Abstract
Chronic kidney disease (CKD) in patients is strongly associated with cardiovascular morbidity and mortality, and prevalent abnormal lipid metabolism. The AIM-HIGH trial examined the benefits of adding extended-release niacin (ERN) to simvastatin in patients with established coronary heart disease. Here we conducted a post-hoc analysis of the AIM-HIGH trial examining whether participants derived cardiovascular or renal benefits when stratified by renal function. Of 3414 participants, 505 had stage 3 CKD at baseline. Among the CKD subset, demographics and cardiovascular disease (CVD) risk factors were well balanced in the ERN and placebo arms. Compared to placebo, CKD participants receiving ERN had a significant decrease in triglycerides by a median of 59.0 mg/dL, and high density lipoprotein-cholesterol significantly increased by a mean of 11.3 mg/dL over a mean follow-up of 3 years. CVD events were similar between CKD participants in both arms. However, all-cause mortality was significantly higher in the ERN group (hazard ratio of 1.73). Mean change in eGFR among ERN-treated CKD participants was not significantly different between study arms. Thus, among AIM-HIGH participants with CKD, the addition of ERN to simvastatin for secondary prevention of CVD improved triglyceride and high density lipoprotein-cholesterol concentrations but did not improve cardiovascular outcomes or kidney function, and was associated with higher all-cause mortality.
Keywords: chronic kidney disease, cardiovascular disease, lipids
Background
Coronary heart disease (CHD) is a leading cause of morbidity and mortality in chronic kidney disease (CKD). Individuals with CKD are at greater risk for major adverse cardiac events (MACE) than the general population (1). Despite this, traditional risk factor reduction strategies that reduce MACE in the general population such as low-density lipoprotein cholesterol (LDL-C) lowering confer less benefit in CKD patients (2). In the Study of Heart and Renal Protection (SHARP), although treatment with simvastatin plus ezetimibe in subjects with CKD led to a 17% reduction in a composite cardiovascular outcome compared to placebo, it did not reduce all-cause or CHD mortality (3).
Low levels of high-density lipoprotein cholesterol (HDL-C) and high triglyceride (TG) levels are both associated with CKD (4). Niacin raises HDL-C and lowers TG levels. However, it is not known whether raising HDL-C and lowering TG translates into improved cardiac outcomes in CKD patients.
Prior studies suggest that lipid lowering therapy may also prevent progression of CKD (5). While animal studies suggest that niacin may protect against GFR loss, (6) to our knowledge, the effect of ERN on longitudinal change in kidney function in humans has not been studied previously. The Atherothrombosis Intervention in Metabolic Syndrome with Low HDL/High Triglycerides: Impact on Global Health Outcomes (AIM-HIGH) tested the hypothesis that ERN, when added to intensive statin therapy reduced MACE in stable CHD patients pre-selected for low baseline HDL-C and elevated triglycerides (TG) as compared with placebo. Ezetimibe could be added to either arm, as needed, to achieve and maintain an on-treatment LDL-C between 40–80 mg/dL. The primary outcome study findings from AIM-HIGH did not show incremental clinical benefit of ERN versus placebo on MACE when added to optimal LDL-C reduction therapy despite a significant improvement in the lipid profile (7).
The present investigation was a post hoc analysis of participants with CKD enrolled in the AIM-HIGH trial. Because the CKD population represents a subset with high cardiac risk, we hypothesized that raising HDL-C and lowering TG with ERN would improve cardiovascular outcomes in these subjects. Secondary objectives were to evaluate the effect of ERN on longitudinal change in kidney function as well as safety and tolerability in participants with CKD.
Results
Baseline characteristics: Of the 3,413 participants with non-missing eGFR in the AIM-HIGH trial, 85.2% were male, 3.4% African Americans, and 4.1% were of Hispanic ethnicity. Five hundred and five study participants (14.8%) had CKD at baseline. Among these, the mean eGFR was 50.3 ± 7.7 ml/min per 1.73m2. 496 participants (98.2%) had an eGFR within the range of 30–59 mL/min per 1.73m2, and 9 (1.8%) had an eGFR < 30 mL/min per 1.73m2.
Approximately 80% of the CKD participants were male, 4 % African Americans, and 3.4% were Hispanics. Compared to participants without CKD, those with CKD were older and more likely to be female, had higher prevalence of diabetes and hypertension, but were less likely to use tobacco. Systolic blood pressures and pulse pressures were slightly higher in CKD participants (Table 1).
Table 1.
Baseline Demographics and Clinical Characteristics Among AIM-HIGH Participants Stratified by CKD Status
| CKD (N=505) |
No CKD (N=2908) |
P-value | ||
|---|---|---|---|---|
| Age (years) | Mean (SD) | 70.7 (7.3) | 62.5 (8.4) | <.001 |
| Gender (male) | N (%) | 408 (80.8) | 2501 (86.0) | 0.002 |
| Race – African American | N (%) | 20 (4.0) | 97 (3.3) | 0.476 |
| Ethnicity-Hispanic | N (%) | 19 (3.8) | 121 (4.2) | 0.676 |
| Current Smoker | N (%) | 58 (11.6) | 564 (19.5) | <.001 |
| Coronary Artery Disease | N (%) | 452 (89.5) | 2694 (92.6) | 0.015 |
| Diabetes | N (%) | 207 (41.0) | 951 (32.7) | <.001 |
| Hypertension | N (%) | 401 (79.4) | 2037 (70.0) | <.001 |
| Diastolic BP (mmHg) | Mean (SD) | 71.4 (10.2) | 74.9 (9.6) | <.001 |
| Systolic BP (mmHg) | Mean (SD) | 130.5 (17.4) | 127.9 (16.1) | <.001 |
| Pulse Pressure (mmHg) | Mean (SD) | 59.1 (16.4) | 53.0 (13.4) | <.001 |
| Use of ACE-Is or ARBs | N (%) | 391 (77.4) | 2137 (73.5) | 0.062 |
Among participants with CKD randomized to ERN (n=254) or placebo (n=251), demographic variables, medical conditions, and CAD risk factors were balanced between treatment arms. Lipid profiles in CKD subjects randomized to placebo or ERN were also similar at baseline (Table 2).
