Abstract
While transposon mutagenesis has been successfully used for Mycoplasma spp. to disrupt and determine non-essential genes, previous attempts with Ureaplasma spp. have been unsuccessful. Using a polyethylene glycol-transformation enhancing protocol, we were able to transform three separate serovars of Ureaplasma parvum with a Tn4001-based mini-transposon plasmid containing a gentamicin resistance selection marker. Despite the large degree of homology between Ureaplasma parvum and Ureaplasma urealyticum, all attempts to transform the latter in parallel failed, with the exception of a single clinical U. urealyticum isolate. PCR probing and sequencing were used to confirm transposon insertion into the bacterial genome and identify disrupted genes. Transformation of prototype serovar 3 consistently resulted in transfer only of sequence between the mini-transposon inverted repeats, but some strains showed additional sequence transfer. Transposon insertion occurred randomly in the genome resulting in unique disruption of genes UU047, UU390, UU440, UU450, UU520, UU526, UU582 for single clones from a panel of screened clones. An intergenic insertion between genes UU187 and UU188 was also characterised. Two phenotypic alterations were observed in the mutated strains: Disruption of a DEAD-box RNA helicase (UU582) altered growth kinetics, while the U. urealyticum strain lost resistance to serum attack coincident with disruption of gene UUR10_137 and loss of expression of a 41 kDa protein. Transposon mutagenesis was used successfully to insert single copies of a mini-transposon into the genome and disrupt genes leading to phenotypic changes in Ureaplasma parvum strains. This method can now be used to deliver exogenous genes for expression and determine essential genes for Ureaplasma parvum replication in culture and experimental models.
Keywords: Ureaplasma parvum, Ureaplasma urealyticum, transposon mutagenesis, gene disruption, RNA helicase
INTRODUCTION
In the United Kingdom and United States about 1 in 12 pregnancies result in premature birth (<32 weeks out of a normal 40 weeks gestation). Between 25–40% of these preterm births are associated with intrauterine infection and Ureaplasma spp. are the organisms most frequently identified. Sixty five percent of very premature newborns (≤ 26 weeks’ gestation) were found to have congenital respiratory Ureaplasma infection [Sung et al. 2011]. These infants often require long-term ventilation and are at an elevated risk of neonatal death and disease. Experimental infection in primates have definitively proven that intrauterine infection of Ureaplasma parvum, as a sole pathogen, induces preterm birth and associated neonatal respiratory disease (Novy et al., 2009); however, the ability to study the role of individual bacterial genes in pathogenesis experimentally has been hampered by a lack of tools to deliver or knock-out genes.
Ureaplasma spp. are one of the smallest self-replicating microorganisms identified to date that belong to the class Mollicutes. This class possesses a number of bacterial species with unusually small genomes (0.75–0.78 Mbp genomes for U. parvum and 0.84–0.95 Mbp genomes for U. urealyticum), (Paralanov et al., 2012) that lack a number of genes resulting in this class’s characteristic failure to make a bacterial cell wall and deficiencies in a number of metabolic pathway enzymes. As a by-product, these deficiencies mediate an inherent resistance to most antibiotics other than macrolides, fluoroquinolones and tetracyclines (Waites et al., 2005). Ureaplasmas utilise urea conversion to ammonium ions for ~95% of their ATP generation (Romano et al., 1980; Smith et al., 1993) and have a pleomorphic microscopic appearance for organisms of approximately 0.1–1.0 μm in diameter (Robertson et al., 2002). Although initially characterised as a single species separated into 14 serovars, Ureaplasma urealyticum was later subdivided into two species (U. parvum and U. urealyticum). These cluster into two distinctive groups when analysed for patterns of antigenic types or key metabolic gene polymorphisms (such as urease) and show a distinctive phylogenetic divergence when comparing the 16S rRNA and 16S–23S rRNA intergenic regions between U. parvum and U. urealyticum (Robertson et al., 2002).
There is evidence of transposon gene delivery into the Ureaplasma spp. genome that occur in nature. The fully sequenced genome for Ureaplasma urealyticum serovar 9 (ATCC strain 33175), which exists as a whole genome shotgun sequence (NZ_AAYQ02000002.1) in the NCBI database, shows the presence of the tetracycline resistance tetM gene (UUR9_0151) adjacent to a conjugal transfer protein (UUR9_0147), transposase (UUR9_0146), and integrase (UUR9_0149). The tetM gene for this genome is accepted to be part of a Tn916 a conjugative transposon also called integrative conjugative element (ICE). Tetracycline resistance for many U. parvum and U. urealyticum genomes have been reported to be due to transposon-associated tetM gene presence in the bacterial genome from isolates from distant countries (Beeton et al., 2009; de Barbeyrac et al., 1996; Govender et al., 2012; Mardassi et al., 2012; Roberts 1990; Taraskina et al., 2002) and donation from transposon-carrying Enterococcus faecalis to a close relative to Ureaplasma (Mycoplasma hominis) during co-culture was demonstrated through mating at a frequency of 10−6 to 10−7 (Roberts and Kenny 1987).
Transposon-carrying plasmids were first successfully used to insert selectable markers in the genomes of Mycoplasma pulmonis, Mycoplasma hyorhinis and Acholeplasma laidlawii in the late 1980’s (Dybvig and Alderete 1988: Dybvig and Cassell 1987; Mahairas and Minion 1989a&b), which showed random genome insertion allowing both investigation of disrupted genes as well as the delivery of exogenous genes. The methods and reagents used to study essential genes and the physiological effects of delivering exogenous genes has continued to expand and be refined (Algire et al., 2009; Paralanov et al., 2012). To date, no report has been made to show successful experimental delivery of transposons or plasmids into Ureaplasma spp.
MATERIALS AND METHODS
Bacterial strains
Escherichia coli
One shot Top10 chemically competent E. coli cells (Invitrogen; Paisley, Scotland, UK), were used as per manufacturer’s instructions. This bacteria has the genotype F-mcrA Δ(mrr-hsdRMS-mcrBC) φ80lacZΔM15 Δlacχ74 recA1 araD139 Δ(ara-leu) 7697 galU galK rpsL (StrR) endA1 nupG λ-.
Ureaplasma spp
Three U. parvum strains for serovar 1 (DFK-1, O10, and HPA78), serovar 3 (HPA5, U6, and HPA56) and serovar 6 (HPA2, HPA61, HPA58) were investigated for the capacity to undergo transposon mutagenesis, and these strains were initially isolated from patients, but have been purified through subcloning and have also been previously characterised and used by our group in previous publications (Beeton et al., 2009 & 2012). Various ATCC strains of U. urealyticum representing serovar 2 (ATCC 27814), 4 (ATCC 27816), 8 (ATCC 27618), 9 (ATCC 33175), and 10 (ATCC 33699) were generously supplied by Dr. Janet Robertson, (University of Alberta, Canada) and these strains as well as a clinical isolate W11 (serovar 12) (Beeton et al., 2009) were investigated in parallel.
