Abstract
Background
Transcription factors that determine retinal development seem to be conserved in different phyla throughout the animal kingdom. In most representatives, however, only a few of the involved transcription factors have been sampled and many animal groups remain understudied. In order to fill in the gaps for the chelicerate group of arthropods, we tested the expression pattern of the candidate genes involved in the eye development in the embryo of the wandering spider Cupiennius salei. One main objective was to profile the molecular development of the eyes and to search for possible variation among eye subtype differentiation. A second aim was to form a basis for comparative studies in order to elucidate evolutionary pathways in eye development.
Results
We screened the spider embryonic transcriptome for retina determination gene candidates and discovered that all except one of the retinal determination genes have been duplicated. Gene expression analysis shows that the two orthologs of all the genes have different expression patterns. The genes are mainly expressed in the developing optic neuropiles of the eyes (lateral furrow, mushroom body, arcuate body) in earlier stages of development (160 to 220 h after egg laying). Later in development (180 to 280 h after egg laying), there is differential expression of the genes in disparate eye vesicles; for example, Cs-otxa is expressed only in posterior-lateral eye vesicles, Cs-otxb, Cs-six1a, and Cs-six3b in all three secondary eye vesicles, Cs-pax6a only in principal eye vesicles, Cs-six1b in posterior-median, and posterior-lateral eye vesicles, and Cs-six3a in lateral and principal eye vesicles.
Conclusions
Principle eye development shows pax6a (ey) expression, suggesting pax6 dependence, although secondary eyes develop independently of pax6 genes and show differential expression of several retinal determination genes. Comparing this with the other arthropods suggests that pax6-dependent median eye development is a ground pattern of eye development in this group and that the ocelli of insects, the median eyes of chelicerates, and nauplius eyes can be homologised. The expression pattern of the investigated genes makes it possible to distinguish between secondary eyes and principal eyes. Differences of gene expression among the different lateral eyes indicate disparate function combined with genetic drift.
Electronic supplementary material
The online version of this article (doi:10.1186/s13227-015-0010-x) contains supplementary material, which is available to authorized users.
Keywords: Eye development, Principal eyes, Retinal determination gene network, Secondary eyes, Spider
Background
A group of transcription factors constituting the retinal determination gene network (RDGN) is involved in the development of many types of animal eyes [1], and these factors probably already existed (albeit with different functions) at the bilaterian base [2]. One of the conserved and essential transcription factors in eye development is pax6, which is required for the formation of the retina in vertebrates [3]. In flies, the pax6 ortholog eyeless (ey) and the pax gene eye gone are necessary to build the entire eye disc (reviewed by [4]). Additionally, ey and another pax6 ortholog, twin of eyeless (toy), are involved in the development of ocelli but not of the Bolwig organ (the larval eye of some flies) (reviewed by [4]). pax6 expression is also present in the early eye anlagen in cephalopods [5,6], planarians [7], nemerteans [8], polychaetes [9], the wasp Nasonia vitripennis [10], and the onychophoran Euperipatoides kanangrensis [11]. pax6 is expressed in putative larval sensory cells in the calcisponges [12]. Nevertheless, it is not universally expressed during bilaterian visual system development. pax6 expression is lacking in eyes or visual organs of an adult polychaete worm (Platynereis dumerilii) [9], a myriapod (Glomeris marginata) [13], amphioxus (Branchiostoma floridae) [14], and the horseshoe crab (Limulus polyphemus) [15]. The earliest target genes of ey have been categorized as ‘early retinal genes’ because their temporal expression extends from stages including the undifferentiated eye primordium to the differentiating retina [4]. This group of RDGN genes includes the six1/2 homeobox gene sine oculis (so), optix orthologs or six3 homeobox genes, the nuclear haloacid dehalogenase group phosphatase eyes absent (eya), and the Ski/Sno-related transcriptional co-factor dachshund (dac) [16]. The eya gene is critical for normal eye development in Drosophila [17]. atonal (atn), a proneuronal gene for photoreceptors in Drosophila, is a downstream RDGN element [18,19]. In the Drosophila eye disc, selection of R8 photoreceptors requires atn gene expression in a subset of proneural cells [19]. Additionally, formation of ocelli requires atn function [19]. Another crucial gene with an equally conserved role in eye and photoreceptor cell development is the orthodenticle/otx gene. In vertebrates, otx orthologs are required for the formation of many retinal cell types [20]. They are also expressed in the photoreceptors of the fly ommatidia, ocelli, and Bolwig organ [21]. The expression of the otx gene has also been documented in eyes and anterior neurogenic regions of different animals such as Dugesia japonica (planarian) [22], Parhyale hawaiensis (amphipod) [23], Euscorpius flavicaudis (scorpion), Tegenaria saeva (spider) [24], and E. kanangrensis (onychophoran) [11]. In Tribolium castaneum, two otd-related genes are present [25]. One of the two genes is expressed in a broad anterior stripe in the blastoderm, and the second gene is expressed in more limited subsets of cells in the anterior brain [25]. Other transcription factors involved in eye development belong to the six family of homeodomain proteins. In Drosophila [26], in planarians [27], and in the polychaete P. dumerilii [9], six1/2 orthologs have an early role in eye specification. The vertebrate six3 gene plays a pivotal role in vertebrate eye formation [28]. It is also involved in polychaete eye formation [9] but shows no expression in either the developing planarian [29] or the onychophoran eyes [11].
The ctenid spider C. salei [30] - a large wandering spider from Central America - has been used as a model in evo-devo studies for many years [31], and recently, its complete development has been described in detail [32]. It has four pairs of well-developed eyes including one pair of principal eyes (PEs; or anterior-median eyes (AME)) and three pairs of secondary eyes (SEs) (posterior-median (PME), anterior-lateral (ALE), and posterior-lateral eyes (PLE)) (Figure 1A,B). The PEs of C. salei consist of a single layer of three to four rhabdomeric photoreceptors [33]. The major anatomical difference between PEs and SEs lies in the photoreceptor rhabdomeres, that is, everted (directed towards the lens) in the PE retina but inverted in the SE retina [33]. In addition, the retinas of PEs are mobile, using two special eye muscles, whereas the SE retinas are immobile [34]. Spiders exhibit three optic neuropils: all photoreceptors of each retina project towards their individual first optic neuropil (ON1); ON1 neurons send retinotopic projections to their individual secondary neuropil (ON2); the third optic neuropil (ON3) is common for all SEs, but a different area serves as the ON3 of the PEs [35-37]. The ON3 of PEs is part of a complex neuropil termed arcuate body [38] or central body [39]; the ON3 serving all SEs was [40] named mushroom body by Hanström [39]. ON1 and ON2 are equivalent to insects’ lamina and medulla, respectively [36].
Figure 1.

Image of a C. salei male showing the details of prosoma (A) frontal view and (B) lateral view. AL anterior-lateral eye, AM anterior-median eye, br brain, ch chelicerae, pd pedipalp, PL posterior-lateral eye, PM posterior-median eye.
Although the innervation and physiology of the eyes of adult C. salei have been extensively studied, the literature describing their detailed development is limited [32,34,38]. Doeffinger et al. [38] described the formation of the optic lobes during the embryonic development of C. salei. The onset of optic lobe formation is initiated as the bilateral grooves form in the lateral-most parts of the head neuroectoderm at 160 to 180 h after egg laying (hAEL). This structure is known as lateral furrows (lf) [32,38]. Later, the lateral grooves assume a kidney-like form at 220 hAEL [32,38]. At 250 hAEL, the lateral invagination has separated from the surface ectoderm, which most likely occurs by the partitioning of the grooves into a dorsal and a ventral vesicle [38]. Doeffinger et al. [38] assumed that these vesicles give rise to the optic ganglia of both lateral eyes (PLE and ALE). Later, the ventromedial neuroectoderm, adjacent to the lateral invaginations, invaginates and forms two vesicles. These, in turn, give rise to the optic ganglia of both median eyes (AME and PME [38]). Due to inconsistencies and incompleteness of the available literature on eye development in C. salei, we provide a more elaborate description of the ontogenesis of this spider’s optic neuropils and eye vesicles.
So far, the molecular mechanisms underlying eye development have not been studied in any spider species. Studying the molecular development of spider eyes is particularly important because they belong to a basal arthropod group, chelicerates, that are relatively understudied regarding molecular processes. Furthermore, spiders possess multiple eye types with different ontogeny and evolutionary history. Potential differences in the molecular patterning of spider eye types can teach us about the evolution of genetic networks in development. Except for a report on the lack of pax6 and atn expression in the developing eyes of the xiphosuran horseshoe crab Limulus [15], the molecular mechanism of eye development in chelicerates remains unknown. We used next-generation RNA sequencing to amplify and characterise candidate genes for eye development in the spider C. salei and tested their expression pattern using whole-mount in situ hybridisation during embryonic development.
Methods
Animal husbandry and staging
Adult C. salei were kept in the breeding stocks in our laboratory animal facility in glass jars at 27°C and a relative humidity of 70% to 80%. They were fed once a week with flies. The cocoons were opened, and relevant embryonic stages (Table 1) were fixed for in situ hybridisation, antibody staining, or preserved in Trizol for RNA extraction.
Table 1.
