Abstract
Background
Sleep disturbance is bi-directionally related to mood de-stabilization in bipolar disorder (BD), and sleep quality differs in men and women. We aimed to determine whether perception of poor sleep quality would have a different effect on mood outcome in men versus women.
Methods
We assessed association between sleep quality (Pittsburgh Sleep Quality Index (PSQI)) at study intake and mood outcome over 2 years in subjects from the Prechter Longitudinal Study of Bipolar Disorder (N=216; 29.6% males). The main outcome measure was the severity, variability, and frequency of mood episodes measured by self-report over 2 years of follow-up. Multivariable linear regression models stratified by sex examined the relationship between PSQI with mood outcomes, while age, stressful life events, mood state and neuroticism at baseline were controlled.
Results
In women, poor sleep quality at baseline predicted increased severity (B=0.28, p<0.001) and frequency of episodes (B=0.32, p<0.001) of depression, and poor sleep quality was a stronger predictor than baseline depression; poor sleep quality predicted increased severity (B=0.19, p<0.05) and variability (B=0.20, p<0.05) of mania, and frequency of mixed episodes (B=0.27, p<0.01). In men, baseline depression and neuroticism were stronger predictors of mood outcome compared to poor sleep quality.
Limitations
We measured perception of sleep quality, but not objective changes in sleep.
Conclusions
In a longitudinal study of BD, women reported poorer perceived sleep quality than men, and poor sleep quality predicted worse mood outcome in BD. Clinicians should be sensitive to addressing sleep complaints in women with BD early in treatment to improve outcome in BD.
Keywords: bipolar disorder, sleep, mood, depression, mania, sex
Introduction
Bipolar disorder (BD) is a recurrent, disabling illness. Though BD is an episodic illness, individuals with BD suffer from depressive symptoms up to 32% of the time, and manic symptoms about 9% of the time (Judd, Akiskal et al. 2002). Subsyndromal and mixed symptoms are prevalent, and contribute to restricting the effort of individuals with BD to achieve life goals in areas such as education, occupation, and personal relationships (Judd, Akiskal et al. 2005).
Poor sleep quality is a state marker and symptom of depressive and manic episodes (Goodwin and Jamison 2007). Lack of sleep can incite mania, and sleep deprivation has been shown since the 1970s to treat depressions (Wu and Bunney 1990, Jackson, Cavanagh et al. 2003), especially in bipolar disorder (Barbini, Colombo et al. 1998, Colombo, Benedetti et al. 1999). Insomnia and poor sleep quality has been linked to worse symptom severity and poor outcome in bipolar disorder (Wu and Bunney 1990, Colombo, Benedetti et al. 1999, Bauer, Grof et al. 2006, Perlman, Johnson et al. 2006, Gruber, Harvey et al. 2009, Gruber, Miklowitz et al. 2011). Also, sleep-disordered breathing, primary insomnia and sleep phase disorders are often comorbid with bipolar disorder, begging the question of these pathologies being related to the underlying mood pathology (Kripke, Mullaney et al. 1978, Wehr 1992, Harvey 2008, Soehner, Kaplan et al. 2013, Naqvi, Wang et al. 2014).
Rates and phenotypes of mood disorders differ between men and women. Women of reproductive age were more prone to depression in the Stanley Foundation bipolar disorder cohort (Altshuler, Kupka et al. 2010), but not in the STEP-BD study (Baldassano, Marangell et al. 2005). In addition, the comorbidity of BD with illnesses that present with gender differences (including anxiety disorders (Baldassano, Marangell et al. 2005, Baldassano 2006, Altshuler, Kupka et al. 2010, Saunders, Fitzgerald et al. 2012), migraine (Fasmer 2001, Low, Cui et al. 2007, Baptista, Uzcategui et al. 2012, Saunders, Nazir et al. 2014), and eating disorders (Baldassano, Marangell et al. 2005, Baldassano 2006, Jen 2013)) has been shown to cause more depression and worse course of illness in BD. Women in the general population report more insomnia than men during the reproductive years at a ratio of 1.4:1.0, (Ohayon 2002, Zhang and Wing 2006, Phillips, Collop et al. 2008, Fernandez-Mendoza, Vgontzas et al. 2012, Singareddy, Vgontzas et al. 2012, Vgontzas, Fernandez-Mendoza et al. 2012), and persistence of insomnia has been associated with depressive disorders, as well as sleep misperception (Fernandez-Mendoza, Calhoun et al. 2011, Fernandez-Mendoza, Vgontzas et al. 2012).
Sleep and gender are both important factors in influencing course of illness in bipolar disorder (Baldassano 2006, Gruber, Harvey et al. 2009, Eidelman, Talbot et al. 2010, Gruber, Miklowitz et al. 2011, Saunders, Fitzgerald et al. 2012, Saunders, Nazir et al. 2014). Women in the general population have more insomnia than men and women are also more prone to mood disorders and different courses of illness in BD. We investigated the relationship between perceived sleep quality and mood outcome in a cohort of patients with BD that were deeply-phenotyped and followed prospectively for two years. We hypothesized women would be more sensitive to the effect of poor sleep quality on mood outcome, and that sleep would differentially affect mood outcome in men and women with bipolar disorder.
Methods
Participants
The Prechter Longitudinal Study of Bipolar Disorder at the University of Michigan (UM) is an IRB approved observational study of outcomes in bipolar disorder (IRBMED HUM000606). Participants in the Prechter Longitudinal Study were evaluated initially for a baseline and study intake assessment described below and followed long-term with self-reported questionnaires and yearly visits. Patients with BD (bipolar disorder type I, bipolar disorder type II, schizoaffective disorder, bipolar type or bipolar disorder NOS, Table 1) and healthy controls (HC) with no personal or family history of mood or psychotic disorders were included.
Table 1.