Expression constructs and the mini-transposon plasmid
The Tn4001-based mini-transposon plasmid (pMT85) has been previously described by Zimmerman and Herrmann (2005) and was generously provided by Prof. R. Herrmann (Heidelberg University, Germany). The gentamicin resistance gene was not codon-usage optimised for Ureaplasma spp. expression.
Transformation of bacteria
Transformation of E. coli One shot Top 10 chemically competent bacteria was performed as per manufacturer’s instructions using heat shock at 42°C and the plasmid containing bacteria selected with 100 mg/l gentamicin. Transformation of Ureaplasma spp. was carried out essentially as outlined for M. mycoides in King and Dybvig (1991) with some modifications. The key aspect of U. parvum growth is the conversion of urea to ammonium ions, which increase the pH of the growth medium from pH =6.2 (yellow) to pH >9 (dark red). Three 96-well plates containing 10-fold serial dilutions of Ureaplasma (200 μl per well, titrated from Rows A–H) were set out the night before the experiment. All of the wells showing pH change consistent with the threshold of detection for phenol red indicator (i.e. last red well, dark orange and first yellow well) were pooled (total volume 10 ml) and utilised for transformation, as these represent U. parvum in log phase growth. This 10 ml routinely gave titrations of 5×108 CFU. Cells were washed three times (centrifuged at 10000xg for 20 min) with 1 ml of 1X Dulbecco’s phosphate-buffered saline with calcium and magnesium (DPBS) (Invitrogen, Paisley, U.K.) at 4°C. The washed pellet was resuspended in a volume of 375 μl of 100 mM CaCl2 on ice for 30 min, then a volume of 100 μl of bacterial cells containing 10 μg of yeast tRNA, 6 μg of pMT85 and 1 ml of 50% PEG-8000 were added at room temperature for 1 min. Five ml of Ureaplasma selective medium (USM; purchased from Mycoplasma experience plc, Surrey, UK) was then added and the cells were allowed to recover at 37°C for 3 h and the growing cells were pelleted by centrifugation at 3600xg for 15 min at 4 °C before being resuspended again in 1 ml USM. A volume of 20 μl cell suspension was diluted in USM in a 1/10 dilution series containing 128 mg/L gentamicin in triplicate (as the endogenous MIC90 for U. parvum is 44 mg/L; unpublished data) and incubated overnight. Control bacteria treated identically (except for the addition of pMT85) were run in parallel and no spontaneous gentamicin resistance was observed. When the first well of transformed bacteria turned the media red, the cells were plated out on Ureaplasma selective agar (Mycoplasma Experience ltd) and individual colonies were examined for presence of gentamicin resistance gene by PCR, prior to further characterisation. In some experiments a dilution series of transformed bacteria were directly plated on to Ureaplasma selective agar plates containing 128 mg/L gentamicin to determine transformation success relative to Ureaplasma selective plates without gentamicin.
Screening of transformed bacteria
Successful transformation of gentamicin resistant bacterial clones was confirmed using PCR and primers designed against the gentamicin resistance gene (aac-aphD; 6′-aminoglycoside N-acetyltransferase); forward 5′-ACATGAATTACACGAGGGC-3′, reverse 5′-GTTCTTCTTCTGACATAGTAG-3′; 401 bp amplicon, Tm=54°C, 35 cycles) using standard PCR methods amplified by Promega GoTaq green DNA polymerase. U. parvum genes disrupted by transposon insertion into the genome were confirmed using primers against Ureaplasma genes: UU390 (hypothetical membrane protein; forward 5′-AGTATTCCCATTGCGACAA-3′, reverse 5′-TATTTATTATCTTTTCTGGAGGTT-3′; 476bp amplicon; Tm=52.4°C); UU450 (hypothetical membrane protein; forward 5′-TTGAATTGAACCCTCAGATCC-3′, reverse 5′-ATTGCTTGATGGAAATGAATCCT-3′ 675bp amplicon; Tm=58°C); UU520 (hypothetical membrane protein; forward 5′-TCTGGAGGGAGTTTGTCTCC-3′; reverse 5- TTTCGCAAAGGTGCTAAACCA-3′; 730bp amplicon; Tm=58°C), UU582 (RNA helicase; forward 5′-TTACCACGACCACTACGTCC-3′, reverse 5′-TTATTGGCGTTGCACCAACAG-3′; 876bp amplicon, Tm=58°C) intergenic insertion between UU187 and UU188 (forward 5′-AGGTCACGATGTTGTTGCTGA-3′, reverse 5′-CAAATATGGGCAACAGGAGCAG-3′; 600bp amplicon, Tm=58°C). Amplifying the ends of the insertion site was performed using one of the above primers in combination with pMT85-specific primers (designed close to the 5′ and 3′ inverted repeat sequence) 195R (5′-CCGTAATCAAGGTCATAGC-3′, Tm=54.5°C) or 3192F (5- TTTGCTGGCCTTTTGCTCAC-3′, Tm=57°C) at the lowest annealing temperature. Amplicons were purified using the Qiaquick PCR clean up kit (Qiagen, Manchester, U.K.) and submitted to Eurofins MWG Operon (Ebersberg, Germany) for sequencing. Primers specific to pMT85 were also used to determine if transposon insertion only utilised sequences between the inverted repeats (1-3437bp) or whether plasmid sequence containing the tnp transposase gene (3438-4820bp) were also present in the genome insertion.
