Abstract
To describe relapsed B-cell lymphoma or leukemia in children/adolescents treated with a “Lymphomes Malins B” regimen and their outcome and to identify prognostic factors for survival, we studied relapses in the LMB89, 96 and 2001 studies of the Société Française d’Oncologie Pédiatrique (Société Française des Cancers de l’Enfant). Therapeutic guidelines at relapse were to obtain a second complete remission and to consolidate the remission with high-dose chemotherapy followed by autologous stem-cell transplantation. Between July 1989 and March 2007, 67 patients of 1322 (5%) relapsed: 57 had Burkitt lymphoma and 10 had large-cell histology. Three patients were initially treated in risk group A, 41 in group B and 23 in group C. Thirty-three patients had a relapse in one site (15 in the central nervous system) and 34 at multiple sites. Sixty-five patients received salvage chemotherapy and 33 achieved complete remission. Forty-one patients also received high-dose chemotherapy followed by autologous (n=33) or allogeneic (n=8) transplantation. With a median follow-up of 6.4 years, the 5-year survival rate was 29.9%. Nineteen patients were still alive, all but one (group A) received consolidation treatment. Multivariate analysis showed the following factors to be significantly associated with better survival: relapse at one site (P=0.0006), large-cell histology (P=0.012), initial prognostic group A or B with lactate dehydrogenase level below twice the normal value (P=0.005), and time to relapse more than 6 months (P=0.04).
Introduction
Survival following childhood B-cell non-Hodgkin lymphoma (B-NHL) [i.e. Burkitt lymphoma (BL) and diffuse large B-cell lymphoma (DLBCL)] or mature B-cell leukemia (B-AL) has increased dramatically from a rate of approximately 35% to 90% during recent decades, particularly in France with the Lymphomes Malins B (LMB) protocols of the Société Française d’Oncologie Pédiatrique (SFOP)/Société Française des Cancers de l’Enfant (SFCE). These protocols include dose escalation of anti-neoplastic agents, such as cyclophosphamide, high-dose (HD) methotrexate and cytarabine.1–6
The few published studies on the prognosis of relapsed B-NHL/B-AL in children7–11 show poor outcomes after intensive initial treatment. However, certain studies have reported that treatment with HD chemotherapy with autologous hematopoietic stem cell transplantation (HSCT) cured some patients with relapsed B-NHL/B-AL.8–10,12–16 The role of adjuvant CD20 monoclonal antibodies remains to be defined.11,17–19
In this study, we retrospectively reviewed relapses in patients enrolled in one of the three recent LMB studies. Cases of primary refractory B-NHL/B-AL were not considered. The aim was to describe the relapses, to analyze the results of the therapeutic strategy, and to identify prognostic factors for survival after relapse.
Methods
Patients
We retrospectively reviewed relapsed B-NHL/B-AL in French, Belgian, and Dutch patients prospectively enrolled in the SFOP/SFCE LMB89,2 French-American-British (FAB)/LMB96,3,5,6 and LMB2001 studies between July 1989 and March 2007. From the FAB/LMB96 study, only SFOP patients were included in the current study. The studies were approved by the SFOP scientific committee or/and National Ethics Committee. Parents/legal guardians provided informed consent for inclusion of their children in the studies in accordance with the Helsinki Declaration. Relapse was defined as any tumor progression after achieving complete remission.
Initial treatment
In each of the three studies, patients were assigned to one of three treatment groups (A, B and C) based on the stage of initial disease,2,3,5,6 and received two (group A), four or five (group B), or eight (group C) courses of chemotherapy. Rituximab was not used in initial treatment. Group A patients (completely resected stage I and abdominal stage II) did not receive central nervous system (CNS) prophylaxis (no intrathecal treatment, no HD methotrexate). Patients in group C (stage IV with CNS involvement and B-AL) received HD methotrexate at a dose of 8 g/m2, and consolidation courses which consisted of HD cytarabine and etoposide (CYVE). In group B (all patients not in group A or group C), patients received HD methotrexate at a dose of 3 g/m2 and cytarabine in a 5-day continuous infusion during consolidation. Group B patients were switched to group C if tumor regression was less than 20% 7 days after the pre-phase COP (cyclophosphamide, oncovin, and prednisone), or if complete remission was not achieved after the first course of consolidation. There were only minor differences among the three studies, allowing the results to be combined and analyzed (see Online Supplementary Material).
Recommendations for treatment of relapse
Although there was no prospective trial for treatment of relapse within the LMB protocols, there were general therapeutic recommendations. The first one was to obtain a second complete remission with salvage chemotherapy, based on the previous therapy: group C therapy/CYVE course for group A patients, and CYVE courses for group B patients. Salvage chemotherapy was more heterogeneous for patients who had already received group C therapy (initially or after switching from group B), depending on the time period of the study and the type of relapse: most patients underwent therapy with VENOMID (vindesine, novantrone, methylprednisolone, ifosfamide), ICN (ifosfamide, carboplatin and novantrone) or ICE (ifosfamide, carboplatin, etoposide, and triple intrathecal therapy). Treatment recommended for CNS relapse was two weekly courses of HD methotrexate (8 or 12 g/m2 as 24-hour infusions), with intrathecal therapy, potentially followed by another chemotherapy regimen.
