Abstract
Objective:
To examine potential sex differences in nonmotor symptoms (NMS) among drug-naive patients with Parkinson disease (PD), and to identify NMS that can best differentiate patients with early PD from controls.
Methods:
Our cross-sectional analysis included 414 newly diagnosed, untreated patients with PD (269 men and 145 women) and 188 healthy controls (121 men and 67 women) in the Parkinson's Progression Markers Initiative Study. NMS were measured using well-validated instruments covering sleep, olfactory, neurobehavioral, autonomic, and neuropsychological domains.
Results:
Male and female patients with PD were fairly comparable on motor presentations but differed on several nonmotor features. Male patients with PD had significantly more pronounced deficits in olfaction (p = 0.02) and in certain cognitive measurements (all p < 0.01) than female patients, whereas female cases experienced higher trait anxiety (p = 0.02). Multiple stepwise logistic regression analysis showed that the combination of NMS measures—University of Pennsylvania Smell Identification Test (UPSIT), Montreal Cognitive Assessment (MoCA), Scales for Outcomes in Parkinson's Disease–Autonomic (SCOPA-AUT), and state anxiety from the State-Trait Anxiety Inventory—effectively differentiated patients with PD from controls with an area under the receiver operating characteristic curve (AUC) of 0.913 (95% confidence interval [CI]: 0.89–0.94). UPSIT, MoCA, and SCOPA-AUT were the most predictive NMS measurements in men (AUC = 0.919; 95% CI: 0.89–0.95) as compared to UPSIT, MoCA, and REM Sleep Behavior Disorder Screening Questionnaire in women (AUC = 0.903; 95% CI: 0.86–0.95).
Conclusions:
Our analysis revealed notable sex differences in several nonmotor features of patients with de novo PD. Furthermore, we found a parsimonious NMS combination that could effectively differentiate de novo cases from healthy controls.
Nonmotor symptoms (NMS) are common among patients with Parkinson disease (PD)1 and contribute greatly to poor quality of life, morbidity, and mortality.2,3 Despite their significant impacts, NMS among patients with PD remain poorly understood and, consequently, undertreated.2 Furthermore, some NMS, such as hyposmia, depression, REM sleep behavior disorder (RBD), and constipation, may even precede PD clinical diagnosis by years.
Clinical and epidemiologic studies have increasingly recognized and investigated the importance of NMS in understanding the natural history, etiology, and clinical care of PD.3,4 However, most previous studies of NMS used hospital-based prevalent PD cases, had small sample sizes, and often lacked a comparable control group. Although a few studies did assess NMS among patients with untreated, de novo PD,5–8 most did not have sufficient sample size to evaluate potential sex differences5–7 or focused executively on one or a limited number of specific symptoms. Therefore, little is known about the burden of the wide spectrum of NMS in patients with de novo, untreated PD.
We therefore investigated 5 major areas of NMS among 414 drug-naive patients with de novo PD and 188 healthy controls from the Parkinson's Progression Markers Initiative (PPMI) Study. We specifically examined potential sex difference in the presence of NMS and identified patterns of NMS that could best differentiate patients with early PD from healthy controls.
METHODS
Study participants.
PPMI is an ongoing, international, multicenter study designed to identify biomarkers of PD progression (http://www.ppmi-info.org/study-design/). Detailed descriptions of the study have been published elsewhere.9 Enrollment began in June 2010 and ended in April 2013. All participants underwent a comprehensive clinical and imaging assessment at the screening and baseline visits. Patients with PD were enrolled in the study if they met the following criteria: (1) an asymmetric resting tremor or asymmetric bradykinesia, or evidence of (either bradykinesia or resting tremor) and rigidity; (2) diagnosed within 2 years; (3) a Hoehn and Yahr stage of I or II; (4) age 30 years or older at diagnosis; (5) evidence of dopamine transporter deficit on DaTscan imaging; and (6) untreated for PD. Demographically comparable healthy subjects also were recruited into the study if they (1) were free of a current or active neurologic disorder; (2) had no first-degree relative with PD; (3) had no detectable dopamine transporter deficit evidence of PD; and (4) had a Montreal Cognitive Assessment (MoCA) score >26. Of the 423 patients with PD and 196 healthy controls enrolled in the study, we excluded from our analysis 9 cases and 8 controls who withdrew from the study. Therefore, the current analyses included 414 patients with de novo PD (269 men and 145 women) and 188 healthy controls (121 men and 67 women). Data for the current study were downloaded from the PPMI database (www.ppmi-info.org/data) according to guidelines.
Clinical evaluations.
Investigators at individual study sites conducted comprehensive clinical examination and used the Movement Disorder Society–sponsored revision of the Unified Parkinson's Disease Rating Scale, Part III–motor ratings and the Hoehn and Yahr scales to evaluate motor dysfunctions and disease severity. We further classified PD phenotypes into tremor dominant, postural instability and gait disturbance, or intermediate-PD according to a published method (appendix e-1 on the Neurology® Web site at Neurology.org).10
NMS assessments.
The NMS assessments at enrollment included an extensive set of tests and structured questionnaires to examine 5 major areas of nonmotor functions, including sleep, olfactory, neurobehavioral, autonomic, and neuropsychological domains (appendix e-2). Sleep disturbance was evaluated using the Epworth Sleepiness Scale and REM Sleep Behavior Disorder Screening Questionnaire (RBDSQ). Olfaction was assessed by the University of Pennsylvania Smell Identification Test (UPSIT). Neurobehavioral assessments included the State-Trait Anxiety Inventory, Questionnaire for Impulsive-Compulsive Disorder in Parkinson's Disease, and the 15-item version of the Geriatric Depression Scale. Autonomic dysfunction was evaluated using the Scales for Outcomes in Parkinson's Disease–Autonomic (SCOPA-AUT). Cognition was evaluated with MoCA as a global cognitive screening test, and a battery of neuropsychological tests to assess 4 cognitive domains: memory, visuospatial, working memory–executive, and attention-processing speed. A detailed list of the neuropsychological tests and their corresponding cognitive domains is shown in appendix e-2. Since the occurrence of impulse control disorders in PD is most likely driven by the use of dopamine agonists,11 and since recent findings from the PPMI cohort show that the prevalence of impulse control and related behavior is equally low both in patients with de novo, untreated PD and in controls,12 we omitted data on the Questionnaire for Impulsive-Compulsive Disorder in Parkinson's Disease assessment from our current analysis.
