ABSTRACT
The glutamate-gated chloride channel (GluCl) is a highly sensitive insecticide target of the avermectin class of insecticides. As an alternative to using chemical insecticides to kill mosquitoes, we tested the effects of purified immunoglobulin G (IgG) targeting the extracellular domain of GluCl from Anopheles gambiae (AgGluCl) on the survivorship of three key mosquito disease vectors: Anopheles gambiae s.s., Aedes aegypti and Culex tarsalis. When administered through a single blood meal, anti-AgGluCl IgG reduced the survivorship of A. gambiae in a dose-dependent manner (LC50: 2.82 mg ml−1, range 2.68–2.96 mg ml−1) but not A. aegypti or C. tarsalis. We previously demonstrated that AgGluCl is only located in tissues of the head and thorax of A. gambiae. To verify that AgGluCl IgG is affecting target antigens found outside the midgut, we injected it directly into the hemocoel via intrathoracic injection. A single, physiologically relevant concentration of anti-AgGluCl IgG injected into the hemocoel equally reduced mosquito survivorship of all three species. To test whether anti-AgGluCl IgG was entering the hemocoel of each of these mosquitoes, we fed mosquitoes a blood meal containing anti-AgGluCl IgG and subsequently extracted their hemolymph. We only detected IgG in the hemolymph of A. gambiae, suggesting that resistance of A. aegypti and C. tarsalis to anti-AgGluCl IgG found in blood meals is due to deficient IgG translocation across the midgut. We predicted that anti-AgGluCl IgG's mode of action is by antagonizing GluCl activity. To test this hypothesis, we fed A. gambiae blood meals containing anti-AgGluCl IgG and the GluCl agonist ivermectin (IVM). Anti-AgGluCl IgG attenuated the mosquitocidal effects of IVM, suggesting that anti-AgGluCl IgG antagonizes IVM-induced activation of GluCl. Lastly, we stained adult, female A. aegypti and C. tarsalis for GluCl expression. Neuronal GluCl expression in these mosquitoes was similar to previously reported A. gambiae GluCl expression; however, we also discovered GluCl staining on the basolateral surface of their midgut epithelial cells, suggesting important physiological differences in Culicine and Anopheline mosquitoes.
KEY WORDS: Aedes aegypti, Anopheles gambiae, Culex tarsalis, GluCl, Mosquitocidal antibody
Highlighted Article: Antibodies against the glutamate-gated chloride channel of A. gambiae reduce the survivorship of three mosquito disease vectors.
INTRODUCTION
Mosquito-borne diseases, such as malaria, dengue and West Nile virus, account for an estimated 1,434,000 deaths annually and 60,056,000 disability adjusted life years (World Health Report, 2012, available at http://www.who.int/gho/publications/world_health_statistics/2012/en/). These diseases are transmitted by Anopheline and Culicine mosquitoes. Historically, the most successful examples of vector-borne disease control have involved targeting mosquito vectors through the use of chemical insecticides. However, cross-cutting strategies that affect multiple mosquito disease vectors are currently limited to broad spectrum insecticides, to which resistance has become widespread (Corbel et al., 2007; Saavedra-Rodriguez et al., 2007; Norris and Norris, 2011; Ranson et al., 2011). Development of novel control strategies is necessary to maintain control of, and further eliminate, the transmission of mosquito-borne diseases.
Current insecticides target critical proteins involved in neuronal signaling. These include voltage-gated sodium channels (targeted by pyrethroids and organochlorines) (Zlotkin, 1999), aceytlcholinesterases (organophosphates and carbamates) (Casida, 1963; Fukuto, 1990) and glutamate-gated chloride channels (avermectins) (Campbell et al., 1983; Cully et al., 1996; Kobylinski et al., 2010, 2011; Sylla et al., 2010). The glutamate-gated chloride channel of Anopheles gambiae (AgGluCl) and other Anopheline mosquitoes has proven to be an exceptionally sensitive target for the insecticidal drug ivermectin (IVM), when introduced through a blood meal (Jones et al., 1992; Gardner et al., 1993; Foley et al., 2000; Fritz et al., 2009; Chaccour et al., 2010; Kobylinski et al., 2010; Sylla et al., 2010). GluCl is a member of the Cys-loop family of ligand-gated ion channels. This channel is only expressed in invertebrates, where it gates an inhibitory chloride current on the post-synaptic membranes of neurons and muscle fibers (Cull-Candy, 1976; Fritz et al., 1979; Janssen et al., 2007, 2010).
Given that GluCl can be targeted by drugs found in a blood meal and that GluCl is not expressed in mammals, we wanted to test the efficacy of AgGluCl as a candidate mosquitocidal vaccine antigen. The concept of using vaccines to kill blood-feeding arthropods gained validity with the success of TickGARDPLUS, an anti-tick vaccine targeting the midgut antigen Bm86 (Jonsson et al., 2000), and has continued with the more recent development of the subolesin/akirin antigens in ticks and also recently in mosquitoes (de la Fuente et al., 2011, 2013; da Costa et al., 2014). Research into mosquitocidal vaccines has been conducted since the 1940s, but with much less success, with most strategies focusing on targeting midgut antigens (Dubin et al., 1948; Hatfield, 1988a; Lal et al., 2001; Foy et al., 2003). In most experiments, animals were immunized against heterogeneous mosquito tissue homogenates, which led to variable reductions in survival and fecundity in multiple mosquito species, including animals immunized against head tissue homogenates where we would expect AgGluCl expression to occur (Almeida and Billingsley, 1998; Foy et al., 2002). Consequently, an efficacious anti-mosquito vaccine has never been developed despite decades of intermittent research (Jacobs-Lorena and Lemos, 1995; Willadsen and Billingsley, 1996; Billingsley et al., 2008).
As GluCl is found outside of the midgut, it is important to understand which mosquito species permit IgG to translocate from the blood meal into the hemolymph. Previous literature shows that antibody translocation is not uniform across mosquito species (Vaughan and Azad, 1988; Jeffers and Michael Roe, 2008). Antibodies have been shown to translocate across the midgut of A. gambiae and other Anopheles spp. for up to 48 h post-ingestion (Vaughan and Azad, 1988; Beier et al., 1989). There are conflicting reports concerning antibody translocation across the midgut of Aedes aegypti, though most reports show that there are barriers to this process (Hatfield, 1988a,b; Ramasamy et al., 1988; Vaughan and Azad, 1988; Jacobs-Lorena and Lemos, 1995). Culex tarsalis has never been tested for this process, but Culex pipiens has been shown to have little to no antibody translocation directly following blood feeding (Vaughan and Azad, 1988).
We administered a polyclonal anti-AgGluCl immunoglobulin G (anti-AgGluCl IgG) to A. gambiae Giles 1902, A. aegypti (Linnaeus) and C. tarsalis Linnaeus 1758 through a blood meal or directly into the hemocoel by intrathoracic injection to determine and quantify its broad mosquitocidal activity across these diverse mosquito species. We also examined the effects on survivorship of co-administering anti-AgGluCl IgG with a known GluCl agonist, IVM, to study the mechanism of action of anti-AgGluCl IgG. In parallel, we compared GluCl tissue expression and antibody translocation into the hemolymph of these three diverse mosquito vectors.
RESULTS
Production and verification of anti-AgGluCl IgG specificity
Polyclonal anti-AgGluCl IgG was generated in rabbits against the bacterially expressed N-terminal extracellular domain of AgGluCl. GluCl is highly conserved across mosquito species, including A. gambiae and A. aegypti (Fig. 1A). Antibody titer and specificity against the recombinant immunized protein was verified by ELISA (1:512,000) and western blot against the recombinant, immunized AgGluCl extracellular antigen, and immunolabeling of C6/36 cells (derived from Aedes albopictus) expressing native, full-length AgGluCl (Fig. 1B,C).
Fig. 1.
