Abstract Abstract
This study sought to determine the prevalence of coronary artery–pulmonary artery collaterals in patients with chronic thromboembolic pulmonary hypertension (CTEPH) and to correlate their presence with the degree of clot burden. CTEPH is a treatable cause of severe pulmonary hypertension and right heart failure. Bronchopulmonary collateral vessels have been used as a supplementary diagnostic and prognostic tool for this disease. Coronary artery–pulmonary artery collaterals in this population have not been described. The coronary angiograms of 300 consecutive patients with CTEPH evaluated for pulmonary thromboendarterectomy (PTE) between January 1, 2007, and May 1, 2014, were examined. Of these patients, 259 (50% male; mean age, 58.3 ± 10.6 years) had cineangiographic images deemed adequate to definitively assess for the presence of coronary artery–pulmonary artery collaterals and were included in the final analyses. Pulmonary angiogram reports were reviewed for extent of pulmonary artery obstruction. The coronary angiograms of 259 age- and sex-matched control patients were also examined. Among 259 CTEPH patients with definitive imaging, 34 coronary artery–pulmonary artery collaterals were found in 28 patients (10.8%), versus 1 coronary artery–pulmonary artery collateral among control subjects (0.4%; P < 0.001). Compared with CTEPH patients without collaterals, patients with collaterals had a significantly higher prevalence of total occlusion of their right or left main pulmonary artery (P < 0.001) or lobar arteries (P < 0.001). In conclusion, the prevalence of coronary artery–pulmonary artery collaterals in CTEPH patients undergoing coronary angiography for possible PTE is approximately 11%. These vessels are associated with more severe pulmonary artery occlusion.
Keywords: collateral vessels, pulmonary circulation, coronary arteries, pulmonary artery obstruction
Chronic thromboembolic pulmonary hypertension (CTEPH) is a major cause of progressive pulmonary hypertension and right heart failure. Approximately 0.5%–3.8% of patients who suffer an acute pulmonary embolism will develop CTEPH, and many investigators believe the true incidence is higher because some patients are eventually diagnosed without a clear history of acute pulmonary embolism.1-6
Unlike other forms of pulmonary hypertension, CTEPH is curable by pulmonary thromboendarterectomy (PTE). Considerations in proceeding with PTE include whether the disease is surgically accessible, whether hemodynamic normalization should be expected given other comorbidities or microvascular disease, and whether operative risks are worthwhile in mildly symptomatic patients knowing that foregoing early PTE allows for the chance that secondary vasculopathy and worsening hemodynamics might develop.1,7
Preoperative evaluation of CTEPH patients includes right heart catheterization and pulmonary angiography. Coronary angiography is often performed to rule out obstructive coronary artery disease. During these workups at the University of California, San Diego (UCSD), Medical Center, coronary artery–pulmonary artery collaterals have been identified. Whereas bronchopulmonary collaterals are a well-established phenomenon in the current CTEPH literature and serve as an auxiliary prognostic indicator,8-10 coronary artery–pulmonary artery collaterals have not previously been described. We sought to determine the prevalence and predictors of coronary artery–pulmonary artery collaterals in patients with CTEPH undergoing workup for possible PTE.
Methods
CTEPH patients
The study included 300 consecutive patients with CTEPH who underwent coronary angiography between January 1, 2007, and May 1, 2014, as part of a preoperative workup for PTE at the UCSD Medical Center. At this institution, coronary angiography is performed to rule out obstructive coronary disease prior to PTE in men more than 40 years of age, women more than 45 years of age, and younger patients who have significant cardiac risk factors.
Each patient’s electronic medical record was reviewed to confirm the diagnosis of CTEPH. This diagnosis was made on the basis of precapillary pulmonary hypertension (mean pulmonary arterial pressure [PAP] of ≥25 mmHg and pulmonary capillary wedge pressure of ≤15 mmHg) as well as findings indicative of chronic occlusive thrombi on ventilation-perfusion scans and pulmonary angiograms.2,11,12 Pulmonary angiogram reports were reviewed for extent of pulmonary artery obstruction. Demographic information, including age, sex, race, and comorbidities, was collected. Functional capacity (World Health Organization class) and hemodynamic data were also obtained.
Collateral vessel analysis
A cardiologist blinded to the pulmonary angiogram results reviewed the coronary angiogram of each subject for evidence of collateral vessels from the coronary arteries to the pulmonary arteries. The images were divided into two categories: (1) adequate (images that provided sufficient visualization of the distal ends of all coronary artery branches) and (2) indeterminate (images that provided insufficient visualization of the ends of all coronary artery branches, e.g., due to failure to pan sufficiently, too-short acquisition, poor opacification of the vessel lumen, or ill-defined heart border).
Adequate images were examined for definite collaterals, visualized on the cineangiogram extending beyond the heart border and into the pulmonary region. Indeterminate images were examined for potential coronary artery–pulmonary artery collaterals, which were subdivided into three groups—probable, possible, and unlikely—as follows: (1) probable collaterals displayed more than one collateral-like characteristic, such as a caliber equal to or larger than other branching vessels, significant tortuosity, and plexus-like appearance,13 but terminated outside the view of the cine images and could not be confirmed to be supplying the pulmonary territory; (2) possible collaterals were equal to or larger than other branching vessels but terminated outside the view of the cine images and could not be confirmed; and (3) unlikely collaterals were small in caliber but terminated outside the view of the cine images and could not be completely excluded.
Operative reports were reviewed for any mention of coronary artery–pulmonary artery collaterals. Intraoperative classification of the CTEPH lesions was also recorded (type 1, proximal clot in the main pulmonary arteries; type 2, thickened intima; type 3, distal disease in the segmental and subsegmental vessels; type 4, inoperable distal vasculopathy not due to pulmonary embolism).7
Control patients
Each of the 259 CTEPH patients with adequate imaging was assigned an age-matched (±3 years) and sex-matched control patient from a procedural database containing patients who underwent coronary angiography for indications other than CTEPH from January 1, 2003, to May 1, 2014. Basic demographic information was collected. The coronary angiogram of each control patient was reviewed for coronary artery–pulmonary artery collaterals. Patients with indeterminate imaging were excluded. Patients with a history of coronary artery disease or significant obstructive disease found during the cardiac catheterization were also excluded.
The study was reviewed and approved by the UCSD Institutional Review Board/Human Research Protections Program.
Statistical analysis
Values are expressed as means and SDs for continuous variables and as counts and percentages for dichotomous variables. To study differences in population characteristics between CTEPH and control patients as well as CTEPH patients with and without collaterals, the Student t test was used for continuous variables, and the χ2 test was used for categorical variables. All tests used a two-sided P value of <0.05 for significance. Analyses were performed using SPSS (ver. 21.0; IBM, Armonk, NY).