Table 2.
Baseline Demographic Features and Clinical Characteristics by Randomization in AIM-HIGH Participants with CKD
| Placebo (N=251) |
Niacin (N=254) |
P-value | ||
|---|---|---|---|---|
| Age (years) | Mean (SD) | 70.8 (7.4) | 70.6 (7.2) | 0.764 |
| Gender (male) | N (%) | 200 (79.7) | 208 (81.9) | 0.529 |
| Race – African American | N (%) | 10 (4.0) | 10 (3.9) | 0.978 |
| Ethnicity-Hispanic | N (%) | 6 (2.4) | 13 (5.1) | 0.107 |
| Current Smoker | N (%) | 33 (13.3) | 25 (10.0) | 0.244 |
| Body Mass Index (kg/m2) | Mean (SD) | 30.4 (5.8) | 30.9 (5.4) | 0.390 |
| Coronary Artery Disease | N (%) | 231 (92.0) | 221 (87.0) | 0.066 |
| Diabetes | N (%) | 102 (40.6) | 105 (41.3) | 0.873 |
| Metabolic Syndrome | N (%) | 206 (83.1) | 214 (84.3) | 0.719 |
| Hypertension | N (%) | 194 (77.3) | 207 (81.5) | 0.243 |
| LDL cholesterol | Mean (SD) | 74.3 (21.2) | 73.8 (21.9) | 0.794 |
| HDL cholesterol | Mean (SD) | 34.5 (6.2) | 34.9 (6.1) | 0.462 |
| Triglycerides | Median (Q1,Q3) | 160 (133, 231) | 175 (132, 222) | 0.839 |
| Diastolic BP (mmHg) | Mean (SD) | 70.9 (10.5) | 71.9 (9.8) | 0.290 |
| Systolic BP (mmHg) | Mean (SD) | 130.1 (16.9) | 130.9 (17.9) | 0.602 |
| Pulse Pressure (mmHg) | Mean (SD) | 59.2 (16.7) | 59.0 (16.2) | 0.920 |
| Use of ACE-Is or ARBs | N (%) | 191 (76.1) | 200 (78.7) | 0.453 |
Effect of ERN on lipids in CKD
Participants from both CKD and non-CKD groups achieved significant increases in HDL-C and decreases in TG concentrations in the ERN arm relative to placebo. In the CKD group, baseline HDL-C concentration was 34.5 mg/dL and 34.9 mg/dL for the placebo and ERN groups respectively. At 3 years, mean HDL-C concentrations were 39.2 (8.2) mg/dL and 45.9 (12.6) mg/dL, respectively (placebo vs ERN, P<0.0001). In the CKD group, median (IQR) baseline TG concentration was 160.0 mg/dL (133.0, 231.0 mg/dL) for the placebo group, and 175.0 mg/dL (132.0, 222.0 mg/dL) for the ERN group. At 3 years, median TG concentrations were 153.0 mg/dL (111.0, 192.0 mg/dL), and 113.0 mg/dL (80.0, 156.0 mg/dL), for placebo vs ERN respectively (P<0.0001). ERN had a greater effect on TG in the CKD group with a median decrease of 59.0 mg/dL, compared to a median decrease of 47.0 mg/dL in participants without CKD, (p=0.031) after 3 years of therapy (Table 3).
Table 3.
Lipid Levels by CKD Status and Treatment: Actual Values and Change from Baseline Values
| Lipid Parameter | Time | CKD* (N=505) |
No CKD** (N = 2908) |
||
|---|---|---|---|---|---|
| Placebo (N = 251) |
Niacin (N = 254) |
Placebo (N = 1444) |
Niacin (N = 1464) |
||
|
Total Cholesterol (mg/dL) |
Baseline | 146.6 (26.0) | 146.1 (26.3) | 144.9 (26.7) | 145.3 (28.5) |
| Mean (SD) | Year 1 | 144.0 (25.1) | 137.0 (25.0) | 143.4 (25.6) | 138.2 (27.3) |
| Change from baseline baseline | −3.1 (27.3) | −8.9 (31.0) | −1.2 (31.1) | −7.0 (32.6) | |
| Year 3 | 140.7 (25.9) | 137.7 (32.1) | 141.6 (23.4) | 136.7 (27.6) | |
| Change from baseline baseline | −8.6 (32.4) | −10.6 (36.8) | −4.7 (28.9) | −10.0 (33.8) | |
|
LDL Cholesterol (mg/dL) |
Baseline | 74.3 (21.2) | 73.8 (21.9) | 73.9 (22.9) | 74.3 (23.7) |
| Mean (SD) | Year 1 | 70.4 (18.1) | 65.4 (20.0) | 70.4 (19.0) | 66.6 (19.9) |
| Change from baseline baseline | −4.8 (21.8) | −8.4 (25.6) | −3.5 (24.8) | −7.9 (26.2) | |
| Year 3 | 67.9 (21.3) | 66.2 (24.1) | 68.4 (19.0) | 65.0 (21.5) | |
| Change from baseline baseline | −8.8 (26.8) | −9.2 (31.2) | −6.6 (24.0) | −10.6 (27.8) | |
|
HDL Cholesterol (mg/dL) |
Baseline | 34.5 (6.2) | 34.9 (6.1) | 35.0 (5.5) | 34.5 (5.6) |
| Mean (SD) | Year 1 | 38.5 (8.4) | 45.5 (12.2) | 38.4 (7.5) | 43.3 (10.6) |
| Change from baseline baseline | 3.9 (5.9) | 10.8 (10.1) | 3.4 (5.5) | 8.8 (8.2) | |
| Year 3 | 39.2 (8.2) | 45.9 (12.6) | 39.1 (7.6) | 43.8 (11.1) | |