The second recommendation was to consolidate the second complete remission with HD chemotherapy and autologous HSCT. The HD chemotherapy was most often either BEAM (BCNU, VP-16, aracytine and melphalan) or BAM (busulfan, aracytine, and melphalan), depending on the histology, time period of the study, and type of relapse. However, some investigators preferred total body irradiation-containing regimens with allogeneic HSCT. The anti-CD20 monoclonal antibody (rituximab) was added to the salvage chemotherapy for some patients after 1996.
Other details on patients, histopathology/immunophenotyping, responses, statistics and HD chemotherapy regimens are presented in the Online Supplementary Methods and Online Supplementary Table SA.
Results
Patients’ initial characteristics
Sixty-seven of 1322 patients (5%) (27/562, 23/383 and 17/377 in LMB89, 96 and 2001, respectively) relapsed: 57 with BL, six with DLBCL and four with primary mediastinal B-cell lymphoma (PMBL). Their characteristics at diagnosis are presented in Table 1. The majority had advanced stage disease. Twenty-five patients had bone marrow and 12 had CNS involvement. Forty-seven (70%) patients had a lactate dehydrogenase (LDH) level > twice the normal level (2N): 0 in group A, 66% in group B and 87% in group C. Three group B patients with stage III and LDH>2N were switched to group C after COP (n=2) or the first consolidation course (n=1).
Table 1.
Patients’ initial characteristics and relapse modalities, overall and according to histology.
Characteristics of relapses
The median age at time of relapse was 9.6 years (range, 1–19.6 years). The median time to relapse after diagnosis was 4.8 months (range, 2.3–14.1 months) in BL and 22.1 months (range, 4.2–32.2 months) in all patients with large-cell histology (DLBCL and PMBL). Relapse occurred in one site in 33 patients, while 34 patients had relapse in multiple sites [including bone marrow (n=25) or CNS (n=10)]. Relapse occurred in both bone marrow and the CNS in eight patients (Table 1).
Salvage chemotherapy
Two FAB/LMB96 patients did not receive salvage chemotherapy: one group B patient considered to need palliative care and one group C patient considered to be in complete remission after excision of the abdominal mass seen at relapse. The other 65 patients received one or more (maximum 3) lines of rescue chemotherapy, depending on the initial treatment group (Table 2). Sixteen patients [11 group B and 5 group C; 12 BL (8 group B, 4 group C), 3 DLBCL, 1 PMBL] also received rituximab (4 in FAB/LMB96 and 12 in LMB2001).
Table 2.
Characteristics of salvage chemotherapy and of consolidation with type of graft.
Response to salvage chemotherapy
One group A patient died of toxicity after the first CYVE course. Thirty-two patients were considered to be in complete remission after salvage chemotherapy, 26 after firstline treatment and six after another line. Including the patient treated by surgery only, 33 patients (49%) were considered to be in complete remission after salvage treatment. Eight patients were in partial remission, two had stable disease and 22 had progressive disease.
First salvage response (complete + partial remission) rates were 67% (2/3 patients) with the group C regimen/CYVE after group A, 66% (19/29 patients) with CYVE after group B and 60% (3/5 patients) with ICE after group C therapy. Of the responding patients, 2/2, 11/19 and 3/3 patients are still alive, respectively. The response rate in group B patients after CYVE was 86% (6/7) in patients with low LDH levels and 60% (12/20) in those with high LDH levels (P=0.36) and complete remission rates were 71% (5/7) and 45% (9/20) (P=0.38), respectively (LDH was missing for 2 patients). Six of the 16 patients who received rituximab were in complete remission after salvage treatment (Table 2).
Consolidation therapy
Forty-one patients (61%) without progressive disease received HD chemotherapy as consolidation therapy. Thirty-three patients received HD chemotherapy followed by autologous HSCT, 27 of whom were in complete remission (1 group A, 15 group B and 11 group C), five in partial remission and one had stable disease. Eight patients (4 with bone marrow involvement at relapse) received HD chemotherapy followed by allogeneic HSCT (6 genoidentical, one using umbilical cord cells and one peripheral stem cells from the father), five were in complete remission, two in partial remission, and one had stable disease (Table 2).
Radiotherapy
Nine patients also received radiotherapy (7 LMB89 and 2 FAB/LMB96). Five out the 31 patients with relapse at the primary site were admnistered local irradiation (3 PMBL, one bone DLBCL, one abdominal BL). Four patients were given cranial irradiation (with or without spinal irradiation) for CNS relapse.
Overall sur vival
The median follow-up time was 6.4 years. All living patients were followed for more than 3 years after relapse (3.2 to 17 years). The 5-year survival rate was 29.9% [95% confidence interval (95% CI): 20.2–41.7%] (Figure 1).
Figure 1.
