Abstract
Our current understanding of cystic fibrosis (CF) has revealed that the biophysical properties of mucus play a considerable role in the pathogenesis of the disease in view of the fact that most mucus-producing organs are affected in CF patients. In this review, we discuss the potential causal relationship between altered cystic fibrosis transmembrane conductance regulator (CFTR) function and the production of mucus with abnormal biophysical properties in the intestine and lungs, highlighting what has been learned from cell cultures and animal models that mimic CF pathogenesis. A similar cascade of events, including mucus obstruction, infection and inflammation, is common to all epithelia affected by impaired surface hydration. Hence, the main structural components of mucus, namely the polymeric, gel-forming mucins, are critical to the onset of the disease. Defective CFTR leads to epithelial surface dehydration, altered pH/electrolyte composition and mucin concentration. Further, it can influence mucin transition from the intracellular to extracellular environment, potentially resulting in aberrant mucus gel formation. While defective HCO3− production has long been identified as a feature of CF, it has only recently been considered as a key player in the transition phase of mucins. We conclude by examining the influence of mucins on the biophysical properties of CF sputum and discuss existing and novel therapies aimed at removing mucus from the lungs.
Keywords: Mucin, Mucus, CF, Goblet cells, CFTR, Mucociliary Clearance, Pathogenesis
1. Introduction
CF is the most common genetic disease, occurring prevalently in the Caucasian population at a rate of 1 in 2,500 newborns of Northern European descent. The most common symptoms of CF include progressive lung disease and chronic digestive conditions, which are the result of mutations of the cystic fibrosis transmembrane conductance regulator (CFTR) (Riordan et al. , 1989). Depending on genetic and environmental factors, symptom severity varies among individuals carrying CFTR mutations. More specifically, CF pathogenesis is characterized by the build-up of thick, sticky mucus in multiple mucin-producing organs, such as the lungs, sinuses, intestine, pancreas and reproductive organs. For this reason, CF is also known as mucoviscidosis, suggesting that polymeric, gel-forming mucins, the large O-linked glycoproteins responsible for the viscoelastic properties of mucus, play a critical role in the disease (Kreda et al. , 2012).
Since the discovery of the defective CF gene in 1989, almost 2000 mutations have been reported and are available in the Cystic Fibrosis Mutation Database1. The CFTR gene encodes for a cyclic adenosine monophosphate (cAMP) regulated chloride channel expressed in apical membranes of various epithelia (Gregory et al. , 1990). In addition to controlling chloride secretion, this channel regulates the function of other membrane proteins, including the epithelial sodium channel (ENaC) (Briel et al. , 1998). Both CFTR and ENaC play an important role in maintaining homeostasis by controlling the movement of water through the epithelium, which is particularly important for mucous membranes. Hence, CFTR malfunction leads to fluid hyperabsorption and subsequent dehydration of the epithelial surface, which, in turn, results in abnormal mucus gel with an increased polymeric mucin concentration and altered biophysical properties (Boucher, 2007, Button et al. , 2012).
Among other tissues, CFTR is expressed in the pancreas, intestine, lungs, and reproductive tract, and each organ-specific phenotype can be related to the production of aberrant mucus with altered biophysical properties (Burgel et al. , 2007, Malmberg et al. , 2006, Reid et al. , 1997). Common symptoms exhibited in mucin-producing organs are blocked ducts and impaired mucosal defence. In this review, we focus on the role of CFTR in the intestine and lungs since these organs have been the subjects of most studies involving the biophysical properties of mucins. By controlling fluid secretion and regulating ion composition in these organs, CFTR plays a critical role in mucosal defence by modulating the biophysical properties of mucus and assisting in bacterial killing (Norkina et al. , 2004a, Pezzulo et al. , 2012, Puchelle et al. , 2002). Abnormal modulation of epithelial inflammation related to CFTR malfunction may further contribute to CF pathophysiology, but the link has yet to be established. CFTR also plays an important role in the transcellular secretion of bicarbonate (HCO3−), an alkalizing agent that plays a crucial physiological role in pH buffering. Although impaired bicarbonate secretion was reported early in the discovery of the disease (Hadorn et al. , 1968), the role of this anion in CF has only recently been a major focus of interest within the mucus/mucin community. As highlighted by Quinton (Quinton, 2008), CF individuals suffer from complications in all mucin-secreting organs, which may be a consequence of defective HCO3− transport. Consistent with this idea, reduced HCO3− secretion in CF may be responsible for lowered epithelial surface pH, which has been shown to impede bacterial killing (Pezzulo et al., 2012) and increase mucus/mucin viscoelasticity (Celli et al. , 2005, Georgiades et al. , 2013). More specifically, there is emerging evidence that HCO3− plays a key role in the expansion of polymeric mucins after their secretion into the extracellular milieu that is essential for normal mucus gel formation and transport (Chen et al. , 2010, Cooper et al. , 2013).
Before the use of physiotherapy (to remove mucus from the lungs) and enzyme therapy (e.g., pancreatic enzymes to correct digestive enzyme insufficiency and inhaled DNase treatment to alter mucus properties), CF was considered to be a fatal childhood disease. However, with improved therapies and strategies for managing the disease, the average life expectancy for CF patients is now 37 years2. In recent years, small molecule therapies directed at restoring CFTR functionality have yielded success with a small number of mutations and are being pursued for other, more common mutations. Indeed, the goal of developing CFTR-specific therapies for all patients is currently a key objective of the Cystic Fibrosis Foundation. In the meantime, alternative approaches are being investigated (e.g., the use of mucolytics, inhibitors of mucin secretion and osmotic agents) to address the problem of mucus accumulation/obstruction in CF patients. In this article, we will review the current understanding of mucus and mucins in CF, describe how inefficient post-secretory mucin expansion may result in mucus with aberrant physical properties, and conclude by discussing therapies aimed at removing mucus from the lungs.
2. Consequences of CFTR mutations for mucus
The molecular mechanisms by which CFTR mutations can disrupt CFTR function include defects in protein synthesis (class I), maturation/trafficking (class II, e.g., ΔF508, the most common mutation with nearly 90% of patients carrying at least one allele), channel gating (class III, e.g. G551D, the target of Kalydeco; see Section 4), altered conductance (class IV) and decreased CFTR abundance (class V) (Ferec and Cutting, 2012). As mentioned above, these defects lead to deficient cAMP-dependent Cl− and HCO3− secretion and enhanced ENaC-mediated Na+ absorption in affected epithelia, resulting in dehydration of the mucus and concentration of its components (e.g., mucins) (Boucher, 2007). As a result, the majority of CF patients require a daily routine of inhaled therapies and exercise to prevent the progression of lung disease or a decrease in lung function. Additionally, most CF patients suffer from gastrointestinal (GI) manifestations regardless of their genotype. A similar pathogenesis cascade of obstruction, infection and inflammation is observed in the airways and intestines and is thought to be the direct result of epithelial surface dehydration and abnormal electrolyte composition.
2.1 Changes in mucus properties in the intestine
Expression of the CFTR gene in the GI tract is low in the stomach and rises in the intestine, displaying a gradient with the highest mRNA levels in the duodenum and lowest levels in the large intestine (Strong et al. , 1994). This pattern of expression may reflect the need for acid neutralization via HCO3− secretion as the proximal intestine receives a highly acidic bolus from the stomach. In addition to exhibiting high levels of CFTR expression, the normal intestine receives large volumes of HCO3−-rich material from the pancreas. Proper pH buffering and fluid secretion in the intestine is essential for the optimal functioning of digestive enzymes and the maintenance of normal bacterial flora. In the case of CF, the combination of prolonged acidity following food uptake and dehydrated intestinal mucus facilitates bacterial growth in the GI tract (De Lisle and Borowitz, 2013, Fridge et al. , 2007, Lisowska et al. , 2009). Mucus adhesion to the intestinal wall may be related to mucin hyperconcentration and/or abnormal mucin expansion upon granule exocytosis (Garcia et al. , 2009, Kesimer et al. , 2010, Verdugo, 2012b); this latter process will be discussed in more depth, below (see Section 3.1). The most serious acute complication of the CF intestinal phenotype is the obstruction of the terminal ileum or proximal large intestine, referred to as meconium ileus, which occurs frequently in CF neonates with severe genotypes (Feingold and Guilloud-Bataille, 1999). Meconium ileus, which is a complex, mucin-rich substance (Schachter and Dixon, 1965), can be surgically removed or washed out with osmotic agents but if untreated can result in the rupture of the intestinal wall and sepsis (De Lisle and Borowitz, 2013). Other consequences of CFTR mutations in the intestine include an abnormal GI microbiota with altered diversity, location and density, and increased inflammation, which leads to ulcerations in 60% of CF patients as revealed by a new endoscopy technique relying on swallowed capsules (PillCam) (Werlin et al. , 2010).