Embryonic stages of C. salei according to Wolf and Hilbrant (2011) that are relevant for eye formation
| Stage | Hours after fertilisation | Days | Name of stage | Description of stage relevant to eye formation |
|---|---|---|---|---|
| Stage 11 | 100 to 130 | 4-5-6 | Prosomal limb buds | Progression in the formation of bilateral cheliceral lobes |
| Stage 12 | 160 to 180 | 6-7-7.5 | Lateral furrow | Formation of kidney-shaped folds lateral to stomodaeum (lf) |
| Stage 14 | 160 to 180 | 6-7-7.5 | Inversion I | Migration of lateral subdivision (ls) in the direction of lf and partly covering it |
| Formation of a crescent-shaped anterior furrow (af) | ||||
| Stage 16 | 180 to 220 | 7.5-9 | Inversion III | Tissue from ls completely covers the lf |
| The growth of medial subdivisions (ms) anteriorly, partly covering the af | ||||
| Stage 18 | 180 to 220 | 7.5-9 | Prosomal shield | Growth of the rim of precheliceral lobes in the direction of mouth opening |
| Formation of eye vesicles | ||||
| Stage 20 | 220 to 280 | 9-11 | Ventral closure | Distinct brain regions such as optic ganglia are evident, all four pairs of eyes are formed but not pigmented |
Transcriptome analysis and gene sequencing
Total RNA extraction was carried out using Trizol reagent according to manufacturer’s instructions from mixed embryonic stages (Life Technologies, Carlsbad, CA, USA). The extracted RNA was sent to Genecore (EMBL, Heidelberg, Germany) for sequencing (Illumina hi-seq, paired-end 100 bp, not normalised). Following quality filtering of reads and de novo transcript assembly using Velvet and Oases v0.2.08 (PMID: 22368243), we searched the resulting transcript database for matches to proteins downloaded from NCBI. BLAST searches and sequence analysis were done with the computer programme Geneious versions 5.6.6-7.1.5 created by Biomatters (http://www.geneious.com/). Primers were constructed based on sequences found in the assembled transcriptome database using the software primer3 [41]. The primers used are listed in Additional file 1. All genes were amplified and sequenced from embryonic cDNA for confirmation (Additional file 2).
Orthology assignment
We used molecular phylogenetic methods to accurately assign the orthology of the genes. The accession numbers of the genes are provided in Tables in Additional files 3-7. Sequences were aligned using the programme ClustalW and MUSCLE implemented in the Geneious programme (http://www.geneious.com/). Bayesian inference on amino acid data using MrBayes v. 3.1.1 was applied for orthology analysis [42,43].
Fixation of embryos and in situ hybridisation and microscopy
Fixation of embryos and in situ hybridisation was carried out according to Damen and Tautz [44]. After in situ hybridisation, the embryos were counter-stained either with SYBR green (Invitrogen, Waltham, MA, USA) or Hoechst (Sigma-Aldrich, St. Louis, MO, USA). The yolk was removed by micro-needles and hairs; the embryos were flat-mounted before taking photographs with an Olympus BX51 microscope (Olympus, Tokyo, Japan) using Cell^D software.
Results
Eye development
In C. salei, the brain ontogeny starts with a series of depressions that fuse together and form deep invaginations. The first differentiation of the precheliceral region and the first event in optic system formation is the formation of a pair of lateral grooves, termed lateral furrows (lf), laterally on the stomodaeum at 160 to 180 hAEL (Table 1 stage 12 according to [32]; Figure 2A,B). lf leads to formation of a lateral subdivision (ls) (Table 1 stage 14 according to [32]; Figure 2C,D). Lateral furrow formation is accompanied by a median invagination. The latter is deeper and leads to a median subdivision (ms, Figure 2D). The ls migrates towards the lf, partly covering it in the middle, forming a figure-eight-like shape (Figure 2D). In the anterior region of the precheliceral lobes, an anterior furrow (af) or semi-lunar groove forms (Figure 2C,D). Later, at 180 to 220 hAEL, the lf is almost completely covered by the tissue from ls, presumably forming the anlagen of ONs of the lateral eyes ([38]; Figure 2D). The ms grows anteriorly, partly covering the af (Table 1 stage 16 according to [32]; Figure 2D). The rim of the precheliceral lobes grows medially towards the mouth opening and starts to cover the brain; this cover is termed the prosomal shield (Table 1 stage 18 according to [32]; Figure 2E,F,G). The eye vesicles of the SEs already form on the prosomal shield before it has reached the position of the optic neuropils, and the eye vesicles migrate with the prosomal shield, eventually ending up on top of the optic neuropil anlagen (Figure 2F,G). At about 180 to 200 hAEL, two pairs of vesicles form in the median subdivision (Table 1 stage 18 according to [32]; Figure 2E,F,G). These vesicles possibly form the optic neuropils (ON 1 to 2) of the posterior- and anterior-median eyes. As mentioned above, the eye vesicles of SEs have formed in the prosomal shield, migrating towards the mouth to cover the optic neuropils. In other spiders (Heteropoda venatoria and Alopecosa spec.), the AMEs or PEs do not form by vesicle invagination but by swelling of cells from the ectoderm in front of the prosoma [34]. The present investigation confirms that this is the case also in C. salei (Figure 2), where the AMEs or PEs form in the rims of the prosomal shield from a median position and move forward by the progress of the prosomal shield (Figure 2H). The dorsal ridge of the af then fuses with the posterior part of the brain, probably forming the arcuate or central body, which is the third neuropil of the PEs (see the ‘Background’ section). The remaining part of the ms probably participates in the formation of ON3 of SEs or the so-called mushroom bodies (Table 1 stage 20 according to [32]). We performed anti-acetylated tubulin antibody staining in order to follow the nerve fibres forming from ONs. This staining demonstrated that no optic fibres form until stage 18 or the prosomal shield stage (Additional file 2). After the ventral closure stage (Additional file 2) due to cuticle formation, whole-mount antibody staining was no longer technically possible.
Figure 2.
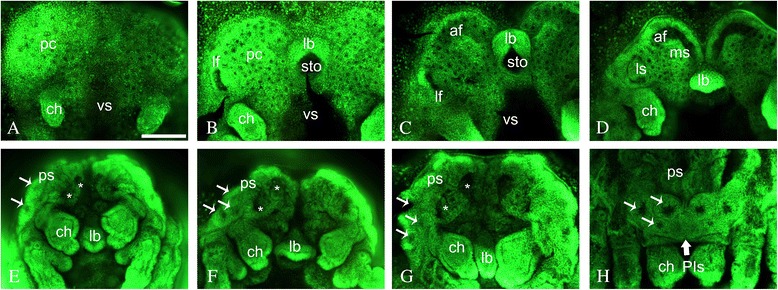
Nuclear staining of different embryonic stages explained in Table 1. (A) prosomal limb buds; (B) lateral furrow; (C) inversion I; (D) inversion III; (E-G) different stages of prosomal shield stage; (H) ventral closure. ch chelicerae, lb labrum, lf lateral furrow, ls lateral subdivision, ms median subdivision, pc precheliceral region or precheliceral lobes, ps prosomal shield, sto stomodaeum, vs ventral sulcus. Scale bar 200 μm.
Orthology of the genes
Phylogenetic analyses of Cs-pax6 genes
We found two orthologs of the pax6 gene named Cs-pax6a and Cs-pax6b. Similar to Yang et al. [16], gene tree reconstruction of the paired domain, homeodomain, and the intervening conserved decapeptide sequence show monophyly of arthropods, lophotrochozoans, and chordates (Additional file 3). Additionally, ey and toy each form a monophylum (pink and yellow box in Additional file 3), except for Anopheles gambiae ey, which groups with toy. The Bayesian likelihood tree categorises Cs-pax6a and Cs-pax6b to the ey and toy monophyla, respectively (Additional file 3). The sequence of Cs-pax6a and the theridiid (cobweb) spider’s pax6.1 from Parasteatoda tepidariorum (Pte-pax6.1) (Schomburg et al. submitted) form a sister group, and Cs-pax6b together with Pte-pax6.2 and Limulus pax6 (Lpo-pax6) form a chelicerate monophylum (Additional file 3). The tree shows that duplication of pax6 possibly occurred before the divergence of chelicerates.
Phylogenetic analyses of Cs-dac genes
Two orthologs of the dachshund gene were found in the spider. The phylogenetic tree of dachshund genes, with ski genes as an outgroup, shows one monophylum each of vertebrates (pink box in Additional file 4) and protostomes (yellow box in Additional file 4). Within the arthropods, arachnids’ dac orthologs appear as a monophylum, showing that spiders’ dac duplication occurred more recently, probably at the base of arachnids (Additional file 4). Cs-daca forms a sister group to Pte-dac1, and Cs-dacb forms a sister group to Pte-dac2 together with both of the eresid (velvet) spider Stegodyphus mimosarum’s Smi-dac orthologs.
Phylogenetic analyses of Cs-atn genes
Two orthologs of the atonal gene were found in the spider. We made a phylogenetic reconstruction of the atonal genes together with other basic helix-loop-helix family genes to clearly demonstrate whether our atonal gene group with atonal genes from the other species. The tree shows a monophylum for neurogenin (ngn), Target of pox neuron (TAP), Scleraxis (scx), and atonal and amos genes (yellow box in Additional file 5). The few exceptions are discussed in Additional file 5. The chelicerates’ atonal genes form a monophylum. Cs-atha and Pte-ath1 as well as Cs-athb and Pte-ath2 each form sister groups (Additional file 5). Limulus has only one atonal ortholog (Lpo-ath) that forms a sister group to both Cs-atha and Pte-ath1 (Additional file 5). This suggests that atonal duplication occurred at the base of arachnids.
