Abstract
Significance: Diabetes is strongly associated with increased incidence of heart disease and mortality due to development of diabetic cardiomyopathy. Even in the absence of cardiovascular disease, cardiomyopathy frequently arises in diabetic patients. Current treatment options for cardiomyopathy in diabetic patients are the same as for nondiabetic patients and do not address the causes underlying the loss of contractility. Recent Advances: Although there are numerous distinctions between Type 1 and Type 2 diabetes, recent evidence suggests that the two disease states converge on mitochondria as an epicenter for cardiomyocyte damage. Critical Issues: Accumulation of dysfunctional mitochondria contributes to cardiac tissue injury in both acute and chronic conditions. Removal of damaged mitochondria by macroautophagy, termed “mitophagy,” is critical for maintaining cardiomyocyte health and contractility both under normal conditions and during stress. However, very little is known about the involvement of mitophagy in the pathogenesis of diabetic cardiomyopathy. A growing interest in this topic has given rise to a wave of publications that aim at deciphering the status of autophagy and mitophagy in Type 1 and Type 2 diabetes. Future Directions: This review summarizes these recent studies with the goal of drawing conclusions about the activation or suppression of autophagy and mitophagy in the diabetic heart. A better understanding of how autophagy and mitophagy are affected in the diabetic myocardium is still needed, as well as whether they can be targeted therapeutically. Antioxid. Redox Signal. 22, 1527–1544.
Introduction
The incidence of both Type 1 and Type 2 diabetes continues to rise worldwide (143). The increase in Type 2 diabetes (T2D), characterized by insulin resistance, is attributed to an unhealthy “Western” diet and obesity. Type 1 diabetes (T1D), in comparison, is largely attributed to genetics and autoimmunity, culminating in the elimination of insulin-secreting pancreatic β-cells (150). It is also clear that environmental factors can contribute to the development of T1D, resulting in earlier onset of the disease and a growing number of juvenile patients (150). Earlier disease onset of both Type 1 and Type 2 diabetes increases treatment duration, cost, and the probability of complications throughout the course of a patient's lifetime.
A longer time course of disease progression also potentiates more severe complications as the number of risk factors is also increased. For instance, the risk of a diabetic patient developing cardiovascular disease increases as risk factors accumulate; aging, high blood pressure, cholesterol, and stress contribute to this. Furthermore, diabetes affects multiple organ systems, including the heart, nervous system, and kidneys. Prevention or effective treatment of complications is of paramount importance for improving patient care and quality of life.
The process of removing organelles and long-lived proteins through vesicular sequestration and lysosomal degradation is known as macroautophagy, hereafter called autophagy. In the context of acute myocardial injury, autophagy has been shown to be a critical cardioprotective process (55, 56, 105). Autophagy is the primary mechanism involved in removing whole mitochondria and is, therefore, essential for mitochondrial quality control. Damaged mitochondria that result from cardiac injury can produce reactive oxygen species (ROS) and release death-inducing factors, augmenting cardiac damage (87). Removal of these mitochondria by “mitophagy” prevents additional cell damage and limits tissue injury. However, prolonged autophagy can be detrimental and leads to cardiac atrophy (137). As diabetes is a disease of altered metabolism with slow, sometimes lifelong progression, cardiac function is at risk of deteriorating as a result of long-term dysregulation of autophagy and mitophagy.
Here, we present a discussion of the various factors involved in the progression of cardiovascular complications in diabetes. Cardiomyopathy affects a significant percentage of diabetic patients and greatly contributes to mortality. We discuss the progression of diabetic cardiomyopathy and the contribution of mitochondria to the pathogenesis. In addition, we examine the state of removal of mitochondria via autophagy in the diabetic heart, and draw conclusions about the feasibility of targeting autophagy for the treatment of diabetic cardiomyopathy.
Mechanisms of Autophagy
Autophagy is the primary process by which the cell degrades long-lived proteins and whole organelles, including mitochondria. Removal of mitochondria by mitophagy is critical to the well-being of cardiomyocytes during acute injury such as ischemia/reperfusion (I/R) (55, 56). By quickly degrading dysfunctional mitochondria, the cell can prevent excessive oxidative damage and streamline ATP production.
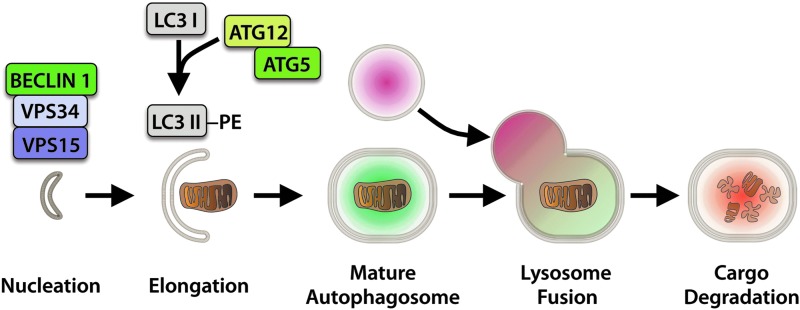
Autophagy begins with an initiation step that involves activation of the BECLIN1-VPS34-VPS15 complex by ULK1/2 (Fig. 1) (77). This is followed by elongation of the isolation membrane by the Autophagy-related (ATG) proteins ATG12 and ATG5, and conjugation of cytosolic LC3I to phosphatidylethanolamine, resulting in membrane-bound LC3II and expansion of the autophagosome membrane to surround the target material (75, 110). The fully formed autophagosome then fuses with a lysosome, resulting in autolysosome formation and cargo degradation, thus allowing for recycling of the subcomponents.
FIG. 1.
Autophagosome formation and degradation. Phagophore nucleation is dependent on BECLIN 1 in complex with VPS34 and VPS15. Elongation of the membrane involves conjugation of LC3I to phosphatidylethanolamine (PE) mediated by ATG12 and ATG5. The fully formed autophagosome subsequently fuses with a lysosome to degrade the cargo. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
In the past, it was believed that lysosomal degradation through autophagy was of low specificity. However, in recent years, it has become clear that sequestration of cargo by an autophagosome is highly specific where individual dysfunctional mitochondria can be sequestered and degraded by targeted mitophagy to ensure a healthy population of mitochondria in the cell.
Mitochondrial Autophagy
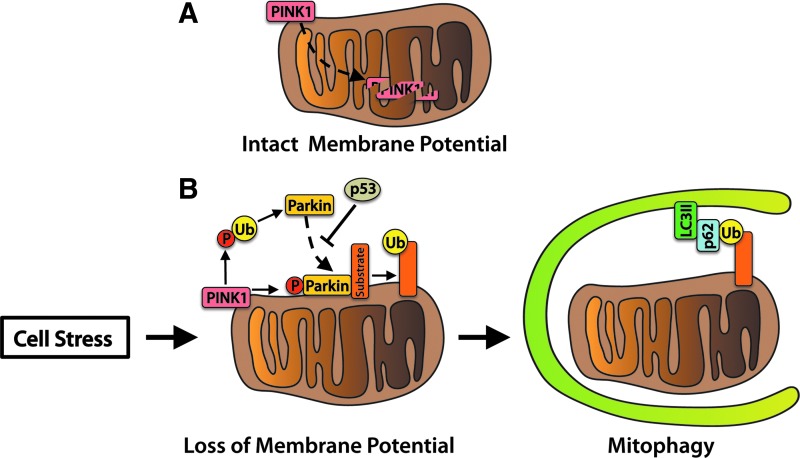
Dysfunctional mitochondria can be detrimental to the health of a cardiomyocyte, necessitating removal of the damaged organelles for myocyte survival (87). There are several pathways that can regulate mitophagy in cells. The first pathway involves labeling of damaged mitochondria for autophagic degradation by PINK1 and PARKIN (Fig. 2). PINK1 is a serine/threonine kinase that is imported and rapidly degraded by mitochondrial proteases in healthy mitochondria (49). Upon loss of mitochondrial membrane potential, PINK1 accumulates on the surface of the mitochondria (73, 116). PINK1-mediated phosphorylation of outer membrane proteins, including MFN2, promotes PARKIN translocation from the cytosol to the mitochondria (22, 104, 116). PARKIN, an E3 ubiquitin ligase, ubiquitinates mitochondrial outer membrane proteins to promote recruitment of the autophagosome (42, 43, 121).
FIG. 2.
PINK1 and PARKIN mediate mitophagy. (A) Healthy mitochondria maintain a high membrane potential and rapidly import and degrade PINK1. (B) Cell stress results in mitochondrial damage and loss of membrane potential. This event causes PINK1 to accumulate on the mitochondrial outer membrane, where it promotes PARKIN translocation and ubiquitin ligase activity by phosphorylating ubiquitin (Ub). Phosphorylated ubiquitin disinhibits PARKIN's ligase activity, resulting in ubiquitination of mitochondrial surface proteins. The scaffolding protein p62 binds ubiquitin and autophagosomal LC3II to anchor mitochondria to the autophagosome. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
Linkage of the ubiquitin-binding protein p62 to ubiquitin on the mitochondrion and lipidated LC3II on the autophagosome provides a physical attachment point (120). Studies in human subjects found that PINK1 transcript levels are suppressed in skeletal muscle tissue of obese or T2D patients, suggesting that mitochondrial quality control via the PINK1/PARKIN pathway is reduced (136). In addition, impaired PARKIN function may contribute to loss of pancreatic β-cells and the development of insulin deficiency. At the onset of diabetes, the tumor suppressor protein p53 accumulates in the cytosol of mouse β-cells and inhibits PARKIN-mediated mitophagy. Mice deficient in p53 have restored mitophagy and are resistant to β-cell loss induced by streptozotocin (STZ) (63). The PINK1/PARKIN pathway plays an important role in mitochondrial quality control in the heart (16, 68, 90). However, its role in diabetic cardiomyopathy is currently unknown.
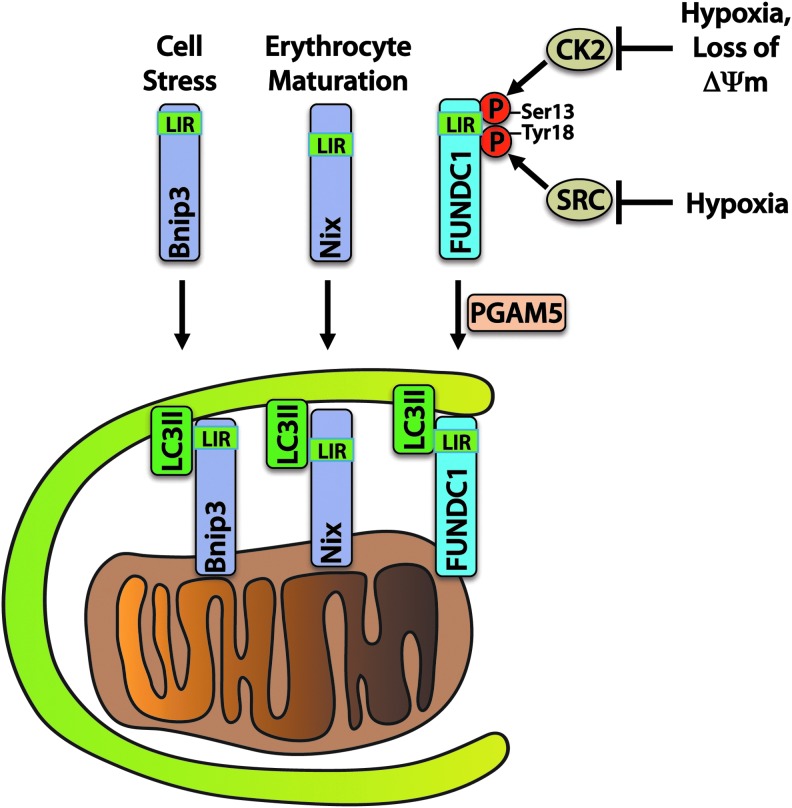
Another important mechanism of mitophagy involves a direct interaction between autophagy receptors on mitochondria and the autophagosome (Fig. 3). The BCL-2 family protein BNIP3 and its homologue NIX are known to participate in mitophagy by providing a direct association between mitochondria and LC3II or gamma-aminobutyric acid receptor-associated protein (GABARAP) on autophagosomes. BNIP3 and NIX play important roles in mitochondrial turnover in the myocardium (31). NIX has also been shown to play a critical role in the removal of mitochondria during erythrocyte maturation (138), and BNIP3 overexpression has been shown to activate mitophagy in myocytes (123). BNIP3 and NIX contain LC3-interacting region (LIR) motifs that bind to LC3 on the autophagosome, thereby allowing for a direct interaction without the need for ubiquitin and p62 (57).
FIG. 3.
BNIP3, NIX, and FUNDC1 are direct receptors for mitophagy. BNIP3 and NIX promote mitochondrial autophagy in response to cell stress or during erythrocyte maturation. Phosphorylation of FUNDC1 by CK2 and SRC kinases maintains FUNDC1 inhibition. In response to hypoxia or mitochondrial damage resulting in loss of membrane potential (ΔΨm), FUNDC1 is activated by dephosphorylation through the phosphatase PGAM5. LC3-interacting region (LIR) motifs bind directly to LC3II on the autophagosome to enable mitophagy. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
BNIP3 may also be involved in quality control of mitochondrial proteins without complete degradation of the mitochondrion. Overexpression of BNIP3 results in the selective reduction of components of the electron transport chain, possibly through activation of mitochondrial proteases (127). BNIP3 and NIX can also directly activate autophagy by displacing BECLIN1 from its inhibitory interaction with BCL-2 (12). Understanding the key roles that these two related proteins play in regulating autophagy and implementing mitophagy in the diabetic myocardium is therefore of great importance.
