Abstract
Host immune response to viral vectors, persistence of nonintegrating vectors, and sustained transgene expression are among the major challenges in gene therapy. To overcome these hurdles, we successfully used minicircle (MC) naked-DNA vectors devoid of any viral or bacterial sequences for the long-term treatment of murine phenylketonuria, a model for a genetic liver defect. MC-DNA vectors expressed the murine phenylalanine hydroxylase (Pah) complementary DNA (cDNA) from a liver-specific promoter coupled to a de novo designed hepatocyte-specific regulatory element, designated P3, which is a cluster of evolutionary conserved transcription factor binding sites. MC-DNA vectors were subsequently delivered to the liver by a single hydrodynamic tail vein (HTV) injection. The MC-DNA vector normalized blood phenylalanine concomitant with reversion of hypopigmentation in a dose-dependent manner for more than 1 year, whereas the corresponding parental plasmid did not result in any phenylalanine clearance. MC vectors persisted in an episomal state in the liver consistent with sustained transgene expression and hepatic PAH enzyme activity without any apparent adverse effects. Moreover, 14–20% of all hepatocytes expressed transgenic PAH, and the expression was observed exclusively in the liver and predominately around pericentral areas of the hepatic lobule, while there was no transgene expression in periportal areas.
Conclusion
This study demonstrates that MC technology offers an improved safety profile and has the potential for the genetic treatment of liver diseases.
Phenylketonuria (PKU, OMIM 261600) is an autosomal recessive metabolic disorder which is caused by a deficiency of the hepatic phenylalanine hydroxylase (PAH, EC 1.14.16.1) enzyme responsible for converting phenylalanine (Phe) to tyrosine in the presence of molecular oxygen and the cofactor tetrahydrobiopterin.1 The PKU mouse Pahenu2/2 is a validated genetic model2,3 to study hepatic gene transfer approaches in a living system, as it offers a direct readout for therapeutic efficacy, i.e., lowering of systemic high blood levels of Phe. We and other investigators have previously shown long-term correction of hyperphenylalaninemia and hypopigmentation of the PKU mouse model with viral gene therapy approaches.3–9
Viruses are attractive tools for gene delivery because they are well adapted to deliver their genetic cargo into cells with high efficiency. Yet, besides gender-dependent effectiveness of liver transduction with adeno-associated viral vectors,10 questions regarding treatment toxicity, immune host response to (repeated) administration, expression stability, and a final risk for insertional mutagenesis following viral vector administration remain largely unresolved issues.11–13 Moreover, the safety requirement for targeting newborn and pediatric patients for potential life-long treatment remains an even higher challenge for viral vector-dependent approaches. In addition, limited cargo capacity of viral vectors and high production costs are hurdles that may preclude their widespread use. In contrast, nonviral vectors have the potential to overcome at least some of these challenges encountered in viral vector-mediated therapy,14–16 which may ultimately overcome safety and manufacturing hurdles, while enabling transfer of also larger transgenes. The barrier limiting the implementation of nonviral vectors for gene therapy hitherto has been the low-level and short duration of gene expression. Minicircle (MC)-DNA vectors are a new form of supercoiled DNA for use in nonviral gene transfer that contain a minimal expression cassette and from which all bacterial DNA, which has been shown to contribute to biological safety problems and transgene silencing,16,17 is removed by intramolecular recombination.18–21 MC-DNA vectors have been demonstrated to sustain and enhance the level of transgene expression 10- to 1,000-fold compared to conventional plasmids in both quiescent tissues in vivo and in vitro.14,21
Here we show efficacy of MC vector-mediated liver-directed gene therapy in an inherited liver enzyme deficiency model, the PAH-deficient phenylketonuric Pahenu2 mouse, and observed long-lasting correction of hyperphenylalaninemia following a single hydrodynamic injection of an MC vector expressing the murine Pah-cDNA from a liver-specific promoter. We found sustained robust therapeutic treatment of the hepatic enzyme deficiency for more than 1 year in Pahenu2 mice, confirming the therapeutic potential of this technology also for other liver defects.
Materials and Methods
Gene Delivery and Animal Experiments
All animal experiments were approved by the State Veterinary Office of Zurich and were carried out according to the guidelines of the Swiss Law of Animal Protection, the Swiss Federal Act on Animal Protection (1978), and the Swiss Animal Protection Ordinance (1981). Young adult (9–15 weeks old; 16–22 g) female and male C57BL/6-Pahenu2(“PKU”) mice were selected for non-viral gene delivery. Mice were maintained on standard chow, and only prior to tail vein bleeding for Phe determination mice were fasted for 4–6 hours. Blood was collected from tail veins on Guthrie filter cards, and Phe concentration was determined using tandem mass spectrometry as described3. Prental plasmid (PP)-DNA or minicircle (MC)-DNA vectors at the indicated doses were injected into mice using HTV injections.22 In brief, mice were rapidly (5–8 seconds) injected under isoflurane anesthesia by way of tail vein with 10% of the body weight of NaCl (0.9%, B. Braun Medical, Sempach, Switzerland) containing the indicated amount of DNA vector using a 27G needle. Following the HTV injection, Rimadyl (Pfizer, New York) was administrated subcutaneously (5 mg/kg body weight). For the F1 generation, a PKU male treated with MC vector was crossbred with two C57BL/6 females. Genomic DNA from tail biopsies from 20 offspring of three different litters was used for examining germ line transmission of the MC vector.
PAH Enzyme Assay
PAH enzyme activity in whole liver extracts was measured according to a highly sensitive and quantitative assay using isotope-dilution liquid chromatography-electrospray ionization tandem mass spectrometry (LC-ESI-MS/MS) developed in our laboratory.23 Frozen tissues were lysed at 4°C in homogenization buffer24 using a Qiagen TissueLyser (Qiagen, Hombrechtikon, Switzerland) according to our publised method.23
More materials and methods are described in the Supporting Materials and Methods.