Results
Demographics
The final analysis included 259 CTEPH patients (50% male; mean age, 58.3 ± 10.6 years) and 259 control patients (mean age, 58.3 ± 10.8 years). Indications for coronary angiography in control patients were chest pain (n = 143), heart failure (n = 21), preoperative evaluation for noncardiac surgery (n = 71), arrhythmia (n = 4), and valve dysfunction (n = 20). Both populations were predominantly white (76% [n = 196] vs. 78% [n = 202], respectively), but the CTEPH population had a higher proportion of blacks (18% [n = 46] vs. 8% [n = 21]). CTEPH patients were characterized as World Health Organization functional class 1 (2%; n = 5), 2 (18%; n = 47), 3 (70%; n = 181), and 4 (10%; n = 26). The control group had a higher prevalence of several comorbidities, including diabetes (P = 0.01), hypertension (P < 0.001), and hyperlipidemia (P < 0.001). Other characteristics are shown in Table 1.
Table 1.
Demographics of patients in this study with adequate imaging
| Characteristic | CTEPH patients (n = 259) | Control patients (n = 259) | P |
|---|---|---|---|
| Age, years | 58.3 ± 10.6 | 58.3 ± 10.8 | 0.98 |
| Sex | |||
| Male | 130 (50.2) | 130 (50.2) | NA |
| Female | 129 (49.8) | 129 (49.8) | |
| Race | |||
| White | 196 (75.7) | 202 (78.0) | |
| African American | 46 (17.8) | 21 (8.1) | 0.001 |
| Asian | 6 (2.3) | 17 (6.6) | |
| Unknown | 11 (4.2) | 19 (7.3) | |
| BMI | 30.8 ± 7.1 | 29.5 ± 7.3 | 0.04 |
| WHO functional class | |||
| 1 | 5 (1.9) | ||
| 2 | 47 (18.1) | NA | NA |
| 3 | 181 (69.9) | ||
| 4 | 26 (10.0) | ||
| Comorbidities | |||
| Diabetes | 56 (21.6) | 82 (31.7) | 0.01 |
| Hypertension | 109 (42.1) | 170 (65.6) | <0.001 |
| Hyperlipidemia | 64 (24.7) | 136 (52.5) | <0.001 |
| Coronary artery disease | 10 (3.9) | 0 (0.0) | 0.001 |
| Chronic kidney disease | 33 (12.7) | 34 (13.1) | 0.92 |
| COPD | 23 (8.9) | 35 (13.5) | 0.09 |
| Asthma | 23 (8.9) | 19 (7.3) | 0.52 |
| Smoking history | |||
| Never smoker | 143 (55.2) | 131 (50.6) | |
| Former smoker | 89 (34.4) | 91 (35.1) | |
| Current smoker | 21 (8.1) | 30 (11.6) | |
| Unknown | 6 (2.3) | 7 (2.7) | 0.53 |
Data are no. (%) or mean ± SD and were compared with the χ2 test or the independent-sample t test, respectively. Boldface type indicates statistical significance. CTEPH: chronic thromboembolic pulmonary hypertension; NA: not applicable; BMI: body mass index; WHO: World Health Organization; COPD: chronic obstructive pulmonary disease.
Prevalence of coronary artery–pulmonary artery collateral vessels
Among the 259 CTEPH patients with cineangiographic images deemed adequate to definitively assess for coronary artery–pulmonary artery collaterals, 34 definite collaterals were observed in 28 CTEPH patients (10.8%, including 22 men and 6 women; Figs. 1–4; Videos 1–5). Six patients had two distinct coronary artery–pulmonary artery collaterals. The remaining 231 CTEPH patients with adequate imaging had no evidence of collaterals. The vessels in the 41 CTEPH patients with angiograms of indeterminate diagnostic quality were classified as probable (n = 10; Fig. S1), possible (n = 11), or unlikely (n = 20) collaterals.
Figure 1.
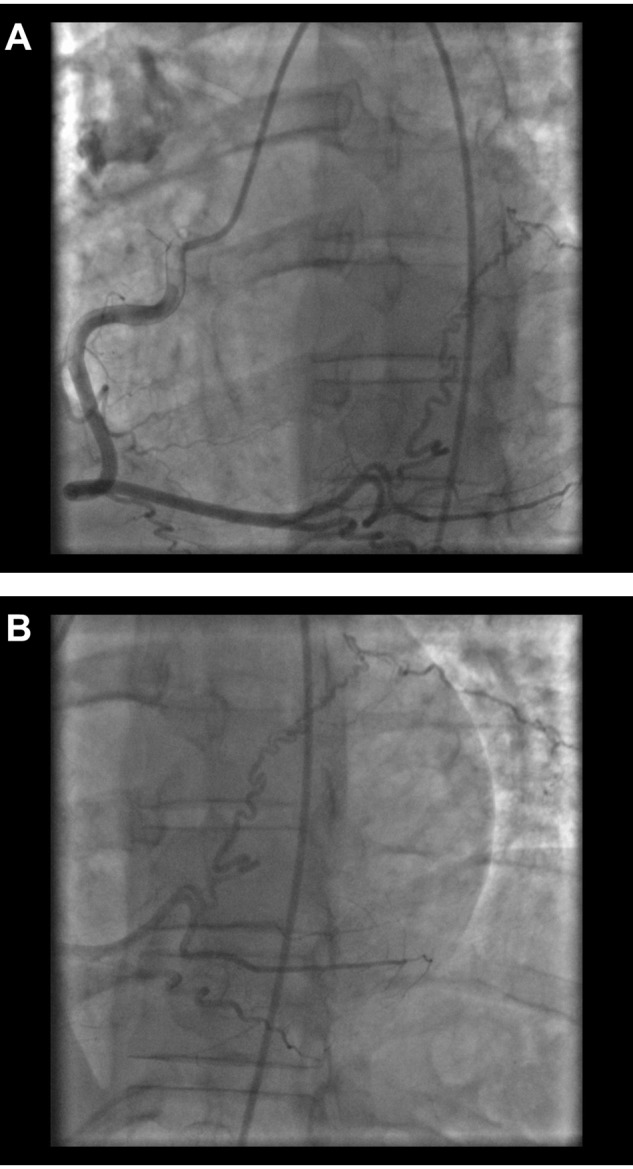
Right posterolateral artery–to–pulmonary artery collateral in a 43-year-old man with chronic thromboembolic pulmonary hypertension. A, Left anterior oblique cranial view. B, Extension of the vessel beyond the heart border in the left anterior oblique cranial view.
Figure 2.
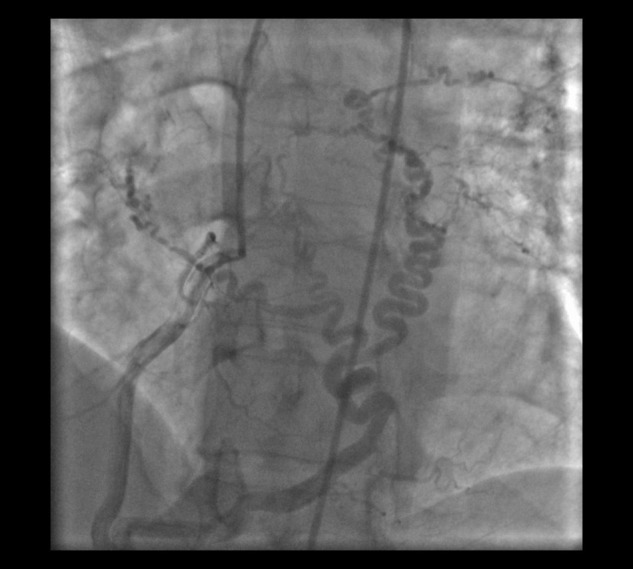
Large corkscrew collateral from the right posterolateral artery to the right and left pulmonary arteries in a 47-year-old man with chronic thromboembolic pulmonary hypertension, shown in the left anterior oblique cranial view.