FUNDC1 is an outer mitochondrial membrane protein that, similar to BNIP3 and NIX, interacts directly with LC3II via an N-terminal LIR. It is maintained in an inactive state through phosphorylation on Tyr 18 and Ser 13 residues by SRC kinase and CK2, respectively. SRC kinase phosphorylation of FUNDC1 decreases during hypoxia, which triggers binding of LC3II on the autophagosome and stimulates mitophagy in HeLa cells (98). Ser 13 is dephosphorylated in response to hypoxia or FCCP treatment by PGAM5 phosphatase, enhancing LC3 binding (20).
Although the function of FUNDC1 has not yet been investigated in the heart, it likely participates in mitophagy in response to stimuli such as ischemia, given that high transcript levels of FUNDC1 are found in the mouse heart (98). Overall, these studies demonstrate that alternative and redundant mechanisms of mitophagy are in place to ensure the removal of dysfunctional mitochondria in cells. Conversely, the degree to which these three mechanisms of mitophagy (PINK1/PARKIN, BNIP3/NIX, and FUNDC1) are involved in the clearance of mitochondria in the diabetic heart has not been determined (84).
An Alternative Autophagy Pathway
Recently, an alternative pathway of autophagy has been identified in cells. Activation of this pathway has been noted in many different tissues, including the heart, and is capable of removing mitochondria in cells (117). Unlike traditional autophagy, the formation of autophagosomes in this pathway is independent of the ATG proteins. Instead, autophagosome expansion is dependent on the small GTPase RAB9, although the identification of other proteins involved in the elongation of the membrane in this pathway remains elusive. In addition, the canonical phagophore membrane has several potential origination sources, including the endoplasm reticulum, mitochondria, and the plasma membrane (5, 53, 125), whereas membranes involved in alternative autophagy are generated from the trans-Golgi network (117). The two autophagy pathways overlap insofar as both traditional and alternative autophagy require ULK-1 and BECLIN-1 for initiation. Currently, very little is known about the proteins involved in regulating alternative autophagy, and additional studies are required to understand the functional role of this pathway in cells.
Upstream Regulators of Autophagy
Autophagy is a highly regulated process that is controlled by several nutrient and energy-sensing pathways. Two main kinases are involved in sensing energy and nutrient status of the cell. These kinases, mTOR and AMPK, dictate whether the cell will activate energy- and amino-acid-consuming anabolic processes such as cellular growth, or energy- and amino-acid-generating catabolic processes such as autophagy, and convey these messages to ULK1/2. In addition, the Forkhead Box O transcription factors and Sirtuin deacetylases also participate in the regulation of autophagy and are important in controlling responses to stress in cardiomyocytes.
Mechanistic target of rapamycin
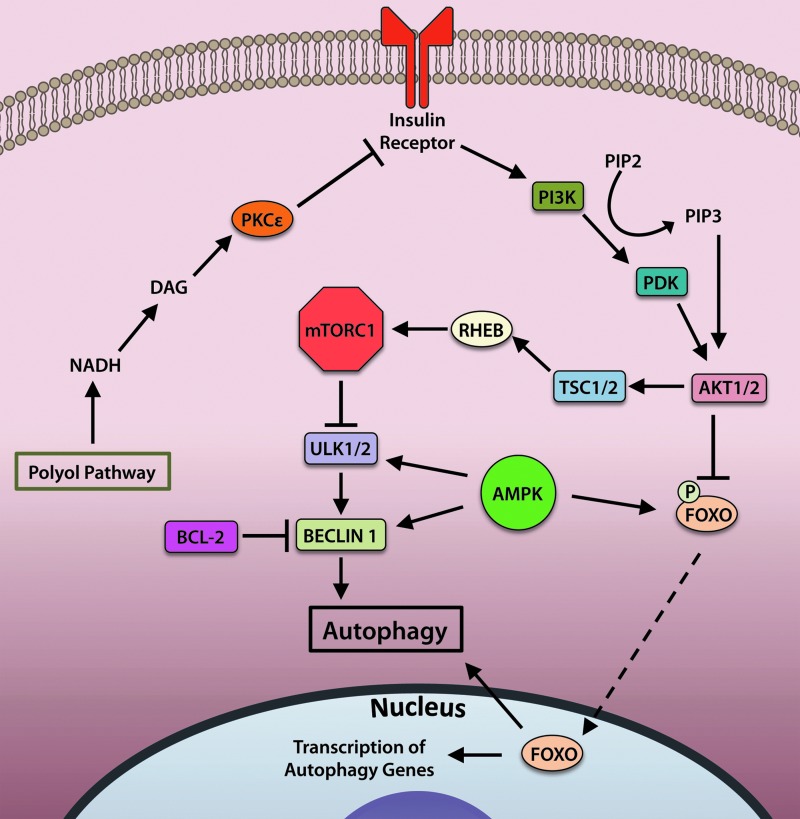
Changes in nutrient or growth factor levels that signal through various cell surface receptors or alterations in amino-acid levels culminate in changes to the activity of Mechanistic Target of Rapamycin (mTOR). mTOR participates in cell survival pathways as a part of mTOR complex 2 (mTORC2), whereas mTOR activity as a part of mTORC1 functions to inhibit autophagy. In addition to mTOR, mTORC1 comprises Regulatory-Associated Protein of mTOR (RAPTOR), Proline-Rich AKT Substrate of 40 kDa (PRAS40), Mammalian Lethal with SEC13 Protein 8 (MLST8), and DEP Domain-Containing mTOR-Interacting Protein (DEPTOR). The complex functions to suppress autophagy and stimulate protein synthesis during times of nutrient sufficiency, and inhibition of mTORC1 via nutrient deprivation shifts the cell away from protein synthesis and toward catabolic autophagy (88).
The activity of mTORC1 is regulated by insulin and growth factors (Fig. 4). Binding of insulin to the insulin receptor stimulates activation of phosphoinositide 3-kinase (PI3K) to mediate conversion of the phospholipid phosphatidylinositol (4,5)-bisphosphate (PIP2) to phosphatidylinositol (3,4,5)-trisphosphate (PIP3), which binds to and activates AKT. Active AKT then inhibits activity of tuberous sclerosis complex (TSC)1/2, a negative regulator of the small GTPase Ras homolog enriched in brain (RHEB) (70). RHEB activates mTOR by binding to its catalytic domain. Activated mTOR inhibits autophagy by phosphorylating and deactivating the serine/threonine kinase ULK1/2 to halt initiation of autophagy (65, 74).
FIG. 4.
Autophagy is regulated by upstream insulin signaling and energy-sensing pathways. Insulin receptor activation activates the PI3K/PDK/AKT pathway, which inhibits autophagy by multiple mechanisms. AKT-mediated phosphorylation of FOXO transcription factors prevents their translocation to the nucleus, thereby suppressing transcription of autophagy-related genes. AKT activation also leads to activation of the mTORC1 complex, which inhibits autophagy by blocking activation of ULK1/2 and BECLIN1. BCL-2 participates in inhibiting BECLIN1 activity by directly binding BECLIN1. Disruption of insulin signaling by polyol pathway products could interfere with insulin receptor signaling, resulting in aberrant autophagy activation. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
mTORC1 also responds to changes in amino-acid levels, which can be altered in the diabetic condition. Amino-acid level sensing and transduction of the status occurs primarily at the lysosome and involves three essential components. A coordinated effort between vacuolar- H+-ATPase (v-ATPase) (164), the Ragulator complex (7), and RAS-related GTP-binding (RAG) GTPases is necessary for mTOR activation (131, 132). mTOR is localized to lysosomes during times of amino-acid sufficiency, presumably to sense nutrient levels. This localization is mediated by active (GTP-bound) RAG complexes (131), which are, in turn, anchored to the lysosome via the scaffolding Ragulator complex (7). v-ATPase responds to accumulating levels of amino acids within the lysosome to activate Ragulator and RAG GTPases, resulting in mTOR activation and localization to the lysosome (164). In the absence of amino acids, v-ATPase associations with Ragulator and RAG proteins are weakened, and mTOR localization is predominantly cytosolic.
AMPK
The energy-sensing kinase AMPK responds to changes in intracellular energy status by activating or deactivating pathways that demand energy input. AMPK is activated during times of cardiomyocyte stress such as ischemia or pressure overload. During states of low energy availability or increased energy demand, such as during exercise (59), glucose deprivation (105), or other conditions in which the AMP/ATP ratio is elevated, AMPK augments autophagy through multiple mechanisms (Fig. 4). AMPK-driven autophagy can occur directly by activation of ULK1/2 or BECLIN 1 (33, 60, 80, 81) or indirectly by acting upstream of mTOR inhibition (71). In addition, AMPK can also promote autophagy via activation of the transcription factors FOXO1 and FOXO3a (discussed in the next section) in cardiomyocytes (103, 141).
FOXO transcription factors
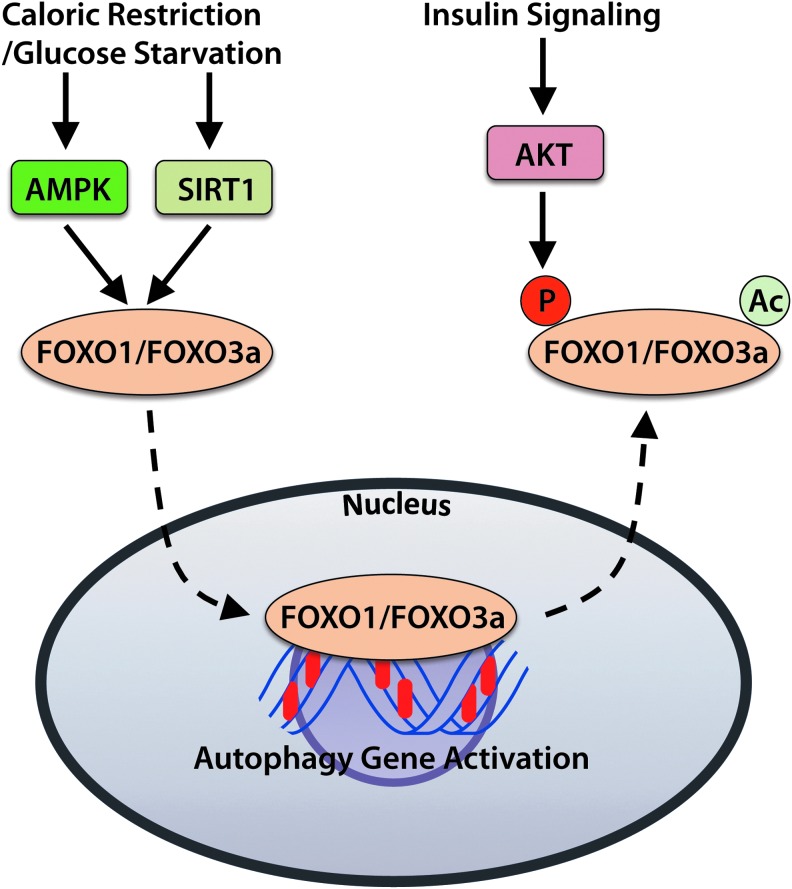
The Forkhead Box O (FOXO) family of transcription factors participates in regulating expression of various factors involved in cell growth, proliferation, and survival. The subfamily members FOXO1 and FOXO3a promote the expression of a number of autophagy genes, including Atg12, Map1lc3A, and Gabarapl1 (Fig. 5) (141). The FOXOs also regulate transcription of BNIP3 and PINK1, which are important regulators of mitophagy (103, 106). Regulation of FOXO activity is controlled by upstream sensors of cellular energy and nutrient levels, as well as by hormones such as insulin.
FIG. 5.
The FOXO transcription factors regulate expression of autophagy genes. Conditions that stimulate autophagy, including caloric restriction or glucose deprivation, result in activation of FOXO1 and FOXO3 by AMPK and SIRT1. Active FOXOs translocate from the cytosol to the nucleus and initiate transcription of autophagy gene products. In contrast, suppression of autophagy and FOXO activity via the insulin receptor/PI3K/AKT signaling pathway leads to acetylation of FOXOs, as well as to phosphorylation of FOXOs by AKT. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
AMPK activates the FOXO proteins when energy levels are low, whereas AKT inhibits FOXOs when growth factors are abundant (18, 50). Phosphorylation of FOXOs by AKT results in deactivation and their export from the nucleus, thereby inhibiting transcription of the genes they control. For instance, AKT-mediated phosphorylation of Foxo3a leads to deactivation and translocation of Foxo3a from the nucleus to the cytosol (18). In contrast, glucose starvation induces FOXO1 and FOXO3 accumulation in the nuclei of neonatal rat cardiomyocytes, which corresponds with elevated autophagy (137, 141).
Sirtuins
The sirtuin (SIRT) family of deacetylases are involved in the regulation of a number of cellular stress responses and metabolic functions. In particular, SIRT1 activates autophagy in response to stimuli such as nutrient deprivation or oxidative stress. In response to caloric restriction (starvation), SIRT1 contributes to the induction of autophagy by deacetylating FOXO transcription factors, activating AMPK, and inhibiting mTOR activity (67, 91). In addition, SIRT1 expression increases during caloric restriction, leading to reduced FOXO3a acetylation and enhanced expression of the autophagy-promoting protein BNIP3 in the kidney (91).
BNIP3 is known to strongly promote mitophagy in cardiomyocytes (123), and evidence of elevated mitophagy was indeed found in kidneys of aged mice subjected to caloric restriction (91). Caloric restriction in the T2D Wistar Fatty (fa/fa) rat model also increases SIRT1 activity and mitophagy in the kidney, and it prevents the development of nephropathy (83). Further, induction of autophagy in response to glucose deprivation is dependent on both SIRT1 and FOXO1 in myocytes (58).
Reactive oxygen species
ROS can be generated from several sites in the cell, including the mitochondria and by NADPH oxidase (NOX) enzymes, and are now recognized as important activators of autophagy (151). In fact, superoxide generated during starvation is believed to be critical for the induction of autophagy in cells (21), and antioxidant treatment can suppress autophagy (149). ROS generated by mitochondria can indirectly activate autophagy by causing oxidative damage to the organelle, which would then require removal via mitophagy (95).