Description of the sample
| ALL (N=216) Mean (SD) |
Male (N=64) Mean (SD) |
Female (N=152) Mean (SD) |
P | |
|---|---|---|---|---|
| Age | 40.25 (12.47) | 41.61 (11.80) | 39.67 (12.74) | 0.30 |
| Body Mass Index | 29.17 (6.90) | 29.43 (5.47) | 29.06 (7.46) | 0.69 |
| BPI | 152 (70%) | 50 (78%) | 102 (67%) | 0.34 |
| BPII | 44 (20%) | 11 (17%) | 33 (22%) | |
| BP NOS | 15 (7%) | 2 (3%) | 13 (9%) | |
| Schizoaffective, BP | 5 (3%) | 1 (2%) | 4 (3%) | |
| Caucasian | 179 (83%) | 57 (90%) | 122 (80%) | 0.42 |
| African-American | 10 (5%) | 3 (5%) | 7 (5%) | |
| Asian | 3 (1%) | 1 (2%) | 2 (1%) | |
| More than one race | 11 (5%) | 1 (2%) | 10 (7%) | |
| Missing | 2 (3%) | 11 (7%) | ||
| Hispanic or Latino | 5 (2%) | 1 (2%) | 4 (3%) | 0.10 |
| Not Hispanic or Latino | 196 (91%) | 59 (92%) | 137 (90%) | |
| Missing | 15 (7%) | 4 (6%) | 11 (7%) | |
| Married | 87 (40%) | 34 (53%) | 53 (35%) | 0.02 |
| Never married | 76 (35%) | 21 (33%) | 55 (36%) | |
| Divorced | 45 (21%) | 7 (11%) | 38 (25%) | |
| Separated | 5 (2%) | 0 (0) | 5 (3%) | |
| Widowed | 3 (1%) | 2 (3%) | 1 (1%) | |
| Employed/student | 129 (60%) | 44 (69%) | 85 (56%) | 0.08 |
| Not employed/disabled | 68 (30%) | 15 (23%) | 53 (35%) | |
| Menopause | N/A | 41 (27%) | ||
| Sleep Quality (PSQI) | 8.45 (4.24) | 8.08 (4.04) | 8.61 (4.32) | 0.40 |
| Stressful events (LEOS) | 1.93 (2.34) | 1.84 (2.51) | 1.98 (2.27) | 0.70 |
| Baseline Depression (HDRS-21 with AT) | 13.62 (11.77) | 11.61 (10.51) | 14.46 (12.19) | 0.10 |
| Baseline Mania (YMRS) | 2.82 (4.13) | 2.72 (4.05) | 2.87 (4.17) | 0.81 |
| Neuroticism | 63.28 (14.41) | 62.97 (13.94) | 63.41 (14.65) | 0.84 |
| Follow-up Depression severity (PHQ-9) | 15.63 (7.23) | 13.77 (7.23) | 16.42 (7.11) | 0.01 |
| Follow-up Depression variability (PHQ-9) | 4.25 (2.42) | 3.99 (2.54) | 4.36 (2.37) | 0.30 |
| Follow-up Depression frequency (PHQ-9) | 0.63 (0.35) | 0.53 (0.37) | 0.68 (0.33) | 5×10−2 |
| Follow-up Mania severity (ASRM) | 7.94 (4.45) | 7.16 (4.20) | 8.27 (4.53) | 0.09 |
| Follow-up Mania variability (ASRM) | 2.56 (1.53) | 2.24 (1.36) | 2.70 (1.58) | 0.04 |
| Follow-up Mania frequency (ASRM) | 0.25 (0.27) | 0.26 (0.29) | 0.24 (0.26) | 0.58 |
| Follow-up Mixed frequency (ASRM) | 0.47 (0.50) | 0.14 (0.22) | 0.15 (0.20) | 0.61 |
Analytic cohort
The clinical sample for this investigation included participants recruited at the University of Michigan between 2005 and 2010, and data were extracted in 2/2012. Inclusion criteria include: diagnosis of BD; exclusion criteria include: mental retardation, active substance dependence, head injury or medical illness causing BD. The cohort selected for analysis included patients with DSM-IV TR BD I, II, NOS or Schizoaffective disorder, bipolar type (n=216) who completed the Pittsburgh Sleep Quality Index (PSQI) at baseline. Participants were excluded in the current analysis if they did not have complete data on the PSQI (n=75).
Process
At baseline, clinicians administered the Diagnostic Interview for Genetic Studies (DIGS) (Nurnberger, Blehar et al. 1994), Hamilton Depression Rating Scale – 21 with Atypical (HDRS) (Hamilton 1960) and Young Mania Rating Scale (YMRS) (Young, Biggs et al. 1978), and recorded height and weight. The DIGS is a clinician-administered diagnostic interview that collects self-reported demographic data including race and ethnicity, and includes the ability to assess the lifetime course of mood disorder as well as associated features of mood illness of the individual, and a comprehensive assessment of psychiatric and medical illnesses. Physicians, psychologists, and masters-level mental health professionals completed standardized training in the study instruments, and a best-estimate procedure was used to verify diagnoses (Leckman, Sholomskas et al. 1982). A baseline set of self-rating questionnaires was completed. Follow-up questionnaires including the Patient Health Questionnaire (PHQ-9) and the Altman Self-Rating Mania Scale (ASRM) were sent to participants every two months. Median number of follow-up questionnaires completed in the 24-month period was 9.0 for both PHQ-9 and ASRM (minimum=2, maximum=13).
Main Outcomes
Our dependent variable of interest was mood outcome over time, characterized by severity, variability, and frequency of clinically significant symptoms of depression or mania through the duration of a two-year follow-up period. Severity of depression was defined for each individual by the maximum PHQ-9 score over the follow up period (maximum = 27.00, minimum = 0); severity of mania was defined for each individual by the maximum ASRM score during follow up (maximum = 20.00, minimum = 0). Variability of depression was defined for each individual by the standard deviation in PHQ-9 scores over the duration of follow up (maximum = 12.73, minimum = 0); variability of mania was defined by the standard deviation in ASRM scores over the duration of follow-up (maximum = 10.31, minimum = 0). The proportion of the follow-up period that the individual had a PHQ-9 or ASRM score over 5 defined the frequency of clinically significant depressive, manic or mixed symptoms (maximum = 1.00, minimum = 0).
