Background
The tissue Doppler-derived surrogate for left ventricular diastolic pressure, E/e′, has been used to prognosticate outcome in a variety of cardiovascular conditions. In this study we determined the relationship of intraoperative E/e′ to the use of inotropic support, duration of mechanical ventilation (MV), length of intensive care unit stay (ICU-LOS) and total hospital stay (H-LOS) in patients requiring cardiac surgery. The records of 245 consecutive patients were retrospectively reviewed to obtain 205 patients who had intraoperative transesophageal echocardiography (TEE) examinations prior to coronary artery bypass grafting (CABG) and/or valvular surgery. Cox proportional hazards and logistic regression models were used to analyze the relation between intraoperative E/e′ or LVEF and early postoperative morbidity (H-LOS, ICU-LOS, and MV) and the probability that a patient would require inotropic support. With adjustments for other predictors (female gender, hypertension, diabetes, history of myocardial infarction, emergency surgery, renal failure, procedure type, length of aortic cross-clamp time), an elevated E/e′ ratio (≥ 8) was significantly associated with an increased ICU-LOS (49 versus 41 median h, P = 0.037) and need for inotropic support (P = 0.002) while baseline LVEF associated with inotropic support alone (P < 0.0001). These data suggest that the tissue Doppler derived-index of left ventricular diastolic filling pressure may be a useful indicator for predicting early morbid events after cardiac surgery, and may even provide additional information from that of baseline LVEF. Further, patients with elevated preoperative E/e′ may need more careful peri- and postoperative management than those patients with E/e′ <8.
Keywords: cardiac surgery, echocardiography, prognosis, length of stay, tissue Doppler
Introduction
Traditional risk factors associated with morbidity and mortality after cardiac surgery include baseline left ventricular ejection fraction (LVEF), multiple left ventricular regional wall motion abnormalities, advanced age, co-morbid conditions, and procedure complexity.1–3 Recently, left ventricular diastolic function has been shown to have value in predicting outcome in patients with cardiovascular disease and those having cardiac surgery. Diastolic dysfunction predicts increased difficulty weaning from cardiopulmonary bypass (CPB),4,5 increased adverse events regardless of LVEF,6 and increased mortality.6 Merello et al. report that the presence of severe diastolic dysfunction, as determined by conventional Doppler measurements, is more accurate in predicting adverse outcomes and mortality after coronary artery bypass grafting (CABG) than other established cardiac operative risk scores.7 However, given that conventional transmitral and pulmonary vein flow patterns are influenced by heart rate and rhythm,8,9 loading conditions,10–12 and other interrelated factors (e.g., mitral regurgitation) that may have confounding effects on the interpretation of pulsed-Doppler flow signals,13,14 tissue Doppler imaging (TDI) could be a valuable addition to the perioperative echocardiographer’s armamentarium.15,16 The early diastolic mitral annular velocity (e′) is less affected by loading conditions than conventional Doppler,17 and the TDI surrogate of LV filling pressure,18 E/e′, has been shown to be relatively independent of systolic function, rhythm abnormalities (such as tachycardia and atrial fibrillation), LV hypertrophy, and functional mitral regurgitation.13,18–23
Accordingly, this retrospective review investigates the association between the intraoperative E/e′ ratio with the requirement of inotropic support, prolonged mechanical ventilation (MV), and increased intensive care unit (ICU-LOS) and hospital lengths of stay (H-LOS) in patients following CABG and/or valvular surgery. We hypothesize that an elevated E/e′ ratio prior to surgical repair predicts early postoperative morbid events.