ROS can also promote opening of the mitochondrial permeability transition pore (MPTP) (134), which results in activation of autophagy and mitophagy (35). ROS can also induce autophagy via activation of HIF-1α-mediated expression of BNIP3, resulting in the release of BECLIN1 from BCL-2 and autophagy initiation (161). BNIP3 itself is also activated by oxidative stress (89). ROS has also been implicated in coordinating with NIX in priming mitochondria for mitophagy, which leads to PARKIN translocation (30).
Etiology and Pathogenesis of Diabetic Cardiomyopathy
Diabetes affects organ systems throughout the body and can lead to nephropathy, neuropathy, retinopathy, and cardiomyopathy (45). Diabetic cardiomyopathy is defined by declining contractility and the development of heart failure independent of confounding factors such as hypertension, obesity, or coronary artery disease (78). In its most severe state, diabetic cardiomyopathy is manifested as concentric hypertrophy, ventricular dilatation, and decreased cardiac output. Systolic dysfunction is not apparent until late in the disease pathogenesis, but recent evidence suggests that diastolic dysfunction may be detectable at earlier time points (see Schilling in this Forum). This dysfunction may be attributed to increased interstitial fibrosis that leads to ventricular wall stiffening (4).
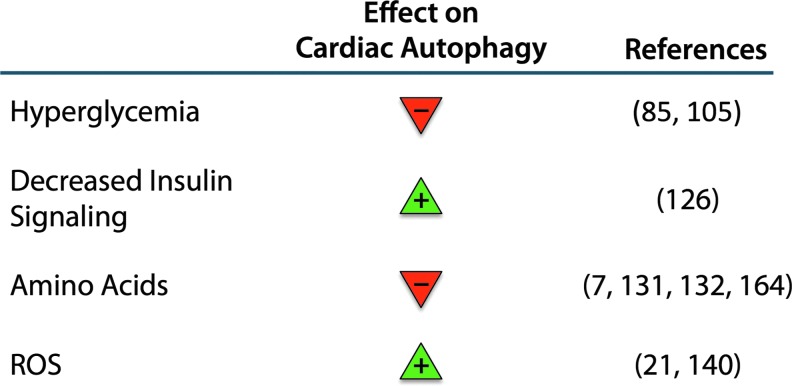
Early detection of diabetic cardiomyopathy remains a challenge, yet it may provide a key to improving outcomes and longevity of diabetic patients. The net effects of various factors that influence autophagy in the heart should be carefully evaluated on a case-by-case basis and are confounded by differing effects on tissue type and individual patients' predispositions. The following analysis of the expected impacts of various factors on autophagy in the diabetic myocardium may help summarize the current understanding (Summarized in Fig. 6).
FIG. 6.
Summary of the effects of metabolic changes in diabetes on autophagy in the heart. Hyperglycemia and elevated amino-acid signaling suppress autophagy, whereas decreased insulin signaling and reactive oxygen species (ROS) generation act to promote autophagy. To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars
The microenvironment enveloping cardiac myocytes in T1D is distinct from that of T2D. This microenvironment can directly impact the state of autophagy in the heart. The distinguishing condition of T1D is insulin insufficiency caused by the death of insulin-producing pancreatic β-cells. This is in contrast with T2D, which is characterized not by a lack of insulin production but instead by systemic insulin resistance. The net result in both disease states is hyperglycemia, and increased circulating glucose contributes to disease pathogenesis via several pathways that will be discussed later in detail. Thus, impaired insulin signaling disrupts tissue homeostasis both directly and indirectly.
A feature distinguishing T1D from T2D is obesity. T2D is frequently associated with obesity, which carries with it a number of risk factors including elevated circulating low-density lipoprotein and triglyceride levels, hypertension, and atherosclerosis/coronary artery disease (79). In addition, elevated circulating amino-acid levels may influence signaling pathways involving key regulators such as AMPK and mTORC1. Still, some risk factors overlap between T1D and T2D. For instance, postprandial reuptake of amino acids is delayed in patients with T1D (36), thereby contributing to similar systemic effects in the absence of obesity. Likewise, circulating lipid levels are also elevated in T1D animal models and patients, and they can be atherogenic (29, 92).
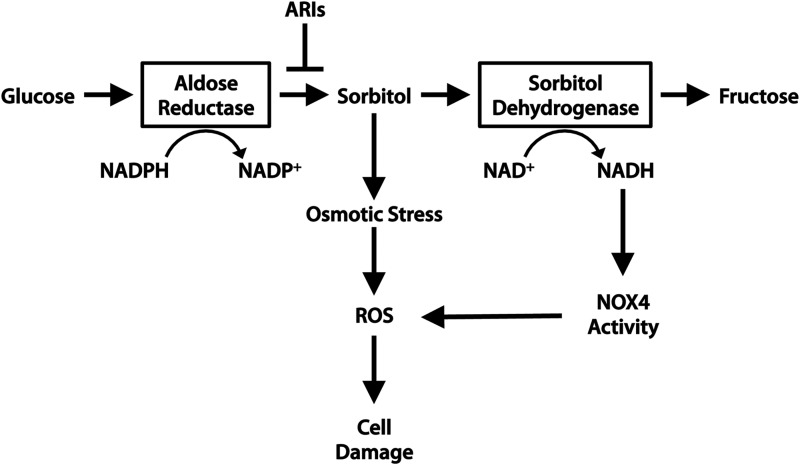
High glucose negatively affects cardiomyocytes in several ways. First, hyperglycemia leads to increased osmotic stress that can lead to myocyte damage and death. Osmotic stress considered on its own, stimulated by high extracellular sorbitol in the absence of hyperglycemia, can induce cardiomyocyte apoptosis (34, 40). Hyperglycemia can lead to osmotic stress through the formation of sugar alcohols, or “polyols” via a pathway involving aldose reductase and sorbitol dehydrogenase. Aldose reductase is a component of the polyol pathway that is responsible for the reduction of monosaccharides. In particular, the reduction of glucose to sorbitol by aldose reductase has been implicated in the development of diabetic complications (45) (Fig. 7). Sorbitol is usually further metabolized by sorbitol dehydrogenase to produce fructose.
FIG. 7.
The polyol pathway contributes to cardiomyocyte damage in diabetes. Hyperglycemia stimulates flux through the polyol pathway, comprising aldose reductase and sorbitol dehydrogenase. Metabolic conversion of glucose to sorbitol by aldose reductase is usually followed by further conversion of sorbitol to fructose. In diabetes, sorbitol accumulates intracellularly and is not cell permeable. Accumulation of sorbitol causes osmotic stress, and NADPH depletion results in oxidative stress and the generation of ROS, leading to cell damage.
In hyperglycemia, sorbitol accumulates within cells and contributes to osmotic stress. Excessive flux through the polyol pathway has also been shown to promote mitochondrial ROS generation. Inhibition of the polyol pathway by aldose reductase or sorbitol dehydrogenase inhibitors (ARIs or SDIs) protects against simulated I/R injury and oxidative modification of SERCA and Ryanodine receptor (RyR) in rat hearts (147), thereby protecting cardiac function. However, further investigation is required to determine whether hyperglycemia leading to polyol pathway flux affects patients with T2D in the same manner as those with T1D (see Aon et al. in this Forum).
High extracellular glucose is also known to induce cardiomyocyte hypertrophy via several pathways. Activation of the PI3K-AKT signaling pathway by high glucose has been shown to increase protein synthesis in neonatal rat cardiomyocytes (160). However, other studies in neonatal rat myocytes found that high glucose alone is insufficient to cause hypertrophy, and protein synthesis is only increased in the presence of aldosterone (135). This suggests that hyperglycemia sensitizes cardiomyocytes to a hypertrophic response, and that addition of a further strain such as osmotic pressure or oxidative stress is needed to activate the hypertrophic response. Consistent with this, cardiomyocytes cultured in high glucose are more sensitive to acute stress such as I/R injury (99). This is likely due to a combination of factors, including mitochondrial sensitization, osmotic stress, oxidation of cellular components by ROS, and decreased autophagy.
Several factors contribute to the formation of ROS in the diabetic heart. Excessive flux through the polyol pathway can result in a surplus of NADH and FADH2 that donate electrons to the mitochondrial electron transport chain. This can overwhelm the electron transport chain componentry and the resident antioxidant superoxide dismutase 2 (SOD2/MnSOD), thereby leading to excessive superoxide production (45). ROS can also be generated by NOX enzymes. NOX4 is of particular interest, because it has been shown to both positively and negatively affect cardiomyocyte health. An increased availability of NADH from polyol pathway flux may stimulate NOX4 activity. Activation of NOX4 by glucose deprivation of cardiomyocytes or fasting in mice induces cardioprotective autophagy (140).
NOX4-generated ROS is known to contribute to oxidative stress during heart failure and in aging hearts (93), which suggests that chronic activation of NOX4 may be detrimental. Mice deficient in NOX4 have improved cardiac function and reduced myocyte apoptosis in response to pressure overload compared with WT mice (93). Moreover, NOX4 expression and activity increase in STZ (T1D) diabetic rat hearts, contributing to oxidative damage and impaired contractility, and in vivo knockdown of NOX4 prevent this functional decline (100). In addition, enhanced NOX4 activity may be linked directly to hyperglycemia, as cardiomyocytes cultured in high glucose have increased NOX4 expression and activity (100). Therefore, while short-term activation of NOX4 may promote beneficial ROS signaling and autophagy, chronic NOX4 activity appears be detrimental.
Mitochondria in Diabetic Cardiomyopathy
Mitochondria play a critical role in the heart by providing the contracting myocytes with ATP through oxidative phosphorylation. The heart is rich in mitochondria due to the high demand for energy. Therefore, it is particularly susceptible to cellular damage caused by dysfunctional mitochondria. Unfortunately, dysfunctional mitochondria can become excessive producers of ROS and release pro-death proteins that can activate cell death pathways (6). Mitochondrial dysfunction and increased production of ROS play key roles in the development of diabetic cardiomyopathy (see Aon et al. in this Forum).
Mitochondrial respiratory chain complexes I and III are primarily responsible for ROS production in the form of superoxide in response to stress such as I/R (86). Superoxide is both a precursor of other ROS and has the potential to directly modify lipids through peroxidation. In addition, the release of ROS by mitochondria can be potentiated by the phenomenon of “ROS-induced-ROS release,” which can result in opening of the MPTP and release of death-promoting factors (165). Electron transport chain dysfunction is known to occur in diabetic hearts.
Studies have found that many of the respiratory chain components are decreased in STZ diabetic rat hearts, but Complex I subunits are the most affected (8, 9). Overexpression of the mitochondria-targeted antioxidant enzyme phospholipid hydroperoxide glutathione peroxidase (mPHGPx) prevents the loss of these proteins and improves contractile function of diabetic rat hearts, suggesting that oxidation of mitochondrial components is responsible (8).
Deficiencies in the electron transport chain are not only limited to the heart but have also been reported in skeletal muscle biopsies of human patients with T2D (128). Under normal conditions, these dysfunctional mitochondria are degraded by mitophagy. However, if the number of dysfunctional mitochondria exceeds the capability of mitophagy or if the process is impaired, then these mitochondria begin to accumulate. How mitophagy is affected in the diabetic myocardium is currently unknown.
Dysfunctional mitochondria can activate the intrinsic pathway of apoptosis that is regulated by the BCL-2 family proteins (52). The BCL-2 family comprises pro- and anti-apoptotic proteins that are maintained in balance in healthy cells. Anti-apoptotic members contain four BCL-2 homology (BH) domains and include BCL-2, BCL-XL, and MCL-1. The pro-apoptotic members include effector proteins BAX and BAK, which form oligomeric pores to permeabilize the outer mitochondrial membrane, and BH3-only proteins such as BID, BIM, and BAD, which directly interact with anti-apoptotic proteins and the effector proteins to maintain an equilibrium (23).
Increased apoptosis of cardiac myocytes has been observed both in human diabetic patients (39) and in animal models of diabetes (19, 60). In addition, a high-fat diet (HFD) alters the BAX:BCL-2 ratio and increases apoptosis in rat myocardium (44). Hyperglycemia can also directly induce release of cytochrome c through mitochondrial permeabilization in cell culture, resulting in apoptosis (19).
Cell death can also be induced via opening of the MPTP, and studies have found that cardiac mitochondria in diabetic animals are more likely to undergo MPTP opening. For instance, mitochondria from STZ diabetic rat hearts have impaired calcium uptake and are more susceptible to calcium-induced MPTP opening (118, 146). The treatment of mitochondria with a reducing agent (dithiothreitol) or an antioxidant peptide (MTP-131) restores the normal threshold for calcium-induced MPTP opening in mitochondria from STZ rat hearts, suggesting that the increased sensitivity to calcium might be due to oxidation of critical mitochondrial proteins (146).
In addition, opening of the MPTP contributes to loss of cells in myocardial I/R injury, and studies have found that mitochondria in the diabetic heart are more susceptible to MPTP opening. Inhibition of the MPTP in T2D rats attenuates tissue injury during ischemia (15). Collectively, these studies suggest that diabetic cardiac mitochondria are unstable and are more likely to activate both apoptotic and necrotic cell death in response to stress.
Mitochondrial Dynamics
Mitochondrial morphology plays an important role regulating bioenergetic efficiency and mitophagy in cells (97). Mitochondrial fusion is mediated by Mitofusin 1 and 2 in the outer mitochondrial membrane and OPA1 in the inner mitochondrial membrane (54). Mitochondrial fission is regulated by fission 1 protein (Fis1), mitochondrial fission factor (Mff), and dynamin-related protein 1 (DRP1). Fis1 and Mff are localized to the outer mitochondrial membrane, whereas cytosolic Drp1 is recruited to mitochondria during fission (54). Studies have found that mitochondrial morphology plays an important role in regulating bioenergetics (see Galloway and Yoon in this Forum).