| Change from baseline baseline | 4.7 (6.3) | 11.3 (11.3) | 4.2 (5.7) | 9.5 (9.0) | |
|
Lipoprotein (a) (nmol/L) |
Baseline | 33.3 (15.3, 105.7) | 34.8 (15.3, 112.8) | 32.3 (12.8, 122.4) | 36.1 (13.4, 127.6) |
| Median (Q1,Q3) | Year 1 | 32.8 (14.8, 112.14) | 24.6 (8.2, 90.0) | 30.1 (9.7, 124.9) | 27.5 (8.4, 110.7) |
| Change from baseline baseline | −0.7 (−9.4, 5.8) | −5.6 (−19.4, 0.0) | −1.3 (−9.4, 3.8) | −6.0 (−20.2, 0.1) | |
|
Triglyceride (mg/dL) |
Baseline | 160.0 (133.0, 231.0) | 175.0 (132.0, 222.0) | 163.0 (131.0, 215.0) | 166.0 (130.5, 218.0) |
| Median (Q1,Q3) | Year 1 | 153.5 (119.0, 213.5) | 112.0 (79.0, 164.0) | 155.0 (118.0, 207.0) | 122.5 (88.0, 172.0) |
| Change from baseline baseline | −10.5 (−37.5, 28.5) | −55.0 (−93.0, −9.0) | −8.0 (−45.0, 30.0) | −43.0 (−80.0, −5.0) | |
| Year 3 | 153.0 (111.0, 192.0) | 113.0 (80.0, 156.0) | 152.0 (115.0, 206.0) | 121.0 (85.0, 174.0) | |
| Change from baseline baseline | −20.0 (−60.0, 22.0) | −59.0 (−111.0, −16.0) | −14.0 (−53.0, 29.0) | −47.0 (−90.0, −6.0) | |
CKD: Year 1-N=454; Year 3-N=233
No CKD: Year 1-N=2660; Year3-N=1505
Effect of ERN on Cardiovascular Outcomes
There was no clinical benefit of randomization to ERN for the composite CVD primary endpoints within the CKD group. Among CKD participants, 60 subjects (23.6%) in the ERN arm and 60 (23.93%) in the placebo arm reached the primary endpoint (ERN vs. Placebo HR 1.02, 95% CI 0.71, 1.45). All-cause mortality was higher for the ERN group with 39 deaths (15.4%) compared to 23 (8.9%) in the placebo group (ERN vs Placebo HR=1.73, 95% CI 1.03, 2.89, P=0.038). However, there was no significant difference in cardiovascular mortality in the CKD group assigned to ERN with 19 CV deaths (7.5%) compared to 12 CV deaths (4.8%) in the placebo group, (ERN vs placebo HR 1.62, 95% CI 0.78, 3.33). We did not observe interaction between ERN, kidney function, and cardiovascular endpoints,in models with/without eGFR stratification (data not shown). Most of the non-cardiovascular deaths were due to cancer (Table 4).
Table 4.
Cardiovascular Endpoint Events and Hazard Ratios in ERN vs. Placebo Treated Stratified by Baseline CKD Status
| CKD | No CKD | |||||
|---|---|---|---|---|---|---|
| Clinical Event | Placebo (N = 251) |
Niacin (N = 254) |
Niacin vs. Placebo HR2 (95% CI) |
Placebo (N = 1444) |
Niacin (N = 1464) |
Niacin vs. Placebo HR (95% CI) |
| Exposure (pt-yr) | 748 | 737 | 4444 | 4477 | ||
| Primary Endpoint1 | 60 (23.9%) | 60 (23.6%) | 1.02 (0.71 – 1.45) | 214 (14.9%) | 222 (15.2%) | 1.03 (0.85 – 1.24) |
| Secondary Endpoints | ||||||
| First occurrence of CHD death, non-fatal MI, ischemic stroke or “high risk” acute coronary syndrome |
40 (15.9%) | 41 (16.1%) | 1.05 (0.68 −1.63) | 118 (8.2%) | 130 (8.9%) | 1.10 (0.85 – 1.41) |
| First occurrence of CHD death, non-fatal MI or ischemic stroke |
35 (13.9%) | 39 (15.4%) | 1.15 (0.73 – 1.82) | 103 (7.1%) | 117 (8.0%) | 1.13 (0.87 – 1.47) |
| Cardiovascular mortality | 12 (4.8%) | 19 (7.5%) | 1.62 (0.78 – 3.33) | 26 (1.8%) | 26 (1.8%) | 0.99 (0.57 – 1.70) |
| Overall Mortality | 23 (9.2%) | 39 (15.4%) | 1.73 (1.03 – 2.89)3 | 59 (4.1%) | 57 (3.9%) | 0.96 (0.67 – 1.38) |
| Cardiac | 12 (4.8%) | 16 (6.3%) | 1.35 (0.64 −2.86) | 22 (1.5%) | 22 (1.5%) | 0.99 (0.55 – 1.79) |
| Vascular, Non-cardiac | 0 (0.0%) | 3 (1.2%) | N/A | 4 (0.3%) | 4 (0.3%) | 0.99 (0.25 – 3.97) |
| Non-cardiovascular | 11 (4.4%) | 18 (7.1%) | 1.67 (0.79 – 3.53) | 32 (2.2%) | 29 (2.0%) | 0.90 (0.54 – 1.49) |
| Cardiovascular death or non-fatal MI | 30 (12.0%) | 34 (13.4%) | 1.16 (0.71 – 1.90) | 91 (6.3%) | 95 (6.5%) | 1.03 (0.78 – 1.38) |
Primary endpoint is defined as first occurrence of CHD death, non-fatal MI, ischemic stroke, hospitalization for acute coronary syndrome or symptom-driven coronary or cerebral revascularization.