Phylogenetic analyses of Cs-otx genes
We found two orthologs of the otx gene in the spider. We constructed the otx tree using aristaless (arx) as an outgroup (Additional file 6). The two orthologs of otx in chelicerates (named otxa and otxb in Cupiennius) do not correspond to otx1 and otx2 of vertebrates, showing that the duplication of the genes in chelicerates occurred independently from duplication of otx in vertebrates. Arthropods’ otx genes do not form a monophylum, and the position of the two chelicerate orthologs within the arthropods is unresolved (Additional file 6).
Phylogenetic analyses of Cs-six genes
The phylogenetic reconstruction of the six family produced a monophylum for each of the six1/2 (Additional file 7 yellow box), six3 (Additional file 7 pink box), and six4 genes (Additional file 7 blue box). The exception is the Apis mellifera six2 gene (which is grouped with six4 genes and is possibly incorrectly assigned to six2). We found two orthologs of six1 and two orthologs of six 3 in the spider. The two orthologs of spiders’ six1 form a sister group to six1 of other arthropods. Cs-six1a forms a sister group to the two other spider six1 genes, namely Pte-so1 and Smi-six1a. Cs-six1b forms a sister group to the spider Smi-six1b, and these two both form a sister group to the spider Pte-so2. This shows that the duplication of six genes probably occurred at the base of the arachnid group. The tree is less well resolved in the case of six3 genes. Arachnid’s six3 genes appear as a monophylum, but the two orthologs do not show a sister relationship. The two copies of Cs-six1 show high similarity of the paired domain, homeodomain, and decapeptide sequences, but areas outside of these domains are unconserved. This results in an only 49% sequence similarity globally; the same holds true for Cs-six3, with 60% similarity globally.
Gene expression patterns
Table 2 summarizes the expression pattern of each gene in different stages of development. Expression has been mainly tested in prosomal limb bud, lateral furrow, prosomal shield, and ventral closure stages. Some of the genes are expressed in structures that are not directly related to the eyes. Examples are expression of Cs-pax6a in the ventral neuroectoderm (not shown) or expression of the genes in other invaginating neural precursor (INP) groups or labrum, which are not discussed here.
Table 2.
Expression of C. salei pax6 , eya , atn , otx , six1 , and six3 in different embryonic stages
| Name of gene | Embryonic stages | |||
|---|---|---|---|---|
| Prosomal limb buds | Lateral furrow | Prosomal shield | Ventral closure | |
| Cs-pax6a | Two symmetric patches at the middle of precheliceral lobes | Medial side of lf | ms in the area of future mushroom body and medial side of lf and other INPs | Vesicles of both PEs |
| Cs-pax6b | _ | An oblique figure-eight-shaped patch in central part of precheliceral lobes and median side of lf | ms in the area of future mushroom body, median INPs | median INPs |
| Cs-eya | Two patches anterior and posterior of future forming lf in precheliceral lobes | Anterior and posterior of lf | Future area of arcuate body of af and forming secondary eye vesicles in the rim of prosomal shields | Vesicles of SEs |
| Cs-daca | _ | _ | In the rim of prosomal shields (forming vesicles of lateral eyes) | _ |
| Cs-dacb | Lateral and medial of lf and INPs in the place of forming af | Prosomal shield and medial INPs | In the rim of prosomal shields around the forming eye vesicles | In the prosomal shields around the forming eye vesicles |
| Cs-atna | In the lateral-most sides of precheliceral lobes, at the site of forming lf | In the forming ms and two symmetric patches under forming af | In the INPs of precheliceral lobes and future mushroom body area of af | _ |
| Cs-atnb | _ | Median side of lf and few INPs in the area of ms | Median side of lf and future mushroom body area of af | INPs of precheliceral lobes |
| Cs-otxa | Two symmetric patches in the lateral and median sides of precheliceral lobes | ls and INPs of ms | Median INPs of precheliceral lobes | Vesicle of PLEs |
| Cs-otxb | Two symmetric patches in the centre of precheliceral lobes | lf, INPs of ms and other INPs | Future mushroom body area of af and INPs at median and lateral sides of precheliceral lobes | Vesicles of all SEs |
| Cs-six1a | _ | Lateral side of lf | In the rim of prosomal shield at the area of forming SEs | All SE vesicles |
| Cs-six1b | _ | Median region of lf and INPs of ms | Future area of arcuate body of af and in the rim of prosomal shields (forming vesicles of posterior eyes) and future mushroom body area of af | Vesicles of PMEs and PLEs, faint expression in the vesicles of PEs |
| Cs-six3a | 3 pairs of patches (two lateral and one medial) in the precheliceral lobes | Median side of lf and future mushroom body area of af | In the rim of prosomal shield (forming eye vesicles of lateral eyes) and ON of PE | Vesicles of PLEs and PMEs and ON of PE |
| Cs-six3b | _ | In the ls and ms in the area of future mushroom body of af | Forming vesicles of SEs in the rim of prosomal shield and median part of af in the region of future mushroom body of af | All SE vesicles |
Expression of Cs-pax6a
In the prosomal limb bud stage, Cs-pax6a is expressed in two symmetric patches at the middle of the precheliceral lobes (Figure 3A1,A2). In the lateral furrow stage, expression is detected in medial side of lf (Figure 3A3). This expression pattern is consistent in the inversion I stage (Figure 3A4). The gene is expressed in ms in the area of future mushroom body and on the medial side of lf and other INPs in the prosomal shield stage (Figure 3A5). In the ventral closure stage, the gene is expressed in vesicles of both PEs (Figure 3A6,A7,A8).
Figure 3.
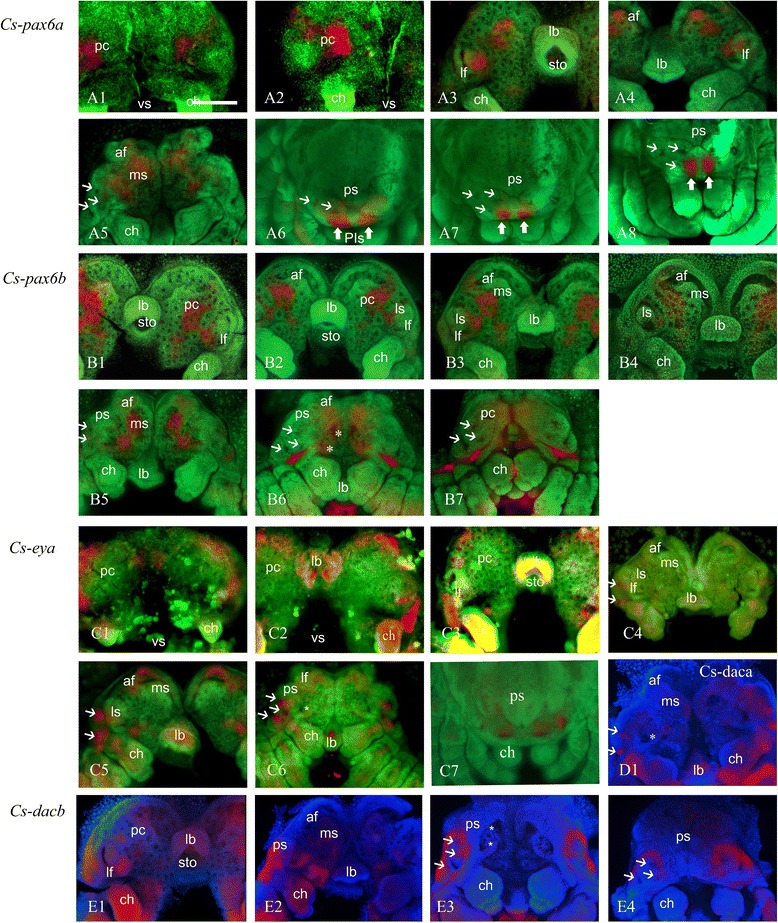
Expression of C. salei pax, eya, and dac in different stages of development. Embryos are flat-mounted and counter stained with SYBR green or Hoechst. (A1-A8) Cs-pax6a, (B1-B7) Cs-pax6b, (C1-C6) Cs-eya, (D1) Cs-dac1, (E1-E4) Cs-dac2. (A1), (A2), (C1), (C2) prosomal limb buds; (A3), (B1), (C3), (E1) lateral furrow; (A4), (B2), (C4) inversion I; (B3), (B4) inversion III; (A5), (B5), (B6), (C5), (C6), (D1), (E2), (E3) prosomal shield; (A6), (A7), (A8), (B7), (C7), (E4) ventral closure. Thin arrows: secondary eye vesicles, Thick arrows: principal eye anlagen, asterisk: ON of median eyes. ch chelicerae, lb labrum, lf lateral furrow, ls lateral subdivision, ms median subdivision, pc precheliceral region or precheliceral lobes, ps prosomal shield, sto stomodaeum, vs ventral sulcus. Scale bar 200 μm.
Expression of Cs-pax6b
Cs-Pax6b is not expressed in the prosomal limb bud stage. The first sign of expression pattern is evident in the lateral furrow stage, where an oblique figure-eight-shaped patch is detectable in central part of the precheliceral lobes and median side of lf (Figure 3B1). This expression remains the same in the inversion I stage (Figure 3B2) and inversion III stage (Figure 3B3,B4). In the prosomal shield stage, the gene is expressed in ms in the area of the future mushroom body and median INPs (Figure 3B4,B5,B6). This expression pattern includes expression of the gene in the anlage of ONs of median eyes (asterisk in Figure 3B6). In the ventral closure stage, the gene is expressed in median INPs.