We assessed sleep quality with the Pittsburgh Sleep Quality Inventory (PSQI)(Buysse, Reynolds et al. 1989). The PSQI obtains a general measure of sleep quality and the total score is derived from seven subscales: sleep quality, sleep latency, sleep duration, sleep efficiency, sleep disturbance, sleep medication, and daytime dysfunction. The Life Events Occurrence Survey (LEOS) assesses for stressful life events in the past 6-month period (McKee SA 2005). The NEO PI is a dimensional measure of personality, which includes Neuroticism, defined as the tendency to experience negative valence emotions including depression and anxiety (Costa and McCrae 1992, McCrae and Costa 2003).
Data and statistical analysis
Values of continuous variables were compared between males and females using two-sample t-tests, and categorical variables were compared using the Pearson chi-square test. All independent variables were standardized, and standardized regression coefficients are reported. Initial models were created to include only PSQI score, gender and the interaction term, which was not significant for any model. Linear multivariate regression models stratified by sex were created to determine the influence of PSQI on mood outcome, accounting for covariates of interest including age, baseline mood symptoms, and recent stressful events and neuroticism. Stress and neuroticism were included because they have been found at baseline to correlate with poor sleep quality independent of mood (Saunders 2013). Models were run on all women, and repeated for all women reported to not be in menopause.
Results
Demographic description of the sample (Table 1)
We followed 216 individuals with BD for 2 years. The majority of the patients were female (152/216, 70%), the average age was 40 years old with a BMI of 29. Age and BMI did not differ between males and females. The majority of both men and women had BPI disorder and were Caucasian. The rates of marriage differed between men and women. About one-quarter of the women had undergone menopause. During the two-year follow-up period, women had greater severity and frequency of depression, and severity and variability of mania. Males and females did not differ by age, sleep quality, stressful life events, baseline depression, baseline mania, or neuroticism.
Gender and Outcome
Because we were interested in the differential impact of outcome by gender, we tested the interaction term for each outcome variable. No interaction terms were significant (data not shown).
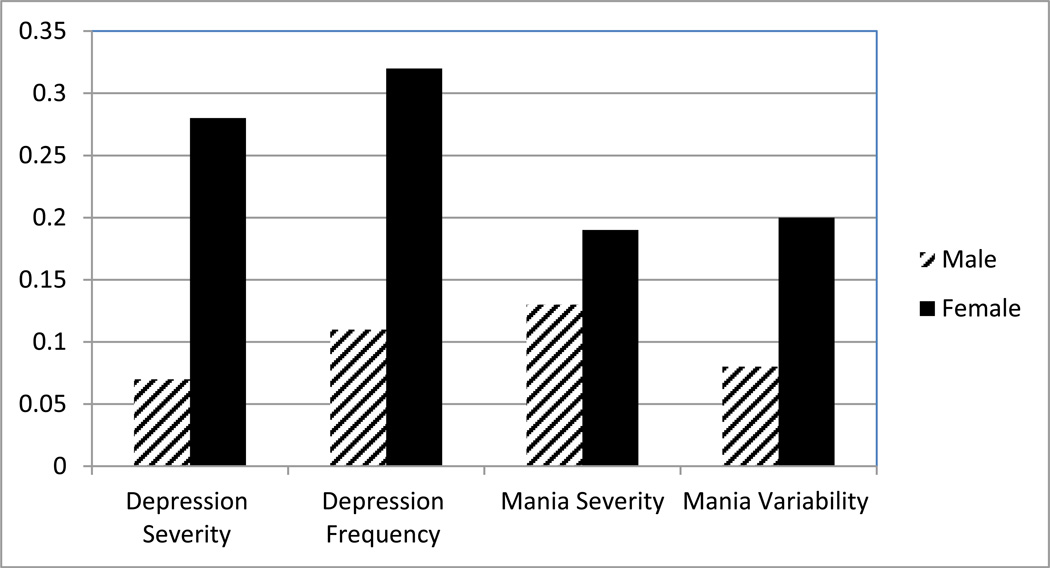
We then tested three clinically-relevant dimensions of the course of mood illness for depression and mania, including severity of symptoms, frequency of symptoms and variability of symptoms each for mania and depression As covariates, we included factors known to effect mood outcome, including age, baseline depressive and manic symptoms. In addition, we included a measure of stress and neuroticism, both factors that were shown to predict poor sleep quality at baseline (Saunders 2013). We ran each model in men and women separately to identify the effect of poor sleep in each group (Figure 1).
Figure 1.
The Effect of Poor Sleep Quality on Mood Outcome over 2 years Differs in Men and Women. Standardize betas presented for multivariable models including sex, age, stressful events, baseline mania, baseline depression, and neuroticism.
Depression (Table 2 & 3; Supplemental Table 1)
Table 2.
Depression Severity Outcome
| Model | Sex | Age | Poor sleep |
Stressful Events |
Baseline Mania |
Baseline Depression |
Neuroticism | Adj R2 |
|---|---|---|---|---|---|---|---|---|
| ALL | ||||||||
| 1 | 0.17* | 0.024 | ||||||
| 2 | 0.17* | 0.06 | 0.023 | |||||
| 3 | 0.14* | 0.06 | 0.48*** | 0.252 | ||||
| 4 | 0.14* | 0.06 | 0.47*** | 0.06 | 0.252 | |||
| 5 | 0.14* | 0.06 | 0.45*** | 0.04 | 0.15* | 0.270 | ||
| 6 | 0.12* | 0.05 | 0.31*** | 0.06 | 0.12* | 0.27*** | 0.319 | |
| 7 | 0.13* | 0.09 | 0.23*** | 0.29 | 0.12* | 0.18** | 0.29*** | 0.380 |
| Male | ||||||||
| 1 | <0.01 | −0.016 | ||||||
| 2 | −0.01 | 0.46*** | 0.182 | |||||
| 3 | >−0.01 | 0.46*** | −0.02 | 0.169 | ||||
| 4 | −0.01 | 0.43** | −0.03 | 0.17 | 0.184 | |||
| 5 | 0.06 | 0.15 | −0.3 | 0.23* | 0.50*** | 0.347 | ||
| 6 | 0.09 | 0.07 | 0.01 | 0.22* | 0.40** | 0.23 | 0.369 | |
| Female | ||||||||
| 1 | 0.09 | 0.001 | ||||||
| 2 | 0.08 | 0.50*** | 0.247 | |||||
| 3 | 0.09 | 0.48*** | 0.10 | 0.265 | ||||
| 4 | 0.09 | 0.46*** | 0.07 | 0.14 | 0.285 | |||
| 5 | 0.07 | 0.36*** | 0.08 | 0.10 | 0.21* | 0.315 | ||
| 6 | 0.10 | 0.28*** | 0.08 | 0.11 | 0.13 | 0.30*** | 0.384 |
p=<0.05
p=<0.01
p=<0.001
Table 3.
