Abstract
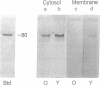
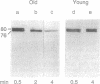
Limited proteolysis by calpain (Ca(2+)-activated protease; EC 3.4.22.17) is believed to regulate the function of membrane enzymes and modify the behavior of membrane structural proteins. Calpain is activated by autolysis. The degradation of band 3 protein by mu-calpain is known to be enhanced in erythrocyte membranes from human individuals > 70 years old (old) as compared with that from individuals 20-30 years old (young). In the present study, monoclonal antibody to mu-calpain was used to study the behavior of calpain in erythrocytes of young and old individuals. Less calpain was found in erythrocyte cytosol and membranes from old than in those from young. Increasing the erythrocyte Ca2+ induced translocation of calpain to the cell membrane and autolysis of the enzyme. Alkylation of erythrocyte thiols also promoted translocation of calpain to the membrane, especially in the presence of Ca2+. When calpain was added to erythrocyte membranes, initial binding was greater and subsequent autolysis faster in old than in young individuals, possibly arising from alterations in cell membranes of old individuals. The enhanced calpain autolysis was accompanied by enhanced degradation of band 3 protein in the old. The results suggest that calpain in old individuals is translocated to the cell membrane and is activated by autolysis, resulting in degradation of certain membrane proteins and loss of calpain. Enhanced calpain-induced membrane proteolysis may play a role in abnormal cell destruction (e.g., shortening the life span of erythrocytes in the aged, neuronal degeneration, etc). The erythrocyte membrane provides a convenient model for the study of age-associated alterations in cell membranes and in calpain behavior.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cottin P., Poussard S., Desmazes J. P., Georgescauld D., Ducastaing A. Free calcium and calpain I activity. Biochim Biophys Acta. 1991 Aug 30;1079(2):139–145. doi: 10.1016/0167-4838(91)90118-j. [DOI] [PubMed] [Google Scholar]
- Croall D. E., DeMartino G. N. Calcium-activated neutral protease (calpain) system: structure, function, and regulation. Physiol Rev. 1991 Jul;71(3):813–847. doi: 10.1152/physrev.1991.71.3.813. [DOI] [PubMed] [Google Scholar]
- Danon D., Marikovsky Y. The aging of the red blood cell. A multifactor process. Blood Cells. 1988;14(1):7–18. [PubMed] [Google Scholar]
- Floyd R. A. Role of oxygen free radicals in carcinogenesis and brain ischemia. FASEB J. 1990 Jun;4(9):2587–2597. [PubMed] [Google Scholar]
- Gershon H., Gershon D. Altered enzyme function and premature sequestration of erythrocytes in aged individuals. Blood Cells. 1988;14(1):93–101. [PubMed] [Google Scholar]
- Glaser T., Kosower N. S. Calpain-calpastatin and fusion. Fusibility of erythrocytes is determined by a protease-protease inhibitor [calpain-calpastatin] balance. FEBS Lett. 1986 Sep 29;206(1):115–120. doi: 10.1016/0014-5793(86)81351-5. [DOI] [PubMed] [Google Scholar]
- Glaser T., Kosower N. S. Fusion of rat erythrocytes by membrane-mobility agent A2C depends on membrane proteolysis by a cytoplasmic calpain. Eur J Biochem. 1986 Sep 1;159(2):387–392. doi: 10.1111/j.1432-1033.1986.tb09880.x. [DOI] [PubMed] [Google Scholar]
- Harman D. The aging process: major risk factor for disease and death. Proc Natl Acad Sci U S A. 1991 Jun 15;88(12):5360–5363. doi: 10.1073/pnas.88.12.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Inomata M., Saito Y., Ito H., Kawashima S. Activation of intracellular calcium-activated neutral proteinase in erythrocytes and its inhibition by exogenously added inhibitors. Biochim Biophys Acta. 1991 Sep 24;1094(3):249–256. doi: 10.1016/0167-4889(91)90083-a. [DOI] [PubMed] [Google Scholar]
- Hayashi M., Saito Y., Kawashima S. Calpain activation is essential for membrane fusion of erythrocytes in the presence of exogenous Ca2+. Biochem Biophys Res Commun. 1992 Jan 31;182(2):939–946. doi: 10.1016/0006-291x(92)91822-8. [DOI] [PubMed] [Google Scholar]
- Inomata M., Hayashi M., Nakamura M., Saito Y., Kawashima S. Properties of erythrocyte membrane binding and autolytic activation of calcium-activated neutral protease. J Biol Chem. 1989 Nov 5;264(31):18838–18843. [PubMed] [Google Scholar]
- Inomata M., Kasai Y., Nakamura M., Kawashima S. Activation mechanism of calcium-activated neutral protease. Evidence for the existence of intramolecular and intermolecular autolyses. J Biol Chem. 1988 Dec 25;263(36):19783–19787. [PubMed] [Google Scholar]