Methods
Patient Population
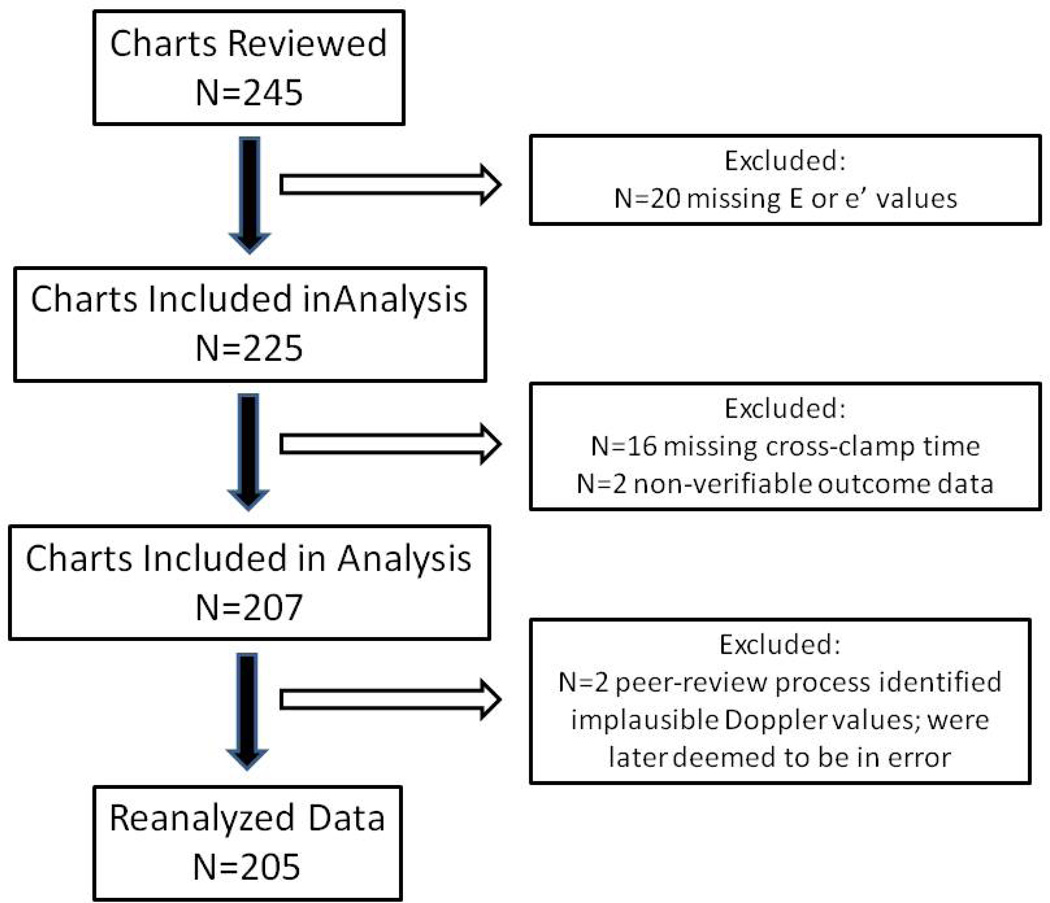
After institutional review board approval, we retrospectively reviewed echocardiographic data from 245 consecutive patients who underwent cardiac surgery between January 2005 and December 2007 at Wake Forest University Baptist Medical Center (Winston-Salem, North Carolina) under the care of two anesthesiologists experienced in transesophageal echocardiography (TEE) (LG and DZ). Included were patients undergoing CABG, valvular, or CABG with valvular surgery. Preoperative characteristics and intraoperative processes of care were obtained from electronic medical records, anesthesia records, and CPB charts. Intensive care unit and H-LOS were obtained from an institutional ICU quality improvement database. Twenty patients were subsequently dropped because of inadequate TEE images and 18 because of incomplete or non-verifiable outcome data. Consequently, the data from 207 patients were used for the initial analysis. However, through the peer-review process, 2 additional patients were identified as having implausible TDI values. As their E/e′ ratios were deemed to be in error (i.e., < 0.5 or >54); these 2 patients were subsequently removed from the overall analysis prior to complete re-estimation of the remaining 205 patients (Figure 1).
Figure 1.
Study inclusion/exclusion flow chart.
Perioperative Care
Anesthesia consisted of an opioid-based technique (fentanyl or sufentanil) with isoflurane and intravenous cisatracurium for muscle relaxation. After anesthetic induction, tracheal intubation, central venous cannulation, and pulmonary artery catheter placement, 2-dimensional, color Doppler, pulsed-wave Doppler, and pulsed-wave TDI studies were performed with a TEE multi-plane probe and echocardiographic unit (SONOS 5500, or iE33, Koninklijke Philips Electronics N.V. Eindhoven, the Netherlands) prior to sternotomy. Cardiac surgery was performed using CPB in the majority of patients with α-stat pH management, and antegrade and retrograde cold blood cardioplegia. Hematocrit was maintained between 20% and 25%, and CPB flow was maintained between 2.0 and 2.5 L/min/m2. The mean perfusion pressure was kept at 60–70 mmHg by the adjustment of a phenylephrine infusion. Patients arrived at the intensive care unit sedated with propofol and mechanically ventilated. When hemodynamic stability and adequate hemostasis were established, awake and alert patients were weaned from mechanical ventilatory support by standard criteria and the discretion of the intensivist.