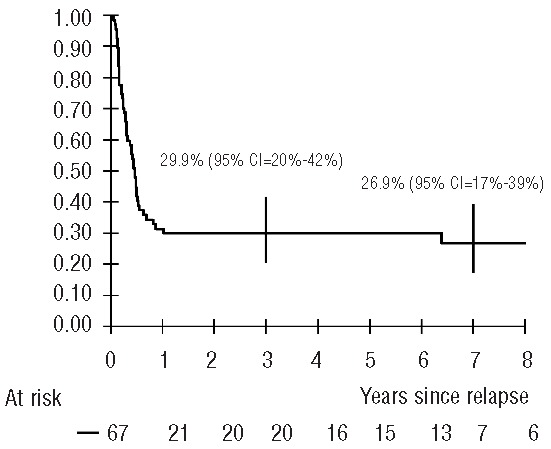
Probability of survival after relapse (vertical bars denote the Rothman 95% confidence interval).
Forty-eight patients died, with all but one of the deaths occurring within 12 months after relapse. Forty-three of the patients died of disease (24 before and 19 after HSCT), while five died of treatment-related toxicity [1 during salvage chemotherapy, 3 after HD chemotherapy (1 BEAM + autologous HSCT, 2 total body irradiation + allogeneic HSCT) and 1 of respiratory failure 5 years after total body irradiation + allogeneic HSCT and several CNS relapses].
Nineteen patients were still alive: one group A patient rescued with the group C regimen without consolidation and 18 after consolidation therapy (1 group A, 14 group B and 3 group C) (Online Supplementary Figure S1). The 5-year survival rates in groups A, B and C were 66.7%, 34.2%, and 17.4%, respectively.
Among patients irradiated after HD chemotherapy, four are still alive after local radiation (1 DLBCL, 2 PMBL, 1 BL) and one with BL after cranio-spinal irradiation for CNS relapse.
Prognostic analysis of overall survival
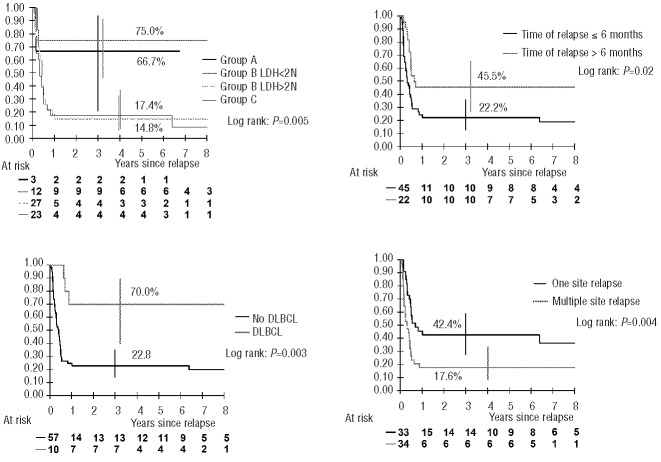
The following factors were significantly associated with survival in the univariate analysis: initial risk group combined with LDH level in group B (group A and group B with LDH ≤2N versus group B with LDH>2N and group C), histology [large-cell (DLBCL and PMBL) versus others], time to relapse (more than 6 months versus within 6 months after diagnosis), number of sites at relapse (relapse in one site versus multiple sites) and type of rescue therapy (CYVE and ICE versus others) (Figure 2 and Table 3).
Figure 2.
Probability of survival after relapse according to the four independent prognostic factors (vertical bars denote the Rothman 95% confidence interval).
Table 3.
Univariate and multivariate prognostic analyses of survival after relapse.
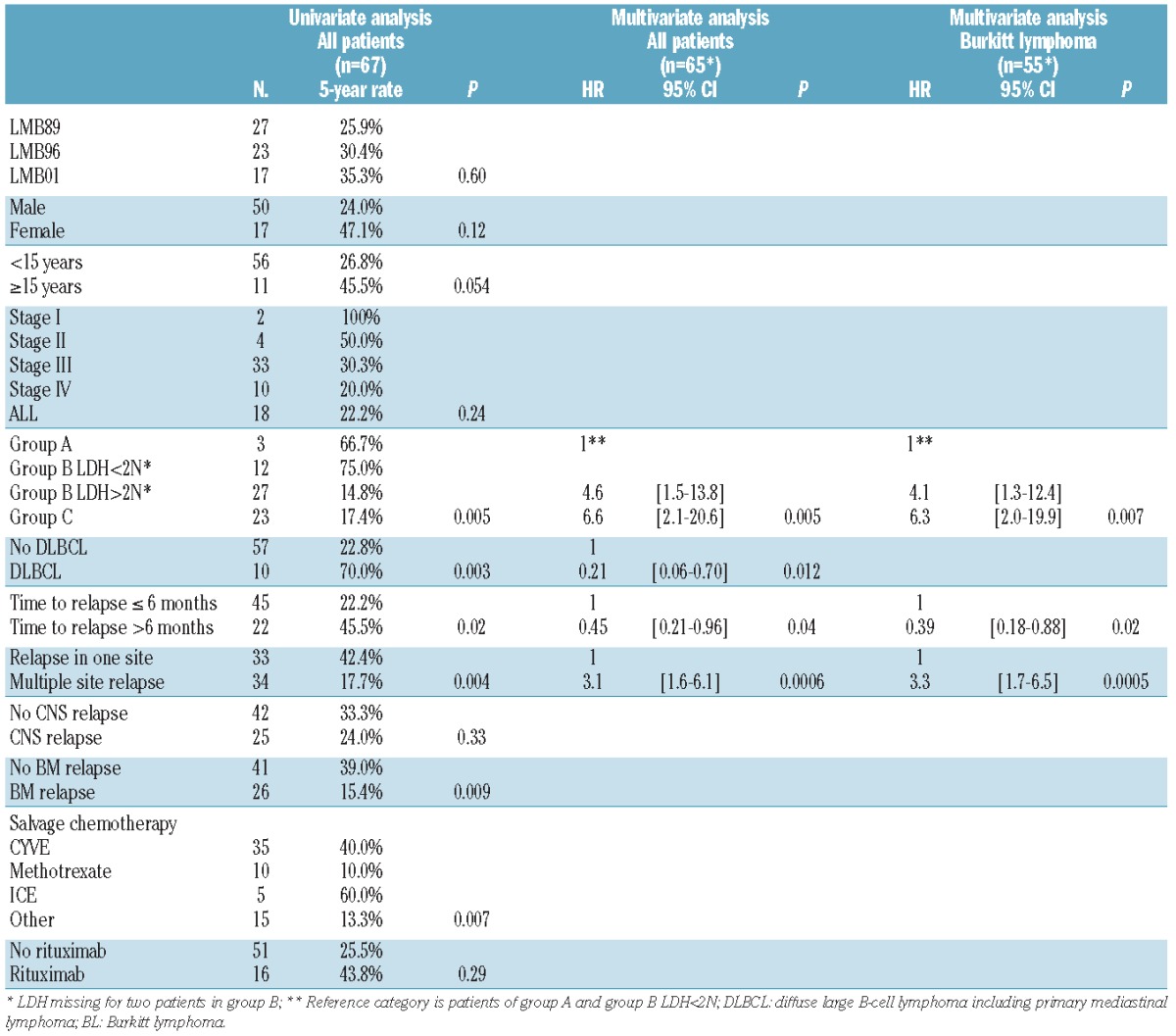
In the multivariate analysis, risk group A or B with LDH ≤2N (P=0.005), large-cell histology (P=0.012), relapse after 6 months (P=0.04) and relapse at one site (P=0.0006) were still independently associated with better survival (Table 3).