- Iwamoto N., Thangnipon W., Crawford C., Emson P. C. Localization of calpain immunoreactivity in senile plaques and in neurones undergoing neurofibrillary degeneration in Alzheimer's disease. Brain Res. 1991 Oct 4;561(1):177–180. doi: 10.1016/0006-8993(91)90766-o. [DOI] [PubMed] [Google Scholar]
- Jennings M. L. Structure and function of the red blood cell anion transport protein. Annu Rev Biophys Biophys Chem. 1989;18:397–430. doi: 10.1146/annurev.bb.18.060189.002145. [DOI] [PubMed] [Google Scholar]
- Kay M. M. Drosophila to bacteriophage to erythrocyte: the erythrocyte as a model for molecular and membrane aging of terminally differentiated cells. Gerontology. 1991;37(1-3):5–32. doi: 10.1159/000213250. [DOI] [PubMed] [Google Scholar]
- Kay M. M., Hughes J., Zagon I., Lin F. B. Brain membrane protein band 3 performs the same functions as erythrocyte band 3. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2778–2782. doi: 10.1073/pnas.88.7.2778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosower N. S., Glaser T., Kosower E. M. Membrane-mobility agent-promoted fusion of erythrocytes: fusibility is correlated with attack by calcium-activated cytoplasmic proteases on membrane proteins. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7542–7546. doi: 10.1073/pnas.80.24.7542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuboki M., Ishii H., Kazama M. Characterization of calpain I-binding proteins in human erythrocyte plasma membrane. J Biochem. 1990 May;107(5):776–780. doi: 10.1093/oxfordjournals.jbchem.a123124. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lane R. D., Allan D. M., Mellgren R. L. A comparison of the intracellular distribution of mu-calpain, m-calpain, and calpastatin in proliferating human A431 cells. Exp Cell Res. 1992 Nov;203(1):5–16. doi: 10.1016/0014-4827(92)90033-5. [DOI] [PubMed] [Google Scholar]
- Lorand L., Bjerrum O. J., Hawkins M., Lowe-Krentz L., Siefring G. E., Jr Degradation of transmembrane proteins in Ca2+-enriched human erythrocytes. An immunochemical study. J Biol Chem. 1983 Apr 25;258(8):5300–5305. [PubMed] [Google Scholar]
- Martínez A., Vitórica J., Bogónez E., Satrústegui J. Differential effects of age on the pathways of calcium influx into nerve terminals. Brain Res. 1987 Dec 1;435(1-2):249–257. doi: 10.1016/0006-8993(87)91608-8. [DOI] [PubMed] [Google Scholar]
- Mellgren R. L. Calcium-dependent proteases: an enzyme system active at cellular membranes? FASEB J. 1987 Aug;1(2):110–115. doi: 10.1096/fasebj.1.2.2886390. [DOI] [PubMed] [Google Scholar]
- Murachi T. Calcium-dependent proteinases and specific inhibitors: calpain and calpastatin. Biochem Soc Symp. 1984;49:149–167. [PubMed] [Google Scholar]
- Pant H. C., Gallant P. E., Gould R., Gainer H. Distribution of calcium-activated protease activity and endogenous substrates in the squid nervous system. J Neurosci. 1982 Nov;2(11):1578–1587. doi: 10.1523/JNEUROSCI.02-11-01578.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontremoli S., Sparatore B., Salamino F., Michetti M., Sacco O., Melloni E. Reversible activation of human neutrophil calpain promoted by interaction with plasma membranes. Biochem Int. 1985 Jul;11(1):35–44. [PubMed] [Google Scholar]
- Saito K., Elce J. S., Hamos J. E., Nixon R. A. Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2628–2632. doi: 10.1073/pnas.90.7.2628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaier A. H., Bradford H. N., Lundberg D., Farber A., Colman R. W. Membrane expression of platelet calpain. Blood. 1990 Mar 15;75(6):1273–1281. [PubMed] [Google Scholar]
- Schwarz-Ben Meir N., Glaser T., Kosower N. S. Band 3 protein degradation by calpain is enhanced in erythrocytes of old people. Biochem J. 1991 Apr 1;275(Pt 1):47–52. doi: 10.1042/bj2750047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman R., Card J. P., Davis L. G. Proteolytic processing of beta-amyloid precursor by calpain I. J Neurosci. 1990 Jul;10(7):2400–2411. doi: 10.1523/JNEUROSCI.10-07-02400.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman E. R., Oliver C. N. Metal-catalyzed oxidation of proteins. Physiological consequences. J Biol Chem. 1991 Feb 5;266(4):2005–2008. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youngman L. D., Park J. Y., Ames B. N. Protein oxidation associated with aging is reduced by dietary restriction of protein or calories. Proc Natl Acad Sci U S A. 1992 Oct 1;89(19):9112–9116. doi: 10.1073/pnas.89.19.9112. [DOI] [PMC free article] [PubMed] [Google Scholar]