Results
Minicircle-Mediated Long-Term Correction of Blood Phenylalanine Concentration in a Dose-Dependent Manner in PKU Mice
Equimolar amounts of MC.PKU20-DNA (MC; 77 μg) vectors and the parental plasmid pMC.PKU20 (PP; 219 μg) vectors (for MC-DNA production, see Supporting Materials and Methods and Supporting Fig. 1), both containing the same transgene cassette expressing murine PAH from a liver-specific promoter designated P3 (minimal transthyretin promoter, TTRmin, coupled to a de novo designed hepatocyte-specific cis-regulatory module 8, HS-CRM8) (see Supporting Materials and Methods for more details),25 were delivered to PKU mouse liver following a single HTV injection.22 We observed a blood Phe decrease from 2,179 ± 293 μmol/L prior to injection to below defined therapeutic levels (≤360 μmol/L) in the MC-treated mice during the entire period of the experiment (Fig. 1) accompanied by complete reversion of hypopigmentation in MC-treated PKU mice (Fig. 2), similar to what we (and others) have observed in previous studies with viral vector approaches.3,5 Although we sacrificed all mice after a period of maximally 6 months for analysis, as shown in Fig. 1 and Table 1, one mouse was kept after MC treatment and lasted with normal blood Phe level for more than 12 months. In contrast, blood Phe from the mice injected with PP vector remained at pretreatment values, indicating a rapid loss of either vector DNA or gene expression or both. Titration studies were also performed to examine the optimal dose of MC vector for gene delivery. Mice receiving a 10-fold lower dose, i.e., 7.7 μg MC.PKU20 vectors, showed less consistent correction of hyperphenylalaninemia, as the Phe values remained near or above therapeutic threshold (Fig. 1). Administration of the lowest dose with 0.77 μg MC.PKU20 vectors had no or only a moderate effect on plasma Phe reduction.
Fig. 1.
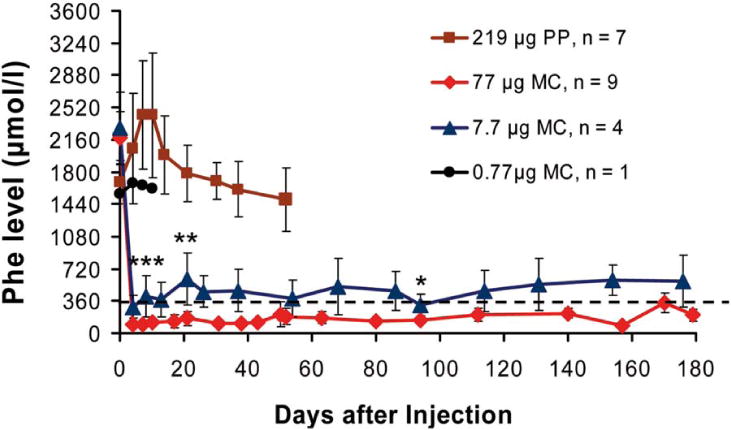
Long-term correction of hyperphenylalaninemia in PKU (Pahenu2) mice after delivery of MC-DNA vectors expressing the Pah-cDNA from the liver-specific P3 promoter. Vectors were delivered to the liver of adult PKU mice by a single HTV injection. Seven mice were injected with the parental plasmid vector pMC.PKU20 (■; 219 μg or 53.7 pmol; 3 × 1013 particles), and 9 mice with an equimolar amount of MC vector MC.PKU20 (◆; 77 μg or 53.7 pmol; 3 × 1013 particles). The minicircle vector MC.PKU20 was also used for titration studies by injecting 4 mice at a dosage of 7.7 μg (▲; 5.37 pmol or 3 × 1012 particles) and 1 mouse with 0.77 μg (●; 0.54 pmol or 3 × 1011 particles). Blood was collected on Guthrie filter cards from tail vein bleedings prior to DNA vector infusion (week 0) and periodically after vector delivery, and depicted as the mean ± SD for each treatment group. Phe concentrations were determined by tandem mass spectrometry. The broken line represents the defined therapeutic levels of blood Phe (≤360 μmol/L). Serum Phe levels in treated mice injected with 77 μg and 7.7 μg MC-DNA vectors were compared for statistically significant difference by one-tailed Student t test, ***P < 0.005 at day 4, **P < 0.05 at day 21, and *P < 0.1 at day 94 following vector administration.
Fig. 2.
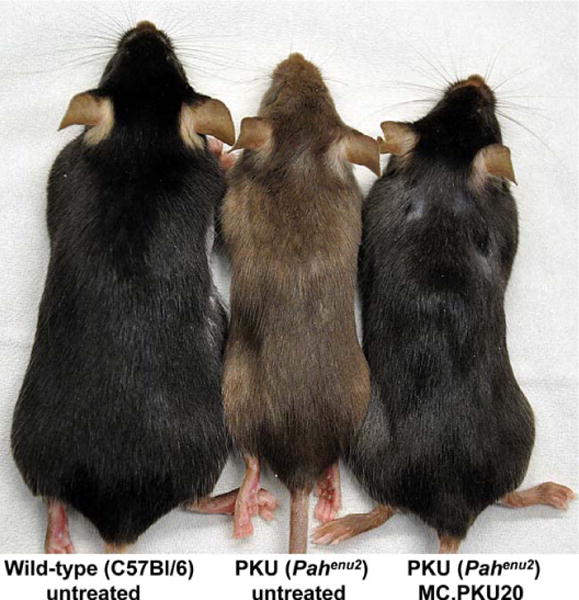
Phenotypic reversion from brown to black coat color of MC-DNA-treated PKU (Pahenu2) mouse. 16 weeks following MC.PKU20-vector injection with the high dose (77 μg; see Fig. 1), the PKU (Pahenu2) mouse shown on the right displayed complete re-pigmentation (from brown to black) in comparison with an age- and gender-matched untreated PKU mouse (middle); an untreated wild-type C57BL/6 mouse is displayed on the left; all mice depicted are females.
Table 1.
Liver Analysis of Treated PKU Mice Sacrificed at Different Timepoints
| Mouse and Treatment | Timepoint of Sacrification (Number of Mice) |
PAH Enzyme Activity
|
Relative Pah-mRNA Gene Expression (fold in %) | DNA Copy Number per Diploid Genome | |
|---|---|---|---|---|---|
| (in mU/mg) | (in % of wt) | ||||
| Wild-type, untreated | (n = 3) | 55.6 ± 10.6 | 100 | n.d. | n.a. |
| PKU, untreated | (n = 3) | < 0.2 | < 0.3 | 100 | n.a. |
| PKU + MC (77 μg) | day 4 (n = 2) | 37.4 ± 25.8 | 67.1 ± 46.4 | 215 ± 24 | 1345 ± 1153 |
| day 10 (n = 3) | 9.0 ± 2.4 | 16.1 ± 4.4 | 217 ± 64 | 119 ± 62 | |
| day 52 (n = 1) | 8.1 | 14.6 | 199 | 16 | |
| day 179 (n = 2) | 5.2 ± 4.4 | 9.3 ± 7.9 | 131 ± 13 | 13 ± 7 | |
| PKU 1 MC (7.7 μg) | day 10 (n = 1) | 1.9 | 3.4 | 112 | 8 |
| PKU 1 MC (0.77 μg) | day 10 (n = 1) | < 0.2 | < 0.2 | 141 | 0.2 |
| PKU 1 PP (219 μg) | day 4 (n = 2) | < 0.2 | < 0.2 | 93 ± 28 | 285 ± 92 |
| day 10 (n = 3) | 0.6 ± 0.1 | 1.0 ± 0.2 | 151 ± 17 | 142 ± 27 | |
| day 52 (n = 2) | < 0.2 | < 0.2 | 123 ± 19 | 13 ± 7 | |
| PKU 1 MC & Stuffer* (219 μg) | day 14 (n = 2) | 1.4 ± 0.9 | 2.4 ± 1.7 | 123 ± 8 | 27 ± 9 |
PAH enzyme activity, Pah-mRNA gene expression and copy numbers of vector genomes in whole liver extracts of PKU (Pahenu2) mice injected with parental plasmid (PP) pMC.PKU20, with minicircle (MC) MC.PKU20, or with MC plus stuffer DNA (pMC.BESPX, empty vector). For blood Phe level values over time see also Figure 2. The amount of vector injected, time point of sacrification and the numbers of mice analyzed are indicated. Note that 219 μg of PP, or 77 μg of MC, is equivalent to 3 × 1013 DNA particles. n.d., not determined; n.a., not applicable.