Figure 3.
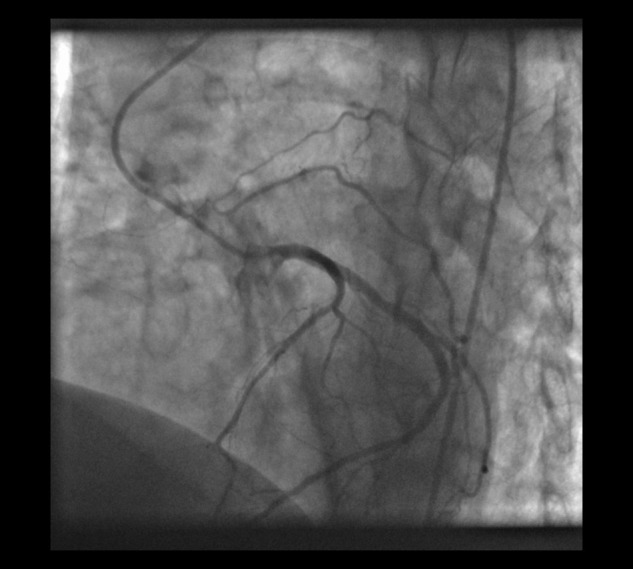
Coronary artery–pulmonary artery collateral arising from the left circumflex artery in a 67-year-old man with chronic thromboembolic pulmonary hypertension, shown in the left anterior oblique cranial view.
Figure 4.
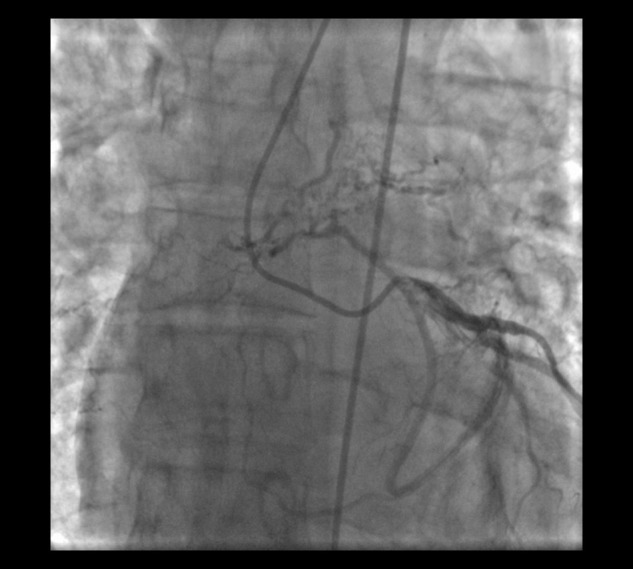
Collateral vessel arising from the proximal left circumflex artery traveling toward the left lung in a 46-year-old man with chronic thromboembolic pulmonary hypertension, shown in the right anterior oblique caudal view.
Video 1.
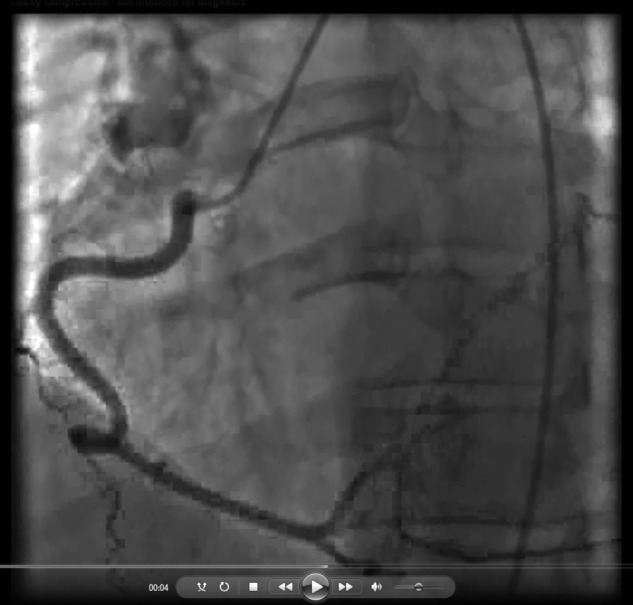
Video 2.
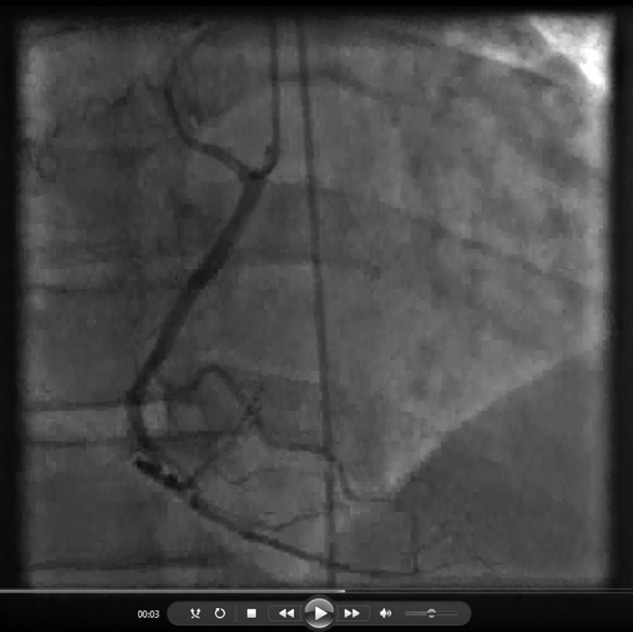
Video 3.
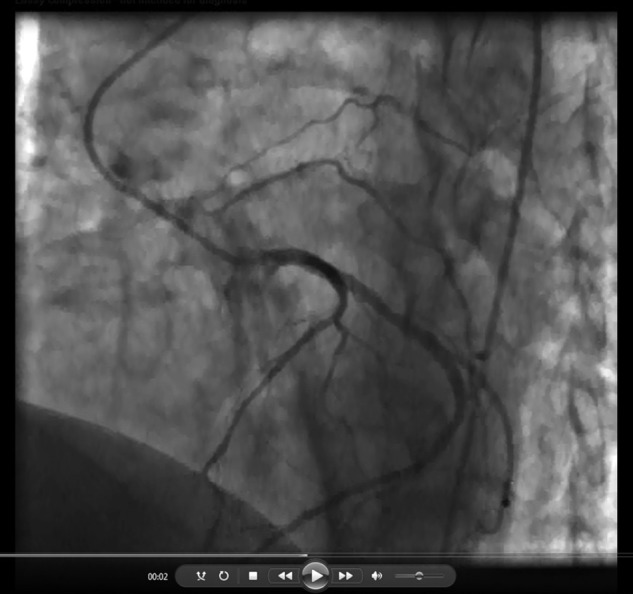
Video 4.