Echocardiographic Examination and Definitions
The TEE examination included assessment of LVEF by Simpson’s method or a global estimation by the attending anesthesiologist (LG or DZ) based on wall motion. In this study, left ventricular systolic function was categorized into three groups: 1) normal was defined as an LVEF >50%; 2) mild-to-moderately reduced was defined as an LVEF ≤50% and ≥35%; and, 3) severely reduced was defined as an LVEF <35%. Regional function was evaluated using the standard 16-segment model as suggested by the American Society of Echocardiography10 and wall motion was scored as normal, hypokinetic, akinetic, or dyskinetic. All valves were thoroughly examined, as was the ascending and descending aorta. The 2-dimensional examination also identified patients with left ventricular hypertrophy and left atrial enlargement (left atrial diameter >40 mm).
Diastolic function was evaluated by conventional pulsed-wave Doppler and pulsed-wave TDI. Peak early (E) and late (A) LV inflow velocities, E/A ratio, and the deceleration time of the E-wave were measured at the mid-esophageal 4-chamber view with the pulsed-wave Doppler sample volume placed between the mitral leaflet tips. Systolic, early diastolic, and late diastolic A-wave pulmonary vein velocities were obtained with the sample site at the entrance of the left upper pulmonary vein viewed from the midesophageal 4-chamber view between 15 and 60 degrees, and the S/D ratio was determined. Peak early diastolic myocardial velocity (e′), or mitral annular displacement, was obtained from the mid-esophageal 4-chamber view with the pulsed-wave TDI sampling volume placed in the middle of the lateral mitral annular wall. The ratio of E/e′ was calculated as a measure of left ventricular filling pressure. In this study, left ventricular diastolic filling pressures were classified into three groups: 1) normal was characterized by E/e′ <8; 2) moderately elevated was characterized by E/e′ ≥8 and ≤15; and 3) severely elevated was characterized by E/e′ >15. Measurements were taken on-line and all images were recorded digitally and stored within the institution’s echocardiography archive.
Data Collection and Definitions
Clinical variables were collected from patient records retrospectively. An emergency procedure was defined as an operation that occurred within 48 h of coronary catheterization because of unstable symptoms not controlled by medical therapy, hemodynamic instability, or failed angioplasty. Hypertension and diabetes were considered to be present in patient’s documented history of hypertension or diabetes necessitating medical treatment. Preoperative renal failure was defined by a history of dialysis. Intraoperative requirements for inotropic support and/or intra-aortic balloon pump were thought to be due to low cardiac output syndrome (cardiac index <2.0 L/min/m2) upon weaning from CPB.
Data Analysis
The number of charts to be reviewed was determined by considering the nature of the conducted statistical model that would be used to evaluate the specified hypotheses. Because the expected degree of relationship of E/e′ with the clinical outcomes was not known prior to the analyses, sample size determination was made based on the 12 a priori selected covariates that would be used in the models. These 12 characteristics were chosen based on prior studies of cardiac surgery outcomes.24,25 Thus, 245 charts were selected to be reviewed in the attempt to obtain ≥200 measurements of the covariate set. This sample size allows ≥18 scores per covariate and is more than the recommended 10% of the number of total observations required to reduce the chances of over-fitting the regression model. Further, this sample size allows ≥80% power to detect even small zero-order correlations (r ≥0.20) using two-tailed significance tests and α=0.05.