Fusion of mitochondria during nutrient deprivation causes an increase in ATP synthesis that helps sustain ATP levels during brief periods of starvation (48). In contrast, excess glucose and fatty acids levels induce mitochondrial fragmentation in β-cells and are associated with increased uncoupled respiration (97). Hyperglycemia has also been reported to cause excessive Drp1-mediated mitochondrial fission in neurons, and reducing fission leads to increased neuronal survival (32). Thus, chronic exposure to excess nutrients leads to excessive fission that is detrimental to cells. Mitochondrial morphology is also altered in diabetic hearts. Type 2 diabetic patients displayed cardiac mitochondrial network fragmentation and a significantly decreased expression of MFN1 (111). However, the functional consequences of altered mitochondrial dynamics in the diabetic myocardium require further investigation (see Galloway and Yoon in this Forum).
Paradoxically, mitochondrial fission is also a prerequisite for mitophagy. Asymmetrical fission of mitochondria generates two bioenergetically different fragments leading to selective sequestration of the mitochondrion with lower mitochondrial membrane potential by mitophagy (148). Recent studies utilizing cardiac specific DRP1 knockout mice have confirmed the importance of mitochondrial fission in mitochondrial clearance (69, 76). These studies demonstrated that mitophagy was abrogated in myocytes lacking DRP1, and this leads to accumulation of dysfunctional mitochondria and development of heart cardiac dysfunction. Conversely, fused mitochondria are protected against mitophagy, possibly because elongated mitochondria are too large to be engulfed by autophagosomes (48).
Since studies indicate that fission is increased in response to an excess nutrient environment, an increase in mitophagy would be anticipated. However, there is an abundance of dysfunctional mitochondria in the diabetic heart (17, 84, 144), suggesting that mitophagy is incapable of clearing these mitochondria. It is likely that chronic exposure to excess nutrients in diabetes will lead to adaptations that interfere with many cellular processes, including autophagy and mitophagy. This adaptation might contribute to the accumulation of dysfunctional mitochondria in myocytes, which can lead to loss of myocytes and the development of diabetic cardiomyopathy.
Autophagy and Mitophagy in the Diabetic Heart
Myocardial autophagy in T1D
Because insulin activates the PI3K/AKT/mTORC1 pathway, it was initially believed that autophagy would be increased in both T1D (insulin deficiency) and T2D (insulin resistance). Consistent with this hypothesis, insulin receptor substrate (IRS) null mice have enhanced autophagic activity in their hearts (126). However, studies have revealed that autophagy is actually suppressed in the hearts of T1D animals (60, 84, 156, 159, 163). For instance, OVE26 mice, a genetic model of T1D, have lower levels of autophagy in their hearts at 5–6 months of age (156, 159). Xu et al. also reported suppressed autophagy after 9 weeks of diabetes in the hearts of STZ mice (159). This study reported that STZ-diabetic mice have lower LC3II/GAPDH ratios than control mice, and experiments with Bafilomycin A1, an inhibitor of lysosomal maturation, indicate impaired formation of autophagosomes in STZ mouse hearts and, consequently, impaired autophagic flux. In addition, the authors report reduced levels of Atg12 and the Atg12-Atg5 complex in the hearts of STZ mice, a potential reason for decreased autophagosome formation (159). The suppression of autophagy in the T1D heart is due to increased mTORC1 activity (159) and/or reduced AMPK activity (156, 159).
In addition, hyperglycemia inhibits autophagy in H9c2 cardiac cells by increasing the interaction between BCL-2 and BECLIN 1 (60). Myocyte apoptosis is greatly elevated in the hearts of diabetic patients: till 85-fold over control patient biopsy samples (39). Because the BCL-2 proteins govern both apoptosis and autophagy, a disruption in their regulation in the diabetic myocardium appears culpable. Evidence of increased binding of BCL-2 to the autophagy inducer BECLIN1 has been observed in animal models of T1D (60), and STZ-diabetic mice show increased cardiomyocyte apoptosis, very much similar to human patients.
He et al. reported that the elevated levels of apoptotic markers in the hearts of STZ-diabetic mice could be ameliorated by treatment with the antidiabetic drug metformin. Metformin treatment was shown to reduce BCL-2 binding to BECLIN1, which has the dual effect of both upregulating autophagy and liberating BCL-2 for apoptosis suppression (60). However, this study only evaluated levels of LC3II in STZ-diabetic mice, and it did not investigate autophagic flux in animals. While the study reports that metformin restores flux in H9c2 cells subjected to high glucose, an in vivo model of chronic diabetes is substantially different from acute glucose treatment of cells. Still, the study raises the possibility that elevated apoptosis and impaired autophagy found in T1D hearts may be related to altered BCL-2 family protein interactions. Overall, these studies indicate that the autophagy pathway is affected at multiple steps in the diabetic myocardium.
The functional role of autophagy in type 1 diabetic hearts
Several studies have investigated whether altered autophagy correlates with development of diabetic cardiomyopathy. Currently, there is a consensus that cardiac autophagy is reduced in type 1 diabetes. However, the functional consequence of this reduction in autophagy remains unclear and controversial. Xie et al. reported that OVE26 and STZ-induced type 1 diabetic mice have reduced autophagy in their hearts, and restoration of autophagic activity with metformin ameliorates diabetic cardiac injury (156). Similarly, He et al. found that activation of AMPK in diabetic mice restores cardiac autophagy and protects against cardiac cell apoptosis, ultimately leading to improvements in cardiac structure and function (60). These results suggest that impaired autophagy contributes to cardiac damage due to reduced clearance of dysfunctional organelles and protein aggregates, and that enhancing autophagy can limit damage in the type 1 diabetic heart.
In contrast, Xu et al. proposed that the reduced cardiac autophagy in type 1 diabetic mice is an adaptive response to prevent excessive autophagic degradation of cellular components. This study found that cardiac damage in STZ and OVE26 type 1 diabetic mice is attenuated in mice deficient in the autophagy proteins BECLIN 1 and ATG16. These mice have significantly improved cardiac function and lower levels of oxidative stress, interstitial fibrosis, and myocyte apoptosis (159). In contrast, cardiac-specific BECLIN 1 transgenic mice have enhanced autophagic activity, resulting in exacerbated diabetes-induced cardiac apoptosis and fibrosis (159). Although type 1 diabetes is associated with mitochondrial dysfunction, this study found that mitophagy proteins PINK1 and PARKIN are significantly reduced in the diabetic hearts, while the mitophagy receptor BNIP3 is unchanged. This suggests that mitophagy is reduced in these hearts despite extensive mitochondrial dysfunction, leading to the accumulation of dysfunctional mitochondria and activation of cell death in myocytes.
Myocardial autophagy in T2D animal models
In contrast to T1D, results from studies on T2D are less consistent and report that cardiac autophagy can be unchanged (94), reduced (27, 51, 139, 158), or even increased (109, 130). However, major inconsistencies in the methods used to assess autophagy between studies likely account for a large portion of the discrepancies in results. For instance, some of these studies report LC3II/I ratios that are unchanged or lower in the hearts of mice with metabolic syndrome. However, inspection of the reported results shows no decrease in LC3II, but instead an increase in LC3I (27, 94), which is not indicative of autophagy suppression but may be due to increased transcription of the LC3 gene. Further, while the ratio of LC3II/LC3I may be informative in cases where only very short-term changes in autophagy are of interest, this ratio alone is insufficient to describe the state of autophagic flux in chronic conditions such as diabetes where transcriptional changes in LC3 may also be occurring.
Some studies speculate on the state of autophagic flux by analyzing degradation of p62, the scaffolding protein that anchors cargo to the LC3II-decorated autophagosome. Guo et al. show that levels of LC3II are decreased while LC3I remains unchanged in the hearts of HFD-fed mice, indicating a lower abundance of autophagosomes (51). They also report that p62 levels are elevated in the HFD group, which is suggestive of decreased autophagic flux. Similarly, Mellor et al. report that the hearts of insulin-resistant fructose-fed mice have an increase in LC3II with no change in LC3I, as well as elevated p62 levels (109), again suggesting impaired autophagic flux. However, because autophagic flux is dynamic, merely evaluating the levels of proteins involved yields only a snapshot of the process. A blockage at the pathway terminus via lysosomal inhibition allows one to evaluate both autophagosome generation and degradation.
Sciarretta et al. found that HFD activates RHEB in the heart, leading to mTORC1 activation and consequent suppression of autophagy (139). Specifically, the authors found reduced levels of LC3II and elevated p62 in HFD-fed mice. Moreover, they found that the number of GFP-LC3-positive autophagosomes in left ventricle cross-sections did not increase in the hearts of HFD-fed mice after administration of the lysosomal-fusion inhibitor chloroquine, suggesting a failure to form autophagosomes rather than increased autophagosome removal.
In contrast, when Xu et al. investigated autophagic flux in the context of insulin resistance and metabolic syndrome, they found that mice fed HFD develop cardiac functional deficits and accumulate LC3II in their hearts, suggesting an increased number of autophagosomes. They also noted the accumulation of p62, indicating impairment in autophagosome clearance. Transmission electron microscopy confirmed the accumulation of autophagosomes and a lack of autolysosomes in the hearts of HFD-fed mice. In addition, HFD-fed mice treated with chloroquine did not accumulate additional LC3II whereas control diet-fed mice did so, confirming that HFD results in impaired clearance of autophagosomes in cardiac tissue (158). Thus, while Sciarretta et al. (139) suggest autophagosome formation is impaired in the hearts of mice fed HFD, Xu et al. (158) suggest impaired autophagosome degradation. Taken together, these studies indicate that the hearts of T2D animals have impaired autophagic flux, although substantial disagreement persists.
While studying autophagy in the hearts of obese animals with insulin resistance is informative because it recapitulates the human disease condition, studies that investigate conditions of insulin resistance in the absence of obesity are equally informative. Mice fed a high fructose diet develop systemic insulin resistance and cardiac dysfunction in the absence of obesity. These mice have markers of impaired clearance of autophagosomes in cardiac tissue, including elevated LC3II/I ratios and accumulated p62 (109). An analysis of flux using pathway inhibition in this model would be of great value to the field, because autophagic flux has not been evaluated in insulin resistance without obesity.
In another model of impaired insulin signaling, mice with cardiac-specific deletions of Irs1 and Irs2 genes have unrestrained autophagic flux, leading to impaired fractional shortening by 4 weeks of age, heart failure, and early mortality. This also demonstrates the importance of endogenous insulin signaling in the suppression of autophagic flux. Notably, these mice show mitochondrial dysfunction that precedes the cardiac functional decline (126). The accumulation of dysfunctional mitochondria suggests a failure of mitophagy despite having enhanced autophagy.
The functional role of autophagy in type 2 diabetic hearts
In contrast to type 1 diabetes, enhancing autophagy in HFD-induced type 2 diabetes appears to be a protective response that reduces cardiac damage. For instance, sustained activation of RHEB and mTORC1 in the hearts of mice on an HFD leads to inhibition of autophagy and exacerbates ischemic injury after a myocardial infarction (139). Many other studies have linked mTORC1 activity to the development of diabetic cardiomyopathy. Völkers et al. found that PRAS40-mediated mTORC1 inhibition prevents the development of HFD-induced diabetic cardiomyopathy (152). Rapamycin, a potent inhibitor of mTOR and inducer of autophagy, improves cardiac function in db/db type 2 diabetic mice (28). In addition, AKT2-deficient mice on HFD have reduced mTORC1 activation and increased autophagy that correlates with reduced hypertrophy and improved cardiac function (158). Of note, knockdown of AKT2 in cancer cells specifically activates mitophagy and can cause cell death from excessive removal of mitochondria (133).
Thus, the cardioprotective effects of AKT2 knockout observed by Xu et al. may be partially explained by an enhancement of mitophagy that expedites removal of dysfunctional mitochondria in the hearts of mice with insulin resistance and metabolic disorder. However, AKT2 knockout mice are known to develop severe diabetes and insulin resistance (24, 41), making systemic inhibition of AKT2 dangerous for patients with T2D. Collectively, these studies demonstrate that enhancing autophagy is cardioprotective in HFD-induced type 2 diabetes. Together, these results highlight the complex role of autophagy in diabetes and the need for additional studies to determine under what conditions autophagy is beneficial versus detrimental in diabetes.
Mitophagy and p53 in diabetes
The tumor suppressor p53 is well known for its role in apoptosis, and loss of p53 is associated with the development of certain cancers (14). Recent studies have also implicated p53 in the development of diabetes. For instance, Hoshino et al. reported that p53-deficient mice were protected against the development of diabetes in models of both STZ-induced type 1 and db/db type 2 diabetes (63). In addition, it is known that hyperglycemia activates p53-mediated cell death in myocytes (37). p53 also promotes cardiac dysfunction in diabetic mice (115). However, p53 has a broad functional role in cells and regulates other processes, including autophagy.
The regulation of autophagy by p53 is very complex, and p53 can both activate and inhibit autophagy in a context-dependent manner (101). Both the intracellular location and levels of p53 can influence its effect on autophagy. For instance, p53 exerts many of its functions by acting as a transcription factor, and nuclear p53 regulates transcription of several genes involved in autophagy, including DRAM (damage-regulated autophagy modulator) and SESTRIN2 (26, 102). Although expression of these genes correlates with increased LC3II levels in cells, the implications of the autophagosome accumulation are not clear. Expression of the p53 target gene DRAM results in increased levels of autophagosomes, as determined by electron microscopy and LC3II levels. However, it is not clear whether DRAM-mediated autophagosome accumulation is the result of increased formation or impaired clearance, as no flux experiments have been completed (26). In addition, DRAM participates in p53-mediated apoptosis, further confounding its role in autophagy (26).