Statistical analysis.
We presented means and SDs as well as medians and interquartile ranges for continuous variables and proportions for categorical variables. To assess differences between groups, we performed the Mann–Whitney U test for continuous variables and χ2 test for categorical variables. For global cognition (MoCA) and all NMS with the exception of neuropsychological assessments, we presented original test scores and used them for group comparison. To allow a more direct comparison among various neuropsychological performances, we first transformed raw scores for neuropsychological variables to T scores according to published normative data. We further calculated a composite score for each cognitive domain by averaging T scores of relevant tests. We assessed sex differences in NMS among patients with PD and among healthy controls using a quantile regression model,13 fitted using PROC QUANTREG in SAS, that included enrollment category (i.e., PD vs healthy controls), separate sex indicator variables for cases and for controls, and age and education as adjustment variables. We further tested potential interactions by reparameterizing the same model: replacing the separate sex indicator variables with a sex main effect and a sex-by-enrollment category interaction. Finally, we examined potential associations between NMS and PD motor subtypes using multivariate logistic regression models, adjusting for age, sex, and education. All statistical analyses were conducted using SAS, version 9.1 (SAS Institute Inc., Cary, NC). Significance tests were 2-tailed, with α = 0.05.
To determine which NMS can best differentiate patients with de novo PD from healthy controls, we conducted a stepwise logistic regression analysis with all NMS variables, age, sex, and education as the predictors. The analyses were first conducted with all samples and then separately by sex. All NMS variables were standardized to z scores to make the β coefficient and odds ratios directly comparable across symptoms. We forced age, sex, and education into all models when appropriate. At each step, NMS variables entered the regression analysis at p < 0.30 and were retained as long as they remained significant at p < 0.05. We determined the predictive accuracy of the final set of predictors in discriminating patients with PD from healthy controls using receiver operating characteristic (ROC) analyses. The area under the ROC curve (AUC) provides an estimated probability that ranges from 0 to 1; an AUC of 0.5 indicates a discriminating power that is no better than chance, whereas a value of 1 implies perfect discrimination. We validated this ROC analysis in 2 ways: first through leave-one-out cross-validation; second, through 1,000 rounds of 3-fold cross-validations where each round used two-thirds of the data for model building (training sample) and the other one-third for cross-validation (validation sample). The ROC 3-fold cross-validation analysis was performed using the R software package (version 3.0.3).
Standard protocol approvals, registrations, and patient consents.
Each participating PPMI site obtained written informed consent from all participants, and received approval from an ethical standards committee on human experimentation.
RESULTS
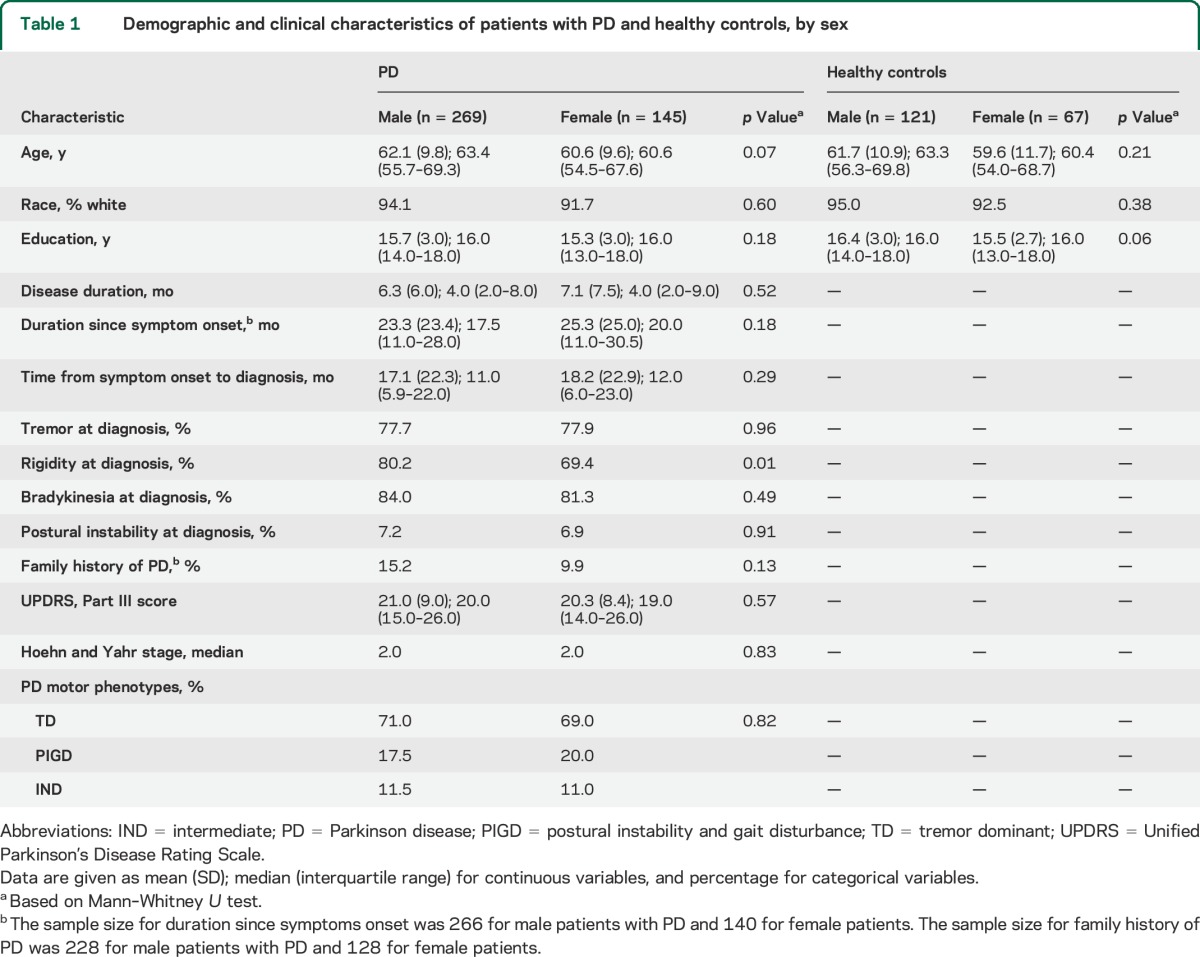
We present sex-specific characteristics of patients with PD and controls in table 1. Overall, there are no sex differences in age, race, and education among either patients with PD or controls. For both male and female patients, the median disease duration at enrollment was about 4 months from diagnosis or about 18 to 20 months since symptom onset. Male and female patients were also comparable in most clinical presentations of motor signs except that male cases were more likely to have reported rigidity at diagnosis.
