The phosphodiesterase 7 inhibitor S14 is able to induce endogenous neuroregenerative processes toward a dopaminergic phenotype. A population of actively dividing cells that give rise to new neurons in the substantia nigra pars compacta of hemiparkinsonian rats after treatment with S14 is described. The data have identified S14 as a novel regulator of dopaminergic neuron generation.
Keywords: Dopaminergic differentiation, Neurogenesis, Parkinson’s disease, Phosphodiesterase 7
Abstract
Parkinson’s disease is characterized by a loss of dopaminergic neurons in a specific brain region, the ventral midbrain. Parkinson’s disease is diagnosed when approximately 50% of the dopaminergic neurons of the substantia nigra pars compacta (SNpc) have degenerated and the others are already affected by the disease. Thus, it is conceivable that all therapeutic strategies, aimed at neuroprotection, start too late. Therefore, an urgent medical need exists to discover new pharmacological targets and novel drugs with disease-modifying properties. In this regard, modulation of endogenous adult neurogenesis toward a dopaminergic phenotype might provide a new strategy to target Parkinson’s disease by partially ameliorating the dopaminergic cell loss that occurs in this disorder. We have previously shown that a phosphodiesterase 7 (PDE7) inhibitor, S14, exerts potent neuroprotective and anti-inflammatory effects in different rodent models of Parkinson’s disease, indicating that this compound could represent a novel therapeutic agent to stop the dopaminergic cell loss that occurs during the progression of the disease. In this report we show that, in addition to its neuroprotective effect, the PDE7 inhibitor S14 is also able to induce endogenous neuroregenerative processes toward a dopaminergic phenotype. We describe a population of actively dividing cells that give rise to new neurons in the SNpc of hemiparkinsonian rats after treatment with S14. In conclusion, our data identify S14 as a novel regulator of dopaminergic neuron generation.
Significance
Parkinson’s disease is a neurodegenerative disorder characterized by the loss of dopaminergic neurons in the ventral midbrain. Currently, no cure and no effective disease-modifying therapy are available for Parkinson’s disease; therefore, an urgent medical need exists to discover new pharmacological targets and novel drugs for the treatment of this disorder. The present study reports that an inhibitor of the enzyme phosphodiesterase 7 (S14) induces proliferation in vitro and in vivo of neural stem cells, promoting its differentiation toward a dopaminergic phenotype and therefore enhancing dopaminergic neuron generation. Because this drug is also able to confer neuroprotection of these cells in animal models of Parkinson’s disease, S14 holds great promise as a therapeutic new strategy for this disorder.
Introduction
Parkinson’s disease (PD) is a chronic and progressive neurodegenerative disorder characterized by motor symptoms (muscular rigidity, resting tremor, and bradykinesia or slowness of movement) and nonmotor symptoms (visual hallucinations and dementia) [1].The hallmark of PD is the gradual loss of dopamine-producing neurons, dopaminergic neurons, in a specific brain region, the ventral midbrain [2]. Although the selective loss of dopaminergic neurons within the substantia nigra pars compacta (SNpc) is the pathological characteristic of this disease, cell loss also occurs in other brain areas, such as the locus ceruleus and dorsal nuclei of the vagus, among others [3].The underlying cause of dopaminergic cell death and the mechanisms implicated remain elusive. Because of the slow progressiveness of this neurodegenerative process [4], PD is usually diagnosed when more than 50% of the dopaminergic neurons of the SNpc have already degenerated and others have already been affected by the disease. Because, to date, no treatments are available that prevent the development of PD or modify its detrimental course (“disease-modifying agents”) and the ones used are only palliative and only lead to temporary improvement of the symptoms, an urgent medical need exists to discover new therapeutic strategies for PD that could slow, halt, or reverse the disease process.
Phosphodiesterases (PDEs) comprise a family of 21 members, which have been so far been classified into 11 groups, according to their sequence homology, cellular distribution, and sensitivity to different PDE inhibitors [5, 6], with some expressed in the central nervous system [7]. PDE7 is a cAMP-specific PDE [5, 8], and it has recently been demonstrated that can be a target for the control of neuroinflammation [9]. PDE7 inhibition has recently emerged as a good therapeutic option for the treatment of different neurodegenerative diseases. Several studies from our group have shown that different inhibitors of PDE7 are potent neuroprotective and anti-inflammatory agents in some animal models of neurodegenerative disorders, including PD [10–13]. Very recent data from our group have shown that PDE7 depletion in the SNpc, using specific short hairpin RNAs for PDE7, significantly protects dopaminergic neurons and improves motor function in lipopolysaccharide (LPS) and 6-hydroxydopamine (6-OHDA) lesioned mice [14].
The ability of the adult central nervous system to produce new neurons is limited, rendering the brain particularly vulnerable to injury and disease. In mammals, most neurons have been born by the prenatal period, but it is well established that neurons continue to arise in two niches of the adult brain, the subventricular zone (SVZ) of the lateral ventricle and the subgranular zone of the dentate gyrus of the hippocampus [15, 16]. The generation of new neurons in these regions might contribute to endogenous repair mechanisms after brain damage and/or chronic disease [17]. It is known that dopamine regulates adult neurogenesis in the SVZ and hippocampus in rodents and humans, and a decrease in new stem cell proliferation has been described in PD (reviewed in [18]). A decreased proliferation has been shown of NSCs in the subventricular zone of human PD brains and in 2 animal models of PD (1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and 6-OHDA) [19–21], as a consequence of dopamine depletion caused by the loss of dopaminergic neurons in the SNpc. Consequently, various therapeutic approaches, in addition to the classic use of dopaminergic agents, are now aimed at manipulating the resident stem/progenitor cells to produce neuroblasts, which could eventually differentiate into dopaminergic cells. The potential of this strategy, inducing endogenous neurogenesis by new therapeutic agents, holds great promise because this increased neurogenesis could help to replace the dopaminergic cells lost in PD and already lost by the time a patient is diagnosed.
The aim of the present study was to investigate the effects of the PDE7 inhibitor S14 on adult neurogenesis in the 6-OHDA model of PD, mimicking a severe dopaminergic striatal deficit. We show that pharmacological manipulation of PDE7 in vivo induces a strong neurogenesis in the SNpc of 6-OHDA-lesioned animals toward a dopaminergic phenotype. In vitro, PDE7 inhibition increased the neuronal differentiation of neurospheres obtained from either the embryonic ventral midbrain or adult SVZ. Next, using PDE7 inhibitors, we could potentially contribute to upregulate endogenous neurogenesis and/or favor integration of new dopaminergic neurons to stimulate neurorepair in PD.
Materials and Methods
Animals
Adult male Wistar rats (8–12 weeks old) were used in this study. All procedures with the rats were specifically approved by the Ethics Committee for Animal Experimentation of the Instituto de Investigaciones Biomedicas (CSIC-UAM) and performed in accordance with the protocols issued, which followed national (normative 1201/2005) and international recommendations (normative 86/609 from the European Communities Council). Adequate measures were taken to minimize the pain or discomfort experienced by the rats.
Embryonic Mesencephalic Precursor Isolation
Cultures were derived from the ventral mesencephalon (VM) of rat embryos at embryonic day 14, as previously described [10]. In brief, ventral mesencephalon was isolated in ice-cold Hanks’ balanced salt solution medium Ca2+ and Mg2+ free, washed several times, and tissue digested 15 minutes at 37°C in trypsin-EDTA plus DNase (0.05%). VM was then gently minced and triturated with a micropipette, and the supernatant was collected and centrifuged at 1,200g for 5 minutes. The pellet was resuspended in Dulbecco’s modified Eagle’s medium (DMEM)/Ham’s-F12 (1:1) containing 0.5 mM glutamine, 200 U/ml penicillin, and 200 μg/ml streptomycin (Gibco, Grand Island, NY, http://www.invitrogen.com), 1% fungizone, 10 ng/ml epidermal growth factor (EGF; PeproTech, London, U.K., http://www.peprotech.com), 10 ng/ml fibroblast growth factor (FGF; PeproTech), and 1× B27 (Gibco). The cells were seeded onto 12-well plates (∼40,000 cells per cm2).
Adult SVZ Precursor Isolation
Primary precursor cultures were prepared from the SVZ of the lateral ventricle of adult Wistar rats, as previously described [22]. In brief, SVZ was microdissected, isolated, and dissociated in DMEM (Invitrogen) containing glutamine, gentamicin, and fungizone. After 15 minutes’ digestion at 37°C in trypsin-EDTA, hyaluronidase, and DNase, myelin was removed using Dulbecco’s phosphate-buffered saline (PBS) (Invitrogen). Isolated precursor cells were seeded into 12-well dishes at a density of ∼40,000 cells per cm2 in DMEM/F12 (1:1; Invitrogen) containing 10 ng/ml EGF, 10 ng/ml FGF, and N2 medium (Gibco).
Neurosphere Culture and Treatment
After 3 days in culture, neural stem cells formed spherical cellular aggregates known as neurospheres (NSs). At that moment, we started to treat free-floating NSs with BRL-50481 (30 μM; Tocris Bioscience, Bristol, U.K., http://www.tocris.com), S14 (10 μM), or vehicle for 7 days. The quinazoline S14 was synthesized following described procedures [23]. The effective dose of compounds was determined from previous studies [10]. For measurements of growth and proliferation, the NSs were counted, and their size was analyzed using the Nikon Digital Sight, SD-L1 software (Nikon, Tokyo, Japan, http://www.nikon.com). The number and diameter of neurospheres were then scored. In each experiment, 6–8 wells per condition were tested and counted, and the radius of 50 neurospheres was determined. For the cell differentiation studies, free-floating NSs cultured for 7 days in the presence or not of the PDE7 inhibitor BRL-50481 or S14 were seeded onto poly-l-lysine (Sigma-Aldrich, St. Louis, MO, http://www.sigmaldrich.com) precoated 6-well plates and/or coverslips. The seeded NSs were further cultured for 3 days in the absence of exogenous growth factors in medium containing 1% fetal bovine serum and in the presence or absence of BRL-50481 or S14.
