigration on interleukin 4-stimulated endothelial cells: A role for endothelium-associated eotaxin-3. J Exp Med 194: 1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeloth N, Schippers A, Wagner N, Muller W, Kuster B, Bernhardt G, Forster R. 2007. Sphingosine-1 phosphate signaling regulates positioning of dendritic cells within the spleen. J Immunol 179: 5855–5863. [DOI] [PubMed] [Google Scholar]
- De Filippo K, Dudeck A, Hasenberg M, Nye E, van Rooijen N, Hartmann K, Gunzer M, Roers A, Hogg N. 2013. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 121: 4930–4937. [DOI] [PubMed] [Google Scholar]
- Devi S, Wang Y, Chew WK, Lima R, A-González N, Mattar CN, Chong SZ, Schlitzer A, Bakocevic N, Chew S, et al. 2013. Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow. J Exp Med 210: 2321–2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVries ME, Kelvin AA, Xu L, Ran L, Robinson J, Kelvin DJ. 2006. Defining the origins and evolution of the chemokine/chemokine receptor system. J Immunol 176: 401–415. [DOI] [PubMed] [Google Scholar]
- Forster R, Schubel A, Breitfeld D, Kremmer E, Renner-Muller I, Wolf E, Lipp M. 1999. CCR7 coordinates the primary immune response by establishing functional microenvironments in secondary lymphoid organs. Cell 99: 23–33. [DOI] [PubMed] [Google Scholar]
- Foussat A, Coulomb-L’Hermine A, Gosling J, Krzysiek R, Durand-Gasselin I, Schall T, Balian A, Richard Y, Galanaud P, Emilie D. 2000. Fractalkine receptor expression by T lymphocyte subpopulations and in vivo production of fractalkine in human. Eur J Immunol 30: 87–97. [DOI] [PubMed] [Google Scholar]
- Gatto D, Wood K, Caminschi I, Murphy-Durland D, Schofield P, Christ D, Karupiah G, Brink R. 2013. The chemotactic receptor EBI2 regulates the homeostasis, localization and immunological function of splenic dendritic cells. Nat Immunol 14: 446–453. [DOI] [PubMed] [Google Scholar]
- Geissmann F, Jung S, Littman DR. 2003. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19: 71–82. [DOI] [PubMed] [Google Scholar]
- Gerszten RE, Garcia-Zepeda EA, Lim YC, Yoshida M, Ding HA, Gimbrone MA Jr, Luster AD, Luscinskas FW, Rosenzweig A. 1999. MCP-1 and IL-8 trigger firm adhesion of monocytes to vascular endothelium under flow conditions. Nature 398: 718–723. [DOI] [PubMed] [Google Scholar]
- Gonzalo JA, Qiu Y, Lora JM, Al-Garawi A, Villeval JL, Boyce JA, Martinez-A C, Marquez G, Goya I, Hamid Q, et al. 2007. Coordinated involvement of mast cells and T cells in allergic mucosal inflammation: Critical role of the CC chemokine ligand 1:CCR8 axis. J Immunol 179: 1740–1750. [DOI] [PubMed] [Google Scholar]
- Groom JR, Luster AD. 2011. CXCR3 ligands: Redundant, collaborative and antagonistic functions. Immunol Cell Biol 89: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groom JR, Richmond J, Murooka TT, Sorensen EW, Sung JH, Bankert K, von Andrian UH, Moon JJ, Mempel TR, Luster AD. 2012. CXCR3 chemokine receptor-ligand interactions in the lymph node optimize CD4+ T helper 1 cell differentiation. Immunity 37: 1091–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulino AV, Moratto D, Sozzani S, Cavadini P, Otero K, Tassone L, Imberti L, Pirovano S, Notarangelo LD, Soresina R, et al. 2004. Altered leukocyte response to CXCL12 in patients with warts hypogammaglobulinemia, infections, myelokathexis (WHIM) syndrome. Blood 104: 444–452. [DOI] [PubMed] [Google Scholar]