Hazard ratios are based on model with baseline eGFR group.
P=0.038
Longitudinal Change in Kidney Function
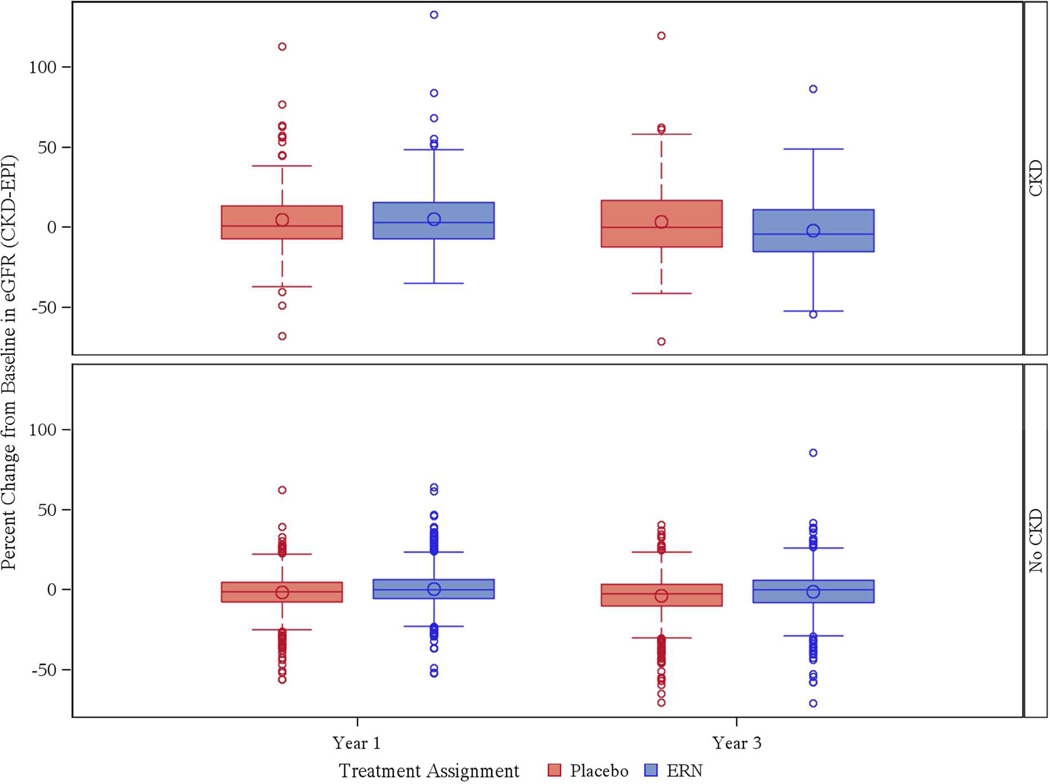
When the entire study sample was evaluated together regardless of CKD status, there was a mean decrease in eGFR of 2.8% (14.0%) in those randomized to placebo (n= 872) and a 1.2% (14.8%) decrease in those treated with ERN (n = 866) over 3 years (p=0.03). There was statistically significant interaction between treatment and CKD status (p-interaction = 0.004) for percent change in eGFR at Year 3. Data examining the subset with and without CKD separately are shown in Table 5 In participants with CKD, there was a 3.3% (24.2%) improvement in eGFR from baseline to year 3 in those randomized to placebo, whereas there was a 1.8% (22.3%) decrease in those randomized to ERN, a result that did not reach statistical significance (p=.10). Conversely, in the subset without CKD, there was a 3.8% (13.6%) decrease in eGFR in those randomized to placebo, whereas those randomized to ERN experienced only a 1.1% (13.5%) decrease in eGFR over 3 years (Figure 1). This finding was statistically significant (p=0.0001). No new incident cases of CKD were observed during the study.
Table 5.
Effect of Randomization to ERN vs. Placebo on Change in eGFR
| CKD | No CKD | ||||||
|---|---|---|---|---|---|---|---|
| Time | Placebo (N = 251) |
Niacin (N = 254) |
P-value | Placebo (N = 1444) |
Niacin (N = 1464) |
P-value | |
| Baseline eGFR | Mean (SD) | 50.5 (7.6) | 50.0 (7.7) | 84.9 (12.6) | 84.4 (12.9) | ||
| Year 1 | N | 191 | 193 | 1093 | 1106 | ||
| Change from baseline in eGFR | Mean (SD) | 2.0 (10.3) | 2.6 (10.1) | 0.5 | −1.4 (9.1) | 0.3 (8.7) | <0.0001 |
| Percent change from baseline in eGFR | Mean % (SD) | 4.6 (21.8) | 5.4 (21.3) | −1.4 (11.3) | 0.6 (11.4) | ||
| Year 3 | N | 123 | 110 | 749 | 756 | ||
| Change from baseline in eGFR | Mean (SD) | 1.5 (11.6) | −0.9 (11.3) | 0.1 | −3.3 (10.9) | −1.1 (10.7) | 0.0001 |
| Percent change from baseline in eGFR | Mean % (SD) | 3.3 (24.2) | −1.8 (22.3) | −3.8 (13.6) | −1.1 (13.5) | ||
Figure 1.