Sanger sequencing of genomic DNA to determine insertion site
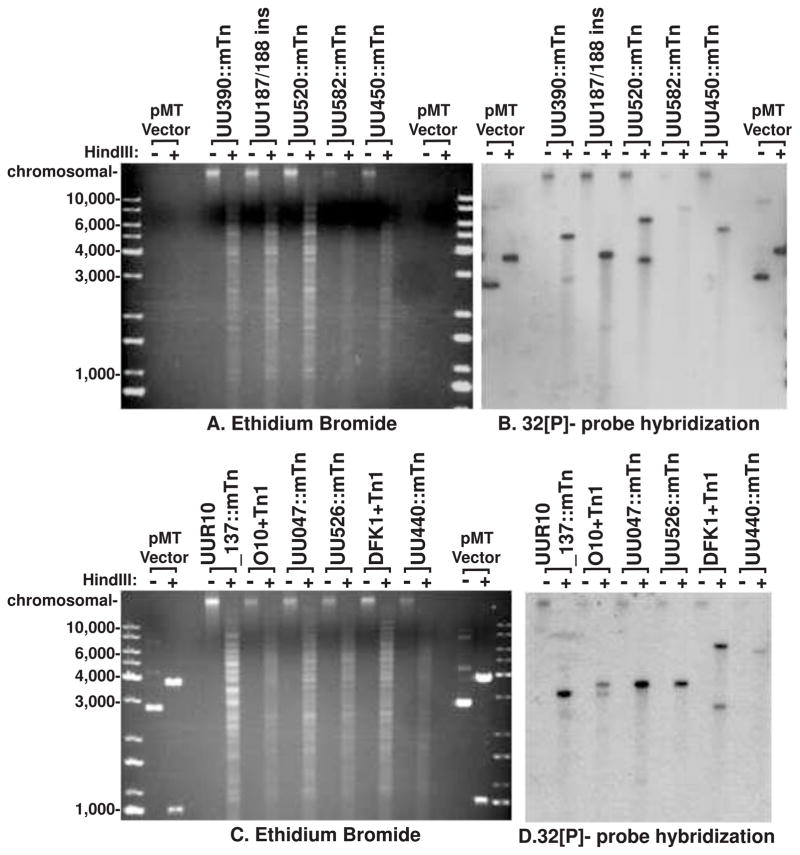
Gentamicin resistant mutants were scaled up to a 100 ml culture, pelleted at 10,000 xg and the genomic DNA extracted utilising the GenElute™ Bacterial Genomic DNA Kit (Sigma-Aldrich), as per the manufacturer’s instructions. DNA from each preparation was either ethanol precipitated, resuspended in 10 μl of molecular grade water and utilised for Sanger sequencing of the purified genomic DNA utilising pMT85 primers 195R or 3192F, or separated on a 1% agarose gel (in TBE) and utilised for in gel radioactive probe hybridization. The genomic DNA and control pMT85 plasmid digestion were performed at 37°C overnight with HindIII (Promega). The buffers were used according to the manufacturer’s recommendations and separated DNA fragments were compared to undigested controls in adjacent lanes. For in gel hybridization: briefly, the gel was dried for 5 h at 50°C and then rehydrated in double-distilled water for 5 min before 30-min incubations in denaturing solution (0.5 M NaOH, 1.5 M NaCl) and neutralizing solution (0.5 M Tris-HCl [pH 7.5], 1.5 M NaCl) at room temperature were performed. The gel was then prehybridized at 65°C using prehybridization solution (20 ml) (6× SSC [1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate], 0.1% [wt/vol] polyvinylpyrrolidone 400, 0.1% [wt/vol] Ficoll, 0.1% [wt/vol] bovine serum albumin [BSA] [Cohn fraction V], 0.5% [wt/vol] SDS, 150 μg/ml denatured calf thymus DNA). An amplicon containing the aac-aphD resistance gene (primers listed above) was purified with a Qiaquick PCR purification kit (Qiagen) and labelled using a random priming kit as per manufacturer’s instructions (Agilent technologies Prime-it Random Primer labelling kit 300385) with [32P]-dCTP. Non-incorporated radio-active nucleotides were then removed using by gel filtration using a G50 column (GE Healthcare). Gels were then washed in 2% SSC followed by 0.1% SSC and bands were visualised by autoradiography at −80°C using X-ray film (Fuji Film).
Immunoblot analysis
Strains for investigation were scaled up to an overnight 5 ml culture in USM and pelleted at 17,000 xg for 20 min in a refrigerated centrifuge. The pellet was then solubilised in 50 μl of NuPAGE® LDS Sample Buffer (Life Technologies, Glasgow, U.K.) as per manufacturer’s instructions, and separated by SDS-PAGE. Following electrophoretic transfer to nitrocellulose membrane, blots were blocked in 5% skim milk dissolved in PBS containing 0.05% Tween20 (PBST) and probed with either human high titre anti-Ureaplasma sera (as per our previous studies (Beeton et al., 2012) or Virostat plc (Portland, ME) monoclonal anti-multiple banded antigen (MBA) antibody (clone 6525). Bound human and mouse antibodies were detected with appropriate peroxidase-conjugated secondary antibodies from Jackson ImmunoResearch Europe ltd. (Newmarket, U.K.) and Pierce ECL detection reagent (Fisher Scientific, Loughborough, U.K.).
Serum killing assay
Serum killing assays were performed as detailed in our previous studies (Beeton et al., 2012), using the previously characterised sera from healthy volunteers (including seronegative sera F1 and F9 as well as seropositive sera F7, M11 and M12). Following 1 hour challenge with 50% serum diluted in complement fixation buffer (Oxoid plc, Basingstoke, U.K.) at 37°C, surviving bacteria were quantified by titration in USM. Killing was determined as the relative decrease in bacterial titre relative to incubation with heat-inactivated serum (56°C, 30 min) as a control. Surviving bacteria were measured after 48 hour incubation at 37°C to enable maximum growth. All killing assays were performed in triplicate and repeated on at least three separate days with a range of characterised seropositive and seronegative human sera.
RESULTS
Transposon mutagenesis of Ureaplasma
Transformation with pMT85 was performed in parallel for eight representative strains of U. parvum and U. urealyticum. Between 1–5 successful transformants survived per 108 U. parvum cells used in the transformation reaction for each experiment; however, all parallel transformations of ATCC strains of U. urealyticum failed while one clinical isolate of U. urealyticum (strain W11; SV12) was successfully transformed. The U. parvum gentamicin MIC90 was determined to be 44 mg/L and for U. urealyticum was 66 mg/L using our previous methods (method detailed in Beeton et al., 2009); therefore, gentamicin selection was performed at 128 mg/L. All resistant clones were found to contain the aac-aphD resistance gene from pMT85, which was not found in untransformed controls (Figure 1). Classical insertion into the genome should only insert the genes bordered by the inverted repeats at position 1bp and 3437 bp in pMT85; therefore, PCR was used to investigate the presence of plasmid sequence beyond the second inverted repeat, including the transposase gene (Figure 1). Prototype serovar 3 strain HPA5 was successfully transformed in 16 separate experiments with pMT85 and only transfer of plasmid DNA bordered by the inverted repeats (i.e. no transposase gene sequence) was observed. We also transformed 3 different strains for each of serovar 1, 3 and 6 of U. parvum; however, not all of them behaved as HPA5. Probing undigested genomic DNA from these isolates found that the aac-aphD gene was located on the chromosome, and probing of HindIII-digested genomic DNA found that with the exception of 3 clones, a single copy was inserted into the genome (Figure 2). Portions of the transposase (trp) gene from pMT85 integrated into the genomes of two strains from serovar 1, one strain from serovar 3 and one strain from serovar 6 (Figure 1 and Supplementary Figure 1). Furthermore, in 3 separate experiments with prototype serovar 1 strain DFK-1 only two out of three followed classical integration (Supplementary Figure 1). Sanger sequencing of purified genomic DNA from strains HPA56 and HPA58 confirmed that genomic integration included transposase sequence. Sequencing with primer 195R (designed to sequence across the IR at pMT85 position 1) confirmed genomic integration with interruption of the gene UU047 (predicted ATP/GTP binding protein) at position 390 bp in the coding region for HPA56 and interruption of the gene UU526 (hypothetical open reading frame) at position 543 bp in the predicted reading frame for HPA58 respectively. However, Sanger sequencing of the genomic DNA with primer 3192F (designed to sequence across the IR at position 3437) showed no integration and the intact presence of the pMT85 transposase gene sequence. However, Sanger sequencing of HPA78 mutant genomic DNA (Supplementary Figure 1) with the same primers, did not confirm the presence of transposase gene as both IR were found to interrupt the gene UU440 for this strain.