Depression Frequency
| Model | Sex | Age | Poor sleep | Stressful Events |
Baseline Mania |
Baseline Depression |
Neuroticism | Adj R2 |
|---|---|---|---|---|---|---|---|---|
| ALL | ||||||||
| 1 | 0.19** | 0.031 | ||||||
| 2 | 0.19** | 0.05 | 0.030 | |||||
| 3 | 0.16** | 0.05 | 0.51*** | 0.291 | ||||
| 4 | 0.16** | 0.05 | 0.51*** | −<0.01 | 0.287 | |||
| 5 | 0.16** | 0.05 | 0.50*** | −0.02 | 0.09 | 0.292 | ||
| 6 | 0.14* | 0.04 | 0.37*** | −0.01 | 0.06 | 0.27*** | 0.344 | |
| 7 | 0.15* | 0.07 | 0.28*** | −0.01 | 0.06 | 0.19* | 0.31*** | 0.412 |
| Male | ||||||||
| 1 | 0.21 | 0.028 | ||||||
| 2 | 0.20 | 0.42*** | 0.196 | |||||
| 3 | 0.19 | 0.45*** | −0.08 | 0.188 | ||||
| 4 | 0.19 | 0.42** | −0.09 | 0.17 | 0.204 | |||
| 5 | 0.25* | 0.19 | −0.09 | 0.22* | 0.40* | 0.306 | ||
| 6 | 0.27* | 0.11 | 0.05 | 0.21 | 0.30* | 0.23 | 0.327 | |
| Female | ||||||||
| 1 | −0.01 | −0.01 | ||||||
| 2 | −0.02 | 0.57*** | 0.311 | |||||
| 3 | −0.01 | 0.56*** | 0.03 | 0.307 | ||||
| 4 | −0.01 | 0.55*** | 0.02 | 0.05 | 0.305 | |||
| 5 | −0.04 | 0.41*** | 0.05 | −0.01 | 0.30*** | 0.365 | ||
| 6 | −0.01 | 0.32*** | 0.04 | 0.004 | 0.21** | 0.33*** | 0.449 |
Depression severity and frequency were predicted by poor sleep in women but not in men. In men, depression severity was predicted by poor sleep until baseline mood symptoms were included, which had a stronger effect than sleep. The most significant predictors of depression frequency in men were baseline depression, baseline mania, and age. In women, the effect of poor sleep remained significant despite inclusion of baseline depression and neuroticism, which each accounted for some of the variance in depression frequency. Depression variability had no gender differential, and poor sleep predicted depression variability until Neuroticism was included, which has a stronger effect.
Mania (Table 4; Supplemental Tables 2 & 3)
Table 4.
Mania Severity
| Model | Sex | Age | Poor sleep | Stressful Events |
Baseline Mania |
Baseline Depression |
Neuroticism | Adj R2 |
|---|---|---|---|---|---|---|---|---|
| ALL | ||||||||
| 1 | 0.11 | 0.008 | ||||||
| 2 | 0.13 | 0.15* | 0.025 | |||||
| 3 | 0.11 | 0.15* | 0.21* | 0.064 | ||||
| 4 | 0.11 | 0.16* | 0.18* | 0.11 | 0.070 | |||
| 5 | 0.11 | 0.16* | 0.16* | 0.08 | 0.19* | 0.101 | ||
| 6 | 0.12 | 0.16* | 0.22* | 0.07 | 0.21* | −0.14 | 0.110 | |
| 7 | 0.13 | 0.18* | 0.17* | 0.08 | 0.21* | −0.18* | 0.16* | 0.126 |
| Male | ||||||||
| 1 | 0.10 | −0.007 | ||||||
| 2 | 0.10 | 0.21 | 0.023 | |||||
| 3 | 0.10 | 0.21 | 0.003 | 0.006 | ||||
| 4 | 0.09 | 0.19 | −0.003 | 0.10 | 0.000 | |||
| 5 | 0.07 | 0.30 | −0.01 | 0.08 | −0.18 | 0.007 | ||
| 6 | 0.12 | 0.13 | 0.07 | 0.04 | −0.39* | 0.48* | 0.135 | |
| Female | ||||||||
| 1 | 0.17* | 0.021 | ||||||
| 2 | 0.16* | 0.21* | 0.057 | |||||
| 3 | 0.18* | 0.18* | 0.15 | 0.072 | ||||
| 4 | 0.18* | 0.15 | 0.11 | 0.22* | 0.114 | |||
| 5 | 0.20* | 0.22* | 0.10 | 0.25* | −0.15 | 0.124 | ||
| 6 | 0.21* | 0.19* | 0.10 | 0.26* | −0.17 | 0.09 | 0.124 |
For women, poor sleep predicted mania severity; however baseline mania and age were stronger factors. Poor sleep was not a predictor of mania severity in men, and the only significant predictors were baseline depression and neuroticism. For women, poor sleep was a predictor of variability manic symptoms, however for men, the only predictors of mania variability were lower baseline depression and neuroticism. Poor sleep was not a predictor of mania frequency, however lower baseline depression scores, baseline mania, and age were predictors.
Mixed (Supplemental Table 4)
The frequency of having clinically-significant mixed symptoms was predicted by poor sleep and baseline manic symptoms in women, but not in men.
Exclusion of women who have completed menopause leads to similar findings in depression outcome variables, however PSQI was no longer a significant predictor of mania variability and frequency.
