Abstract
Achromatopsia (ACHM) is caused by a progressive loss of cone photoreceptors leading to color blindness and poor visual acuity. Animal studies and human clinical trials have shown that gene replacement therapy with adeno-associate virus (AAV) is a viable treatment option for this disease. Although there have been successful attempts to optimize capsid proteins for increased specificity, it is simpler to restrict expression via the use of cell type-specific promoters. To target cone photoreceptors, a chimeric promoter consisting of an enhancer element of inter-photoreceptor retinoid-binding protein promoter and a minimal sequence of the human transducin alpha-subunit promoter (IRBPe/GNAT2) was created. Additionally, a synthetic transducin alpha-subunit promoter (synGNAT2/GNAT2) containing conserved sequence blocks located downstream of the transcriptional start was created. The strength and specificity of these promoters were evaluated in murine retina by immunohistochemistry. The results showed that the chimeric, (IRBPe/GNAT2) promoter is more efficient and specific than the synthetic, synGNAT2/GNAT2 promoter. Additionally, IRBPe/GNAT2-mediated expression was found in all cone subtypes and it was improved over existing promoters currently used for gene therapy of achromatopsia.
Keywords: Cone photoreceptors, Chimeric promoter, Adeno-associated virus, Targeted expression, Gene replacement therapy, Achromatopsia
87.1 Introduction
Achromatopsia (ACHM) is an autosomal recessive disease affecting about 1:30,000 humans. It is characterized by poor visual acuity, loss of central vision, photophobia, color blindness, and reduced photopic electroretinographic amplitudes due to loss of cone photoreceptors. In humans there are three distinct subclasses of cone photoreceptors, each named for the specific wavelength of light to which they respond. L-cones respond to long wavelength light, whereas M-cones and S-cones respond to medium and short wavelength light, respectively. Spectral sensitivity is mediated by the specific form of cone opsin that each cone subclass expresses. Gene therapy-based treatments for a number of diseases affecting cone photoreceptors are currently under development [1–6].
Although ACHM is a relatively rare disorder, it presents itself as a good target for gene therapy as the causative genes are known. Additionally, proof-of-concept gene replacement studies in animal models have shown clear success [1, 3–5, 7, 8]. ACHM affects all classes of photoreceptors, including S cones. Recent evidence from case studies of patients with ACHM suggests that ACHM is progressive, with cones degenerating over time [9]. Therefore, early intervention with a therapy that targets all subclasses of cone photoreceptors would be ideal.
In recent years, adeno-associated virus (AAV) has emerged as the most efficient viral vector for gene therapy due to its ability to transduce dividing and non-dividing cells with minimal immune response[10]. Although there have been successful attempts to optimize AAV capsid proteins for increased cell specificity, a simpler way to restrict expression is through the use of cell type-specific promoters. To deliver genes safely and efficiently to cone photoreceptors of ACHM-affected individuals, therapeutic vectors should ideally utilize promoters which; (1) are capable of expressing transgene both efficiently and selectively in cones with little or no off-target expression in other retinal cell types, such as rod or retinal pigment epithelial (RPE) cells, (2) are small enough in size to accommodate the transgene within the ~ 4.8 kb (kilobases) packaging limit of AAV (3) are able to drive gene expression in all subclasses of cone photoreceptors.
To date, cone targeting promoters used in proof-of-concept gene therapy studies for ACHM have been deficient in one or more of these criteria. In studies by Alexander et al. [7] and Komaromy et al. [8]. A 2100 bp (base pair) version of the human red/green opsin promoter (PR2.1) was used to drive therapeutic transgene expression. In mouse, expression was limited primarily to cones with sparse expression in rods [7]. In canine, expression was highly selective for M and L cones whereas expression in S cones was not detected [8]. Furthermore the PR2.1 promoter is relatively large in size. In the case of CNGB3 gene replacement, the AAV capsid is barely able to accommodate the PR2.1 promoter and CNGB3 cDNA (~ 2.4 kb). Such vectors may experience reduced packaging efficiency because of the oversized nature of the genome.
In addition to the PR2.1 promoter, respective, homologous regulatory regions of human and mouse S cone opsin have been tested as promoters in animal models of ACHM. In rodent, a 569 bp human S opsin promoter (HB569) led to reporter gene expression in all cone subclasses but the expression was weaker in comparison to the PR2.1 promoter [11]. In dog, the HB569 promoter performed poorly in terms of both specificity and efficiency, with relatively few L/M cones expressing the trans-gene and with leaky expression in rods and RPE [12].
A 500 bp version of the mouse S opsin (mBP) promoter has been tested in the context of gene replacement for CNGA3 and performed well [3]. However it is likely that it, like the closely related human S opsin promoter, will perform poorly in higher order mammals, such as dog and human.