Statistical analyses were conducted using SPSS 15.0 (SPSS, Inc., Chicago, IL). Prior to conducting the analyses, the distributions of all variables were examined using histograms and descriptive statistics. Three of the clinical outcomes (total ICU h, total hospital days, and total MV h) were positively skewed so were expressed as medians and 95% confidence intervals. Differences in possible predictive factors among E/e′ groups were assessed using Kruskal-Wallis tests for continuous or ordinal variables (age, ejection fraction, mitral annular velocity, aortic cross-clamp time) and by Chi-square tests for dichotomous variables (male gender, hypertension, diabetes, history of myocardial infarction, emergency surgery, renal failure, procedure type). Post-hoc testing was conducted using Mann-Whitney U tests or Chi-square tests, and all post-hoc analyses were evaluated using a Bonferroni adjusted level of significance (p<0.017) to account for the multiple comparisons across E/e′ groups. Although there were no censored cases (i.e., exact LOS was known for all of the participants in the study), a Cox proportional hazards model was used to analyze the relation between early postoperative morbidity (LOS-H, LOS-ICU, and MV time) and preoperative E/e′. A logistic regression model was used to examine the probability that a patient would leave the OR with inotropic support. Two models were conducted for each outcome variable, one with only E/e′ group (unadjusted), and one with E/e′ group while controlling for selected covariates in Table I (adjusted). A similar analysis was undertaken to describe the relation between the same outcome variables and preoperative LVEF. Where appropriate, all analyses are two-tailed with statistical significance determined at p<0.05.
Table I.
Sample Characteristics and Clinical Predictors
| Predictor | E/e′ <8 (n = 72) |
8 ≥ E/e′ 8 ≤15 (n = 98) |
E/e′ >15 (n = 35) |
p |
|---|---|---|---|---|
| Age, years | 58.5 [27 – 82] | 66 [33 – 84]† | 67 [38 – 89]† | 0.002 |
| OPCAB | 0 (0%) | 3 (3.1%) | 2 (5.7%) | 0.17 |
| Cross-clamp time, minutes | 80.5 [11 – 170] | 82.5 [0 – 194] | 94 [0 – 238]† | 0.017 |
| Hypertension | 49 (68.1%) | 83 (84.7%)† | 26 (74.3%) | 0.035 |
| Status: Elective | 56 (77.8%) | 79 (80.6%) | 34 (97.1%)† | 0.04 |
| Diabetes Mellitus | 17 (23.6%) | 36 (36.7%) | 9 (25.7%) | 0.15 |
| Dialysis | 1 (1.4%) | 2 (2%) | 4 (11.4%)† | 0.016 |
| History MI | 23 (31.9%) | 40 (40.8%) | 10 (28.6%) | 0.31 |
| Gender: male | 65 (90.3%) | 72 (73.5%)† | 16 (45.7%)†‡ | < 0.0001 |
| Operation: | 0.002 | |||
| CABG | 43 (59.7%) | 55 (56.1%) | 7 (20%)†‡ | |
| Valve | 6 (8.3%) | 10 (10.2%) | 8 (22.9%) | |
| Multiple | 23 (31.9%) | 33 (33.7%) | 20 (57.1%)†‡ | |
| Ejection Fraction, % | 59 [35 – 80] | 55 [15 – 83]† | 52 [20 –85] | 0.003 |
| e′ (cm/sec) | 10 [5.5 – 17]† | 7 [3.3 – 17.9]† | 5 [3.2 – 7.6]‡ | < 0.0001 |
| E/e′ | 6.3 [3.9 – 7.9] | 10 [8 – 14.8] | 18.5 [15.4 – 37.4] | NA |
Values are presented as median [range], or frequency (%), as appropriate
e′ = preoperative lateral mitral annular descent; E/e′ = left ventricular filling pressure.
Differs from E/e′ <8, p <0.017
Differs from 8 ≥ E/e′ ≤15, p < 0.017
Results
Clinical Characteristics
Of the 205 study patients, the TDI measurements of LV filling pressures were normal (E/e′ <8) in 35%, moderately elevated (8 ≤ E/e′ <15) in 48%, and severely elevated (E/e′) in 17% of the group. The clinical characteristics and predictors of the study population categorized according to E/e′ are displayed in Table I. Although the incidence of diabetes mellitus, history of myocardial infarction, and off-pump coronary artery bypass (OPCAB) were similar among the three E/e′ groups, female gender, renal failure, procedural urgency, and combination valvular/ CABG surgery were more frequent in the high filling pressure group (E/e′ >15) when compared to the normal filling pressure group (E/e′<8). As expected, the high E/e′ group was also older and had longer aortic cross-clamp times than the lowest E/e′ group. Correspondingly, the patients in the intermediate filling pressure group, 8 ≥ E/e′ ≤ 15, were also more likely to be older, female, and with a history of hypertension than those in the normal filling pressure group.