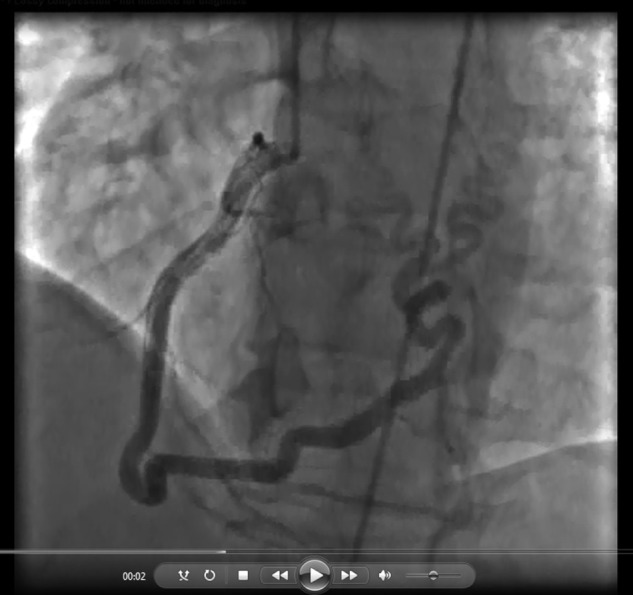
Video 5.
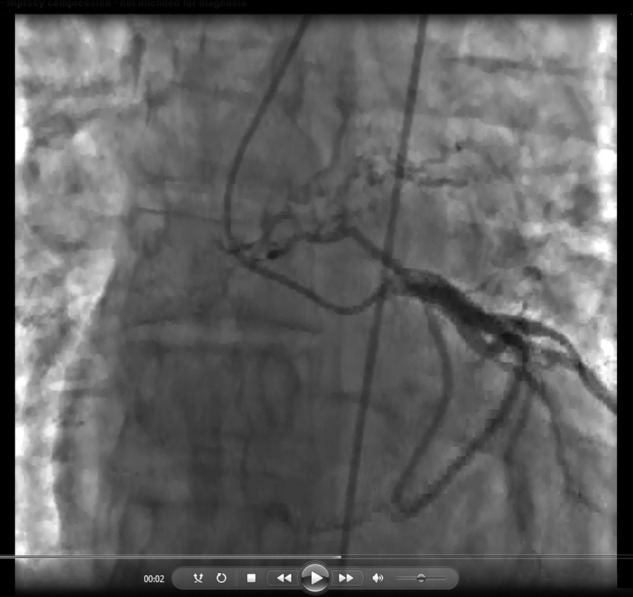
The prevalence of coronary artery–pulmonary artery collaterals was significantly lower in the control group than in the CTEPH group (0.4% [n = 1] vs. 10.8% [n = 28]; P < 0.001). The sole control patient with a collateral had cystic fibrosis and was undergoing evaluation for lung transplantation (Fig. S2).
Characteristics of CTEPH patients with coronary artery–pulmonary artery collaterals
As shown in Table 2, CTEPH patients with coronary artery–pulmonary artery collaterals were younger than those without them (mean age, 50.3 ± 9.1 vs. 59.3 ± 10.4 years; P < 0.001). They were predominantly male (79% [n = 22 of 28] vs. 46% [n = 107 of 231]; P = 0.001) and had a lower prevalence of chronic kidney disease (0% [n = 0 of 28] vs. 14% [n = 33 of 231]; P = 0.03). One patient (3.6%) had a history of coronary artery disease.
Table 2.
Demographics of chronic thromboembolic pulmonary hypertension (CTEPH) patients with and without collateral vessels
| Characteristic | CTEPH patients with collaterals (n = 28) | CTEPH patients without collaterals (n = 231) | P |
|---|---|---|---|
| Age, years | 50.3 ± 9.1 | 59.3 ± 10.4 | <0.001 |
| Sex | |||
| Male | 22 (78.6) | 107 (46.3) | 0.001 |
| Female | 6 (21.4) | 124 (53.7) | |
| Race | |||
| White | 16 (57.1) | 180 (77.9) | |
| African American | 9 (32.1) | 37 (16.0) | 0.04 |
| Asian | 2 (7.1) | 4 (1.7) | |
| Unknown | 1 (3.6) | 10 (4.3) | |
| BMI | 29.8 ± 4.7 | 33.0 ± 14.3 | 0.46 |
| WHO functional class | |||
| 1 | 1 (3.6) | 4 (1.7) | |
| 2 | 9 (32.1) | 38 (16.5) | 0.14 |
| 3 | 17 (60.7) | 164 (71.0) | |
| 4 | 1 (3.6) | 25 (10.8) | |
| Comorbidities | |||
| Diabetes | 9 (32.1) | 47 (20.3) | 0.16 |
| Hypertension | 12 (42.9) | 97 (42.0) | 0.95 |
| Hyperlipidemia | 7 (25.0) | 57 (24.7) | 0.98 |
| Coronary artery disease | 1 (3.6) | 9 (3.9) | 0.93 |
| Chronic kidney disease | 0 (0.0) | 33 (14.3) | 0.03 |
| COPD | 2 (7.1) | 21 (9.1) | 0.73 |
| Asthma | 3 (10.7) | 20 (8.7) | 0.72 |
| Smoking history | |||
| Never smoker | 16 (57.1) | 127 (55.0) | |
| Former smoker | 7 (25.0) | 82 (35.5) | 0.47 |
| Current smoker | 4 (14.3) | 17 (7.4) | |
| Unknown | 1 (3.6) | 5 (2.2) |
Data are no. (%) or mean ± SD and were compared with the χ2 test or the independent-sample t test, respectively. Boldface type indicates statistical significance. BMI: body mass index; WHO: World Health Organization; COPD: chronic obstructive pulmonary disease.
CTEPH patients with collaterals had a significantly higher incidence of total occlusion of their right or left main pulmonary artery or lobar arteries. In 8 patients (29% of those with collaterals), complete occlusion of the main pulmonary artery was noted on the side toward which the collateral traveled, whereas only 1.3% (n = 3 of 231) of those without collaterals had complete occlusion (P < 0.001). Additionally, 46% (n = 13 of 28) of those with collaterals versus 15% (n = 35 of 231) of those without collaterals had subtotal occlusion completely limiting flow to one or more lobes of the ipsilateral lung (P < 0.001).
Hemodynamic data in the CTEPH group with collaterals revealed a mean PAP of 41 ± 10 mmHg, a mean cardiac index of 2.2 ± 0.7 L/min/m2, a mean pulmonary vascular resistance (PVR) of 590 ± 294 dyn-s/cm5, and a mean pulmonary arterial saturation of 65% ± 9%. The CTEPH group without collaterals had comparable hemodynamics. The groups were similar in baseline arterial oxygen saturation and oxygen requirements (Table 3).