Gene-targeted animal models (e.g., mice, pigs and ferrets) lacking CFTR or Cftr expression develop meconium ileus at birth, confirming the importance of CFTR in the GI tract (Rogers et al. , 2008, Snouwaert et al. , 1992, Sun et al. , 2010). Cftr mutant mouse models, which include both Cftr point mutations (e.g., Cftr!F508/!F508) and complete Cftr knockouts (e.g., Cftr−/−), have greatly contributed to our understanding of the GI manifestations of CF. However, Cftr mutant mice develop only mild lung phenotypes, likely due to compensation mechanisms that stimulate other Cl− channels, such that these mice are not as effective for studying CF lung pathogenesis. The major intestinal phenotypes in CF mouse models are obstruction, bacterial colonization and inflammation (Guilbault et al. , 2007, Lynch et al. , 2013). Mucus adhesion contributes to bacterial colonization in CF mice, which can be alleviated by laxative treatment (De Lisle et al. , 2007). Although a direct causal relationship between bacterial colonization and inflammation has not yet been established, CF mice exhibit increased levels of neutrophils, mast-cells and inflammatory markers (Norkina et al. , 2004b). The slow intestinal transit due to abnormal mucus biophysical properties appears to initiate a cascade of events that promote chronic bacterial infection and inflammation.
2.2 Impact on mucociliary clearance in the lungs
Although CFTR mutations affect several organs, the progression of lung disease can be particularly life-threatening. Despite much improvement in therapies, pulmonary complications are still the major cause of morbidity and mortality in CF patients. CF patients experiencing a rapid decline in lung function typically display poor survival rate unless they undergo lung transplantation (Rosenbluth et al. , 2004). The production of “abnormal” mucus with aberrant rheological properties in the lungs leads to a cascade of events that involve mucus adhesion to epithelial surfaces, airway plugging, chronic bacterial infection and inflammation (Button et al. , 2012, Mall et al. , 2004, Stoltz et al. , 2010). Once these early events (i.e., mucus stasis and/or bacterial infection) take place in the lungs, complex feedback mechanisms ensue. For instance, bacterial infection stimulates goblet cell hyperplasia and the recruitment of neutrophils (Cash et al. , 1979), which worsen mucus viscosity and reduce clearance via the release of extracellular DNA (Lethem et al. , 1990, Shak et al. , 1990). Impaired clearance facilitates bacterial growth and biofilm formation, triggering more inflammation (Davies, 2002, Stoltz et al. , 2010). Recently, much effort from the scientific community has focused on the specific mechanisms underlying the onset of lung disease in CF patients; i.e., whether mucin concentration or impaired bacterial killing alone can prompt CF lung pathogenesis.
In CF, airway dehydration leads to airway surface liquid (ASL) volume depletion, increased mucus concentration and reduced mucociliary clearance (MCC) (Boucher, 2007, Matsui et al. , 1998). Specifically, increased mucin concentration causes the osmotic pressure of the mucus layer to increase, which draws water osmotically from the periciliary layer (PCL) that is occupied, in part, by cell-surface tethered mucins (Button et al. , 2012, Kesimer et al. , 2013). Beyond a critical concentration of 5% solids, the osmotic pressure of the mucus layer exceeds the osmotic pressure of the PCL, initiating the collapse of the PCL. As a result of this collapse, cilia are compressed and unable to beat. The mucus layer adheres to the cell surfaces of the epithelium, causing MCC to cease. Mucus stasis eventually leads to airway plugging, chronic bacterial infection, inflammation and airway tissue damage (bronchiectasis).
As mentioned earlier, Cftr mutant mice develop only mild lung phenotypes. However, it was still possible to validate the hypothesis that airway dehydration promotes mucus plugging and triggers inflammation using a mouse model that overexpresses the β subunit of the Na+ channel in the lungs, also called the βENaC mouse (Mall et al. , 2004). This model has demonstrated that accelerated Na+ transport alone produces a CF-like lung phenotype that exhibits PCL collapse, slowed MCC rate, mucus obstruction and neutrophilic inflammation (Figure 1). βENaC-overexpressing neonates show a temporary increase in bacterial burden that appears to resolve by adulthood (Livraghi-Butrico et al. , 2012). Interestingly, βENaC-overexpressing animals born and housed in germ-free (gnotobiotic) and LPS-free conditions developed and maintained an inflammatory phenotype, suggesting that mucus dehydration alone is sufficient to trigger airway inflammation (Livraghi-Butrico et al. , 2012). These results imply that the epithelium responds to an abnormal mucus layer by initiating the recruitment of neutrophils. These early events instigate a vicious cycle in the lungs as dying neutrophils entrapped in thick mucus release their DNA and worsen the rheological properties of mucus (Lethem et al. , 1990, Shak et al. , 1990). This thick, immobile mucus provides an optimal environment for bacterial growth and, consequently, stimulates further mucin release and neutrophil infiltration (Knowles and Boucher, 2002, Smith, 1997, Wanner et al. , 1996).
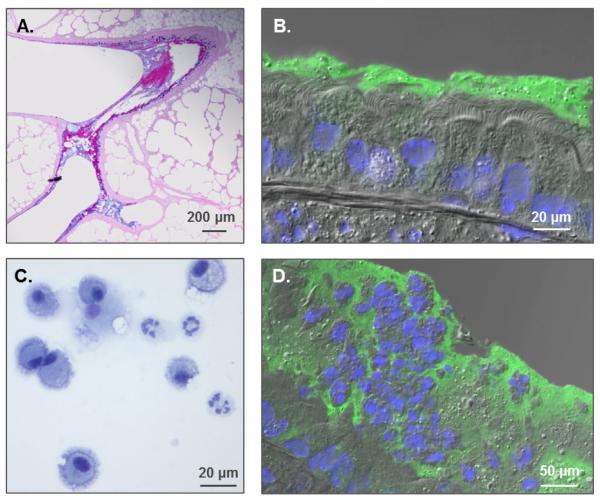
Fig. 1. Formation of mucus plaques and neutrophilic inflammation in the • ENaC mouse model.
(A.) AB-PAS stain of βENaC lungs revealing mucus plugs at airway branching. (B.) Immunohistochemistry (IHC) displaying PCL collapse and mucin accumulation on airway surfaces (green=Muc5b, Blue=DAPI). (C.) Inflammatory cells from a βENaC mouse bronchoalveolar lavage showing a mixture of macrophages and neutrophils. (D.) IHC exhibiting inflammatory cells trapped in mucus.
In contrast with the mouse, the CF pig model that expresses a mutant form (-/! 508) spontaneously develops the features of CF lung disease, including infection (Stoltz et al. , 2010). In this model, airways can be completely occluded with tenacious, adherent mucus, resembling mucus observed in human CF lungs. Histopathology has revealed that these mucus plaques exhibit a lamellar appearance, suggesting that the progressive deposition of mucus is the result of slowed MCC. Within hours of birth (6-12h), CF pigs show no inflammation but increased bacterial burden. The impaired ability to eradicate bacteria is related to lowered ASL pH, which can be directly linked to the inability of CF airways to secrete HCO3− (Pezzulo et al. , 2012, Stoltz et al. , 2010). Although HCO3− plays a critical role in bacterial killing, the mucus obstructive phenotype is not exclusively related to defective HCO3− secretion since the βENaC mouse model develops mucus plugging subsequent to airway dehydration.
3. Polymeric, gel-forming mucins
The molecular framework and biophysical properties of mucus are provided in large part by the high-molecular-weight (Mr 2-100MDa) polymeric gel-forming mucins (Sheehan et al. 1986). The products of 5 genes (MUC genes), MUC2, MUC5AC, MUC5B, MUC6 and MUC19 in the human and Muc2, Muc5ac, Muc5b, Muc6 and Muc19 in the mouse (Chen et al. , 2004, Escande et al. , 2004, Thornton et al. , 2008), make up this family of closely related O-linked glycoproteins (Figure 2). Of particular relevance to this review are MUC2/Muc2 (Gum et al. , 1994), the major intestinal mucin, and MUC5AC/Muc5ac and MUC5B/Muc5b, the major respiratory mucins (Thornton et al. , 2008). The physicochemical and biological properties of these mucins are dictated by their heavily glycosylated mucin domains. The dense glycosylation of these molecules (up to 80% in mass) with neutral and negatively-charged O-glycans stiffens the mucin polypeptide, resulting in a high volume occupancy in solution which is important for gel-formation (Gerken, 1993). Furthermore, the extensive glycosylation protects the underlying mucin polypeptide from degradation by host and pathogen-proteases produced during inflammation and infection (Hasnain et al. , 2012, Henke et al. , 2011, Innes et al. , 2009) and provides specific ligands for bacterial adhesins that influence the interaction of the host with commensal and pathogenic organisms (McGuckin et al. , 2011).