Expression of Cs-eya
In the prosomal limb bud stage, Cs-eya is expressed in two patches anterior and posterior of future lf in precheliceral lobes (Figure 3C1,C2). The expression is detected anterior and posterior of lf in the lateral furrow stage (Figure 3C3). This expression is consistent in the inversion I stage (Figure 3C4). The future area of the arcuate body of af and the forming secondary eye vesicles in the rim of prosomal shields are the areas which express Cs-eya in the prosomal shield stage (Figure 3C5,C6). In the ventral closure stage, the gene expression is seen in the vesicles of SEs (Figure 3C7).
Expression of Cs-daca
Cs-daca is expressed only in the rim of prosomal shields (forming vesicles of lateral eyes) in the prosomal shield stage (Figure 3D1).
Expression of Cs-dacb
In the lateral furrow stage, Cs-dacb is expressed lateral and medial of lf and INPs at the site of forming af (Figure 3E1). In the prosomal shield stage, the gene is expressed in prosomal shield and medial INPs (Figure 3E2,E3). The gene expression is observed in the rim of prosomal shields around the forming eye vesicles in the ventral closure stage (Figure 3E4).
Expression of Cs-atna
In the prosomal limb bud stage, Cs-atna is expressed in the lateral-most sides of precheliceral lobes, at the site of forming lf (Figure 4A1). In the lateral furrow stage, the gene expression is detected in the forming ms and two symmetric patches under forming af (Figure 4A2). The gene is expressed in the INPs of precheliceral lobes and future mushroom body area of af in the prosomal shield stage (Figure 4A3). The gene is not expressed in the ventral closure stage.
Figure 4.
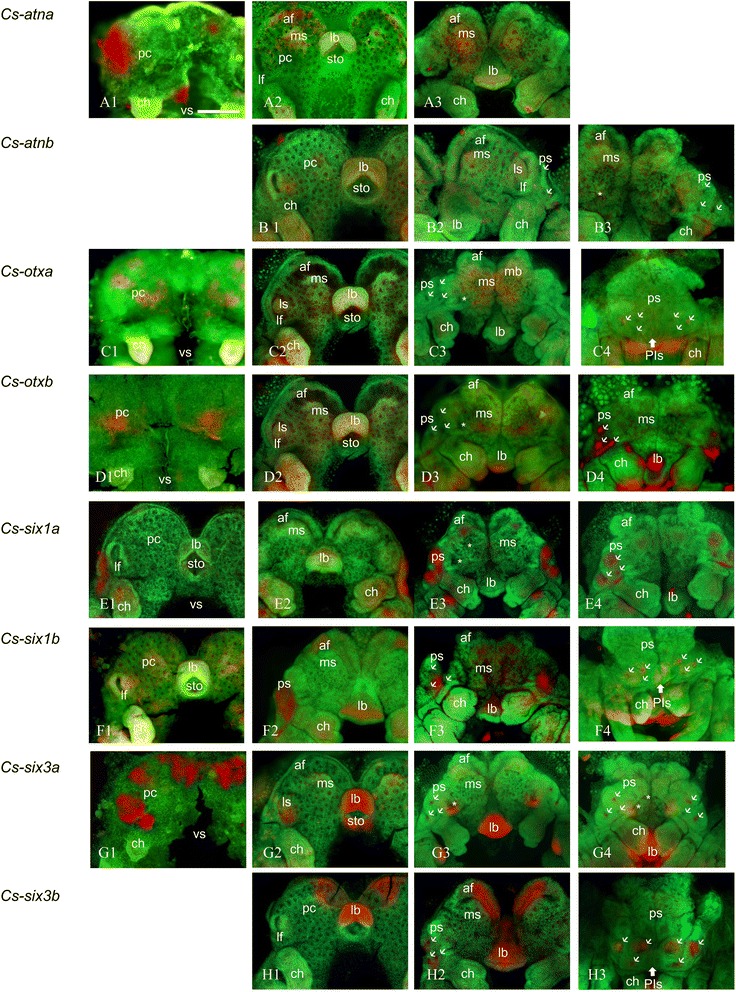
Expression of C. salei atn, otx, six1, and six2 in different stages of development. Embryos are flat-mounted and counter stained with SYBR green. (A1-A3) Cs-atna, (B1-B3) Cs-atnb, (C1-C4) Cs-otxa, (D1-D4) Cs-otxb, (E1-E4) Cs-six1a, (F1-F4) Cs-six1b, (G1-G4) Cs-six3a, (H1-H3) Cs-six3b. (A1), (C1), (D1), (G1) prosomal limb buds; (A2), (B1), (E1), (F1), (G2), (H1) lateral furrow; (C2), (D2), (E2) inversion I; (F3) inversion III; (A3), (B2), (B3), (C3), (D3), (E3), (F3), (G3), (G4), (H2) prosomal shield; (C4), (D4), (E4), (F4), (H3) ventral closure. Thin arrows: secondary eye vesicles, Thick arrows: principal eye anlagen, asterisk: ON of median eyes. ch chelicerae, lb labrum, lf lateral furrow, ls lateral subdivision, ms median subdivision, pc precheliceral region or precheliceral lobes, ps prosomal shield, sto stomodaeum, vs ventral sulcus. Scale bar 200 μm.
Expression of Cs-atnb
Cs-atnb is not expressed in the prosomal shield stage. The first sign of expression appears at the lateral furrow stage in median side of lf and few INPs in the area of ms (Figure 4B1). In the prosomal shield stage, the gene is expressed in median side of lf and future mushroom body area of af (Figure 4B2,B3). The gene is not expressed in the ventral closure stage (data not shown).
Expression of Cs-otxa
In the prosomal limb bud stage, Cs-otxa is expressed in two symmetric patches in the lateral and median sides of precheliceral lobes (Figure 4C1). In the lateral furrow stage, the gene is expressed in ls and INPs of ms (Figure 4C2). The expression is detected in median INPs of precheliceral lobes in the prosomal shield stage (Figure 4C3). In the ventral closure stage, the pattern is detected in the vesicle of PLEs.
Expression of Cs-otxb
In the prosomal limb bud stage, Cs-otxb is expressed in two symmetric patches in the centre of precheliceral lobes (Figure 4D1). In the lateral furrow stage, the pattern is detected in lf, INPs of ms, and other INPs (Figure 4D2). In the prosomal shield stage, the gene is expressed in the future mushroom body area of af and INPs at median and lateral sides of precheliceral lobes (Figure 4D3). The pattern is detectable in the vesicles of all SEs in the ventral closure stage (Figure 4D4).
Expression of Cs-six1a
Cs-six1a is not expressed in the prosomal limb bud stage (data not shown). In the lateral furrow stage, the first sign of expression pattern is detectable in lateral side of lf (Figure 4E1). The expression is the same in the inversion I stage (Figure 4E2). In the prosomal shield stage, the gene is expressed in the rim of the prosomal shield at the area of forming SEs (Figure 4E3). In the ventral closure stage, the gene is expressed in the vesicles of all SEs (Figure 4E4).
Expression of Cs-six1b
Cs-six1b is not expressed in the prosomal shield stage (data not shown). The median region of lf and INPs of ms is the areas that express Cs-six1b in the lateral furrow stage (Figure 4F1). The gene is expressed in lateral side of lf in the inversion III stage (Figure 4F2). The gene expression is detected in future area of the arcuate body of af and in the rim of prosomal shields (forming vesicles of posterior eyes) and future mushroom body area of af in the prosomal shield stage (Figure 4F3). In the ventral closure stage, vesicles of PMEs and PLEs show expression of Cs-six1b. In addition, we detected a faint expression of the gene in the PE vesicles (Figure 4F4).
Expression of Cs-six3a
In the prosomal limb bud stage, three pairs of patches (two lateral and one medial) in the precheliceral lobes express Cs-six3a (Figure 4G1). In the lateral furrow stage, the gene is expressed in median side of lf and future mushroom body area of af (Figure 4G2). The rim of the prosomal shield (forming eye vesicles of lateral eyes) and ON of PE are the places that express Cs-six3a in the prosomal shield stage (Figure 4G3). In the ventral closure stage, the gene expression appears in the vesicles of PLEs and PMEs and ON of PE (Figure 4G4).
Expression of Cs-six3b
Cs-six3b is not expressed in the prosomal limb bud stage. The expression in the lateral furrow stage occurs in the ls and ms in the area of the future mushroom body of af (Figure 4H1). In the prosomal shield stage, the gene is expressed in forming vesicles of SEs in the rim of the prosomal shield and in the region of the future mushroom body of af (Figure 4H2). In the ventral closure stage, the gene is detectable in all SE vesicles (Figure 4H3).