In contrast, cytosolic p53 can inhibit autophagy (101), and it can directly inhibit mitophagy in the heart by interacting with PARKIN, thereby preventing it from translocating to dysfunctional mitochondria. As a result, the removal of damaged mitochondria is abrogated and dysfunctional mitochondria accumulate, leading to cardiac functional decline (64). Inhibition of PARKIN-mediated mitophagy by p53 has also been linked to impaired function of pancreatic β-cells in T1D (63). However, whether cytosolic p53 contributes to the development of diabetic cardiomypathy by inhibiting PARKIN-mediated mitophagy in myocytes remains to be determined.
The study by Hoshino et al. found that pharmacological inhibition of p53 with pifithrin-alpha restores mitophagy and ameliorates accumulation of dysfunctional mitochondria in β-cells in both T1D and T2D (63). Although T1D and T2D have distinct mechanisms, mitochondrial dysfunction in myocytes and other cells is a common characteristic for both types. Thus, it is possible that activation of p53 in the hearts of diabetic mice contributes to loss of myocytes through both activation of apoptosis and inhibition of autophagy. Additional studies are needed to determine whether preserving or enhancing PARKIN-mediated mitophagy represents a potential therapeutic target for both T1D and T2D.
Autophagy in Cell Types Beyond the Cardiomyocyte
Metabolic changes associated with diabetes will impact many other cell types and tissues that can influence cardiac function. For instance, obesity is the result of excess fat storage in the form of white adipose tissue. Because adipocytes regulate fat storage and can release pro-inflammatory cytokines (13), they can have profound effects on cardiac function. Recent studies have found that autophagy and mitophagy play important roles in adipogenesis, and inhibition of autophagy reduces adipose tissue mass (47, 145, 162). Adipocyte-specific Atg7 knockout mice are lean due to reduced white adipose tissue (145, 162), and these mice are also resistant to HFD-induced obesity (162). Abrogation of autophagy in adipocytes results in altered cell morphology and a large number of mitochondria compared with normal white adipocytes (47), which indicates that mitophagy is important for adipocyte maturation. Thus, these studies suggest that activation of autophagy and mitophagy in adipocytes can indirectly contribute to the development of T2D by promoting obesity.
Cardiac fibrosis is a major feature of diabetic cardiomyopathy, where excessive production and deposition of extracellular matrix (ECM) proteins leads to increased myocardial stiffness and subsequent cardiac dysfunction (4). Fibroblasts are responsible for producing the ECM in the heart, and recent evidence suggests that autophagy is an important modulator of fibrosis. Autophagy is involved in the intracellular degradation of type I collagen for the prevention of excess ECM deposition (3, 72, 82). There is also evidence that impaired autophagy promotes differentiation of fibroblasts to myofibroblasts, the profibrotic phenotype of fibroblasts (2, 96). Although studies have reported increased activation of cardiac fibroblasts and fibrosis in diabetic hearts, no studies have examined whether autophagy plays a role in this response. However, considering that autophagy is reduced in T1D hearts (60, 156, 159, 163), it is possible that autophagy plays a role in preventing fibrosis. Hyperglycemia may also participate in the enhanced fibrosis in diabetic hearts, as it both causes activation of cultured fibroblasts (142) and reduces autophagy in cardiac myocytes (85). Thus, several factors may contribute to fibrosis in diabetic cardiomyopathy.
Diabetic patients are also at an increased risk of developing vascular diseases due to defects in endothelial and vascular smooth muscle cell (SMC) function (25). The role of autophagy in atherosclerosis is still poorly understood, and few studies have focused on the role of autophagy in vascular cells in the setting of diabetes. Studies indicate a protective role of autophagy against atherosclerosis in SMCs. Autophagy protects SMCs in vitro against oxidized lipids and excess free cholesterol, while inhibition of autophagy triggers cell death (62, 157).
In addition, sustained hyperglycemia results in the generation of advanced glycation end products (AGEs) that can damage endothelial cells (46). Hou et al. reported that AGEs increase both the number of autophagosomes in endothelial cells and cell death (66). Inhibition of autophagy, however, enhanced AGE-mediated cell death in endothelial cells (155), suggesting that autophagy is functioning to protect against AGE-mediated cellular damage. Although these in vitro studies suggest that enhanced autophagy protects against stress in vascular cells and could potentially influence the stability of atherosclerotic plaques, additional studies are needed to characterize how diabetes affects autophagy and mitophagy in these cells.
Therapies for Diabetes and Diabetic Cardiomyopathy
Many treatment options are available for diabetes and diabetic cardiomyopathy, and it is possible that several of these therapies could affect the status of autophagy. Although no treatments exist that specifically target mitophagy, the effects of available therapies on autophagy and mitophagy have not yet been adequately researched. Several established and experimental treatments may alter autophagy, either positively or negatively, including angiotensin receptor blockers (ARBs), the AMP analog AICAR, ARIs, FOXO1 inhibitors, and resveratrol.
ARBs are first-line therapies for hypertension and heart failure that function to modulate the renin-angiotensin-aldosterone system via inhibition of angiotensin II (AngII) type 1 (AT1) receptors. Recently, a link between AT1 receptors and autophagy has been made. Stimulation of neonatal rat cardiomyocytes overexpressing AT1 with AngII results in hypertrophy and elevated markers of autophagy, both of which are completely abrogated by the ARB candesartan (122). Moreover, AngII has been shown to contribute to insulin resistance in skeletal muscle and SMCs (38, 153, 154). AngII not only has been shown to activate AMPK in vascular SMCs to inhibit proliferation but also has been found to inhibit AMPK in H9c2 rat cardiomyoblast cells to induce hypertrophy (61, 114). However, the effects of AngII and ARBs on mitophagy remain to be determined, and whether ARBs can be utilized to modulate autophagy remains unexplored.
The AMP analog AICAR is not only known to be cardioprotective during I/R injury though inhibition of adenosine kinase and adenosine deaminase, but it has also been shown to induce translocation of the GLUT-4 glucose transporter to the surface of rat ventricular papillary myocytes in an AMPK-dependent manner via a pathway that is independent of insulin signaling and PI3K activation (129). This is an exciting prospect for patients with T2D who may have impaired cardiac glucose uptake. However, the effects of AICAR-mediated AMPK activation on autophagy and mitophagy in the myocardium are not fully understood. Although AICAR potently activates AMPK in rat hepatocytes, autophagy is actually inhibited (108). Radioactive pulse-chase assays were used to measure proteolytic degradation of long-lived proteins by autophagy in the rat hepatocytes. Despite robust activation of AMPK, proteolysis was actually found to be inhibited by AICAR to the same extent as the autophagy inhibitor 3-MA (108).
One possible explanation is that ATP was depleted to such an extent that activation of the highly-ATP dependent process of autophagy became unfeasible despite activation of AMPK (107). Thus, the use of AICAR for the treatment of diabetic cardiomyopathy should be investigated carefully to determine under what circumstances AMPK activation is beneficial versus detrimental.
ARIs aim at inhibiting the polyol pathway and reducing oxidative and osmotic stress in a variety of cell types. Animal studies using ARIs for the treatment or prevention of diabetic neuropathy have shown great promise, but clinical trials conducted since the mid 1980s and ongoing have found ARIs to be not efficacious and potentially toxic to human patients. Nevertheless, ARIs have been shown to be cardioprotective to both diabetic and nondiabetic rats during simulated I/R injury (124), and the activity of aldose reductase may affect autophagy levels in the heart through the accumulation of diacylglycerol (DAG) caused by excess NADH production from polyol pathway flux. DAG-mediated activation of protein kinase C results in inhibition of the insulin receptor signaling pathway and decreased AKT activity, culminating in activation of FOXO transcription factors and increased transcription of autophagy genes (1). Thus, aldose reductase inhibition could help suppress aberrantly activated autophagy and prevent ATP depletion, although no studies have been conducted to determine the effects of aldose reductase inhibition on cardiac autophagy.
Recently, developed inhibitors of FOXO1 aim at lowering circulating glucose levels by reducing hepatic FOXO1-driven gluconeogenesis. In this respect, these inhibitors have shown efficacy in the T2D db/db mouse model (113). Battiprolu et al. also found that FOXO1 and FOXO3 are aberrantly active in hearts of high-fat diet-induced and genetic (db/db) models of T2D. Excessive FOXO1 and FOXO3 activity in T2D may contribute to the development of contractile dysfunction, as cardiomyocyte-specific FOXO1 knockout mice are resistant to HFD-induced cardiac hypertrophy and have improved heart function after prolonged HFD feeding (10). Pharmacologic inhibition of FOXOs may prove to be a viable means of modulating autophagy in the diabetic heart in the future, but the effects of FOXO1 inhibitors on cardiac function or autophagy have not yet been investigated.
Resveratrol is a plant-derived polyphenol that is purported to have health benefits, including improving cardiovascular health, reducing the risk of cancer, reducing severity of diabetic complications, and promoting longevity (Sung et al. in this Forum). Although several of the earlier studies on the health benefits of resveratrol may have been overstated, the compound has the potential to promote autophagy via SIRT1 activation (112). Other studies indicate that resveratrol does not directly activate SIRT1, but instead may act on intermediates such as AMPK to promote activation (11, 119). Therefore, while resveratrol may have beneficial effects in some circumstances, the precise mechanism of action remains unclear.
Concluding Remarks
Inconsistencies in animal models and assays used to evaluate autophagy and mitophagy in diabetes have yielded convoluted and often conflicting results. However, as evidence accumulates, the distinction between Type 1 and Type 2 diabetes grows more apparent. A concerted effort to standardize animal models for future studies should be made to clarify results and refine conclusions. Part of this involves understanding the strengths and limitations of animal models that do not precisely recapitulate the human condition. Findings from studies in animal models of type 1 diabetes tend to be more in accord than findings from type 2 diabetic animals. This may be due to a greater degree of consistency in type 1 animal models, and it also underscores the complexity of the disease state in type 2 diabetes. More refined models of type 2 diabetes would help enhance our understanding of the state of autophagy and mitophagy in the heart, as well as the contributions of autophagy to the pathogenesis of cardiomyopathy.
One hypothesis regarding the state of autophagy in the diabetic myocardium is that T1D may have impaired autophagosome formation, while T2D may have impaired autophagosome clearance. Although this appears to be an undesirable feature of these conditions, it allows for more individualized therapeutic approaches. In the correct context, the ability to selectively upregulate autophagosome formation or enhance autophagosome clearance may prove to be greatly beneficial. The discrepancies between T1D and T2D and the status of autophagy in each may also account for why so many treatments that appear promising in animal models fail to show efficacy in clinical trials. It is therefore of critical importance that future studies on the status of autophagy in diabetes focus on clarifying the state of autophagic flux.
A lack of sufficient studies on mitophagy in the heart and other tissues leaves many uncertainties about the contribution of improper mitochondrial clearance in diabetes. However, a growing interest in the field of mitophagy in diabetes promises to shed more light on the significance of mitophagy in the development of diabetic cardiomyopathy. Targeting mitophagy to combat diabetic cardiomyopathy by enriching the heart's population of mitochondria with healthier organelles is a novel approach and should be investigated more thoroughly with the prospect of decreasing patient mortality and improving quality of life.