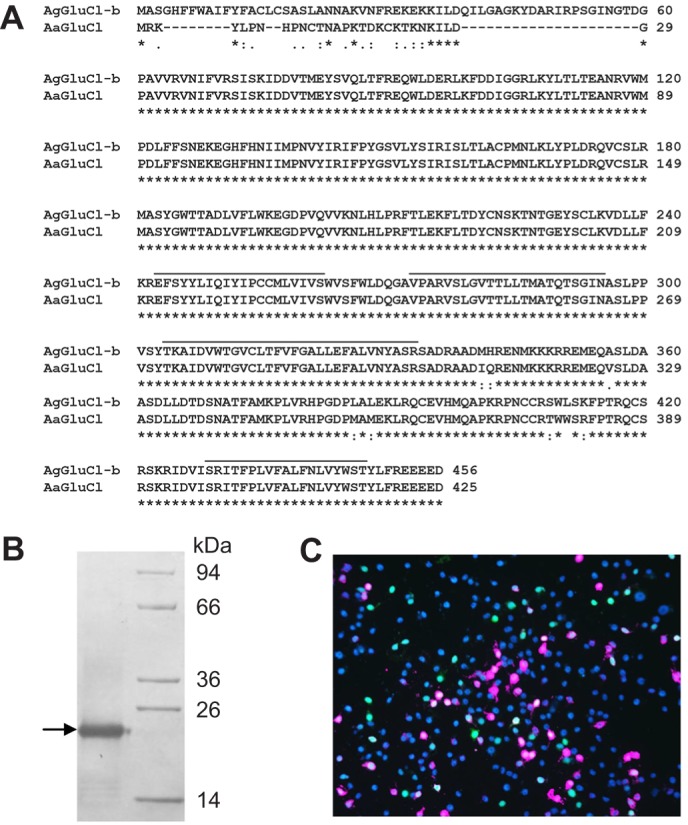
Mosquito GluCl alignment and anti-AgGluCl IgG specificity. (A) ClustalW alignment of predicted amino acid sequences for the glutamate-gated chloride channels AgGluCl-b (AGAP001434) from Anopheles gambiae and AaGluCl (AAEL003003) from Aedes aegypti. Black bars denote transmembrane domains, asterisks indicate identical residues, and colons and full-stops indicate conservation between groups of strongly and weakly similar properties, respectively. (B) Western blot of anti-AgGluCl IgG against the AgGluCl extracellular domain. (C) C6/36 cells transfected with AgGluCl and eGFP (green). Cells were immunostained for anti-AgGluCl IgG (pink) and DAPI (blue) (Habluetzel et al., 1997).
Anti-AgGluCl IgG kills A. gambiae when administered through a blood meal or intrathoracic injection
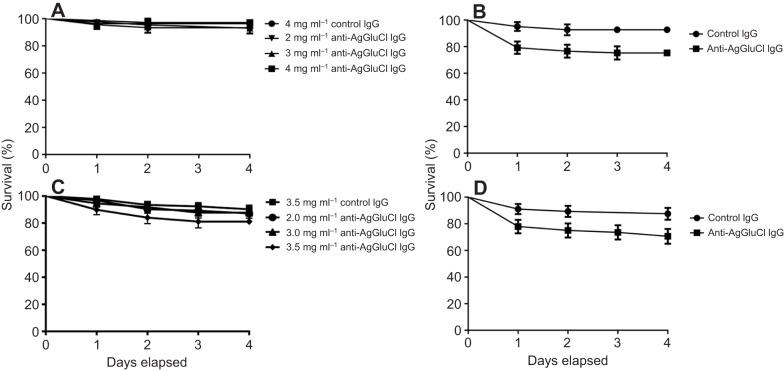
To measure the effects of anti-AgGluCl IgG on A. gambiae survivorship, we fed mosquitoes on blood meals containing anti-AgGluCl IgG ranging from 1.0 to 3.0 mg ml−1. Blood feeding of five different anti-AgGluCl IgG concentrations showed that anti-AgGluCl IgG induces a strong, dose-dependent mosquitocidal effect (Fig. 2A). Non-specific polyclonal (control) rabbit IgG, when blood fed at the highest concentration tested, did not affect mosquito mortality. These results were consistent across three replicates. We calculated the LC50 (the concentration of anti-AgGluCl IgG that was lethal to 50% of the mosquitoes) of anti-AgGluCl IgG to be 2.82 mg ml−1 (2.68, 2.96 mg ml−1; N=1499) (Fig. 2A).
Fig. 2.
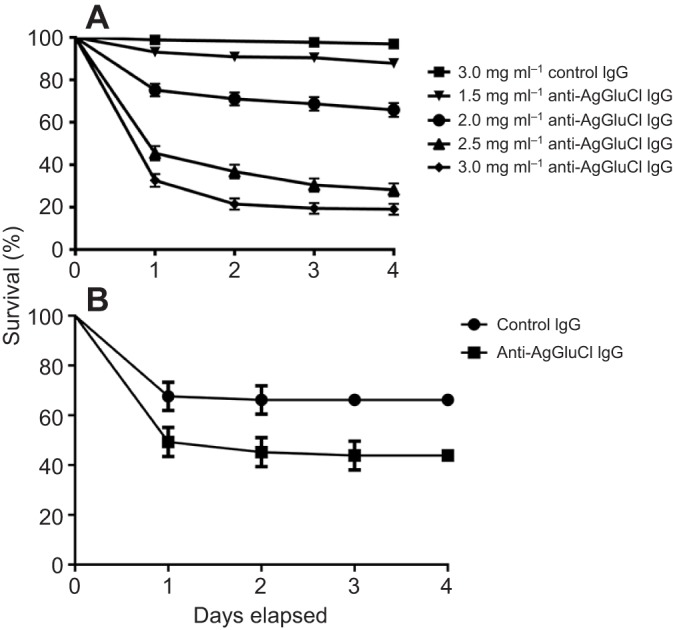
Anti-AgGluCl IgG kills A. gambiae when administered through a blood meal or directly into the hemocoel. (A) Survival curves of A. gambiae after feeding on a single blood meal containing anti-AgGluCl IgG or non-specific polyclonal (control) IgG. (B) Survival curves of A. gambiae after a single intrathoracic injection of anti-AgGluCl IgG . Data are presented as means and s.e.
To verify that anti-AgGluCl IgG was affecting survivorship by translocating across the midgut and binding to AgGluCl, which we found was only expressed in the hemocoel (see the companion paper, Meyers et al., 2015), we injected a physiologically relevant concentration (958 ng ml−1) of anti-AgGluCl IgG (Vaughan et al., 1990) or control rabbit IgG directly into the hemocoel by intrathoracic injection. A single injection of anti-AgGluCl IgG significantly reduced A. gambiae survivorship over 4 days [P=0.0083; hazard ratio: 2.322 (1.242, 4.30), N=141] compared with control (Fig. 2B).
Anti-AgGluCl IgG antagonizes AgGluCl activity
We hypothesized that the mode of action of anti-AgGluCl IgG is to antagonize channel function by binding to regions on the extracellular domain of AgGluCl that are critical for channel opening. To test this, we co-administered anti-AgGluCl IgG with IVM, a known GluCl agonist. We fed A. gambiae a single blood meal containing the LC75 of IVM (Kobylinski et al., 2010) and a physiologically relevant concentration (282 µg ml−1) of anti-AgGluCl IgG (Stoute et al., 1997), and monitored survivorship over 4 days (Fig. 3). IVM alone and IVM mixed with control non-specific rabbit IgG produced significant mosquitocidal effects relative to control groups [IVM versus PBS: P<0.0001, hazard ratio: 8.930 (5.765, 11.99), N=208; IVM+control IgG versus PBS: P<0.0001, hazard ratio: 8.641 (6.576, 14.31), N=193]. Control IgG had no effect on IVM toxicity [IVM versus IVM+control IgG: P=0.9213, hazard ratio: 0.9890 (0.7497, 1.290), N=225]. A single blood meal containing a physiologically relevant concentration of anti-AgGluCl IgG did not significantly reduce mosquito survivorship though the data were trending towards significance [anti-AgGluCl IgG versus PBS: P=0.0621, hazard ratio: 1.837 (0.9894, 3.531), N=183]. However, the combination of IVM and anti-AgGluCl IgG attenuated the mosquitocidal effects of IVM alone [IVM versus IVM+anti-AgGluCl IgG: P<0.0001, hazard ratio: 2.022 (1.842, 3.471), N=117], suggesting anti-AgGluCl IgG partially blocks IVM-induced activation of AgGluCl.
Fig. 3.