Clinical Outcomes
Table II presents the clinical outcomes stratified by E/e′. Without adjusting for other predictors (univariate model), elevated E/e′ ratios (≥8) were significantly associated with longer ICU-LOS and LOS-H, increased duration of MV and a greater need for inotropic support when compared to E/e′<8. Interestingly, in the multivariate model that evaluated E/e′ while adjusting for the effects of age, gender, hypertension, diabetes mellitus, history of myocardial infarction, operation type, elective status, and aortic cross-clamp duration, E/e′ ratios ≥8 (e.g., moderately elevated and severely elevated E/e′ class of filling pressures) were still independently related to increased ICU LOS and need for inotropic support (p-values <0.037), but were not significantly associated with H-LOS or duration of MV. As expected, when the same clinical outcome variables were stratified by baseline LVEF, the lowest LVEF class (<35%) was more likely to need inotropic support than those patients in the mild -to-moderate systolic dysfunction group, regardless of other clinical predictors (Table III).
Table II.
Clinical Outcomes Stratified by E/e′
| Clinical Outcomes | E/e′ < 8 (n = 72) |
8 ≥ E/e′ 8 ≤ 15 (n = 98) |
E/e′ > 15 (n = 35) |
p | p adjusted* |
|---|---|---|---|---|---|
| ICU-LOS (hr) | 41 [28.5 – 53.5] | 49 [35.8 – 62.2]† | 71 [66.4 – 75.6]†‡ | <0.0001 | 0.037 |
| H-LOS (days) | 5 [4.4 – 5.6] | 7 [6.4 – 7.6]† | 7 [4.1 – 8.9]† | 0.003 | 0.43 |
| MV duration (hr) | 9 [5.4 – 12.6] | 14 [12.2 – 15.8]† | 18 [15.1 – 20.9]† | 0.012 | 0.220 |
| Inotropes leaving OR | 8 (11.1%) | 36 (36.7%)† | 17 (48.6%)† | <0.0001 | 0.002 |
Values are presented as median [95% CI], or frequency (%), as appropriate
e′ = preoperative lateral mitral annular descent; E/e′ = left ventricular filling pressure.
E/e′ group differences after adjusting for age, cross-clamp time, hypertension, elective status, iabetes mellitus, dialysis, history of MI, gender, and operation type