Table 1.
Demographic and clinical characteristics of patients with PD and healthy controls, by sex
As expected, patients with PD scored significantly worse than controls in nearly all NMS measurements (p < 0.05 for all) except for daytime sleepiness and working memory–executive and visuospatial cognitive domains. We also examined whether the presence of specific NMS was related to the age at onset or time from symptom onset to diagnosis. Applying commonly used cutoffs for hyposmia (UPSIT <34), RBD (RBDSQ ≥5), daytime sleepiness (Epworth Sleepiness Scale score ≥10), depression (Geriatric Depression Scale ≥5), and poor cognition (MoCA <26), we did not observe any statistically significant differences for time from symptom onset to PD diagnosis between patients with and without these specific NMS. However, the median age at onset was older in patients with hyposmia (61.1 vs 54.3 years, p = 0.0006) and poor cognition (64 vs 59.7, p = 0.0009) compared with patients without (data not shown for other symptoms).
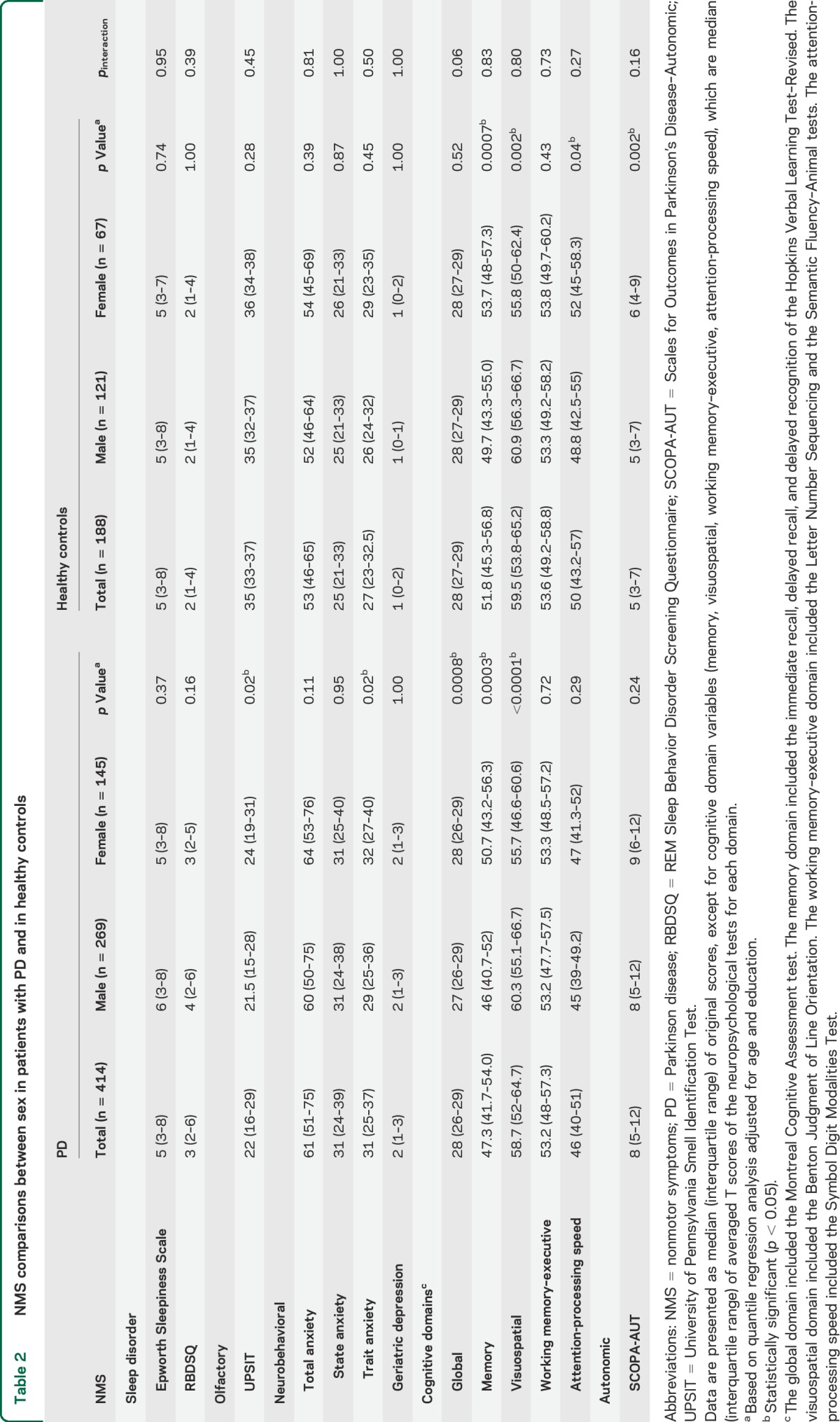
We observed several sex differences for NMS presentations in patients with PD as well as in controls (table 2). Male patients performed significantly worse than female patients on odor identification. While female cases experienced significantly higher trait anxiety, they outperformed their male counterparts on global cognition (MoCA) and memory domain, but underperformed for visuospatial domain. Detailed scores of individual cognitive tests are provided in table e-1. Among the healthy control group, we observed similar sex differences in cognitive domains for memory, visuospatial, and attention-processing speed, but not in global cognition. We also found a significant sex difference on SCOPA-AUT among controls, but not for cases. We further examined potential interactions between sex and case-control status on the presentation of NMS and did not find any significant interaction.