Expression in eye vesicles
Several of the genes we studied are expressed in the forming vesicles of the eyes, which start at the prosomal shield stage (180 to 220 hAEL) and continue to the ventral closure stage (220 to 280 hAEL). The genes are expressed differentially in different vesicles (Figures 3 and 4). Cs-pax6a is expressed in the area where the anlagen of PEs delaminates (Figure 3A6,A7,A8). This is not the case for Cs-pax6b, which is expressed in two patches of INPs in ms (Figure 3B5), which later corresponds to the ON of the median eyes (Figure 3B6). In addition, Cs-six1b and Cs-eya show a faint expression pattern in the vesicles of PEs (Figures 4F4 and 5A2). Cs-dacb is not expressed in the eye vesicles themselves but in an area surrounding the vesicles of SEs (Figure 3E2,E3,E4). Cs-eya (Figure 3C5,C6; Figure 5A1,A2), Cs-otxb (Figure 4D4; Figure 5D), Cs-six1a (Figure 4E3,E4; Figure 5E1,E2), and Cs-six3b (Figure 4H2,H3; Figure 5H1,H2) are expressed in the forming SE vesicles, Cs-daca (Figure 3D1; Figure 5B) PLEs and ALEs, Cs-otxa in the vesicles of PLEs (Figure 4C4; Figure 5C), and Cs-six3a (Figure 4G3,G4; Figure 5G1,G2) and Cs-six1b in the vesicles of PMEs and PLEs (Figure 4F3,F4; Figure 5F).
Figure 5.
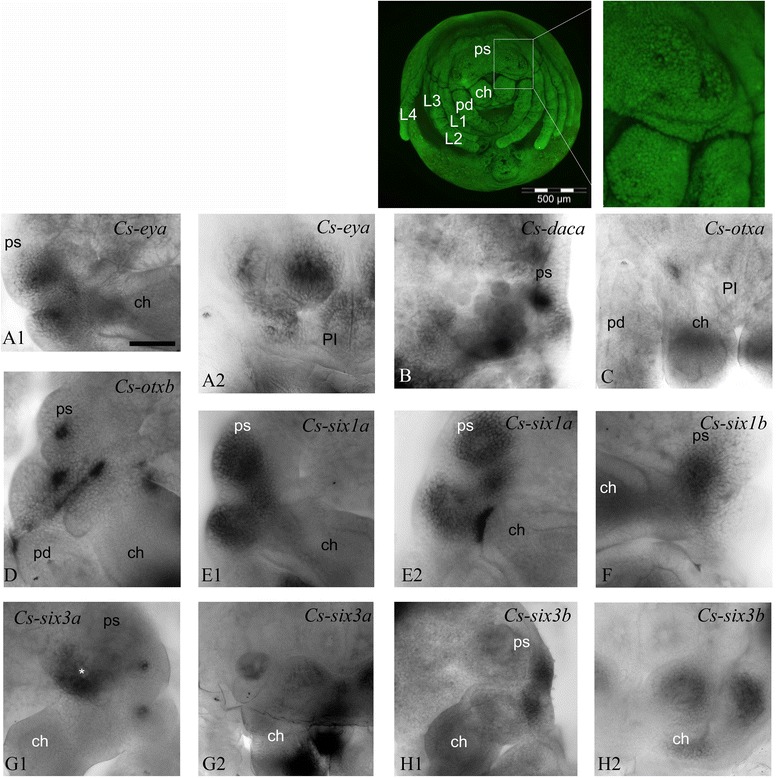
Expression of the RDGN elements in eye vesicles in prosomal shield and ventral closure stages. (A1), (A2) Cs-eya; (B) Cs-daca; (C) Cs-otxa; (D) Cs-otxb; (E1), (E2) Cs-six1a; (F) Cs-six1b; (G1), (G2) Cs-six3a; (H1), (H2) Cs-six3b. (A1), (B), (E1), (F), (G1), (H1), prosomal shield; (A2), (C), (G2), (H2), ventral closure. ch chelicerae, pd pedipalps, ps prosomal shield, asterisk: ON of median eyes. Scale bar 100 μm.
Discussion
Expression around the lateral furrow
The expression pattern of most of the genes starts before the onset of eye development at the prosomal limb bud stage, 100 to 130 hAEL (Table 2, Figures 3 and 4). Some of the genes, however, do not show any expression at this stage (Table 2, Figures 3 and 4). At the prosomal limb bud stage, Cs-pax6a shows two symmetric patches of expression at the centre of the precheliceral area (Figure 3A1,A2); Cs-eya shows two symmetric patches of expression anterior and posterior of future forming lf (Figure 3C1,C2); Cs-atha shows two symmetric patches in the lateral-most part of precheliceral lobes at the site of future lf (Figure 4A1); Cs-dacb (Figure 3E1) and Cs-otxa (Figure 4C1) show two pairs of symmetric patches on the lateral and medial sides of the precheliceral lobes; Cs-otxb shows two symmetric patches in the middle of the precheliceral lobes (Figure 4D1); and Cs-six3a shows three pairs of expression patches (two in the lateral and one in the medial regions of the precheliceral lobes; Figure 4G1). These genes are later (160 to 180 hAEL) expressed around the area of lf. Cs-pax6a (Figure 3A3) and b (Figure 3B1,B2,B3,B4), Cs-atnb (Figure 4B1), Cs-otxa (Figure 4C2), Cs-six1b (Figure 4F1), and Cs-six3a (Figure 4G2) show overlapping expression in the median side of the lateral furrow where the lateral subdivision is forming. Cs-eya is visible as two symmetric pairs of expression areas anterior and posterior of lf (Figure 3C2,C3). Cs-six1a is expressed in the lateral side of lf (Figure 4E1). Cs-six3b shows no pattern of expression around lf at this stage (Figure 4H1).
Expression in optic neuropils of the median eyes
Cs-dacb (Figure 3E1) and Cs-six3a (Figure 4G3) are the only two genes whose expression corresponds to the area of the forming first and second optic neuropil anlagen of the PEs. Cs-pax6a (Figure 3A5) and Cs-pax6b expression corresponds to the anlage of ONs of both median eyes (asterisks on Figure 3B6).
Expression in the area of the mushroom body anlage of the anterior furrow
At 180 to 220 hAEL, the ms grows towards the af and partly covers it to form the mushroom body or ON3 of SEs. Several of the genes including Cs-pax6a (Figure 3A4,A5) and b (Figure 3B5), Cs-atna (Figure 4A3) and b (Figure 4B2), Cs-otxa (Figure 4C3) and b (Figure 4D3), Cs-six1a (Figure 4E3), and Cs-six3a (Figure 4G3) are expressed in this area at this stage.
Expression in the area of the arcuate body anlage of the anterior furrow
Cs-eya (Figure 3C4,C5) as well as Cs-six1b (Figure 4F2) and 3b (Figure 4H2) are expressed in the area of the anterior furrow in the region of the forming arcuate body or ON3 of PEs at 180 to 220 hAEL.
Eye development and differential expression of the genes
Eye development in the spider C. salei is somewhat unconventional, initiating with the formation of the ON anlagen in the differentiating neuroectoderm. Anti-acetylated tubulin staining does not show any optic fibres forming until stage 18. This contradicts the description of ON formation by Doeffinger et al. [38], who note ONs already at 160 to 180 hAEL. We corroborate Doeffinger et al. in stating that the ‘anlagen’ of optic neuropils form from lf, medial invaginations, and af. ONs of the lateral eyes form earlier than the ONs of the median eyes. The ONs of the lateral eyes form with the lf, and the ONs of the median eyes form when two pairs of vesicles are separated from the median subdivision. Afterwards, af forms what will give rise to the arcuate body or ON3 of PEs; the growth of ms over the posterior part of af forms the mushroom body or ON3 of SEs. Only then do the vesicles of SEs form in the prosomal shield and start migrating over ON to make contact with them. This contradicts the observation of Doeffinger et al. [38], who assume that lf separates from the surface ectoderm and forms the optic vesicles of lateral eyes. This unconventional process of eye formation may explain why several of the RDGN genes initiate to show their expression in ON anlagen. At the prosomal limb bud stage, the area of the future lateral furrow is discernible by overlapping expression of several RDGN genes (Figure 6A). In addition to this area, Cs-six3a marks the area of the future anterior furrow (Figure 6A). Cs-eya and Cs-six1a mark the lateral-most side of the prosomal lobes, where the prosomal shield later forms (Figure 6A,B). In the ON anlagen of lateral eyes (lateral furrow), most of the elements of RDGN demonstrate expression around this region (Figure 6B). Expressions of several of the genes map to the area where lateral subdivision takes place (Figure 6B). Cs-pax6a and Cs-pax6b show complementary non-overlapping expression in this area (Figure 6B). In contrast, Cs-pax6a and Cs-pax6b have overlapping expression in the ONs of the median eye (Figure 6C). Different sets of genes are expressed in ON3 of PEs and SEs (Figure 6). In earlier stages of development, the expressions of RDGN genes in P. tepidariorum (with the exception of Pte-so genes) are similar to the expression of the Cupiennius homologs (Schomburg et al. submitted). In Limulus, Lp-pax6 and Lp-atn are not expressed during the formation of different eyes [15]. According to our phylogenetic analyses, Lp-pax6 cluster together with Cs-pax6b in the toy monophylum. Cs-pax6b, similar to Lp-pax6, is not directly expressed in the eyes, but rather in the ON of the median eyes and other brain regions. The same applies for Cs-atn, which has an expression similar to Lp-atn expression patterns in brain centres rather than directly in the eyes [15]. The lack of an ey ortholog in Limulus could reflect deficiency in PCR screening; this requires further investigation to finalize the possible role of ey in median eye development in Limulus.
Figure 6.