Among the patients who relapsed in the CNS, 4/15 with an isolated relapse (1 group A, 2 group B and 1 group C) and 1/10 with combined relapse (group B) are alive. Their salvage included HD methotrexate with or without HD cytarabine. The four surviving patients with isolated relapse received either BEAM (n=2) or BAM (n=2) consolidation followed by autologous HSCT. One other patient with an isolated CNS relapse died from a late complication 5 years following allogeneic HSCT. The only surviving patient with a combined relapse (bone marrow + CNS) had a DLBCL and received total body irradiation-cyclophosphamide with an allograft (Online Supplementary Table SB).
Rituximab administration was not significantly associated with survival (P=0.29 in univariate analysis and P=0.10 after adjustment for the other risk factors identified). Survival was similar among patients with BL not given (n=45) or given (n=12) rituximab (5-year survival rates 22.2% and 25.0%, respectively, P=0.82, P=0.22 after adjustment). Four of the ten patients who had large-cell histology received rituximab. Their 5-year survival rate was 100%, while the survival rate of the six patients who did not receive rituximab was 50% (P=0.11).
The ten patients with large cell histology had better survival than the other patients. The small sample size prohibited a prognostic analysis, but it is worth noting that this subgroup more often had a low LDH level, relapse at one site, and late relapse (Online Supplementary Table SC). More importantly, the multivariate prognostic analysis that included the 55 patients who did not have large-cell histology showed the same prognostic factors as those of the whole cohort.
Factors associated with sur vival after consolidation with hematopoietic stem cell transplantation
The survival rate of the 41 patients who received consolidation HD chemotherpay with HSCT was 46.3% (95% CI: 32–61%). Eighteen patients were still alive [16/33 after autologous HSCT (BEAM 12/23; busulfan-based 3/9; other 1/1) and 2/8 after allogeneic HSCT]. The type of graft and the response status at the time of HSCT were not significantly associated with survival (Figure 3); however, the power of the analyses was low due to the small sample size. The three patients in partial remission who were still alive after autologous HSCT were in fact in very good partial remission without histological study of the residual mass (2 PMBL and 1 cervical BL). They could have been considered as having “unconfirmed” complete remission. The survival of the 35 patients who received transplants in definitive or unconfirmed complete remission was significantly better than that of the six other patients (54.3% versus 0%, P=0.016).
Figure 3.