Table 2.
NMS comparisons between sex in patients with PD and in healthy controls
In our exploratory analysis of NMS in relation to PD clinical subtypes (table e-2), excessive daytime sleepiness and memory cognitive domain scores were associated with the tremor dominant phenotype. While both excessive daytime sleepiness and SCOPA-AUT scores were associated with the postural instability and gait disturbance subtype, SCOPA-AUT was the only nonmotor feature associated with the intermediate-PD phenotype.
The multiple stepwise logistic regression analysis that included both males and females identified 4 NMS measurements as significant predictors for PD: UPSIT, state anxiety, SCOPA-AUT, and MoCA (table 3). The sex-specific stepwise analyses identified as significant predictors UPSIT, SCOPA-AUT, and MoCA scores for males, and UPSIT, RBDSQ, and MoCA scores for females. We assessed the predictive performance of each model in differentiating patients with PD from healthy controls by ROC curves (figure 1, A–C). The AUC estimates from the leave-one-out cross-validation analyses were 0.913 for all samples combined, 0.919 for males, and 0.903 for females. We obtained similar AUC estimates from the 1,000 rounds of 3-fold cross-validations using two-thirds of the total sample for model building and the remaining one-third for validation (figure e-1).
Table 3.
Significant NMS predictors of PD from multiple stepwise logistic regression analysis
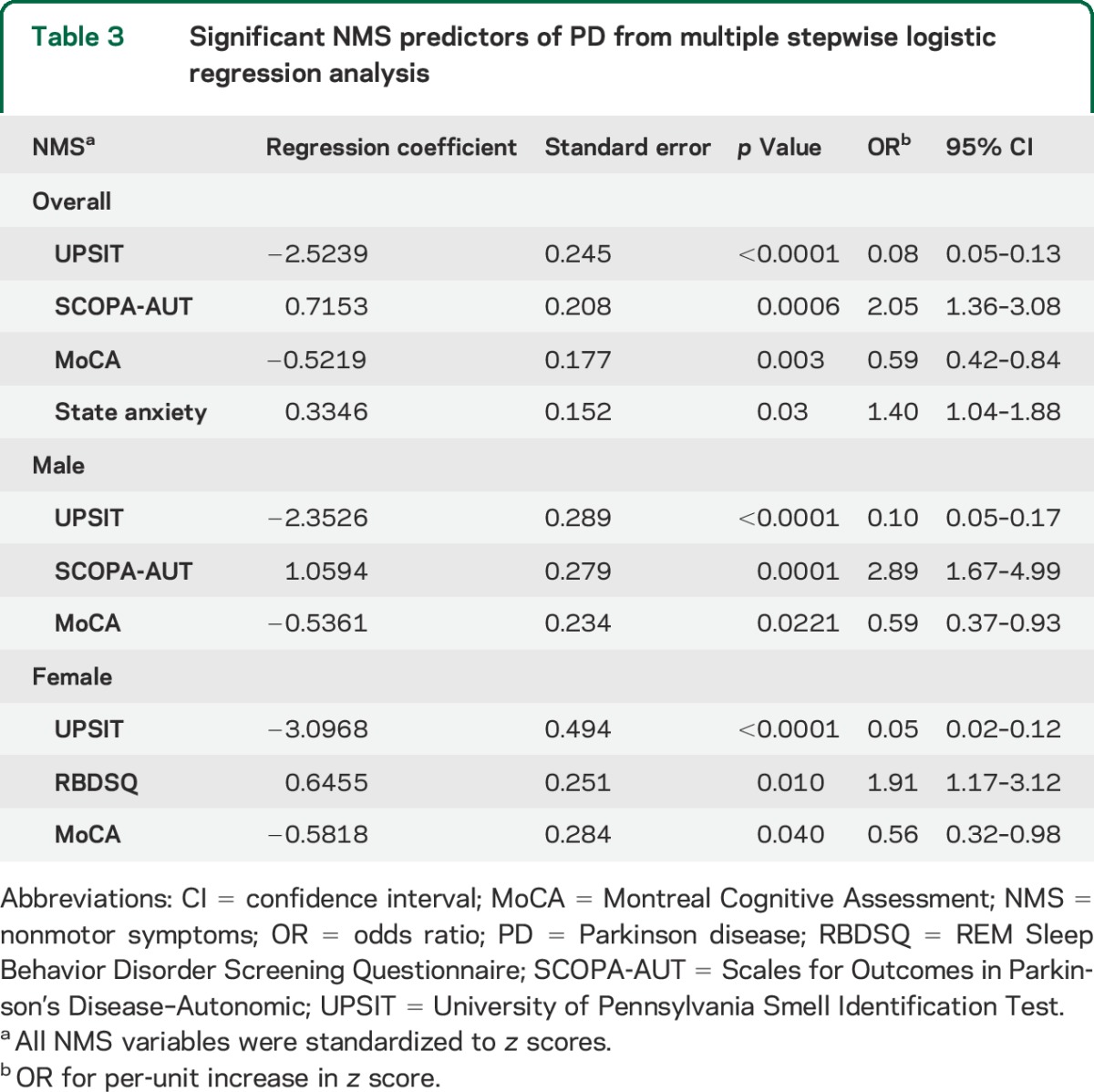
Figure 1. ROC curves with AUC of the nonmotor symptom assessments for overall samples combined (A) and for men (B) and women (C).