Figure 1.
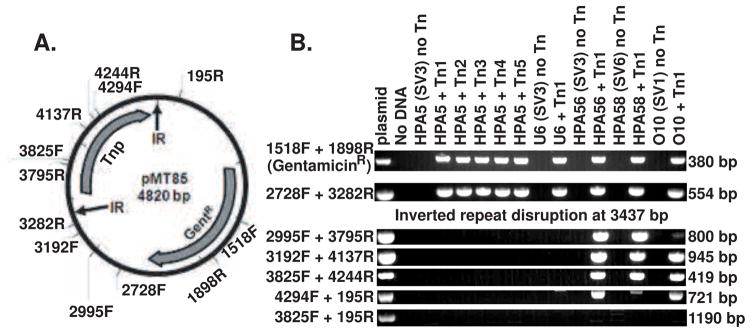
PCR mapping of regions of mini-transposon plasmid pMT85 (A) present in the genomes of gentamicin resistant selected clonal strains (B). Presence of the gentamicin resistance gene (primers 1518F to 1898R) was only found in transposon mutated strains. PCR probing for different regions of the plasmid identified that strains such as HPA5 and U6 only contain plasmid DNA from between the inverted repeat (IR) regions. Whereas, other transposon mutated strains (HPA56, HPA56 and O10) from different serovars (SV) contain mini-transposon plasmid DNA that include some of the transposase (Tnase) gene. No Tn indicates parental strain, while different numbers after Tn indicate clones from separate experiments. Expected amplicon size is indicated to the right of the figure.
Figure 2.
In gel hybridization detection of gentamicin selection gene. Agarose gel separation of total genomic DNA extracted from transposon mutated Ureaplasma strains. Comparing HindIII digested and undigested genomic DNA. A and C show ethidium bromide visualisation of the DNA prior to probing with the gentamicin resistance gene (visualised by autoradiography in B and D). Three mutants (UU350::mTn, DFK1+Tn1, and O10+Tn1) show 2 bands suggesting a mixed colony or 2 insertion sites. No undigested samples show any extra-chromosomal plasmid DNA. All the remaining examined isolates show a single insertion site into the genome. HindIII-digested and undigested pMT85 vector is shown along with the KAPA Universal DNA ladder for fragment size comparison.
Interruption of U. parvum genes by random genomic insertion
Primer pairs were designed based on the genomic sequence for U. parvum ATCC serovar 3 strain 700970 for genes UU390, UU450, UU520, UU582 and intergenic region between UU187 and UU188. These primers successfully amplified these genes by PCR in all parent strains of Ureaplasma, while single failure for each primer set to amplify these genes in mutated strains (Supplementary figure 2) was due to transposon integration and disruption of these genes. These disruptions were then, confirmed by sequencing the integration interface (Figure 3) Sequencing of the junctions between transposon insertion for these genes found 8 bp direct repeats that were unique for each clone, adjacent to the inverted repeat from pMT85 (Figure 3). The 8 bp direct repeat for the intergenic insert between UU187 and UU188 replicated the last two bases of the TAA stop codon from UU187 ensuring that UU187 was not disrupted..
Figure 3.
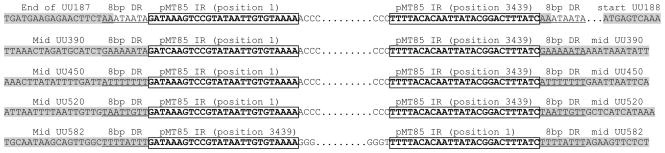
Sequence alignment of transposon insertion boundaries for HPA5 187/188 intergenic insertion, UU390::mTn, UU450::mTn, UU520::mTn, and UU582::mTn. Inverted repeats are highlighted in green, coding regions of genes are highlighted in grey. The Tn insertion sites are bordered by 8-basepair direct repeats, with 100% identity intra-strain, but unique when compared inter-strain (except being very AT-rich). The direct repeat for the 187/188 insertion shows that the direct repeat replicates the stop codon for UU187 (TAA), therefore no disruption of UU187 coding region occurred.
Screening Ureaplasma mutants for altered expression of the major surface antigen
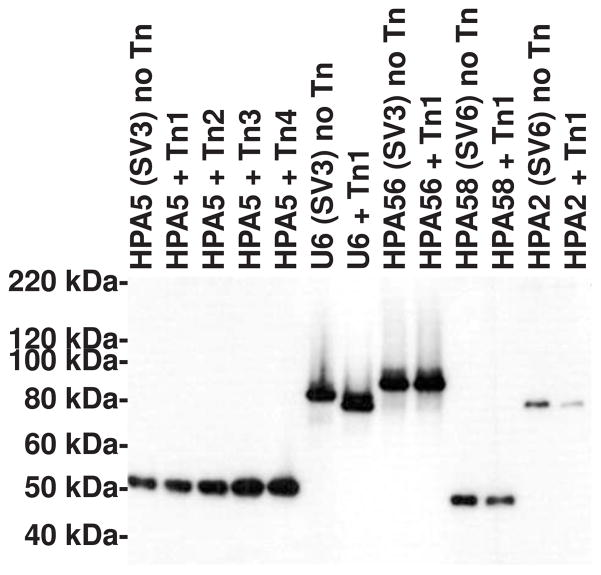
Immunoblot analysis using monoclonal anti-multiple banded antigen (MBA; UU375) antibody was carried out to examine if any of the mutated strains had altered size or expression of the major surface antigen, MBA, relative to the untransformed parent strain. In 27 transformed clones from four different Ureaplasma serovars, MBA-negative clones (phase variation) were never observed, and only one clone showed a small alteration to the MBA mass (Figure). The site of genomic integration for this strain (U6) is known to be at the predicted integral membrane protein gene UU450 (Figure 3), which is not close to the gene encoding the MBA, therefore the observed MBA mass alteration for this strain is not due to direct interference with the coding gene.