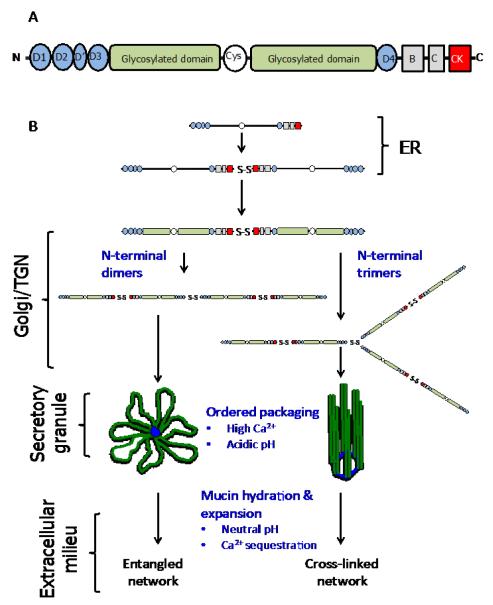
Fig. 2. Cartoon of mucin structure and assembly.
(A) Polymeric, gel-forming mucins all share a common structural architecture. The N- and C-terminal domains have a high cysteine content and these form intra- and intermolecular disulphide bonds. The central highly-glycosylated domains (mucin domains) are enriched in serine, threonine and proline residues and the O-glycans are covalently attached, via the linkage sugar N-acetylgalactosamine, to serine and threonine residues. The O-glycan chains are comprised of N-acetylglucosamine, galactose, N-acetylgalactosamine, fucose and sialic acid; galactose can be modified by sulphation. The number, length and amino acid sequence of these glycosylated domains differ between mucins. The glycosylated domains are interrupted with cys-domains, and the number of these cysteine-rich regions differs between mucins. For more detailed reviews on the primary structure of MUC2, MUC5AC and MUC5B the reader is referred to the following articles - Dekker et al., 2002 and Rose and Voynow, 2006. (B) The early steps in polymeric mucin assembly are well accepted. In the endoplasmic reticulum (ER), the non-O-glycosylated polypeptide forms dimers via disulphide bonds formed between the CK-domains. In the golgi/trans-golgi network (TGN), mucin dimers are O-glycosylated and then multimerise by disulphide bonds formed between N-terminal D3 domains. There are two mechanisms proposed for this step, multimers form from dimers of dimers (MUC5B, Ridley et al. , 2011, Round et al. , 2004, Sheehan et al. , 2004, Thornton et al. , 1990) or trimers of dimers (MUC2, Ambort et al., 2012). The mucin multimers are then packged in an ordered state within secretory granules. The acidic pH inside the granules and their high Ca2+ content facilitates mucin organisation via non-covalent interactions between mucin N-termini (Ambort et al., 2012). After secretion mucins hydrate and expand to form mucus.
Flanking the central glycosylated region are the N- and C-terminal cysteine-rich domains of the mucin monomers, which are important for intracellular disulphide-linked polymerization (Asker et al. , 1998a, b, Asker et al. , 1995, Axelsson et al. , 1998, Perez-Vilar and Hill, 1999); their role in this process will be discussed in more detail below. Other cysteine-rich domains (cys-domains) interrupt the central glycosylated regions of the mucin monomers and their number is different between mucins; MUC2, MUC5B and MUC5AC contain 2, 7 and 9 respectively (Rose and Voynow, 2006). The different patterns of interruption of the glycosylated domains coupled with the tissue-specific expression of the mucins (intestinal tract for MUC2 and respiratory tract for MUC5AC and MUC5B) implies different functions for MUC2 compared with MUC5AC and MUC5B. It has been suggested that cys-domains have a role in non-covalent, dynamic cross-linking of mucin polymers within mucus (Ambort et al. , 2011). One could hypothesise that their location between the stiffened, glycosylated regions of the mucin polypeptide would provide points of flexibility within the mucin polymers that might be important for the dynamic behaviour of the mucin polymers in mucus, and/or during their intracellular assembly. Moreover, cys-domains may provide sites for proteolytic cleavage for normal mucin turnover or degradation during infection/inflammation. Further research is needed to fully elucidate the function of these domains.
At the mucosal surfaces, mucins are produced mainly from specialised secretory cells: surface epithelial goblet cells (in the intestine and lungs) and glandular mucous cells (in the lungs). In these cells, the pre-assembled mucin polymers are stored, condensed and dehydrated within secretory granules (Verdugo, 1991 ). The release of mucin granules is regulated either by a baseline mechanism or a purinergic-induced secretory pathway (Davis and Dickey, 2008). Once secreted into the extracellular environment, mucins hydrate and expand to form a mucus gel with viscoelastic properties that depend on the entanglement of large mucin polymer chains (Georgiades et al. , 2013, Raynal et al. , 2002, Verdugo, 2012b, Verdugo et al. , 1987). Other factors will influence the viscoelastic properties of mucus, including hydration status (i.e., mucin concentration), ionic environment, pH, and the degree of cross-linking (covalent and non-covalent bonds) between mucin polymeric chains and between mucins and other components of mucus (i.e., globular proteins) (Ambort et al. , 2012, Ambort et al. , 2011, Georgiades et al. , 2013, Raynal et al. , 2003). Thus, CFTR malfunction and the resultant consequences for the epithelial surface (e.g., altered mucin and electrolyte concentrations and abnormal pH) will have major effects on the propensity of mucus gel-formation. In particular, a current focus of mucin-related research in CF is the mechanism of intracellular mucin polymer formation and the role of HCO3− in their transition from an intracellular to extracellular form.
3.1 Intracellular mucin polymer formation and post-secretion expansion
The intracellular assembly of polymeric mucins, as detailed in Figure 2, is a complex process. Disulphide-linkage between C-terminal domains yields dimers, which are subsequently polymerised via N-terminal disulphide bonding (Asker et al. , 1998a, b, Asker et al. , 1995, Axelsson et al. , 1998, Perez-Vilar and Hill, 1999). The molecular details of intracellular mucin polymer formation have best been described for the intestinal mucin, MUC2, and have yet to be fully elucidated for the respiratory mucins, MUC5AC and MUC5B. In brief, MUC2 dimers are reported to polymerise via N-terminal trimerisation to yield branched polymers (Ambort et al. , 2012, Godl et al. , 2002), whereas MUC5B and MUC5AC dimers polymerise via N-terminal dimerisation to form linear polymers (Ridley et al. , 2011, Round et al. , 2004, Sheehan et al. , 2004, Thornton et al. , 1990). Whatever the mechanism of polymer assembly, it has become increasingly clear that mucin polymers are highly organised within the secretory granules (Ambort et al. , 2012, Kesimer et al. , 2010), and that this is necessary in order to control their rapid expansion after secretion into the extracellular environment to form mucus (see Verdugo 2012a for an in-depth discussion of the biophysics and dynamics of post-secretory mucin expansion).
It has been proposed that Ca2+ ions have critical roles in the intragranular organisation of mucins. It has long been accepted that shielding the negative charge associated with the O-glycans (sialic acid and sulphate groups) by Ca2+ ions facilitates mucin compaction (Kuver and Lee, 2004, Verdugo et al. , 1987). More recently, it has been suggested that Ca2+ ions, in the acidic pH environment of the granule, organise the N-termini of MUC2 into 6-membered ring-like structures that facilitate efficient packing (Ambort et al. , 2012). These authors proposed a novel model for the highly organised packing of MUC2 within the granule, and how this facilitates the unpacking necessary to form the lamellar structure reported for MUC2-rich mucin gels (Round et al. , 2012). However, this model remains controversial and is incompatible with the swelling kinetics and behaviour of mucins as they expand after secretion (Verdugo, 2012a, b). Moreover, whether this model can be generalized to MUC5AC and MUC5B, which form linear rather than branched polymers, has yet to be determined. Electron microscopy images of salivary mucins suggest that intragranular MUC5B is not packaged as a random coil but exists as a cross-linked structure that is organised around ‘nodes’ containing the terminal protein domains of the mucin polypeptide (Kesimer et al. , 2010). However, the involvement of Ca2+ ions in these ‘nodes’ has not been determined.
Upon mucin exocytosis onto the hydrated epithelial surface, Ca2+ ions are exchanged for Na+ ions and pH is increased, leading to a rapid expansion of the mucin polymers (Verdugo, 1991, 2012b). Although we currently have an incomplete understanding of the mechanism controlling this critical transition, it has been shown that HCO3− ions in the luminal milieu play an important role in mucin expansion by sequestering Ca2+ ions and promoting alkalinization, thereby facilitating efficient expansion and hydration of the glycosylated mucin domains (Chen et al. , 2010, Gustafsson et al. , 2012, Quinton, 2010). This suggests that a critical function of CFTR is regulating of the viscoelastic properties of mucus, and that HCO3− is in short supply during the course of mucin granule exocytosis in patients with CF. Defective HCO3−-mediated post-secretory mucin expansion coupled with defective hydration likely causes intestinal blockage (Garcia et al. , 2009) and airway obstruction (Cooper et al. , 2013). One of the striking phenotypes exhibited by CF mice, besides mecomium ileus, is the production of abnormal ileal mucus that is adherent to the epithelium. This mucus is two or three times denser than mucus of wild-type (WT) mice and less penetrable to beads the size of bacteria (Gustafsson et al. , 2012). Remarkably, in this mouse model, CF mucus properties appear to be normalised when mucins are secreted into buffers containing 115 mM HCO3− or 20 mM EDTA (Gustafsson et al. , 2012), likely due to sequestration of Ca2+ ions that allow mucins to expand. In addition, the volume of CF mucus (but not WT mucus) increases in a HCO3−-enriched milieu, suggesting that CF mucus is incompletely expanded (Ambort et al. , 2012).