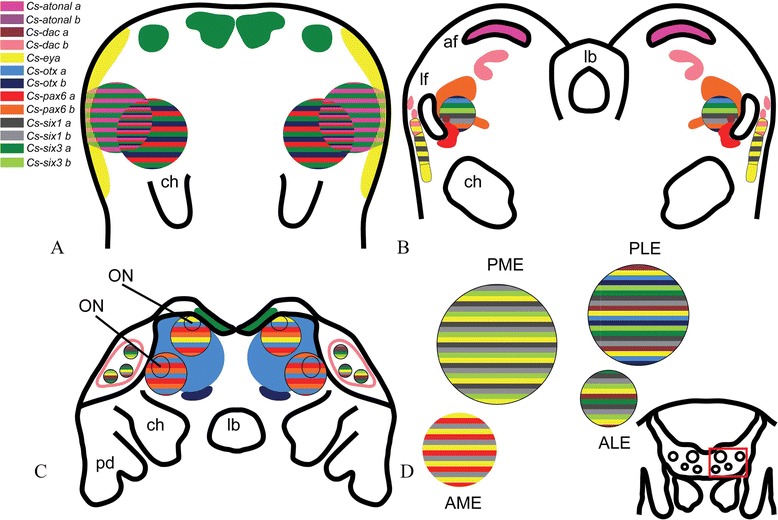
Scheme summarizing the expression of RDGN genes in the main embryonic stages and the vesicles of the eyes. (A) Prosomal limb buds. (B) Lateral furrow. (C) Prosomal shield. (D) Ventral closure. af anterior furrow, ALE anterior-lateral eyes, AME anterior-median eyes, ch chelicerae, lb labrum, lf lateral furrow, ON optic neuropil, pd pedipalps, PLE posterior-lateral eyes, PME posterior-median eyes.
The RDGN genes also show differential expression in the eye vesicles, so that each developing eye can be distinguished by a specific set of transcription factors (Figures 7 and 6D). For example, Cs-pax6a, Cs-six1b, and Cs-eya determine the PEs (Figures 7 and 6D). This is the same as principal eye determination in the spider P. tepidariorum, with the difference that, in that species, Pte-Otd genes also are expressed in the PEs, which is not the case in C. salei (Schomburg et al. submitted). Cs-eya, Cs-otxb, Cs-six1a, Cs-six3b, and Cs-daca determine ALEs (Figures 7 and 6D). Excluding otxb, the same genes determine the ALEs in P. tepidariorum, with the difference that, in this spider, Pt-so2 is also expressed in ALEs (Schomburg et al. submitted). The same set of genes as ALEs together with Cs-otxa, Cs-six1b, and Cs-six3a distinguish PLEs (Figures 7 and 6D). This is different from PLEs in P. tepidariorum. The only similar genes in this pair of eyes are eya, six1a or so1, and six3a or six3.2. Cs-eya, Cs-otxb, Cs-six1a, and Cs-six1b together with Cs-six3a and Cs-six3b determine PMEs (Figure 6 and 6D). In P. tepidariorum, in addition to these genes also dac orthologs are expressed in the PMEs. Each eye pair is apparently specified by a different combination of RDGN transcription factors, and recruitment of the genes in different eyes was at least partly independent in the two spiders. The reason for differential gene expression among the secondary eyes is probably the diverging function of the adult eyes combined with genetic drift [45]. The analysis of gene expression pattern, however, also makes it possible to distinguish the secondary from the principal eyes. The lateral eyes share the expression of four out of a total of eight genes expressed in secondary eyes, whereas all eyes (principal + secondary) have only one gene in common (Figure 7). To further assess how these genes interact with each other and how the gene networks have evolved in each eye, we aim to perform knockdown experiments of the key genes in each eye vesicle and follow how the expression of the other RDGN genes is affected.
Figure 7.
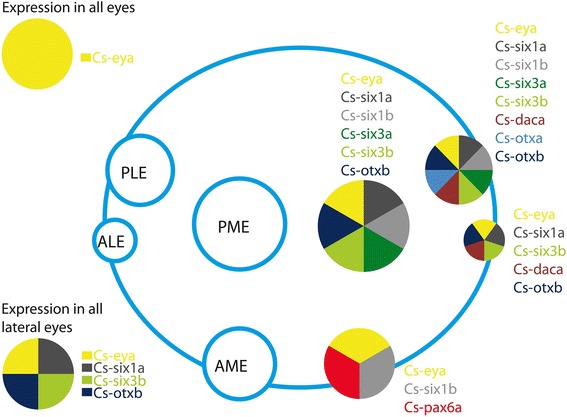
Schematic expression of the genes in the eye vesicles. ALEs anterior-lateral eyes, AMEs anterior-median eyes, PLEs posterior-lateral eyes, PMEs posterior-median eyes.
Origin of ey and toy in arthropods
Yang et al. (2009) suggested that the duplication generating ey and toy occurred before the diversification of the major arthropod subgroups Pancrustacea, Myriapoda, and Chelicerata because there are two pax6 genes in the insects Drosophila and Tribolium and in the myriapod Glomeris. Our data support this hypothesis by demonstrating the existence of both homologs of ey (Cs-pax6a and Pte-pax6.1) and toy (Cs-pax6band Pte-pax6.2) in the two arachnids, with possible different functions due to different gene expression patterns. The lack of two pax6 genes in Limulus [15] may merely reflect technical deficiency of PCR screening.
pax-dependent and pax-independent gene networks regulating principal and secondary eye formation
ey and toy are among the earliest selector genes expressed in the eye-antennal imaginal disc in Drosophila. Their expression becomes specific to the eyefield during the second larval instar (reviewed by [4]). Both are also present in the region of the eye disc, where the ocelli develop (reviewed by [4]). It remains unclear, however, whether this expression is down regulated once ocellus differentiation initiates. Expression of toy has not been observed in the Bolwig organ, and reports considering the expression of ey are conflicting (reviewed by [4]). In Tribolium, knockdown of ey and toy affects larval eye development strongly but adult eye development only mildly. Compound eye loss was reported in the combination of ey, toy, and dac knockdown [16]. The homolog of ey in the spider Cs-pax6a is expressed in the area of the lateral furrow and median subdivision, where most of the ey downstream genes in the RDGN are expressed. Cs-pax6a is the sole gene of those investigated that is expressed in the PE vesicles of Cupiennius (Figure 5). Cs-eya and Cs-six1b show a weak expression in this pair of eyes (Figure 7). The need for pax6 activation during PE development is consistent with the data that consider a universal role of pax6 in animal eye development [46]. This expression in PEs may activate other genes such as eya and six. Future knockdown experiments may identify the molecules functioning downstream of pax6 genes in the PEs. It is also possible that, in Cupiennius, RDGN genes downstream of pax6 function only in the optic neuropil instead of the retina. Another possibility is that Cs-pax6a is involved in transcriptional control of eye differentiation in other ways, such as activation of opsin and the pigmentation process, as was also suggested in Platynereis eye development [9]. The direct role of the pax6 gene in photoreceptor cell differentiation [46] clearly does not apply for all spider eyes. The SEs differentiate in the absence of Cs-pax. How then can the redundancy of pax6 genes for SEs be explained? Co-existence of distinct eye types, differentiating with or without pax6, has also been shown for other groups, for example, adult eye differentiation in the absence of pax6 in polychaetes [9], squid [6], horseshoe crabs [15], myriapods [13], and the Joseph cells and Hesse organs of lancet fish [14]. The difference between eye determination pathways in the PEs and SEs can also be explained by the ways in which the eyes develop. PE development involves swelling of the epidermis rather than vesicle formation and yields the so-called inverted eyes, which exhibit everse retina despite the reversal of the epidermal layer [47]. These two eye types are also functionally different: the forward-facing pair of PEs have narrow fields of view but high resolution that probably detect shape, whereas the three pairs of SEs have wide fields of view and function as motion detectors [48].
Homology of spider eyes with eyes of other arthropods
The last common ancestor of euarthropods is thought to have been equipped with a pair of lateral facetted eye and two pairs of median ocelli-type eyes. This eye ground pattern changed in the different lineages, yielding the great variability in visual equipment present today among euarthropods [47,49]. Arachnids have two externally visible PEs that are regarded as median eyes (reviewed [47]). Works of Homann suggest that the PEs are homologous to ocelli (reviewed by [47]). Onychophoran eyes show a mixture of annelid and arthropod characters [47], and a homology of the onychophoran eyes with the median or lateral ocelli of euarthropods has been suggested [50,51]. The consensus is thus that the median eyes of chelicerates, the crustacean nauplius eyes, and the insect ocelli are homologous. All these eye types demonstrate a pax-dependent development pathway that further supports their homology at the molecular level.
Spiders lack lateral facetted eyes but instead have up to three pairs of ocelli-type lateral eyes that are believed to have evolved from a facetted eye of the type present in Limulus. One suggestion is that the original facetted eye splits up into separate units, each with a common cuticular lens, a condition present among present day scorpions; this ommatidial organization was subsequently lost in the spider lineage. It is also possible that the lateral eyes of spiders evolved from three separate pairs of ommatidia [47]. The present study shows that each of the four eye pairs of spiders expresses a unique combination of RDGN genes. The separate RDGN profile of the spider eyes suggests another evolutionary scenario, namely a de novo origin of each of the lateral and/or median eyes in the arachnid lineage (except scorpions). If the lateral eyes had evolved from a single facetted eye, they would be expected to share a common molecular patterning mechanism. This is especially the case because, at least in C. salei, all lateral eyes seem to have the same function [48] and express the same set of opsins [11,52]. Nonetheless, further molecular characterisation of the different lateral eyes might reveal differences that are currently unrecognisable. Hence, we cannot rule out the possibility of common origin of the lateral eyes with subsequent differentiation of function accompanied by a gradual shift in the RDGN.