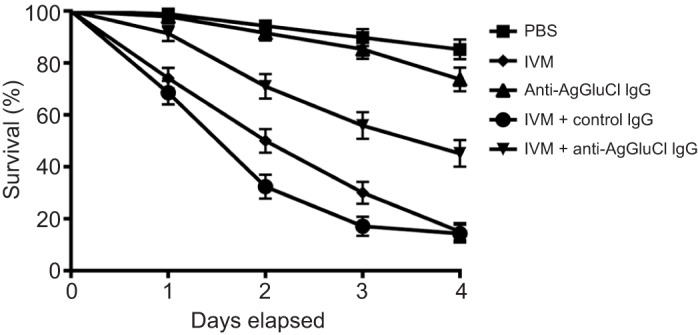
Survival curves of A. gambiae after feeding on a single blood meal containing anti-AgGluCl IgG plus the GluCl agonist ivermectin (IVM). Data are presented as means and s.e.
Anti-AgGluCl IgG kills A. aegypti and C. tarsalis when administered through intrathoracic injection but not through a blood meal
As GluCl is highly conserved across multiple mosquito species (percentage homology A. gambiae versus A. aegypti: 92.47%) (Megy et al., 2012), we tested to see whether anti-AgGluCl IgG had the same mosquitocidal properties in A. aegypti and C. tarsalis as we observed in A. gambiae. When introduced through a blood meal, anti-AgGluCl IgG had no effect on survivorship in A. aegypti up to a concentration of 4.0 mg ml−1 [P=0.6124, hazard ratio: 0.4526 (0.06213, 3.297), N=80] (Fig. 4A). Culex tarsalis blood fed on anti-AgGluCl IgG up to 3.5 mg ml−1 also showed no effect on survivorship [P=0.0867, hazard ratio: 2.131 (0.8966, 5.066), N=161] (Fig. 4C).
Fig. 4.
Anti-AgGluCl IgG kills Aedes aegypti and Culextarsalis when administered directly into the hemocoel, but not through a blood meal. Survival curves of A. aegypti (A) and C. tarsalis (C) after feeding on a single blood meal containing anti-AgGluCl IgG and of A. aegypti (B) and C. tarsalis (D) after a single intrathoracic injection of anti-AgGluCl IgG. Data are presented as means and s.e.
To test whether anti-AgGluCl IgG could affect mosquito survivorship if it was artificially made to cross the midgut, we intrathoracically injected A. aegypti and C. tarsalis with anti-AgGluCl IgG. When a physiological relevant concentration of anti-AgGluCl IgG (958 ng ml−1) (Vaughan et al., 1990) was administered through a single intrathoracic injection, it reduced survivorship in both A. aegypti [P=0.0221, hazard ratio: 2.969 (1.169, 7.537), N=122] and C. tarsalis [P=0.0237, hazard ratio: 2.536 (1.132, 5.682), N=124] over 4 days, equal to the reduction in survivorship of A. gambiae that underwent the same treatment (Fig. 4B,D and Fig. 2B).
IgG is found in the hemolymph of A. gambiae, but not A. aegypti or C. tarsalis, following a blood meal containing anti-AgGluCl IgG
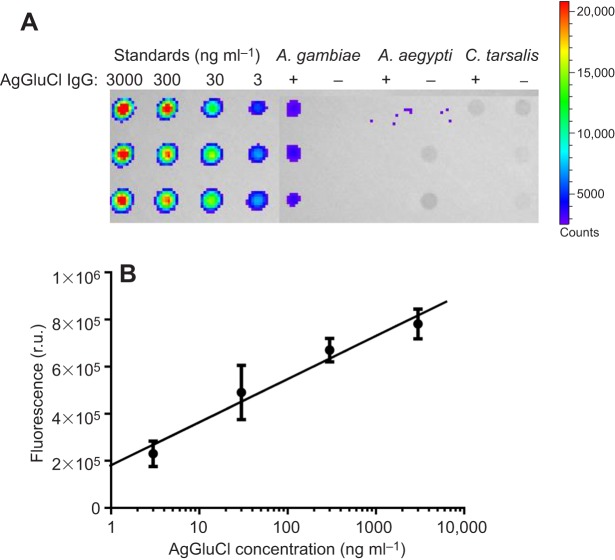
To determine whether IgG introduced through a blood meal is able to traverse the midgut into the hemocoel of these three mosquito disease vectors, we fed mosquitoes a blood meal containing anti-AgGluCl IgG and extracted their hemolymph at 3 h post-blood feeding. Hemolymph, diluted in PBS from the extraction procedure, was blotted for the rabbit-derived anti-AgGluCl IgG. Anti-AgGluCl IgG was detected in the diluted hemolymph of A. gambiae that had fed on anti-AgGluCl IgG-containing blood meals, but not in A. aegypti or C. tarsalis (Fig. 5A). A standard curve of anti-AgGluCl IgG was created from 3 ng ml−1 to 3 µg ml−1 to estimate the amount of anti-AgGluCl IgG that had diffused into the A. gambiae hemolymph. From this standard, we calculated 12.92 ng ml−1 anti-AgGluCl IgG in the hemolymph of A. gambiae following a blood meal containing anti-AgGluCl IgG (Fig. 5B). This calculation compensated for the dilution of hemolymph in PBS.
Fig. 5.
Dot blot of mosquito hemolymph. (A) Dot blot of anti-AgGluCl IgG standards and hemolymph extracted from A. gambiae, A. aegypti and C. tarsalis after feeding on an anti-AgGluCl IgG-containing blood meal (+) or a normal blood meal (–). (B) Anti-AgGluCl IgG standard curve: y=183,300x+180,344; r2:0.8816. r.u., relative units.
GluCl expression in A. aegypti and C. tarsalis
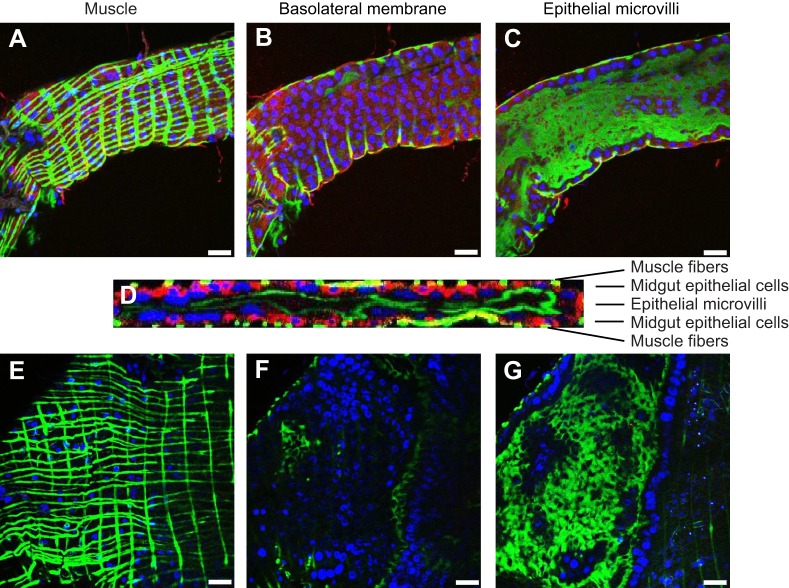
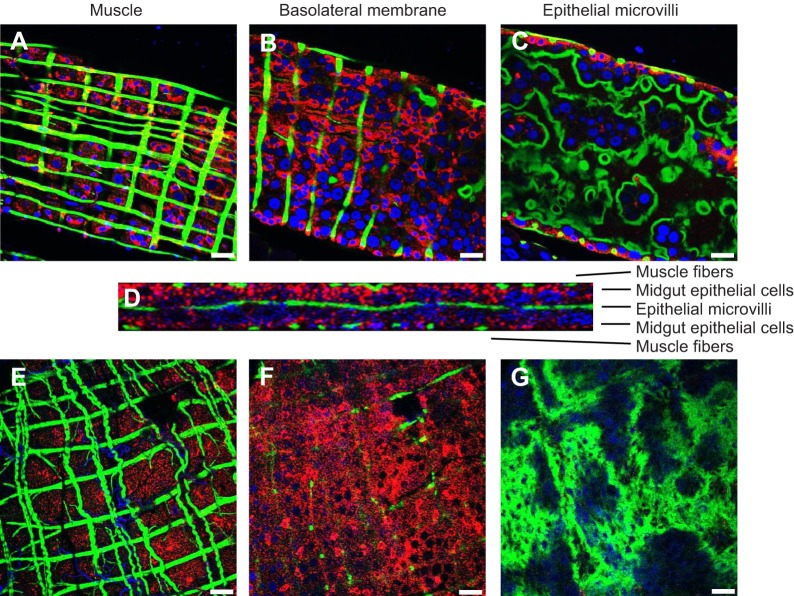
Given that anti-AgGluCl IgG reduces the survivorship of A. gambiae, A. aegypti and C. tarsalis when introduced into the hemocoel, we wanted to examine GluCl tissue expression to understand what physiological systems anti-AgGluCl IgG could be affecting. In the accompanying study, we found that AgGluCl is expressed in antenna segments, the Johnston's organ, the head and the thoracic ganglia of adult, female A. gambiae (Table 1) (Meyers et al., 2015). We stained sagittal sections of whole adult female mosquitoes for GluCl and a neuronal marker (Jan and Jan, 1982) to distinguish neuronal tissue. Immunolabeling of A. aegypti and C. tarsalis showed GluCl expression in the antennal segments, Johnston's organ, optic lobe, supraesophageal ganglion and thoracic ganglia, just as we found in A. gambiae (Table 1) (Meyers et al., 2015). There were small puncta of GluCl staining on antennal segments localized at the base of hair sensillae (supplementary material Fig. S1A, Fig. S2A). The Johnston's organ, a mechanosensory organ in the pedicel associated with audition and flight coordination, had specific GluCl staining in scolopidea (supplementary material Fig. S1B, Fig. S2B). GluCl staining was present in neuronal tissues found in the head (supplementary material Fig. S1C, Fig. S2C). Lastly, there was GluCl staining on neuronal cell bodies found in all three thoracic ganglia (supplementary material Fig. S1D, Fig. S2D). Surprisingly, A. aegypti and C. tarsalis exhibited unique GluCl staining on the midgut epithelium, which was not present in A. gambiae. Midguts were dissected and individually stained to visualize the precise location of GluCl in the midgut. In A. aegypti, GluCl expression was restricted to the anterior midgut and was not present in the posterior midgut (Fig. 6). In C. tarsalis, GluCl expression was found throughout the anterior and posterior midgut (Fig. 7). In both A. aegypti and C. tarsalis, GluCl expression was confined to the basolateral side of the midgut epithelial cells and was not found on the epithelial microvilli that project into the midgut lumen (Fig. 6D and Fig. 7D).