The use of CF mice has provided a better understanding of, and new insights into the importance of HCO3− in the expansion of mucins, particularly MUC2/Muc2. Whether this new knowledge can be generalised to respiratory mucins is not yet clear because the mechanisms of polymerisation for MUC5AC and MUC5B have yet to be determined. Intestinal mucus has been described as a chemical gel where the mucin matrix is formed from branched polymers (as a result of N-terminal trimerisation; Figure 2), resulting in a covalently cross-linked gel network (Ambort et al. , 2011, Hong et al. , 2005). In contrast, respiratory mucus is a physical gel in which linear polymers (as a result of N-terminal dimerization; Figure 2) are entangled and maintained in close proximity by electrostatic and hydrophobic bonds (Sheehan et al. , 2004, Thornton et al. , 2008). These different mucin network organisations may be related to the different functional requirements of their respective organs. In the intestine, a tight mucin network with limited swelling capacity might provide the best protection against the passage of large volumes of gastric juice and partially digested food. On the other hand, the respiratory tract might benefit from airway mucus that is more dynamic, allowing for the precise tuning of its rheological properties for optimal MCC and, at the same time, ensuring bacterial trapping.
3.2 Mucins in CF sputum
MUC5AC and MUC5B are the major gel-forming mucins produced in the lungs. Traditionally, their function has been viewed as a ‘catch-all’ barrier to trap inhaled pathogens, particulates that are continuously removed from the lungs by the coordinated beating of cilia. The specific roles of MUC5AC and MUC5B have been largely undetermined until recently. Further, the O-glycans coating these mucins may have diverged, evolutionary, to suit the needs of different species. Nonetheless, mouse models generated to examine the role of mucins have revealed that Muc5ac provides protection against viral infection by acting as a decoy for viral receptors (Ehre et al. , 2012), while Muc5b is essential for MCC and controlling bacterial infection (Muc5ac appears dispensable for these latter functions) (Roy et al. , 2013).
Analysis of CF sputum mucin content has revealed increased MUC5AC and MUC5B concentrations (especially following exacerbations) with MUC5B being the predominant mucin (Henke et al. , 2007, Horsley et al. , 2013, Kirkham et al. , 2002). Likewise, immunohistochemical analysis has demonstrated increased concentrations MUC5AC and MUC5B in the mucus plugs of CF airways when compared to normal controls, again, with a higher relative abundance of MUC5B (Burgel et al. , 2007). Analysis of the macromolecular properties of polymeric mucins in CF sputum has shown that they are, on average, smaller compared to normal mucins, likely due to the increased proteolytic activity of CF sputum (Davies et al. , 2002, Gupta and Jentoft, 1992, Rose et al. , 1987, Thornton et al. , 1991). Intriguingly, a significant genetic association was found between the length of the gene region encoding the MUC5AC variable number of tandem repeats (VNTR) and CF lung disease severity. However, the mechanisms underlying this association remain obscure (Guo et al. , 2011).
While CF mucus exhibits an altered composition of mucins, there are no major changes in the macromolecular properties of the mucins that might explain the sub-optimal mucus transport properties. Mucins isolated from expectorated sputum have not been reported to contain cross-linked species that could represent the unexpanded, intragranular form. However, this molecular form may be more highly represented in adherent mucus plaques that are not expectorated. Detailed molecular analysis of the lamellar-structured plaques found in the CF pig airways might shed light on this issue.
In contrast to normal respiratory mucus, the physical properties of CF sputum are not wholly due to polymeric mucins but arise from a complex interaction between the mucins and other sputum components, including cell surface mucins, DNA and actin, as well as inflammatory cells, bacteria, viruses and exoproducts (Matthews et al. , 1963, Voynow and Rubin, 2009). As a result, the airways clearance of mucus that occurs through the coordinated beating of cilia is compromised; however, the influence of these different components on the aberrant rheological properties of sputum is incompletely understood. The importance of mucins as opposed to other macromolecules, particularly DNA, in determining the viscoelastic properties of CF sputum has been questioned (Henke et al. , 2004). However, the biophysical properties of mucins in CF secretions may have been underestimated as a result of proteolytic degradation, rendering rheological assays inaccurate and difficult to perform (Horsley et al. , 2013). Moreover, proteolysis would explain observations suggesting mucin levels were lower in CF sputum than in healthy controls (Henke et al. , 2004). Nonetheless, the clinical benefits associated with treatments aimed at improving mucus clearance have been clearly demonstrated in CF patients. Typically, removal of thickened, adhered, mucus relies on physical (i.e., physiotherapy) and/or chemical (i.e., orally administered or inhaled medications) therapies. Indeed, agents aimed at improving mucin solubilisation will have an important role in the treatment of CF lung disease.
4. Therapies aimed at removing mucus from the lungs
Therapeutic strategies targeting the underlying cause of CF (i.e., CFTR malfunction) offer important benefits for CF patients as compared to treating a wide range of symptoms. Thus, for the first 10 to 15 years following the discovery of the gene defect in CF, research towards correcting the defect by gene therapy was a major focus of the CF community. However, it was eventually realised that gene transfer into diseased lungs was going to be more difficult than originally anticipated, likely due to a physical barrier produced by persistent mucus production and the short-lived expression of the delivered therapeutic gene (Griesenbach et al. , 2003). This led to greater exploration of alternative approaches for correcting CFTR malfunction. More recently, high-throughput screening programs for the identification of compounds capable of restoring CFTR function (see Section 2) were initiated by the Cystic Fibrosis Foundation and lead to the discovery of Ivacaftor (Kalydeco or VX-770) by Vertex Pharmaceuticals Inc. In vitro, Ivacaftor was shown to increase Cl−-transport activity of cell lines expressing the G551D-CFTR mutation by increasing the time the channel remained open at the cell surface (i.e., it is a CFTR potentiator) (Van Goor et al. , 2009). Approximately 4 to 5% of CF patients carry the G551D mutation, limiting the impact of this oral drug on the global CF population (McKone et al. , 2003). Nevertheless, the treatment of G551D patients was associated with rapid and sustained improvement in lung function (FEV1), reduced pulmonary exacerbations and respiratory symptoms, weight gain and increased sweat chloride concentration (Accurso et al. , 2010, Ramsey et al. , 2011). In addition, remarkable improvements in mucociliary clearance and ventilation defects have been shown during treatment with Ivacaftor, suggesting that restitution of CFTR function is sufficient to normalise the aberrant properties of mucus (Altes et al. , 2012, Donaldson et al. , 2013). Currently, two large clinical trials testing the effects of Ivacaftor in combination with a CFTR corrector (VX-809) in patients carrying the most common mutation (! F508) are being conducted, based upon promising results in earlier studies, including a significant improvement in lung function (Boyle et al. , 2012).
Although correcting the underlying cause of CF is an overarching goal and appears to restore mucociliary clearance (Altes et al. , 2012, Donaldson et al. , 2013), alternative therapies that reduce airway mucus obstruction (mucolytics) have been used for decades and novel therapies with this particular aim are currently being investigated. The discovery that CF secretions contain substantial amounts of extracellular DNA led to the development of a recombinant human DNase I (rhDNase or Dornase α or Pulmozyme®) by Genentech Inc. in the 1990s. Initially, in vitro experiments confirmed that rhDNase decreased the viscosity of CF sputum (Shak et al. , 1990). Hence, rhDNase was tested in patients via inhalation and resulted in reduced exacerbations and respiratory symptoms, as well as improved FEV1 (Fuchs et al. , 1994, Ramsey et al. , 1993). Over the past two decades, rhDNase has been routinely used for CF patient care as a means of clearing mucopurulent mucus, but alone is not completely effective at alleviating the problems associated with mucus overproduction and airway obstruction.
Since breaking down large DNA molecules improves respiratory symptoms, it is reasonable to postulate that reducing the size of mucin polymers may also benefit airway clearance. The use of N-acetylcysteine (NAC), a reducing agent that targets disulphide bonds between mucin monomers and results in depolymerisation, was approved in the 1960s and has been used as both an oral and inhaled therapy. Surprisingly, inhalation of 20% NAC (1.2M) has not yielded clinical benefits, as reviewed by two independent Cochrane studies (Nash et al. , 2009, Tam et al. , 2013). It could be argued that the lack of clinical benefits with NAC is related to decreased mucin concentration and that DNA is the major contributor to the properties of mucus in CF (Henke and Ratjen, 2007). Alternatively, NAC might simply be a weak reducing agent that is ineffective at the concentrations deposited in the lower airways. Although this notion needs to be further investigated, the development of more potent reducing agents with increased efficacy may present important clinical benefits for CF patients.