Immunoblot Analysis
Proteins were isolated from the cell cultures using standard methods. In brief, the cells were resuspended in ice-cold cell lysis buffer (Cell Signaling Technology, Beverly, MA, http://www.cellsignal.com) with a protease inhibitor cocktail (Roche, Indianapolis, IN, http://www.roche.com) and incubated for 15–30 minutes on ice. A total amount of 30 µg of protein was loaded on a 10% or 12% SDS-polyacrylamide gel electrophoresis gel and transferred to nitrocellulose membranes (Protran, Whatman; Sigma-Aldrich). The membranes were blocked in Tris-buffered saline with 0.05% Tween-20 and 5% skimmed milk, incubated with primary and secondary antibodies, and washed according to standard procedures. The primary antibodies were PDE7A (rabbit; Santa Cruz Biotechnology Inc., Santa Cruz, CA, http://www.scbt.com), PDE7B (rabbit; Proteintech Group, Chicago, IL, http://www.proteintech.com), phosphorylated cAMP response element-binding protein (p-CREB) (rabbit; Cell Signaling), CREB (rabbit; Cell Signaling), Musashi1 (rabbit; Abcam, Cambridge, U.K., http://www.abcam.com), β-II-tubulin (Tuj-1 clone, rabbit; Abcam), microtubule-associated protein 2 (MAP-2) (mouse; Sigma-Aldrich), Nurr1 (rabbit; Santa Cruz Biotechnology), tyrosine hydroxylase (TH) (rabbit; EMD Millipore, Billerica, MA, http://www.emdmillipore.com), and α-tubulin (mouse; Sigma-Aldrich). The secondary peroxidase-conjugated antibodies were donkey anti-rabbit (Amersham Biosciences, GE Healthcare, Piscataway, NJ, http://www.amersham.com) and rabbit anti-goat and rabbit anti-mouse antibodies (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, http://www.jacksonimmuno.com). The data presented in the figures are the average of the quantification of at least three independent experiments corresponding to three different samples.
Immunocytochemistry
NS cultures were examined immunocytochemically, as previously described [24]. In brief, after 1 hour of incubation with the corresponding primary antibody, the cells were washed with PBS and incubated with the appropriate Alexa Fluor-labeled secondary antibody (Alexa Fluor-488 or Alexa Fluor-647; Molecular Probes, Leiden, The Netherlands, http://probes.invitrogen.com) for 45 minutes at 37°C. Later, the images were obtained using a LSM710 laser scanning spectral confocal microscope (Carl Zeiss, Jena, Germany, http://www.zeiss.com). The confocal microscope settings were adjusted to produce the optimum signal/noise ratio. Primary antibodies were directed against the following: β-III-tubulin (TuJ-1 clone; rabbit; Abcam), MAP-2 (mouse; Sigma-Aldrich), Nurr1 (rabbit; Santa Cruz Biotechnology), and TH (rabbit; EMD Millipore). Staining of nuclei was performed using 4′,6-diamidino-2-phenylindole.
Neurogenic Studies In Vivo
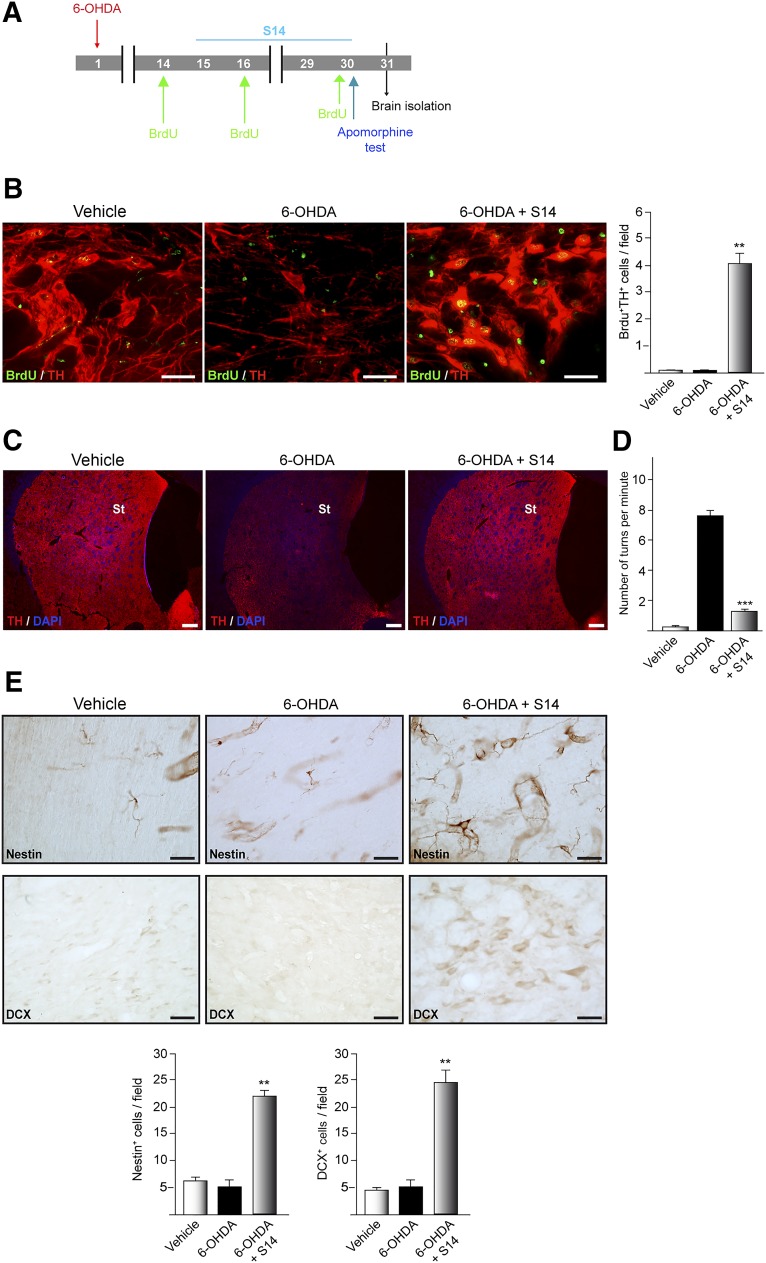
To determine the neurogenic effects of S14 in a model of dopaminergic cell loss, the rats were properly anesthetized and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, http://www.kopfinstruments.com). Rats (n = 5 per group) were injected into the right side of the striatum with 6-OHDA (9 μg in 2.5 μl PBS containing 0.02% ascorbic acid) based on the following coordinates: from bregma, posterior −3.0 mm, lateral +1.0 mm, ventral +5.0 mm, according to the atlas of Paxinos [25]. Control rats of the same age were injected with PBS. The rats were then housed individually to recover and 15 days after lesioning S14 compound (10 mg/kg body weight) was intragastrically administered in a sodium carboxy methyl cellulose suspension. This dose was chosen because of its effectiveness in different previously published works [10, 13]. The rats were sacrificed 1 month after lesioning. To label the entire population of proliferating cells, the rats were intraperitoneally injected with 5-bromo-2-deoxyuridine (BrdU; 50 mg/kg) at the indicated times (1, 14, and 16 days) before sacrifice.
Immunohistochemistry
The animals previously anesthetized were perfused transcardially with 4% paraformaldehyde, and brains were obtained, postfixed in the same solution at 4°C overnight, cryoprotected, and frozen. Finally, 30-μm coronal sections were obtained in a cryostat. Free-floating sections were immunostained using immunofluorescence analysis or the diaminobenzidine method, as previously described [22]. In brief, for BrdU detection, the samples were first incubated with 2 M HCl for 30 minutes at 37°C before blocking for 1 hour in PBS containing 5% normal serum, 0.1 M lysine, and 0.1% Triton X-100. The sections were then incubated with anti-BrdU mouse monoclonal (Dako, Glostrup, Denmark, http://www.dako.com), anti-nestin rabbit polyclonal (Abcam), anti-TH rabbit polyclonal (EMD Millipore), anti-glial fibrillary acidic protein mouse monoclonal (GFAP; Sigma-Aldrich), and Texas Red Lycopersicon esculentum (tomato-lectin; Vector Laboratories, Burlingame, CA, http://www.vectorlabs.com) antibodies at 4°C overnight, washed 3 times and incubated with Alexa Fluor-488 or Alexa Fluor-647 secondary antibodies for 1 hour at room temperature. After rinsing, the sections were mounted with Vectashield (Vector Laboratories). Images were obtained using a LSM710 laser scanning spectral confocal microscope (Carl Zeiss). Confocal microscope settings were adjusted to produce the optimum signal/noise ratio. For doublecortin (DCX), CREB, and p-CREB detection floating sections were immersed in 3% hydrogen peroxide to inactivate endogenous peroxidase, blocked for 2 hours at room temperature in 5% normal horse serum in PBS, containing 4% bovine serum albumin, 0.1 M lysine, and 0.1% Triton X-100. Next, the sections were incubated overnight with an anti-DCX goat (Santa Cruz Biotechnology), anti-p-CREB polyclonal rabbit (Abcam) or anti-CREB (Cell Signaling) polyclonal rabbit antibody. After several rinses, the sections were incubated for 1 hour with the corresponding biotinylated secondary antibody and then processed following the avidin-biotin protocol (ABC, Vectastain kit; Vector Laboratories). The slides were examined with a Nikon eclipse 90i microscope, equipped with a DS-Fi1 digital camera (Nikon). Five rats from each experimental group were analyzed.