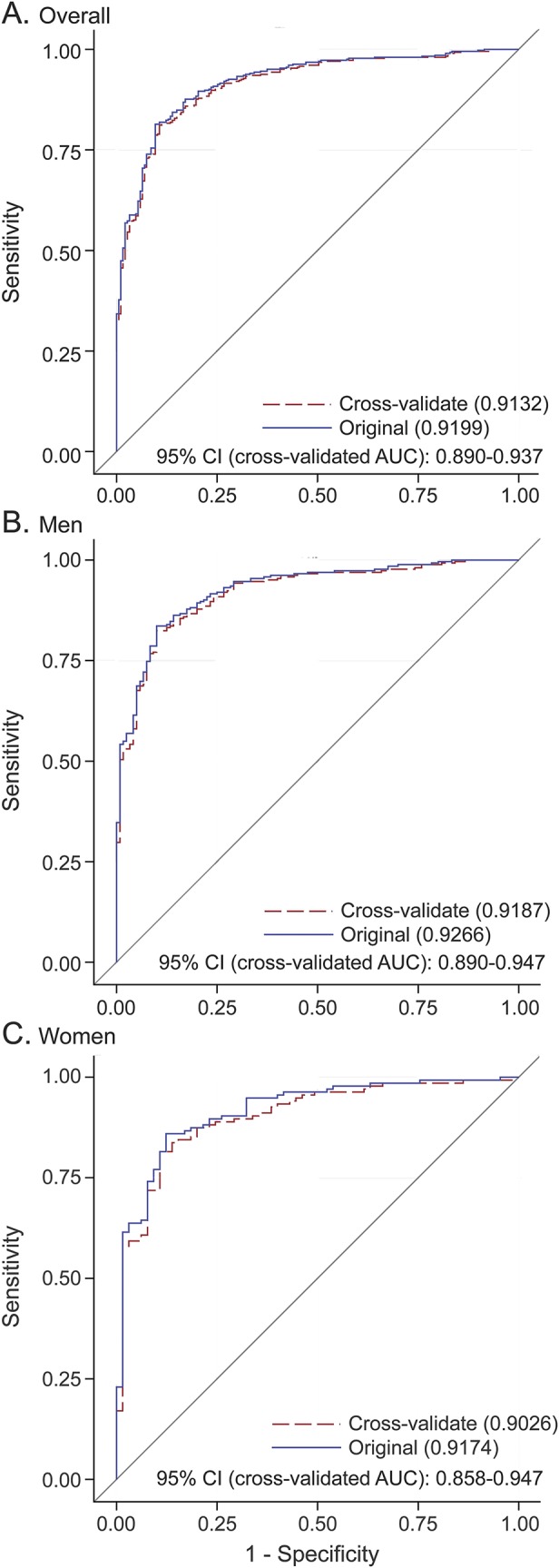
The 2 AUC estimates are from leave-one-out cross-validation and from the original ROC curve. AUC = area under the ROC curve; CI = confidence interval; ROC = receiver operating characteristic.
DISCUSSION
To our knowledge, this study is the largest to date to simultaneously evaluate multiple well-measured NMS among newly diagnosed, drug-naive patients with PD and healthy controls. As expected, patients with PD performed substantially worse than controls on nearly all NMS measurements.14 Male and female patients with PD were fairly comparable on motor presentations but differed on several nonmotor features. Of interest, we also saw similar but often smaller sex differences in cognition among controls, suggesting these are likely intrinsic sex differences that may be further exacerbated by the onset of PD. Furthermore, our analyses support the notion that a few of these NMS, sense of smell in particular, could effectively differentiate newly diagnosed patients with PD from controls. If this could be extrapolated to prodromal PD as the Braak hypothesis postulates,15 studies of these NMS may help characterize high-risk populations for PD.
PD disproportionately affects men,16 with a male to female incidence ratio of approximately 1.5.17 Furthermore, male patients tend to develop motor symptoms earlier and have faster progression18; female patients, however, are more prone to developing dyskinesia.18 In addition, potential sex differences have been reported for PD risk factors such as plasma urate,19 caffeine intake,20 and dairy consumption,21 all of which showed stronger evidence for an association in men than in women. Finally, sex differences in health care–seeking behaviors have also been observed among patients with PD, showing that female patients have delayed access to a movement disorder specialist compared with male patients.22
Several previous studies8,23–28 also investigated potential sex differences in NMS, albeit mostly among treated patients with PD. While findings varied widely across these studies, several of them found higher prevalence or severity of fatigue,23,25,26,28 apathy,24,26 depression,23–26 anxiety,23–26 and pain8,23–25,27 in female patients, whereas sexual dysfunction8,24–28 was more prevalent in male patients. Several potential limitations complicate interpretation of these results. Nearly all studies23–28 exclusively assessed treated patients with PD, whose NMS presentation might have been affected by treatment and disease progression.5,14,29 Furthermore, many previous studies lacked a comparison group,23–25 making it impossible to distinguish PD-specific sex differences from intrinsic sex differences not related to the disease. To our knowledge, only one previous study8 examined potential sex differences in NMS prevalence among patients with de novo PD and controls. That study, however, used only the 30-item PD Non-Motor Symptoms Questionnaire to screen symptoms with single questions. They found that male patients with PD were more likely to report taste/smell and sexual difficulties.