Finally, like the S opsin promoters described above, regulatory regions of cone arrestin identified by Craft et al. has been utilized as a promoter in AAV transduction experiments and later in gene replacement studies in ACHM animal models [1, 13]. In experiments performed in mice aimed at characterizing gene expression, respective 500 bp mouse and human cone arrestin promoters (mCAR and hCAR) drove strong expression in retina (see Results section). However specificity was poor, with rods and RPE clearly being transduced. In experiments utilizing mCAR that were performed in dog, the same general expression pattern was seen, with strong expression observed in all classes of cones and off-target expression in rods and RPE (Komáromy and Boye, unpublished).
In this work, we present data on novel cone-targeted promoters showing improved efficiency and specificity compared to existing promoters.
87.2 Materials and Methods
87.2.1 Promoter Construction
A chimeric promoter (IRBPe/GNAT2) was constructed by fusing an enhancer element (IRBPe) from position − 1619 to − 1411 of the interphotoreceptor retinoid-binding protein (IRBP) to a core promoter of human transducin alpha-subunit (GNAT2) ranging from position − 151 to + 126. Additionally a synthetic promoter (synGNAT2/GNAT2) was created by aligning the GNAT2 regulatory regions of multiple taxa (human, canine, mouse, and rat) using ClustalW and selecting conserved areas to add to the core promoter region. Both promoters were cloned in a vector plasmid to drive the expression of green fluorescent protein (GFP). Additionally a 0.5 kb version of the human and mouse cone arrestin promoter was created [1, 13]. The arrangement of the promoter constructs used in this study are depicted in Fig. 87.1.
Fig. 87.1.
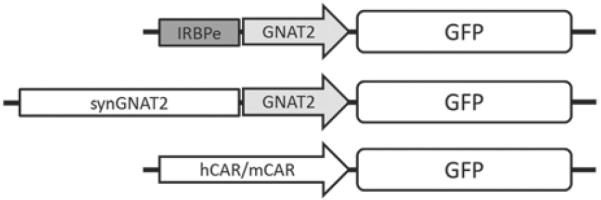
Schematic representation of the IRBPe/GNAT2 chimeric promoter containing the IRBPe enhancer element and the minimal GNAT2 promoter (top), the synthetic (synGNAT2) addition of conserved areas to the core GNAT2 promoter (middle) and the cone arrestin promoter regions of human (hCAR) and mouse (mCAR) species (bottom)
87.2.2 Virus Packaging and Injection
AAV vectors were packaged, purified, and titered according to previously published methods [14, 15]. C57BL/6 mice were injected subretinally with 1 μl of at least 5 × 1012 virus genomes/ml of AAV5 vectors. Mice were injected at 4 to 5 weeks of age and promoter expression and tropism were analyzed by confocal microscopy 4 weeks post-injection.
All experiments were approved by the University of Florida's IACUC and conducted in accordance with the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and NIH regulations.
87.2.3 Histology
Injected mice and uninjected controls were sacrificed 4 weeks post-injection and the eyes were surgically removed and fixed in 4 % paraformaldehyde. Eyes were then treated with sucrose buffer of increasing concentrations of sucrose (from 5 to 20 %) in phosphate buffered saline (PBS). After removing cornea and lens, the remaining eye cups were embedded in OCT (Sakura Finetek, Torrance, CA) and frozen tissue was then sectioned for analysis with a spinning disk confocal microscope (Olympus).
87.3 Results
To determine the efficiency and specificity of the promoters, C57BL/6 wild-type mice were injected with AAV5 containing the respective promoters driving GFP expression. Figure 87.2a, b show strong expression of GFP in the outermost nuclear region of the outer nuclear layer of the mouse retina which is known to contain the cell bodies of cone photoreceptor cells. The synthetic human transducin alpha-subunit (synGNAT2/GNAT2) promoter, however, shows transduction in a relatively fewer number of cells suggesting that parts of the synGNAT2 sequence act as a negative regulator in some cells (Fig. 87.2c, d). Expression in RPE or the inner retinal cell layers was not observed with either promoter. To evaluate whether IRBPe/GNAT2 would promote expression in S-cones, Nrl−/− mice were injected with AAV-IRBPe/GNAT-GFP vector. The retinas of Nrl−/− mice are composed exclusively of S cone-like photoreceptors [16, 17]. The strong, photoreceptor exclusive expression observed in Nrl −/− mice clearly support that IRBPe/GNAT2 is capable of transducing mouse S-cones (Fig. 87.2e). In contrast to the GNAT2 promoters, the mouse and human cone arrestin promoters (Fig. 87.3) resulted in strong off-target expression in the retinal pigment epithelium.
Fig. 87.2.