Table 1.
GluCl expression in Anophelesgambiae, Aedesaegypti and Culextarsalis
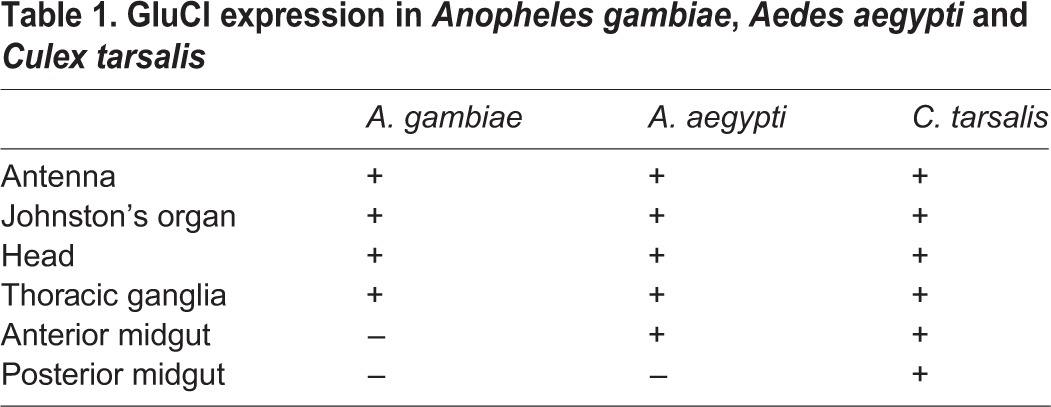
Fig. 6.
Immunostaining of dissected A. aegypti anterior and posterior midgut. Confocal slices of the muscle, basolateral membrane and epithelial microvilli of the anterior (A–C) and posterior (E–G) midgut. (D) Confocal stack through the thickness of the anterior midgut. Red: GluCl; green: actin; blue: DAPI. Scale bars: 50 µm.
Fig. 7.
Immunostaining of dissected C. tarsalis anterior and posterior midgut. Confocal slices of the muscle, basolateral membrane and epithelial microvilli of the anterior (A–C) and posterior (E–G) midgut. (D) Confocal stack through the thickness of the posterior midgut. Red: GluCl; green: actin; blue: DAPI. Scale bars: 50 µm.
DISCUSSION
In the present study, we have shown that IgG targeting the N-terminal extracellular domain of AgGluCl reduces A. gambiae survivorship when mixed with calf blood and administered through an artificial membrane feeder. To our knowledge, this is the first example of a single antigen that can be targeted by IgG in a mosquito blood meal to consistently and significantly reduce mosquito survivorship. Previous vaccine development strategies targeting mosquitoes have immunized animals with whole mosquito tissues, which could never be employed as a vaccine antigen (Alger and Cabrera, 1972; Almeida and Billingsley, 1998), or against a single midgut antigen. These resulted in small and highly variable reductions in survivorship and fecundity and differed between mosquito species, immunized hosts and trials (Dubin et al., 1948; Alger and Cabrera, 1972; Hatfield, 1988a,b; Ramasamy et al., 1988; Vaughan and Azad, 1988; Srikrishnaraj et al., 1993; Noden et al., 1995; Almeida and Billingsley, 1998, 2002; Lal et al., 2001; Foy et al., 2003; Billingsley et al., 2008; da Costa et al., 2014). In contrast, blood meals containing anti-AgGluCl IgG consistently reduced A. gambiae survivorship across all replicates in a dose-dependent manner.
The LC50 concentration of anti-AgGluCl IgG in a blood meal and the range of anti-AgGluCl IgG concentrations that reduced A. gambiae survivorship are greater than IgG concentrations found in an immunized human host (Shawler et al., 1985; Stoute et al., 1997). Such a vaccine would not benefit from natural boosting as the host is not naturally exposed to AgGluCl epitopes, so any vaccine would depend on relatively high antibody titers. Our anti-AgGluCl IgG was created against the large 244 amino acid extracellular domain. AgGluCl epitope optimization targeting highly antigenic regions of this domain or passive immunization of monoclonal antibodies against targeted extracellular AgGluCl epitopes could improve anti-AgGluCl IgG mosquito toxicity. Also, serial blood feeding might result in accumulation of anti-AgGluCl IgG, causing an increase in mortality over time. Though IgG in the mosquito hemolymph has been shown to be cleared by 48 h post-feeding, those experiments were done with IgG that had no hemocoelic targets (Vaughan and Azad, 1988). If anti-AgGluCl IgG persists in the hemocoel by binding to GluCl-expressing tissues, there could be aggregate effects from serial blood feeding over time on a physiological concentration of anti-AgGluCl IgG on A. gambiae survivorship and fecundity. Furthermore, evidence from the field suggests very frequent biting by A. gambiae, approximately every 1–2 days, providing multiple opportunities for anti-AgGluCl IgG ingestion (Beier, 1996; Scott et al., 2006). The concentration of anti-AgGluCl IgG used for intrathoracic injections is within the range of IgG concentrations found in Anopheles stephensi hemolymph 3 h after feeding on rats immunized against Plasmodium falciparum circumsporozoite protein (Vaughan et al., 1990). This suggests that A. gambiae survivorship might be affected when fed directly on an AgGluCl-immunized host. The mosquitocidal effects of anti-AgGluCl IgG injected into the hemocoel also suggest that anti-AgGluCl IgG is binding to antigens found outside of the midgut to affect A. gambiae survivorship.
When we co-administered anti-AgGluCl IgG with the potent GluCl agonist IVM, we found an attenuation of IVM-induced mortality. This suggests that anti-AgGluCl IgG works by antagonizing AgGluCl activity. AgGluCl antagonism would disrupt inhibitory glutamatergic synaptic communication and potentially cause neuronal hyper-excitation. It is still unclear how anti-AgGluCl IgG antagonizes AgGluCl activity; it could directly block channel activity or indirectly affect activity by causing AgGluCl to be internalized into intracellular vesicles. The likely explanation is that anti-AgGluCl IgG binding to multiple extracellular epitopes of AgGluCl antagonizes the channel and prevents IVM-induced opening of the channel. Though the conformation of AgGluCl would change with IVM binding, it seems unlikely that this would affect the binding of the polyclonal anti-AgGluCl IgG because the antibodies likely bind to multiple epitopes on the extracellular domain of AgGluCl, while IVM binding occurs in between subunits on residues found on the AgGluCl transmembrane domain (Hibbs and Gouaux, 2011). Auto-immune antibodies against other channels have been shown to antagonize channel activity in mammals, which is likely the same mechanism of action of anti-AgGluCl IgG (Weber et al., 2000; Wang et al., 2007; Briani et al., 2008; Vernino et al., 2008). However, GluCl is not expressed in any mammalian species, making it unlikely that anti-AgGluCl IgG will have auto-immune effects in humans or livestock. The mammalian receptors most closely related to the insect GluCl are the GABA-gated chloride channel and the glycine-gated chloride channel (Lynagh and Lynch, 2012) but their amino acid similarity with AgGluCl is <35%. Nevertheless, anti-AgGluCl IgG reactivity to these channels and other neurotransmitter receptors should be tested in the design of any vaccine that would use AgGluCl epitopes as antigens.