Cognitive impairment is common in PD, and recent evidence suggests that it may occur even in the earliest stages of the disease30 despite the fact that early severe dementia is often an exclusion criterion of PD diagnosis according to the Queen Square Brain Bank criteria. Our results, however, clearly documented poorer performance in multiple cognitive domains around PD diagnosis, particularly in men. Our findings are consistent with the few previous studies reporting statistically significant dysfunctions across a range of cognitive domains in untreated patients with newly diagnosed PD, particularly in the domains of visuospatial31,32 and memory.31–35 The findings are also consonant with reports that as many as one-quarter to one-third of patients have mild cognitive impairment at or near the time of diagnosis.36 Other cognitive dysfunctions reported in studies of untreated patients with early PD include deficits in random motor behavior,37 psychomotor speed,31,34 set-formation,33 executive function,31–35,37 visuomotor construction,33 working memory,33,37 attention,31,34 and language.33 Furthermore, mild cognitive impairment within the first year of PD diagnosis predicts a highly increased risk of early dementia.38 Taken together, these reports suggest that cognitive impairment may be an important NMS to measure in early PD and even in its prodromal stage.
Longitudinal follow-up of newly diagnosed patients with PD is necessary to understand the progression of NMS and to evaluate treatment strategies. The longitudinal data collection in the PPMI study is ongoing, and a preliminary analysis with 96 patients with PD and 83 controls showed that most NMS including depression, anxiety, fatigue, excessive daytime sleepiness, and impulse control disorders remained relatively stable over the first 2 years of the disease.14 Although patients with PD experienced increases in symptoms of apathy and psychosis and a slight decline in global cognition over time, these changes were comparable to those in healthy controls. The initiation of dopamine replacement therapy, however, was associated with a decrease in fatigue and an increase in impulsive control disorders and excessive daytime sleepiness.14 A few smaller studies have reported similar results.5,6
One important prospect of NMS research is its potential to characterize high-risk populations for PD. Because few of these symptoms are specific to PD, such research may require simultaneous measurements of multiple NMS in large prospective studies and lengthy follow-up. While such research efforts are currently under way, for example, in the Prospective Evaluation of Risk Factors for Idiopathic Parkinson's Syndrome Study39 and the Parkinson At-Risk Syndrome Study,40 careful examinations on NMS among patients with de novo PD may be a cost-effective approach to search for specific NMS or combinations of NMS that can best differentiate patients with PD from controls. Our regression analysis showed that only a few NMS measures in combination could effectively differentiate patients with PD from controls. Of note, in both males and females, smell identification appeared to be the most important single measurement differentiating PD from controls. We know of only one previous study that examined the discriminating power of NMS in patients with de novo PD. In the DeNoPa Study,7 the inclusion of SCOPA-AUT and olfaction tests along with the Non-Motor Symptoms Questionnaire, ECG, and serum cholesterol gave an AUC of 0.913. That study, however, did not examine the data separately for males and for females.
The current study builds on the strength of the PPMI cohort, a landmark international effort to systematically search for clinical, biochemical, and neuroimaging biomarkers for PD progression in drug-naive patients. The assessment of a wide range of NMS and their severity with well-validated instruments at the study baseline offers a unique opportunity to understand NMS among patients with early, untreated PD. Furthermore, by not dichotomizing these variables in our data analysis, we could use the full spectrum of the measurements and evaluate symptom severity. Finally, the study's recruitment of a large number of patients with PD and a well-characterized healthy control group provided a sufficient sample size for meaningful sex-specific analyses and between-group comparisons.
Our analysis also has several limitations. First, our analysis is cross-sectional, using the PPMI baseline data among de novo patients. However, as the cohort is currently being followed with annual reassessments of NMS, further investigation of sex differences using longitudinal data will be feasible in the near future. Second, the PPMI cohort comprises predominately white volunteers who were committed to multiple comprehensive clinical examinations, neuroimaging, and longitudinal follow-ups. Hence, results from this cohort may not be readily generalizable to late-onset sporadic PD or to individuals without PD in general. However, the clinical characteristics of PPMI patients are typical of general patients with PD, and the PPMI investigators' commitment to detailed clinical examinations will ensure high-quality data collection.
Our analysis confirmed the predominance of multiple NMS in patients with de novo, untreated PD and found notable sex differences in sense of smell, cognitive domains, and anxiety. Furthermore, a few NMS, such as sense of smell and overall cognition, could effectively differentiate de novo cases from healthy controls. Future studies should further systematically investigate NMS before disease diagnosis to prospectively examine their potential in characterizing high-risk populations and in measuring disease progression.
Supplementary Material
ACKNOWLEDGMENT
Data used in the preparation of this article are from the Parkinson's Progression Markers Initiative (PPMI) database (www.ppmi-info.org/data). For up-to-date information on the study, visit www.ppmi-info.org.