- Halim TY, Steer CA, Matha L, Gold MJ, Martinez-Gonzalez I, McNagny KM, McKenzie AN, Takei F. 2014. Group 2 innate lymphoid cells are critical for the initiation of adaptive T helper 2 cell-mediated allergic lung inflammation. Immunity 40: 425–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halova I, Draberova L, Draber P. 2012. Mast cell chemotaxis—Chemoattractants and signaling pathways. Front Immunol 3: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hancock WW, Gao W, Csizmadia V, Faia KL, Shemmeri N, Luster AD. 2001. Donor-derived IP-10 initiates development of acute allograft rejection. J Exp Med 193: 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Liu R, Piao W, Zhou Q, Vollmer TL, Campagnolo DI, Xiang R, La Cava A, Van Kaer L, Shi FD. 2010. Central nervous system (CNS)-resident natural killer cells suppress Th17 responses and CNS autoimmune pathology. J Exp Med 207: 1907–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez PA, Gorlin RJ, Lukens JN, Taniuchi S, Bohinjec J, Francois F, Klotman ME, Diaz GA. 2003. Mutations in the chemokine receptor gene CXCR4 are associated with WHIM syndrome, a combined immunodeficiency disease. Nat Genet 34: 70–74. [DOI] [PubMed] [Google Scholar]
- Hickman HD, Li L, Reynoso GV, Rubin EJ, Skon CN, Mays JW, Gibbs J, Schwartz O, Bennink JR, Yewdell JW. 2011. Chemokines control naive CD8+ T cell selection of optimal lymph node antigen presenting cells. J Exp Med 208: 2511–2524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillyer P, Male D. 2005. Expression of chemokines on the surface of different human endothelia. Immunol Cell Biol 83: 375–382. [DOI] [PubMed] [Google Scholar]
- Hokeness KL, Kuziel WA, Biron CA, Salazar-Mather TP. 2005. Monocyte chemoattractant protein-1 and CCR2 interactions are required for IFN-α/β-induced inflammatory responses and antiviral defense in liver. J Immunol 174: 1549–1556. [DOI] [PubMed] [Google Scholar]
- Humbles AA, Lu B, Friend DS, Okinaga S, Lora J, Al-Garawi A, Martin TR, Gerard NP, Gerard C. 2002. The murine CCR3 receptor regulates both the role of eosinophils and mast cells in allergen-induced airway inflammation and hyperresponsiveness. Proc Natl Acad Sci 99: 1479–1484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagnoff MF, Eckmann L. 1997. Epithelial cells as sensors for microbial infection. J Clin Invest 100: 6–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenmuller W, Torabi-Parizi P, Subramanian N, Lammermann T, Germain RN. 2012. A spatially organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell 150: 1235–1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenmuller W, Brandes M, Wang Z, Herz J, Egen JG, Germain RN. 2013. Peripheral prepositioning and local CXCL9 chemokine-mediated guidance orchestrate rapid memory CD8+ T cell responses in the lymph node. Immunity 38: 502–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan IA, Thomas SY, Moretto MM, Lee FS, Islam SA, Combe C, Schwartzman JD, Luster AD. 2006. CCR5 is essential for NK cell trafficking and host survival following Toxoplasma gondii infection. PLoS Pathog 2: e49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ND, Chou RC, Seung E, Tager AM, Luster AD. 2006. A unique requirement for the leukotriene B4 receptor BLT1 for neutrophil recruitment in inflammatory arthritis. J Exp Med 203: 829–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurth I, Willimann K, Schaerli P, Hunziker T, Clark-Lewis I, Moser B. 2001. Monocyte selectivity and tissue localization suggests a role for breast and kidney-expressed chemokine (BRAK) in macrophage development. J Exp Med 194: 855–861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuziel WA, Morgan SJ, Dawson TC, Griffin S, Smithies O, Ley K, Maeda N. 1997. Severe reduction in leukocyte adhesion and monocyte extravasation in mice deficient in CC chemokine receptor 2. Proc Natl Acad Sci 94: 12053–12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lammermann T, Afonso PV, Angermann BR, Wang JM, Kastenmuller W, Parent CA, Germain RN. 2013. Neutrophil swarms require LTB4 and integrins at sites of cell death in vivo. Nature 498: 371–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landsman L, Bar-On L, Zernecke A, Kim KW, Krauthgamer R, Shagdarsuren E, Lira SA, Weissman IL, Weber C, Jung S. 2009. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood 113: 963–972. [DOI] [PubMed] [Google Scholar]