Table 3.
Angiographic and preoperative hemodynamic characteristics of chronic thromboembolic pulmonary hypertension (CTEPH) patients with and without collaterals
| Characteristic | CTEPH patients with collaterals (n = 28) | CTEPH patients without collaterals (n = 231) | P |
|---|---|---|---|
| Total occlusion of main pulmonary artery | 8 (28.6) | 3 (1.3) | <0.001 |
| Total occlusion of lobar artery or all its segmental branches | 13 (46.4) | 35 (15.2) | <0.001 |
| RA pressure, mmHg | |||
| Mean | 10.4 ± 7.3 | 9.4 ± 4.9 | 0.34 |
| RV pressure, mmHg | |||
| Systolic | 69.6 ± 18.1 | 72.9 ± 22.3 | 0.45 |
| Diastolic | 14.8 ± 7.3 | 12.6 ± 5.6 | 0.07 |
| PAP, mmHg | |||
| Systolic | 69.8 ± 18.1 | 73.3 ± 22.1 | 0.41 |
| Diastolic | 25.4 ± 7.6 | 24.5 ± 8.9 | 0.63 |
| Mean | 41.1 ± 10.4 | 42.4 ± 12.5 | 0.60 |
| PCWP, mmHg | 10.6 ± 3.3 | 11.2 ± 4.4 | 0.55 |
| LV pressure, mmHg | |||
| Systolic | 118.5 ± 18.5 | 122.8 ± 20.8 | 0.32 |
| End-diastolic | 14.7 ± 4.5 | 14.8 ± 5.0 | 0.89 |
| Aortic pressure, mmHg | |||
| Systolic | 118.0 ± 17.7 | 122.5 ± 20.3 | 0.28 |
| Diastolic | 74.0 ± 9.1 | 72.3 ± 11.5 | 0.47 |
| Mean | 90.9 ± 11.6 | 92.6 ± 14.3 | 0.56 |
| CI, L/min/m2 | 2.2 ± 0.7 | 2.3 ± 0.7 | 0.54 |
| PVR, dyn-s/cm5 | 589.8 ± 294.0 | 621.3 ± 389.6 | 0.68 |
| Pulmonary arterial saturation, % | 65.0 ± 8.8 | 64.6 ± 8.9 | 0.82 |
| Arterial saturation, % | 94.3 ± 4.4 | 95.1 ± 2.9 | 0.23 |
| Supplemental oxygen requirement, L by nasal cannula | 1.0 ± 2.0 | 0.7 ± 1.5 | 0.30 |
Data are no. (%) or mean ± SD and were compared with the χ2 test or the independent-sample t test, respectively. Boldface type indicates statistical significance. RA: right atrium; RV: right ventricle; PAP: pulmonary arterial pressure; PCWP: pulmonary capillary wedge pressure; LV: left ventricle; CI: cardiac index; PVR: pulmonary vascular resistance.
Anatomic distribution of collateral vessels
Collateral vessels most often originated from the right coronary artery (RCA; 52.9%, n = 18) and the left circumflex artery (LCx; 35.3%, n = 12; Table 4). They also most commonly traveled toward the right lung. All 28 patients with collaterals underwent surgery. Three operative reports commented on significant collateral flow from the coronary circulation. Two other operative reports mentioned heavy collateral supply but did not specify the origin.
Table 4.
Relationship among collateral vessel origin, pulmonary destination, and clot burden
| Pulmonary artery destination | Total occlusion of main pulmonary artery | Jamieson type of lesion on ipsilateral lung | ||||
|---|---|---|---|---|---|---|
| Collateral origin | Left | Right | 1 | 2 | 3 | |
| RCA (n = 18) | 3 | 15 | 4 | 9 | 7 | 2 |
| LCx (n = 12) | 4 | 8 | 4 | 9 | 2 | 1 |
| LAD (n = 1) | 1 | 0 | 0 | 0 | 1 | 0 |
| PL (n = 3)a | 3 | 1 | 1 | 0 | 3 | 1 |
RCA: right coronary artery; LCx: left circumflex artery; LAD: left anterior descending artery; PL: posterolateral branch of the RCA.
One collateral arising from the PL traveled to both the left and the right pulmonary artery.
Type 1 (or proximal) disease was the most common lesion found intraoperatively in the lung toward which the collateral traversed. In the lone control patient with a coronary artery–pulmonary artery collateral vessel, the collateral arose from the LCx and supplied the left lung territory.
Operative and postoperative outcomes
The 28 CTEPH patients with collaterals and the 208 patients without collaterals who underwent PTE did not differ significantly in total circulatory arrest time (P = 0.48). Postoperative hemodynamic data in the CTEPH group with collaterals revealed a significantly lower mean PAP compared with that in the group without collaterals (21.0 ± 7 vs. 24.1 ± 7 mmHg; P = 0.03). Final mean PVR in the patients with collaterals was 189.9 ± 64.2 dyn-s/cm5, compared with 233.7 ± 117.4 dyn-s/cm5 in those without them (P = 0.05). This change represented a nonsignificant reduction in PVR (60.6% ± 19.6% vs. 53.5% ± 27.2%, respectively; P = 0.19). The prevalence of reperfusion injury was also not significantly different between groups (10.7% vs. 16.3%, respectively; P = 0.16; Table 5).
Table 5.
Postoperative characteristics of chronic thromboembolic pulmonary hypertension (CTEPH) patients with and without collaterals
| Characteristic | CTEPH patients with collaterals (n = 28) | CTEPH patients without collaterals (n = 208) | P |
|---|---|---|---|
| Total circulatory arrest time, minutes | 50.0 ± 21.3 | 47.2 ± 18.1 | 0.48 |
| Mean PAP, mmHg | 21.0 ± 7.0 | 24.1 ± 7.0 | 0.03 |
| PVR, dyn-s/cm5 | 189.9 ± 64.2 | 233.7 ± 117.4 | 0.05 |
| ΔPVR, % | 60.6 ± 19.6 | 53.5 ± 27.2 | 0.19 |
| Reperfusion injury | 3 (10.7) | 34 (16.3) | 0.16 |
Data are no. (%) or mean ± SD and were compared with the χ2 test or the independent-sample t test, respectively. Boldface type indicates statistical significance. PAP: pulmonary arterial pressure; PVR: pulmonary vascular resistance.