Controlling the rate of mucin secretion has also been considered as a way to alleviate airway obstruction in CF. Understanding the regulation of mucin granule exocytosis is essential for this approach, and studies have shown similarities between the exocytic mechanism in mucin-producing cells and other secretory cells, such as neurons (Burgoyne and Morgan, 2003, Davis and Dickey, 2008). Goblet cell secretagogues include P2Y2 receptor agonists (ATP and UTP) and other agents that cause intracellular Ca2+ mobilization (Davis and Dickey, 2008, Kreda et al. , 2007). The identification of downstream effectors of these secretagogues is essential to advancing a mucin regulatory approach. Inhibition of key components of the mucin exocytotic pathway, such as Munc13-2, VAMP8, ROCK, MARCKS, can inhibit the release of mucin granules and are currently being tested as potential drug targets (Jones et al. , 2012, Kreda, Davis, 2012, Kreda et al. , 2010, Singer et al. , 2004, Zhu et al. , 2008).
Perhaps the simplest approach to removing mucus from the lungs is the use of osmotically active agents that rehydrate mucus. Inhalation of hypertonic saline has been shown to reduce pulmonary exacerbation, as well as improve FEV1 and MCC (Donaldson et al. , 2006, Elkins et al. , 2006). Another osmotically active agent, mannitol, has recently been shown to yield similar benefits (Bilton et al. , 2013, Daviskas et al. , 2010). Given the diversity of CFTR mutations, mucolytics, regulators of mucin secretion and osmotic agents may present several advantages over CFTR correctors/potentiators, such as being cheaper and generic for all CFTR mutations. Additional benefits related to the removal of mucus, besides improved airflow, might include reduced bacterial infection and inflammation.
5. Conclusions
Although much work is still needed to fully understand the causal relationship between defective CFTR function and the production of aberrant mucus, significant progress towards this goal has been made in recent years. Better insights of the CF pathogenesis cascade have arisen from a variety of sources, including primary cell culture, animal models, and also human patients, with studies performed on native tissues or intact organs. Hence, in some cases, translation of these findings to the general CF population may not be a straightforward process. In this review, we have tentatively summarised this progress, taking into account some inadvertent omissions and misconceptions. The relationship between impaired CFTR function and the production of sticky, viscous mucus in several organs is not always obvious but can be related to two physiological roles of the channel. By controlling water movement and HCO3− secretion, CFTR plays an essential role in the hydration and expansion of the mucins that are normally and continuously produced to protect mucosal surfaces. In CF, a water/electrolyte imbalance compromises the formation of the mucus gel that lines the mucosa and triggers a cascade of events with complex feedback mechanisms. In the lungs, the onset of mucus obstruction, infection and inflammation can be life-threatening. Hence, removal of mucus and mucopurulent material from the lungs is crucial for CF patients. Currently, translational research is being conducted to identify a number of compounds that offer promising pharmacotherapy for CF. More specifically, inhaled mucolytic agents have the potential to improve airflow, prevent bacterial growth and reduce inflammation and tissue damage. Targeting mucin intramolecular disulphide bonds using reducing agents is an interesting approach that could also benefit patients suffering from chronic obstructive pulmonary disease (COPD). Conceptually, this type of treatment would be used as an acute therapy (e.g., for exacerbation) rather than on a daily basis. Importantly, safety issues related to the reduction of other disulphide bonds would have to be considered. For example, the specificity of the target (i.e., mucins) over other luminal proteins will be difficult to achieve and necessitate monitoring. However, the side effects of such a treatment might be outweighed by its clinical benefits if it can effectively “clean” the lungs.
Acknowledgements
The authors wish to thank the NIH (NHLBI R01-HL116228) and the Cystic Fibrosis Foundation Therapeutics, Inc. (EHRE07XX0 and THORNTO7XX0).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no conflict of interest
References
- Accurso FJ, Rowe SM, Clancy JP, Boyle MP, Dunitz JM, Durie PR, et al. Effect of VX-770 in persons with cystic fibrosis and the G551D-CFTR mutation. N Engl J Med. 2010;363:1991–2003. doi: 10.1056/NEJMoa0909825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altes T, Johnson MA, Miller GW, Mugler JP, Flors L, Mata J, et al. Hyperpolarized gas MRI of Ivacaftor therapy in persons with cystic fibrosis and the G551D-CFTR mutation. Pediatric Pulmonology. 2012;(Supplement 35):291. [Google Scholar]
- Ambort D, Johansson ME, Gustafsson JK, Nilsson HE, Ermund A, Johansson BR, et al. Calcium and pH-dependent packing and release of the gel-forming MUC2 mucin. Proc Natl Acad Sci U S A. 2012;109:5645–50. doi: 10.1073/pnas.1120269109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambort D, van der Post S, Johansson ME, Mackenzie J, Thomsson E, Krengel U, et al. Function of the CysD domain of the gel-forming MUC2 mucin. Biochem J. 2011;436:61–70. doi: 10.1042/BJ20102066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asker N, Axelsson MA, Olofsson SO, Hansson GC. Dimerization of the human MUC2 mucin in the endoplasmic reticulum is followed by a N-glycosylation-dependent transfer of the mono- and dimers to the Golgi apparatus. J Biol Chem. 1998a;273:18857–63. doi: 10.1074/jbc.273.30.18857. [DOI] [PubMed] [Google Scholar]
- Asker N, Axelsson MA, Olofsson SO, Hansson GC. Human MUC5AC mucin dimerizes in the rough endoplasmic reticulum, similarly to the MUC2 mucin. Biochem J. 1998b;335(Pt 2):381–7. doi: 10.1042/bj3350381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asker N, Baeckstrom D, Axelsson MA, Carlstedt I, Hansson GC. The human MUC2 mucin apoprotein appears to dimerize before O-glycosylation and shares epitopes with the ‘insoluble’ mucin of rat small intestine. Biochem J. 1995;308(Pt 3):873–80. doi: 10.1042/bj3080873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelsson MA, Asker N, Hansson GC. O-glycosylated MUC2 monomer and dimer from LS 174T cells are water-soluble, whereas larger MUC2 species formed early during biosynthesis are insoluble and contain nonreducible intermolecular bonds. J Biol Chem. 1998;273:18864–70. doi: 10.1074/jbc.273.30.18864. [DOI] [PubMed] [Google Scholar]
- Bilton D, Daviskas E, Anderson SD, Kolbe J, King G, Stirling RG, et al. Phase 3 randomized study of the efficacy and safety of inhaled dry powder mannitol for the symptomatic treatment of non-cystic fibrosis bronchiectasis. Chest. 2013;144:215–25. doi: 10.1378/chest.12-1763. [DOI] [PubMed] [Google Scholar]
- Boucher RC. Cystic fibrosis: a disease of vulnerability to airway surface dehydration. Trends Mol Med. 2007;13:231–40. doi: 10.1016/j.molmed.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Boyle MP, Bell SC, Konstan MW, McColley SA, Kang L, Patel N. The investigational CFTR corrector, VX-809 (Lumafactor) co-administered with the oral potentiator Ivacaftor improved CFTR and lung function in F508Del homozygous patients: Phase II study resutls. Pediatric Pulmonology. 2012 Abstract #260. [Google Scholar]
- Briel M, Greger R, Kunzelmann K. Cl− transport by cystic fibrosis transmembrane conductance regulator (CFTR) contributes to the inhibition of epithelial Na+ channels (ENaCs) in Xenopus oocytes co-expressing CFTR and ENaC. J Physiol. 1998;508(Pt 3):825–36. doi: 10.1111/j.1469-7793.1998.825bp.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgel PR, Montani D, Danel C, Dusser DJ, Nadel JA. A morphometric study of mucins and small airway plugging in cystic fibrosis. Thorax. 2007;62:153–61. doi: 10.1136/thx.2006.062190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgoyne RD, Morgan A. Secretory granule exocytosis. Physiol Rev. 2003;83:581–632. doi: 10.1152/physrev.00031.2002. [DOI] [PubMed] [Google Scholar]