Discussion
We found that poor sleep quality at baseline prospectively predicted poor mood outcome in bipolar disorder above and beyond baseline depression in women, but not in men. Women had increased depression severity and frequency, increased mania severity and variability, and increased frequency of mixed episodes if, at the intake baseline, sleep was of poor quality. Why would we see an effect of sex in the relationship of quality of sleep to outcome in bipolar disorder? Sleep abnormalities have been linked to poor outcome in bipolar disorder: in a one-year follow-up study from the STEP-BD cohort, Gruber et al. found lower total sleep time was associated with higher mania scores, but a relationship of total sleep time to depression scores was not found, however sleep variability was associated with depression scores over time (Gruber, Miklowitz et al. 2011). Sex differences, including an increased prevalence of BPII in women, has been shown in the STEP-BD (Baldassano, Marangell et al., 2005). A study in the Stanley Foundation Bipolar Treatment Outcome Network showed women spent a higher proportion of visits in a depressive episode than men (Altshuler, Kupka et al., 2010). Findings from our study corroborate the findings from the Altshuler et al. study and suggest that poor sleep quality may influence longitudinal depressive symptoms in women.
Women in the general population are more likely to report insomnia than men, and the subjective sleep complaints have been suggested to be more associated with psychological distress than with physiological consequences of objective sleep loss (Ohayon 2002, Zhang and Wing 2006, Phillips, Collop et al. 2008, Fernandez-Mendoza, Calhoun et al. 2011, Fernandez-Mendoza, Vgontzas et al. 2012, Singareddy, Vgontzas et al. 2012, Vgontzas, Fernandez-Mendoza et al. 2012). In adolescents, insomnia and daytime sleepiness were associated with anxiety and depression and not with objective sleep disturbance (Calhoun, Vgontzas et al. 2011). While subjective sleep is poor in BD as noted above, BD is also associated with objective sleep disturbance. Studies in euthymic BP subjects using actigraphy, an objective measurement of sleep disturbance, have shown decreased sleep efficiency (Harvey, Schmidt et al. 2005), more variability in circadian activity (Jones, Hare et al. 2005), longer sleep onset latency, longer sleep duration and variability of sleep duration and less day-to-day stability relative to comparison subjects (Millar, Espie et al. 2004), demonstrating that sleep abnormalities are present not only in mood episodes, but at baseline euthymia as well.
While women in general report more subjective sleep complaints than men, studies suggest healthy women of reproductive age without sleep complaints objectively sleep better than men and have more resilience to effect of sleep-loss induced cytokines (Vgontzas, Zoumakis et al. 2004). Healthy women of reproductive age have been shown to sleep physiologically better (Redline, Kirchner et al. 2004, Walsleben, Kapur et al. 2004, Bixler, Papaliaga et al. 2009), and have some protection against disruption of sleep by external awakenings (Bixler, Papaliaga et al. 2009). A robust ability to survive sleep interruption and deprivation during the phase of life in which a woman may care for an infant may be a result of evolutionary adaption. If a hormonal influence on sleep is protective and adaptive, we would hypothesize that disruption of the sleep-wake regulation system in women would require a stronger stimulus than to disrupt the sleep-wake system in men. Therefore, if mood disorder is disrupting sleep in women, and we are detecting mood disorder outcome, we would expect to detect worse outcome in women because the threshold for disrupting the sleep regulation system is higher. However, unipolar depression is more prevalent in reproductive-aged women than in men (Kessler, McGonagle et al. 1993, Kessler, Berglund et al. 2005), which may counter this hypothesis or indicate that systems other than the interaction with sleep are at play.
These objective sleep differences in BD between men and women are likely to have several driving biological mechanisms. The circadian clock has long been hypothesized to be altered in bipolar disorder (Georgi 1947, Kripke, Mullaney et al. 1978, Wehr, Goodwin et al. 1982, Nievergelt, Kripke et al. 2005, Nievergelt, Kripke et al. 2006, Roybal, Theobold et al. 2007), and may be altered by ovarian hormones (Murphy, Pezuk et al. 2013, Bailey and Silver 2014). Alterations in the sleep-wake system as a part of the genetic and neurobiological differences that predispose to bipolar disorder may alter the way in which ovarian hormones interact with the circadian system, thus altering the phenotype of sleep in bipolar disorder. The sex differences and altered sleep provide powerful opportunities to study dynamic patterns in related biological mechanisms asking specific questions aimed towards understanding perturbations of the circadian patterns.
Limitations
The measurements used in this study are self-report of sleep quality. The PSQI is a well-validated, reliable instrument for measuring self-reported quality of sleep, and is valid for capturing accurate reports of sleep for up to one month (Buysse, Reynolds et al. 1989, Broderick, Junghaenel et al. 2013). However, we do not have objective sleep data to correlate with subjective report. Thus the sex difference in sleep quality may be due to a difference in perception of sleep quality rather than underlying differences in sleep architecture. Women with BD may perceive poor sleep quality due to higher sensitivity to negative internal states (Fernandez-Mendoza, Calhoun et al. 2011). Objective measures of sleep and activity levels such as actigraphy or polysomnography will quantify these parameters but require substantial resources. Detailed and repetitive sleep studies in variable mood states would be ideal.
Conclusion
Sleep quality differentially affected mood outcome in bipolar disorder by sex. Baseline sleep quality predicted worse mood outcome in women. These data suggest that particular attention to sleep quality in BD women during clinical treatment would be beneficial. Subjective and biological factors may be involved, and further studies of the hormonal influence on the sleep-wake cycle may elucidate the relationship between sex differences in sleep and mood in BD.
Supplementary Material
Acknowledgements
We would like to thank the participants of the Prechter Bipolar Research Studies for dedicating time and effort to the study of bipolar disorder, and the dedicated research team of the Prechter Bipolar Group for support for this work.
Sources of funding: We would like to thank Mrs. Heinz C. Prechter and the Prechter Bipolar Research Fund for generously supporting this research. In addition, EFHS was supported by the National Center for Research Resources, GrantKL2 RR033180, and is now at the National Center for Advancing Translational Sciences, Grant KL2 TR000126. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Role of funders: The sponsors of this research did not have direct influence over the collection, analysis or interpretation of data.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions: EFHS was responsible for the idea, study design and wrote the first draft of the paper. EFHS had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. JFM, MK, SA and MM participated in interpretation of data and reviewed the manuscript.