Percent Change from Baseline in eGFR (%) by CKD Status and Treatment
Adverse Events and Treatment Discontinuation
Within the CKD group, there was a significantly higher rate of discontinuation of ERN compared to placebo (32.7% vs. 22.7%, p = 0.01). In the ERN arm, higher rates of flushing, increased glucose, and gastrointestinal symptoms were observed compared to the placebo arm. Specifically for symptomatic flushing/itching, these symptoms accounted for near 30% of the primary reason for drug discontinuation in the ERN arm compared to 14% in the placebo arm in the CKD group (Table 6).
Table 6.
Reasons for Drug Discontinuation by Treatment Assignment
| CKD | No CKD | ||||||
|---|---|---|---|---|---|---|---|
| Placebo | Niacin | P-value | Placebo | Niacin | P-value | ||
| N | 251 | 254 | 1444 | 1464 | |||
| Discontinued study drug | N (%) | 57 (22.7%) | 83 (32.7%) | 0.012 | 284 (19.7%) | 353 (24.2%) | 0.004 |
|
Primary reason for drug discontinuation |
Flushing, itching | 8 (14.0%) | 25 (30.1%) | 35 (12.3%) | 79 (22.4%) | ||
| Liver function test abnormality | 0 (0.0%) | 1 (1.2%) | 5 (1.8%) | 4 (1.1%) | |||
| Patient request | 22 (36.8%) | 24 (28.9%) | 115 (40.5%) | 102 (29.0%) | |||
| Non-study physician request | 8 (14.0%) | 9 (10.8%) | 27 (9.5%) | 40 (11.3%) | |||
| Other clinical reason to discontinue | 16 (28.1%) | 13 (15.7%) | 79 (27.8%) | 83 (23.5%) | |||
| Increased glucose | 2 (3.5%) | 5 (6.0%) | 12 (4.2%) | 24 (6.8%) | |||
| Gastrointestinal symptoms | 1 (1.8%) | 5 (6.0%) | 11 (3.9%) | 21 (5.9%) | |||
Discussion
In this secondary analysis from AIM-HIGH, there was no incremental benefit of adding ERN to simvastatin-versus placebo in the important, high-risk subset of CKD patients with respect to secondary prevention of cardiovascular events. As expected, the CKD patients had a much higher primary end-point than the non-CKD group indicating a very high residual CVD risk in this subgroup. It is also important to note that most of the non-cardiovascular deaths were due to cancer, but no association between niacin treatment and cancer was observed.
It is not clear why AIM-HIGH results do not demonstrate the cardiovascular benefit of improvement in HDL-C and TG levels in CKD patients. There are several possibilities: 1) There is no different impact of ERN on cardiovascular outcomes in different levels of renal function in secondary prevention. 2) The impact of the low HDL-C and elevated TG levels is reduced in the context of aggressively treated LDL-C in this subset of patients. 3) Perhaps HDL-C is not the best marker to study clinical outcomes during ERN therapy. In addition, there is still some degree of uncertainty on whether HDL-C is a modifiable cardiac risk factor. 4) It is conceivable that other factors related to decreased kidney function such as elevated FGF-23 levels or other unidentified uremic factors play a more important role on cardiovascular outcomes (8).
From the early stages of CKD, abnormal apolipoprotein metabolism can be demonstrated (9). Oxidative stress is part of the complex interplay between CKD and heart disease (10), and is linked to endothelial dysfunction (11), that is highly prevalent in patients with moderate to severe CKD (12, 13). Patients with CKD are at high risk for CV events (1). It is conceivable that drugs such as niacin could have a cardio protective effect in CKD considering their anti-oxidant properties and effects on endothelial function (9, 14).
Recent studies targeting LDL-C for primary prevention in CKD patients who are not on renal replacement therapy such as the SHARP trial, demonstrated that LDL-C reduction is cardio protective (3). A sub study of the TNT (Treating to New Targets) trial demonstrated a survival benefit with lowering of LDL cholesterol with atorvastatin in CKD (15). Triglycerides-lowering agents such as gemfibrozil and fenofibrate have been shown to be cardio protective in non-dialytic CKD patients (16, 17). These results suggest that LDL plays a more important role than HDL-C in cardiovascular disease in CKD, and that there may be a role for TG although we could not demonstrate in our study. Studies testing inhibition of cholesteryl ester transfer protein (CETP) such as torcetrapib and dalcetrapib failed to demonstrate improvement in cardiovascular outcomes despite successfully increasing HDL-C levels (18, 19). The recently completed HPS-2 THRIVE (Second Heart Protection Study Treatment of HDL to Reduce Incidence of Vascular Events) study is the largest prospective randomized trial testing niacin in patients at high-risk for CV events to date, and failed to demonstrate cardioprotective effects (20).
Another important endpoint of this study was to examine the effects of ERN on longitudinal change in kidney function and to assess the tolerability in CKD patients. We observed a slower rate of decline in eGFR in ERN treated patients when all AIM-HIGH participants were examined together. In non- CKD patients, ERN therapy was also associated with a slower rate of eGFR decline than placebo. These data indicate that ERN slows decline in eGFR in a human trial extending observations in animals that niacin beneficially affects renal function. However, there was no difference in rates of eGFR decline in this small subgroup of CKD patients comparing ERN to placebo-treated patients. These results cannot exclude a possible benefit of ERN in kidney function in CKD because the study was not powered to detect definitive changes in kidney function, and certainly raise interest for further prospective studies examining ERN as possible nephroprotective agent since this is the largest prospective human data published so far demonstrating such effect. Furthermore, in a recent substudy of the AIM-HIGH trial, Guyton et al observed a trend toward cardiovascular benefit in the sub-group with the lowest HDL-C and highest TG levels treated with ERN (21). Whether CKD subjects with these characteristics would receive cardiovascular benefit from ERN is not known.