Sum of MC.PKU20 (77 μg) plus stuffer DNA (142 μg; pMC.BESPX, empty vector); blood Phe levels dropped from 2150 ± 109 μmol/L (before injection) to 768 ± 161 μmol/L at day 5 postinjection, and to 536 ± 231 μmol/L at day 14 postinjection.
Molecular Analysis of PAH Expression in Liver of Treated Mice
PAH enzyme activity and Pah gene expression were determined in whole-liver extracts from sacrificed PKU mice at different timepoints following MC or PP vector injections. As seen in Fig. 1 and Table 1, PAH activity in liver of high-dose (77 μg) MC.PKU20-vector-treated mice was equivalent to 67% of wild-type activity 4 days after the infusion. This activity decreased rapidly to 16% at day 10 but remained stable from 9% to 15% between day 52 to day 179. The considerable standard deviation (SD) at day 179 is because one mouse exhibited normal Phe levels and 8.3 mU/mg (16%) of PAH activity, while the other mouse had fluctuating and moderately elevated Phe levels and a PAH activity of 2.1 mU/mg (4%). These observations are in agreement with previous experience where we3 and others26 found that PAH activity as low as ~5% of wild-type may be sufficient to maintain blood Phe below therapeutic threshold (≤360 μmol/L).26 In accordance with these observations, we measured in PKU mice treated with a 10-fold lower dose, i.e., 7.7 μg of MC.PKU20, and with blood Phe slightly above the therapeutic threshold (Fig. 1), 3% of wild-type liver PAH activity at day 10. The mouse treated with the lowest dose, 0.77 μg of MC.PKU20, expressed no detectable PAH activity, similar to what was found for all mice treated with PP pMC.PKU20 at all timepoints investigated (<1% of wild-type liver PAH).
The maximal amount of DNA applied to these mice is considerable, although comparable to other HTV studies with naked DNA.27,28 Nevertheless, to rule out any potential liver toxicity of such high doses of DNA, we analyzed two mice 14 days after infusion with a total of 219 μg DNA, the sum of the therapeutic dose of 77 μg of MC.PKU20-DNA plus additional 142 μg of corresponding backbone, which is termed “stuffer DNA.” As also seen in Table 1, analysis of blood Phe levels, hepatic PAH enzyme activity, and vector genome copy number per hepatocyte (see below) were similar to the result observed after injection with 7.7 μg of MC.PKU20. We interpret this result as competition between the stuffer DNA to the therapeutic MC vector DNA for hepatocyte transfection.
In parallel, hepatic Pah-mRNA expression was analyzed by quantitative reverse-transcription real-time polymerase chain reaction (PCR) in all mice (Table 1). Since the PKU mouse expresses endogenous Pah-mRNA and (inactive) mutant PAH protein from both Pahenu2 alleles, we could only compare the relative transgene expression level between treated and untreated PKU mice (the latter was set as 100%). Therapeutic correction of blood Phe in PKU mice was associated with ~2-fold overexpression of the Pah-mRNA, although there is considerable variability in the Pah-mRNA level between groups of treated mice.
Number of (Episomal) DNA Vector in Liver of Treated Mice
To determine if vector loss was responsible for the transient PAH expression in PKU mice infused with PP vectors, we determined the average number of MC or PP vector genomes per diploid genome in all liver cells. As displayed in Table 1, we detected 1,345 ± 1,153 genome copies per hepatocyte at day 4 postinjection of 77 μg of MC.PKU20 vector but 119 ± 62 genome copies at day 10 postinfusion, and 13 to 16 copies between day 52 to day 179. In liver from mice infused with lower doses of MC vectors, i.e., 7.7 μg or 0.77 μg of DNA, we detected only 0.2–8 vector genome copies per diploid hepatocyte. Obviously, a few vector copies per hepatocyte expressed sufficient PAH to maintain blood Phe concentration at therapeutic levels. Animals receiving equimolar copies of PP vectors that are 3 times larger than MC vectors showed 285 ± 92 copies per diploid genome at day 4, 142 ± 27 copies at day 10, and 13 ± 7 at day 52 postinfusion. As shown above, mice treated with PP vectors exhibited neither PAH enzyme activity (<1% of wt) nor reduction of blood Phe levels. These data establish that the vector copy numbers detected for MC or PP vectors cannot explain the differences in PAH transgene expression or blood Phe levels in treated PKU mice, similar to that reported by others.29
Although liver is the organ with the highest reported efficacy of gene transfer mediated by hydrodynamic infusion, we detected between 0.3–0.02 copies per diploid genome in kidney at day 179 postinfusion with a corresponding PAH enzyme activity close to the detection limit (<0.2 mU/mg; <0.2% of wild-type activity in kidney). In all other organs tested, i.e., heart, lung, spleen, muscle, and brain, the copy number was close to zero (<0.01 copies per diploid genome). Our results are in agreement with observations reported by others that found ~ 1,000-fold lower expression in kidney, spleen, lung, and heart compared to liver, with the highest expression in kidney.22
P3 Promoter of the Injected MC or PP Vector Is Not Methylated and Vector DNA Remains Episomal
The presence of innate mechanisms to identify and eliminate foreign DNA from mammalian cells seriously limits the efficacy of gene therapy with bacterially derived DNA which can be swiftly epigenetically “silenced” through methylation of CpG islands. We elected to analyze the CpG island present in the P3 promoter as epigenetic modification of this site would have the most significant effect on transgene expression. Figure 3 illustrates that whether the animal has been treated with MC or PP vector, the complete double digestion with EcoRI and the methylation-sensitive enzyme AscI pattern achieved at different timepoints after administration suggested that the CpG island in the P3 promoter remained unmethylated, which is consistent with findings from others.29 Furthermore, as the restriction enzyme digest with EcoRI cuts the MC- or PP-vector only once, the persistence of single vector-size bands established that vector DNA in liver remained as monocircular episomes. Besides nonintegration in liver, we observed a complete absence of MC vector in the F1 generation of treated mice upon saturation PCR (for details see Supporting Materials and Methods).