Conclusions
Here, we report for the first time the duplication of several genes of the RDGN in an arachnid. The two pax6 genes show homology to ey and toy and demonstrate divergent expression patterns. This differential expression pattern is also true for the remaining duplicated genes, namely six1, six3, atn, otx, and dac. The elements of the RDGN demonstrate expression patterns differentially in different eye vesicles. Moreover, PE development shows pax6a (ey) expression, suggesting pax6 dependence, although secondary eyes develop independently of pax6 genes and show differential expression of several RDGN genes. Comparing this with the other arthropods suggests that pax6-dependent median eye development is a ground pattern of eye development in this group and that the ocelli of insects, the median eyes of chelicerates, and nauplius eyes can be homologised. The expression pattern also makes it possible to distinguish between secondary eyes and principal eyes, but differences among the secondary eyes are probably due to functional divergence and genetic drift. The expression of the genes extends to the anlagen of ONs of the eyes, and different eye ONs show different combinations of the transcription factors. For example, ON anlagen of median eyes is developed with pax6b expression, but lateral eyes show a combination of different genes independent of pax6. Similarly, different combinations of the RDGN genes pattern the mushroom bodies and arcuate bodies, which are considered to be the third optic neuropil of secondary and principal eyes, respectively.
Acknowledgements
This work was funded by the Austrian Research Council (FWF) (grant numbers M1296-B17, P26228-B24) to BJE. David Fredman is thanked for assembling the transcriptome.
Abbreviations
- af
anterior furrow
- ALEs
anterior-lateral eyes
- AMEs
anterior-median eyes
- INPs
invaginating neural precursors
- lf
lateral furrow
- ls
lateral subdivision
- ms
medial subdivision
- ON
optic neuropil
- PEs
principal eyes
- PLEs
posterior-lateral eyes
- PMEs
posterior-median eyes
- ps
prosomal shield
- RDGN
retinal determination gene network
Additional files
Primer sequences used for amplification of the genes.
Anti-alpha-acetylated tubulin and nuclear staining in prosomal shield (A) and ventral closure (B) stages. These are the first stages showing any sign of nerve fibre formation in the embryos. We therefore assume that optic neuropils are formed later during development when whole-mount antibody staining is no longer technically possible anymore because the cuticle inhibits antibody penetration.
Phylogenetic tree of bilaterian pax6 genes based on the paired domain, the homeodomain, and the conserved regions in the linker between the two DNA binding domains. Arthropod pax6 genes do cluster in two groups of eyeless- and toy-like genes as previously shown by Callaerts et al. 2006. Csa-pax6a (black arrow) clusters together with Pte-pax6.1 as the sister group to ey genes (the pink box) Csa-pax6b (black arrow) clearly clusters together with toy-like genes as the sister group to Pte-Pax6.2 gene (the yellow box) with the exception of Aga-ey.
Phylogenetic tree of bilaterian dachshund genes. dac1 of vertebrates and dac of protostomes form a monophylum each (purple and yellow boxes, respectively).
Phylogenetic tree of bilaterian bHLH genes with twist as outgroup. The tree shows a monophylum for neurogenin (ngn), Target of pox neuron (TAP), Scleraxis (scx), and atonal and amos genes (yellow box) with the exception of Ceratitis capitata ngn1, Musca domestica scx, and Nasonia vitripennis dmd which cluster together with atonal and amos. This is probably due to mislabeling of the genes in automated annotation methods. Chelicerates’ atonal forms a monophylum with Cs-atha as the sister group to Pte-ath1. These two form a sister group to Limulus ath and Cs-athb forms a sister group to Pte-ath2. The sister relationship between atonal and amos is not clear. Other Cupiennius bHLH genes (Cs-bHLH a, b, and c) cluster together with atonal 8.
Phylogenetic tree of bilaterian otx genes with aristaless ( arx ) as outgroup. The two orthologs of otx in chelicerates (named otxa and otxb in Cupiennius) do not correspond to otx1 and otx2 of vertebrates showing that the duplication of the genes in chelicerates had happened independently from duplication of otx in vertebrates. Arthropods’ otx genes do not form a monophylum, and position of chelicerates’ two orthologs within the arthropods is unresolved.
Phylogenetic tree of bilaterian six1/2, six3, and six4 genes based on the six protein-protein interaction domain and the homeodomain. The tree is rooted with six4. six1/2 and six3 genes form monophyla (purple and yellow, respectively). Spider’s six1a and b group with six1/2 monophylum forming a sister relationship with each other and six3a and b group with six3 genes forming a sister relationship with each other.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
LS performed the experiments and phylogenetic analyses and wrote the manuscript. BJE designed and supervised the experiments, performed the transcriptome analyses, illustrated the schemes, and contributed to writing the manuscript. AS contributed to writing the manuscript. All authors have read and approved of the final version of the manuscript.
Contributor Information
Leyli Samadi, Email: leili.samadi@univie.ac.at.
Axel Schmid, Email: axel.schmid@univie.ac.at.
Bo Joakim Eriksson, Email: joakim.eriksson@univie.ac.at.
References
- 1.Arendt D. Evolution of eyes and photoreceptor cell types. Int J Dev Biol. 1993;47:563–71. [PubMed] [Google Scholar]
- 2.Lamb TD, Arendt D, Collin SP. The evolution of phototransduction and eyes. Phil Trans Roy Soc B Biol Sci. 2009;364(1531):2791–3. doi: 10.1098/rstb.2009.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Marquardt T, Ashery-Padan R, Andrejewski N, Scardigli R, Guillemot F, Gruss P. Pax6 is required for the multipotent state of retinal progenitor cells. Cell. 2001;105(1):43–55. doi: 10.1016/S0092-8674(01)00295-1. [DOI] [PubMed] [Google Scholar]
- 4.Friedrich M. Ancient mechanisms of visual sense organ development based on comparison of the gene networks controlling larval eye, ocellus, and compound eye specification in Drosophila. Arthropod Struct Dev. 2006;35(4):357–78. doi: 10.1016/j.asd.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 5.Hartmann B, Lee PN, Kang YY, Tomarev S, de Couet HG, Callaerts P. Pax6 in the sepiolid squid Euprymna scolopes: evidence for a role in eye, sensory organ and brain development. Mech Dev. 2003;120(2):177–83. doi: 10.1016/S0925-4773(02)00456-2. [DOI] [PubMed] [Google Scholar]
- 6.Tomarev SI, Callaerts P, Kos L, Zinovieva R, Halder G, Gehring W, et al. Squid Pax-6 and eye development. Proc Natl Acad Sci U S A. 1997;94(6):2421–6. doi: 10.1073/pnas.94.6.2421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Callaerts P, Munoz-Marmol AM, Glardon S, Castillo E, Sun H, Li W-H, et al. Isolation and expression of a Pax-6 gene in the regenerating and intact Planarian Dugesia(G)tigrina. Proc Natl Acad Sci. 1999;96(2):558–63. doi: 10.1073/pnas.96.2.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Loosli F, Kmita-Cunisse M, Gehring WJ. Isolation of a Pax-6 homolog from the ribbonworm Lineus sanguineus. Proc Natl Acad Sci. 1996;93(7):2658–63. doi: 10.1073/pnas.93.7.2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arendt D, Tessmar K, de Campos-Baptista M-IM, Dorresteijn A, Wittbrodt J. Development of pigment-cup eyes in the polychaete Platynereis dumerilii and evolutionary conservation of larval eyes in Bilateria. Development. 2002;129(5):1143–54. doi: 10.1242/dev.129.5.1143. [DOI] [PubMed] [Google Scholar]
- 10.Keller RG, Desplan C, Rosenberg MI. Identification and characterization of Nasonia Pax genes. Insect Mol Biol. 2010;19:109–20. doi: 10.1111/j.1365-2583.2009.00921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Eriksson B, Samadi L, Schmid A. The expression pattern of the genes engrailed, pax6, otd and six3 with special respect to head and eye development in Euperipatoides kanangrensis Reid 1996 (Onychophora: Peripatopsidae) Dev Genes Evol. 2013;223(4):237–46. doi: 10.1007/s00427-013-0442-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fortunato S, Leininger S, Adamska M. Evolution of the Pax-Six-Eya-Dach network: the calcisponge case study. EvoDevo. 2014;5(1):23. doi: 10.1186/2041-9139-5-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prpic N-M. Duplicated Pax6 genes in Glomeris marginata (Myriapoda: Diplopoda), an arthropod with simple lateral eyes. Zoology. 2005;108(1):47–53. doi: 10.1016/j.zool.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 14.Glardon S, Holland LZ, Gehring WJ, Holland ND. Isolation and developmental expression of the amphioxus Pax-6 gene (AmphiPax-6): insights into eye and photoreceptor evolution. Development. 1998;125(14):2701–10. doi: 10.1242/dev.125.14.2701. [DOI] [PubMed] [Google Scholar]