Cell Counts
The total number of cells stained with a particular marker was determined, as previously described [10], with some modifications. Immunoreactive cells in the SNpc, SVZ, or midbrain aqueduct were counted in 1-in-6 series of 30-μm coronal sections (microtome setting) from the rostrocaudal extent of the SNpc, SVZ, or aqueduct. The boundaries of each nervous system region were determined with reference to internal anatomic landmarks [25]. For each area of interest, 3,3′-diaminobenzidine-stained images were analyzed under a light microscope (Eclipse 80i; Nikon). Double labeling was examined with a confocal laser scanning microscope (Carl Zeiss). Five well-defined high magnification (×400) fields per rat were analyzed using computer-assisted image analysis software (Soft Imaging System Corporation, Lakewood, CO, http://www.soft-imaging.com). Positive cells, which intersected the uppermost focal plane (exclusion plane) and the lateral exclusion boundaries of the counting frames, were not counted. Five rats per group were used.
Rotation Behavior Analysis
The rats were tested for apomorphine-induced contralateral rotations, as previously described [10, 14]. In brief, 30 days after unilateral 6-OHDA injection into the striatum, apomorphine (Sigma-Aldrich, Madrid, Spain) was subcutaneously administered at 0.5 mg/kg. After apomorphine injection, the rats were individually set into hemispherical glass bowls (diameter 20 cm). Starting 10 minutes after apomorphine application, the numbers of full contralateral rotations were monitored for 40 minutes. Analysis of the completed (360°) rotations was done offline and are expressed as the number of contralateral net turns per minute. Three different experiments with at least 12 rats per experimental group were performed.
Statistics Analysis
Statistical comparisons for significance were performed using analysis of variance using the SPSS statistical software package, version 20.0, for Windows (IBM Corporation, Armonk, NY, http://www.ibm.com) followed by the Newman-Keuls test. Differences were considered statistically significant at p < .05.
Results
Effect of PDE7 Inhibition on the Levels of PDE7 and p-CREB in Neurosphere Cultures
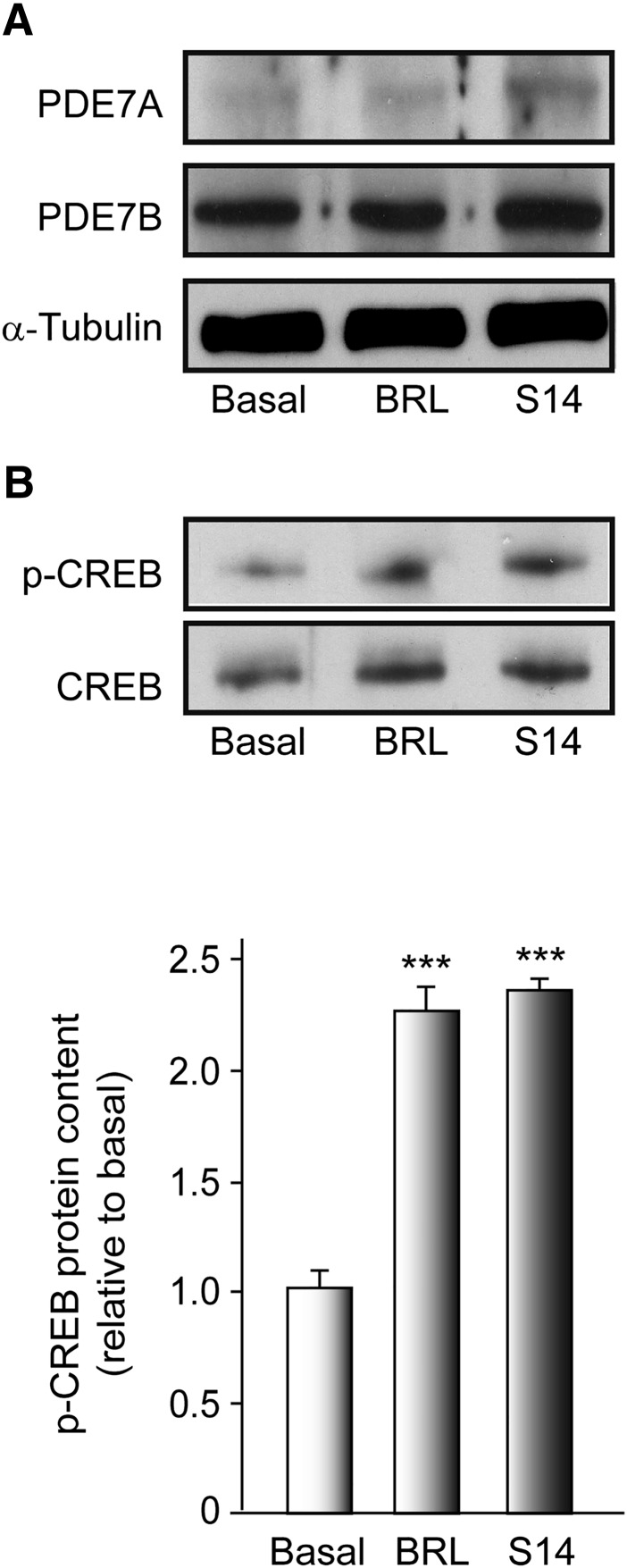
We first performed Western blot analysis to determine the levels of PDE7 and CREB phosphorylation in vitro, using neural progenitors isolated from the embryonic VM. The progenitor cells divide, forming small proliferating NSs, visible after 3 days in culture. Because PDE7 comprises two genes, PDE7A and PDE7B, both isoforms were analyzed. As shown in Figure 1A, both isoforms were expressed in VM-derived NS, with the levels of PDE7B more prominent than those of PDE7A. No effect of S14 or BRL50481 (a commercially available PDE7 inhibitor used as a standard reference) on PDE7A and PDE7B expression was observed. We next examined the effects of S14 and BRL50481 on the phosphorylation levels of the CREB, a well-known target of the cAMP signaling pathway, to confirm that effectively the S14 compound is acting through inhibition of PDE7 and subsequent induction of the cAMP pathway. To analyze this, NS cultures were treated or not with BRL50481 or S14 for 7 days, and the phosphorylation of CREB was determined. The results presented in Figure 1B clearly show that treatment of NS with BRL50481 or S14 promoted a significant increase in the levels of p-CREB.
Figure 1.
PDE7 expression in embryonic ventral mesencephalic (VM) neurospheres. VM-derived neurospheres were cultured as indicated in Materials and Methods and treated with vehicle, BRL (30 µM), or S14 (10 µM) for 7 days. (A): Representative Western blot showing the levels of PDE7A and PDE7B. (B): Western blot showing the levels of p-CREB. Quantification analysis is also shown. Results are mean ± SD from 3 independent experiments. ∗∗∗, p ≤ .001 versus vehicle-treated (basal) cultures. Abbreviations: BRL, BRL50481; CREB, cAMP response element-binding protein; p-CREB, phosphorylated CREB; PDE, phosphodiesterase.