- Button B, Cai LH, Ehre C, Kesimer M, Hill DB, Sheehan JK, et al. A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science. 2012;337:937–41. doi: 10.1126/science.1223012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cash HA, Woods DE, McCullough B, Johanson WG, Jr., Bass JA. A rat model of chronic respiratory infection with Pseudomonas aeruginosa. Am Rev Respir Dis. 1979;119:453–9. doi: 10.1164/arrd.1979.119.3.453. [DOI] [PubMed] [Google Scholar]
- Celli J, Gregor B, Turner B, Afdhal NH, Bansil R, Erramilli S. Viscoelastic properties and dynamics of porcine gastric mucin. Biomacromolecules. 2005;6:1329–33. doi: 10.1021/bm0493990. [DOI] [PubMed] [Google Scholar]
- Chen EY, Yang N, Quinton PM, Chin WC. A new role for bicarbonate in mucus formation. Am J Physiol Lung Cell Mol Physiol. 2010;299:L542–9. doi: 10.1152/ajplung.00180.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhao YH, Kalaslavadi TB, Hamati E, Nehrke K, Le AD, et al. Genome-wide search and identification of a novel gel-forming mucin MUC19/Muc19 in glandular tissues. Am J Respir Cell Mol Biol. 2004;30:155–65. doi: 10.1165/rcmb.2003-0103OC. [DOI] [PubMed] [Google Scholar]
- Cooper JL, Quinton PM, Ballard ST. Mucociliary transport in porcine trachea: differential effects of inhibiting chloride and bicarbonate secretion. Am J Physiol Lung Cell Mol Physiol. 2013;304:L184–90. doi: 10.1152/ajplung.00143.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies JC. Pseudomonas aeruginosa in cystic fibrosis: pathogenesis and persistence. Paediatr Respir Rev. 2002;3:128–34. doi: 10.1016/s1526-0550(02)00003-3. [DOI] [PubMed] [Google Scholar]
- Davies JR, Herrmann A, Russell W, Svitacheva N, Wickstrom C, Carlstedt I. Respiratory tract mucins: structure and expression patterns. Novartis Found Symp. 2002;248:76–88. discussion -93, 277-82. [PubMed] [Google Scholar]
- Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol. 2008;70:487–512. doi: 10.1146/annurev.physiol.70.113006.100638. [DOI] [PubMed] [Google Scholar]
- Daviskas E, Anderson SD, Jaques A, Charlton B. Inhaled mannitol improves the hydration and surface properties of sputum in patients with cystic fibrosis. Chest. 2010;137:861–8. doi: 10.1378/chest.09-2017. [DOI] [PubMed] [Google Scholar]
- De Lisle RC, Borowitz D. The cystic fibrosis intestine. Cold Spring Harb Perspect Med. 2013;3:a009753. doi: 10.1101/cshperspect.a009753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lisle RC, Roach E, Jansson K. Effects of laxative and N-acetylcysteine on mucus accumulation, bacterial load, transit, and inflammation in the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2007;293:G577–84. doi: 10.1152/ajpgi.00195.2007. [DOI] [PubMed] [Google Scholar]
- Donaldson SH, Bennett WD, Zeman KL, Knowles MR, Tarran R, Boucher RC. Mucus clearance and lung function in cystic fibrosis with hypertonic saline. N Engl J Med. 2006;354:241–50. doi: 10.1056/NEJMoa043891. [DOI] [PubMed] [Google Scholar]
- Donaldson SH, Zeman K, Laube B, Corcoran T, Locke LW, Pilewski J, et al. Effect of Ivacaftor on mucociliary clearance and mucus rheology in patients with G551D-CFTR mutation. Pediatric Pulmonology. 2013;(Supplement 36) [Google Scholar]
- Ehre C, Worthington EN, Liesman RM, Grubb BR, Barbier D, O’Neal WK, et al. Overexpressing mouse model demonstrates the protective role of Muc5ac in the lungs. Proc Natl Acad Sci U S A. 2012;109:16528–33. doi: 10.1073/pnas.1206552109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins MR, Robinson M, Rose BR, Harbour C, Moriarty CP, Marks GB, et al. A controlled trial of long-term inhaled hypertonic saline in patients with cystic fibrosis. N Engl J Med. 2006;354:229–40. doi: 10.1056/NEJMoa043900. [DOI] [PubMed] [Google Scholar]
- Escande F, Porchet N, Bernigaud A, Petitprez D, Aubert JP, Buisine MP. The mouse secreted gel-forming mucin gene cluster. Biochim Biophys Acta. 2004;1676:240–50. doi: 10.1016/j.bbaexp.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Feingold J, Guilloud-Bataille M. Genetic comparisons of patients with cystic fibrosis with or without meconium ileus. Clinical Centers of the French CF Registry. Ann Genet. 1999;42:147–50. [PubMed] [Google Scholar]
- Ferec C, Cutting GR. Assessing the Disease-Liability of Mutations in CFTR. Cold Spring Harb Perspect Med. 2012;2:a009480. doi: 10.1101/cshperspect.a009480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fridge JL, Conrad C, Gerson L, Castillo RO, Cox K. Risk factors for small bowel bacterial overgrowth in cystic fibrosis. J Pediatr Gastroenterol Nutr. 2007;44:212–8. doi: 10.1097/MPG.0b013e31802c0ceb. [DOI] [PubMed] [Google Scholar]
- Fuchs HJ, Borowitz DS, Christiansen DH, Morris EM, Nash ML, Ramsey BW, et al. Effect of aerosolized recombinant human DNase on exacerbations of respiratory symptoms and on pulmonary function in patients with cystic fibrosis. The Pulmozyme Study Group. N Engl J Med. 1994;331:637–42. doi: 10.1056/NEJM199409083311003. [DOI] [PubMed] [Google Scholar]
- Garcia MA, Yang N, Quinton PM. Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J Clin Invest. 2009;119:2613–22. doi: 10.1172/JCI38662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiades P, Pudney PD, Thornton DJ, Waigh T. Particle tracking microrheology of purified gastrointestinal mucins. Biopolymers. 2013 doi: 10.1002/bip.22372. [DOI] [PubMed] [Google Scholar]
- Gerken TA. Biophysical approaches to salivary mucin structure, conformation and dynamics. Crit Rev Oral Biol Med. 1993;4:261–70. doi: 10.1177/10454411930040030201. [DOI] [PubMed] [Google Scholar]
- Godl K, Johansson ME, Lidell ME, Morgelin M, Karlsson H, Olson FJ, et al. The N terminus of the MUC2 mucin forms trimers that are held together within a trypsin-resistant core fragment. J Biol Chem. 2002;277:47248–56. doi: 10.1074/jbc.M208483200. [DOI] [PubMed] [Google Scholar]
- Gregory RJ, Cheng SH, Rich DP, Marshall J, Paul S, Hehir K, et al. Expression and characterization of the cystic fibrosis transmembrane conductance regulator. Nature. 1990;347:382–6. doi: 10.1038/347382a0. [DOI] [PubMed] [Google Scholar]
- Griesenbach U, Geddes DM, Alton EW. Update on gene therapy for cystic fibrosis. Curr Opin Mol Ther. 2003;5:489–94. [PubMed] [Google Scholar]
- Guilbault C, Saeed Z, Downey GP, Radzioch D. Cystic fibrosis mouse models. Am J Respir Cell Mol Biol. 2007;36:1–7. doi: 10.1165/rcmb.2006-0184TR. [DOI] [PubMed] [Google Scholar]
- Gum JR, Jr., Hicks JW, Toribara NW, Siddiki B, Kim YS. Molecular cloning of human intestinal mucin (MUC2) cDNA. Identification of the amino terminus and overall sequence similarity to prepro-von Willebrand factor. J Biol Chem. 1994;269:2440–6. [PubMed] [Google Scholar]
- Guo X, Pace RG, Stonebraker JR, Commander CW, Dang AT, Drumm ML, et al. Mucin variable number tandem repeat polymorphisms and severity of cystic fibrosis lung disease: significant association with MUC5AC. PLoS One. 2011;6:e25452. doi: 10.1371/journal.pone.0025452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R, Jentoft N. The structure of tracheobronchial mucins from cystic fibrosis and control patients. J Biol Chem. 1992;267:3160–7. [PubMed] [Google Scholar]
- Gustafsson JK, Ermund A, Ambort D, Johansson ME, Nilsson HE, Thorell K, et al. Bicarbonate and functional CFTR channel are required for proper mucin secretion and link cystic fibrosis with its mucus phenotype. J Exp Med. 2012;209:1263–72. doi: 10.1084/jem.20120562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadorn B, Zoppi G, Shmerling DH, Prader A, McIntyre I, Anderson CM. Quantitative assessment of exocrine pancreatic function in infants and children. J Pediatr. 1968;73:39–50. doi: 10.1016/s0022-3476(68)80037-x. [DOI] [PubMed] [Google Scholar]
- Hasnain SZ, McGuckin MA, Grencis RK, Thornton DJ. Serine protease(s) secreted by the nematode Trichuris muris degrade the mucus barrier. PLoS Negl Trop Dis. 2012;6:e1856. doi: 10.1371/journal.pntd.0001856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke MO, John G, Germann M, Lindemann H, Rubin BK. MUC5AC and MUC5B mucins increase in cystic fibrosis airway secretions during pulmonary exacerbation. Am J Respir Crit Care Med. 2007;175:816–21. doi: 10.1164/rccm.200607-1011OC. [DOI] [PubMed] [Google Scholar]