Abbreviations Used
- AGEs
advanced glycation end products
- ARBs
angiotensin receptor blockers
- ARI
aldose reductase inhibitor
- ATG
autophagy-related
- BNIP3
BCL-2/adenovirus E1B nineteen kDa protein-interacting protein 3
- DEPTOR
DEP domain-containing mTOR-interacting protein
- ECM
extracellular matrix
- FOXO
forkhead Box subclass O
- GABARAP
gamma-aminobutyric acid receptor-associated protein
- HFD
high fat diet
- I/R
ischemia/reperfusion
- IRS
insulin receptor substrate
- LC3
microtubule-associated protein 1 light chain 3
- LIR
LC3-interacting region
- MFN1/2
mitofusin-1/2
- MLST8
mammalian lethal with SEC13 protein
- MPTP
mitochondrial permeability transition pore
- mTOR
mechanistic target of rapamycin
- NOX
NADPH oxidase
- PE
phosphatidylethanolamine
- PRAS40
proline-rich AKT substrate, 40 kDa
- RAG
ras-related GTP-binding
- RAPTOR
regulatory-associated protein of mTOR
- RHEB
ras homolog enriched in brain
- ROS
reactive oxygen species
- SDI
sorbitol dehydrogenase inhibitor
- SIRT
sirtuin
- SMC
smooth muscle cell
- STZ
streptozotocin
- ULK
Unc-51-like autophagy activating kinase
- VPS
vacuolar protein sorting
References
- 1.Abdillahi M, Ananthakrishnan R, Vedantham S, Shang L, Zhu Z, Rosario R, Zirpoli H, Bohren KM, Gabbay KH, and Ramasamy R. Aldose reductase modulates cardiac glycogen synthase kinase-3β phosphorylation during ischemia-reperfusion. Am J Physiol Heart Circ Physiol 303: H297–H308, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Araya J, Kojima J, Takasaka N, Ito S, Fujii S, Hara H, Yanagisawa H, Kobayashi K, Tsurushige C, Kawaishi M, Kamiya N, Hirano J, Odaka M, Morikawa T, Nishimura SL, Kawabata Y, Hano H, Nakayama K, and Kuwano K. Insufficient autophagy in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol 304: L56–L69, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Aránguiz-Urroz P, Canales J, Copaja M, Troncoso R, Vicencio JM, Carrillo C, Lara H, Lavandero S, and Díaz-Araya G. Beta(2)-adrenergic receptor regulates cardiac fibroblast autophagy and collagen degradation. Biochim Biophys Acta 1812: 23–31, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Asbun J. and Villarreal FJ. The pathogenesis of myocardial fibrosis in the setting of diabetic cardiomyopathy. J Am Coll Cardiol 47: 693–700, 2006 [DOI] [PubMed] [Google Scholar]
- 5.Axe EL, Walker SA, Manifava M, Chandra P, Roderick HL, Habermann A, Griffiths G, and Ktistakis NT. Autophagosome formation from membrane compartments enriched in phosphatidylinositol 3-phosphate and dynamically connected to the endoplasmic reticulum. J Cell Biol 182: 685–701, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baines CP. The cardiac mitochondrion: nexus of stress. Annu Rev Physiol 72: 61–80, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Bar-Peled L, Schweitzer LD, Zoncu R, and Sabatini DM. Ragulator is a GEF for the rag GTPases that signal amino acid levels to mTORC1. Cell 150: 1196–1208, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baseler WA, Dabkowski ER, Jagannathan R, Thapa D, Nichols CE, Shepherd DL, Croston TL, Powell M, Razunguzwa TT, Lewis SE, Schnell DM, and Hollander JM. Reversal of mitochondrial proteomic loss in Type 1 diabetic heart with overexpression of phospholipid hydroperoxide glutathione peroxidase. Am J Physiol Regul Integr Comp Physiol 304: R553–R565, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baseler WA, Dabkowski ER, Williamson CL, Croston TL, Thapa D, Powell MJ, Razunguzwa TT, and Hollander JM. Proteomic alterations of distinct mitochondrial subpopulations in the type 1 diabetic heart: contribution of protein import dysfunction. Am J Physiol Regul Integr Comp Physiol 300: R186–R200, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Battiprolu PK, Hojayev B, Jiang N, Wang ZV, Luo X, Iglewski M, Shelton JM, Gerard RD, Rothermel BA, Gillette TG, Lavandero S, and Hill JA. Metabolic stress-induced activation of FoxO1 triggers diabetic cardiomyopathy in mice. J Clin Invest 122: 1109–1118, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beher D, Wu J, Cumine S, Kim KW, Lu S-C, Atangan L, and Wang M. Resveratrol is not a direct activator of SIRT1 enzyme activity. Chem Biol Drug Des 74: 619–624, 2009 [DOI] [PubMed] [Google Scholar]
- 12.Bellot G, Garcia-Medina R, Gounon P, Chiche J, Roux D, Pouysségur J, and Mazure NM. Hypoxia-induced autophagy is mediated through hypoxia-inducible factor induction of BNIP3 and BNIP3L via their BH3 domains. Mol Cell Biol 29: 2570–2581, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berg AH. and Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res 96: 939–949, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Berkers CR, Maddocks ODK, Cheung EC, Mor I, and Vousden KH. Metabolic regulation by p53 family members. Cell Metab 18: 617–633, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhamra GS, Hausenloy DJ, Davidson SM, Carr RD, Paiva M, Wynne AM, Mocanu MM, and Yellon DM. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res Cardiol 103: 274–284, 2008 [DOI] [PubMed] [Google Scholar]
- 16.Billia F, Hauck L, Konecny F, Rao V, Shen J, and Mak TW. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proc Natl Acad Sci USA 108: 9572–9577, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boudina S, Bugger H, Sena S, O'Neill BT, Zaha VG, Ilkun O, Wright JJ, Mazumder PK, Palfreyman E, Tidwell TJ, Theobald H, Khalimonchuk O, Wayment B, Sheng X, Rodnick KJ, Centini R, Chen D, Litwin SE, Weimer BE, and Abel ED. Contribution of impaired myocardial insulin signaling to mitochondrial dysfunction and oxidative stress in the heart. Circulation 119: 1272–1283, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, and Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell 96: 857–868, 1999 [DOI] [PubMed] [Google Scholar]
- 19.Cai L, Li W, Wang G, Guo L, Jiang Y, and Kang YJ. Hyperglycemia-Induced Apoptosis in Mouse Myocardium: Mitochondrial Cytochrome c-Mediated Caspase-3 Activation Pathway. Diabetes 51: 1938–1948, 2002 [DOI] [PubMed] [Google Scholar]
- 20.Chen G, Han Z, Feng D, Chen Y, Chen L, Wu H, Huang L, Zhou C, Cai X, Fu C, Duan L, Wang X, Liu L, Liu X, Shen Y, Zhu Y, and Chen Q. A regulatory signaling loop comprising the PGAM5 phosphatase and CK2 controls receptor-mediated mitophagy. Mol Cell 54: 362–377, 2014 [DOI] [PubMed] [Google Scholar]
- 21.Chen Y, Azad MB, and Gibson SB. Superoxide is the major reactive oxygen species regulating autophagy. Cell Death Differ 16: 1040–1052, 2009 [DOI] [PubMed] [Google Scholar]
- 22.Chen Y. and Dorn GW. PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science 340: 471–475, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, and Green DR. The BCL-2 family reunion. Mol Cell 37: 299–310, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cho H, Mu J, Kim JK, Thorvaldsen JL, Chu Q, Crenshaw EB, Kaestner KH, Bartolomei MS, Shulman GI, and Birnbaum MJ. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta). Science 292: 1728–1731, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Creager MA, Lüscher TF, Cosentino F, and Beckman JA. Diabetes and vascular disease: pathophysiology, clinical consequences, and medical therapy: Part I. Circulation 108: 1527–1532, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Crighton D, Wilkinson S, O'Prey J, Syed N, Smith P, Harrison PR, Gasco M, Garrone O, Crook T, and Ryan KM. DRAM, a p53-induced modulator of autophagy, is critical for apoptosis. Cell 126: 121–134, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Cui M, Yu H, Wang J, Gao J, and Li J. Chronic caloric restriction and exercise improve metabolic conditions of dietary-induced obese mice in autophagy correlated manner without involving AMPK. J Diabetes Res 2013: 852754, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Das A, Durrant D, Koka S, Salloum FN, Xi L, and Kukreja RC. Mammalian target of rapamycin (mTOR) inhibition with rapamycin improves cardiac function in type 2 diabetic mice: potential role of attenuated oxidative stress and altered contractile protein expression. J Biol Chem 289: 4145–4160, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Degenhardt TP, Alderson NL, Arrington DD, Beattie RJ, Basgen JM, Steffes MW, Thorpe SR, and Baynes JW. Pyridoxamine inhibits early renal disease and dyslipidemia in the streptozotocin-diabetic rat. Kidney Int 61: 939–950, 2002 [DOI] [PubMed] [Google Scholar]
- 30.Ding W-X, Ni H-M, Li M, Liao Y, Chen X, Stolz DB, Dorn GW, and Yin X-M. Nix is critical to two distinct phases of mitophagy, reactive oxygen species-mediated autophagy induction and Parkin-ubiquitin-p62-mediated mitochondrial priming. J Biol Chem 285: 27879–27890, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dorn GW. Mitochondrial pruning by Nix and BNip3: an essential function for cardiac-expressed death factors. J Cardiovasc Trans Res 3: 374–383, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Edwards JL, Quattrini A, Lentz SI, Figueroa-Romero C, Cerri F, Backus C, Hong Y, and Feldman EL. Diabetes regulates mitochondrial biogenesis and fission in mouse neurons. Diabetologia 53: 160–169, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Egan DF, Shackelford DB, Mihaylova MM, Gelino S, Kohnz RA, Mair W, Vasquez DS, Joshi A, Gwinn DM, Taylor R, Asara JM, Fitzpatrick J, Dillin A, Viollet B, Kundu M, Hansen M, and Shaw RJ. Phosphorylation of ULK1 (hATG1) by AMP-activated protein kinase connects energy sensing to mitophagy. Science 331: 456–461, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eisner V, Quiroga C, Criollo A, Eltit JM, Chiong M, Parra V, Hidalgo K, Toro B, Díaz-Araya G, and Lavandero S. Hyperosmotic stress activates p65/RelB NFκB in cultured cardiomyocytes with dichotomic actions on caspase activation and cell death. FEBS Lett 580: 3469–3476, 2006 [DOI] [PubMed] [Google Scholar]
- 35.Elmore SP, Qian T, Grissom SF, and Lemasters JJ. The mitochondrial permeability transition initiates autophagy in rat hepatocytes. FASEB J 15: 2286–2287, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Felig P, Wahren J, Sherwin R, and Palaiologos G. Amino acid and protein metabolism in diabetes mellitus. Arch Intern Med 137: 507–513, 1977 [PubMed] [Google Scholar]
- 37.Fiordaliso F, Leri A, Cesselli D, Limana F, Safai B, Nadal-Ginard B, Anversa P, and Kajstura J. Hyperglycemia activates p53 and p53-regulated genes leading to myocyte cell death. Diabetes 50: 2363–2375, 2001 [DOI] [PubMed] [Google Scholar]
- 38.Folli F, Kahn CR, Hansen H, Bouchie JL, and Feener EP. Angiotensin II inhibits insulin signaling in aortic smooth muscle cells at multiple levels. A potential role for serine phosphorylation in insulin/angiotensin II crosstalk. J Clin Invest 100: 2158–2169, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Frustaci A, Kajstura J, Chimenti C, Jakoniuk I, Leri A, Maseri A, Nadal-Ginard B, and Anversa P. Myocardial cell death in human diabetes. Circ Res 87: 1123–1132, 2000 [DOI] [PubMed] [Google Scholar]
- 40.Galvez AS, Ulloa JA, Chiong M, Criollo A, Eisner V, Barros LF, and Lavandero S. Aldose reductase induced by hyperosmotic stress mediates cardiomyocyte apoptosis: differential effects of sorbitol and mannitol. J Biol Chem 278: 38484–38494, 2003 [DOI] [PubMed] [Google Scholar]
- 41.Garofalo RS, Orena SJ, Rafidi K, Torchia AJ, Stock JL, Hildebrandt AL, Coskran T, Black SC, Brees DJ, Wicks JR, McNeish JD, and Coleman KG. Severe diabetes, age-dependent loss of adipose tissue, and mild growth deficiency in mice lacking Akt2/PKB beta. J Clin Invest 112: 197–208, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AHV, and Taanman JW. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Hum Mol Genet 19: 4861–4870, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Geisler S, Holmström KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, and Springer W. PINK1/Parkin-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nat Cell Biol 12: 119–131, 2010 [DOI] [PubMed] [Google Scholar]
- 44.Ghosh S, An D, Pulinilkunnil T, Qi D, Lau HCS, Abrahani A, Innis SM, and Rodrigues B. Role of dietary fatty acids and acute hyperglycemia in modulating cardiac cell death. Nutrition 20: 916–923, 2004 [DOI] [PubMed] [Google Scholar]
- 45.Giacco F. and Brownlee M. Oxidative stress and diabetic complications. Circ Res 107: 1058–1070, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Goldin A, Beckman JA, Schmidt AM, and Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation 114: 597–605, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Goldman SJ, Zhang Y, and Jin S. Autophagic degradation of mitochondria in white adipose tissue differentiation. Antioxid Redox Signal 14: 1971–1978, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gomes LC, Di Benedetto G, and Scorrano L. During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol 13: 589–598, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Greene AW, Grenier K, Aguileta MA, Muise S, Farazifard R, Haque ME, McBride HM, Park DS, and Fon EA. Mitochondrial processing peptidase regulates PINK1 processing, import and Parkin recruitment. EMBO Rep 13: 378–385, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greer EL, Oskoui PR, Banko MR, Maniar JM, Gygi MP, Gygi SP, and Brunet A. The energy sensor AMP-activated protein kinase directly regulates the mammalian FOXO3 transcription factor. J Biol Chem 282: 30107–30119, 2007 [DOI] [PubMed] [Google Scholar]
- 51.Guo R, Zhang Y, Turdi S, and Ren J. Adiponectin knockout accentuates high fat diet-induced obesity and cardiac dysfunction: role of autophagy. Biochim Biophys Acta 1832: 1136–1148, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gustafsson AB. and Gottlieb RA. Bcl-2 family members and apoptosis, taken to heart. Am J Physiol Cell Physiol 292: C45–C51, 2007 [DOI] [PubMed] [Google Scholar]