As GluCl is highly conserved across multiple mosquito species, we tested to see whether blood meals containing anti-AgGluCl IgG could affect the survivorship of two other important disease vectors, A. aegypti and C. tarsalis. Anti-AgGluCl IgG did not affect A. aegypti or C. tarsalis survivorship when administered through a blood meal. When a physiologically relevant concentration of anti-AgGluCl IgG was directly injected into the hemocoel, it reduced the survivorship in both A. aegypti and C. tarsalis. Surprisingly, all three mosquito species had the same reduction in survivorship to intrathoracic injections of anti-AgGluCl IgG. This suggests that anti-AgGluCl IgG has a similar affinity for the GluCl across these mosquito species and that GluCl plays a similar, critical physiological role in all three species.
Previous research has shown that whole IgG passes through the midgut of A. gambiae (Vaughan and Azad, 1988). There are conflicting reports on antibody translocation in A. aegypti, but most work has shown that this process does not occur (Vaughan and Azad, 1988; Jacobs-Lorena and Lemos, 1995). There have been no reports on antibody passage across the midgut of C. tarsalis, though other Culex species have been shown to have little to no antibody passage across the midgut (Vaughan and Azad, 1988). In agreement with previous work, we only detected anti-AgGluCl IgG in the diluted hemolymph of A. gambiae but not A. aegypti, nor did we find anti-AgGluCl IgG in the diluted hemolymph of C. tarsalis post-blood feeding. These results provide an explanation for the sensitivity of these three mosquito species to blood meals containing anti-AgGluCl IgG. Using our standard curve, we detected 12.92 ng ml−1 of anti-AgGluCl IgG in A. gambiae hemolymph. This is well below the concentration found in A. stephensi following blood feeding on an immunized rat (900–958 ng ml−1) (Vaughan et al., 1990). However, in our experiment we added rabbit-derived anti-AgGluCl IgG to calf blood that already contained calf IgG. We believe the anti-AgGluCl IgG concentration we detected was low because the rabbit-derived anti-AgGluCl IgG comprises only a small portion of the total IgG in the blood meal. It is also possible that a major portion of anti-AgGluCl IgG remained bound to target antigens throughout the hemocoel, sequestering it from our hemolymph extraction. Though the concentration of anti-AgGluCl IgG in the hemolymph may not reflect what would be present in the hemolymph following a blood meal from an AgGluCl-immunized host, it still verifies the presence of IgG in the hemolymph and confirms that IgG is able to translocate across the A. gambiae midgut. It is not clear how IgG in a blood meal translocates into the hemocoel of A. gambiae, nor is it known what the barriers are to this process in A. aegypti or C. tarsalis. Further research is required to examine this key divergence in midgut physiology between Anopheline and Culicine mosquitoes.
To begin to understand what physiological role GluCl has in A. aegypti and C. tarsalis, we stained sagittal slices of whole, adult female mosquitoes for GluCl and found a similar neuronal GluCl expression pattern to that in A. gambiae (Meyers et al., 2015). There was only one tissue that had disparate GluCl expression between these three mosquito species; this was the midgut, which exhibited GluCl staining in A. aegypti and C. tarsalis but not in A. gambiae (Meyers et al., 2015). GluCl expression in the midgut came as a surprise because A. aegypti and C. tarsalis were not sensitive to anti-AgGluCl IgG when administered through a blood meal. Closer examination of GluCl midgut expression through direct immunostaining of dissected midguts showed that GluCl expression only occurred on the basolateral surface of the midgut epithelial cells and was not present on the epithelial microvilli found in the midgut lumen of both A. aegypti and C. tarsalis. This means that GluCl antigens are not directly exposed to the blood meal and that IgG would need to translocate across midgut epithelial cells to bind to extracellular epitopes of GluCl found on the basolateral surface of the midgut epithelium. Our data show that IgG is unable to translocate into the hemocoel in A. aegypti and C. tarsalis, which is why GluCl on the basolateral surface of the midgut is not affected by anti-AgGluCl IgG-laden blood meals. At present, there is no evidence that anti-AgGluCl IgG insensitivity in A. aegypti and C. tarsalis is related to GluCl expression on the midgut. Though GluCl on the midgut could be acting as a ‘sink’ and binding anti-AgGluCl IgG from a blood meal before it could reach neuronally expressed GluCl in the head and thorax, it has been previously shown that anti-Rickettsia typhii antibodies also do not translocate from the blood meal to the hemolymph in A. aegypti and C. pipiens, giving further evidence that this refractory phenotype to anti-AgGluCl IgG-laden blood meals is due to a barrier in antibody translocation (Vaughan and Azad, 1988). GluCl expression is confined to the anterior midgut of A. aegypti and not present in the posterior midgut, whereas GluCl expression was detected in both the anterior and posterior midgut of C. tarsalis. It is unclear what role GluCl has on the midgut epithelium or why it is differentially expressed across these three mosquito species, but its role in chloride ion transport suggests it might be involved in maintaining ionic balance during midgut alkanization and digestion (del Pilar Corena et al., 2005).
Targeting GluCl through a vaccine strategy will not be effective at killing Culicine mosquitoes because IgG is unable to translocate into the hemocoel following a blood meal. However, a mosquitocidal vaccine targeting the highly conserved AgGluCl has major potential against multiple Anopheles spp. which have previously been shown to pass IgG through their midgut, including Anopheles arabiensis, Anopheles stephensi, Anopheles funestus and Anopheles albimanus (Vaughan and Azad, 1988; Beier et al., 1989). Further research testing the effects of anti-AgGluCl IgG on mosquito survivorship and the fecundity of A. gambiae and other Anopheles spp. after feeding on animals immunized against extracellular AgGluCl epitopes will be required to discern the potential of AgGluCl as a target antigen of a pan-Anopheles vaccine and as an intervention to control malaria parasite transmission.
MATERIALS AND METHODS
Mosquitoes
Anopheles gambiae s.s. G3 strain (origin The Gambia), Aedes aegypti (Vergel) and Culex tarsalis (California) were raised at 28–31°C, 80% relative humidity on a 14 h:10 h light:dark cycle. Larvae were fed ground Tetramin® fish food daily. Adults were provided with water and 10% sucrose solution ad libitum. Colony mosquitoes were blood fed every 3–4 days on defibrinated calf blood.
Production and verification of anti-AgGluCl IgG specificity
Polyclonal anti-AgGluCl IgG was prepared by GenScript USA, Inc. (Piscataway, NJ, USA). Briefly, two rabbits were immunized against the recombinant N-terminal extracellular domain of AgGluCl produced in Escherichia coli (244 amino acids in length). Rabbit serum was collected and affinity purified to isolate polyclonal rabbit IgG. Antibody specificity to AgGluCl was verified via ELISA, western blot and immunostaining of C6/36 cells (derived from Aedes albopictus) (Singh and Paul, 1969) transfected with AgGluCl cloned into the pIB/V5-His TOPO® plasmid (Life Technologies™, Grand Island, NY, USA) (Fig. 1B,C).
Blood feed with artificial membrane feeder
Two to 4 day post-emergence mosquitoes were fed defibrinated calf blood spiked with purified rabbit anti-AgGluCl IgG (GenScript), purified rabbit control IgG (GenScript), IVM or vehicle via glass bell feeders (Lillie Glass Feeders, Symrna, GA, USA) sealed with pig sausage casing and heated to 37°C. Mosquitoes were given 30–45 min to feed, after which they were knocked down briefly at 4°C for sorting and removal of unfed and partially fed mosquitoes. Fully engorged mosquitoes were monitored for survivorship for 4 days following the blood meal.