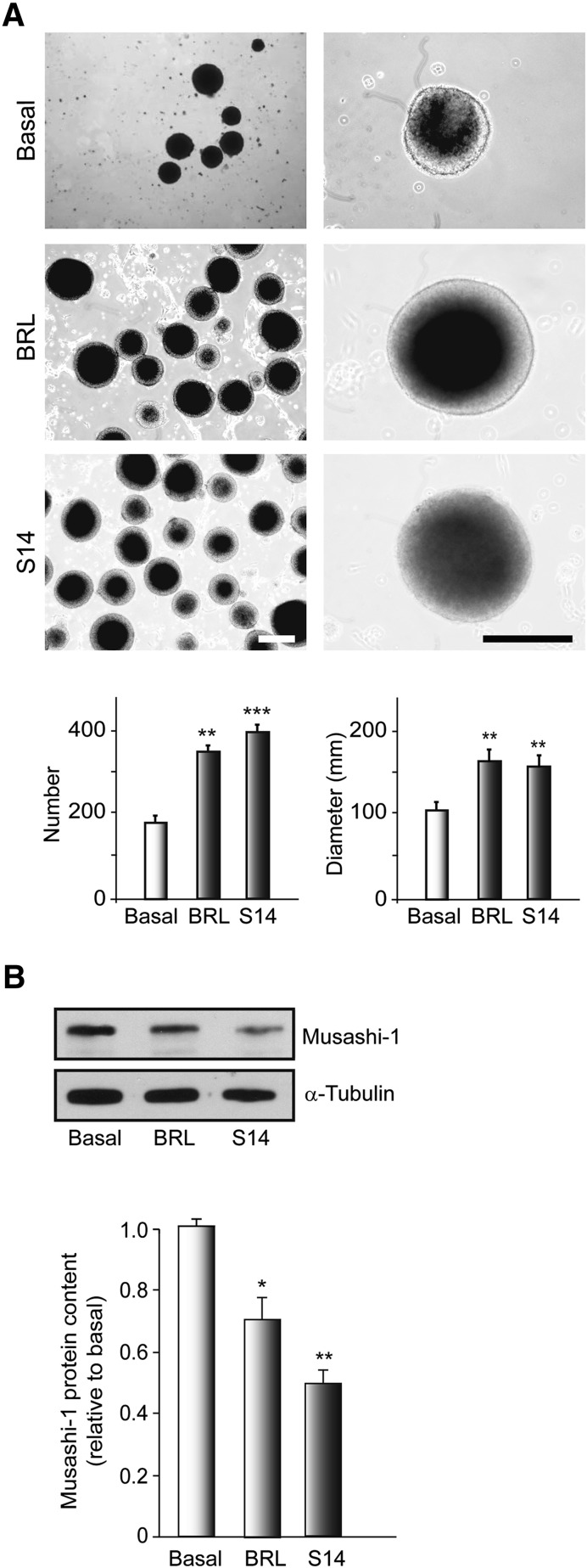
PDE7 Inhibition Induces Proliferation and Growth of Embryonic Ventral Mesencephalic-Derived Neurospheres
Next, we analyzed whether S14 affected the neural stem cell proliferation of NS cultures. To assess the involvement of PDE7 inhibition in neurosphere formation, NSs were cultured in floating conditions on nonadhesive dishes and cultured for 7 days in the presence or absence of BRL50481 or S14, and the number and diameter of the neurospheres were evaluated. The results depicted in Figure 2A show that PDE7 inhibition increased the rate of NS formation and their size. Treatment with BRL50481 and S14 significantly increased the number of NSs derived from embryonic VM (350 ± 11 and 401 ± 16), respectively, compared with vehicle-treated cultures (180 ± 13). The size of the NSs also increased after BRL50481 (162.5 ± 12.5 µm) or S14 (156.5 ± 11 µm) treatment compared with nontreated cultures (101 ± 9 µm). Later, we analyzed the levels of musashi-1, a known marker of the NS undifferentiated state, in NSs cultured under proliferative conditions in the presence or absence of PDE7 inhibitors for 7 days. The Western blot analysis shown in Figure 2B clearly shows a decrease in the amount of musashi-1 protein in those cultures treated with BRL50481 or S14, suggesting a diminution of the maintenance of NS stemness in those cultures treated with both compounds. Taking together, these results indicate that BRL50481 and S14 treatment promoted an increase in the number and size of neurospheres derived from embryonic VM, suggesting that PDE7 inhibition controls the proliferation and growth of neural progenitors in this area of the brain.
Figure 2.
Effects of phosphodiesterase 7 inhibition on embryonic ventral mesencephalic (VM) neurosphere formation. VM-derived neurospheres were cultured, treated with vehicle, BRL (30 µM), or S14 (10 µM) for 7 days, and the number and size were determined as indicated in Materials and Methods. (A): Representative phase-contrast micrographs and quantification analysis showing the number and size of the neurospheres. Scale bars = 100 μm. (B): Representative Western blot and quantification analysis showing expression levels of the precursor cell marker Musashi-1. Results are mean ± SD from 3 independent experiments. ∗, p ≤ .05; ∗∗, p ≤ .01; ∗∗∗, p ≤ .001 versus vehicle-treated (basal) cultures. Abbreviation: BRL, BRL50481.
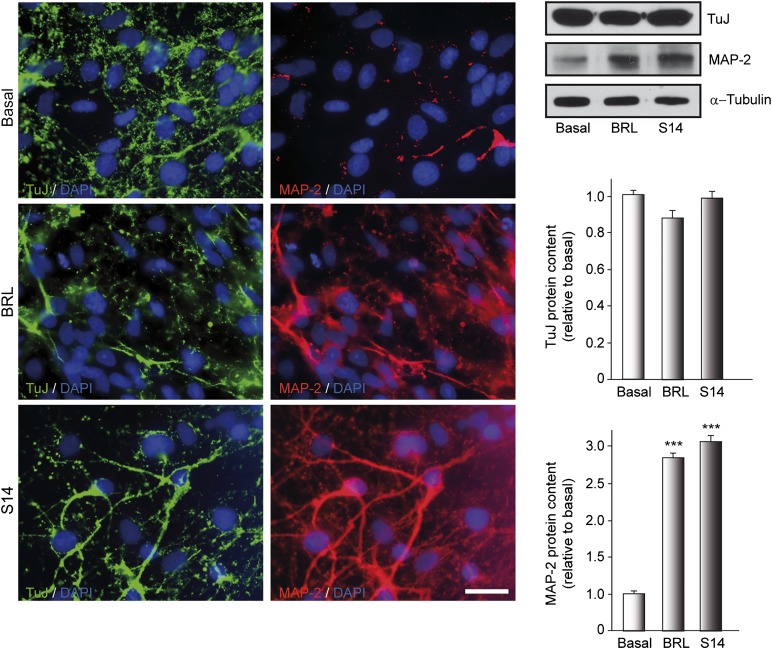
PDE7 Inhibition Upregulates MAP-2 Expression in Neural Stem Cells Derived From Ventral Mesencephalon
To investigate whether BRL50481 and S14 influenced neuronal differentiation after adhesion of NSs, we analyzed using immunocytochemistry the expression of 2 neuronal markers, β-III-tubulin (a classic marker of immature neurons) and MAP-2 (a marker of more mature neurons). To this end, NSs treated with BRL50481 or S14 for 7 days adhered to a substrate and allowed to differentiate for 3 days. Next, immunocytochemical analysis using specific antibodies was performed. Some cultures were used for Western blot analysis and quantification of protein levels. Immunocytochemical analysis showed no differences in β-III-tubulin expression between the control and treated cultures (Fig. 3) in the outgrowth of the NSs. In contrast, inhibition of PDE7 with BRL50481 and S14 resulted in an increase in the number of MAP-2-positive cells compared with basal levels. Western blot analysis confirmed that PDE7 inhibition significantly promoted cell maturation of embryonic neural stem cells toward a neuronal phenotype.
Figure 3.
Inhibition of phosphodiesterase 7 promotes neurogenesis of embryonic ventral mesencephalic neurospheres. Neurospheres were cultured in the presence of vehicle, BRL (30 µM), or S14 (10 µM) for 7 days and later adhered for 3 days to allow differentiation in the presence of inhibitors. Immunocytochemistry and Western blot were performed on differentiated neurospheres as indicated in Materials and Methods. Immunofluorescence images show the expression of the neuronal markers TuJ clone (early neurogenesis) in green and MAP-2 (mature neurons) in red. DAPI was used for nuclear staining. Scale bar = 20 μm. Western blot and quantification analysis are also shown. ∗∗∗, p ≤ .001 versus vehicle-treated (basal) cultures. Abbreviations: BRL, BRL50481; DAPI, 4′,6-diamidino-2-phenylindole; MAP-2, microtubule-associated protein 2; TuJ, β-III-tubulin.
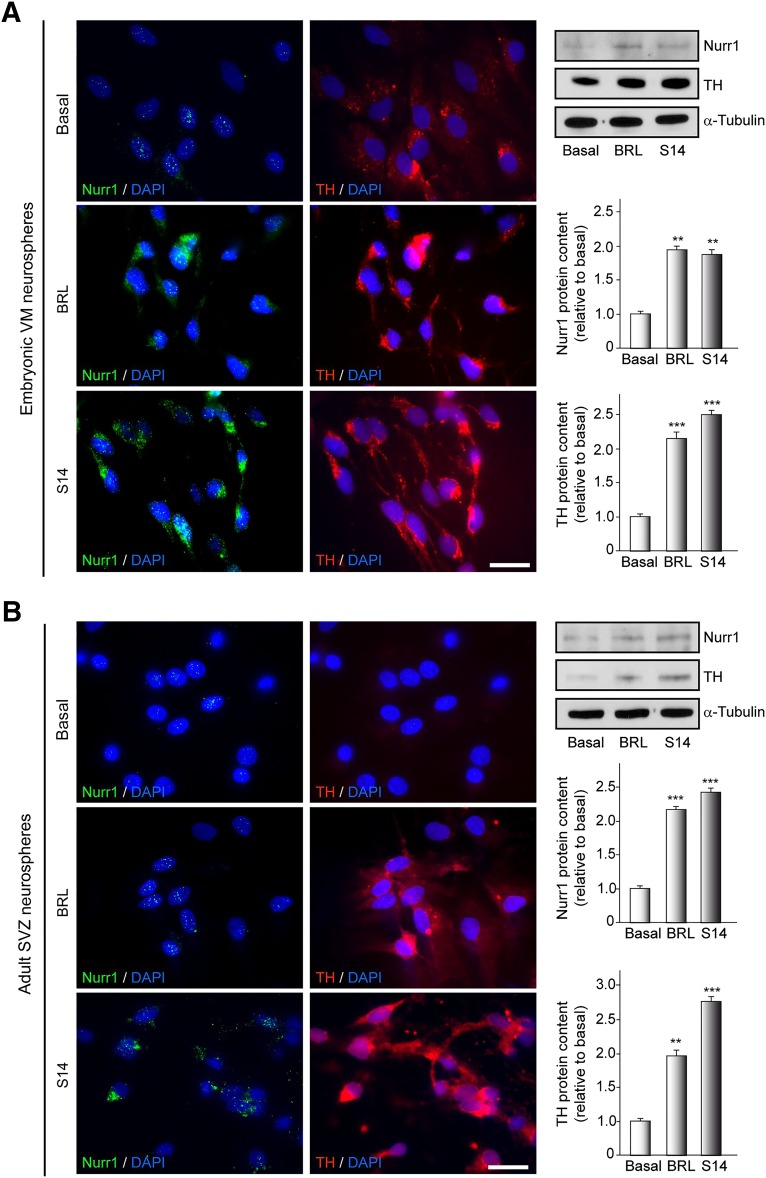
PDE7 Inhibition Upregulates Nurr1 and TH Expression in Neural Stem Cells Derived From Ventral Mesencephalon and SVZ
Considering the potential neurogenic effect of PDE7 inhibition, we next investigated whether treatment with the PDE7 inhibitors BRL50481 and S14 could specifically promote the differentiation of NSs toward dopaminergic neurons. For this purpose, we analyzed the expression levels of Nurr1 (a marker of dopaminergic precursors) and TH (the rate-limiting enzyme in dopamine synthesis and marker of dopaminergic neurons) in NS cultures derived from both embryonic midbrain and adult SVZ. NSs treated or not with BRL50481 or S14 during 7 days were adhered to poly-l-lysine-coated plates and allowed to differentiate for 3 days. Next, immunocytochemical and Western blot analysis using specific antibodies were performed. The results presented in Figure 4A show that the number of Nurr1- and TH-positive stained cells in embryonic VM cultures is greatly increased in those cultures treated with either BRL50481 or S14 compounds, with respect to the controls. Furthermore, this increase was also observed in NS cultures derived from a well-established adult neurogenic niche, the SVZ (Fig. 4B). These results clearly indicate that PDE7 inhibition promotes the differentiation of neural stem cells in vitro toward a dopaminergic phenotype.