Fig. 3.
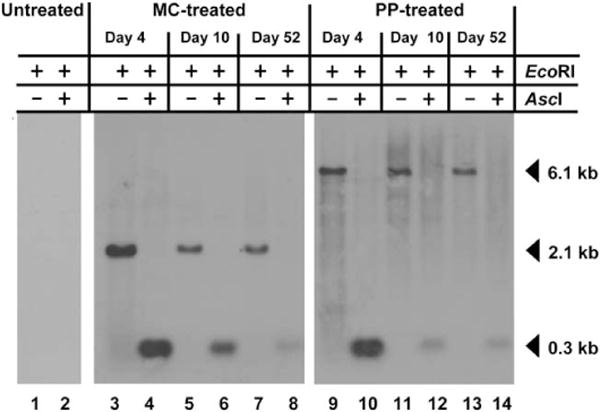
Southern analysis demonstrates that DNA vectors remain episomal and unmethylated on CpG island in the P3 promoter. Total DNA was prepared from the livers of PKU mice at a range of timepoints following hydrodynamic vector infusion. Genomic DNA was digested with EcoRI either alone or with the CpG methylation sensitive enzyme AscI. The blotted DNA was probed with 32P labeled DNA amplified from the P3 promoter, which is common to both constructs. In the single EcoRI digest, the vectors are linearized and the probe detects a vector-sized band (6.1 kb PP pMC.PKU20 or 2.1 kb MC MC.PKU20). If the DNA is not methylated, then the double digest using EcoRI and AscI removes the P3 promoter sequence and the probe detects a smaller 0.3 kb fragment. Lanes 3 to 8 represent MC-treated PKU mouse livers (77 μg), while lanes 9 to 14 represent PP-treated PKU mouse livers (219 μg). Lanes 1 and 2, PKU untreated; lanes 3, 4, 9, and 10, day 4; lanes 5, 6, 11, and 12, day 10; lanes 7, 8, 13, and 14, day 52.
Assessment of Liver Toxicity and Inflammation Upon HTV Injection of MC Vectors
No adverse effects were detected in any treated mice following HTV injection. Nevertheless, hydrodynamic injection of vector DNA can cause acute liver injury due to the rapid injection of a large fluid volume resulting in rapid liver expansion.31 To evaluate these potential effects, we measured two markers of liver injury, aspartate aminotransferase (AST) and alanine aminotransferase (ALT) activities. As shown in Table 2, regardless of whether saline alone or saline plus vector DNA (PP or MC) had been injected, both serum AST and ALT activities had returned to baseline by 14 days posttreatment.
Table 2.
Liver Toxicity and Inflammatory Parameters in Serum of Treated PKU Mice
| Treatment
|
|||||
|---|---|---|---|---|---|
| Serum Parameter | Saline Only | PP | MC | MC & Stuffer DNA | |
| AST (IU/L) | 2 weeks before injection | 105 ± 64 | 98 ± 4 | 68 ± 4 | 68 ± 11 |
| 1 day after injection | 1810 ± 1598 | 2885 ± 1075 | 1258 ± 4 | 1925 ± 304 | |
| 2 weeks after injection | 88 ± 25 | 85 ± 21 | 73 ± 11 | 83 ± 18 | |
| ALT (IU/L) | 2 weeks before injection | 58 ± 18 | 78 ± 32 | 40 ± 7 | 63 ± 4 |
| 1 day after injection | 3130 ± 2178 | 4958 ± 2110 | 2240 ± 976 | 3195 ± 382 | |
| 2 weeks after injection | 65 ± 21 | 48 ± 11 | 18 ± 4 | 50 ± 14 | |
| IL-12 p40 (pg/ml) | 2 weeks before injection | 96 ± 11 | 134 ± 58 | 210 ± 71 | 130 ± 23 |
| 1 day after injection | 68 ± 8 | 92 ± 47 | 157 ± 45 | 103 ± 36 | |
| 2 weeks after injection | 103 ± 31 | 133 ± 40 | 186 ± 36 | 139 ± 26 | |
| TNF-α (pg/ml) | 2 weeks before injection | 2.2 ± 1.1 | 1.5 ± 0.7 | 1.1 ± 0.1 | 1.4 ± 0.5 |
| 1 day after injection | 2.3 ± 0.6 | 3.7 ± 1.5 | 1.6 ± 0.9 | 4.6 ± 0.4 | |
| 2 weeks after injection | 1.6 ± 0.9 | 1.3 ± 0.4 | <1 | 4.4 ± 1.3 | |
| IFN-γ (pg/ml) | 2 weeks before injection | <3 | <3 | <3 | <3 |
| 1 day after injection | <3 | <3 | <3 | <3 | |
| 2 weeks after injection | <3 | <3 | <3 | <3 | |
The concentration of liver specific enzymes including aspartate aminotransferase (AST) and alanine aminotransferase (ALT), as well as the inflammatory biomarkers interleukin-12 (IL-12 p40), tumor necrosis factor-alpha (TNF-α) and interferon-gamma (IFN-γ) were assessed 2 weeks before, and 1 day or 2 weeks after hydrodynamic delivery of saline only, parental plasmid (PP) pMC.PKU20, minicircle (MC) MC.PKU20, or minicircle (MC) MC.PKU20 plus stuffer DNA (pMC.BESPX, empty vector). Except for the MC treatment, equal amount of DNA was delivered to the mice, i.e. 219 μg of PP or 77 μg MC or 77 μg MC plus 142 μg stuffer DNA. All vector preparations used were supposed to contain < 0.1 endotoxin units per μg DNA according to the Qiagen endofree plasmid purification kit (Qiagen AG, Hombrechtikon, Switzerland).
AST, aspartate aminotransferase; ALT, alanine aminotransferase; IL-12, interleukin-12 p40; TNF-α, tumor necrosis factor-alpha; IFN-γ, interferon-gamma; 2 mice were measured in each group; data represent mean ± SD.