Intrathoracic injection of anti-AgGluCl IgG
Adult female mosquitoes were briefly knocked down at 4°C for intrathoracic injection. Glass capillaries (3-00-203-G/XL, Drummond Scientific, Broomall, PA, USA) were pulled using a Flaming/Brown Micropipette Puller Model P-87 (Sutter Instruments, Novato, CA, USA) to create microcapillary injection needles. Mosquitoes were injected with 69 nl of either 958 ng ml−1 anti-AgGluCl IgG or control IgG using a Drummond Nanoject II Automatic Injector (3-000-204, Drummond Scientific) and glass microcapillary injection needles. Following injection, mosquitoes were maintained for 4 days and survivorship was monitored.
Survivorship analysis and anti-AgGluCl IgG LC50 determination
Replicates were pooled and analyzed by log-rank test (Mantel-Haenzel method; proportioned hazards model) with 95% confidence intervals using Prism software (GraphPad, La Jolla, CA, USA). LC50 determination followed previously described analysis (Kobylinski et al., 2010). Briefly, survivorship replicates were pooled into a non-linear mixed model with probit analysis, which accounted for background mortality in the control group and assessed replicate effects, to calculate the lethal concentration that killed 50% of mosquitoes (LC50). Wing lengths from 20 mosquitoes from each group were measured and compared across groups to ensure mosquito size did not affect survivorship (Lounibos et al., 1995).
Hemolymph extraction and dot blot
Hemolymph samples were taken from A. gambiae, A. aegypti and C. tarsalis 3 h following blood feeding on de-fibrinated calf blood (control) or de-fibrinated calf blood containing 3.0 mg ml−1 rabbit-derived anti-AgGluCl IgG. Hemolymph was extracted by hemocoel perfusion as previously described (Vaughan et al., 1990). Briefly, a small perforation was made on the dorsal portion of the abdomen between the 7th and 8th segment. Mosquitoes were intrathoracically injected with PBS, using glass microcapillary injection needles, until a drop (∼0.5 µl) of hemolymph protruded from the previously made abdominal tear. This hemolymph was collected into a glass microcapillary, extruded into a microcentrifuge tube and stored at −80°C. Each sample contained pools of 10 mosquito hemolymph extractions.
Fifty microliters of anti-AgGluCl IgG standards (3 ng ml−1 to 3 µg ml−1) and extracted mosquito hemolymph were blotted onto nitrocellulose paper using the Bio-Rad Bio-Dot SF Microfiltration Apparatus (Bio-Rad Laboratories, Hercules, CA, USA) following the manufacturer’s protocol. As mosquito hemolymph extractions yielded very little volume, we diluted 5 µl of hemolymph with 45 µl PBS for blotting onto the nitrocellulose paper. Blots were blocked with 3% BSA in 0.05% Tween-20 Tris-based buffer solution (TTBS) for 1 h on a rocker. Blots were treated with a 1:50,000 dilution of goat anti-rabbit IgG (H+L) HRP conjugate (Thermo-Scientific, Waltham, MA, USA) in 1% BSA TTBS solution for 3 h on a shaker and then washed for 15 min in TTBS. Antibodies were visualized using SuperSignal West Femto Maximum Sensitivity Substrate (Thermo-Scientific) as per the manufacturer’s protocol. Fluorescence was measured using IVIS-200 Optical Imaging System (Xenogen, St Hopkinton, MA, USA) and quantified using Living Image 3.0 software (Caliper Life Science, Waltham, MA, USA). Anti-AgGluCl IgG standards were fitted with a linear regression curve, which was used to estimate the concentration of anti-AgGluCl IgG in the mosquito hemolymph.
Immunohistochemistry (IHC)
Whole mosquito specimens were prepared from female blood-fed mosquitoes aged 2–4 days post-emergence. After blood feeding, engorged females were injected intrathoracically (Drummond Nanoject) with 46 nl of 4% paraformaldehyde. Specimens were briefly washed in 70% ethanol before overnight fixation in 4% paraformaldehyde at 4°C. After fixation, specimens were paraffin embedded, cut into 5 µm thick slices and mounted onto slides (Colorado HistoPrep, Fort Collins, CO, USA). Slides were heated at 65°C for 10 min and treated with xylene to remove the paraffin layer and then re-hydrated with graded washes in ethanol and PBS. Specimens were treated with graded washes of methanol and PBS to reduce autofluorescence. Slides were blocked for 2 h with 10% non-fat dry milk and 0.1% Triton-X in PBS. Primary antibody staining consisted of 1:500 anti-AgGluCl IgG (prepared by GenScript) and 1:500 goat anti-HRP (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) incubated overnight at 4°C. Specimens were washed with 0.05% TTBS and then incubated with 1:1000 donkey anti-rabbit Alexa 555 (Invitrogen, Waltham, MA, USA) and 1:1000 donkey anti-goat Alexa 488 (Invitrogen) for 3 h at room temperature. For midgut staining, midguts were dissected from 2–4 day old females and fixed in 4% paraformaldehyde overnight. Midguts were blocked with 3% bovine serum albumin (BSA) in TTBS for 1 h and stained with 1:500 anti-AgGluCl IgG for 3 h. Specimens were subsequently washed in TTBS and stained with 1:1000 donkey anti-rabbit Alexa 555 (Invitrogen) and Acti-stain 488 phalloidin (Cytoskeleton Inc., Denver, CO, USA) for 1 h. Slides from either preparation were mounted with VectaShield® containing DAPI (Vector Laboratories, Burlingame, CA, USA). Negative controls underwent the same procedure, but substituting histidine affinity-purified polyclonal rabbit IgG from non-immunized rabbits as the primary antibody.
Supplementary Material
Acknowledgements
This work is dedicated to the late Ines Marques da Silva for her assistance with this project. We thank C. Nguyen, T. Burton, J. Seaman and J. Donkoh for their laboratory assistance.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
J.I.M. and B.D.F. conceived the study and designed the experiments. J.I.M. and M.G. performed the experiments. J.I.M. and B.D.F. analyzed the data and wrote the manuscript.
Funding
This study was supported by the National Institutes of Health [R01AI94349-01A1 to B.D.F.] and the Colorado State University Infectious Disease Supercluster [1-32613 to B.D.F and K.M.P.]. Deposited in PMC for release after 12 months.