Figure 4.
Inhibition of phosphodiesterase 7 promotes dopaminergic neurogenesis on embryonic VM and adult SVZ neurospheres. Neurospheres were cultured in the presence of vehicle, BRL (30 µM), or S14 (10 µM) for 7 days and later adhered for 3 days to allow differentiation in the presence of inhibitors. Immunocytochemistry and Western blot were performed on differentiated neurospheres from VM (A) or SVZ (B). Immunofluorescence images show the expression of Nurr1 in green and TH in red. DAPI was used for nuclear staining. Scale bars = 20 μm. Representative Western blots and quantification analysis are shown. ∗∗, p ≤ .01; ∗∗∗, p ≤ .001 versus vehicle-treated (basal) cultures. Abbreviations: BRL, BRL50481; DAPI, 4′,6-diamidino-2-phenylindole; SVZ, subventricular zone; TH, tyrosine hydroxylase; VM, ventral mesencephalic.
S14 Induces Proliferation and Differentiation of Adult Progenitor Cells In Vivo in the SNpc
Given the in vitro results showing a neurogenic effect of PDE7 inhibition and demonstrating an increase in the number of de novo dopaminergic neurons, we next assessed the efficacy of the PDE7 inhibitor S14 in vivo. It has been suggested that neurogenesis is impaired in PD; therefore, any treatment able to enhance endogenous neurogenesis might have relevant disease-modifying effects in this disorder.
To analyze in vivo neurogenesis, adult rats were lesioned with 6-OHDA and 15 days later received a daily oral dose of S14, which is known to cross the blood-brain barrier [13, 26] for another 15 days, following the experimental approach shown in Figure 5A. The rats were also intraperitoneally injected with BrdU. To test whether neurons are generated in the SNpc after S14 treatment, coronal sections containing the SNpc were doubled-immunostained using anti-TH- and anti-BrdU-specific antibodies. Vehicle-treated rats presented with BrdU+ cells throughout the entire SNpc (Fig. 5B). These results are in agreement with those previously described by others [27], indicating that proliferating cells are present in this region. In accordance with these results, we found BrdU-labeled cells predominantly in doublets, suggesting that the cells had divided locally. Rats lesioned with 6-OHDA also presented with some scattered BrdU-labeled cells. When the 6-OHDA-lesioned rats were treated with S14, in addition to the BrdU+ cells, we also observed cells double-stained for BrdU and TH, suggesting that this compound elicited an increase in the generation of new dopaminergic cells in the injured SNpc of adult rats. In accordance with the increase in TH+ cells elicited by S14 in the SNpc, a parallel increase in TH staining was observed in the striatum of the treated animals (Fig. 5C). Concerning neuroinflammation, an event that occurs after a brain injury, we observed a significant reduction in glial activation (astrocytes and microglial cells) in those rats lesioned with 6-OHDA and treated with S14 (supplemental online Fig. 1). Because of these results, we next studied whether the new generation of dopaminergic cells in the SNpc, together with the reinnervation observed in the striatum and the anti-inflammatory effect of S14, was associated with an improvement of the motor alterations induced by 6-OHDA injection. To this end, 30 days after lesioning, the rats were tested for apomorphine-induced contralateral rotations. Apomorphine subcutaneous administration induces contralateral rotational behavior in denervated animals. Our results clearly showed a significant increase in the number of contralateral turns per minute after apomorphine administration in 6-OHDA-lesioned rats (Fig. 5D) compared with that in the control animals. S14 administration led to substantial attenuation of the asymmetric motor behavior in the lesioned animals.
Figure 5.
Effect of the inhibition of phosphodiesterase 7 on neurogenesis in the substantia nigra pars compacta (SNpc) in an animal model of Parkinson disease. (A): Experimental approach. 6-OHDA (9 µg) or vehicle was injected unilaterally into the striatum of adult rats. Two weeks after lesion inducement, the rats received a daily intragastric dose of S14 (10 mg/kg) until brain isolation. Before sacrifice, the rats were intraperitoneally injected with BrdU (50 mg/kg) at the indicated times. (B): Immunofluorescence images and quantification showing double expression of BrdU+ (green) and TH+ (red) in cells in the SNpc. Scale bars = 25 μm. (C): Ipsilateral coronal sections processed for TH immunoreactivity labeling dopaminergic St fiber density (red). Scale bars = 100 μm. DAPI was used for nuclear staining. (D): On day 30 after injury, apomorphine-induced rotation tests were performed. Values represent the mean ± SD from 3 different experiments. At least 12 rats per experimental group were evaluated. ∗∗∗, p ≤ .001 versus 6-OHDA-injected rats. (E): Neural stem cell marker expression in the SNpc. Representatives images showing nestin- and DCX-expressing cells on immunohistochemistry. Scale bars = 25 μm. Quantification of the number of nestin- and DCX-positive cells in the SNpc is shown. Values in all quantifications represent the mean ± SD from 3 different experiments and 5 rats per experiment per experimental group. ∗∗, p ≤ .01 versus 6-OHDA-injected rats. Abbreviations: 6-OHDA, 6-hydroxydopamine; BrdU, 5-bromo-2-deoxyuridine; DAPI, 4′,6-diamidino-2-phenylindole; DCX, doublecortin; St, striatal; TH, tyrosine hydroxylase.
The newborn cells found in the SNpc could originate from precursors already present in this area or from precursor cells existing in other areas of the brain. Therefore, we first examined the presence of possible neural progenitor cells (NPCs) in the SNpc by analyzing the occurrence of nestin and DCX-stained cells. Nestin is commonly used as a reliable biological marker of NPCs in vitro and in vivo [28–31]. DCX is a microtubule-associated protein and a valuable endogenous marker for dividing neuroblasts and immature neurons [32, 33]. The results shown in Figure 5E indicate the presence of nestin- and DCX-labeled cells in the SNpc of the vehicle- and 6-OHDA-injected animals. The presence of nestin-positive cells suggest that, as indicated by other investigators, the SNpc of adult animals can also contain neural precursor cells. Figure 5E also shows that rats lesioned with 6-OHDA and treated with S14 presented with an increase in the number of nestin-positive cells. Also, the number of cells expressing DCX was increased in the S14-treated animals.
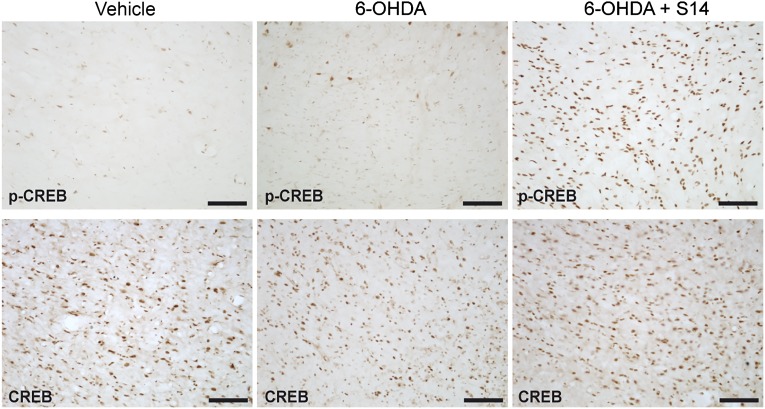
It is known that PDE7 inhibition increases cAMP levels. Consequently, we next analyzed the phosphorylation state of CREB, a well-known target of the cAMP signaling pathway. The results shown in Figure 6 clearly indicate that S14 oral administration increases the levels of p-CREB in the SNpc in this animal model of PD. Immunodetection of the total amount of CREB protein was used as a control of the induction of the phosphorylation state.
Figure 6.