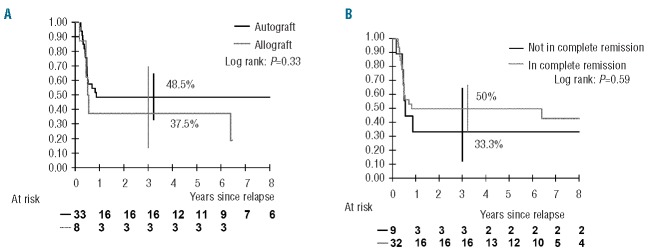
Probability of survival after relapse among the 41 patients treated with hematopoietic stem cell transplantation according to (A) the type of graft and (B) the response status at the time of high-dose chemotherapy (vertical bars denote the Rothman 95% confidence interval)
Discussion
Relapse of childhood B-NHL following the SFOP/SFCE LMB89, 96 and 2001 protocols has been rare (5% of patients). The overall survival rate was 29.9%, despite salvage chemotherapy in 97% of patients, and HD chemotherapy with HSCT in 61%. This study showed that relapse of BL occurred earlier (median time to relapse 5 months after diagnosis) compared to that of DLBCL (27 months). It also showed that Burkitt histology and initial therapeutic group C and B with LDH >2N were poor prognostic factors, as were early relapse (<6 months) and relapse at multiple sites.
Few studies have specifically focused on relapse in childhood B-NHL4,7,9–11,15–17,20–23 Survival was generally less than 30%. It is generally accepted that a good response to salvage treatment before HD chemotherapy is essential and that patients with progressive disease should not receive HD chemotherapy.4,10 In our study, the number of patients treated with each type of rescue chemotherapy was too small to be able to compare the response rates between rescue types with adequate power. However, the CYVE regimen24 seemed to be effective rescue for patients initially treated with the group A regimen and group B patients with LDH ≤2N. Rescue chemotherapy and the second complete remission rate should be improved for other patients, while the R-ICE regimen may be a promising rescue chemotherapy.25–27 The benefit of rituximab in combination with HSCT has been shown in adults with relapsed DLBCL.26,28 Some case reports and a recent UK series have suggested beneficial effects of rituximab in relapsed children.11,18,19,29 However, only 16 patients received rituximab in our study, thus, the power was inadequate to evaluate the effects of rituximab. The role of local radiotherapy was also not assessable, but may be of interest in some cases of local relapse of DLBCL.
Philip et al. reported that relapsed patients have subsequent relapses if intensification of treatment is not administered.4 The survival of HSCT recipients varies, depending mainly on the status at the time of the transplant, with better outcome for patients in second complete remission.4,7–9,12–14 We could not demonstrate that being in second complete remission at the time of HDC was significantly associated with survival, but overall, patients in second or unconfirmed complete remission had better survival than others.
The type of HSCT had no impact on outcome. Allogeneic HSCT was not more beneficial than autologous HSCT (survival rate of 38% versus 49%, respectively) and caused more toxicity. A graft-versus-lymphoma effect has not been shown in BL.9,15,30–32 In particular, the review published by Gross et al. showed similar event-free survival rates in BL (n=41) and DLBCL (n=52) with autologous HSCT and allogeneic HSCT (27% versus 31% and 52% versus 50%, respectively), which is in contrast to the clear advantage of allogeneic HSCT in lymphoblastic lymphoma.15 BEAM and busulfan-based regimens were both administered before autologous HSCT, but a conclusion could not be drawn regarding the benefits of each regimen, which were not randomized and administered at the investigators’ discretion. Nevertheless, the consolidation regimen for high-risk patients needs to be improved.
Previous studies on BL found that one-third of relapses occurred in the CNS, one-third at the primary site and one-third at other sites.14 We observed a comparable distribution in our study (22% isolated CNS, 27% unifocal and 51% multifocal). Survival differed according to the site of relapse, in contrast with previously published results.4 Relapse at one site was significantly associated with better survival (42% versus 18% at multiple sites). CNS relapse has been shown to be curable.4,9,33 In our study, four out of 15 patients with isolated CNS relapse were still alive.
Although no differences in survival in DLBCL and BL were observed in the LMB studies or in the BFM studies,2,3,34,35 large-cell histology was associated with better survival after relapse (70% versus23%) and lower risk characteristics, which is consistent with the fact that they are different entities.35,36
Advanced disease and LDH level are recognized as poor prognostic factors at diagnosis; interestingly, they were significant prognostic factors at relapse.11,37 Thus, group A and group B patients with low LDH levels had better survival (67% and 75%, respectively) than group B patients with high LDH levels and group C patients (15% and 17%, respectively). It should be noted that initial risk stratification was not only indicative of initial tumor burden, but also of treatment burden before relapse. This emphasizes the need for an effective first-line treatment and the necessity to be very cautious with any reduction of this first-line therapy because of the lack of a solid second chance in these groups of patients.