Our findings of high rates of ERN discontinuation in the CKD arm (32%) should be carefully considered in future clinical trials examining the role of ERN in dyslipidemias in patients who are in other stages of CKD. It is possible that worse tolerance of niacin among CKD participants (i.e. niacin-related altered food intake) contributed to our findings.
Strengths of this study include the prospective, randomized, placebo controlled design, and a large cohort of participants with stable CKD, and longitudinal assessment of renal function.
This study has several limitations: 1) AIM-HIGH excluded patients with serum creatinine > 2.5 mg/dL, thus whether or not the results would generalize to patients with more advanced CKD remains unknown. 2) All study participants had prevalent cardiovascular disease at baseline and were aggressively treated with statins to target LDL levels. Whether or not ERN would improve primary prevention of CV events in CKD patients is unknown. 3) We lacked data on albuminuria to test effects of ERN vs. placebo in early stages of CKD. The majority of the participants were on Renin- Angiotensin-Aldosterone System blockade, besides adequately controlled LDL levels. These factors may have contributed to a slower than expected rate of decline in eGFR irrespective of the lipid lowering effect. 4) The number of patients with both baseline and three year assessment of creatinine, n=241, is not large, limiting the inference that can be made on eGFR based on these numbers. 5) CKD is more prevalent in African Americans who represented less than 4% of the participants limiting the generalizability of these results beyond this subset. 6) We have limited data on apolipoprotein levels. Previous publications suggest that targeting apolipoprotein levels including ApoB and apoB/apoA1 ratio could provide better correlation with cardiovascular outcomes compared to HDL-C (22, 23).
There is a major need in conducting prospective randomized trials in patients with early stages of CKD in order to improve cardiovascular outcomes. In fact, the new KDIGO (Kidney Disease improving Global Outcomes) report persuasively argues for more research on the treatment of dyslipidemias in patients with CKD who have either elevated levels of TG or low levels of HDL-C (24). Although AIM-HIGH is one of the largest prospective studies evaluating the use of combined statin-ERN treatment for secondary cardiovascular prevention that included a sizeable proportion of patients with CKD, the sample size and number of deaths were relatively small. These factors limit inferences on differences and causes of mortality we can draw from this study.
Moreover it is not known if a larger sample size or a longer follow-up would lead to different results, therefore a possible benefit cannot be definitively excluded with the present data.
In summary, in CKD patients with atherogenic dyslipidemia from AIM-HIGH treated with ERN conferred no significant benefit on cardiovascular events despite significant increases in HDL-C and reduction of TG. Renal function decline was significantly slowed in AIM-HIGH patients as a whole, but no significant cardiovascular benefit was seen in the CKD sub-group. These findings coupled with a similar lack of cardioprotective benefits in the overall AIM-HIGH trial and in the much larger HPS2-THRIVE trial, raise doubts regarding the utility of this treatment strategy in the statin era.
Methods
Study Design
The AIM-HIGH study design and baseline characteristics of the study population have been described in detail previously (7, 25). Briefly, AIM-HIGH was a multi-center, prospective, randomized, double-blind, placebo-controlled trial in men or women age 45 years or older with established, stable cardiovascular disease and atherogenic dyslipidemia defined as low baseline HDL-C (< 40 mg/dL for men and < 50 mg/dL for women), high TG (100 – 400 mg/dL) and LDL-C < 180 mg/dL (adjusted for statin treatment at entry). Participants were recruited from 92 centers in the United States and Canada. The hypothesis was that raising HDL-C (as well as lowering TG, LDL-c, and lipoprotein a with extended- release niacin (ERN, Niaspan™, AbbVie Inc.) would decrease the rate of a composite primary endpoint (coronary artery disease mortality, non-fatal myocardial infarction, ischemic stroke, hospitalization for acute coronary syndrome or symptom-driven coronary or cerebral revascularization), during a projected mean 55 month follow-up. Participants with serum creatinine ≥ 2.5 mg/dL at screening were excluded. During a 4 – 8 week open label run-in period, all patients were treated with 40 mg of simvastatin and doses of ERN increasing weekly from 500 mg/day to 2,000 mg/day. Patients tolerating at least 1,500 mg/day of ERN were randomly assigned to ERN or placebo. The placebo tablets contained a small dose (50 mg) of immediate-release niacin in each 500-mg or 1000-mg tablet to mask the identity of the blinded treatment to patients and study personnel. All participants were treated with simvastatin (20–80 mg daily) with dose adjusted as needed to achieve a target LDL-C of 40–80 mg/dl. Ezetimibe 10 mg daily could be added as needed to subjects in either blinded treatment arm to achieve the LDL-C target. The trial was stopped on the recommendation of the Data and Safety Monitoring Board after a mean of 36 months of follow-up, based on the observation of lack of efficacy of ERN in reducing the composite primary endpoint (26). All living participants were followed to a final close-out visit, which was conducted between June and September 2011.