S14 oral administration induces the phosphorylation of CREB in an animal model of Parkinson's disease. Adult rats were treated using the experimental approach described in Figure 5A. Serial coronal sections were made, and consecutive sections containing the substantia nigra pars compacta were immunostained using anti-p-CREB or anti-CREB antibodies. Representative images of ipsilateral sections showing p-CREB (upper) or CREB (lower) are shown. Scale bars = 100 μm. Abbreviations: 6-OHDA, 6-hydroxydopamine; CREB, cAMP response element-binding protein; p-CREB, phosphorylated CREB.
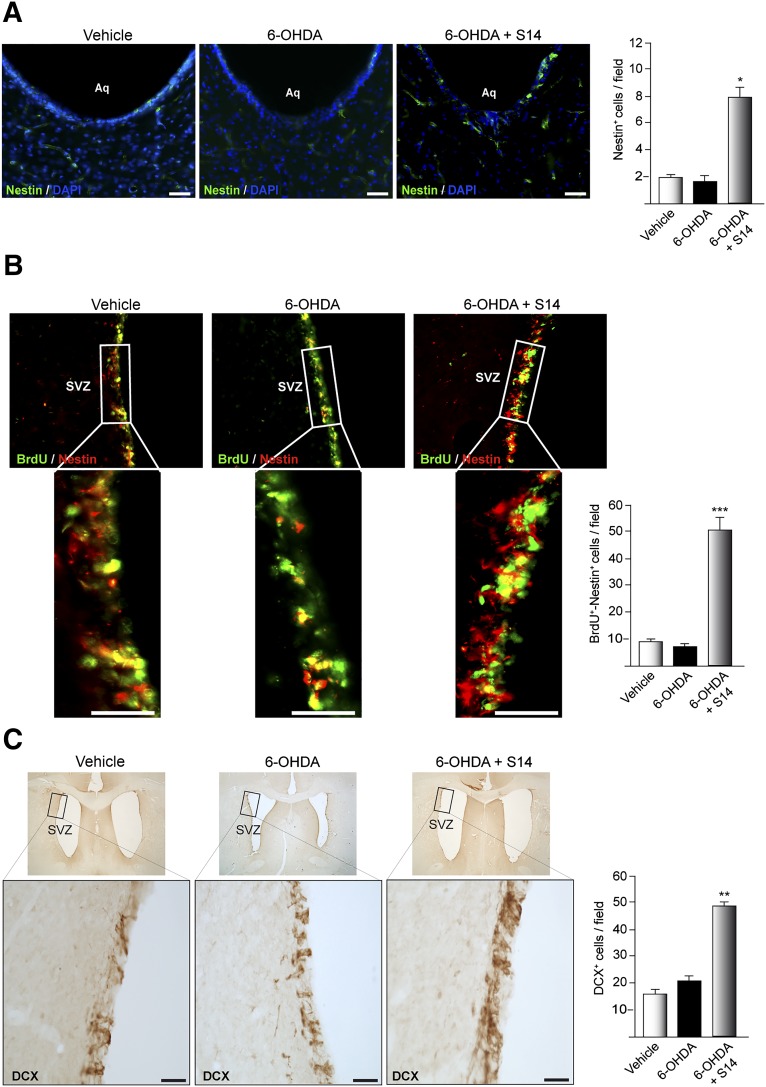
S14 Increases the Number of Nestin-Positive Cells in the Mesencephalic Aqueduct and Induces Proliferation and Differentiation of Adult Progenitor Cells In Vivo in the SVZ
As noted, the newborn cells found in the SNpc could also have originated from precursors present in other brain areas such as the mesencephalic aqueduct and could have migrated to the SNpc, such as has been previously suggested [27, 34]. Also, several reports have shown impaired neurogenesis in the SVZ in patients with PD and in several animal models of PD. Consequently, we finally analyzed the presence of possible NPCs in both regions (Fig. 7). Figure 7A shows that S14 treatment considerably increased the number of nestin-positive cells in the mesencephalic aqueduct of rats lesioned with 6-OHDA and treated later with S14, compared with the control vehicle-treated animals. In the SVZ, S14 treatment considerably increased the number of nestin/BrdU double-stained cells (Fig. 7B). These results suggest that S14 stimulates the proliferation of new progenitors in the mesencephalic aqueduct and in the SVZ of adult rats. We also analyzed the presence of DCX-stained cells in this neurogenic niche. As shown in Figure 7C, the increase in proliferating cells in the SVZ after S14 treatment correlated with a notable increase in the number of DCX+ cells in this area. An increase in the migrating chain of cells was also observed.
Figure 7.
Effect of the inhibition of PDE7 on neurogenesis in the adult midbrain Aq and SVZ in an animal model of Parkinson disease. The experimental approach used was that described in Figure 5A. (A): Brain coronal sections showing nestin-expressing cells in the Aq. Scale bars = 100 μm. (B): Coronal sections of SVZ showing the immunofluorescence expression of BrdU-positive (green) and nestin-positive (red) cells. (C): DCX-expressing cells in the SVZ. Insets show higher magnifications of representatives selected areas. Scale bars = 75 μm. Quantification of the number of nestin-positive cells in the Aq (A) and BrdU-positive/nestin-positive cells (B) and DCX-positive cells (C) in the SVZ is shown. Values represent the mean ± SD from 3 different experiments and 5 rats per experiment per experimental group. ∗, p ≤ .05; ∗∗, p ≤ .01; ∗∗∗, p ≤ .001 versus 6-OHDA-injected control rats. Abbreviations: 6-OHDA, 6-hydroxydopamine; Aq, aqueduct; BrdU, 5-bromo-2-deoxyuridine; DAPI, 4′,6-diamidino-2-phenylindole; DCX, doublecortin; SVZ, subventricular zone.
Discussion
Similar to other neurodegenerative diseases, PD is diagnosed when more than 50% of the dopaminergic neurons of the SNpc have already degenerated. Currently, no cure and no effective disease-modifying therapy are available; dopamine replacement treatment is only palliative, leading to temporarily limited improvement of the clinical symptoms. Also, chronic treatment with dopaminergic drugs, such as l-DOPA, results in severe side effects such as dyskinesia [35]. Consequently, new approaches to treat PD are being developed. Grafts of dopamine neurons derived from induced pluripotent or embryonic stem cells have been used to test the clinical potential of differentiated stem cells in PD. However, their value is largely questioned by data from transplanted patients that indicate that the grafted neurons have compromised function and eventually acquire disease [36–40]. Another approach to achieve clinical benefit is an indirect method by activating precursor cells already present in the brain. In the present study, we investigated the potential effect of PDE7 inhibition on the promotion of dopaminergic cells in the 6-OHDA animal model of PD. Our results demonstrated a unique role for the PDE7 inhibitor S14 as a regulator of dopamine precursor cell proliferation and differentiation in the SNpc of 6-OHDA-lesioned adult rats, which could have potential implications for future innovative therapies in PD.
Previous work from our group has shown that the PDE7 inhibitor S14 significantly protects dopaminergic neurodegeneration and improves motor function in LPS-lesioned animals [10]. In the present study, we show that S14 regulates the expansion and differentiation of the stem cell population derived from VM. This is evident in vitro by an enhanced number and size of neurospheres and an induction of MAP2-positive cells. More interestingly, we also found a significant increase in the number of Nurr1- and TH-positive cells, indicating that the S14 compound can elicit differentiation of VM stem cells toward a dopaminergic phenotype. We also observed an increase in Nurr1- and TH-labeled cells in the NS cultures derived from the SVZ of adult rats treated with the compound.
Our work shows that PDE7 inhibition by S14 has a dual function in neural stem cells: induction of proliferation and differentiation, and suggests that this compound is not only mitogenic for neural stem cells, but also is an inducer of neuronal differentiation. Inhibition of PDE7 can promote both proliferation and differentiation. However, precedents for this phenomenon have been seen in other cases, such as leukotriene B4 [41], mild hypoxia [42], bone morphogenetic proteins [43], EGF/FGF2 [44], nerve growth factor/brain-derived neurotrophic factor/basic fibroblast growth factor [45], and the transcription factors Lmx1a and Lmx1b [46]. Thus, we suggest that inhibition of PDE7 could represent a new strategy for restoring neurogenesis.
The mechanism of action of this compound seems to be an inhibition of PDE7, the subsequent activation of the cAMP/PKA signaling pathway, and the activation of the transcription factor CREB by phosphorylation. These results are in accordance with different studies showing that p-CREB plays a considerable role in adult neurogenesis [47], in particular, in the hippocampus [48, 49]. Our results add new and important data suggesting that activation of CREB after PDE7 inhibition results in the generation of new neurons with a dopaminergic phenotype.
Several studies have suggested that neurogenesis in the SVZ is impaired in PD, which might be due to the lack of dopamine in the subventricular zone [19, 20, 50]. An impairment in neurogenesis could have negative consequences for the development of new therapeutic approaches, because neural stem cells are a potential source for endogenous repair of the lost dopaminergic neurons. Our in vivo studies demonstrate an enhancement of neural stem cell proliferation and a larger population of new TH-positive cells in the SNpc of rats lesioned with 6-OHDA and treated with S14. Our findings that endogenous neurogenesis can be induced by PDE7 inhibition after a 6-OHDA lesion raises the question of whether this can be used therapeutically in neurodegenerative diseases, and specifically PD. Other studies in rodents have suggested that promoting cell proliferation in the subventricular zone can have a positive effect in models of PD, probably mediated by a neurotrophic effect on the nigrostriatal system [51, 52]. Our results also show an increase, induced by the treatment with S14, in the number of double-labeled nestin/BrdU cells in the SVZ of lesioned rats, indicating an increase in the generation of new progenitor cells in this neurogenic niche. It has been shown that in adult humans, new neurons integrate in the striatum, which is adjacent to the SVZ niche [53]. It is known that in the SVZ of humans, the generation of neuronal precursors occurs; however, unlike rodents, these new neurons are not added in the olfactory bulb of adult humans [54, 55]. This fact poses the question of whether neuroblasts can migrate to another location close to the ventricle (e.g., the striatum). Our data suggest that the newly generated neurons found in the SNpc of adult rats after S14 treatment might originate from the SVZ, such as has been described in humans. In line with this notion, data have shown that, although the vast majority of neurons generated in the SVZ in rodents integrate in the olfactory bulb, a number of striatal neurons are also generated from the SVZ in both rodents and monkeys after a stroke [56–58].