Disruption of RNA helicase (UU582) gene altered Ureaplasma growth kinetics
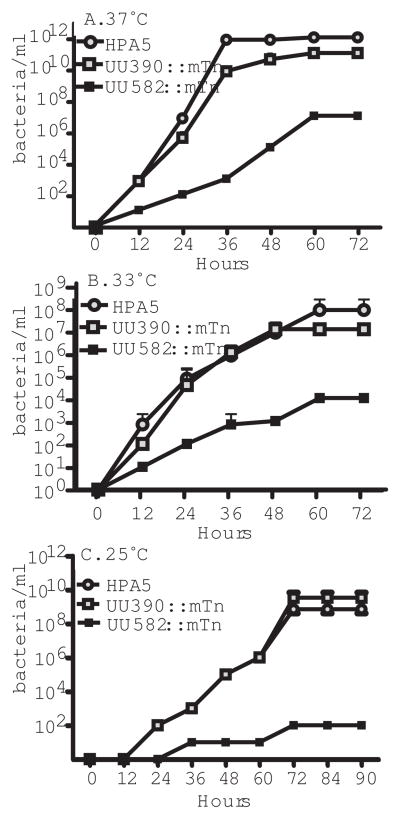
Random transposon insertion was found to disrupt the gene UU582 as detailed in figure 3 and supplementary figure 2. UU582 encodes the only copy of an ATP-dependent DeaD-box bacterial RNA-helicase in the U. parvum genome and disruption of this gene was found to alter final titre and growth kinetics for the bacteria at a range of incubation temperatures. Of all the transposon mutated Ureaplasma strains, only the disruption of gene 582 (UU582::mTn) had this effect. Maximum bacterial titre was obtained for parent and all other transposon mutated strains by 36 hr at 37°C (Figure 5), while UU582::mTn did not reach maximum titre until 60 hr and had a 3 to 4 log reduction in final bacterial titre. As DEADbox RNA-helicase mutants are reported to be incapable of replication at lower temperatures (Owttrim 2013) we also investigated growth kinetics at 33°C and 25°C (an example of growth for the parent strain at these temperatures is shown in supplementary figure 3). Under these conditions the maximum titre for other Ureaplasma strains took longer to attain and UU582::mTn titres were 108-fold lower at 25°C.
Figure 5.
Growth kinetics for U. parvum parent strain (HPA5) compared to membrane protein disruption (UU390::mTn) or DEADbox RNA-helicase gene disruption (UU582::mTn) when incubated at 37°C (A.), 33°C (B.), or 25°C (C.). Strains were titrated out in a 10-fold dilution series and growth measured at time points indicated by urease conversion of urea to ammonium ions. Ureaplasma growth is shown as colour (pH indicator) changing units per ml. Mean and standard deviation of dilutions performed in triplicate. Results were consistent through three repeated experiments.
Altered complement susceptibility for mutated U. urealyticum strain
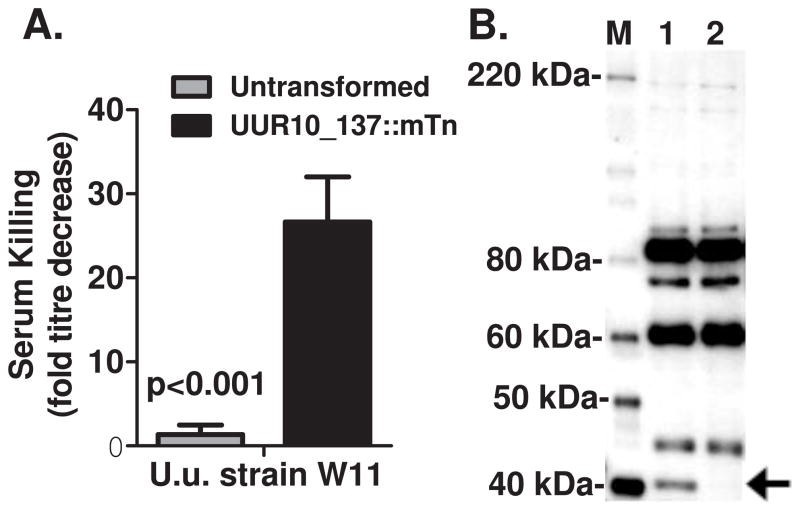
While no alteration in serum killing (either susceptible or resistant) was observed for any of the U. parvum strains (data not shown) following transposon mutagenesis, the U. urealyticum strain (W11) became significantly more susceptible to complement-mediated attack following transposon mutagenesis (Figure 6). W11 remained resistant to seronegative serum attack (sera that did not show reactivity by immunoblot), but were readily killed by sera containing anti-Ureaplasma antibodies. Immunoblot analysis using a high-titre seropositive serum identified a coincident loss of a 41 kDa mass bacterial protein in the mutant strain relative to the serum-resistant parent strain. Genomic DNA analysis identified a single genomic insertion site by in gel hybridisation for the aac-aphD gene (data not shown) and direct genomic sequencing of the W11 mutant found that the gene UUR10_0137 (ATCC strain 33699 serovar 10 numbering) was disrupted at amino acid 126 of 231.
Figure 6.
Serum killing (A) and immunoblot analysis using human high titre seropositive serum (B) for parental U. urealyticum strain W11 and following successful transposon mutagenesis (+Tn). Serum killing of increases significantly following 1 h challenge with human serum containing anti-Ureaplasma antibodies and analysis of this serum shows the serum-sensitive transposon mutated strain has lost a 41 kDa band that was immunoreactive with the challenging serum. Bar graph shows mean +/− SEM of experiments performed in triplicate. Representative immunoblot from three repeat experiments shown.
DISCUSSION
Here we report the first successful transposon delivery of gentamicin resistance gene 6′-aminoglycoside N-acetyltransferase in Ureaplasma. While U. parvum and U. urealyticum are very closely related, the refined protocol we utilised was consistently successful for U. parvum, but failed to deliver the antibiotic selection marker to all ATCC strains of U. urealyticum except for one clinical U. urealyticum strain. Genomic sequencing of several transposon insertions in U. parvum mutants showed that classical inverted repeat-bordered insertion was consistently achieved for some strains, but use of only 1 inverted repeat with retention of the transposase in the insert for a minority of other strains. Investigation of disrupted genes further showed that usually only one insertion of the transposon occurred in the genome at random locations.
We utilised a PEG-based transformation protocol when more recent transposon mutagenesis of M. bovis, M. agalactiae, M. mobile, M, pneumoniae, and M. hyopneumoniae have favoured electroporation (Maglennon et al. 2013, Baranowski et al. 2014, Shimizu et al. 2014, Sharma et al. 2014). However, Voelker and Dybvig (1996) found similar efficiencies in comparing transposon delivery via conjugation, PEG-based transformation and electroporation of M. arthritidis, including one strain where PEG-based transformation was successful when electroporation failed. However, they also found a wide variation in efficiency between strains. Our attempts at transferring tetracycline resistance to Ureaplasma spp. using conjugation with a tetM-positive Tn916-positive E. faecalis failed (data not shown) and we did not attempt electroporation methods for comparison.