GLOSSARY
- AUC
area under the curve
- CI
confidence interval
- MoCA
Montreal Cognitive Assessment
- NMS
nonmotor symptoms
- PD
Parkinson disease
- PPMI
Parkinson's Progression Markers Initiative
- RBD
REM sleep behavior disorder
- RBDSQ
REM Sleep Behavior Disorder Screening Questionnaire
- ROC
receiver operating characteristic
- SCOPA-AUT
Scales for Outcomes in Parkinson's Disease–Autonomic
- UPSIT
University of Pennsylvania Smell Identification Test
Footnotes
Editorial, page 2102
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Rui Liu: study conceptualization and design, data analysis, interpretation of data, drafting of the manuscript, critical revision of the manuscript for important intellectual content, and final approval of the version to be published. David M. Umbach: data analysis, interpretation of data, critical revision of the manuscript for important intellectual content, and final approval of the version to be published. Shyamal D. Peddada: interpretation of data, critical revision of the manuscript for important intellectual content, and final approval of the version to be published. Zongli Xu: data analysis, interpretation of data, critical revision of the manuscript for important intellectual content, and final approval of the version to be published. Alexander I. Tröster: interpretation of data, critical revision of the manuscript for important intellectual content, and final approval of the version to be published. Xuemei Huang: interpretation of data, critical revision of the manuscript for important intellectual content, and final approval of the version to be published. Honglei Chen: study conceptualization and design, study supervision, critical revision of the manuscript for important intellectual content, and final approval of the version to be published.
STUDY FUNDING
Parkinson's Progression Markers Initiative, a public-private partnership, is funded by the Michael J. Fox Foundation for Parkinson's Research and funding partners, including AbbVie, Avid Radiopharmaceuticals, Biogen Idec, Bristol-Myers Squibb, Covance, Élan, GE Healthcare, Genentech, GSK-GlaxoSmithKline, Lilly, Merck, MSD-Meso Scale Discovery, Piramal, Pfizer, Roche, and UCB (www.ppmi-info.org/fundingpartners). R.L., D.M.U., S.D.P., Z.X., and H.C. are supported by the Intramural Research Program of the NIH, the National Institute of Environmental Health Sciences (Z01-ES-101986). H.X. is supported by NIH grants NS060722, ES019672, and NS08215.
DISCLOSURE
R. Liu, D. Umbach, S. Peddada, Z. Xu, and A. Tröster report no disclosures relevant to the manuscript. X. Huang has served as a consultant for Easton Associate, Public Healthcare, Teva Pharmaceutical Industries Ltd., and the National Institute of Environmental Health Sciences; holds patent US 6,916,823 (issued 2005): Method of treatment of dopamine-related dysfunction (plus foreign patents) and has filed a patent regarding Early detection of Parkinson's disease using novel motor signs; receives research support from the NIH (NS060722, ES019672, and NS08215), the Pennsylvania Tobacco Settlement Fund, and Huck Institute of Penn State University; and holds stock in BioValve Technologies, Inc. H. Chen receives NIH intramural funding (Z01-ES-101986) and serves on the editorial boards of the American Journal of Epidemiology, International Journal of Molecular Epidemiology and Genetics, and American Journal of Neurodegenerative Disease. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Hinnell C, Chaudhuri KR. The effect of non-motor symptoms on quality of life in Parkinson's disease. Eur Neurol Rev 2009;4:29–33. [Google Scholar]
- 2.Chaudhuri KR, Healy DG, Schapira AH. Non-motor symptoms of Parkinson's disease: diagnosis and management. Lancet Neurol 2006;5:235–245. [DOI] [PubMed] [Google Scholar]
- 3.Chen H, Burton EA, Ross GW, et al. Research on the premotor symptoms of Parkinson's disease: clinical and etiological implications. Environ Health Perspect 2013;121:1245–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berg D, Marek K, Ross GW, Poewe W. Defining at-risk populations for Parkinson's disease: lessons from ongoing studies. Mov Disord 2012;27:656–665. [DOI] [PubMed] [Google Scholar]
- 5.Erro R, Picillo M, Vitale C, et al. Non-motor symptoms in early Parkinson's disease: a 2-year follow-up study on previously untreated patients. J Neurol Neurosurg Psychiatry 2013;84:14–17. [DOI] [PubMed] [Google Scholar]
- 6.Kim HJ, Park SY, Cho YJ, et al. Nonmotor symptoms in de novo Parkinson disease before and after dopaminergic treatment. J Neurol Sci 2009;287:200–204. [DOI] [PubMed] [Google Scholar]
- 7.Mollenhauer B, Trautmann E, Sixel-Doring F, et al. Nonmotor and diagnostic findings in subjects with de novo Parkinson disease of the DeNoPa cohort. Neurology 2013;81:1226–1234. [DOI] [PubMed] [Google Scholar]
- 8.Picillo M, Amboni M, Erro R, et al. Gender differences in non-motor symptoms in early, drug naive Parkinson's disease. J Neurol 2013;260:2849–2855. [DOI] [PubMed] [Google Scholar]
- 9.Marek K, Jennings D, Lasch S, et al. The Parkinson progression marker initiative (PPMI). Prog Neurobiol 2011;95:629–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jankovic J, McDermott M, Carter J, et al. Variable expression of Parkinson's disease: a base-line analysis of the DATATOP cohort. The Parkinson Study Group. Neurology 1990;40:1529–1534. [DOI] [PubMed] [Google Scholar]
- 11.Garcia-Ruiz PJ, Martinez Castrillo JC, Alonso-Canovas A, et al. Impulse control disorder in patients with Parkinson's disease under dopamine agonist therapy: a multicentre study. J Neurol Neurosurg Psychiatry 2014;85:840–844. [DOI] [PubMed] [Google Scholar]