Although some of the newly generated neurons found in the SNpc can be generated in the SVZ, other origins cannot be excluded. The presence of progenitor cells in non-neurogenic regions, such as the cortex, septum, spinal cord, ventricular extension, and SN has also been demonstrated but at a less appreciable level compared with the established neurogenic regions [59]. In the present study, we report that cells expressing the uncommitted neural precursor marker nestin are present not only in the SVZ but also in the SNpc and in the midbrain aqueduct. Similarly, we have found DCX+ cells, a marker associated with dividing neuroblasts, in the SNpc after treatment with S14. We furthermore report a significant increase in the SNpc neurons in rats lesioned with 6-OHDA after treatment with S14. Similar results have been previously reported by others, describing the presence of dopaminergic neurons with BrdU-positive nuclei in the SNpc, suggesting that these cells could have migrated from the midbrain aqueduct or arisen from precursors already existing in the SNpc [34, 60, 61]. However, other investigators have not been able to reproduce these results, and the occurrence of neurogenesis in the SNpc remains controversial [18, 27]. These discrepancies among the different studies could have resulted from the different methodologies used. Our findings suggest that the newly generated dopaminergic cells, in response to the S14 treatment, can originate from precursor cells migrating either from the SVZ or the midbrain aqueduct toward the SNpc and/or from precursor cells already present in the SNpc.
Conclusion
Together, these observations suggest that PDE7 inhibition could represent a method of replacing neurons lost in the SNpc of PD patients and consequently could confer therapeutic benefit in this disease. The PDE7 inhibitor S14 holds great promise as a therapeutic new strategy for PD, because this compound, in addition to inducing the replacement of dopaminergic neurons, is also able to induce significant neuroprotection of the remaining cells.
Supplementary Material
Acknowledgments
This work was supported by MICINN (Grant SAF2010-16365 to A.P.-C.) and by MINECO and FEDER funds (European Union program) (Grant IPT-2012-0762-300000). Centro de Investigación Biomédica en Red sobre Enfermedades Neurodegenerativas (CIBERNED) is funded by the Instituto de Salud Carlos III. J.A.M.-G. is a postdoctoral fellow from CIBERNED.
Author Contributions
J.A.M.-G., A.S., and A.P.-C.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; S.A.-G.: data analysis and interpretation; C.G. and A.M.: collection and/or assembly of data, provision of study materials, manuscript writing.
Disclosure of Potential Conflicts of Interest
C.G. and A.M. have uncompensated intellectual property rights and compensated research funding. The other authors indicated no potential conflicts of interest.
References
- 1.Dawson TM, Dawson VL. Molecular pathways of neurodegeneration in Parkinson’s disease. Science. 2003;302:819–822. doi: 10.1126/science.1087753. [DOI] [PubMed] [Google Scholar]
- 2.Schapira AH. Neurobiology and treatment of Parkinson’s disease. Trends Pharmacol Sci. 2009;30:41–47. doi: 10.1016/j.tips.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 3.Damier P, Hirsch EC, Agid Y, et al. The substantia nigra of the human brain. II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain. 1999;122:1437–1448. doi: 10.1093/brain/122.8.1437. [DOI] [PubMed] [Google Scholar]
- 4.Braak H, Del Tredici K, Rüb U, et al. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- 5.Conti M, Beavo J. Biochemistry and physiology of cyclic nucleotide phosphodiesterases: Essential components in cyclic nucleotide signaling. Annu Rev Biochem. 2007;76:481–511. doi: 10.1146/annurev.biochem.76.060305.150444. [DOI] [PubMed] [Google Scholar]
- 6.Bender AT, Beavo JA. Cyclic nucleotide phosphodiesterases: Molecular regulation to clinical use. Pharmacol Rev. 2006;58:488–520. doi: 10.1124/pr.58.3.5. [DOI] [PubMed] [Google Scholar]
- 7.Kleppisch T. Phosphodiesterases in the central nervous system. Handb Exp Pharmacol. 2009:71–92. doi: 10.1007/978-3-540-68964-5_5. [DOI] [PubMed] [Google Scholar]
- 8.Mehats C, Andersen CB, Filopanti M, et al. Cyclic nucleotide phosphodiesterases and their role in endocrine cell signaling. Trends Endocrinol Metab. 2002;13:29–35. doi: 10.1016/s1043-2760(01)00523-9. [DOI] [PubMed] [Google Scholar]
- 9.Giembycz MA, Smith SJ. Phosphodiesterase 7A: A new therapeutic target for alleviating chronic inflammation? Curr Pharm Des. 2006;12:3207–3220. doi: 10.2174/138161206778194123. [DOI] [PubMed] [Google Scholar]
- 10.Morales-Garcia JA, Redondo M, Alonso-Gil S, et al. Phosphodiesterase 7 inhibition preserves dopaminergic neurons in cellular and rodent models of Parkinson disease. PLoS One. 2011;6:e17240. doi: 10.1371/journal.pone.0017240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Redondo M, Brea J, Perez DI, et al. Effect of phosphodiesterase 7 (PDE7) inhibitors in experimental autoimmune encephalomyelitis mice: Discovery of a new chemically diverse family of compounds. J Med Chem. 2012;55:3274–3284. doi: 10.1021/jm201720d. [DOI] [PubMed] [Google Scholar]
- 12.Redondo M, Zarruk JG, Ceballos P, et al. Neuroprotective efficac of quinazoline type phosphodiesterase 7 inhibitors in cellular cultures and experimental stroke model. Eur J Med Chem. 2012;47:175–185. doi: 10.1016/j.ejmech.2011.10.040. [DOI] [PubMed] [Google Scholar]
- 13.Perez-Gonzalez R, Pascual C, Antequera D, et al. Phosphodiesterase 7 inhibitor reduced cognitive impairment and pathological hallmarks in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2013;34:2133–2145. doi: 10.1016/j.neurobiolaging.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 14.Morales-Garcia JA, Aguilar-Morante D, Hernandez-Encinas E, et al. Silencing phosphodiesterase 7B gene by lentiviral-shRNA interference attenuates neurodegeneration and motor deficits in hemiparkinsonian mice. Neurobiol Aging. 2015;36:1160–1173. doi: 10.1016/j.neurobiolaging.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 15.Gage FH, Kempermann G, Palmer TD, et al. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol. 1998;36:249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 16.Luskin MB, Zigova T, Soteres BJ, et al. Neuronal progenitor cells derived from the anterior subventricular zone of the neonatal rat forebrain continue to proliferate in vitro and express a neuronal phenotype. Mol Cell Neurosci. 1997;8:351–366. doi: 10.1006/mcne.1996.0592. [DOI] [PubMed] [Google Scholar]
- 17.Curtis MA, Faull RL, Eriksson PS. The effect of neurodegenerative diseases on the subventricular zone. Nat Rev Neurosci. 2007;8:712–723. doi: 10.1038/nrn2216. [DOI] [PubMed] [Google Scholar]
- 18.Borta A, Höglinger GU. Dopamine and adult neurogenesis. J Neurochem. 2007;100:587–595. doi: 10.1111/j.1471-4159.2006.04241.x. [DOI] [PubMed] [Google Scholar]
- 19.Baker SA, Baker KA, Hagg T. Dopaminergic nigrostriatal projections regulate neural precursor proliferation in the adult mouse subventricular zone. Eur J Neurosci. 2004;20:575–579. doi: 10.1111/j.1460-9568.2004.03486.x. [DOI] [PubMed] [Google Scholar]
- 20.Höglinger GU, Rizk P, Muriel MP, et al. Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci. 2004;7:726–735. doi: 10.1038/nn1265. [DOI] [PubMed] [Google Scholar]
- 21.Lennington JB, Pope S, Goodheart AE, et al. Midbrain dopamine neurons associated with reward processing innervate the neurogenic subventricular zone. J Neurosci. 2011;31:13078–13087. doi: 10.1523/JNEUROSCI.1197-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morales-Garcia JA, Luna-Medina R, Alfaro-Cervello C, et al. Peroxisome proliferator-activated receptor γ ligands regulate neural stem cell proliferation and differentiation in vitro and in vivo. Glia. 2011;59:293–307. doi: 10.1002/glia.21101. [DOI] [PubMed] [Google Scholar]
- 23.Castaño T, Wang H, Campillo NE, et al. Synthesis, structural analysis, and biological evaluation of thioxoquinazoline derivatives as phosphodiesterase 7 inhibitors. ChemMedChem. 2009;4:866–876. doi: 10.1002/cmdc.200900043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Morales-Garcia JA, Luna-Medina R, Alonso-Gil S, et al. Glycogen synthase kinase 3 inhibition promotes adult hippocampal neurogenesis in vitro and in vivo. ACS Chem Neurosci. 2012;3:963–971. doi: 10.1021/cn300110c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Paxinos CW. The Rat Brain in Stereotaxic Coordinates. San Diego, CA: Academic Press; 1998. [Google Scholar]