Naked DNA injection can induce innate immune responses predominantly triggered by the presence of (unmethylated) CpG dinucleotide motifs in the vector sequence or by contaminating bacterial endotoxin or genomic DNA.17,32,33 We evaluated the immunostimulatory potential of MC vector-treated mice by measuring interleukin-12 (IL-12 p40), tumor necrosis factor-alpha (TNF-α), and interferon-gamma (IFN-γ; see Table 2). As seen in Table 2, no differences in serum cytokine concentrations were detected between the saline controls and any treatment group at 1 or 14 days postinjection. We conclude that inflammatory response following MC or PP injection is negligible, that neither the size nor amount of the vector injected limits efficacy, and that an inflammatory response is not involved in the suppression of transgene expression from PP.
Histochemical Analysis of PAH-Transgene Expression
For a detailed distribution analysis of PAH transgene expression on a cellular level in liver (and kidney), PKU mice were infused with 77 μg MC-vector expressing Flag-tagged PAH (MC.PKU37; for details see Supporting Materials and Methods), which is equivalent to the high dosing with vector MC.PKU20, resulting in normalization of blood Phe levels as observed in Fig. 1. Quantitative histochemical analysis 12 days postinfusion revealed that the Flag-PAH protein was expressed in 16.7% ± 2.9 of the cells compared to control mice treated with saline only (Fig. 4A,B). The majority of the transfected cells were clustered around central veins, while there were almost no positive hepatocytes in periportal areas (Fig. 4C,D). Hematoxylin and eosin staining illustrates that no liver damage or necrosis was observed accompanied with normal AST and ALT values (Fig. 4E,F). Because we detected trace amounts of MC vector genome and PAH activity in kidney from treated mice, we performed immunohistochemical analysis for the presence of Flag-tagged PAH protein in sections of renal tissue where we did not observe any Flag-PAH staining and found preserved integrity of glomeruli and tubuli (Fig. 4G,H).
Fig. 4.
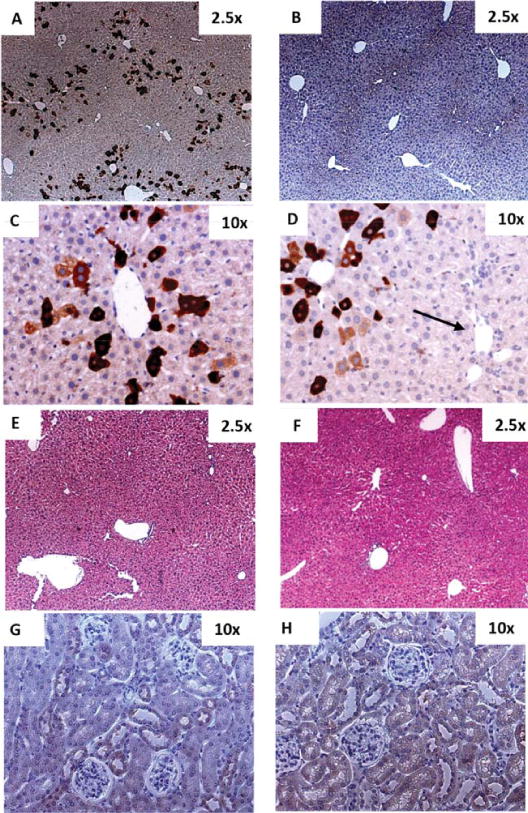
Heterogeneous distribution of PAH expression in mouse liver and kidney upon HTV injection of MCs. 77 μg or 53.7 pmol (3 3 × 1013 particles) of MC-DNA vector MC.PKU37 expressing an N-terminal Flag-tagged PAH was infused into the liver of 6 adult female PKU mice by way of hydrodynamic tail vein (HTV) injection. All treated mice were sacrificed on day 12. One PKU adult female PKU mouse was infused with saline only by way of HTV, and was sacrificed on day 12 as a negative control. Sections (A, B, C, and D) were stained with anti-Flag antibody followed by hematoxylin counterstain. Representative examples of a random field in 2.53× magnification in (A) a PKU mouse liver after delivery of MC.PKU37 demonstrating 16.7% ± 2.9* positively stained liver cells which were distributed heterogeneously, and (B) PKU mouse liver injected with saline only. PAH-expressing cells in a PKU mouse liver injected with MC.PKU37 were predominately expressed in the pericentral area (C,D), whereas hepatocytes around the portal triad (arrow in D) do not show any positive staining for PAH transgene expression. Liver sections were examined histologically by hematoxylin and eosin staining from (E) a PKU mouse liver infused with MC.PKU37, and (F) a negative control mouse liver. Kidney sections stained with anti-Flag antibody followed by hematoxylin counterstain were obtained from mice injected with (G) saline only or (H) MC.PKU37 (original magnification 10-fold). *The mean ± SD was calculated according to the percentage of the positively stained cells from five random fields in 2.5× magnification of each mouse.
Discussion
Nonviral naked-DNA gene transfer vectors have several advantages over viral vectors, and MC vectors in particular, which are free of plasmid bacterial sequences, have been shown to be capable of persistent and prolonged transgene expression in several animal models,14–16 and have the potential for a much better safety profile than for viral vectors. The MC.PKU20 vector presented here contains our simplest version of a transgene cassette, the Pah-cDNA containing a liver-specific promoter25 plus a Kozak sequence at its 5′ end, and a polyA signal at its 3′ end without further modification to the native cDNA sequence. Some studies have proposed that smaller molecules, including MC-DNA vectors, have greater gene transfer efficiency than larger complexes, as they might have better bioavailability characteristics due to more efficient vector uptake by cells34 and higher diffusion coefficient.18 In this context, the MC-DNA consisting of only the expression cassette plus the 36 bp attR hybrid site is about one-third the size of the PP. We injected up to 3 × 1013 copies, equivalent to 77 μg MC-DNA or 219 μg PP, and found a rapid decline of DNA copy numbers for both vectors during the first few days, which has also been observed by others.35 Our findings are in agreement with the published data in that injection of MC-DNA vector yielded greater numbers of vectors per hepatocyte than injection of the larger PP, at least 4 days after injection. The rapid decline of vector during an initial period following hydrodynamic injection may correspond to “wash out” due to systemic application, elimination of transfected but damaged cells during the HTV administration process, and to the degradation of DNA vectors before they reach the nucleus. Interestingly, after this initial clearing phase, transgene expression remained stable over a period of more than 12 months. Furthermore, we have no evidence for integration events of MC vector DNA, which is also in agreement with reports from others showing MCs are retained in a passive episomal state with no indication of integration.36,37
Besides efficient hepatocyte targeting and vector dosing, high-level and hepatocyte-restricted transgene expression from a liver-specific promoter is another critical parameter for therapeutic efficacy. We included in our vector a de novo designed, hepatocyte specific regulatory element combined with a liver-specific promoter,25 and observed PAH expression controlled by the liver promoter exclusively in hepatocytes, although trace amounts of vector were present in kidneys. Optimization of the expression cassette, such as codon optimization and/or inclusion of a 5′ hybrid intron, may lead to further improvements in PAH expression and allow for therapeutic efficacy at lower vector doses; experiments to investigate these possibilities are underway. Moreover, because the size of MC vectors is theoretically not limited, the use of this system permits the evaluation of (larger) natural endogenous promoters to drive transgene expression.