- 53.Hailey DW, Rambold AS, Satpute-Krishnan P, Mitra K, Sougrat R, Kim PK, and Lippincott-Schwartz J. Mitochondria supply membranes for autophagosome biogenesis during starvation. Cell 141: 656–667, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hall AR, Burke N, Dongworth RK, and Hausenloy DJ. Mitochondrial fusion and fission proteins: novel therapeutic targets for combating cardiovascular disease. Br J Pharmacol 171: 1890–1906, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hamacher-Brady A, Brady NR, and Gottlieb RA. Enhancing macroautophagy protects against ischemia/reperfusion injury in cardiac myocytes. J Biol Chem 281: 29776–29787, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Hamacher-Brady A, Brady NR, Logue SE, Sayen MR, Jinno M, Kirshenbaum LA, Gottlieb RA, and Gustafsson AB. Response to myocardial ischemia/reperfusion injury involves Bnip3 and autophagy. Cell Death Differ 14: 146–157, 2007 [DOI] [PubMed] [Google Scholar]
- 57.Hanna RA, Quinsay MN, Orogo AM, Giang K, Rikka S, and Gustafsson AB. Microtubule-associated protein 1 light chain 3 (LC3) interacts with Bnip3 protein to selectively remove endoplasmic reticulum and mitochondria via autophagy. J Biol Chem 287: 19094–19104, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hariharan N, Maejima Y, Nakae J, Paik J, DePinho RA, and Sadoshima J. Deacetylation of FoxO by Sirt1 plays an essential role in mediating starvation-induced autophagy in cardiac myocytes. Circ Res 107: 1470–1482, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.He C, Bassik MC, Moresi V, Sun K, Wei Y, Zou Z, An Z, Loh J, Fisher J, Sun Q, Korsmeyer S, Packer M, May HI, Hill JA, Virgin HW, Gilpin C, Xiao G, Bassel-Duby R, Scherer PE, and Levine B. Exercise-induced BCL2-regulated autophagy is required for muscle glucose homeostasis. Nature 481: 511–515, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.He C, Zhu H, Li H, Zou M-H, and Xie Z. Dissociation of Bcl-2-Beclin1 complex by activated AMPK enhances cardiac autophagy and protects against cardiomyocyte apoptosis in diabetes. Diabetes 62: 1270–1281, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hernández JS, Barreto-Torres G, Kuznetsov AV, Khuchua Z, and Javadov S. Crosstalk between AMPK activation and angiotensin II-induced hypertrophy in cardiomyocytes: the role of mitochondria. J Cell Mol Med 18: 709–720, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hill BG, Haberzettl P, Ahmed Y, Srivastava S, and Bhatnagar A. Unsaturated lipid peroxidation-derived aldehydes activate autophagy in vascular smooth-muscle cells. Biochem J 410: 525–534, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Hoshino A, Ariyoshi M, Okawa Y, Kaimoto S, Uchihashi M, Fukai K, Iwai-Kanai E, Ikeda K, Ueyama T, Ogata T, and Matoba S. Inhibition of p53 preserves Parkin-mediated mitophagy and pancreatic β-cell function in diabetes. Proc Natl Acad Sci USA 111: 3116–3121, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hoshino A, Mita Y, Okawa Y, Ariyoshi M, Iwai-Kanai E, Ueyama T, Ikeda K, Ogata T, and Matoba S. Cytosolic p53 inhibits Parkin-mediated mitophagy and promotes mitochondrial dysfunction in the mouse heart. Nat Comms 4: 2308, 2013 [DOI] [PubMed] [Google Scholar]
- 65.Hosokawa N, Hara T, Kaizuka T, Kishi C, Takamura A, Miura Y, Iemura S-I, Natsume T, Takehana K, Yamada N, Guan J-L, Oshiro N, and Mizushima N. Nutrient-dependent mTORC1 association with the ULK1-Atg13-FIP200 complex required for autophagy. Mol Biol Cell 20: 1981–1991, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hou X, Hu Z, Xu H, Xu J, Zhang S, Zhong Y, He X, and Wang N. Advanced glycation endproducts trigger autophagy in cadiomyocyte via RAGE/PI3K/AKT/mTOR pathway. Cardiovasc Diabetol 13: 78, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, Cohen RA, and Zang M. SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem 283: 20015–20026, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Huang C, Andres AM, Ratliff EP, Hernandez G, Lee P, and Gottlieb RA. Preconditioning involves selective mitophagy mediated by Parkin and p62/SQSTM1. PLoS One 6: e20975, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ikeda Y, Shirakabe A, Maejima Y, Zhai P, Sciarretta S, Toli J, Nomura M, Mihara K, Egashira K, Ohishi M, Abdellatif M, and Sadoshima J. Endogenous Drp1 mediates mitochondrial autophagy and protects the heart against energy stress. Circ Res 116: 264–278, 2015 [DOI] [PubMed] [Google Scholar]
- 70.Inoki K, Li Y, Zhu T, Wu J, and Guan K-L. TSC2 is phosphorylated and inhibited by Akt and suppresses mTOR signalling. Nat Cell Biol 4: 648–657, 2002 [DOI] [PubMed] [Google Scholar]
- 71.Inoki K, Zhu T, and Guan K-L. TSC2 mediates cellular energy response to control cell growth and survival. Cell 115: 577–590, 2003 [DOI] [PubMed] [Google Scholar]
- 72.Ishida Y, Yamamoto A, Kitamura A, Lamandé SR, Yoshimori T, Bateman JF, Kubota H, and Nagata K. Autophagic elimination of misfolded procollagen aggregates in the endoplasmic reticulum as a means of cell protection. Mol Biol Cell 20: 2744–2754, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jin SM, Lazarou M, Wang C, Kane LA, Narendra DP, and Youle RJ. Mitochondrial membrane potential regulates PINK1 import and proteolytic destabilization by PARL. J Cell Biol 191: 933–942, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jung CH, Jun CB, Ro S-H, Kim Y-M, Otto NM, Cao J, Kundu M, and Kim D-H. ULK-Atg13-FIP200 complexes mediate mTOR signaling to the autophagy machinery. Mol Biol Cell 20: 1992–2003, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kabeya Y, Mizushima N, Yamamoto A, Oshitani-Okamoto S, Ohsumi Y, and Yoshimori T. LC3, GABARAP and GATE16 localize to autophagosomal membrane depending on form-II formation. J Cell Sci 117: 2805–2812, 2004 [DOI] [PubMed] [Google Scholar]
- 76.Kageyama Y, Hoshijima M, Seo K, Bedja D, Sysa-Shah P, Andrabi SA, Chen W, Höke A, Dawson VL, Dawson TM, Gabrielson K, Kass DA, Iijima M, and Sesaki H. Parkin-independent mitophagy requires Drp1 and maintains the integrity of mammalian heart and brain. EMBO J 33: 2798–2813, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kang R, Zeh HJ, Lotze MT, and Tang D. The Beclin 1 network regulates autophagy and apoptosis. Cell Death Differ 18: 571–580, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kannel WB. and McGee DL. Diabetes and cardiovascular disease. The Framingham study. JAMA 241: 2035–2038, 1979 [DOI] [PubMed] [Google Scholar]
- 79.Kannel WB. Risk stratification in hypertension: new insights from the Framingham Study. Am J Hypertens 13: 3S–10S, 2000 [DOI] [PubMed] [Google Scholar]
- 80.Kim J, Kim YC, Fang C, Russell RC, Kim JH, Fan W, Liu R, Zhong Q, and Guan K-L. Differential regulation of distinct Vps34 complexes by AMPK in nutrient stress and autophagy. Cell 152: 290–303, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kim J, Kundu M, Viollet B, and Guan K-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat Cell Biol 13: 132–141, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kim SI, Na H-J, Ding Y, Wang Z, Lee SJ, and Choi ME. Autophagy promotes intracellular degradation of type I collagen induced by transforming growth factor (TGF)-β1. J Biol Chem 287: 11677–11688, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kitada M, Takeda A, Nagai T, Ito H, Kanasaki K, and Koya D. Dietary restriction ameliorates diabetic nephropathy through anti-inflammatory effects and regulation of the autophagy via restoration of Sirt1 in diabetic Wistar fatty (fa/fa) rats: a model of type 2 diabetes. Exp Diabetes Res 2011: 908185, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kobayashi S. and Liang Q. Autophagy and mitophagy in diabetic cardiomyopathy. Biochim Biophys Acta 1852: 252–261, 2015 [DOI] [PubMed] [Google Scholar]
- 85.Kobayashi S, Xu X, Chen K, and Liang Q. Suppression of autophagy is protective in high glucose-induced cardiomyocyte injury. Autophagy 8: 577–592, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Korge P, Ping P, and Weiss JN. Reactive oxygen species production in energized cardiac mitochondria during hypoxia/reoxygenation: modulation by nitric oxide. Circ Res 103: 873–880, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kubli DA. and Gustafsson AB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res 111: 1208–1221, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Kubli DA. and Gustafsson AB. Cardiomyocyte health: adapting to metabolic changes through autophagy. Trends Endocrinol Metab 25: 156–164, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kubli DA, Quinsay MN, Huang C, Lee Y, and Gustafsson AB. Bnip3 functions as a mitochondrial sensor of oxidative stress during myocardial ischemia and reperfusion. Am J Physiol Heart Circ Physiol 295: H2025–H2031, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kubli DA, Zhang X, Lee Y, Hanna RA, Quinsay MN, Nguyen CK, Jimenez R, Petrosyan S, Murphy AN, and Gustafsson AB. Parkin protein deficiency exacerbates cardiac injury and reduces survival following myocardial infarction. J Biol Chem 288: 915–926, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Kume S, Uzu T, Horiike K, Chin-Kanasaki M, Isshiki K, Araki S-I, Sugimoto T, Haneda M, Kashiwagi A, and Koya D. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest 120: 1043–1055, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kunjathoor VV, Wilson DL, and LeBoeuf RC. Increased atherosclerosis in streptozotocin-induced diabetic mice. J Clin Invest 97: 1767–1773, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kuroda J, Ago T, Matsushima S, Zhai P, Schneider MD, and Sadoshima J. NADPH oxidase 4 (Nox4) is a major source of oxidative stress in the failing heart. Proc Natl Acad Sci USA 107: 15565–15570, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lancel S, Montaigne D, Marechal X, Marciniak C, Hassoun SM, Decoster B, Ballot C, Blazejewski C, Corseaux D, Lescure B, Motterlini R, and Neviere R. Carbon monoxide improves cardiac function and mitochondrial population quality in a mouse model of metabolic syndrome. PLoS One 7: e41836, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lemasters JJ. Selective mitochondrial autophagy, or mitophagy, as a targeted defense against oxidative stress, mitochondrial dysfunction, and aging. Rejuvenation Res 8: 3–5, 2005 [DOI] [PubMed] [Google Scholar]
- 96.Li C-J, Lv L, Li H, and Yu D-M. Cardiac fibrosis and dysfunction in experimental diabetic cardiomyopathy are ameliorated by alpha-lipoic acid. Cardiovasc Diabetol 11: 73, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Liesa M. and Shirihai OS. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab 17: 491–506, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liu L, Feng D, Chen G, Chen M, Zheng Q, Song P, Ma Q, Zhu C, Wang R, Qi W, Huang L, Xue P, Li B, Wang X, Jin H, Wang J, Yang F, Liu P, Zhu Y, Sui S, and Chen Q. Mitochondrial outer-membrane protein FUNDC1 mediates hypoxia-induced mitophagy in mammalian cells. Nat Cell Biol 14: 177–185, 2012 [DOI] [PubMed] [Google Scholar]
- 99.Luan R, Liu S, Yin T, Lau WB, Wang Q, Guo W, Wang H, and Tao L. High glucose sensitizes adult cardiomyocytes to ischaemia/reperfusion injury through nitrative thioredoxin inactivation. Cardiovasc Res 83: 294–302, 2009 [DOI] [PubMed] [Google Scholar]
- 100.Maalouf RM, Eid AA, Gorin YC, Block K, Escobar GP, Bailey S, and Abboud HE. Nox4-derived reactive oxygen species mediate cardiomyocyte injury in early type 1 diabetes. Am J Physiol Cell Physiol 302: C597–C604, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Maiuri MC, Galluzzi L, Morselli E, Kepp O, Malik SA, and Kroemer G. Autophagy regulation by p53. Curr Opin Cell Biol 22: 181–185, 2010 [DOI] [PubMed] [Google Scholar]
- 102.Maiuri MC, Malik SA, Morselli E, Kepp O, Criollo A, Mouchel P-L, Carnuccio R, and Kroemer G. Stimulation of autophagy by the p53 target gene Sestrin2. Cell Cycle 8: 1571–1576, 2009 [DOI] [PubMed] [Google Scholar]
- 103.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, Burden SJ, Di Lisi R, Sandri C, Zhao J, Goldberg AL, Schiaffino S, and Sandri M. FoxO3 controls autophagy in skeletal muscle in vivo. Cell Metab 6: 458–471, 2007 [DOI] [PubMed] [Google Scholar]
- 104.Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou Y-S, Saiki S, Kawajiri S, Sato F, Kimura M, Komatsu M, Hattori N, and Tanaka K. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol 189: 211–221, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, and Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 100: 914–922, 2007 [DOI] [PubMed] [Google Scholar]
- 106.Mei Y, Zhang Y, Yamamoto K, Xie W, Mak TW, and You H. FOXO3a-dependent regulation of Pink1 (Park6) mediates survival signaling in response to cytokine deprivation. Proc Natl Acad Sci USA 106: 5153–5158, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Meijer AJ. and Codogno P. AMP-activated protein kinase and autophagy. Autophagy 3: 238–240, 2007 [DOI] [PubMed] [Google Scholar]
- 108.Meley D, Bauvy C, Houben-Weerts JHPM, Dubbelhuis PF, Helmond MTJ, Codogno P, and Meijer AJ. AMP-activated protein kinase and the regulation of autophagic proteolysis. J Biol Chem 281: 34870–34879, 2006 [DOI] [PubMed] [Google Scholar]
- 109.Mellor KM, Bell JR, Young MJ, Ritchie RH, and Delbridge LMD. Myocardial autophagy activation and suppressed survival signaling is associated with insulin resistance in fructose-fed mice. J Mol Cell Cardiol 50: 1035–1043, 2011 [DOI] [PubMed] [Google Scholar]
- 110.Mizushima N, Noda T, and Ohsumi Y. Apg16p is required for the function of the Apg12p-Apg5p conjugate in the yeast autophagy pathway. EMBO J 18: 3888–3896, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Montaigne D, Marechal X, Coisne A, Debry N, Modine T, Fayad G, Potelle C, Arid El J-M, Mouton S, Sebti Y, Duez H, Preau S, Remy-Jouet I, Zerimech F, Koussa M, Richard V, Neviere R, Edme J-L, Lefebvre P, and Staels B. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation 130: 554–564, 2014 [DOI] [PubMed] [Google Scholar]