Differs from E/e′ < 8, p < 0.017
Differs from 8 ≥ E/e′ ≤ 15 p < 0.017
Table III.
Clinical Outcomes Stratified by Ejection Fraction (EF)
| Clinical Outcomes | EF ≤ 35 (n = 22) |
35 > EF ≤ 50 (n = 55) |
EF > 50 (n = 128) |
p | p adjusted* |
|---|---|---|---|---|---|
| ICU-LOS (hr) | 45 [40.4 – 49.6] | 47 [43.4 – 50.6] | 46 [41.1 – 50.9] | 0.89 | 0.56 |
| H-LOS (days) | 6 [5 –7] | 7 [6.4 – 7.6] | 6 [5.4 – 6.6] | 0.83 | 0.76 |
| MV duration (hr) | 15 [9.6 – 20.4] | 16 [13.9 – 18.1] | 13 [9.9 – 16.1] | 0.67 | 0.64 |
| Inotropes leaving OR | 18 (81.8%) | 24 (43.6%)† | 19 (14.8%)† | <0.0001 | <0.0001 |
Values are presented as median [95% CI], or frequency (%), as appropriate
EF group differences after adjusting for age, cross-clamp time, hypertension, elective status, diabetes mellitus, dialysis, history of MI, gender, and operation type
Differs from EF < 35, p < 0.017
Differs from 35 ≥ EF ≤ 50 p < 0.017
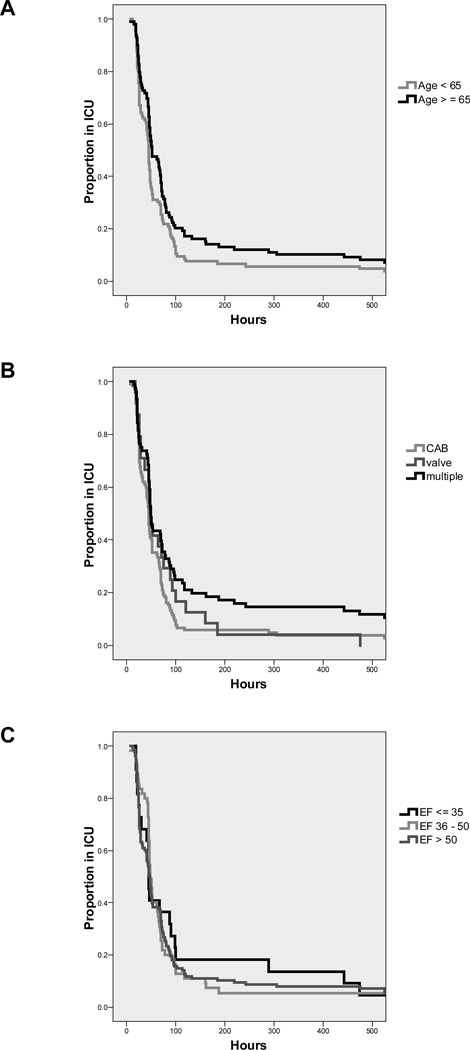
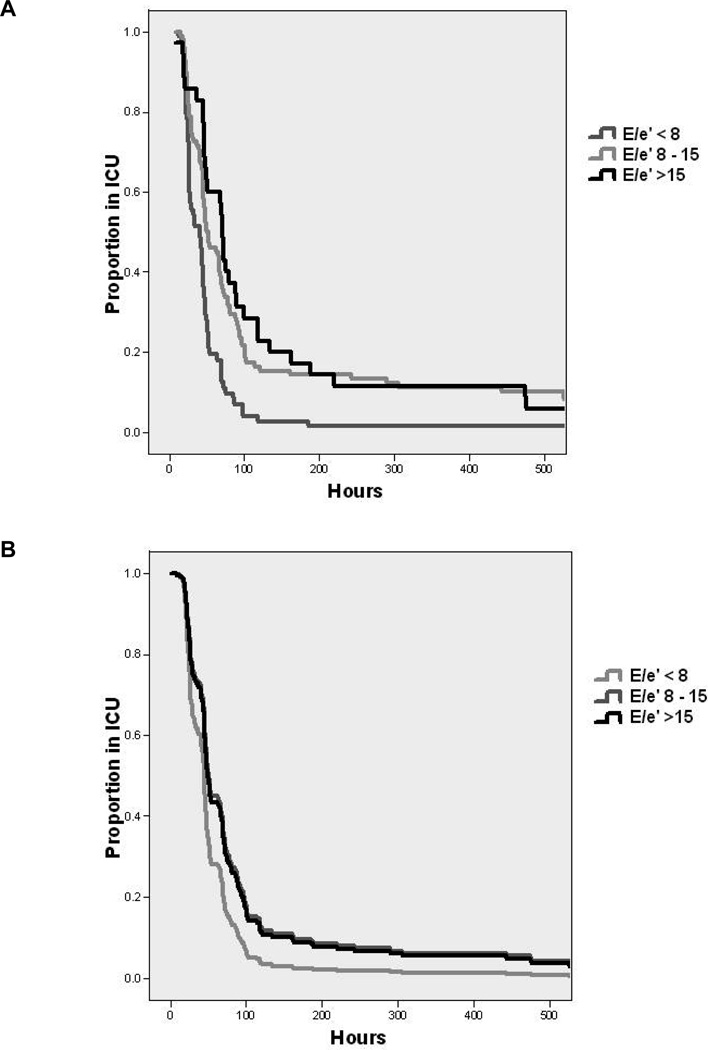
Figure 2 presents the effect of age, LVEF, and procedure type on the hazard rate of ICU-LOS. Without adjusting for other predictors (univariate model), age >65 (median time in ICU 51.0 h [95% CI: 37.2 – 64.8] versus age <65, 45 h [42.1 – 47.9]), and multiple procedures (CABG: 44 h [40.9 – 47.1], valve: 47 h [39.3 – 54.7], and multiple: 49 h [45.1 – 52.9]) were significantly associated with prolonged ICU-LOS. Figure 3 plots the ICU-LOS according to E/e′ class. Importantly, the multivariate model examining E/e′ class with the other clinical predictors including gender, hypertension, diabetes, history of myocardial infarction, emergency surgery, renal failure, procedure type, and aortic cross-clamp time showed that E/e′ ≥8 was significantly related to an increased proportion of patients requiring prolonged ICU stay compared to those patients with E/e′ <8 (HR 0.64 [95% CI: 0.45 – 0.90]).