While PP vector genomes are persistent in livers of hydrodynamically injected PKU mice, we found no detectable liver PAH activity even at day 4 posttreatment. Expression of the transgene cassette in the PP must therefore be transcriptionally blocked or very rapidly suppressed while the PP itself is not lost. Other investigators have previously observed this phenomenon of plasmid-derived transcription blocking or silencing in liver (and other organs) compared to permissive expression from MC devoid of any plasmid backbone DNA.28,29,35,38 and references therein Unmethylated lated CpG dinucleotides are present at a higher frequency in bacterial genomes and plasmids than in vertebrate DNA, and unmethylated DNA may yet be recognized as foreign by the cell and subsequently be silenced through methylation.39 Published analyses on the role of CpG DNA methylation in plasmid DNA transcriptional suppression are divergent, as some reports indeed found CpG methylation to be responsible for suppression of expression,28 including direct methylation of the transgene coding sequence,40 while other studies,29 including our study, ruled out a role for CpG DNA methylation-dependent transgene silencing. Work led by the group of Dr. Kay reported that histone modification of extragenic DNA flanking the expression cassette might be responsible for transcriptional silencing of plasmid-mediated transgene expression.41,42 Such mechanisms might be the reason for transgene suppression also for our PP, although we have not addressed experimentally histone modification or any other mechanism at this point. Our study, however, eliminated an anti-DNA vector or transgene inflammatory response as the cause of transcriptional silencing. Moreover, a potential silencing mechanism seems not to be limited to the 5′ promoter regions but could also be due to methylation of the downstream coding sequences, termed intragenic methylation.40
Several studies, including ours, demonstrate that HTV injection generally transduces not more than 20% of hepatocytes in mouse liver with a heterogeneous impact at the cellular level with more vector genomes distributed to the pericentral region than to the periportal region of the hepatic acinus.22,43 Our findings further confirm that the HTV injection method likely delivers MC-DNA by way of the central hepatic veins, through intercellular fenestrations, and finally to the surrounding hepatocytes. While the hydrodynamic injection method of naked DNA vectors is an effective gene delivery method for hepatocytes in mice, achieving physiologically relevant hepatocyte transduction in larger animals or humans remains a major challenge. Nevertheless, modifications to achieve efficient delivery of naked DNA to the liver upon hydrodynamic injections are being explored in larger animal models as well as in humans.44–46 As mentioned before, MC DNA does not integrate into the host liver genome and remains episomal. The low turnover rate of adult hepatocytes allows for long-term sustained MC-mediated transgene expression, but very gradual loss of MC vector genomes from liver is to be expected. Liver (but also skeletal muscle tissue5) is a favorable targeting organ not only to treat PKU, but also other genetic liver defects, because of a low turnover rate of hepatocytes which allows the nonintegrating vector to be maintained as a stable episome. Moreover, even though an episomal nonviral vector may be gradually lost during the natural turnover of cells with time, various investigators have examined the possibility of repeated vector administration using the hydrodynamic gene delivery method.22,47 Predictions of the immune response in humans following viral vector administration based on the innate and antigen-dependent immune responses seen in animal models have not been accurate.12 This is likely because the diversity of the immune system between mice and humans is generally underestimated, which makes extrapolation from experimental results in mice to expected outcomes in humans very difficult. Nevertheless, we propose, based on the results presented here, including long-term vector genome persistence and sustained transgene expression without any apparent adverse effects in a murine PKU model, that MC technology offers an improved safety profile over viral vectors and has the potential for successful gene therapy of heritable liver disorders.
Supplementary Material
Acknowledgments
We thank Merima Mustedanagic for pMC.PKU37, the mass spectrometry units of the Divisions of Clinical Chemistry and Biochemistry and Newborn Screening for Phe measurements, the animal facilities for advice, and Felix H. Sennhauser for continuous support.
Supported by grants from the Children’s Research Center Zurich (to H.M.V.), the Swiss National Science Foundation (no. 310030-122045 to B.T.), the National Institute of Health (research grant no. 1R01HD057033 to C.O.H. and B.T.), and the Stiftung für wissenschaftliche Forschung der Universitüt Zürich (to B.T.).
Abbreviations
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- HS-CRM8
hepatocyte-specific cis-regulatory module 8
- HTV
hydrodynamic tail vein injection
- LC-ESI-MS/MS
liquid chromatography-electrospray ionization tandem mass spectrometry
- MC
minicircle-DNA vector
- Pah
phenylalanine hydroxylase
- Phe
phenylalanine
- PKU
phenylketonuria
- PP
parental plasmid
- TTRmin
minimal transthyretin promoter
Footnotes
Additional Supporting Information may be found in the online version of this article at the publisher’s website.
Potential conflict of interest: Nothing to report
View this article online at wileyonlinelibrary.com.