Large scale analysis of random genome integration has been used for Mycoplasma spp. to determine the essential genes of a minimum genome, the underlying presumption being that essential genes cannot be disrupted. M. genitalium has the smallest genome and saturating Tn mutagenesis on this organism led to the proposal of a between 265-350 genes as being the minimum required to sustain self-replicating independent life (Hutchison et al., 1999). Further refinement of this method identified all 43 RNA-coding genes to be essential and 382 of 482 M. genitalium protein-coding genes to be essential for culture growth of M. genitalium (Glass et al., 2006). These studies were hugely influential in the construction of the minimum synthetically assembled genome based on M. genitalium in 2008 (Gibson et al., 2008). However, comparison of the smallest Mycoplasma and Ureaplasma spp., which vary in their use of glycolysis, arginine-metabolism and urea-metabolism for survival, showed U. parvum, M. hominis and M. genitalium only have an overlap of 247 coding sequences (Pereyre et al., 2009). These observations suggest that, much is yet to be learned from minimum genome analysis of the other mollicutes.
Comparison of 19 sequenced genomes for human Ureaplasma spp. have identified an average of 608 predicted genes for each U. parvum genome and 664 for U. urealyticum (Paralanov et al., 2012). A total of 1020 possible predicted protein coding genes, including singletons, were identified with a core conserved genome of 515 genes. Now that transposon mutagenesis is routinely successful with U. parvum, determination of non-essential genes in a microbe that does not use the glycolysis pathway will add empirical verification to the composition of a hypothetical minimum gene set. However, we acknowledge that our studies have not examined whether transposon insertion into the genome had altered gene expression. Two of the transposon-mutated strains showed phenotypic alteration. Further, we would predict that all of the other identified open-reading frame disruptions that we identified should result in a failure to express fully functional proteins, as the predicted open reading frames were disrupted between 150–704bp into the expected coding region. However, as they are hypothetical open reading frames of unknown function, they may not be expressed in parent strains. The one exception may be the integration between UU187(rpoB)) and UU188 (rpoC), both of which are predicted to be homologues of DNA-directed RNA polymerase subunit beta. The stop codon of UU187 and the intergenic region between the genes was conserved, thus expression of both of these genes may be conserved. In any event, no phenotypic alteration was observed for this mutant. Most of the disrupted genes were of unknown function and only listed as predicted open-reading frames. Genes UU390, UU450, and UU520 may encode membrane proteins and UU047 is predicted to encode a conserved hypothetical ATP/GTP-binding protein. UU526 is predicted to encode an MBA paralogue that should be expressed as a surface-associated membrane lipoprotein, as is UU440, which is also predicted to be a hypothetical membrane lipoprotein. While associated with an alteration in serum resistance, the gene UUR10_0137 is a predicted protein of unknown function.
We have also identified an intermediate level of gene class here, where disruption of the only annotated RNA helicase in the U. parvum genome (UU582) resulted in a significant physiological growth alteration that would likely affect the capacity of the resultant strain to survive in vivo. Removal of the DEAD RNA helicase gene from Mycoplasma mycoides subspecies capri also results in a viable but slow growing phenotype (jglass data not shown). No mutant in the orthologous gene was found for M, genitalium, but one was found in M. pneumoniae and M. pulmonis transposon bombardment studies. RNA helicases largely belong to superfamily two of the six families of nucleic acid helicases. The U. parvum RNA helicase in particular belongs to the DEAD-box family based on the signature sequence, Asp-Glu-Ala-Asp (Fairman-Williams et al., 2010). While many bacteria encode a few RNA helicases, a substantial number of sequenced bacterial genomes only contain a single DEAD-box helicase (Lu et al., 1999). Although E. coli encodes 5 RNA helicases, assessment following individual disruption of each found that the ΔdeaD mutation was primarily responsible for observed growth defects at 37°C including increased doubling time. The ΔdeaD (as well as the ΔsrmB) mutation in E. coli also exhibited a cold sensitive phenotype (Jagessar and Jain 2010), very similar to our observations for U. parvum.
We were unable to successfully deliver the mini-transposon to a range of U. urealyticum strains despite being performed in parallel with the same conditions and reagents used to successfully mutagenise U. parvum. The only exception was a single experiment where a clinical strain (W11) was successfully transformed resulting in delivery of the gentamicin resistance gene. This mutant exhibited our second observed phenotypic change: altered survival following serum challenge. Previously we have characterised the complement sensitivity of U. parvum strains and found that some strains (such as HPA5) are very sensitive and killed by seronegative serum, some strains are readily killed only by serum containing anti-Ureaplasma antibodies (such as HPA2 and DFK-1), and others are inherently resistant (Beeton et al., 2012). However, we did not observe any alteration of serum sensitivity or resistance of the mutagenised U. parvum strains compared to the parent strains in this study (data not shown). While we have not finished extending our studies of serum resistance of all U. urealyticum serovars as yet, characterisation of the W11 strain found it to be completely resistant to all previously characterised anti-Ureaplasma antibody containing (seropositive) sera. However, following transposon integration into the W11 genome, the resultant strain was sensitive to killing by seropositive sera. The altered phenotype was co-incident with loss of a 41 kDa protein detected by the human high titre anti-Ureaplasma serum used to challenge the Ureaplasma strains and the single transposon insertion site was found to disrupt the hypothetical gene UUR10_0137 (ATCC 33699 serovar 10 gene annotation numbering). This is a predicted membrane protein that is highly conserved in all U. parvum and U.u realyticum serovars. Further, there are highly homologous predicted open-reading frames in all the other non-haemoplasma members of the Mycoplasma pneumoniae group of mycoplasmas. The predicted mass of this open reading frame is 27 kDa, but the size may be increased by post-translational modification. It is also possible that the disruption of this gene may not have any direct bearing on the loss of the expression of the 41 kDa protein or alteration of serum resistance. Further experiments are required to determine if isolated expression of this gene is capable of solely mediating serum resistance.
The major surface antigen MBA (gene UU375 in ATCC strain 700970) has also previously been shown to be susceptible to phase variation following bacterial stress (Zimmerman et al., 2009 & 2011) or alteration of size (Robinson et al., 2013). We only observed one mutant with an alteration in MBA size and no mutants with loss of MBA expression. Therefore, the temperature-shock and selection in gentamicin associated with transformation do not appear to trigger phase variation and we would expect that loss of MBA expression would require gene disruption.