- 15.Blackburn DC, Conley KW, Plachetzki DC, Kempler K, Battelle B-A, Brown NL. Isolation and expression of Pax6 and atonal homologues in the American horseshoe crab, Limulus polyphemus. Dev Dynam. 2008;237(8):2209–19. doi: 10.1002/dvdy.21634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang X, ZarinKamar N, Bao R, Friedrich M. Probing the Drosophila retinal determination gene network in Tribolium (I): The early retinal genes dachshund, eyes absent and sine oculis. Dev Biol. 2009;333(1):202–14. doi: 10.1016/j.ydbio.2009.02.040. [DOI] [PubMed] [Google Scholar]
- 17.Zimmerman JE, Bui QT, Liu H, Bonini NM. Molecular genetic analysis of Drosophila eyes absent mutants reveals an eye enhancer element. Genetics. 2000;154(1):237–46. doi: 10.1093/genetics/154.1.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jarman AP, Grell EH, Ackerman L, Jan LY, Jan YN. atonal is the proneural gene for Drosophila photoreceptors. Nature. 1994;369(6479):398–400. doi: 10.1038/369398a0. [DOI] [PubMed] [Google Scholar]
- 19.Melicharek D, Shah A, DiStefano G, Gangemi AJ, Orapallo A, Vrailas-Mortimer AD, et al. Identification of novel regulators of atonal expression in the developing Drosophila retina. Genetics. 2008;180(4):2095–110. doi: 10.1534/genetics.108.093302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Viczian AS, Vignali R, Zuber ME, Barsacchi G, Harris WA. XOtx5b and XOtx2 regulate photoreceptor and bipolar fates in the Xenopus retina. Development. 2003;130(7):1281–94. doi: 10.1242/dev.00343. [DOI] [PubMed] [Google Scholar]
- 21.Vandendries ER, Johnson D, Reinke R. Orthodenticle is required for photoreceptor cell development in the Drosophila eye. Dev Biol. 1996;173(1):243–55. doi: 10.1006/dbio.1996.0020. [DOI] [PubMed] [Google Scholar]
- 22.Simonnet F, Célérier M-L, Quéinnec E. Orthodenticle and empty spiracles genes are expressed in a segmental pattern in chelicerates. Dev Genes Evol. 2006;216(7–8):467–80. doi: 10.1007/s00427-006-0093-4. [DOI] [PubMed] [Google Scholar]
- 23.Browne W, Schmid BM, Wimmer E, Martindale M. Expression of otd orthologs in the amphipod crustacean, Parhyale hawaiensis. Dev Gene Evol. 2006;216(10):581–95. doi: 10.1007/s00427-006-0074-7. [DOI] [PubMed] [Google Scholar]
- 24.Umesono Y, Watanabe K, Agata K. Distinct structural domains in the planarian brain defined by the expression of evolutionarily conserved homeobox genes. Dev Genes Evol. 1999;209(1):31–9. doi: 10.1007/s004270050224. [DOI] [PubMed] [Google Scholar]
- 25.Li Y, Brown SJ, Hausdorf B, Tautz D, Denell RE, Finkelstein R. Two orthodenticle-related genes in the short-germ beetle Tribolium castaneum. Dev Genes Evol. 1996;206(1):35–45. doi: 10.1007/s004270050028. [DOI] [PubMed] [Google Scholar]
- 26.Serikaku MA, O’Tousa JE. sine oculis is a homeobox gene required for Drosophila visual system development. Genetics. 1994;138(4):1137–50. doi: 10.1093/genetics/138.4.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pineda D, Gonzalez J, Callaerts P, Ikeo K, Gehring WJ, Salo E. Searching for the prototypic eye genetic network: sine oculis is essential for eye regeneration in planarians. Proc Natl Acad Sci. 2000;97(9):4525–9. doi: 10.1073/pnas.97.9.4525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.López-Ríos J, Tessmar K, Loosli F, Wittbrodt J, Bovolenta P. Six3 and Six6 activity is modulated by members of the groucho family. Development. 2003;130(1):185–95. doi: 10.1242/dev.00185. [DOI] [PubMed] [Google Scholar]
- 29.Saló E, Pineda D, Marsal M, Gonzalez J, Gremigni V, Batistoni R. Genetic network of the eye in Platyhelminthes: expression and functional analysis of some players during planarian regeneration. Gene. 2002;287(1–2):67–74. doi: 10.1016/S0378-1119(01)00863-0. [DOI] [PubMed] [Google Scholar]
- 30.Lachmuth U, Grasshoff M, Barth FG. Taxonomishe Revision der Gattung Cupiennius Simon 1891. Senkenbergiana Biol. 1985;65:329–72. [Google Scholar]
- 31.McGregor AP, Hilbrant M, Pechmann M, Schwager EE, Prpic N-M, Damen WGM. Cupiennius salei and Achaearanea tepidariorum: spider models for investigating evolution and development. Bioessays. 2008;30(5):487–98. doi: 10.1002/bies.20744. [DOI] [PubMed] [Google Scholar]
- 32.Wolff C, Hilbrant M. The embryonic development of the central American wandering spider Cupiennius salei. Front Zool. 2011;8(1):15. doi: 10.1186/1742-9994-8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Land M, Barth FG. The quality of vision in the ctenid spider Cupiennius salei. J Exp Biol. 1992;164:227–42. [Google Scholar]
- 34.Homan H. Die Augen der Araneae. Z Morphol Tiere. 1971;69:201–72. doi: 10.1007/BF00277623. [DOI] [Google Scholar]
- 35.Babu KS, Barth FG. Neuroanatomy of central nervous system of the wandering spider Cupiennius salei. Zoomorphology. 1984;104:344–59. doi: 10.1007/BF00312185. [DOI] [Google Scholar]
- 36.Hanström B. Untersuchung über die relative Größe der Gehirnzentren verschiedener Arthropoden unter Berüchsichtigung der Lebensweise. Z Mikr Anat Forsch. 1926;7:139–90. [Google Scholar]
- 37.Strausfeld NJ, Babu KS. Two visual systems in one brain: neuropils serving the principal eyes of the spider Cupiennius salei. J Comp Neurol. 1993;328:63–75. doi: 10.1002/cne.903280105. [DOI] [PubMed] [Google Scholar]
- 38.Doeffinger C, Hartenstein V, Stollewerk A. Compartmentalization of the precheliceral neuroectoderm in the spider Cupiennius salei: development of the arcuate body, optic ganglia, and mushroom body. J Comp Neurol. 2010;518(13):2612–32. doi: 10.1002/cne.22355. [DOI] [PubMed] [Google Scholar]
- 39.Hanström B. Fortgesetzte Untersuchung über das Araneengehirn. Zool Jahrb Abt Ontog Tiere Anat. 1935;59:455–78. [Google Scholar]
- 40.Strausfeld NJ, Barth FG. Two visual systems in one brain: neuropils serving the secondary eyes of the spider Cupiennius salei. J Comp Neurol. 1993;328:43–62. doi: 10.1002/cne.903280104. [DOI] [PubMed] [Google Scholar]
- 41.Rozen S, Skaletsky H. Primer3 on the WWW for general users and for biologist programmers. Methods Mol Biol. 2000;132:365–86. doi: 10.1385/1-59259-192-2:365. [DOI] [PubMed] [Google Scholar]
- 42.Huelsenbeck JP, Ronquist F. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics. 2001;17(8):754–5. doi: 10.1093/bioinformatics/17.8.754. [DOI] [PubMed] [Google Scholar]
- 43.Ronquist F, Huelsenbeck JP. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19(12):1572–4. doi: 10.1093/bioinformatics/btg180. [DOI] [PubMed] [Google Scholar]
- 44.Damen WGM, Tautz D. A Hox class 3 orthologue from the spider Cupiennius salei is expressed in a Hox-gene-like fashion. Dev Genes Evol. 1998;208(10):586–90. doi: 10.1007/s004270050218. [DOI] [PubMed] [Google Scholar]
- 45.Weiss KM, Fullerton SM. Phenogenetic drift and the evolution of genotype–phenotype relationships. Theor Popul Biol. 2000;57(3):187–95. doi: 10.1006/tpbi.2000.1460. [DOI] [PubMed] [Google Scholar]
- 46.Gehring WJ, Ikeo K. Pax 6: mastering eye morphogenesis and eye evolution. Trends Genet. 1999;15(9):371–7. doi: 10.1016/S0168-9525(99)01776-X. [DOI] [PubMed] [Google Scholar]
- 47.Paulus HF. Eye structure and the monophyly of Arthropoda. In: Gupta AP, editor. Arthropod Phylogeny. London: Van Nostrand Reinhold Company; 1979. pp. 299–383. [Google Scholar]
- 48.Zurek DB, Taylor AJ, Evans CS, Nelson XJ. The role of the anterior lateral eyes in the vision-based behaviour of jumping spiders. J Exp Biol. 2010;213(14):2372–8. doi: 10.1242/jeb.042382. [DOI] [PubMed] [Google Scholar]
- 49.Harzsch S, Vilpoux K, Blackburn DC, Platchetzki D, Brown NL, Melzer R, et al. Evolution of arthropod visual systems: development of the eyes and central visual pathways in the horseshoe crab Limulus polyphemus Linnaeus, 1758 (Chelicerata, Xiphosura) Dev Dyn. 2006;235(10):spc1–1. doi: 10.1002/dvdy.20866. [DOI] [PubMed] [Google Scholar]
- 50.Dakin WJ. The eye of Peripatus. Q J Microsc Sci. 1921;65:163–72. [Google Scholar]
- 51.Mayer G. Structure and development of onychophoran eyes: what is the ancestral visual organ in arthropods? Arthropod Struct Dev. 2006;35(4):231–45. doi: 10.1016/j.asd.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 52.Zopf LM, Schmid A, Fredman D, Eriksson BJ. Spectral sensitivity of the ctenid spider Cupiennius salei. J Exp Biol. 2013;216(21):4103–8. doi: 10.1242/jeb.086256. [DOI] [PMC free article] [PubMed] [Google Scholar]