In conclusion, our series is one of the largest cohorts of relapsed pediatric patients to date. We confirmed that early relapse (<6 months) was a significant factor contributing to inferior outcome, and that the source of stem cells (autologous versus allogeneic) did not affect outcome. Moreover, we observed that initial low-risk disease at diagnosis, large-cell histology, and localized relapse are associated with better outcome. For the patients with unfavorable characteristics, (i.e., those initially in group B with high LDH levels, those in group C, and those with early and multi-site relapse), new treatment combinations are necessary to improve the second complete remission rate before HD chemotherapy and HSCT. New drugs, including antibodies and targeted therapy, should be investigated, as should be a double HSCT rescue strategy. In CNS relapses the place of intrathecal/intraventricular antibodies also needs to be investigated. Due to the small number of relapsing patients, these investigations should be conducted on an international level.
Acknowledgments
The authors thank the data managers of the LMB89 and FAB/LMB96 (N Dupouy) and LMB2001 (Y Oubouzar) studies, the members of the DSMC of the FAB/LMB96 trial: Michael Link, Alfred Reiter, David Harrington, and Robert Souhami, the members of the LMB89 histo-cytology review panel (MJ Terrier Lacombe, M Raphael, C Bayle, P Feldman) and of the international FAB/LMB96 review panel (M Raphael, S Perkins, K McCarthy, MJ Terrier-Lacombe, M Lones, and A Wotherspoon) and all the investigators who treated patients and participated in the study, as listed below. They also thank Nita Nguyen for language editing.
Footnotes
The online version of this article has a Supplementary Appendx.
SFOP/SFCE institutions and investigators in order according to number of registered patients
Gustave Roussy, Villejuif (C Patte, L Brugieres, J Grill, O Hartmann, C Kalifa, O Oberlin, D Valteau, C Dufour, V Minard-Colin); CHU Pellegrin, Bordeaux (Y Perel, A Notz, N Aladjidi); Emma Kinderziekenhuis, Amsterdam (H Behrendt, J Zsiros); Hopital Debrousse, Lyon (Y Bertrand, C Pondarré); Hopital De La Timone, Marseille (C Coze, JC Gentet, N André); CHRU Hopital Sud, Rennes (E Le Gall, V Gandemer); Hopital Trousseau, Paris (G Leverger, J Landmann-Parker, D Tabone, A Auvrignon); Centre Leon Berard, Lyon (D Frapppaz, C Bergeron, P Marrec); Hopital Huriez, Lille (B Nelken, F Mazingue, A Lambilliote); Hopital Fabiola, Bruxelles, Belgique (C Devalck, E Sariban); CHU et Hopital Lenval, Nice (N Sirvens, A Deville, C Soler); Institut Curie, Paris (J Michon, F Doz, H Pacquement, D Orbach); CHRU Nancy (P Chastagner, C Schmitt, P Bordigoni); CHU, Nantes (F Mechinaud, C Thomas, N Corradini); CHU Purpan, Toulouse (A Robert, H Rubie, AI Bertozzi); Hopital Clocheville, Tours (O Lejars, A Jourdain); Hopital C. Nicolle, Rouen (JP Vannier); CHU Dupuytren, Limoges (L De Lumley, Piguet); CHU Cote de Nacre, Caen (P Boutard, O Minckes); Hopital Michalon, Grenoble (D Plantaz); Hopital St Charles, Montpellier (G Margeritte, JL Bernard); Hopital Robert Debre, Paris (E Vilmer,); Hopital J. Bernard, Poitiers (F Millot); Hospices Civils, Strasbourg (P Lutz); Hopital St Louis, Paris (A Baruchel, T Leblanc); Hopital Nord, St Etienne (JL Stephan, C Berger); Hotel Dieu, Clermont Ferrand (F Demeocq); Hopital St Jacques, Besancon (E Plouvier, V Laitier)
Funding
Supported by grants from the Institut-Gustave-Roussy, from Association pour la Recherche contre le Cancer, La Ligue Nationale Contre le Cancer (FAB/LMB96), and Société Française des Cancers de l’Enfant et de l’Adolescent & Enfants et Santé (LMB2001).