Supplementary material
Supplementary material available online at http://jeb.biologists.org/lookup/suppl/doi:10.1242/jeb.118596/-/DC1
References
- Alger N. E. and Cabrera E. J. (1972). An increase in death rate of Anopheles stephensi fed on rabbits immunized with mosquito antigen. J. Econ. Entomol. 65, 165-168. 10.1093/jee/65.1.165 [DOI] [PubMed] [Google Scholar]
- Almeida A. P. G. and Billingsley P. F. (1998). Induced immunity against the mosquito Anopheles stephensi Liston (Diptera: Culicidae): effects on mosquito survival and fecundity. Int. J. Parasitol. 28, 1721-1731. 10.1016/S0020-7519(98)00149-0 [DOI] [PubMed] [Google Scholar]
- Almeida A. P. and Billingsley P. F. (2002). Induced immunity against the mosquito Anopheles stephensi (Diptera: Culicidae): effects of cell fraction antigens on survival, fecundity, and plasmodium berghei (Eucoccidiida: Plasmodiidae) transmission. J. Med. Entomol. 39, 207-214. 10.1603/0022-2585-39.1.207 [DOI] [PubMed] [Google Scholar]
- Beier J. C. (1996). Frequent blood-feeding and restrictive sugar-feeding behavior enhance the malaria vector potential of Anopheles gambiae s.l. and An. funestus (Diptera:Culicidae) in western Kenya. J. Med. Entomol. 33, 613-618. 10.1093/jmedent/33.4.613 [DOI] [PubMed] [Google Scholar]
- Beier J. C., Oster C. N., Koros J. K., Onyango F. K., Githeko A. K., Rowton E., Koech D. K. and Roberts C. R. (1989). Effect of human circumsporozoite antibodies in Plasmodium-infected Anopheles (Diptera: Culicidae). J. Med. Entomol. 26, 547-553. 10.1093/jmedent/26.6.547 [DOI] [PubMed] [Google Scholar]
- Billingsley P. F., Foy B. and Rasgon J. L. (2008). Mosquitocidal vaccines: a neglected addition to malaria and dengue control strategies. Trends Parasitol. 24, 396-400. 10.1016/j.pt.2008.06.003 [DOI] [PubMed] [Google Scholar]
- Briani C., Doria A., Ruggero S., Toffanin E., Luca M., Albergoni M. P., D'Odorico A., Grassivaro F., Lucchetta M., De Lazzari F. et al. (2008). Antibodies to muscle and ganglionic acetylcholine receptors (AchR) in celiac disease. Autoimmunity 41, 100-104. 10.1080/08916930701619987 [DOI] [PubMed] [Google Scholar]
- Campbell W. C., Fisher M. H., Stapley E. O., Albers-Schonberg G. and Jacob T. A. (1983). Ivermectin: a potent new antiparasitic agent. Science 221, 823-828. 10.1126/science.6308762 [DOI] [PubMed] [Google Scholar]
- Casida J. E. (1963). Mode of action of carbamates. Annu. Rev. Entomol. 8, 39-58. 10.1146/annurev.en.08.010163.000351 [DOI] [PubMed] [Google Scholar]
- Chaccour C., Lines J. and Whitty C. J. M. (2010). Effect of ivermectin on Anopheles gambiae mosquitoes fed on humans: the potential of oral insecticides in malaria control. J. Infect. Dis. 202, 113-116. 10.1086/653208 [DOI] [PubMed] [Google Scholar]
- Corbel V., N'Guessan R., Brengues C., Chandre F., Djogbenou L., Martin T., Akogbéto M., Hougard J. M. and Rowland M. (2007). Multiple insecticide resistance mechanisms in Anopheles gambiae and Culex quinquefasciatus from Benin, West Africa. Acta Tropica 101, 207-216. 10.1016/j.actatropica.2007.01.005 [DOI] [PubMed] [Google Scholar]
- Cull-Candy S. G. (1976). Two types of extrajunctional L-glutamate receptors in locust muscle fibres. J. Physiol. 255, 449-464. 10.1113/jphysiol.1976.sp011289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cully D. F., Paress P. S., Liu K. K., Schaeffer J. M. and Arena J. P. (1996). Identification of a Drosophila melanogaster glutamate-gated chloride channel sensitive to the antiparasitic agent avermectin. J. Biol. Chem. 271, 20187-20191. 10.1074/jbc.271.33.20187 [DOI] [PubMed] [Google Scholar]
- da Costa M., Pinheiro-Silva R., Antunes S., Moreno-Cid J. A., Custódio A., Villar M., Silveira H., de la Fuente J. and Domingos A. (2014). Mosquito Akirin as a potential antigen for malaria control. Malaria J. 13, 470 10.1186/1475-2875-13-470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de la Fuente J., Moreno-Cid J. A., Canales M., Villar M., de la Lastra J. M. P., Kocan K. M., Galindo R. C., Almazán C. and Blouin E. F. (2011). Targeting arthropod subolesin/akirin for the development of a universal vaccine for control of vector infestations and pathogen transmission. Vet. Parasitol. 181, 17-22. 10.1016/j.vetpar.2011.04.018 [DOI] [PubMed] [Google Scholar]
- de la Fuente J., Moreno-Cid J. A., Galindo R. C., Almazan C., Kocan K. M., Merino O., Perez de la Lastra J. M., Estrada-Peña A. and Blouin E. F. (2013). Subolesin/Akirin vaccines for the control of arthropod vectors and vectorborne pathogens. Transbound. Emerg. Dis. 60 Suppl. 2, 172-178. 10.1111/tbed.12146 [DOI] [PubMed] [Google Scholar]
- del Pilar Corena M., VanEkeris L., Salazar M. I., Bowers D., Fiedler M. M., Silverman D., Tu C. and Linser P. J. (2005). Carbonic anhydrase in the adult mosquito midgut. J. Exp. Biol. 208, 3263-3273. 10.1242/jeb.01739 [DOI] [PubMed] [Google Scholar]
- Dubin I. N., Reese J. D. and Seamans L. A. (1948). Attempt to produce protection against mosquitoes by active immunization. J. Immunol. 58, 293-297. [PubMed] [Google Scholar]
- Foley D. H., Bryan J. H. and Lawrence G. W. (2000). The potential of ivermectin to control the malaria vector Anopheles farauti. Trans. R. Soc. Trop. Med. Hyg. 94, 625-628. 10.1016/S0035-9203(00)90211-6 [DOI] [PubMed] [Google Scholar]
- Foy B. D., Killeen G. F., Magalhaes T. and Beier J. C. (2002). Immunological targeting of critical insect antigens. Am. Entomol. 48, 150-162. 10.1093/ae/48.3.150 [DOI] [Google Scholar]
- Foy B. D., Magalhaes T., Injera W. E., Sutherland I., Devenport M., Thanawastien A., Ripley D., Cardenas-Freytag L. and Beier J. C. (2003). Induction of mosquitocidal activity in mice immunized with Anopheles gambiae midgut cDNA. Infect. Immun. 71, 2032-2040. 10.1128/IAI.71.4.2032-2040.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz L. C., Wang C. C. and Gorio A. (1979). Avermectin B1a irreversibly blocks postsynaptic potentials at the lobster neuromuscular junction by reducing muscle membrane resistance. Proc. Natl. Acad. Sci. USA 76, 2062-2066. 10.1073/pnas.76.4.2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz M. L., Siegert P. Y., Walker E. D., Bayoh M. N., Vulule J. R. and Miller J. R. (2009). Toxicity of bloodmeals from ivermectin-treated cattle to Anopheles gambiae s.l. Ann. Trop. Med. Parasitol. 103, 539-547. 10.1179/000349809X12459740922138 [DOI] [PubMed] [Google Scholar]
- Fukuto T. R. (1990). Mechanism of action of organophosphorus and carbamate insecticides. Environ. Health Perspect. 87, 245-254. 10.1289/ehp.9087245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner K., Meisch M. V., Meek C. L. and Biven W. S. (1993). Effects of ivermectin in canine blood on Anopheles quadrimaculatus, Aedes albopictus and Culex salinarius. J. Am. Mosq. Control Assoc. 9, 400-402. [PubMed] [Google Scholar]
- Habluetzel A., Diallo D. A., Esposito F., Lamizana L., Pagnoni F., Lengeler C., Traore C. and Cousens S. N. (1997). Do insecticide-treated curtains reduce all-cause child mortality in Burkina Faso? Trop. Med. Int. Health 2, 855-862. 10.1046/j.1365-3156.1997.d01-413.x [DOI] [PubMed] [Google Scholar]
- Hatfield P. R. (1988a). Anti-mosquito antibodies and their effects on feeding, fecundity and mortality of Aedes aegypti. Med. Vet. Entomol. 2, 331-338. 10.1111/j.1365-2915.1988.tb00205.x [DOI] [PubMed] [Google Scholar]
- Hatfield P. R. (1988b). Detection and localization of antibody ingested with a mosquito bloodmeal. Med. Vet. Entomol. 2, 339-345. 10.1111/j.1365-2915.1988.tb00206.x [DOI] [PubMed] [Google Scholar]
- Hibbs R. E. and Gouaux E. (2011). Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 474, 54-60. 10.1038/nature10139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs-Lorena M. and Lemos F. J. A. (1995). Immunological strategies for control of insect disease vectors: a critical assessment. Parasitol. Today 11, 144-147. 10.1016/0169-4758(95)80134-0 [DOI] [PubMed] [Google Scholar]