References
- 1.Blau N, van Spronsen FJ, Levy HL. Phenylketonuria. Lancet. 2010;376:1417–1427. doi: 10.1016/S0140-6736(10)60961-0. [DOI] [PubMed] [Google Scholar]
- 2.Shedlovsky A, McDonald JD, Symula D, Dove WF. Mouse models of human phenylketonuria. Genetics. 1993;134:1205–1210. doi: 10.1093/genetics/134.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding Z, Georgiev P, Thöny B. Administration-route and gender-independent long-term therapeutic correction of phenylketonuria (PKU) in a mouse model by recombinant adeno-associated virus 8 pseudotyped vector-mediated gene transfer. Gene Ther. 2006;13:587–593. doi: 10.1038/sj.gt.3302684. [DOI] [PubMed] [Google Scholar]
- 4.Yagi H, Ogura T, Mizukami H, Urabe M, Hamada H, Yoshikawa H, et al. Complete restoration of phenylalanine oxidation in phenylketonuria mouse by a self-complementary adeno-associated virus vector. J Gene Med. 2011;13:114–122. doi: 10.1002/jgm.1543. [DOI] [PubMed] [Google Scholar]
- 5.Ding Z, Harding CO, Rebuffat A, Elzaouk L, Wolff JA, Thöny B. Correction of murine PKU following AAV-mediated intramuscular expression of a complete phenylalanine hydroxylating system. Mol Ther. 2008;16:673–681. doi: 10.1038/mt.2008.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harding CO, Gillingham MB, Hamman K, Clark H, Goebel-Daghighi E, Bird A, et al. Complete correction of hyperphenylalaninemia following liver-directed, recombinant AAV2/8 vector-mediated gene therapy in murine phenylketonuria. Gene Ther. 2006;13:457–462. doi: 10.1038/sj.gt.3302678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oh HJ, Park ES, Kang S, Jo I, Jung SC. Long-term enzymatic and phenotypic correction in the phenylketonuria mouse model by adeno-associated virus vector-mediated gene transfer. Pediatr Res. 2004;56:278–284. doi: 10.1203/01.PDR.0000132837.29067.0E. [DOI] [PubMed] [Google Scholar]
- 8.Mochizuki S, Mizukami H, Ogura T, Kure S, Ichinohe A, Kojima K, et al. Long-term correction of hyperphenylalaninemia by AAV-mediated gene transfer leads to behavioral recovery in phenylketonuria mice. Gene Ther. 2004;11:1081–1086. doi: 10.1038/sj.gt.3302262. [DOI] [PubMed] [Google Scholar]
- 9.Rebuffat A, Harding CO, Ding Z, Thony B. Comparison of adeno-associated virus pseudotype 1, 2, and 8 vectors administered by intramuscular injection in the treatment of murine phenylketonuria. Hum Gene Ther. 2010;21:463–477. doi: 10.1089/hum.2009.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Voutetakis A, Zheng C, Wang J, Goldsmith CM, Afione S, Chiorini JA, et al. Gender differences in serotype 2 adeno-associated virus biodistribution after administration to rodent salivary glands. Hum Gene Ther. 2007;18:1109–1118. doi: 10.1089/hum.2007.072. [DOI] [PubMed] [Google Scholar]
- 11.Asokan A, Schaffer DV, Samulski RJ. The AAV vector toolkit: poised at the clinical crossroads. Mol Ther. 2012;20:699–708. doi: 10.1038/mt.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mingozzi F, High KA. Therapeutic in vivo gene transfer for genetic disease using AAV: progress and challenges. Nat Rev Genet. 2011;12:341–355. doi: 10.1038/nrg2988. [DOI] [PubMed] [Google Scholar]
- 13.Donsante A, Miller DG, Li Y, Vogler C, Brunt EM, Russell DW, et al. AAV vector integration sites in mouse hepatocellular carcinoma. Science. 2007;317:477. doi: 10.1126/science.1142658. [DOI] [PubMed] [Google Scholar]
- 14.Kay MA. State-of-the-art gene-based therapies: the road ahead. Nat Rev Genet. 2011;12:316–328. doi: 10.1038/nrg2971. [DOI] [PubMed] [Google Scholar]
- 15.Ehrhardt A, Haase R, Schepers A, Deutsch MJ, Lipps HJ, Baiker A. Episomal vectors for gene therapy. Curr Gene Ther. 2008;8:147–161. doi: 10.2174/156652308784746440. [DOI] [PubMed] [Google Scholar]
- 16.Gill DR, Pringle IA, Hyde SC. Progress and prospects: the design and production of plasmid vectors. Gene Ther. 2009;16:165–171. doi: 10.1038/gt.2008.183. [DOI] [PubMed] [Google Scholar]
- 17.Hyde SC, Pringle IA, Abdullah S, Lawton AE, Davies LA, Varathalingam A, et al. CpG-free plasmids confer reduced inflammation and sustained pulmonary gene expression. Nat Biotechnol. 2008;26:549–551. doi: 10.1038/nbt1399. [DOI] [PubMed] [Google Scholar]
- 18.Darquet AM, Cameron B, Wils P, Scherman D, Crouzet J. A new DNA vehicle for nonviral gene delivery: supercoiled minicircle. Gene Ther. 1997;4:1341–1349. doi: 10.1038/sj.gt.3300540. [DOI] [PubMed] [Google Scholar]
- 19.Bigger BW, Tolmachov O, Collombet JM, Fragkos M, Palaszewski I, Coutelle C. An araC-controlled bacterial cre expression system to produce DNA minicircle vectors for nuclear and mitochondrial gene therapy. J Biol Chem. 2001;276:23018–23027. doi: 10.1074/jbc.M010873200. [DOI] [PubMed] [Google Scholar]
- 20.Mayrhofer P, Blaesen M, Schleef M, Jechlinger W. Minicircle-DNA production by site specific recombination and protein-DNA interaction chromatography. J Gene Med. 2008;10:1253–1269. doi: 10.1002/jgm.1243. [DOI] [PubMed] [Google Scholar]
- 21.Kay MA, He CY, Chen ZY. A robust system for production of minicircle DNA vectors. Nat Biotechnol. 2010;28:1287–1289. doi: 10.1038/nbt.1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liu F, Song Y, Liu D. Hydrodynamics-based transfection in animals by systemic administration of plasmid DNA. Gene Ther. 1999;6:1258–1266. doi: 10.1038/sj.gt.3300947. [DOI] [PubMed] [Google Scholar]
- 23.Heintz C, Troxler H, Martinez A, Thony B, Blau N. Quantification of phenylalanine hydroxylase activity by isotope-dilution liquid chromatography-electrospray ionization tandem mass spectrometry. Mol Genet Metab. 2012;105:559–565. doi: 10.1016/j.ymgme.2011.12.025. [DOI] [PubMed] [Google Scholar]
- 24.Elzaouk L, Leimbacher W, Turri M, Ledermann B, Bürki K, Blau N, et al. Dwarfism and low insulin-like growth factor-1 due to dopamine depletion in Pts−/− mice rescued by feeding neurotransmitter precursors and H4-biopterin. J Biol Chem. 2003;278:28303–28311. doi: 10.1074/jbc.M303986200. [DOI] [PubMed] [Google Scholar]