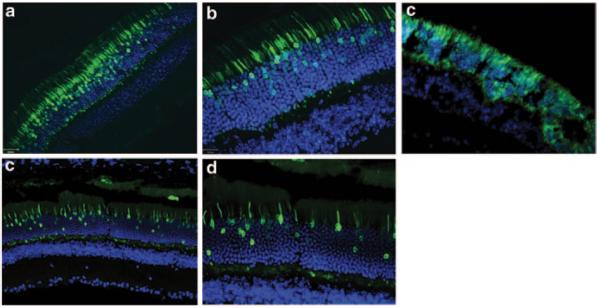
a–d show sections of C57BL/6 mouse retina treated subretinally with AAV5-IRBPe/GNAT2-GFPat 20 × (a) and 40 × (b) magnification and treated with AAV5-synGNAT2/GNAT2-GFP at 20 × (c) and 40 × (d) magnification. (e) Sections of Nrl−/− mouse treated subretinally with AAV5-IRBPe/GNAT2-GFP
Fig. 87.3.
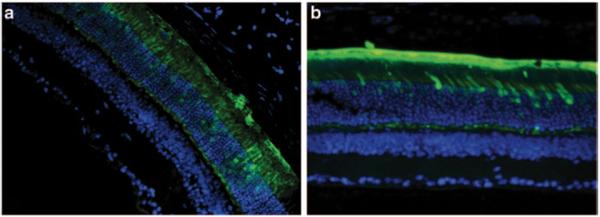
Sections of mouse retina were treated with AAV5-mCAR-GFP (a) and hCAR-GFP (b) at 20 × magnification
87.4 Discussion
In this study, an enhancer element of the interphotoreceptor retinoid-binding protein was combined with a minimal human transducin alpha-subunit promoter (IRBPe/GNAT2) and used to achieve selective expression in cone photoreceptors. Ying et al. [18] created a transgenic mouse line in which the same elements in a different configuration were used to drive chloramphenicol acetyltransferase to ablate cone photoreceptors [19]. The resulting transgenic mouse lacked cone photoreceptors. However, in ventral retina, rod photoreceptors were also absent [20]. The region-specific absence of rod photoreceptors was reported as a consequence of developmental defect due to lack of cones. However, given that only ~ 2.5 % of photoreceptors are cones, loss of rods was more likely due to mis-expression of the diphtheria toxin in rods. In contrast, when the IRBP enhancer element is fused upstream of the minimal GNAT2 promoter to create a chimeric promoter, expression in rods is reduced. Like IRBPe/GNAT2, a synthetic transducin alpha-subunit promoter fused to the minimal promoter (synGNAT2/GNAT2) also transduced cones, but not as efficiently. Both promoters exhibit some off-target expression in a small number of rods but not in retinal pigment epithelium or inner retinal layers. The relatively small size the IRBPe/GNAT2 chimeric promoter (less than 500 bp) and its ability to promote strong, specific expression in all cone subclasses improve on existing promoters like PR2.1. Other promoters like the cone arrestin promoters are an improvement in size and strength, however they mediate strong off-target transgene expression.
Even though both versions of the GNAT2 promoter show activity in a small number of rod photoreceptors, higher selectivity of cone expression may be likely in higher mammals. In summary, both promoters are useful tools for gene replacement therapy targeting cone photoreceptor cells in human patients.
Acknowledgment
The authors thank Seok-Hong Min, James Peterson, and Jingfen Sun for their excellent technical assistance. They also acknowledge grants from the Foundation Fighting Blindness, Research to Prevent Blindness, Inc. for partial support of this work.
References
- 1.Carvalho LS, Xu J, Pearson RA, Smith AJ, Bainbridge JW, Morris LM, et al. Long-term and age-dependent restoration of visual function in a mouse model of CNGB3-associated achromatopsia following gene therapy. Hum Mol Genet. 2011;20(16):3161–3175. doi: 10.1093/hmg/ddr218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cideciyan AV, Hauswirth WW, Aleman TS, Kaushal S, Schwartz SB, Boye SL, et al. Human RPE65 gene therapy for Leber congenital amaurosis: persistence of early visual improvements and safety at 1 year. Hum Gene Ther. 2009;20(9):999–1004. doi: 10.1089/hum.2009.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Michalakis S, Muhlfriedel R, Tanimoto N, Krishnamoorthy V, Koch S, Fischer MD, et al. Restoration of cone vision in the CNGA3−/− mouse model of congenital complete lack of cone photoreceptor function. Mol Ther. 2010;18(12):2057–2063. doi: 10.1038/mt.2010.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michalakis S, Muhlfriedel R, Tanimoto N, Krishnamoorthy V, Koch S, Fischer MD, et al. Gene therapy restores missing cone-mediated vision in the CNGA3−/− mouse model of achromatopsia. Adv Exp Med Biol. 2012;723:183–189. doi: 10.1007/978-1-4614-0631-0_25. [DOI] [PubMed] [Google Scholar]