Author access to data: The Heinz C. Prechter Bipolar Research Fund supported the collection of the data for the Prechter Longitudinal Study of Bipolar Disorder and the Prechter Bipolar Genetic Repository. The data reside at the University of Michigan, and can be requested through inquiries addressed to Dr. Melvin G. McInnis, MD, Department of Psychiatry, University of Michigan School of Medicine, Ann Arbor, MI, 48109.
Potential Conflicts of Interest: Disclaimer: E. Saunders–Projects in Knowledge CME (consultant); M. McInnis – Merck Pharmaceuticals (Honoraria for Speaker’s bureau); M Kamali has received research grant support from Janssen Pharmaceutical and Assurex Health.
Prior presentation of data: These data have not been presented elsewhere.
Contributor Information
Julio Fernandez-Mendoza, Email: jfernandezmendoza@hmc.psu.edu.
Masoud Kamali, Email: masoud@med.umich.edu.
Shervin Assari, Email: assari@umich.edu.
Melvin G. McInnis, Email: mmcinnis@umich.edu.
Reference
- Altshuler LL, Kupka RW, Hellemann G, Frye MA, Sugar CA, McElroy SL, Nolen WA, Grunze H, Leverich GS, Keck PE, Zermeno M, Post RM, Suppes T. Gender and depressive symptoms in 711 patients with bipolar disorder evaluated prospectively in the Stanley Foundation bipolar treatment outcome network. Am J Psychiatry. 2010;167(6):708–715. doi: 10.1176/appi.ajp.2009.09010105. [DOI] [PubMed] [Google Scholar]
- Bailey M, Silver R. Sex differences in circadian timing systems: implications for disease. Front Neuroendocrinol. 2014;35(1):111–139. doi: 10.1016/j.yfrne.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldassano CF. Illness course, comorbidity, gender, and suicidality in patients with bipolar disorder. The Journal of clinical psychiatry. 2006;67(Suppl 11):8–11. Journal Article. [PubMed] [Google Scholar]
- Baldassano CF, Marangell LB, Gyulai L, Nassir Ghaemi S, Joffe H, Kim DR, Sagduyu K, Truman CJ, Wisniewski SR, Sachs GS, Cohen LS. Gender differences in bipolar disorder: retrospective data from the first 500 STEP-BD participants. Bipolar disorders. 2005;7(5):465–470. doi: 10.1111/j.1399-5618.2005.00237.x. [DOI] [PubMed] [Google Scholar]
- Baptista T, Uzcategui E, Arape Y, Serrano A, Mazzarella X, Quiroz S, Ramirez CI, Padron de Freytez A. Migraine life-time prevalence in mental disorders: concurrent comparisons with first-degree relatives and the general population. Invest Clin. 2012;53(1):38–51. [PubMed] [Google Scholar]
- Barbini B, Colombo C, Benedetti F, Campori E, Bellodi L, Smeraldi E. The unipolar-bipolar dichotomy and the response to sleep deprivation. Psychiatry Res. 1998;79(1):43–50. doi: 10.1016/s0165-1781(98)00020-1. [DOI] [PubMed] [Google Scholar]
- Bauer M, Grof P, Rasgon N, Bschor T, Glenn T, Whybrow PC. Temporal relation between sleep and mood in patients with bipolar disorder. Bipolar Disord. 2006;8(2):160–167. doi: 10.1111/j.1399-5618.2006.00294.x. [DOI] [PubMed] [Google Scholar]
- Bixler EO, Papaliaga MN, Vgontzas AN, Lin HM, Pejovic S, Karataraki M, Vela-Bueno A, Chrousos GP. Women sleep objectively better than men and the sleep of young women is more resilient to external stressors: effects of age and menopause. J Sleep Res. 2009;18(2):221–228. doi: 10.1111/j.1365-2869.2008.00713.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broderick JE, Junghaenel DU, Schneider S, Pilosi JJ, Stone AA. Pittsburgh and Epworth sleep scale items: accuracy of ratings across different reporting periods. Behav Sleep Med. 2013;11(3):173–188. doi: 10.1080/15402002.2012.654549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2748771):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Calhoun SL, Vgontzas AN, Fernandez-Mendoza J, Mayes SD, Tsaoussoglou M, Basta M, Bixler EO. Prevalence and risk factors of excessive daytime sleepiness in a community sample of young children: the role of obesity, asthma, anxiety/depression, and sleep. Sleep. 2011;34(4):503–507. doi: 10.1093/sleep/34.4.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo C, Benedetti F, Barbini B, Campori E, Smeraldi E. Rate of switch from depression into mania after therapeutic sleep deprivation in bipolar depression. Psychiatry Res. 1999;86(3):267–270. doi: 10.1016/s0165-1781(99)00036-0. [DOI] [PubMed] [Google Scholar]
- Costa PT, Jr, McCrae RR. Revised NEO Personality Inventory (NEO PI-R) and NEO Five-Factor inventory (NEO-FFI) professional manual. Odessa, FL: Psychological Assessment Resources; 1992. [Google Scholar]
- Eidelman P, Talbot LS, Gruber J, Harvey AG. Sleep, illness course, and concurrent symptoms in inter-episode bipolar disorder. Journal of Behavior Therapy and Experimental Psychiatry. 2010;41(2):145–149. doi: 10.1016/j.jbtep.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasmer OB. The prevalence of migraine in patients with bipolar and unipolar affective disorders. Cephalalgia. 2001;21(9):894–899. doi: 10.1046/j.1468-2982.2001.00279.x. [DOI] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Calhoun SL, Bixler EO, Karataraki M, Liao D, Vela-Bueno A, Jose Ramos-Platon M, Sauder KA, Basta M, Vgontzas AN. Sleep misperception and chronic insomnia in the general population: role of objective sleep duration and psychological profiles. Psychosomatic medicine. 2011;73(1):88–97. doi: 10.1097/PSY.0b013e3181fe365a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Mendoza J, Vgontzas AN, Bixler EO, Singareddy R, Shaffer ML, Calhoun SL, Karataraki M, Vela-Bueno A, Liao D. Clinical and polysomnographic predictors of the natural history of poor sleep in the general population. Sleep. 2012;35(5):689–697. doi: 10.5665/sleep.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgi F. Psychophysische Korrelationen: III. Psychiatrische Probleme im Lichte der Rhythmusforschung. Schweiz Med Wochenschr. 1947;77(49):1276–1280. [PubMed] [Google Scholar]