Discussion
Collateral vessels are a distinguishing feature of CTEPH compared with other forms of pulmonary hypertension.1,14,15 Numerous studies suggest that bronchopulmonary collaterals possess significant physiological and prognostic value.8-10,16-18 These vessels have been likened to a CTEPH biomarker14,16 owing to their potential to help differentiate CTEPH from idiopathic pulmonary arterial hypertension8 in the midst of nearly identical small pulmonary artery histopathology19 as well as their association with distal arteriolar sparing.9 Other sources of collateral flow in CTEPH patients include the inferior phrenic, intercostal, and internal mammary arteries.18 Pulmonary artery–to–pulmonary artery collaterals have also been noted.20
To our knowledge, coronary artery–pulmonary artery collaterals have not been previously described in the CTEPH literature. They are found, however, in various pathologies, such as chronic obstructive pulmonary disease and atrial fibrillation,21 Takayasu’s arteritis with severe pulmonary hypertension,22 and pulmonary atresia with ventricular septal defect.23 They are also found, usually asymptomatically, in the general population. The prevalence was approximated at 0.1%–0.2% in two large-scale studies of more than 10,000 coronary angiograms.24,25 Drawbacks of this diagnostic modality include contrast dilution, flow limitation, and two-dimensional viewing.26 When studied with multidetector computed tomography (CT) in 5,372 consecutive patients, the prevalence was 0.32%,26 similar to the prevalence that we report in our control group.
The increased prevalence of coronary artery–pulmonary artery collaterals in our CTEPH group compared with our control group suggests that these vessels may help maintain pulmonary parenchymal viability, especially among CTEPH patients with a more significant degree of pulmonary artery obstruction.
Studies of bronchial collaterals in CTEPH patients support the idea that coronary artery–pulmonary artery collaterals may preserve tissue oxygenation in areas distal to the occluded pulmonary artery. The bronchial circulation component of the dual blood supply to the lungs is responsible for nourishing the lung parenchyma. Delivering oxygenated blood from the systemic circulation, two bronchial arteries to each lung commonly originate from the thoracic aorta or intercostal arteries, although anatomic variation exists.8,27 Some of these vessels follow the large airways outside the lungs, while others enter the lung parenchyma.27 Vessels outside the lungs drain into the bronchial veins, azygos vein, or superior vena cava and subsequently into the right atrium, whereas vessels that pierce the pleura form a network of capillaries that drain into the pulmonary veins and into the left atrium.8,27 The latter is deemed bronchopulmonary collateral flow.8,28
Normally, bronchial venous flow accounts for about 1% of the systemic cardiac output.29 In CTEPH patients, however, bronchopulmonary collaterals can account for up to 30% of the blood flow draining directly into the pulmonary veins.8 Animal models of chronic microemboli as well as unilateral pulmonary artery obstruction also demonstrate that markedly dilated bronchial arteries supply areas of decreased pulmonary artery flow.30-32
Collateral flow may play the most substantial role in patients with high-grade proximal lesions. Large bronchial collateral circulation is commonly associated with proximal occlusion and operable disease.14 In a study of 59 CTEPH patients, Shimizu et al.17 found that the median total cross-sectional area of bronchial arteries was significantly larger in patients with thrombi of the lobar or main arteries than in patients with thrombi of the segmental or distal arteries. A dual-energy pulmonary CT angiography study also sought to correlate the extent of pulmonary artery obstruction with collaterals, finding that 64% of completely occluded lobes still displayed evidence of blood flow, suggestive of significant collateral supply.16 Although the origin of the flow was not examined in that study, this statistic parallels our analysis associating coronary artery–pulmonary artery collaterals with more severe pulmonary artery obstruction: of 11 patients in our study with total occlusion of a main pulmonary artery, 8 (73%) had definite collaterals.
In addition to their potential in delineating more proximal and operative disease, collaterals may serve as a marker for postoperative success. Both bleeding and reperfusion lung injury are significant causes of death after PTE surgery.1,2 Kauczor et al.10 found a lower postoperative mortality rate in CTEPH patients with dilated bronchial arteries. This finding correlates with the significant risk for poor hemodynamic response after PTE in the absence of these collaterals.9 Our data revealed a significantly lower postoperative mean PAP in patients with collaterals but no significant difference in postoperative PVR or prevalence of reperfusion injury. Whether the presence of coronary artery–pulmonary artery collaterals confers a long-term morbidity and mortality benefit after PTE is unknown and merits future study.
One theory for collateral formation and their role in hemodynamic outcome is rooted in the pathogenesis of CTEPH, which involves microvasculature remodeling distal to both obstructed and nonobstructed vessels.1,3,19,33 Patients without bronchopulmonary collaterals may have greater amounts of distal arteriopathy or lesions, leading to higher postoperative PVR. On the other hand, patients with proximal disease and less distal CTEPH or vasculopathy may tend to have a greater pressure difference between the systemic circulation and the pulmonary circulation distal to the occlusions, which could serve as a stimulus for bronchopulmonary (and coronary artery–pulmonary artery) collateralization.8,9 The absence of collaterals in patients with primary pulmonary artery hypertension, which affects smaller vessels, complements this theory.8
The origin of coronary artery–pulmonary artery collaterals in CTEPH patients may be explained by the extension of the sinoatrial (SA) nodal artery toward the occluded pulmonary artery. The SA nodal artery is a major contributor of blood supply to the atrial myocardium34 and is the largest atrial artery.35 Two studies of cadaveric hearts show strikingly similar proportions of the SA nodal artery origin compared with the proportions of the origin of the collaterals in our CTEPH patients. In these studies, approximately 35% of SA nodal arteries arose from the LCx, and approximately 65% arose from the RCA.35,36 In our study, 35% of the collaterals arose from the LCx, and 53% arose from the RCA. Albeit rarely, the SA nodal artery may also originate from the posterolateral branch of the RCA: an evaluation of 1,500 coronary angiograms revealed a prevalence of 0.8%,37 similar to the percentage of patients (1%) with collaterals arising from the posterolateral branch among our 259 CTEPH patients with definitive imaging.
Overall, these comparisons are limited because some of our CTEPH patients had collaterals arising from multiple sources (both the RCA and the LCx, for example), and the actual origins of their SA nodal arteries were not studied. Further investigation is needed to understand the stimuli for coronary artery–pulmonary artery collateralization in CTEPH, whether they be pressure gradients or vascular damage that results from obstruction, as has been proposed in studies of other collateral vessels in CTEPH.8,20
Our study has a few limitations. It did not evaluate whether coronary artery–pulmonary artery collaterals are associated with bronchopulmonary collaterals. Although most CTEPH patients are evaluated with CT pulmonary angiography, the timing of these scans is optimized to detect contrast filling the pulmonary arteries rather than the thoracic aorta or intercostal arteries from which bronchial arteries typically originate. The study also included only CTEPH patients who underwent preoperative coronary angiography for PTE. Although unlikely, it is possible that the subset of CTEPH patients who did not undergo coronary angiography has a different prevalence of coronary artery–pulmonary artery collaterals. Additionally, several angiograms were indeterminate for determining the presence of collateral vessels. The true prevalence could actually be slightly higher than we have reported if any of the probable collaterals are indeed true coronary artery–pulmonary artery collaterals. Finally, since this study was performed retrospectively, we were unable to confirm the presence of collaterals intraoperatively for the majority of patients.
This study also has several strengths, particularly the large number of CTEPH patients included. Furthermore, each patient underwent extensive and uniform characterization of their lesions and hemodynamics via both invasive and noninvasive means, performed by a team of physicians expert in assessing CTEPH patients.