Figure 2.
The effect of age, baseline left ventricular ejection fraction, and procedure type on the hazard rates of ICU length of stay. Without adjusting for other predictors (univariate model), age >65 and multiple cardiac procedures (e.g., CABG and valve surgery) were significantly associated with prolonged ICU-LOS while a reduced preoperative LVEF was not associated with ICU-LOS. For presentation, data are truncated to 500 h (95% of values were at or below 500 h of ICU stay).
Figure 3.
The relationship between E/e′ class and ICU length of stay, without adjustments for other predictors (univariate model, left) and with adjustments for age, gender, hypertension, diabetes mellitus, history of myocardial infarction, operation type, elective status, and aortic cross clamp duration (multivariate model, right). In both the univariate and multivariate models, an E/e′ value ≥8 was significantly associated with prolonged ICU-LOS. For presentation, data are truncated to 500 h (95% of values were at or below 500 h of ICU stay).
Discussion
Our data show that TDI-derived estimations of left ventricular diastolic filling pressure (E/e′) obtained with TEE prior to surgical repair was significantly related to ICU-LOS, H-LOS, duration of MV, and the use of inotropes. When adjusted for patient and operative characteristics, E/e′ was significantly associated with ICU-LOS and the use of inotropic support after CABG and/or valvular surgery.
It is well established that complications following cardiac surgery are encountered in patients who are advanced in age, female gender, have diminished systolic function and multiple comorbidities and who have prolonged aortic cross-clamp times.1–3,24 Because there remain those individuals who do not necessarily display these predictive characteristics but still experience a difficult hospital course, a non- invasive, readily available, and easily obtainable clinical predictor could be very useful. Recently, several studies demonstrated the clinical efficacy of TDI echocardiographic examination of left ventricular longitudinal motion in various pathologic conditions such as acute myocardial infarction and non-valvular atrial fibrillation.19,26 If TDI echocardiography offers additional information beyond clinical judgement and baseline systolic function in the perioperative setting, it too could be used to identify potential at-risk cardiac surgical patients, and thus, may be a very useful minimally invasive tool for the perioperative care provider.
The ratio of transmitral E wave velocity to TDI measured mitral annular velocity (E/e′) relates early transmitral left ventricular filling to myocardial relaxation and is used to estimate mean left atrial pressure,18,27 which may be elevated in various cardiac pathologies, and specifically in advanced diastolic dysfunction. Although e′ and E/e′ measurements alone are not diagnostic of diastolic dysfunction per se, they aid in the assessment of its severity when combined with other measurements.28 Both e′ and E/e′ are related to the intrinsic pathophysiology of diastolic dysfunction; specifically, reduced myocardial relaxation and elevated LV filling pressures are reflective of physiologic derangements that may indicate more advanced diastolic heart disease. In the estimation of elevated LV diastolic pressure, E/e′ values >15 represent elevated LV filling pressure, and <8 reflect normal filling pressure.18,27 Unlike conventional mitral and pulmonary venous flow velocity indices of LV filling pressures (e.g., a short mitral deceleration time (<140 ms) and/or an increased E/A ratio (>2.5), the accuracy of E/e′ in estimating LV filling pressures is relatively independent of rate and rhythm abnormalities (such as sinus tachycardia and atrial fibrillation), LV hypertrophy, and functional mitral regurgitation.13,21–23,29
However, there are several caveats to TDI measurements that warrant mention. Importantly, patients with relatively normal hearts with higher baseline tissue Doppler velocities will have more preload dependence compared to patients with marked impairment in myocardial relaxation.30–32 Age influences TDI values as well; e′ decreases in a linear fashion with increasing age, and thus E/e′ increases with age.33,34 Although e′ is used to relate to global indices of LV relaxation, it is a regional index. Errors can, therefore, occur in patients with regional wall motion abnormalities at the Doppler sampling site, unless an average of two sites is provided. Another important consideration when using TDI is the position of the sample area within the wall. Diastolic velocities measured in the lateral wall are higher than velocities measured in the septum, as the septum is tethered to the right ventricle and other structures in the middle of the heart.27,35 We have used the lateral mitral annular velocity because of its accessibility with TEE, and ease of Doppler beam alignment. Indeed, lateral mitral annular velocity may be less sensitive to regional wall motion abnormalities than septal annular motion,36 as well as less influenced by preload variation.37