- 112.Morselli E, Maiuri MC, Markaki M, Megalou E, Pasparaki A, Palikaras K, Criollo A, Galluzzi L, Malik SA, Vitale I, Michaud M, Madeo F, Tavernarakis N, and Kroemer G. Caloric restriction and resveratrol promote longevity through the Sirtuin-1-dependent induction of autophagy. Cell Death Dis 1: e10, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nagashima T, Shigematsu N, Maruki R, Urano Y, Tanaka H, Shimaya A, Shimokawa T, and Shibasaki M. Discovery of novel forkhead box O1 inhibitors for treating type 2 diabetes: improvement of fasting glycemia in diabetic db/db mice. Mol Pharmacol 78: 961–970, 2010 [DOI] [PubMed] [Google Scholar]
- 114.Nagata D, Takeda R, Sata M, Satonaka H, Suzuki E, Nagano T, and Hirata Y. AMP-activated protein kinase inhibits angiotensin II-stimulated vascular smooth muscle cell proliferation. Circulation 110: 444–451, 2004 [DOI] [PubMed] [Google Scholar]
- 115.Nakamura H, Matoba S, Iwai-Kanai E, Kimata M, Hoshino A, Nakaoka M, Katamura M, Okawa Y, Ariyoshi M, Mita Y, Ikeda K, Okigaki M, Adachi S, Tanaka H, Takamatsu T, and Matsubara H. p53 promotes cardiac dysfunction in diabetic mellitus caused by excessive mitochondrial respiration-mediated reactive oxygen species generation and lipid accumulation. Circ Heart Fail 5: 106–115, 2012 [DOI] [PubMed] [Google Scholar]
- 116.Narendra DP, Jin SM, Tanaka A, Suen D-F, Gautier CA, Shen J, Cookson MR, and Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol 8: e1000298, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y, and Shimizu S. Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 461: 654–658, 2009 [DOI] [PubMed] [Google Scholar]
- 118.Oliveira PJ, Seiça R, Coxito PM, Rolo AP, Palmeira CM, Santos MS, and Moreno AJM. Enhanced permeability transition explains the reduced calcium uptake in cardiac mitochondria from streptozotocin-induced diabetic rats. FEBS Lett 554: 511–514, 2003 [DOI] [PubMed] [Google Scholar]
- 119.Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, and Pabst B. SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem 285: 8340–8351, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Øvervatn A, Bjørkøy G, and Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282: 24131–24145, 2007 [DOI] [PubMed] [Google Scholar]
- 121.Poole AC, Thomas RE, Yu S, Vincow ES, and Pallanck L. The mitochondrial fusion-promoting factor mitofusin is a substrate of the PINK1/parkin pathway. PLoS One 5: e10054, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Porrello ER, D'Amore A, Curl CL, Allen AM, Harrap SB, Thomas WG, and Delbridge LMD. Angiotensin II type 2 receptor antagonizes angiotensin II type 1 receptor-mediated cardiomyocyte autophagy. Hypertension 53: 1032–1040, 2009 [DOI] [PubMed] [Google Scholar]
- 123.Quinsay MN, Thomas RL, Lee Y, and Gustafsson AB. Bnip3-mediated mitochondrial autophagy is independent of the mitochondrial permeability transition pore. Autophagy 6: 855–862, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ramasamy R, Oates PJ, and Schaefer S. Aldose reductase inhibition protects diabetic and nondiabetic rat hearts from ischemic injury. Diabetes 46: 292–300, 1997 [DOI] [PubMed] [Google Scholar]
- 125.Ravikumar B, Moreau K, Jahreiss L, Puri C, and Rubinsztein DC. Plasma membrane contributes to the formation of pre-autophagosomal structures. Nat Cell Biol 12: 747–757, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Riehle C, Wende AR, Sena S, Pires KM, Pereira RO, Zhu Y, Bugger H, Frank D, Bevins J, Chen D, Perry CN, Dong XC, Valdez S, Rech M, Sheng X, Weimer BC, Gottlieb RA, White MF, and Abel ED. Insulin receptor substrate signaling suppresses neonatal autophagy in the heart. J Clin Invest 123: 5319–5333, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rikka S, Quinsay MN, Thomas RL, Kubli DA, Zhang X, Murphy AN, and Gustafsson AB. Bnip3 impairs mitochondrial bioenergetics and stimulates mitochondrial turnover. Cell Death Differ 18: 721–731, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ritov VB, Menshikova EV, Azuma K, Wood R, Toledo FGS, Goodpaster BH, Ruderman NB, and Kelley DE. Deficiency of electron transport chain in human skeletal muscle mitochondria in type 2 diabetes mellitus and obesity. Am J Physiol Endocrinol Metab 298: E49–E58, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Russell RR, Bergeron R, Shulman GI, and Young LH. Translocation of myocardial GLUT-4 and increased glucose uptake through activation of AMPK by AICAR. Am J Physiol 277: H643–H649, 1999 [DOI] [PubMed] [Google Scholar]
- 130.Russo SB, Baicu CF, Van Laer A, Geng T, Kasiganesan H, Zile MR, and Cowart LA. Ceramide synthase 5 mediates lipid-induced autophagy and hypertrophy in cardiomyocytes. J Clin Invest 122: 3919–3930, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, and Sabatini DM. Ragulator-rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell 141: 290–303, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, and Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320: 1496–1501, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Santi SA. and Lee H. Ablation of Akt2 induces autophagy through cell cycle arrest, the downregulation of p70S6K, and the deregulation of mitochondria in MDA-MB231 cells. PLoS One 6: e14614, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Saotome M, Katoh H, Yaguchi Y, Tanaka T, Urushida T, Satoh H, and Hayashi H. Transient opening of mitochondrial permeability transition pore by reactive oxygen species protects myocardium from ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol 296: H1125–H1132, 2009 [DOI] [PubMed] [Google Scholar]
- 135.Sato A. and Funder JW. High glucose stimulates aldosterone-induced hypertrophy via type I mineralocorticoid receptors in neonatal rat cardiomyocytes. Endocrinology 137: 4145–4153, 1996 [DOI] [PubMed] [Google Scholar]
- 136.Scheele C, Nielsen AR, Walden TB, Sewell DA, Fischer CP, Brogan RJ, Petrovic N, Larsson O, Tesch PA, Wennmalm K, Hutchinson DS, Cannon B, Wahlestedt C, Pedersen BK, and Timmons JA. Altered regulation of the PINK1 locus: a link between type 2 diabetes and neurodegeneration? FASEB J 21: 3653–3665, 2007 [DOI] [PubMed] [Google Scholar]
- 137.Schips TG, Wietelmann A, Höhn K, Schimanski S, Walther P, Braun T, Wirth T, and Maier HJ. FoxO3 induces reversible cardiac atrophy and autophagy in a transgenic mouse model. Cardiovasc Res 91: 587–597, 2011 [DOI] [PubMed] [Google Scholar]
- 138.Schweers RL, Zhang J, Randall MS, Loyd MR, Li W, Dorsey FC, Kundu M, Opferman JT, Cleveland JL, Miller JL, and Ney PA. NIX is required for programmed mitochondrial clearance during reticulocyte maturation. Proc Natl Acad Sci USA 104: 19500–19505, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sciarretta S, Zhai P, Shao D, Maejima Y, Robbins J, Volpe M, Condorelli G, and Sadoshima J. Rheb is a critical regulator of autophagy during myocardial ischemia: pathophysiological implications in obesity and metabolic syndrome. Circulation 125: 1134–1146, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sciarretta S, Zhai P, Shao D, Zablocki D, Nagarajan N, Terada LS, Volpe M, and Sadoshima J. Activation of NADPH oxidase 4 in the endoplasmic reticulum promotes cardiomyocyte autophagy and survival during energy stress through the protein kinase RNA-activated-like endoplasmic reticulum kinase/eukaryotic initiation factor 2α/activating transcription factor 4 pathway. Circ Res 113: 1253–1264, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sengupta A, Molkentin JD, and Yutzey KE. FoxO transcription factors promote autophagy in cardiomyocytes. J Biol Chem 284: 28319–28331, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Shamhart PE, Luther DJ, Adapala RK, Bryant JE, Petersen KA, Meszaros JG, and Thodeti CK. Hyperglycemia enhances function and differentiation of adult rat cardiac fibroblasts. Can J Physiol Pharmacol 92: 598–604, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Shaw JE, Sicree RA, and Zimmet PZ. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87: 4–14, 2010 [DOI] [PubMed] [Google Scholar]
- 144.Shen X, Zheng S, Thongboonkerd V, Xu M, Pierce WM, Klein JB, and Epstein PN. Cardiac mitochondrial damage and biogenesis in a chronic model of type 1 diabetes. Am J Physiol Endocrinol Metab 287: E896–E905, 2004 [DOI] [PubMed] [Google Scholar]
- 145.Singh R, Xiang Y, Wang Y, Baikati K, Cuervo AM, Luu YK, Tang Y, Pessin JE, Schwartz GJ, and Czaja MJ. Autophagy regulates adipose mass and differentiation in mice. J Clin Invest 119: 3329–3339, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sloan RC, Moukdar F, Frasier CR, Patel HD, Bostian PA, Lust RM, and Brown DA. Mitochondrial permeability transition in the diabetic heart: contributions of thiol redox state and mitochondrial calcium to augmented reperfusion injury. J Mol Cell Cardiol 52: 1009–1018, 2012 [DOI] [PubMed] [Google Scholar]
- 147.Tang WH, Kravtsov GM, Sauert M, Tong XY, Hou XY, Wong TM, Chung SK, and Man Chung SS. Polyol pathway impairs the function of SERCA and RyR in ischemic-reperfused rat hearts by increasing oxidative modifications of these proteins. J Mol Cell Cardiol 49: 58–69, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Twig G, Elorza A, Molina AJA, Mohamed H, Wikstrom JD, Walzer G, Stiles L, Haigh SE, Katz S, Las G, Alroy J, Wu M, Py BF, Yuan J, Deeney JT, Corkey BE, and Shirihai OS. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J 27: 433–446, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Underwood BR, Imarisio S, Fleming A, Rose C, Krishna G, Heard P, Quick M, Korolchuk VI, Renna M, Sarkar S, García-Arencíbia M, O'Kane CJ, Murphy MP, and Rubinsztein DC. Antioxidants can inhibit basal autophagy and enhance neurodegeneration in models of polyglutamine disease. Hum Mol Genet 19: 3413–3429, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.van Belle TL, Coppieters KT, and Herrath von MG. Type 1 diabetes: etiology, immunology, and therapeutic strategies. Physiol Rev 91: 79–118, 2011 [DOI] [PubMed] [Google Scholar]
- 151.Vernon PJ. and Tang D. Eat-me: autophagy, phagocytosis, and reactive oxygen species signaling. Antioxid Redox Signal 18: 677–691, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Völkers M, Doroudgar S, Nguyen N, Konstandin MH, Quijada P, Din S, Ornelas L, Thuerauf DJ, Gude N, Friedrich K, Herzig S, Glembotski CC, and Sussman MA. PRAS40 prevents development of diabetic cardiomyopathy and improves hepatic insulin sensitivity in obesity. EMBO Mol Med 6: 57–65, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Wei Y, Sowers JR, Clark SE, Li W, Ferrario CM, and Stump CS. Angiotensin II-induced skeletal muscle insulin resistance mediated by NF-kappaB activation via NADPH oxidase. Am J Physiol Endocrinol Metab 294: E345–E351, 2008 [DOI] [PubMed] [Google Scholar]
- 154.Wei Y, Sowers JR, Nistala R, Gong H, Uptergrove GM-E, Clark SE, Morris EM, Szary N, Manrique C, and Stump CS. Angiotensin II-induced NADPH oxidase activation impairs insulin signaling in skeletal muscle cells. J Biol Chem 281: 35137–35146, 2006 [DOI] [PubMed] [Google Scholar]
- 155.Xie Y, You S-J, Zhang Y-L, Han Q, Cao Y-J, Xu X-S, Yang Y-P, Li J, and Liu C-F. Protective role of autophagy in AGE-induced early injury of human vascular endothelial cells. Mol Med Rep 4: 459–464, 2011 [DOI] [PubMed] [Google Scholar]
- 156.Xie Z, Lau K, Eby B, Lozano P, He C, Pennington B, Li H, Rathi S, Dong Y, Tian R, Kem D, and Zou M-H. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes 60: 1770–1778, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xu K, Yang Y, Yan M, Zhan J, Fu X, and Zheng X. Autophagy plays a protective role in free cholesterol overload-induced death of smooth muscle cells. J Lipid Res 51: 2581–2590, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xu X, Hua Y, Nair S, Sreejayan N, Zhang Y, and Ren J. Akt2 knockout preserves cardiac function in high-fat diet-induced obesity by rescuing cardiac autophagosome maturation. J Mol Cell Biol 5: 61–63, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Xu X, Kobayashi S, Chen K, Timm D, Volden P, Huang Y, Gulick J, Yue Z, Robbins J, Epstein PN, and Liang Q. Diminished autophagy limits cardiac injury in mouse models of type 1 diabetes. J Biol Chem 288: 18077–18092, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Yeshao W, Gu J, Peng X, Nairn AC, and Nadler JL. Elevated glucose activates protein synthesis in cultured cardiac myocytes. Metab Clin Exp 54: 1453–1460, 2005 [DOI] [PubMed] [Google Scholar]
- 161.Zhang H, Bosch-Marce M, Shimoda LA, Tan YS, Baek JH, Wesley JB, Gonzalez FJ, and Semenza GL. Mitochondrial autophagy is an HIF-1-dependent adaptive metabolic response to hypoxia. J Biol Chem 283: 10892–10903, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 162.Zhang Y, Goldman S, Baerga R, Zhao Y, Komatsu M, and Jin S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proc Natl Acad Sci USA 106: 19860–19865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Zhao Y, Zhang L, Qiao Y, Zhou X, Wu G, Wang L, Peng Y, Dong X, Huang H, Si L, Zhang X, Zhang L, Li J, Wang W, Zhou L, and Gao X. Heme oxygenase-1 prevents cardiac dysfunction in streptozotocin-diabetic mice by reducing inflammation, oxidative stress, apoptosis and enhancing autophagy. PLoS One 8: e75927, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zoncu R, Bar-Peled L, Efeyan A, Wang S, Sancak Y, and Sabatini DM. mTORC1 senses lysosomal amino acids through an inside-out mechanism that requires the vacuolar H(+)-ATPase. Science 334: 678–683, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zorov DB, Juhaszova M, and Sollott SJ. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol Rev 94: 909–950, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]