- Henke MO, John G, Rheineck C, Chillappagari S, Naehrlich L, Rubin BK. Serine proteases degrade airway mucins in cystic fibrosis. Infect Immun. 2011;79:3438–44. doi: 10.1128/IAI.01252-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henke MO, Ratjen F. Mucolytics in cystic fibrosis. Paediatr Respir Rev. 2007;8:24–9. doi: 10.1016/j.prrv.2007.02.009. [DOI] [PubMed] [Google Scholar]
- Henke MO, Renner A, Huber RM, Seeds MC, Rubin BK. MUC5AC and MUC5B Mucins Are Decreased in Cystic Fibrosis Airway Secretions. Am J Respir Cell Mol Biol. 2004;31:86–91. doi: 10.1165/rcmb.2003-0345OC. [DOI] [PubMed] [Google Scholar]
- Hong Z, Chasan B, Bansil R, Turner BS, Bhaskar KR, Afdhal NH. Atomic force microscopy reveals aggregation of gastric mucin at low pH. Biomacromolecules. 2005;6:3458–66. doi: 10.1021/bm0505843. [DOI] [PubMed] [Google Scholar]
- Horsley A, Rousseau K, Ridley C, Flight W, Jones A, Waigh TA, et al. Reassessment of the importance of mucins in determining sputum properties in cystic fibrosis. J Cyst Fibros. 2013 doi: 10.1016/j.jcf.2013.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Innes AL, Carrington SD, Thornton DJ, Kirkham S, Rousseau K, Dougherty RH, et al. Ex vivo sputum analysis reveals impairment of protease-dependent mucus degradation by plasma proteins in acute asthma. Am J Respir Crit Care Med. 2009;180:203–10. doi: 10.1164/rccm.200807-1056OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LC, Moussa L, Fulcher ML, Zhu Y, Hudson EJ, O’Neal WK, et al. VAMP8 is a vesicle SNARE that regulates mucin secretion in airway goblet cells. J Physiol. 2012;590:545–62. doi: 10.1113/jphysiol.2011.222091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesimer M, Ehre C, Burns KA, Davis CW, Sheehan JK, Pickles RJ. Molecular organization of the mucins and glycocalyx underlying mucus transport over mucosal surfaces of the airways. Mucosal Immunol. 2013;6:379–92. doi: 10.1038/mi.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesimer M, Makhov AM, Griffith JD, Verdugo P, Sheehan JK. Unpacking a gel-forming mucin: a view of MUC5B organization after granular release. Am J Physiol Lung Cell Mol Physiol. 2010;298:L15–22. doi: 10.1152/ajplung.00194.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkham S, Sheehan JK, Knight D, Richardson PS, Thornton DJ. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem J. 2002;361:537–46. doi: 10.1042/0264-6021:3610537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles MR, Boucher RC. Mucus clearance as a primary innate defense mechanism for mammalian airways. J Clin Invest. 2002;109:571–7. doi: 10.1172/JCI15217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreda SM, Davis CW, Rose MC. CFTR, mucins, and mucus obstruction in cystic fibrosis. Cold Spring Harb Perspect Med. 2012;2:a009589. doi: 10.1101/cshperspect.a009589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreda SM, Okada SF, van Heusden CA, O’Neal W, Gabriel S, Abdullah L, et al. Coordinated release of nucleotides and mucin from human airway epithelial Calu-3 cells. J Physiol. 2007;584:245–59. doi: 10.1113/jphysiol.2007.139840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreda SM, Seminario-Vidal L, van Heusden CA, O’Neal W, Jones L, Boucher RC, et al. Receptor-promoted exocytosis of airway epithelial mucin granules containing a spectrum of adenine nucleotides. J Physiol. 2010;588:2255–67. doi: 10.1113/jphysiol.2009.186643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuver R, Lee SP. Calcium binding to biliary mucins is dependent on sodium ion concentration: relevance to cystic fibrosis. Biochem Biophys Res Commun. 2004;314:330–4. doi: 10.1016/j.bbrc.2003.12.088. [DOI] [PubMed] [Google Scholar]
- Lethem MI, James SL, Marriott C, Burke JF. The origin of DNA associated with mucus glycoproteins in cystic fibrosis sputum. Eur Respir J. 1990;3:19–23. [PubMed] [Google Scholar]
- Lisowska A, Wojtowicz J, Walkowiak J. Small intestine bacterial overgrowth is frequent in cystic fibrosis: combined hydrogen and methane measurements are required for its detection. Acta Biochim Pol. 2009;56:631–4. [PubMed] [Google Scholar]
- Livraghi-Butrico A, Kelly EJ, Klem ER, Dang H, Wolfgang MC, Boucher RC, et al. Mucus clearance, MyD88-dependent and MyD88-independent immunity modulate lung susceptibility to spontaneous bacterial infection and inflammation. Mucosal Immunol. 2012;5:397–408. doi: 10.1038/mi.2012.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch SV, Goldfarb KC, Wild YK, Kong W, De Lisle RC, Brodie EL. Cystic fibrosis transmembrane conductance regulator knockout mice exhibit aberrant gastrointestinal microbiota. Gut Microbes. 2013;4:41–7. doi: 10.4161/gmic.22430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mall M, Grubb BR, Harkema JR, O’Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–93. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- Malmberg EK, Noaksson KA, Phillipson M, Johansson ME, Hinojosa-Kurtzberg M, Holm L, et al. Increased levels of mucins in the cystic fibrosis mouse small intestine, and modulator effects of the Muc1 mucin expression. Am J Physiol Gastrointest Liver Physiol. 2006;291:G203–10. doi: 10.1152/ajpgi.00491.2005. [DOI] [PubMed] [Google Scholar]
- Matsui H, Randell SH, Peretti SW, Davis CW, Boucher RC. Coordinated clearance of periciliary liquid and mucus from airway surfaces. J Clin Invest. 1998;102:1125–31. doi: 10.1172/JCI2687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews LW, Spector S, Lemm J, Potter JL. Studies on Pulmonary Secretions. I. The over-All Chemical Composition of Pulmonary Secretions from Patients with Cystic Fibrosis, Bronchiectasis, and Laryngectomy. Am Rev Respir Dis. 1963;88:199–204. doi: 10.1164/arrd.1963.88.2.199. [DOI] [PubMed] [Google Scholar]
- McGuckin MA, Linden SK, Sutton P, Florin TH. Mucin dynamics and enteric pathogens. Nat Rev Microbiol. 2011;9:265–78. doi: 10.1038/nrmicro2538. [DOI] [PubMed] [Google Scholar]
- McKone EF, Emerson SS, Edwards KL, Aitken ML. Effect of genotype on phenotype and mortality in cystic fibrosis: a retrospective cohort study. Lancet. 2003;361:1671–6. doi: 10.1016/S0140-6736(03)13368-5. [DOI] [PubMed] [Google Scholar]
- Nash EF, Stephenson A, Ratjen F, Tullis E. Nebulized and oral thiol derivatives for pulmonary disease in cystic fibrosis. Cochrane Database Syst Rev. 2009:CD007168. doi: 10.1002/14651858.CD007168.pub2. [DOI] [PubMed] [Google Scholar]
- Norkina O, Burnett TG, De Lisle RC. Bacterial overgrowth in the cystic fibrosis transmembrane conductance regulator null mouse small intestine. Infect Immun. 2004a;72:6040–9. doi: 10.1128/IAI.72.10.6040-6049.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norkina O, Kaur S, Ziemer D, De Lisle RC. Inflammation of the cystic fibrosis mouse small intestine. Am J Physiol Gastrointest Liver Physiol. 2004b;286:G1032–41. doi: 10.1152/ajpgi.00473.2003. [DOI] [PubMed] [Google Scholar]
- Perez-Vilar J, Hill RL. The structure and assembly of secreted mucins. J Biol Chem. 1999;274:31751–4. doi: 10.1074/jbc.274.45.31751. [DOI] [PubMed] [Google Scholar]
- Pezzulo AA, Tang XX, Hoegger MJ, Alaiwa MH, Ramachandran S, Moninger TO, et al. Reduced airway surface pH impairs bacterial killing in the porcine cystic fibrosis lung. Nature. 2012;487:109–13. doi: 10.1038/nature11130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puchelle E, Bajolet O, Abely M. Airway mucus in cystic fibrosis. Paediatr Respir Rev. 2002;3:115–9. doi: 10.1016/s1526-0550(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Quinton PM. Cystic fibrosis: impaired bicarbonate secretion and mucoviscidosis. Lancet. 2008;372:415–7. doi: 10.1016/S0140-6736(08)61162-9. [DOI] [PubMed] [Google Scholar]
- Quinton PM. Role of epithelial HCO3(−) transport in mucin secretion: lessons from cystic fibrosis. Am J Physiol Cell Physiol. 2010;299:C1222–33. doi: 10.1152/ajpcell.00362.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsey BW, Astley SJ, Aitken ML, Burke W, Colin AA, Dorkin HL, et al. Efficacy and safety of short-term administration of aerosolized recombinant human deoxyribonuclease in patients with cystic fibrosis. Am Rev Respir Dis. 1993;148:145–51. doi: 10.1164/ajrccm/148.1.145. [DOI] [PubMed] [Google Scholar]