- Goodwin FK, Jamison KR. Manic-Depressive Illness: Bipolar Disorders and Recurrent Depression. New York, NY: Oxford University Press; 2007. [Google Scholar]
- Gruber J, Harvey AG, Wang PW, Brooks JO, 3rd, Thase ME, Sachs GS, Ketter TA. Sleep functioning in relation to mood, function, and quality of life at entry to the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD) Journal of affective disorders. 2009;114(1–3):41–49. doi: 10.1016/j.jad.2008.06.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber J, Miklowitz DJ, Harvey AG, Frank E, Kupfer D, Thase ME, Sachs GS, Ketter TA. Sleep matters: sleep functioning and course of illness in bipolar disorder. J Affect Disord. 2011;134(1–3):416–420. doi: 10.1016/j.jad.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton M. A rating scale for depression. Journal of neurology, neurosurgery, and psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. Journal Article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harvey AG. Sleep and circadian rhythms in bipolar disorder: seeking synchrony, harmony, and regulation. Am J Psychiatry. 2008;165(7):820–829. doi: 10.1176/appi.ajp.2008.08010098. [DOI] [PubMed] [Google Scholar]
- Harvey AG, Schmidt DA, Scarna A, Semler CN, Goodwin GM. Sleep-related functioning in euthymic patients with bipolar disorder, patients with insomnia, and subjects without sleep problems. The American Journal of Psychiatry. 2005;162(1):50–57. doi: 10.1176/appi.ajp.162.1.50. [DOI] [PubMed] [Google Scholar]
- Jackson A, Cavanagh J, Scott J. A systematic review of manic and depressive prodromes. Journal of affective disorders. 2003;74(3):209–217. doi: 10.1016/s0165-0327(02)00266-5. [DOI] [PubMed] [Google Scholar]
- Jen A, Saunders EFH, Ornstein RM, Kamali M, McInnis MG. Impulsivity, anxiety, and alcohol misuse in bipolar disorder comorbid with eating disorders. International Journal of Bipolar Disorders. 2013;1(13):1–9. doi: 10.1186/2194-7511-1-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones SH, Hare DJ, Evershed K. Actigraphic assessment of circadian activity and sleep patterns in bipolar disorder. Bipolar Disord. 2005;7(2):176–186. doi: 10.1111/j.1399-5618.2005.00187.x. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Schettler PJ, Endicott J, Leon AC, Solomon DA, Coryell W, Maser JD, Keller MB. Psychosocial disability in the course of bipolar I and II disorders: a prospective, comparative, longitudinal study. Arch Gen Psychiatry. 2005;62(12):1322–1330. doi: 10.1001/archpsyc.62.12.1322. [DOI] [PubMed] [Google Scholar]
- Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, Leon AC, Rice JA, Keller MB. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Archives of General Psychiatry. 2002;59(6):530–537. doi: 10.1001/archpsyc.59.6.530. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62(6):593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Kessler RC, McGonagle KA, Swartz M, Blazer DG, Nelson CB. Sex and depression in the National Comorbidity Survey. I: Lifetime prevalence, chronicity and recurrence. J Affect Disord. 1993;29(2–3):85–96. doi: 10.1016/0165-0327(93)90026-g. [DOI] [PubMed] [Google Scholar]
- Kripke DF, Mullaney DJ, Atkinson M, Wolf S. Circadian rhythm disorders in manic-depressives. Biol Psychiatry. 1978;13(3):335–351. [PubMed] [Google Scholar]
- Leckman JF, Sholomskas D, Thompson WD, Belanger A, Weissman MM. Best estimate of lifetime psychiatric diagnosis: a methodological study. Archives of General Psychiatry. 1982;39(8):879–883. doi: 10.1001/archpsyc.1982.04290080001001. [DOI] [PubMed] [Google Scholar]
- Low NC, Cui L, Merikangas KR. Sex differences in the transmission of migraine. Cephalalgia. 2007;27(8):935–942. doi: 10.1111/j.1468-2982.2007.01378.x. [DOI] [PubMed] [Google Scholar]
- McCrae RR, Costa PT. Personality in adulthood : a five-factor theory perspective. New York: Guilford Press; 2003. [Google Scholar]
- McKee SAAM, Kochetkova A, Maciejewski P, O’Malley S, Krishnan-Sarin S, Mazure CM. A new measure for assessing the impact of stressful events on smoking behavior; Prague, CZ. Annual Meeting of the Society for Research on Nicotine and Tobacco; 2005. p. 16. [Google Scholar]
- Millar A, Espie CA, Scott J. The sleep of remitted bipolar outpatients: a controlled naturalistic study using actigraphy. Journal of affective disorders. 2004;80(2–3):145–153. doi: 10.1016/S0165-0327(03)00055-7. [DOI] [PubMed] [Google Scholar]
- Murphy ZC, Pezuk P, Menaker M, Sellix MT. Effects of ovarian hormones on internal circadian organization in rats. Biol Reprod. 2013;89(2):35. doi: 10.1095/biolreprod.113.109322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqvi HA, Wang D, Glozier N, Grunstein RR. Sleep-disordered breathing and psychiatric disorders. Curr Psychiatry Rep. 2014;16(12):519. doi: 10.1007/s11920-014-0519-z. [DOI] [PubMed] [Google Scholar]