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Patte C, Philip T, Rodary C, et al. High survival rate in advanced-stage B-cell lymphomas and leukemias without CNS involvement with a short intensive polychemotherapy: results from the French Pediatric Oncology Society of a randomized trial of 216 children. J Clin Oncol. 1991; 9(1):123–132. [DOI] [PubMed] [Google Scholar]
- 2.Patte C, Auperin A, Michon J, et al. The Société Française d’Oncologie Pédiatrique LMB89 protocol: highly effective multiagent chemotherapy tailored to the tumor burden and initial response in 561 unselected children with B-cell lymphomas and L3 leukemia. Blood. 2001;97(11):3370–3379. [DOI] [PubMed] [Google Scholar]
- 3.Patte C, Auperin A, Gerrard M, et al. Results of the randomized international FAB/LMB96 trial for intermediate risk B-cell non-Hodgkin lymphoma in children and adolescents: it is possible to reduce treatment for the early responding patients. Blood. 2007;109(7):2773–2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Philip T, Hartmann O, Pinkerton R, et al. Curability of relapsed childhood B-cell non-Hodgkin’s lymphoma after intensive first line therapy: a report from the Société Française d’Oncologie Pédiatrique. Blood. 1993;81(8):2003–2006. [PubMed] [Google Scholar]
- 5.Gerrard M, Cairo MS, Weston C, et al. Excellent survival following two courses of COPAD chemotherapy in children and adolescents with resected localized B-cell non-Hodgkin’s lymphoma: results of the FAB/LMB 96 international study. Br J Haematol. 2008;141(6):840–847. [DOI] [PubMed] [Google Scholar]
- 6.Cairo MS, Gerrard M, Sposto R, et al. Results of a randomized international study of high-risk central nervous system B non-Hodgkin lymphoma and B acute lymphoblastic leukemia in children and adolescents. Blood. 2007;109(7):2736–2743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atra A, Gerrard M, Hobson R, Imeson JD, Hann IM, Pinkerton CR. Outcome of relapsed or refractory childhood B-cell acute lymphoblastic leukaemia and B-cell non-Hodgkin’s lymphoma treated with the UKCCSG 9003/9002 protocols. Br J Haematol. 2001;112(4):965–968. [DOI] [PubMed] [Google Scholar]
- 8.Philip T, Biron P, Maraninchi D, et al. Massive chemotherapy with autologous bone marrow transplantation in 50 cases of bad prognosis non-Hodgkin’s lymphoma. Br J Haematol. 1985;60(4):599–609. [DOI] [PubMed] [Google Scholar]
- 9.Ladenstein R, Pearce R, Hartmann O, Patte C, Goldstone T, Philip T. High-dose chemotherapy with autologous bone marrow rescue in children with poor-risk Burkitt’s lymphoma: a report from the European Lymphoma Bone Marrow Transplantation Registry. Blood. 1997;90(8):2921–2930. [PubMed] [Google Scholar]
- 10.Fujita N, Mori T, Mitsui T, et al. The role of hematopoietic stem cell transplantation with relapsed or primary refractory childhood B-cell non-Hodgkin lymphoma and mature B-cell leukemia: a retrospective analysis of enrolled cases in Japan. Pediatr Blood Cancer. 2008;51(2):188–192. [DOI] [PubMed] [Google Scholar]
- 11.Anoop P, Sankpal S, Stiller C, et al. Outcome of childhood relapsed or refractory mature B-cell non-Hodgkin lymphoma and acute lymphoblastic leukemia. Leuk Lymphoma. 2012;53(10):1882–1888. [DOI] [PubMed] [Google Scholar]
- 12.Hartmann O, Pein F, Beaujean F, et al. Highdose polychemotherapy with autologous bone marrow transplantation in children with relapsed lymphomas. J Clin Oncol. 1984;2(9):979–985. [DOI] [PubMed] [Google Scholar]
- 13.Hartmann O, Benhamou E, Beaujean F, et al. High-dose busulfan and cyclophosphamide with autologous bone marrow transplantation support in advanced malignancies in children: a phase II study. J Clin Oncol. 1986;4(12):1804–1810. [DOI] [PubMed] [Google Scholar]
- 14.Loiseau HA, Hartmann O, Valteau D, et al. High-dose chemotherapy containing busulfan followed by bone marrow transplantation in 24 children with refractory or relapsed non-Hodgkin’s lymphoma. Bone Marrow Transplant. 1991;8(6):465–472. [PubMed] [Google Scholar]
- 15.Gross TG, Hale GA, He W, et al. Hematopoietic stem cell transplantation for refractory or recurrent non-Hodgkin lymphoma in children and adolescents. Biol Blood Marrow Transplant. 2010;16(2):223–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim H, Park ES, Lee SH, et al. Clinical outcome of relapsed or refractory Burkitt lymphoma and mature B-cell lymphoblastic leukemia in children and adolescents. Cancer Res Treat. 2014;46(4):358–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Grossbard ML, Gribben JG, Freedman AS, et al. Adjuvant immunotoxin therapy with anti-B4-blocked ricin after autologous bone marrow transplantation for patients with B-cell non-Hodgkin’s lymphoma. Blood. 1993;81(9):2263–2271. [PubMed] [Google Scholar]
- 18.Bay A, Dogan M, Acikgoz M, Oner AF. Rituximab in a child with relapsed Burkitt lymphoma. Pediatr Blood Cancer. 2007;49(2):218. [DOI] [PubMed] [Google Scholar]