- Le Borgne M, Etchart N, Goubier A, Lira SA, Sirard JC, van Rooijen N, Caux C, Ait-Yahia S, Vicari A, Kaiserlian D, et al. 2006. Dendritic cells rapidly recruited into epithelial tissues via CCR6/CCL20 are responsible for CD8+ T cell crosspriming in vivo. Immunity 24: 191–201. [DOI] [PubMed] [Google Scholar]
- Leon B, Ballesteros-Tato A, Browning JL, Dunn R, Randall TD, Lund FE. 2012. Regulation of TH2 development by CXCR5+ dendritic cells and lymphotoxin-expressing B cells. Nat Immunol 13: 681–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maghazachi AA. 2010. Role of chemokines in the biology of natural killer cells. Curr Top Microbiol Immunol 341: 37–58. [DOI] [PubMed] [Google Scholar]
- Marone G, Triggiani M, de Paulis A. 2005. Mast cells and basophils: Friends as well as foes in bronchial asthma? Trends Immunol 26: 25–31. [DOI] [PubMed] [Google Scholar]
- Marshall JS. 2004. Mast-cell responses to pathogens. Nat Rev Immunol 4: 787–799. [DOI] [PubMed] [Google Scholar]
- Martin C, Burdon PC, Bridger G, Gutierrez-Ramos JC, Williams TJ, Rankin SM. 2003. Chemokines acting via CXCR2 and CXCR4 control the release of neutrophils from the bone marrow and their return following senescence. Immunity 19: 583–593. [DOI] [PubMed] [Google Scholar]
- Martin-Fontecha A, Thomsen LL, Brett S, Gerard C, Lipp M, Lanzavecchia A, Sallusto F. 2004. Induced recruitment of NK cells to lymph nodes provides IFN-γ for TH1 priming. Nat Immunol 5: 1260–1265. [DOI] [PubMed] [Google Scholar]
- Mattes J, Yang M, Mahalingam S, Kuehr J, Webb DC, Simson L, Hogan SP, Koskinen A, McKenzie AN, Dent LA, et al. 2002. Intrinsic defect in T cell production of interleukin (IL)-13 in the absence of both IL-5 and eotaxin precludes the development of eosinophilia and airways hyperreactivity in experimental asthma. J Exp Med 195: 1433–1444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald B, Pittman K, Menezes GB, Hirota SA, Slaba I, Waterhouse CC, Beck PL, Muruve DA, Kubes P. 2010. Intravascular danger signals guide neutrophils to sites of sterile inflammation. Science 330: 362–366. [DOI] [PubMed] [Google Scholar]
- Menzies-Gow A, Robinson DS. 2001. Eosinophil chemokines and chemokine receptors: Their role in eosinophil accumulation and activation in asthma and potential as therapeutic targets. J Asthma 38: 605–613. [DOI] [PubMed] [Google Scholar]
- Mercier FE, Ragu C, Scadden DT. 2012. The bone marrow at the crossroads of blood and immunity. Nat Rev Immunol 12: 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuter S, Schaerli P, Roos RS, Brandau O, Bosl MR, von Andrian UH, Moser B. 2007. Murine CXCL14 is dispensable for dendritic cell function and localization within peripheral tissues. Mol Cell Biol 27: 983–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra A, Hogan SP, Lee JJ, Foster PS, Rothenberg ME. 1999. Fundamental signals that regulate eosinophil homing to the gastrointestinal tract. J Clin Invest 103: 1719–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moatti D, Faure S, Fumeron F, Amara Mel W, Seknadji P, McDermott DH, Debre P, Aumont MC, Murphy PM, de Prost D, et al. 2001. Polymorphism in the fractalkine receptor CX3CR1 as a genetic risk factor for coronary artery disease. Blood 97: 1925–1928. [DOI] [PubMed] [Google Scholar]