- Nievergelt CM, Kripke DF, Barrett TB, Burg E, Remick RA, Sadovnick AD, McElroy SL, Keck PE, Jr, Schork NJ, Kelsoe JR. Suggestive evidence for association of the circadian genes PERIOD3 and ARNTL with bipolar disorder. American journal of medical genetics.Part B, Neuropsychiatric genetics : the official publication of the International Society of Psychiatric Genetics. 2006;141B(3):234–241. doi: 10.1002/ajmg.b.30252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievergelt CM, Kripke DF, Remick RA, Sadovnick AD, McElroy SL, Keck PE, Jr, Kelsoe JR. Examination of the clock gene Cryptochrome 1 in bipolar disorder: mutational analysis and absence of evidence for linkage or association. Psychiatric genetics. 2005;15(1):45–52. doi: 10.1097/00041444-200503000-00008. [DOI] [PubMed] [Google Scholar]
- Nurnberger JI, Jr, Blehar MC, Kaufmann CA, York-Cooler C, Simpson SG, Harkavy-Friedman J, Severe JB, Malaspina D, Reich T. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Archives of General Psychiatry. 1994;51(11):849–859. doi: 10.1001/archpsyc.1994.03950110009002. discussion 863-844. [DOI] [PubMed] [Google Scholar]
- Ohayon MM. Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev. 2002;6(2):97–111. doi: 10.1053/smrv.2002.0186. [DOI] [PubMed] [Google Scholar]
- Perlman CA, Johnson SL, Mellman TA. The prospective impact of sleep duration on depression and mania. Bipolar Disord. 2006;8(3):271–274. doi: 10.1111/j.1399-5618.2006.00330.x. [DOI] [PubMed] [Google Scholar]
- Phillips BA, Collop NA, Drake C, Consens F, Vgontzas AN, Weaver TE. Sleep disorders and medical conditions in women. Proceedings of the Women & Sleep Workshop, National Sleep Foundation, Washington, DC, March 5–6, 2007. J Womens Health (Larchmt) 2008;17(7):1191–1199. doi: 10.1089/jwh.2007.0561. [DOI] [PubMed] [Google Scholar]
- Redline S, Kirchner HL, Quan SF, Gottlieb DJ, Kapur V, Newman A. The effects of age, sex, ethnicity, and sleep-disordered breathing on sleep architecture. Arch Intern Med. 2004;164(4):406–418. doi: 10.1001/archinte.164.4.406. [DOI] [PubMed] [Google Scholar]
- Roybal K, Theobold D, Graham A, DiNieri JA, Russo SJ, Krishnan V, Chakravarty S, Peevey J, Oehrlein N, Birnbaum S, Vitaterna MH, Orsulak P, Takahashi JS, Nestler EJ, Carlezon WA, Jr, McClung CA. Mania-like behavior induced by disruption of CLOCK. Proc Natl Acad Sci U S A. 2007;104(15):6406–6411. doi: 10.1073/pnas.0609625104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders EF, Fitzgerald KD, Zhang P, McInnis MG. Clinical features of bipolar disorder comorbid with anxiety disorders differ between men and women. Depress Anxiety. 2012;29(8):739–746. doi: 10.1002/da.21932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders EF, Nazir R, Kamali M, Ryan KA, Evans S, Langenecker S, Gelenberg AJ, McInnis MG. Gender differences, clinical correlates, and longitudinal outcome of bipolar disorder with comorbid migraine. J Clin Psychiatry. 2014;75(5):512–519. doi: 10.4088/JCP.13m08623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders ENDM, Fernandez-Mendoza J, Kamali MK, Ryan A, Langenecker SA, Gelenberg AJ, McInnis MG. Sleep quality during euthumia in bipolar diosrder: the role of clinical features, personality traits and stressful life events. International Journal of Bipolar Disorders. 2013;1(16) doi: 10.1186/2194-7511-1-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singareddy R, Vgontzas AN, Fernandez-Mendoza J, Liao D, Calhoun S, Shaffer ML, Bixler EO. Risk factors for incident chronic insomnia: a general population prospective study. Sleep Med. 2012;13(4):346–353. doi: 10.1016/j.sleep.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soehner AM, Kaplan KA, Harvey AG. Insomnia comorbid to severe psychiatric illness. Sleep Med Clin. 2013;8(3):361–371. doi: 10.1016/j.jsmc.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Fernandez-Mendoza J, Bixler EO, Singareddy R, Shaffer ML, Calhoun SL, Liao D, Basta M, Chrousos GP. Persistent insomnia: the role of objective short sleep duration and mental health. Sleep. 2012;35(1):61–68. doi: 10.5665/sleep.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vgontzas AN, Zoumakis E, Bixler EO, Lin HM, Follett H, Kales A, Chrousos GP. Adverse effects of modest sleep restriction on sleepiness, performance, and inflammatory cytokines. J Clin Endocrinol Metab. 2004;89(5):2119–2126. doi: 10.1210/jc.2003-031562. [DOI] [PubMed] [Google Scholar]
- Walsleben JA, Kapur VK, Newman AB, Shahar E, Bootzin RR, Rosenberg CE, O’Connor G, Nieto FJ. Sleep and reported daytime sleepiness in normal subjects: the Sleep Heart Health Study. Sleep. 2004;27(2):293–298. doi: 10.1093/sleep/27.2.293. [DOI] [PubMed] [Google Scholar]
- Wehr TA. Improvement of depression and triggering of mania by sleep deprivation. JAMA. 1992;267(4):548–551. [PubMed] [Google Scholar]
- Wehr TA, Goodwin FK, Wirz-Justice A, Breitmaier J, Craig C. 48-hour sleep-wake cycles in manic-depressive illness: naturalistic observations and sleep deprivation experiments. Arch Gen Psychiatry. 1982;39(5):559–565. doi: 10.1001/archpsyc.1982.04290050037008. [DOI] [PubMed] [Google Scholar]
- Wu JC, Bunney WE. The biological basis of an antidepressant response to sleep deprivation and relapse: review and hypothesis. Am J Psychiatry. 1990;147(1):14–21. doi: 10.1176/ajp.147.1.14. [DOI] [PubMed] [Google Scholar]
- Young RC, Biggs JT, Ziegler VE, Meyer DA. A rating scale for mania: reliability, validity and sensitivity. Br J Psychiatry. 1978;133(Journal Article):429–435. doi: 10.1192/bjp.133.5.429. [DOI] [PubMed] [Google Scholar]
- Zhang B, Wing YK. Sex differences in insomnia: a meta-analysis. Sleep. 2006;29(1):85–93. doi: 10.1093/sleep/29.1.85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.