- 19.De Vries MJ, Veerman AJP, Zwaan CM. Rituximab in three children with relapsed/refractory B-cell acute lymphoblastic leukaemia/Burkitt non-Hodgkin’s lymphoma. Br J Haematol. 2004;125(3):414–415. [DOI] [PubMed] [Google Scholar]
- 20.Kobrinsky NL, Sposto R, Shah NR, et al. Outcomes of treatment of children and adolescents with recurrent non-Hodgkin’s lymphoma and Hodgkin’s disease with dexamethasone, etoposide, cisplatin, cytarabine, and L-asparaginase, maintenance chemotherapy, and transplantation: Children’s Cancer Group Study CCG-5912. J Clin Oncol. 2001;19(9):2390–2396. [DOI] [PubMed] [Google Scholar]
- 21.Won SC, Han JW, Kwon SY, et al. Autologous peripheral blood stem cell transplantation in children with non-Hodgkin’s lymphoma: a report from the Korean Society of Pediatric Hematology-Oncology. Ann Hematol. 2006;85(11):787–794. [DOI] [PubMed] [Google Scholar]
- 22.Attarbaschi A, Dworzak M, Steiner M, et al. Outcome of children with primary resistant or relapsed non-Hodgkin lymphoma and mature B-cell leukemia after intensive firstline treatment: a population-based analysis of the Austrian Cooperative Study Group. Pediatr Blood Cancer. 2005;44(1):70–76. [DOI] [PubMed] [Google Scholar]
- 23.Sandlund JT, Bowman L, Heslop HE, et al. Intensive chemotherapy with hematopoietic stem-cell support for children with recurrent or refractory NHL. Cytotherapy. 2002;4(3):253–258. [DOI] [PubMed] [Google Scholar]
- 24.Gentet JC, Patte C, Quintana E, et al. Phase II study of cytarabine and etoposide in children with refractory or relapsed non-Hodgkin’s lymphoma: a study of the French Society of Pediatric Oncology. J Clin Oncol. 1990;8(4):661–665. [DOI] [PubMed] [Google Scholar]
- 25.Hertzberg MS, Crombie C, Benson W, Taper J, Gottlieb D, Bradstock KF. Outpatient fractionated ifosfamide, carboplatin and etoposide as salvage therapy in relapsed and refractory non-Hodgkin’s and Hodgkin’s lymphoma. Ann Oncol. 2006;17(Suppl 4):iv25–30. [DOI] [PubMed] [Google Scholar]
- 26.Kewalramani T, Zelenetz AD, Nimer SD, et al. Rituximab and ICE as second-line therapy before autologous stem cell transplantation for relapsed or primary refractory diffuse large B-cell lymphoma. Blood. 2004;103(10):3684–3688. [DOI] [PubMed] [Google Scholar]
- 27.Griffin TC, Weitzman S, Weinstein H, et al. A study of rituximab and ifosfamide, carboplatin, and etoposide chemotherapy in children with recurrent/refractory B-cell (CD20+) non-Hodgkin lymphoma and mature B-cell acute lymphoblastic leukemia: a report from the Children’s Oncology Group. Pediatr Blood Cancer. 2009;52(2): 177–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gisselbrecht C, Glass B, Mounier N, et al. Salvage regimens with autologous transplantation for relapsed large B-cell lymphoma in the rituximab era. J Clin Oncol. 2010;28(27):4184–4190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Culi S, Culi V, Armanda V, Kuljis D, Pesuti-Pisac V, Jankovi S. Anti-CD20 monoclonal antibody (Rituximab) for therapy of mediastinal CD20-positive large B-cell non-Hodgkin lymphoma with a local tumor extension into the lung of a 10-year-old girl. Pediatr Hematol Oncol. 2003;20(4):339–344. [DOI] [PubMed] [Google Scholar]
- 30.Schmitz N, Dreger P, Glass B, Sureda A. Allogeneic transplantation in lymphoma: current status. Haematologica. 2007;92(11): 1533–1548. [DOI] [PubMed] [Google Scholar]
- 31.Grigg AP, Seymour JF. Graft versus Burkitt’s lymphoma effect after allogeneic marrow transplantation. Leuk Lymphoma. 2002;43(4):889–892. [DOI] [PubMed] [Google Scholar]
- 32.Harris RE, Termuhlen AM, Smith LM, et al. Autologous peripheral blood stem cell transplantation in children with refractory or relapsed lymphoma: results of Children’s Oncology Group study A5962. Biol Blood Marrow Transplant. 2011;17(2):249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sariban E, Edwards B, Janus C, Magrath I. Central nervous system involvement in American Burkitt’s lymphoma. J Clin Oncol. 1983;1(11):677–681. [DOI] [PubMed] [Google Scholar]
- 34.Cairo MS, Sposto R, Gerrard M, et al. Advanced stage, increased lactate dehydrogenase, and primary site, but not adolescent age (≥ 15 years), are associated with an increased risk of treatment failure in children and adolescents with mature B-cell non-Hodgkin’s lymphoma: results of the FAB LMB 96 study. J Clin Oncol. 2012;30(4): 387–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reiter A, Klapper W. Recent advances in the understanding and management of diffuse large B-cell lymphoma in children. Br J Haematol. 2008;142(3):329–347. [DOI] [PubMed] [Google Scholar]
- 36.Rosenwald A, Ott G. Burkitt lymphoma versus diffuse large B-cell lymphoma. Ann Oncol. 2008;19(Suppl 4):iv67–69. [DOI] [PubMed] [Google Scholar]
- 37.Cairo MS, Sposto R, Fan J, et al. Survival in children and adolescents with mature B-NHL progressing or relapsing from treatment on FAB/LMB 96: tumor burden and time to relapse are poor prognostic factors. Ped Blood Cancer. 2009; 855 (abstract). [Google Scholar]