Conclusions
The prevalence of coronary artery–pulmonary artery collaterals in patients with CTEPH undergoing coronary angiography is approximately 11%. These vessels are associated with proximal lesions and more significant pulmonary artery obstruction. Their diagnostic and prognostic significance is an area for future investigation.
Supplement.
Figure S1.
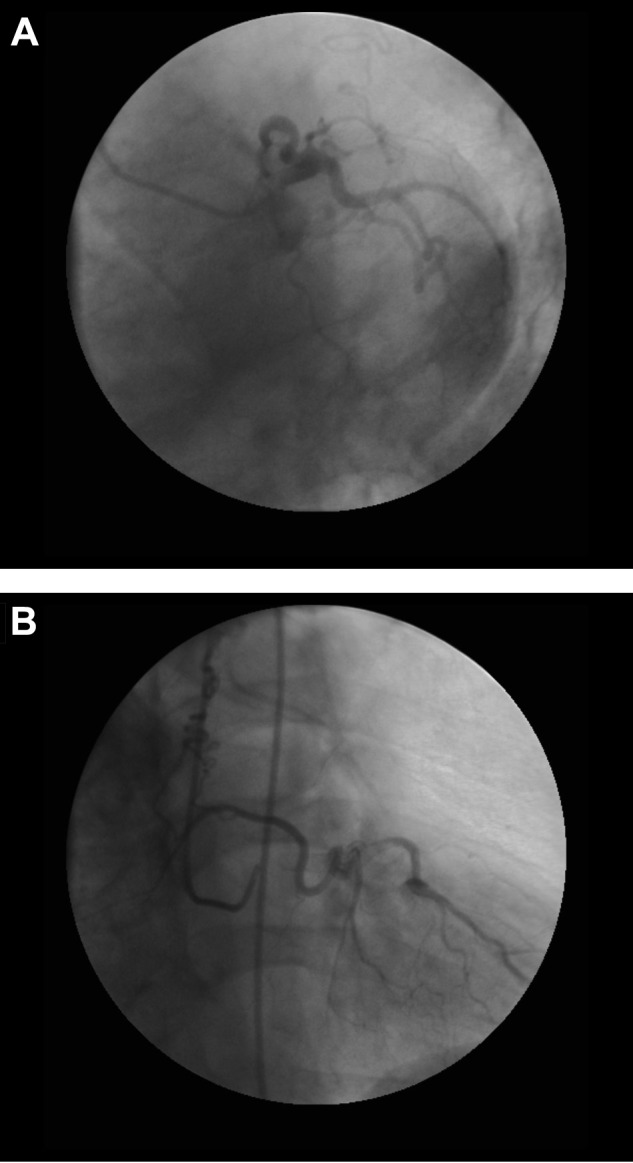
A probable collateral arising from the left circumflex artery displaying collateral-like properties of large caliber and significant tortuosity, shown in left anterior oblique caudal (A) and right anterior oblique cranial (B) views.
Figure S2.
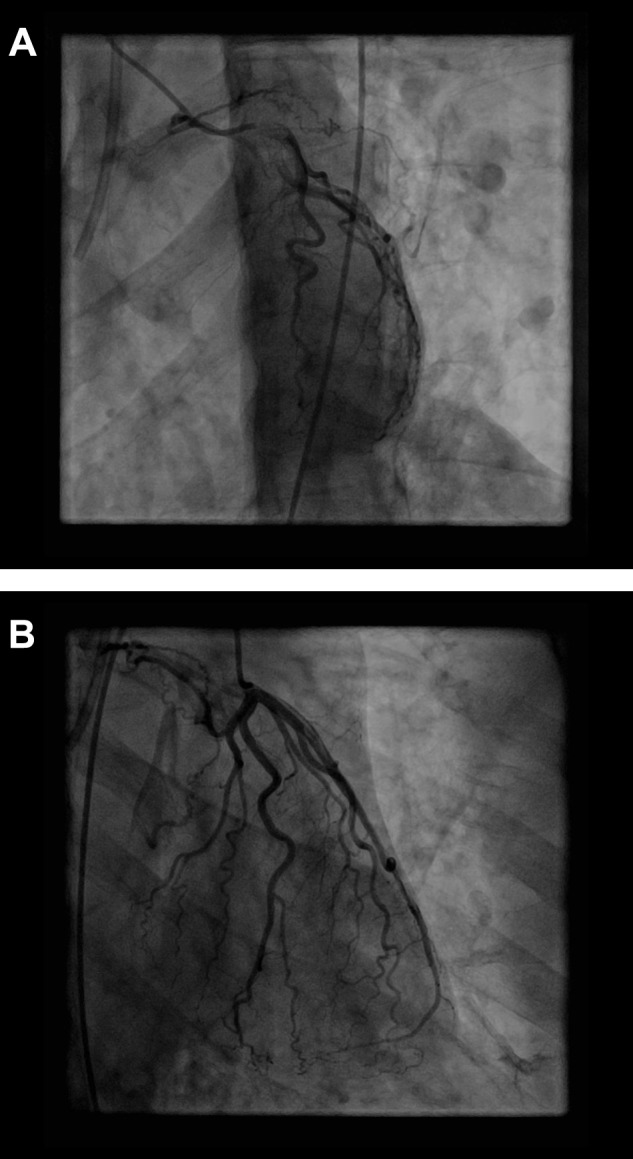
Collateral vessel arising from the left circumflex artery in a patient with cystic fibrosis, shown in left anterior oblique caudal (A) and right anterior oblique cranial (B) views.
Source of Support: Nil.
Conflict of Interest: None declared.