- Nilsson G, Johnell M, Hammer CH, Tiffany HL, Nilsson K, Metcalfe DD, Siegbahn A, Murphy PM. 1996. C3a and C5a are chemotaxins for human mast cells and act through distinct receptors via a pertussis toxin-sensitive signal transduction pathway. J Immunol 157: 1693–1698. [PubMed] [Google Scholar]
- Nomiyama H, Osada N, Yoshie O. 2010. The evolution of mammalian chemokine genes. Cytokine Growth Factor Rev 21: 253–262. [DOI] [PubMed] [Google Scholar]
- Ochi H, Hirani WM, Yuan Q, Friend DS, Austen KF, Boyce JA. 1999. T helper cell type 2 cytokine-mediated comitogenic responses and CCR3 expression during differentiation of human mast cells in vitro. J Exp Med 190: 267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oghumu S, Dong R, Varikuti S, Shawler T, Kampfrath T, Terrazas CA, Lezama-Davila C, Ahmer BM, Whitacre CC, Rajagopalan S, et al. 2013. Distinct populations of innate CD8+ T cells revealed in a CXCR3 reporter mouse. J Immunol 190: 2229–2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohl L, Mohaupt M, Czeloth N, Hintzen G, Kiafard Z, Zwirner J, Blankenstein T, Henning G, Forster R. 2004. CCR7 governs skin dendritic cell migration under inflammatory and steady-state conditions. Immunity 21: 279–288. [DOI] [PubMed] [Google Scholar]
- Palframan RT, Collins PD, Williams TJ, Rankin SM. 1998. Eotaxin induces a rapid release of eosinophils and their progenitors from the bone marrow. Blood 91: 2240–2248. [PubMed] [Google Scholar]
- Palframan RT, Jung S, Cheng G, Weninger W, Luo Y, Dorf M, Littman DR, Rollins BJ, Zweerink H, Rot A, et al. 2001. Inflammatory chemokine transport and presentation in HEV: A remote control mechanism for monocyte recruitment to lymph nodes in inflamed tissues. J Exp Med 194: 1361–1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pflicke H, Sixt M. 2009. Preformed portals facilitate dendritic cell entry into afferent lymphatic vessels. J Exp Med 206: 2925–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piqueras B, Connolly J, Freitas H, Palucka AK, Banchereau J. 2006. Upon viral exposure, myeloid and plasmacytoid dendritic cells produce 3 waves of distinct chemokines to recruit immune effectors. Blood 107: 2613–2618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pope SM, Zimmermann N, Stringer KF, Karow ML, Rothenberg ME. 2005. The eotaxin chemokines and CCR3 are fundamental regulators of allergen-induced pulmonary eosinophilia. J Immunol 175: 5341–5350. [DOI] [PubMed] [Google Scholar]
- Possot C, Schmutz S, Chea S, Boucontet L, Louise A, Cumano A, Golub R. 2011. Notch signaling is necessary for adult, but not fetal, development of RORγt+ innate lymphoid cells. Nat Immunol 12: 949–958. [DOI] [PubMed] [Google Scholar]
- Proebstl D, Voisin MB, Woodfin A, Whiteford J, D’Acquisto F, Jones GE, Rowe D, Nourshargh S. 2012. Pericytes support neutrophil subendothelial cell crawling and breaching of venular walls in vivo. J Exp Med 209: 1219–1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot AE, Handel TM, Johnson Z, Lau EK, LiWang P, Clark-Lewis I, Borlat F, Wells TN, Kosco-Vilbois MH. 2003. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci 100: 1885–1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruenster M, Mudde L, Bombosi P, Dimitrova S, Zsak M, Middleton J, Richmond A, Graham GJ, Segerer S, Nibbs RJ, et al. 2009. The Duffy antigen receptor for chemokines transports chemokines and supports their promigratory activity. Nat Immunol 10: 101–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randolph GJ, Ochando J, Partida-Sanchez S. 2008. Migration of dendritic cell subsets and their precursors. Annu Rev Immunol 26: 293–316. [DOI] [PubMed] [Google Scholar]
- Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, Mitchell AJ, Tay SS, Jain R, Forbes-Blom E, et al. 2013. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol 14: 564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadik CD, Kim ND, Iwakura Y, Luster AD. 2012. Neutrophils orchestrate their own recruitment in murine arthritis through C5aR and FcγR signaling. Proc Natl Acad Sci 109: E3177–E3185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallusto F, Schaerli P, Loetscher P, Schaniel C, Lenig D, Mackay CR, Qin S, Lanzavecchia A. 1998. Rapid and coordinated switch in chemokine receptor expression during dendritic cell maturation. Eur J Immunol 28: 2760–2769. [DOI] [PubMed] [Google Scholar]
- Sawa S, Cherrier M, Lochner M, Satoh-Takayama N, Fehling HJ, Langa F, Di Santo JP, Eberl G. 2010. Lineage relationship analysis of RORγt+ innate lymphoid cells. Science 330: 665–669. [DOI] [PubMed] [Google Scholar]
- Schaerli P, Willimann K, Ebert LM, Walz A, Moser B. 2005. Cutaneous CXCL14 targets blood precursors to epidermal niches for Langerhans cell differentiation. Immunity 23: 331–342. [DOI] [PubMed] [Google Scholar]
- Semmling V, Lukacs-Kornek V, Thaiss CA, Quast T, Hochheiser K, Panzer U, Rossjohn J, Perlmutter P, Cao J, Godfrey DI, et al. 2010. Alternative cross-priming through CCL17-CCR4-mediated attraction of CTLs toward NKT cell-licensed DCs. Nat Immunol 11: 313–320. [DOI] [PubMed] [Google Scholar]
- Serbina NV, Pamer EG. 2006. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol 7: 311–317. [DOI] [PubMed] [Google Scholar]
- Shi C, Pamer EG. 2011. Monocyte recruitment during infection and inflammation. Nat Rev Immunol 11: 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi C, Jia T, Mendez-Ferrer S, Hohl TM, Serbina NV, Lipuma L, Leiner I, Li MO, Frenette PS, Pamer EG. 2011. Bone marrow mesenchymal stem and progenitor cells induce monocyte emigration in response to circulating toll-like receptor ligands. Immunity 34: 590–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sojka DK, Plougastel-Douglas B, Yang L, Pak-Wittel MA, Artyomov MN, Ivanova Y, Zhong C, Chase JM, Rothman PB, Yu J, et al. 2014. Tissue-resident natural killer (NK) cells are cell lineages distinct from thymic and conventional splenic NK cells. eLife 3: e01659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark K, Eckart A, Haidari S, Tirniceriu A, Lorenz M, von Bruhl ML, Gartner F, Khandoga AG, Legate KR, Pless R, et al. 2013. Capillary and arteriolar pericytes attract innate leukocytes exiting through venules and “instruct” them with pattern-recognition and motility programs. Nat Immunol 14: 41–51. [DOI] [PubMed] [Google Scholar]
- Struyf S, Proost P, Schols D, De Clercq E, Opdenakker G, Lenaerts JP, Detheux M, Parmentier M, De Meester I, Scharpe S, et al. 1999. CD26/dipeptidyl-peptidase IV down-regulates the eosinophil chemotactic potency, but not the anti-HIV activity of human eotaxin by affecting its interaction with CC chemokine receptor 3. J Immunol 162: 4903–4909. [PubMed] [Google Scholar]
- Suratt BT, Petty JM, Young SK, Malcolm KC, Lieber JG, Nick JA, Gonzalo JA, Henson PM, Worthen GS. 2004. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood 104: 565–571. [DOI] [PubMed] [Google Scholar]