We have succeeded in developing a methodology that is capable of delivering a mini-transposon to the U. parvum genome, which results in random gene disruption. This report shows that analysis of transposon mutated Ureaplasma strains is now possible to determine non-essential genes, especially important for the numerous hypothetical open-reading frames identified in the analysis of 19 sequenced Ureaplasma spp. genomes (Paralanov et al., 2012). This methodology can also now be utilised to determine the minimal genome contingent for a bacterial class that do not utilise glycolysis to survive and shed further light on core essential genes. Characterisation of disrupted gene mutants with pathogenesis studies in experimental in utero model infections will also be a key to identifying pathogenic markers within the U. parvum genome, as it has been shown to initiate preterm labour and chronic lung disease in preterm neonates experimentally as a sole pathogen (Novy et al., 2009). Transposon mutagenesis will also be valuable in enabling delivery and expression of exogenous genes to U. parvum for in vivo tracking and possibly as a mucosal vaccine delivery tool of the future.
Supplementary Material
Figure 4.
Immunoblot analysis of SDS solubilised total bacterial protein probed with monoclonal anti-multiple banded antigen antibody. Of 26 individual transformation experiments only one strain (U6) showed altered mobility of the MBA following transposon mutagenesis (+Tn). Representative gel of several experiments is shown showing 3 strains of serovar (SV) 3 and 2 strains of serovar 6.
Acknowledgments
This research was supported by the Libyan Higher Education (Sebha University) through a PhD studentship to Mr. Aboklaish, and a grant from the US National Institute of Allergy and Infectious Diseases to Dr. Glass (R21 AI098057-02). These studies were supported funded by funding initiatives by the National Institute for Social Care and Health Research (NISCHR; research support from the Welsh Government) via the registered research group Microbial and Infection Translational Research Group (MITReG).
Footnotes
Disclosure of Conflict of Interests: The authors have no conflicts of interests to declare.
References
- Algire MA, Lartigue C, Thomas DW, Assad-Garcia N, Glass JI, Merryman C. New selectable marker for manipulating the simple genomes of Mycoplasma species. Antimicrob Agents Chemother. 2009;53(10):4429–32. doi: 10.1128/AAC.00388-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranowski E, Bergonier D, Sagné E, Hygonenq MC, Ronsin P, Berthelot X, Citti C. Experimental infections with Mycoplasma agalactiae identify key factors involved in host-colonization. PLoS One. 2014;9(4):e93970. doi: 10.1371/journal.pone.0093970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeton ML, Chalker VJ, Maxwell NC, Kotecha S, Spiller OB. Concurrent titration and determination of antibiotic resistance in ureaplasma species with identification of novel point mutations in genes associated with resistance. Antimicrob Agents Chemother. 2009;53(5):2020–7. doi: 10.1128/AAC.01349-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beeton ML, Daha MR, El-Shanawany T, Jolles SR, Kotecha S, Spiller OB. Serum killing of Ureaplasma parvum shows serovar-determined susceptibility for normal individuals and common variable immuno-deficiency patients. Immunobiology. 2012;217(2):187–94. doi: 10.1016/j.imbio.2011.07.009. [DOI] [PubMed] [Google Scholar]
- de Barbeyrac B, Dupon M, Rodriguez P, Renaudin H, Bébéar C. A Tn1545-like transposon carries the tet(M) gene in tetracycline resistant strains of Bacteroides ureolyticus as well as Ureaplasma urealyticum but not Neisseria gonorrhoeae. J Antimicrob Chemother. 1996;37(2):223–32. doi: 10.1093/jac/37.2.223. [DOI] [PubMed] [Google Scholar]
- Dybvig K, Alderete J. Transformation of Mycoplasma pulmonis and Mycoplasma hyorhinis: transposition of Tn916 and formation of cointegrate structures. Plasmid. 1988;20(1):33–41. doi: 10.1016/0147-619x(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Dybvig K, Cassell GH. Transposition of gram-positive transposon Tn916 in Acholeplasma laidlawii and Mycoplasma pulmonis. Science. 1987;235(4794):1392–4. doi: 10.1126/science.3029869. [DOI] [PubMed] [Google Scholar]
- Fairman-Williams ME, Guenther UP, Jankowsky E. SF1 And SF2 helicases: family matters. Curr Opin Structr Biol. 2010;20:313–24. doi: 10.1016/j.sbi.2010.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DG, Benders GA, Andrews-Pfannkoch C, Denisova EA, Baden-Tillson H, Zaveri J, Stockwell TB, Brownley A, Thomas DW, Algire MA, Merryman C, Young L, Noskov VN, Glass JI, Venter JC, Hutchison CA, 3rd, Smith HO. Complete chemical synthesis, assembly, and cloning of a Mycoplasma genitalium genome. Science. 2008;319(5867):1215–20. doi: 10.1126/science.1151721. [DOI] [PubMed] [Google Scholar]
- Glass JI, Assad-Garcia N, Alperovich N, Yooseph S, Lewis MR, Maruf M, Hutchison CA, 3rd, Smith HO, Venter JC. Essential genes of a minimal bacterium. Proc Natl Acad Sci U S A. 2006;103(2):425–30. doi: 10.1073/pnas.0510013103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govender S, Gqunta K, le Roux M, de Villiers B, Chalkley LJ. Antibiotic susceptibilities and resistance genes of Ureaplasma parvum isolated in South Africa. J Antimicrob Chemother. 2012;67(12):2821–4. doi: 10.1093/jac/dks314. [DOI] [PubMed] [Google Scholar]
- Hutchison CA, Peterson SN, Gill SR, Cline RT, White O, Fraser CM, Smith HO, Venter JC. Global transposon mutagenesis and a minimal Mycoplasma genome. Science. 1999;286(5447):2165–9. doi: 10.1126/science.286.5447.2165. [DOI] [PubMed] [Google Scholar]
- Jagessar KL, Jain C. Functional and molecular analysis of Eschericia coli strains lacking multiple DEAD-box helicases. RNA. 2010;16(7):1386–92. doi: 10.1261/rna.2015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King KW, Dybvig K. Plasmid transformation of Mycoplasma mycoides subspecies mycoides is promoted by high concentrations of polyethylene glycol. Plasmid. 1991;26(2):108–15. doi: 10.1016/0147-619x(91)90050-7. [DOI] [PubMed] [Google Scholar]
- Lu J, Aoki H, Ganoza MC. Molecular characterization of a prokaryotic translation factor homologues to the eukaryotic initiation factor eIF4A. Int J Biochem Cell Biol. 1999;31:215–29. doi: 10.1016/s1357-2725(98)00142-3. [DOI] [PubMed] [Google Scholar]