SupplementPulmCirc-005-313.s006.pdf (1.6MB, pdf)
References
- 1.Fedullo P, Kerr KM, Kim NH, Auger WR. Chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med 2011;183:1605–1613. [DOI] [PubMed]
- 2.Haythe J. Chronic thromboembolic pulmonary hypertension: a review of current practice. Prog Cardiovasc Dis 2012;55:134–143. [DOI] [PubMed]
- 3.McNeil K, Dunning J. Chronic thromboembolic pulmonary hypertension (CTEPH). Heart 2007;93:1152–1158. [DOI] [PMC free article] [PubMed]
- 4.Pengo V, Lensing AW, Prins MH, et al. Incidence of chronic thromboembolic pulmonary hypertension after pulmonary embolism. N Engl J Med 2004;350:2257–2264. [DOI] [PubMed]
- 5.Becattini C, Agnelli G, Pesavento R, et al. Incidence of chronic thromboembolic pulmonary hypertension after a first episode of pulmonary embolism. Chest 2006;130:172–175. [DOI] [PubMed]
- 6.Klok FA, van Kralingen KW, van Dijk AP, Heyning FH, Vliegen HW, Huisman MV. Prospective cardiopulmonary screening program to detect chronic thromboembolic pulmonary hypertension in patients after acute pulmonary embolism. Haematologica 2010;95:970–975. [DOI] [PMC free article] [PubMed]
- 7.Kim NH. Assessment of operability in chronic thromboembolic pulmonary hypertension. Proc Am Thorac Soc 2006;3:584–588. [DOI] [PubMed]
- 8.Endrys J, Hayat N, Cherian G. Comparison of bronchopulmonary collaterals and collateral blood flow in patients with chronic thromboembolic and primary pulmonary hypertension. Heart 1997;78:171–176. [DOI] [PMC free article] [PubMed]
- 9.Heinrich M, Uder M, Tscholl D, Grgic A, Kramann B, Schafers HJ. CT scan findings in chronic thromboembolic pulmonary hypertension: predictors of hemodynamic improvement after pulmonary thromboendarterectomy. Chest 2005;127:1606–1613. [DOI] [PubMed]
- 10.Kauczor HU, Schwickert HC, Mayer E, Schweden F, Schild HH, Thelen M. Spiral CT of bronchial arteries in chronic thromboembolism. J Comput Assist Tomogr 1994;18:855–861. [DOI] [PubMed]
- 11.Galiè N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2009;34:1219–1263. [DOI] [PubMed]
- 12.Wilkens H, Lang I, Behr J, et al. Chronic thromboembolic pulmonary hypertension (CTEPH): updated recommendations of the Cologne Consensus Conference 2011. Int J Cardiol 2011;154(suppl):S54–S60. [DOI] [PubMed]
- 13.Gibson CM, Ryan K, Sparano A, et al. Angiographic methods to assess human coronary angiogenesis. Am Heart J 1999;137:169–179. [DOI] [PubMed]
- 14.Delcroix M, Vonk Noordegraaf A, Fadel E, Lang I, Simonneau G, Naeije R. Vascular and right ventricular remodelling in chronic thromboembolic pulmonary hypertension. Eur Respir J 2013;41:224–232. [DOI] [PubMed]
- 15.Hoeper MM, Mayer E, Simonneau G, Rubin LJ. Chronic thromboembolic pulmonary hypertension. Circulation 2006;113:2011–2020. [DOI] [PubMed]
- 16.Hoey ET, Mirsadraee S, Pepke-Zaba J, Jenkins DP, Gopalan D, Screaton NJ. Dual-energy CT angiography for assessment of regional pulmonary perfusion in patients with chronic thromboembolic pulmonary hypertension: initial experience. AJR Am J Roentgenol 2011;196:524–532. [DOI] [PubMed]
- 17.Shimizu H, Tanabe N, Terada J, et al. Dilatation of bronchial arteries correlates with extent of central disease in patients with chronic thromboembolic pulmonary hypertension. Circ J 2008;72:1136–1141. [DOI] [PubMed]
- 18.Remy-Jardin M, Duhamel A, Deken V, Bouaziz N, Dumont P, Remy J. Systemic collateral supply in patients with chronic thromboembolic and primary pulmonary hypertension: assessment with multi-detector row helical CT angiography. Radiology 2005;235:274–281. [DOI] [PubMed]
- 19.Moser KM, Bloor CM. Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest 1993;103:685–692. [DOI] [PubMed]
- 20.Hodson J, Graham A, Hughes JM, Gibbs JS, Jackson JE. Pulmonary artery–to–pulmonary artery anastomoses: angiographic demonstration in patients with chronic thromboembolic pulmonary hypertension. Clin Radiol 2006;61:259–263. [DOI] [PubMed]
- 21.Smith SC, Adams DF, Herman MV, Paulin S. Coronary-to-bronchial anastomoses: an in vivo demonstration by selective coronary arteriography. Radiology 1972;104:289–290. [DOI] [PubMed]
- 22.Choi HM, Kim HK, Shin HS, et al. Total occlusion of right main pulmonary artery in a patient with Takayasu’s arteritis and severe pulmonary hypertension. J Cardiovasc Ultrasound 2012;20:189–192. [DOI] [PMC free article] [PubMed]
- 23.Amin Z, McElhinney DB, Reddy VM, Moore P, Hanley FL, Teitel DF. Coronary to pulmonary artery collaterals in patients with pulmonary atresia and ventricular septal defect. Ann Thorac Surg 2000;70:119–123. [DOI] [PubMed]
- 24.Said SA, Landman GH. Coronary-pulmonary fistula: long-term follow-up in operated and non-operated patients. Int J Cardiol 1990;27:203–210. [DOI] [PubMed]
- 25.Hobbs RE, Millit HD, Raghavan PV, Moodie DS, Sheldon WC. Coronary artery fistulae: a 10-year review. Cleve Clin Q 1982;49:191–197. [DOI] [PubMed]
- 26.Kim MS, Jung JI, Chun HJ. Coronary to pulmonary artery fistula: morphologic features at multidetector CT. Int J Cardiovasc Imaging 2010;26:273–280. [DOI] [PubMed]
- 27.Marchand P, Gilroy JC, Wilson VH. An anatomical study of the bronchial vascular system and its variations in disease. Thorax 1950;5:207–221. [DOI] [PMC free article] [PubMed]
- 28.McCullagh A, Rosenthal M, Wanner A, Hurtado A, Padley S, Bush A. The bronchial circulation—worth a closer look: a review of the relationship between the bronchial vasculature and airway inflammation. Pediatr Pulmonol 2010;45:1–13. [DOI] [PubMed]
- 29.Fritts HW Jr., Harris P, Chidsey CA 3rd, Clauss RH, Cournand A. Estimation of flow through bronchial-pulmonary vascular anastomoses with use of T-1824 dye. Circulation 1961;23:390–398. [DOI] [PubMed]
- 30.Sherrier RH, Chiles C, Newman GE. Chronic multiple pulmonary emboli: regional response of the bronchial circulation. Invest Radiol 1989;24:437–441. [DOI] [PubMed]
- 31.Weibel ER. Early stages in the development of collateral circulation to the lung in the rat. Circ Res 1960;8:353–376. [DOI] [PubMed]
- 32.Charan NB, Carvalho P. Angiogenesis in bronchial circulatory system after unilateral pulmonary artery obstruction. J Appl Physiol (1985) 1997;82:284–291. [DOI] [PubMed]
- 33.Kim H, Yung GL, Marsh JJ, et al. Endothelin mediates pulmonary vascular remodelling in a canine model of chronic embolic pulmonary hypertension. Eur Respir J 2000;15:640–648. [DOI] [PubMed]
- 34.Nerantzis CE, Toutouzas P, Avgoustakis D. The importance of the sinus node artery in the blood supply of the atrial myocardium: an anatomical study of 360 cases. Acta Cardiol 1983;38:35–47. [PubMed]
- 35.James TN, Burch GE. The atrial coronary arteries in man. Circulation 1958;17:90–98. [DOI] [PubMed]
- 36.Hutchinson MC. A study of the atrial arteries in man. J Anat 1978;125:39–54. [PMC free article] [PubMed]
- 37.Okmen AS, Okmen E. Sinoatrial node artery arising from posterolateral branch of right coronary artery: definition by screening consecutive 1500 coronary angiographies. Anadolu Kardiyol Derg 2009;9:481–485. [PubMed]