- Tal O, Lim HY, Gurevich I, Milo I, Shipony Z, Ng LG, Angeli V, Shakhar G. 2011. DC mobilization from the skin requires docking to immobilized CCL21 on lymphatic endothelium and intralymphatic crawling. J Exp Med 208: 2141–2153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas SY, Scanlon ST, Griewank KG, Constantinides MG, Savage AK, Barr KA, Meng F, Luster AD, Bendelac A. 2011. PLZF induces an intravascular surveillance program mediated by long-lived LFA-1-ICAM-1 interactions. J Exp Med 208: 1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uguccioni M, Mackay CR, Ochensberger B, Loetscher P, Rhis S, LaRosa GJ, Rao P, Ponath PD, Baggiolini M, Dahinden CA. 1997. High expression of the chemokine receptor CCR3 in human blood basophils. Role in activation by eotaxin, MCP-4, and other chemokines. J Clin Invest 100: 1137–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varona R, Villares R, Carramolino L, Goya I, Zaballos A, Gutierrez J, Torres M, Martinez AC, Marquez G. 2001. CCR6-deficient mice have impaired leukocyte homeostasis and altered contact hypersensitivity and delayed-type hypersensitivity responses. J Clin Invest 107: R37–R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald O, Weiss ID, Wald H, Shoham H, Bar-Shavit Y, Beider K, Galun E, Weiss L, Flaishon L, Shachar I, et al. 2006. IFN-γ acts on T cells to induce NK cell mobilization and accumulation in target organs. J Immunol 176: 4716–4729. [DOI] [PubMed] [Google Scholar]
- Walker JA, Barlow JL, McKenzie AN. 2013. Innate lymphoid cells—How did we miss them? Nat Rev Immunol 13: 75–87. [DOI] [PubMed] [Google Scholar]
- Wang Y, Cui L, Gonsiorek W, Min SH, Anilkumar G, Rosenblum S, Kozlowski J, Lundell D, Fine JS, Grant EP. 2009. CCR2 and CXCR4 regulate peripheral blood monocyte pharmacodynamics and link to efficacy in experimental autoimmune encephalomyelitis. J Inflamm (Lond) 6: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber C, Weber KS, Klier C, Gu S, Wank R, Horuk R, Nelson PJ. 2001. Specialized roles of the chemokine receptors CCR1 and CCR5 in the recruitment of monocytes and TH1-like/CD45RO+ T cells. Blood 97: 1144–1146. [DOI] [PubMed] [Google Scholar]
- Weber M, Hauschild R, Schwarz J, Moussion C, de Vries I, Legler DF, Luther SA, Bollenbach T, Sixt M. 2013. Interstitial dendritic cell guidance by haptotactic chemokine gradients. Science 339: 328–332. [DOI] [PubMed] [Google Scholar]
- Wehr A, Baeck C, Heymann F, Niemietz PM, Hammerich L, Martin C, Zimmermann HW, Pack O, Gassler N, Hittatiya K, et al. 2013. Chemokine receptor CXCR6-dependent hepatic NK T cell accumulation promotes inflammation and liver fibrosis. J Immunol 190: 5226–5236. [DOI] [PubMed] [Google Scholar]
- Wong CK, Hu S, Cheung PF, Lam CW. 2010. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: Implications in allergic inflammation. Am J Respir Cell Mol Biol 43: 305–315. [DOI] [PubMed] [Google Scholar]
- Worbs T, Bode U, Yan S, Hoffmann MW, Hintzen G, Bernhardt G, Forster R, Pabst O. 2006. Oral tolerance originates in the intestinal immune system and relies on antigen carriage by dendritic cells. J Exp Med 203: 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan S, Gessner R, Dietel C, Schmiedek U, Fan H. 2012. Enhanced ovalbumin-induced airway inflammation in CD26−/− mice. Eur J Immunol 42: 533–540. [DOI] [PubMed] [Google Scholar]
- Yuan Q, Campanella GS, Colvin RA, Hamilos DL, Jones KJ, Mathew A, Means TK, Luster AD. 2006. Membrane-bound eotaxin-3 mediates eosinophil transepithelial migration in IL-4-stimulated epithelial cells. Eur J Immunol 36: 2700–2714. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Raoof M, Chen Y, Sumi Y, Sursal T, Junger W, Brohi K, Itagaki K, Hauser CJ. 2010. Circulating mitochondrial DAMPs cause inflammatory responses to injury. Nature 464: 104–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O, Nomiyama H. 2006. The chemokine and chemokine receptor superfamilies and their molecular evolution. Genome Biol 7: 243. [DOI] [PMC free article] [PubMed] [Google Scholar]