Fasting specimens of blood total cholesterol (TC),TG, HDL-C and LDL-C were measured at baseline, at months 1, 3 and 6, and at annual intervals; while lipoprotein (a) [Lp(a)] was measured only at baseline and year 1. Per protocol, serum creatinine concentrations were obtained at screening, year 1 and 3. Blood lipids and creatinine concentrations were measured in a central laboratory (Northwest Lipid Metabolism and Diabetes Research Laboratory, University of Washington). All endpoint events were reviewed by an independent clinical events adjudication committee, masked to the identity of treatment. Primary endpoints for this post-hoc analysis were identical to the study proper, as noted above. In addition, we compared annual changes in estimated GFR (eGFR) and incident CKD rates between treatment arms and by kidney function group. CKD was defined as estimated GFR of < 60 mL/min/1.73 m2 Study participants that were not classified as CKD at baseline but had an eGFR < 60 mL/min/1.73 m2 at the end of the study were counted as incident CKD cases. Study participants were evaluated for adverse effects in clinic or by phone on a quarterly basis. We compared rates of serious adverse events and drop-out rates by treatment arm and by kidney function group.
Statistical Analysis
Assessment of the kidney function was computed with the CKD-EPI formula (27) to assess estimated glomerular filtration rate (eGFR). Patients with eGFR < 60 mL/min per 1.73m2 were considered to have CKD (28).
Baseline demographic and clinical characteristics were compared between the ERN and placebo groups using independent two-sample t-test (pooled variance) for continuous variables such as body mass index (BMI), blood pressures, and baseline lipids. The Wilcoxon rank sum test was used for continuous variables not normally distributed, while chi-square test was used for categorical variables such as smoking status, history of CHD, diabetes, hypertension, and use of inhibitors of the renin-angiotensin system. Results of the Wilcoxon rank sum test were expressed as median and interquartile range (Q1,Q3). Descriptive statistics for lipids, and eGFR were summarized over scheduled study time by treatment assignment within eGFR groups. Cox proportional hazards models were used to generate hazard ratios for the primary outcome and other cardiovascular events of interest. Primary and secondary endpoint rates were based on an intention to treat analysis. Participant observation was censored at their last follow-up visit or death. The effect of ERN on kidney function analyses at 1 and 3 years was limited to participants with serum creatinine values available at those time points. ANCOVA model was used to test for interaction between eGFR group and the effect of ERN at 3 years of treatment. In addition, we conducted test for interaction without eGFR stratification in the statistical model. Adverse event rates were monitored routinely as a safety indicator throughout the trial and a chi-square test was used to compare the two treatment arms. Values are expressed in mean ± SD unless otherwise specified. Data management and statistical analysis were performed at the data coordinating center (Axio Research, Seattle). Statistical analysis was performed using SAS 9.3 statistical software (Cary, NC, USA).
Acknowledgment
Funding:
This study was funded by the National Heart, Lung, and Blood Institute (HL 081649 and HL 081616) and by an unrestricted grant from Abbott Laboratories. Abbott Laboratories donated the extended-release niacin, the matching placebo, and the ezetimibe; Merck donated the simvastatin. Neither of these companies had any role in the oversight or design of the study or in the analysis or interpretation of the data.
Disclosures: JHI is funded by the NIH/NIDDK (1RO1DK101720-01), and has served as consultant and received honoraria from Shire Pharmaceuticals and Keryx Biopharmaceuticals, and has served as a consultant for Astra Zeneca. RSK has received research support from Wyeth and Bristol Myers Squibb.
References
- 1.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu C. Chronic Kidney Disease and the Risks of Death, Cardiovascular Events and Hospitalization. N Engl J Med. 2004;351:1296–1305. doi: 10.1056/NEJMoa041031. [DOI] [PubMed] [Google Scholar]
- 2.Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomized trials. Lancet. 2010;376:1670–168. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Biagent C, Landray MJ, Reith C, et al. The effects of lowering LDL cholesterol with simvastatin plus ezetimibe in patients with chronic kidney disease (Study of Heart and Renal Protection): a randomized placebo-controlled trial. Lancet. 2011;377:2181–2192. doi: 10.1016/S0140-6736(11)60739-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vaziri ND. Dyslipidemia of chronic renal failure: the nature, mechanisms, and potential consequences. Am J Physiol. 2006;290:F262–F272. doi: 10.1152/ajprenal.00099.2005. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd J, Kastelein J, Bittner V, Deedwania P, Dobson S, et al. Effect of intensive lipid lowering with atorvastatin on renal function in patients with coronary artery disease: The Treating to New Targets (TN) Study. Clin J Am Soc Nephrol. 2007;2:1131–1139. doi: 10.2215/CJN.04371206. [DOI] [PubMed] [Google Scholar]
- 6.Cho K, Kim H, Rodriguez-Iturbe B, Vaziri ND. Niac in ameliorates oxidative stress, inflammation, proteinuria, and hypertension in rats with chronic renal failure. Am J Physiol Renal Physiol. 2009;297:F106–F113. doi: 10.1152/ajprenal.00126.2009. [DOI] [PubMed] [Google Scholar]