- Maglennon GA, Cook BS, Deeney AS, Bossé JT, Peters SE, Langford PR, Maskell DJ, Tucker AW, Wren BW, Rycroft AN. Transposon mutagenesis in Mycoplasma hyopneumoniae using a novel mariner-based system for generating random mutations. Vet Res. 2013;44:124. doi: 10.1186/1297-9716-44-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahairas GG, Minion FC. Random insertion of the gentamicin resistance transposon Tn4001 in Mycoplasma pulmonis. Plasmid. 1989a;21(1):43–7. doi: 10.1016/0147-619x(89)90085-1. [DOI] [PubMed] [Google Scholar]
- Mahairas GG, Minion FC. Transformation of Mycoplasma pulmonis: demonstration of homologous recombination, introduction of cloned genes, and preliminary description of an integrating shuttle system. J Bacteriol. 1989b;171(4):1775–80. doi: 10.1128/jb.171.4.1775-1780.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardassi BB, Aissani N, Moalla I, Dhahri D, Dridi A, Mlik B. Evidence for the predominance of a single tet(M) gene sequence type in tetracycline-resistant Ureaplasma parvum and Mycoplasma hominis isolates from Tunisian patients. J Med Microbiol. 2012;61(Pt 9):1254–61. doi: 10.1099/jmm.0.044016-0. [DOI] [PubMed] [Google Scholar]
- Novy MJ, Duffy L, Axthelm MK, Sadowsky DW, Witkin SS, Gravett MG, Cassell GH, Waites KB. Ureaplasma parvum or Mycoplasma hominis as sole pathogens cause chorioamnionitis, preterm delivery, and fetal pneumonia in rhesus macaques. Reprod Sci. 2009;16(1):56–70. doi: 10.1177/1933719108325508. [DOI] [PubMed] [Google Scholar]
- Owttrim GW. RNA helicases: diverse roles in prokaryotic response to abiotic stress. RNA Biol. 2013;10(1):96–110. doi: 10.4161/rna.22638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paralanov V, Lu J, Duffy LB, Crabb DM, Shrivastava S, Methé BA, Inman J, Yooseph S, Xiao L, Cassell GH, Waites KB, Glass JI. Comparative genome analysis of 19 Ureaplasma urealyticum and Ureaplasma parvum strains. BMC Microbiol. 2012;30(12):88. doi: 10.1186/1471-2180-12-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereyre S, Sirand-Pugnet P, Beven L, Charron A, Renaudin H, Barré A, Avenaud P, Jacob D, Couloux A, Barbe V, de Daruvar A, Blanchard A, Bébéar C. Life on arginine for Mycoplasma hominis: clues from its minimal genome and comparison with other human urogenital mycoplasmas. PLoS Genet. 2009;5(10):e1000677. doi: 10.1371/journal.pgen.1000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson JW, Dando SJ, Nitsos I, Newnham J, Polglase GR, Kallapur SG, Pillow JJ, Kramer BW, Jobe AH, Payton D, Knox CL. Ureaplasma parvum serovar 3 multiple banded antigen size variation after chronic intra-amniotic infection/colonization. PLoS One. 2013;8(4):e62746. doi: 10.1371/journal.pone.0062746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MC, Kenny GE. Conjugal transfer of transposon Tn916 from Streptococcus faecalis to Mycoplasma hominis. J Bacteriol. 1987;169(8):3836–9. doi: 10.1128/jb.169.8.3836-3839.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts MC. Characterization of the Tet M determinants in urogenital and respiratory bacteria. Antimicrob Agents Chemother. 1990;34(3):476–8. doi: 10.1128/aac.34.3.476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson JA, Stemke GW, Davis JW, Jr, Harasawa R, Thirkell D, Kong F, Shepard MC, Ford DK. Proposal of Ureaplasma parvum sp. nov. and amended description of Ureaplasma urealyticum. Int J Syst Evol Microbiol. 2002;52(Pt 2):587–97. doi: 10.1099/00207713-52-2-587. [DOI] [PubMed] [Google Scholar]
- Romano N, Tolone G, Ajello F, La Licata R. Adenosine 5′-triphosphate synthesis induced by urea hydrolysis in Ureaplasma urealyticum. J Bacteriol. 1980;144(2):830–2. doi: 10.1128/jb.144.2.830-832.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma S, Markham PF, Browning GF. Genes Found Essential in Other Mycoplasmas Are Dispensable in Mycoplasma bovis. PLoS One. 2014;9(6):e97100. doi: 10.1371/journal.pone.0097100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T, Kimura Y, Kida Y, Kuwano K, Tachibana M, Hashino M, Watarai M. Cytadherence of Mycoplasma pneumoniae Induces Inflammatory Responses through Autophagy and Toll-Like Receptor 4. Infect Immun. 2014;82(7):3076–3086. doi: 10.1128/IAI.01961-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DG, Russell WC, Ingledew WJ, Thirkell D. Hydrolysis of urea by Ureaplasma urealyticum generates a transmembrane potential with resultant ATP synthesis. J Bacteriol. 1993;175(11):3253–8. doi: 10.1128/jb.175.11.3253-3258.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung TJ, Xiao L, Duffy L, Waites KB, Chesko KL, Viscardi RM. Frequency of ureaplasma serovars in respiratory secretions of preterm infants at risk for bronchopulmonary dysplasia. Pediatr Infect Dis J. 2011;30(5):379–83. doi: 10.1097/INF.0b013e318202ac3a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraskina AE, Savicheva AM, Akopian TA, Soroka AE, Momynaliev KT, Govorun VM. Drift of tetM determinant in urogenital microbiocenosis containing mycoplasmas during treatment with a tetracycline antibiotic. Bull Exp Biol Med. 2002;134(1):60–3. doi: 10.1023/a:1020664807029. [DOI] [PubMed] [Google Scholar]
- Voelker LL, Dybvig K. Characterization of the lysogenic bacteriophage MAV1 from Mycoplasma arthritidis. J Bacteriol. 1996;178(20):6078–81. doi: 10.1128/jb.180.22.5928-5931.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waites KB, Katz B, Schelonka RL. Mycoplasmas and ureaplasmas as neonatal pathogens. Clin Microbiol Rev. 2005 Oct;18(4):757–89. doi: 10.1128/CMR.18.4.757-789.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]; J Bacteriol. 175(11):3253–8. doi: 10.1128/jb.175.11.3253-3258.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman CU, Herrmann R. Synthesis of a small, cysteine-rich, 29 amino acids long peptide in Mycoplasma pneumoniae. FEMS Microbiol Lett. 2005;253(2):315–21. doi: 10.1016/j.femsle.2005.09.054. [DOI] [PubMed] [Google Scholar]
- Zimmerman CU, Rosengarten R, Spergser J. Ureaplasma antigenic variation beyond MBA phase variation: DNA inversions generating chimeric structures and switching in expression of the MBA N-terminal paralogue UU172. Mol Microbiol. 2011;79(3):663–76. doi: 10.1111/j.1365-2958.2010.07474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman CU, Stiedl T, Rosengarten R, Spergser J. Alternate phase variation in expression of two major surface membrane proteins (MBA and UU376) of Ureaplasma parvum serovar 3. FEMS Microbiol Lett. 2009;292(2):187–93. doi: 10.1111/j.1574-6968.2009.01505.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.