- 26.Paterniti I, Mazzon E, Gil C, et al. PDE 7 inhibitors: New potential drugs for the therapy of spinal cord injury. PLoS One. 2011;6:e15937. doi: 10.1371/journal.pone.0015937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lie DC, Dziewczapolski G, Willhoite AR, et al. The adult substantia nigra contains progenitor cells with neurogenic potential. J Neurosci. 2002;22:6639–6649. doi: 10.1523/JNEUROSCI.22-15-06639.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Englund U, Björklund A, Wictorin K. Migration patterns and phenotypic differentiation of long-term expanded human neural progenitor cells after transplantation into the adult rat brain. Brain Res Dev Brain Res. 2002;134:123–141. doi: 10.1016/s0165-3806(01)00330-3. [DOI] [PubMed] [Google Scholar]
- 29.Lendahl U. Gene regulation in the formation of the central nervous system. Acta Paediatr Suppl. 1997;422:8–11. doi: 10.1111/j.1651-2227.1997.tb18337.x. [DOI] [PubMed] [Google Scholar]
- 30.Mazurová Y, Rudolf E, Látr I, et al. Proliferation and differentiation of adult endogenous neural stem cells in response to neurodegenerative process within the striatum. Neurodegener Dis. 2006;3:12–18. doi: 10.1159/000092087. [DOI] [PubMed] [Google Scholar]
- 31.Mitsuhashi T, Aoki Y, Eksioglu YZ, et al. Overexpression of p27Kip1 lengthens the G1 phase in a mouse model that targets inducible gene expression to central nervous system progenitor cells. Proc Natl Acad Sci USA. 2001;98:6435–6440. doi: 10.1073/pnas.111051398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown JP, Couillard-Després S, Cooper-Kuhn CM, et al. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- 33.Couillard-Despres S, Winner B, Schaubeck S, et al. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- 34.Zhao M, Momma S, Delfani K, et al. Evidence for neurogenesis in the adult mammalian substantia nigra. Proc Natl Acad Sci USA. 2003;100:7925–7930. doi: 10.1073/pnas.1131955100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Huot P, Johnston TH, Koprich JB, et al. The pharmacology of l-DOPA-induced dyskinesia in Parkinson’s disease. Pharmacol Rev. 2013;65:171–222. doi: 10.1124/pr.111.005678. [DOI] [PubMed] [Google Scholar]
- 36.Hagell P, Piccini P, Björklund A, et al. Dyskinesias following neural transplantation in Parkinson’s disease. Nat Neurosci. 2002;5:627–628. doi: 10.1038/nn863. [DOI] [PubMed] [Google Scholar]
- 37.Olanow CW, Goetz CG, Kordower JH, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann Neurol. 2003;54:403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 38.Kordower JH, Chu Y, Hauser RA, et al. Transplanted dopaminergic neurons develop PD pathologic changes: A second case report. Mov Disord. 2008;23:2303–2306. doi: 10.1002/mds.22369. [DOI] [PubMed] [Google Scholar]
- 39.Li JY, Englund E, Holton JL, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 40.Ma Y, Tang C, Chaly T, et al. Dopamine cell implantation in Parkinson's disease: Long-term clinical and (18)F-FDOPA PET outcomes. J Nucl Med. 2010;51:7–15. doi: 10.2967/jnumed.109.066811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wada K, Arita M, Nakajima A, et al. Leukotriene B4 and lipoxin A4 are regulatory signals for neural stem cell proliferation and differentiation. FASEB J. 2006;20:1785–1792. doi: 10.1096/fj.06-5809com. [DOI] [PubMed] [Google Scholar]
- 42.Cai M, Zhou Y, Zhou B, et al. Hypoxic conditioned medium from rat cerebral cortical cells enhances the proliferation and differentiation of neural stem cells mainly through PI3-K/Akt pathways. PLoS One. 2014;9:e111938. doi: 10.1371/journal.pone.0111938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen HL, Panchision DM. Concise review: Bone morphogenetic protein pleiotropism in neural stem cells and their derivatives—Alternative pathways, convergent signals. Stem Cells. 2007;25:63–68. doi: 10.1634/stemcells.2006-0339. [DOI] [PubMed] [Google Scholar]
- 44.Bressan RB, Melo FR, Almeida PA, et al. EGF-FGF2 stimulates the proliferation and improves the neuronal commitment of mouse epidermal neural crest stem cells (EPI-NCSCs) Exp Cell Res. 2014;327:37–47. doi: 10.1016/j.yexcr.2014.05.020. [DOI] [PubMed] [Google Scholar]
- 45.Chen SQ, Cai Q, Shen YY, et al. Combined use of NGF/BDNF/bFGF promotes proliferation and differentiation of neural stem cells in vitro. Int J Dev Neurosci. 2014;38:74–78. doi: 10.1016/j.ijdevneu.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 46.Yan CH, Levesque M, Claxton S, et al. Lmx1a and Lmx1b function cooperatively to regulate proliferation, specification, and differentiation of midbrain dopaminergic progenitors. J Neurosci. 2011;31:12413–12425. doi: 10.1523/JNEUROSCI.1077-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nakagawa S, Kim JE, Lee R, et al. Regulation of neurogenesis in adult mouse hippocampus by cAMP and the cAMP response element-binding protein. 2002;22:3673–3682. doi: 10.1523/JNEUROSCI.22-09-03673.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Merz K, Herold S, Lie DC. CREB in adult neurogenesis—Master and partner in the development of adult-born neurons? Eur J Neurosci. 2011;33:1078–1086. doi: 10.1111/j.1460-9568.2011.07606.x. [DOI] [PubMed] [Google Scholar]
- 49.Zhu DY, Lau L, Liu SH, et al. Activation of cAMP-response-element-binding protein (CREB) after focal cerebral ischemia stimulates neurogenesis in the adult dentate gyrus. Proc Natl Acad Sci USA. 2004;101:9453–9457. doi: 10.1073/pnas.0401063101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Freundlieb N, Francois C, Tande D, et al. Dopaminergic substantia nigra neurons project topographically organized to the subventricular zone and stimulate precursor cell proliferation in aged primates. J Neurosci. 2006;26:2321–2325. doi: 10.1523/JNEUROSCI.4859-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Androutsellis-Theotokis A, Rueger MA, Park DM, et al. Targeting neural precursors in the adult brain rescues injured dopamine neurons. Proc Natl Acad Sci USA. 2009;106:13570–13575. doi: 10.1073/pnas.0905125106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zachrisson O, Zhao M, Andersson A, et al. Restorative effects of platelet derived growth factor-BB in rodent models of Parkinson's disease. J Parkinsons Dis. 2011;1:49–63. doi: 10.3233/JPD-2011-0003. [DOI] [PubMed] [Google Scholar]
- 53.Ernst A, Alkass K, Bernard S, et al. Neurogenesis in the striatum of the adult human brain. Cell. 2014;156:1072–1083. doi: 10.1016/j.cell.2014.01.044. [DOI] [PubMed] [Google Scholar]
- 54.Bergmann O, Liebl J, Bernard S, et al. The age of olfactory bulb neurons in humans. Neuron. 2012;74:634–639. doi: 10.1016/j.neuron.2012.03.030. [DOI] [PubMed] [Google Scholar]
- 55.Sanai N, Nguyen T, Ihrie RA, et al. Corridors of migrating neurons in the human brain and their decline during infancy. Nature. 2011;478:382–386. doi: 10.1038/nature10487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hou SW, Wang YQ, Xu M, et al. Functional integration of newly generated neurons into striatum after cerebral ischemia in the adult rat brain. Stroke. 2008;39:2837–2844. doi: 10.1161/STROKEAHA.107.510982. [DOI] [PubMed] [Google Scholar]
- 57.Tonchev AB, Yamashima T, Sawamoto K, et al. Enhanced proliferation of progenitor cells in the subventricular zone and limited neuronal production in the striatum and neocortex of adult macaque monkeys after global cerebral ischemia. J Neurosci Res. 2005;81:776–788. doi: 10.1002/jnr.20604. [DOI] [PubMed] [Google Scholar]
- 58.Wei B, Nie Y, Li X, et al. Emx1-expressing neural stem cells in the subventricular zone give rise to new interneurons in the ischemic injured striatum. Eur J Neurosci. 2011;33:819–830. doi: 10.1111/j.1460-9568.2010.07570.x. [DOI] [PubMed] [Google Scholar]
- 59.Aponso PM, Faull RL, Connor B. Increased progenitor cell proliferation and astrogenesis in the partial progressive 6-hydroxydopamine model of Parkinson’s disease. Neuroscience. 2008;151:1142–1153. doi: 10.1016/j.neuroscience.2007.11.036. [DOI] [PubMed] [Google Scholar]
- 60.Kawano H, Ohyama K, Kawamura K, et al. Migration of dopaminergic neurons in the embryonic mesencephalon of mice. Brain Res Dev Brain Res. 1995;86:101–113. doi: 10.1016/0165-3806(95)00018-9. [DOI] [PubMed] [Google Scholar]
- 61.Meyer AK, Maisel M, Hermann A, et al. Restorative approaches in Parkinson’s disease: Which cell type wins the race? J Neurol Sci. 2010;289:93–103. doi: 10.1016/j.jns.2009.08.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.