- 25.Nair N, Rincon MY, Evens H, Sarcar S, Dastidar S, Samara-Kuko E, et al. Computationally designed liver-specific transcriptional CIS-regulatory modules and hyper-functional factor IX improve liver-targeted gene therapy for hemophilia B. Blood. 2014 doi: 10.1182/blood-2013-10-534032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hamman K, Clark H, Montini E, Al-Dhalimy M, Grompe M, Finegold M, et al. Low therapeutic threshold for hepatocyte replacement in murine phenylketonuria. Mol Ther. 2005;12:337–344. doi: 10.1016/j.ymthe.2005.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Crespo A, Peydro A, Dasi F, Benet M, Calvete JJ, Revert F, et al. Hydrodynamic liver gene transfer mechanism involves transient sinusoidal blood stasis and massive hepatocyte endocytic vesicles. Gene Ther. 2005;12:927–935. doi: 10.1038/sj.gt.3302469. [DOI] [PubMed] [Google Scholar]
- 28.Schuttrumpf J, Milanov P, Abriss D, Roth S, Tonn T, Seifried E. Transgene loss and changes in the promoter methylation status as determinants for expression duration in nonviral gene transfer for factor IX. Hum Gene Ther. 2011;22:101–106. doi: 10.1089/hum.2009.212. [DOI] [PubMed] [Google Scholar]
- 29.Chen ZY, Riu E, He CY, Xu H, Kay MA. Silencing of episomal transgene expression in liver by plasmid bacterial backbone DNA is independent of CpG methylation. Mol Ther. 2008;16:548–556. doi: 10.1038/sj.mt.6300399. [DOI] [PubMed] [Google Scholar]
- 30.Jenke AC, Scinteie MF, Stehle IM, Lipps HJ. Expression of a transgene encoded on a non-viral episomal vector is not subject to epigenetic silencing by cytosine methylation. Mol Biol Rep. 2004;31:85–90. doi: 10.1023/b:mole.0000031363.35839.46. [DOI] [PubMed] [Google Scholar]
- 31.Suda T, Gao X, Stolz DB, Liu D. Structural impact of hydrodynamic injection on mouse liver. Gene Ther. 2007;14:129–137. doi: 10.1038/sj.gt.3302865. [DOI] [PubMed] [Google Scholar]
- 32.Sakurai H, Kawabata K, Sakurai F, Nakagawa S, Mizuguchi H. Innate immune response induced by gene delivery vectors. Int J Pharm. 2008;354:9–15. doi: 10.1016/j.ijpharm.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 33.Wooddell CI, Subbotin VM, Sebestyen MG, Griffin JB, Zhang G, Schleef M, et al. Muscle damage after delivery of naked plasmid DNA into skeletal muscles is batch dependent. Hum Gene Ther. 2011;22:225–235. doi: 10.1089/hum.2010.113. [DOI] [PubMed] [Google Scholar]
- 34.Chabot S, Orio J, Schmeer M, Schleef M, Golzio M, Teissie J. Minicircle DNA electrotransfer for efficient tissue-targeted gene delivery. Gene Ther. 2013;20:62–68. doi: 10.1038/gt.2011.215. [DOI] [PubMed] [Google Scholar]
- 35.Jacobs F, Snoeys J, Feng Y, Van Craeyveld E, Lievens J, Armentano D, et al. Direct comparison of hepatocyte-specific expression cassettes following adenoviral and nonviral hydrodynamic gene transfer. Gene Ther. 2008;15:594–603. doi: 10.1038/sj.gt.3303096. [DOI] [PubMed] [Google Scholar]
- 36.Argyros O, Wong SP, Fedonidis C, Tolmachov O, Waddington SN, Howe SJ, et al. Development of S/MAR minicircles for enhanced and persistent transgene expression in the mouse liver. J Mol Med (Berl) 2011;89:515–529. doi: 10.1007/s00109-010-0713-3. [DOI] [PubMed] [Google Scholar]
- 37.Chen ZY, He CY, Ehrhardt A, Kay MA. Minicircle DNA vectors devoid of bacterial DNA result in persistent and high-level transgene expression in vivo. Mol Ther. 2003;8:495–500. doi: 10.1016/s1525-0016(03)00168-0. [DOI] [PubMed] [Google Scholar]
- 38.Osborn MJ, McElmurry RT, Lees CJ, DeFeo AP, Chen ZY, Kay MA, et al. Minicircle DNA-based gene therapy coupled with immune modulation permits long-term expression of alpha-L-iduronidase in mice with mucopolysaccharidosis type I. Mol Ther. 2011;19:450–460. doi: 10.1038/mt.2010.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doerfler W. On the biological significance of DNA methylation. Biochemistry (Mosc) 2005;70:505–524. doi: 10.1007/s10541-005-0145-9. [DOI] [PubMed] [Google Scholar]
- 40.Maunakea AK, Nagarajan RP, Bilenky M, Ballinger TJ, D’Souza C, Fouse SD, et al. Conserved role of intragenic DNA methylation in regulating alternative promoters. Nature. 2010;466:253–257. doi: 10.1038/nature09165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu J, Zhang F, Xu S, Fire AZ, Kay MA. The extragenic spacer length between the 5′ and 3′ ends of the transgene expression cassette affects transgene silencing from plasmid-based vectors. Mol Ther. 2012;20:2111–2119. doi: 10.1038/mt.2012.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gracey Maniar LE, Maniar JM, Chen ZY, Lu J, Fire AZ, Kay MA. Minicircle DNA vectors achieve sustained expression reflected by active chromatin and transcriptional level. Mol Ther. 2013;21:131–138. doi: 10.1038/mt.2012.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang G, Budker V, Wolff JA. High levels of foreign gene expression in hepatocytes after tail vein injections of naked plasmid DNA. Hum Gene Ther. 1999;10:1735–1737. doi: 10.1089/10430349950017734. [DOI] [PubMed] [Google Scholar]
- 44.Kamimura K, Suda T, Xu W, Zhang G, Liu D. Image-guided, lobe-specific hydrodynamic gene delivery to swine liver. Mol Ther. 2009;17:491–499. doi: 10.1038/mt.2008.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Khorsandi SE, Bachellier P, Weber JC, Greget M, Jaeck D, Zacharoulis D, et al. Minimally invasive and selective hydrodynamic gene therapy of liver segments in the pig and human. Cancer Gene Ther. 2008;15:225–230. doi: 10.1038/sj.cgt.7701119. [DOI] [PubMed] [Google Scholar]
- 46.Izsvak Z, Hackett PB, Cooper LJ, Ivics Z. Translating Sleeping Beauty transposition into cellular therapies: victories and challenges. Bioessays. 2010;32:756–767. doi: 10.1002/bies.201000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Argyros O, Wong SP, Niceta M, Waddington SN, Howe SJ, Coutelle C, et al. Persistent episomal transgene expression in liver following delivery of a scaffold/matrix attachment region containing non-viral vector. Gene Ther. 2008;15:1593–1605. doi: 10.1038/gt.2008.113. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