- 7.AIM-HIGH Investigators. The role of niacin in raising high-density lipoprotein cholesterol to reduce cardiovascular events in patients with atherosclerotic cardiovascular disease and optimally treated low-density lipoprotein cholesterol: baseline characteristics of study participants. The Atherothrombosis Intervention in Metabolic syndrome with low HDL/high triglycerides: impact on Global Health outcomes (AIM-HIGH) trial. Am Heart J. 2011 Mar;161(3):538–543. doi: 10.1016/j.ahj.2010.12.007. Epub 2011 Feb 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Isakova T, Xie H, Schwartz S, Lo J, Ojo A, Sondheimer J, Hsu C, Lash J, et al. Fibroblast growth factor 23 and risks of mortality and end-stage renal disease in patients with chronic kidney disease. JAMA. 2011;23:2432–2439. doi: 10.1001/jama.2011.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaziri ND, Dicus M, Ho ND, Boroujerdi-Rad L, Sindhu RK. Oxidative stress and dysregulation of superoxide dismutase and NADPH oxidase in renal insufficiency. Am J Kidney Dis. 2011 Nov;5(6):357–372. doi: 10.1046/j.1523-1755.2003.00702.x. [DOI] [PubMed] [Google Scholar]
- 10.Himmelfarb J, Hakim R. Oxidative stress in uremia. Curr Opin Nephrol Hypertens. 2003;12(6):593–598. doi: 10.1097/00041552-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Heitzer T, Schlinzig T, Krohn K, Meinertz T, Munzel T. Endothelial dysfunction, Oxidative Stress, and Risk of Cardiovascular Events in Patients with Coronary Artery Disease. Circulation. 2001;104:2673–2678. doi: 10.1161/hc4601.099485. [DOI] [PubMed] [Google Scholar]
- 12.Yilmaz MI, Saglam M, Caglar K, Cakir E, Sonmez A, et al. The Determinants of Endothelial Dysfunction in CKD: Oxidative Stress and Asymmetric Dimethylarginine. Am J Kidney Dis. 2006;47(1):42–50. doi: 10.1053/j.ajkd.2005.09.029. [DOI] [PubMed] [Google Scholar]
- 13.Kalil R, Flanigan, Stanford W, Haynes WG. Dissociation between progression of coronary artery calcification and endothelial function in hemodialysis patients: A prospective pilot study. Clin Nephrol. 2011;78(1):1–9. doi: 10.5414/CN106830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Warnholtz A, Wild P, Ostad MA, Elsner V, Stieber F, et al. Effects of oral niacin on endothelial dysfunction in patients with coronary artery disease: Results of the randomized, double-blind, placebo-controlled INEF study. Atherosclerosis. 2009;209:216–221. doi: 10.1016/j.atherosclerosis.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 15.Shepherd J, Lastelein JJ, Bittner V, Deedwania P, Breazna A, Dobson S, et al. Intensive Lipid Lowering with Atorvastatin in Patients with Coronary Heart Disease and Chronic Kidney Disease; The TNT Study. J Am Coll Cardiol. 2008;51:1448–1451. doi: 10.1016/j.jacc.2007.11.072. [DOI] [PubMed] [Google Scholar]
- 16.Ting R, Keech A, Drury P, Donoghoe M, Hedley J, Jenkins A, et al. Benefits and safety of long-term fenofibrate therapy in people with type 2 diabetes and renal impairment. Diabetes Care. 2012;35:218–225. doi: 10.2337/dc11-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tonelli M, Collins D, Robins S, Bloomfield H, Curham G. for the Veterans Affairs High-Densisty Lipoprotein Intervention Trial (VA-HIT) Investigators. Kidney Int. 2004;66:1123–1130. doi: 10.1111/j.1523-1755.2004.00862.x. [DOI] [PubMed] [Google Scholar]
- 18.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JP, Komajda M, et al. Effects of Torcetrapib in patients at high risk for coronary evens. N J Engl Med. 357:2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 19.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. Effects of Dalcetrapib in patients with recent acute coronary syndrome. N Engl J Med. 2012;367:2089–2099. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 20.Effects of extended-release niacin with laropiprant in high-risk patients. The HPS2-THRIVE Collaborative Group. N Engl J Med. 2014;371:203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 21.Guyton J, Slee A, Anderson T, Fleg J, Goldberg R, Kashyap M, et al. Relationship of lipoprotein to cardiovascular events. The AIM-HIGH Trial. JACC. 2013;62:1580–1584. doi: 10.1016/j.jacc.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walldius G, Jungner I, Aastveit A, Holme I, Furberg C, Sniderman A. The apoB/apoA ratio is better than the cholesterol ratios to estimate the balance between plasma proatherogenic antiatherogenic lipoproteins and to predict coronary risk. Clin CHem Lab Med. 2004;42(12):1355–1363. doi: 10.1515/CCLM.2004.254. [DOI] [PubMed] [Google Scholar]
- 23.Bockholdt S, Arsenault B, Mora S, Pedersen T, LaRosa J, Nestel P, Simes R, et al. Association of LDL cholesterol, non-HDL cholesterol, and apolipoprotein B levels with risk of cardiovascular events among patients treated with statins. A meta-analysis. JAMA. 307:1302–1309. doi: 10.1001/jama.2012.366. [DOI] [PubMed] [Google Scholar]
- 24.KDIGO Clinical Practice Guideline for Lipid Management in Chronic Kidney Disease. Kidney International Supplements. 2013 Nov; [Google Scholar]
- 25.AIM-HIGH Investigators. The role of niacin in raising high-density lipoprotein cholesterol to reduce cardiovascular events in patients with atherosclerotic cardiovascular disease and optimally treated low-density lipoprotein cholesterol: baseline characteristics of study participants. The Atherothrombosis Intervention in Metabolic syndrome with low HDL/high triglycerides: impact on Global Health outcomes (AIM-HIGH) trial. Am Heart J. 2011 Mar;161(3):538–543. doi: 10.1016/j.ahj.2010.12.007. Epub 2011 Feb 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.The AIM-HIGH Investigators. Niacin in Patients with Low HDL Cholesterol Levels Receiving Intensive Statin Therapy. N Engl J Med. 2011;365(24):2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 27.Levey AS, Stevens LA, Schmid CH, Zhang Y, Castro IIIAF, et al. A New Equation to Estimate Glomerular Filtration Rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Definition and classification of CKD. KDIGO. Kidney Int Suppl. 2013;3:19. doi: 10.1038/kisup.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]