- Jan L. Y. and Jan Y. N. (1982). Antibodies to horseradish peroxidase as specific neuronal markers in Drosophila and in grasshopper embryos. Proc. Natl. Acad. Sci. USA 79, 2700-2704. 10.1073/pnas.79.8.2700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen D., Derst C., Buckinx R., Van den Eynden J., Rigo J.-M. and Van Kerkhove E. (2007). Dorsal unpaired median neurons of Locusta migratoria express ivermectin- and fipronil-sensitive glutamate-gated chloride channels. J. Neurophysiol. 97, 2642-2650. 10.1152/jn.01234.2006 [DOI] [PubMed] [Google Scholar]
- Janssen D., Derst C., Rigo J. M. and Van Kerkhove E. (2010). Cys-loop ligand-gated chloride channels in dorsal unpaired median neurons of Locusta migratoria. J. Neurophysiol. 103, 2587-2598. 10.1152/jn.00466.2009 [DOI] [PubMed] [Google Scholar]
- Jeffers L. A. and Michael Roe R. (2008). The movement of proteins across the insect and tick digestive system. J. Insect Physiol. 54, 319-332. 10.1016/j.jinsphys.2007.10.009 [DOI] [PubMed] [Google Scholar]
- Jones J. W., Meisch M. V., Meek C. L. and Bivin W. S. (1992). Lethal effects of ivermectin on Anopheles quadrimaculatus. J. Am. Mosq. Control Assoc. 8, 278-280. [PubMed] [Google Scholar]
- Jonsson N. N., Matschoss A. L., Pepper P., Green P. E., Albrecht M. S., Hungerford J. and Ansell J. (2000). Evaluation of tickGARD(PLUS), a novel vaccine against Boophilus microplus, in lactating Holstein-Friesian cows. Vet. Parasitol. 88, 275-285. 10.1016/S0304-4017(99)00213-7 [DOI] [PubMed] [Google Scholar]
- Kobylinski K. C., Deus K. M., Butters M. P., Hongyu T., Gray M., da Silva I. M., Sylla M. and Foy B. D. (2010). The effect of oral anthelmintics on the survivorship and re-feeding frequency of anthropophilic mosquito disease vectors. Acta Tropica 116, 119-126. 10.1016/j.actatropica.2010.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobylinski K. C., Sylla M., Chapman P. L., Sarr M. D. and Foy B. D. (2011). Ivermectin mass drug administration to humans disrupts malaria parasite transmission in Senegalese villages. Am. J. Trop. Med. Hyg. 85, 3-5. 10.4269/ajtmh.2011.11-0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal A. A., Patterson P. S., Sacci J. B., Vaughan J. A., Paul C., Collins W. E., Wirtz R. A. and Azad A. F. (2001). Anti-mosquito midgut antibodies block development of Plasmodium falciparum and Plasmodium vivax in multiple species of Anopheles mosquitoes and reduce vector fecundity and survivorship. Proc. Natl. Acad. Sci. USA 98, 5228-5233. 10.1073/pnas.091447398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lounibos L. P., Nishimura N., Conn J. and Lourenço-de-Oliveira R. (1995). Life history correlates of adult size in the malaria vector Anopheles darlingi. Mem. Inst. Oswaldo Cruz 90, 769-774. 10.1590/S0074-02761995000600020 [DOI] [PubMed] [Google Scholar]
- Lynagh T. and Lynch J. W. (2012). Ivermectin binding sites in human and invertebrate Cys-loop receptors. Trends Pharmacol. Sci. 33, 432-441. 10.1016/j.tips.2012.05.002 [DOI] [PubMed] [Google Scholar]
- Megy K., Emrich S. J., Lawson D., Campbell D., Dialynas E., Hughes D. S. T., Koscielny G., Louis C., MacCallum R. M., Redmond S. N. et al. (2012). VectorBase: improvements to a bioinformatics resource for invertebrate vector genomics. Nucleic Acids Res. 40, D729-D734. 10.1093/nar/gkr1089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers J. I., Gray M., Kuklinski W., Johnson L. B., Snow C. D., Black W. C. IV, Partin K. M. and Foy B. D. (2015). Characterization of the target of ivermectin, the glutamate-gated chloride channel, from Anopheles gambiae. J. Exp. Biol. 218, 1478-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noden B. H., Vaughan J. A., Imbrahim M. S. and Beier J. C. (1995). An immunological factor that effects Anopheles gambiae survival. J. Am. Mosq. Contr Assn. 11, 45-49. [PubMed] [Google Scholar]
- Norris L. C. and Norris D. E. (2011). Insecticide resistance in Culex quinquefasciatus mosquitoes after the introduction of insecticide-treated bed nets in Macha, Zambia. J. Vector Ecol. 36, 411-420. 10.1111/j.1948-7134.2011.00182.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasamy M. S., Ramasamy R., Kay B. H. and Kidson C. (1988). Anti-mosquito antibodies decrease the reproductive capacity of Aedes aegypti. Med. Vet. Entomol. 2, 87-93. 10.1111/j.1365-2915.1988.tb00053.x [DOI] [PubMed] [Google Scholar]
- Ranson H., N'Guessan R., Lines J., Moiroux N., Nkuni Z. and Corbel V. (2011). Pyrethroid resistance in African Anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 27, 91-98. 10.1016/j.pt.2010.08.004 [DOI] [PubMed] [Google Scholar]
- Saavedra-Rodriguez K., Urdaneta-Marquez L., Rajatileka S., Moulton M., Flores A. E., Fernandez-Salas I., Bisset J., Rodriguez M., McCall P. J., Donnelly M. J. et al. (2007). A mutation in the voltage-gated sodium channel gene associated with pyrethroid resistance in Latin American Aedes aegypti. Insect Mol. Biol. 16, 785-798. 10.1111/j.1365-2583.2007.00774.x [DOI] [PubMed] [Google Scholar]
- Scott T. W., Githeko A. K., Fleisher A., Harrington L. C. and Yan G. (2006). DNA profiling of human blood in anophelines from lowland and highland sites in Western Kenya. Am. J. Trop. Med. Hyg. 75, 231-237. [PubMed] [Google Scholar]
- Shawler D. L., Bartholomew R. M., Smith L. M. and Dillman R. O. (1985). Human immune response to multiple injections of murine monoclonal IgG. J. Immunol. 135, 1530-1535. [PubMed] [Google Scholar]
- Singh K. R. and Paul S. D. (1969). Isolation of Dengue viruses in Aedes albopictus cell cultures. Bull. World Health Organ. 40, 982-983. [PMC free article] [PubMed] [Google Scholar]
- Srikrishnaraj K. A., Ramasamy R. and Ramasamy M. S. (1993). Fecundity of Anopheles tessellatus reduced by the ingestion of murine anti-mosquito antibodies. Med. Vet. Entomol. 7, 66-68. 10.1111/j.1365-2915.1993.tb00653.x [DOI] [PubMed] [Google Scholar]
- Stoute J. A., Slaoui M., Heppner D. G., Momin P., Kester K. E., Desmons P., Wellde B. T., Garçon N., Krzych U., Marchand M. et al. (1997). A preliminary evaluation of a recombinant circumsporozoite protein vaccine against plasmodium falciparum malaria. N. Engl. J. Med. 336, 86-91. 10.1056/NEJM199701093360202 [DOI] [PubMed] [Google Scholar]
- Sylla M., Kobylinski K. C., Gray M., Chapman P. L., Sarr M. D., Rasgon J. L. and Foy B. D. (2010). Mass drug administration of ivermectin in south-eastern Senegal reduces the survivorship of wild-caught, blood fed malaria vectors. Malar. J. 9, 365 10.1186/1475-2875-9-365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaughan J. A. and Azad A. F. (1988). Passage of host immunoglobulin G from blood meal into hemolymph of selected mosquito species (Diptera: Culicidae). J. Med. Entomol. 25, 472-474. 10.1093/jmedent/25.6.472 [DOI] [PubMed] [Google Scholar]
- Vaughan J. A., Wirtz R. A., do Rosario V. E. and Azad A. F. (1990). Quantitation of antisporozoite immunoglobulins in the hemolymph of Anopheles stephensi after bloodfeeding. Am. J. Trop. Med. Hyg. 42, 10-16. [DOI] [PubMed] [Google Scholar]
- Vernino S., Lindstrom J., Hopkins S., Wang Z. and Low P. A. (2008). Characterization of ganglionic acetylcholine receptor autoantibodies. J. Neuroimmunol. 197, 63-69. 10.1016/j.jneuroim.2008.03.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Low P. A., Jordan J., Freeman R., Gibbons C. H., Schroeder C., Sandroni P. and Vernino S. (2007). Autoimmune autonomic ganglionopathy: IgG effects on ganglionic acetylcholine receptor current. Neurology 68, 1917-1921. 10.1212/01.wnl.0000263185.30294.61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber F., Rudel R., Aulkemeyer P. and Brinkmeier H. (2000). Anti-GM1 antibodies can block neuronal voltage-gated sodium channels. Muscle Nerve 23, 1414-1420. [DOI] [PubMed] [Google Scholar]
- Willadsen P. and Billingsley P. F. (1996). Immune intervention against blood-feeding insects. In Biology of the Insect Midgut (ed. Lehane M. J. and Billingsley P. F.), pp. 323-344. London: Chapman & Hall. [Google Scholar]
- Zlotkin E. (1999). The insect voltage-gated sodium channel as target of insecticides. Annu. Rev. Entomol. 44, 429-455. 10.1146/annurev.ento.44.1.429 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.