- 12.Weintraub D, Papay K, Siderowf A. Screening for impulse control symptoms in patients with de novo Parkinson disease: a case-control study. Neurology 2013;80:176–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Koenker R, Hallock K. Quantile regression: an introduction. J Econ Perspect 2001;15:143–156. [Google Scholar]
- 14.de la Riva P, Smith K, Xie SX, Weintraub D. Course of psychiatric symptoms and global cognition in early Parkinson disease. Neurology 2014;83:1096–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Braak H, Bohl JR, Muller CM, Rub U, de Vos RA, Del Tredici K. Stanley Fahn Lecture 2005: the staging procedure for the inclusion body pathology associated with sporadic Parkinson's disease reconsidered. Mov Disord 2006;21:2042–2051. [DOI] [PubMed] [Google Scholar]
- 16.Miller IN, Cronin-Golomb A. Gender differences in Parkinson's disease: clinical characteristics and cognition. Mov Disord 2010;25:2695–2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Taylor KS, Cook JA, Counsell CE. Heterogeneity in male to female risk for Parkinson's disease. J Neurol Neurosurg Psychiatry 2007;78:905–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haaxma CA, Bloem BR, Borm GF, et al. Gender differences in Parkinson's disease. J Neurol Neurosurg Psychiatry 2007;78:819–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen H, Mosley TH, Alonso A, Huang X. Plasma urate and Parkinson's disease in the Atherosclerosis Risk in Communities (ARIC) Study. Am J Epidemiol 2009;169:1064–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ascherio A, Zhang SM, Hernan MA, et al. Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann Neurol 2001;50:56–63. [DOI] [PubMed] [Google Scholar]
- 21.Chen H, O'Reilly E, McCullough ML, et al. Consumption of dairy products and risk of Parkinson's disease. Am J Epidemiol 2007;165:998–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Saunders-Pullman R, Wang C, Stanley K, Bressman SB. Diagnosis and referral delay in women with Parkinson's disease. Gend Med 2011;8:209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barone P, Antonini A, Colosimo C, et al. The PRIAMO Study: a multicenter assessment of nonmotor symptoms and their impact on quality of life in Parkinson's disease. Mov Disord 2009;24:1641–1649. [DOI] [PubMed] [Google Scholar]
- 24.Guo X, Song W, Chen K, et al. Gender and onset age-related features of non-motor symptoms of patients with Parkinson's disease: a study from Southwest China. Parkinsonism Relat Disord 2013;19:961–965. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Martin P, Falup Pecurariu C, Odin P, et al. Gender-related differences in the burden of non-motor symptoms in Parkinson's disease. J Neurol 2012;259:1639–1647. [DOI] [PubMed] [Google Scholar]
- 26.Solla P, Cannas A, Ibba FC, et al. Gender differences in motor and non-motor symptoms among Sardinian patients with Parkinson's disease. J Neurol Sci 2012;323:33–39. [DOI] [PubMed] [Google Scholar]
- 27.Szewczyk-Krolikowski K, Tomlinson P, Nithi K, et al. The influence of age and gender on motor and non-motor features of early Parkinson's disease: initial findings from the Oxford Parkinson Disease Center (OPDC) discovery cohort. Parkinsonism Relat Disord 2014;20:99–105. [DOI] [PubMed] [Google Scholar]
- 28.Krishnan S, Sarma G, Sarma S, Kishore A. Do nonmotor symptoms in Parkinson's disease differ from normal aging? Mov Disord 2011;26:2110–2113. [DOI] [PubMed] [Google Scholar]
- 29.Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol 2009;8:464–474. [DOI] [PubMed] [Google Scholar]
- 30.Kehagia AA, Barker RA, Robbins TW. Neuropsychological and clinical heterogeneity of cognitive impairment and dementia in patients with Parkinson's disease. Lancet Neurol 2010;9:1200–1213. [DOI] [PubMed] [Google Scholar]
- 31.Aarsland D, Bronnick K, Larsen JP, Tysnes OB, Alves G. Cognitive impairment in incident, untreated Parkinson disease: the Norwegian ParkWest Study. Neurology 2009;72:1121–1126. [DOI] [PubMed] [Google Scholar]
- 32.Erro R, Santangelo G, Picillo M, et al. Link between non-motor symptoms and cognitive dysfunctions in de novo, drug-naive PD patients. J Neurol 2012;259:1808–1813. [DOI] [PubMed] [Google Scholar]
- 33.Cooper JA, Sagar HJ, Jordan N, Harvey NS, Sullivan EV. Cognitive impairment in early, untreated Parkinson's disease and its relationship to motor disability. Brain 1991;114:2095–2122. [DOI] [PubMed] [Google Scholar]
- 34.Elgh E, Domellof M, Linder J, Edstrom M, Stenlund H, Forsgren L. Cognitive function in early Parkinson's disease: a population-based study. Eur J Neurol 2009;16:1278–1284. [DOI] [PubMed] [Google Scholar]
- 35.Poletti M, Frosini D, Pagni C, et al. Mild cognitive impairment and cognitive-motor relationships in newly diagnosed drug-naive patients with Parkinson's disease. J Neurol Neurosurg Psychiatry 2012;83:601–606. [DOI] [PubMed] [Google Scholar]
- 36.Litvan I, Aarsland D, Adler CH, et al. MDS Task Force on Mild Cognitive Impairment in Parkinson's Disease: critical review of PD-MCI. Mov Disord 2011;26:1814–1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Miah IP, Olde Dubbelink KT, Stoffers D, Deijen JB, Berendse HW. Early-stage cognitive impairment in Parkinson's disease and the influence of dopamine replacement therapy. Eur J Neurol 2012;19:510–516. [DOI] [PubMed] [Google Scholar]
- 38.Pedersen KF, Larsen JP, Tysnes OB, Alves G. Prognosis of mild cognitive impairment in early Parkinson disease: the Norwegian ParkWest Study. JAMA Neurol 2013;70:580–586. [DOI] [PubMed] [Google Scholar]
- 39.Berg D, Godau J, Seppi K, et al. The PRIPS Study: screening battery for subjects at risk for Parkinson's disease. Eur J Neurol 2013;20:102–108. [DOI] [PubMed] [Google Scholar]
- 40.Siderowf A, Jennings D, Eberly S, et al. Impaired olfaction and other prodromal features in the Parkinson At-Risk Syndrome Study. Mov Disord 2012;27:406–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