- 5.Pang JJ, Deng WT, Dai X, Lei B, Everhart D, Umino Y, et al. AAV-mediated cone rescue in a naturally occurring mouse model of CNGA3-achromatopsia. PLoS.One. 2012;7(4):e35250. doi: 10.1371/journal.pone.0035250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mancuso K, Hauswirth WW, Li Q, Connor TB, Kuchenbecker JA, Mauck MC, et al. Gene therapy for red-green colour blindness in adult primates. Nature. 2009;461(7265):784–787. doi: 10.1038/nature08401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alexander JJ, Umino Y, Everhart D, Chang B, Min SH, Li Q, et al. Restoration of cone vision in a mouse model of achromatopsia. Nat Med. 2007;13(6):685–687. doi: 10.1038/nm1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Komaromy AM, Alexander JJ, Rowlan JS, Garcia MM, Chiodo VA, Kaya A, et al. Gene therapy rescues cone function in congenital achromatopsia. Hum Mol Genet. 2010;19(13):2581–2593. doi: 10.1093/hmg/ddq136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thiadens AA, Somervuo V, van den Born LI, Roosing S, van Schooneveld MJ, Kuijpers RW, et al. Progressive loss of cones in achromatopsia: an imaging study using spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2010;51(11):5952–5957. doi: 10.1167/iovs.10-5680. [DOI] [PubMed] [Google Scholar]
- 10.Daya S, Berns KI. Gene therapy using adeno-associated virus vectors. Clin Microbiol Rev. 2008;21(4):583–593. doi: 10.1128/CMR.00008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glushakova LG, Timmers AM, Pang J, Teusner JT, Hauswirth WW. Human blue-opsin promoter preferentially targets reporter gene expression to rat s-cone photoreceptors. Invest Ophthalmol Vis Sci. 2006;47(8):3505–3513. doi: 10.1167/iovs.05-1670. [DOI] [PubMed] [Google Scholar]
- 12.Komaromy AM, Alexander JJ, Cooper AE, Chiodo VA, Glushakova LG, Acland GM, et al. Targeting gene expression to cones with human cone opsin promoters in recombinant AAV. Gene Ther. 2008;15(14):1049–1055. doi: 10.1038/gt.2008.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li A, Zhu X, Craft CM. Retinoic acid upregulates cone arrestin expression in retinoblastoma cells through a Cis element in the distal promoter region. Invest Ophthalmol Vis Sci. 2002;43(5):1375–1383. [PubMed] [Google Scholar]
- 14.Jacobson SG, Acland GM, Aguirre GD, Aleman TS, Schwartz SB, Cideciyan AV, et al. Safety of recombinant adeno-associated virus type 2-RPE65 vector delivered by ocular sub-retinal injection. Mol Ther. 2006;13(6):1074–1084. doi: 10.1016/j.ymthe.2006.03.005. [DOI] [PubMed] [Google Scholar]
- 15.Zolotukhin S, Potter M, Zolotukhin I, Sakai Y, Loiler S, Fraites TJ, Jr, et al. Production and purification of serotype 1, 2, and 5 recombinant adeno-associated viral vectors. Methods. 2002;28(2):158–167. doi: 10.1016/s1046-2023(02)00220-7. [DOI] [PubMed] [Google Scholar]
- 16.Daniele LL, Lillo C, Lyubarsky AL, Nikonov SS, Philp N, Mears AJ, et al. Cone-like morphological, molecular, and electrophysiological features of the photoreceptors of the Nrl knockout mouse. Invest Ophthalmol Vis Sci. 2005;46(6):2156–2167. doi: 10.1167/iovs.04-1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nikonov SS, Daniele LL, Zhu X, Craft CM, Swaroop A, Pugh EN., Jr Photoreceptors of Nrl −/− mice coexpress functional S- and M-cone opsins having distinct inactivation mechanisms. J Gen Physiol. 2005;125(3):287–304. doi: 10.1085/jgp.200409208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ying S, Fong SL, Fong WB, Kao CW, Converse RL, Kao WW. A CAT reporter construct containing 277 bp GNAT2 promoter and 214 bp IRBP enhancer is specifically expressed by cone photoreceptor cells in transgenic mice. Curr Eye Res. 1998;17(8):777–782. [PubMed] [Google Scholar]
- 19.Ying S, Jansen HT, Lehman MN, Fong SL, Kao WW. Retinal degeneration in cone photoreceptor cell-ablated transgenic mice. Mol Vis. 2000;6:101–108. [PubMed] [Google Scholar]
- 20.Fong SL, Criswell MH, Belecky-Adams T, Fong WB, McClintick JN, Kao WW, et al. Characterization of a transgenic mouse line lacking photoreceptor development within the ventral retina. Exp Eye Res. 2005;81(4):376–388. doi: 10.1016/j.exer.2005.06.007. [DOI] [PubMed] [Google Scholar]