- Ramsey BW, Davies J, McElvaney NG, Tullis E, Bell SC, Drevinek P, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N Engl J Med. 2011;365:1663–72. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raynal BD, Hardingham TE, Sheehan JK, Thornton DJ. Calcium-dependent protein interactions in MUC5B provide reversible cross-links in salivary mucus. J Biol Chem. 2003;278:28703–10. doi: 10.1074/jbc.M304632200. [DOI] [PubMed] [Google Scholar]
- Raynal BD, Hardingham TE, Thornton DJ, Sheehan JK. Concentrated solutions of salivary MUC5B mucin do not replicate the gel-forming properties of saliva. Biochem J. 2002;362:289–96. doi: 10.1042/0264-6021:3620289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid CJ, Hyde K, Ho SB, Harris A. Cystic fibrosis of the pancreas: involvement of MUC6 mucin in obstruction of pancreatic ducts. Mol Med. 1997;3:403–11. [PMC free article] [PubMed] [Google Scholar]
- Ridley C, Hughes GA, Bonser L, Ford RC, Thornton DJ. MUC5B Mucin Assembly and Extracellular Organization. Pediatric Pulmonology. 2011 [Google Scholar]
- Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science. 1989;245:1066–73. doi: 10.1126/science.2475911. [DOI] [PubMed] [Google Scholar]
- Rogers CS, Stoltz DA, Meyerholz DK, Ostedgaard LS, Rokhlina T, Taft PJ, et al. Disruption of the CFTR gene produces a model of cystic fibrosis in newborn pigs. Science. 2008;321:1837–41. doi: 10.1126/science.1163600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MC, Brown CF, Jacoby JZ, 3rd, Lynn WS, Kaufman B. Biochemical properties of tracheobronchial mucins from cystic fibrosis and non-cystic fibrosis individuals. Pediatr Res. 1987;22:545–51. doi: 10.1203/00006450-198711000-00015. [DOI] [PubMed] [Google Scholar]
- Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–78. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- Rosenbluth DB, Wilson K, Ferkol T, Schuster DP. Lung function decline in cystic fibrosis patients and timing for lung transplantation referral. Chest. 2004;126:412–9. doi: 10.1378/chest.126.2.412. [DOI] [PubMed] [Google Scholar]
- Round AN, Berry M, McMaster TJ, Corfield AP, Miles MJ. Glycopolymer charge density determines conformation in human ocular mucin gene products: an atomic force microscope study. J Struct Biol. 2004;145:246–53. doi: 10.1016/j.jsb.2003.10.029. [DOI] [PubMed] [Google Scholar]
- Round AN, Rigby NM, Garcia de la Torre A, Macierzanka A, Mills EN, Mackie AR. Lamellar structures of MUC2-rich mucin: a potential role in governing the barrier and lubricating functions of intestinal mucus. Biomacromolecules. 2012;13:3253–61. doi: 10.1021/bm301024x. [DOI] [PubMed] [Google Scholar]
- Roy MG, Livraghi-Butrico A, Fletcher AA, McElwee MM, Evans SE, Boerner RM, et al. Muc5b is required for airway defence. Nature. 2013 doi: 10.1038/nature12807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachter H, Dixon GH. A Comparative Study of the Proteins in Normal Meconium and in Meconium from Meconium Ileus Patients. Can J Biochem. 1965;43:381–97. doi: 10.1139/o65-046. [DOI] [PubMed] [Google Scholar]
- Shak S, Capon DJ, Hellmiss R, Marsters SA, Baker CL. Recombinant human DNase I reduces the viscosity of cystic fibrosis sputum. Proc Natl Acad Sci U S A. 1990;87:9188–92. doi: 10.1073/pnas.87.23.9188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan JK, Kirkham S, Howard M, Woodman P, Kutay S, Brazeau C, et al. Identification of molecular intermediates in the assembly pathway of the MUC5AC mucin. J Biol Chem. 2004;279:15698–705. doi: 10.1074/jbc.M313241200. [DOI] [PubMed] [Google Scholar]
- Singer M, Martin LD, Vargaftig BB, Park J, Gruber AD, Li Y, et al. A MARCKS-related peptide blocks mucus hypersecretion in a mouse model of asthma. Nat Med. 2004;10:193–6. doi: 10.1038/nm983. [DOI] [PubMed] [Google Scholar]
- Smith A. Pathogenesis of bacterial bronchitis in cystic fibrosis. Pediatr Infect Dis J. 1997;16:91–5. doi: 10.1097/00006454-199701000-00030. discussion 5-6, 123-6. [DOI] [PubMed] [Google Scholar]
- Snouwaert JN, Brigman KK, Latour AM, Malouf NN, Boucher RC, Smithies O, et al. An animal model for cystic fibrosis made by gene targeting. Science. 1992;257:1083–8. doi: 10.1126/science.257.5073.1083. [DOI] [PubMed] [Google Scholar]
- Stoltz DA, Meyerholz DK, Pezzulo AA, Ramachandran S, Rogan MP, Davis GJ, et al. Cystic fibrosis pigs develop lung disease and exhibit defective bacterial eradication at birth. Sci Transl Med. 2010;2:29ra31. doi: 10.1126/scitranslmed.3000928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strong TV, Boehm K, Collins FS. Localization of cystic fibrosis transmembrane conductance regulator mRNA in the human gastrointestinal tract by in situ hybridization. J Clin Invest. 1994;93:347–54. doi: 10.1172/JCI116966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Sui H, Fisher JT, Yan Z, Liu X, Cho HJ, et al. Disease phenotype of a ferret CFTR-knockout model of cystic fibrosis. J Clin Invest. 2010;120:3149–60. doi: 10.1172/JCI43052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam J, Nash EF, Ratjen F, Tullis E, Stephenson A. Nebulized and oral thiol derivatives for pulmonary disease in cystic fibrosis. Cochrane Database Syst Rev. 2013;7:CD007168. doi: 10.1002/14651858.CD007168.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton DJ, Davies JR, Kraayenbrink M, Richardson PS, Sheehan JK, Carlstedt I. Mucus glycoproteins from ‘normal’ human tracheobronchial secretion. Biochem J. 1990;265:179–86. doi: 10.1042/bj2650179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton DJ, Rousseau K, McGuckin MA. Structure and function of the polymeric mucins in airways mucus. Annu Rev Physiol. 2008;70:459–86. doi: 10.1146/annurev.physiol.70.113006.100702. [DOI] [PubMed] [Google Scholar]
- Thornton DJ, Sheehan JK, Lindgren H, Carlstedt I. Mucus glycoproteins from cystic fibrotic sputum. Macromolecular properties and structural ‘architecture’. Biochem J. 1991;276(Pt 3):667–75. doi: 10.1042/bj2760667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Goor F, Hadida S, Grootenhuis PD, Burton B, Cao D, Neuberger T, et al. Rescue of CF airway epithelial cell function in vitro by a CFTR potentiator, VX-770. Proc Natl Acad Sci U S A. 2009;106:18825–30. doi: 10.1073/pnas.0904709106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdugo P. Mucin exocytosis. Am Rev Respir Dis. 1991;144:S33–7. doi: 10.1164/ajrccm/144.3_pt_2.S33. [DOI] [PubMed] [Google Scholar]
- Verdugo P. Mucus supramolecular topology: an elusive riddle. Proc Natl Acad Sci U S A. 2012a;109:E2956. doi: 10.1073/pnas.1211117109. author reply E7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdugo P. Supramolecular dynamics of mucus. Cold Spring Harb Perspect Med. 2012b;2 doi: 10.1101/cshperspect.a009597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdugo P, Aitken M, Langley L, Villalon MJ. Molecular mechanism of product storage and release in mucin secretion. II. The role of extracellular Ca++ Biorheology. 1987;24:625–33. doi: 10.3233/bir-1987-24615. [DOI] [PubMed] [Google Scholar]
- Voynow JA, Rubin BK. Mucins, mucus, and sputum. Chest. 2009;135:505–12. doi: 10.1378/chest.08-0412. [DOI] [PubMed] [Google Scholar]
- Wanner A, Salathe M, O’Riordan TG. Mucociliary clearance in the airways. Am J Respir Crit Care Med. 1996;154:1868–902. doi: 10.1164/ajrccm.154.6.8970383. [DOI] [PubMed] [Google Scholar]
- Werlin SL, Benuri-Silbiger I, Kerem E, Adler SN, Goldin E, Zimmerman J, et al. Evidence of intestinal inflammation in patients with cystic fibrosis. J Pediatr Gastroenterol Nutr. 2010;51:304–8. doi: 10.1097/MPG.0b013e3181d1b013. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Ehre C, Abdullah LH, Sheehan JK, Roy M, Evans CM, et al. Munc13-2-/-baseline secretion defect reveals source of oligomeric mucins in mouse airways. J Physiol. 2008;586:1977–92. doi: 10.1113/jphysiol.2007.149310. [DOI] [PMC free article] [PubMed] [Google Scholar]