The present study found a significant relationship between elevated E/e′ and H-LOS, ICU-LOS, the use of inotropic support, and the duration of MV. Further, when adjusted for the effect of patient and operative characteristics, E/e′ maintained a significant relationship between ICU-LOS and the use of inotropic support. The magnitude of this relationship was substantial; in particular, we found that those patients with moderately elevated (E/e′ ≥8 and ≤15) and severely elevated (E/e′ >15) tissue-Doppler derived filling pressures spent on average between 9 and 30 h longer in the ICU than those with an E/e′ ratio <8. In addition, 48% of the severely elevated E/e′ group and 36% in the moderately elevated received postoperative inotropic support, compared to only 11% of those with E/e′ <8. A second analysis was performed to examine the relationship between baseline LVEF and the outcome variables, in order to ensure consistency with previous reports.24 Indeed, our data confirm that preoperative systolic dysfunction associates with a greater propensity for inotropic use but not H-LOS, ICU-LOS, or MV duration after cardiac surgery. Taken together, these findings suggest that the TDI-derived index of left ventricular diastolic filling pressure may provide additional prognostic information from that of baseline systolic function.
There are several limitations in the present study. First, this was a small, retrospective study of patients who underwent CABG and/or valvular cardiac surgery utilizing CPB. While underpowered to detect a significant difference in mortality between those patients with normal and abnormal E/e′, the current group of patients was large enough to determine the primary outcomes LOS-ICU, LOS-H, the use of inotropic support, and duration of MV. Ideally, these results should be verified prospectively in a study also powered to detect in-hospital and long-term mortality. Second, the decision to include all patients undergoing cardiac surgery, rather than to separate CABG and valvular procedures, was made in an attempt to examine the utility of E/e′ in our overall practice of cardiac surgery. Indeed, this may have introduced bias in that patients with valvular pathology exhibit different physiology than those with coronary artery disease. There has been some validation of TDI in those individuals with valvular disease, as E/e′ correlates well with invasive measure of filling pressures in patients with moderate to severe aortic stenosis,38 and in those with secondary mitral regurgitation, but not primary mitral regurgitation.13 Although a complete examination of valvular structure and function was performed during the intraoperative TEE, we did not include the presence and/or severity of mitral regurgitation in our data set. Certainly, severe mitral regurgitation could have influenced the tissue-Doppler derived index of filling pressure,39,40 and thus, these data should be interpreted with caution. Third, while we have attempted to consider a large number of demographic variables, as reflected in Table I, this list of covariates was limited in number by our sample size, and does not consider every potential patient variable. Also, as the study was a retrospective analysis, the use of inotropic support in the study population was left to the discretion of the anesthesiologist and surgeon, which may have introduced bias. However, it is our practice to initiate inotropes upon weaning from CPB, or shortly thereafter, for low cardiac output syndrome (e.g., cardiac index <2.0 L/min/m2) and not for preoperative elevations in diastolic filling pressure.
In conclusion, this study adds to the growing body of evidence indicating that TDI is of clinical relevance in the perioperative setting. Specifically, the tissue-Doppler derived surrogate of diastolic filling pressure, E/e′, obtained intraoperatively by TEE or perhaps even by a preoperative transthoracic echocardiographic examination41,42 may be a useful indicator for predicting early morbid events after cardiac surgery that may even provide information beyond that of baseline LVEF. Importantly, patients with an elevated preoperative E/e′ may need more careful peri- and postoperative management than those patients with E/e′ <8.
Acknowledgments
Funded in part by grants to Dr. Groban from the Dennis Jahnigen Career Development Award, the National Institutes of Health grants KO8-AG026764-04 Paul Beeson Award, and Hartford Foundation Project, American Geriatrics Society, Anesthesia Initiative on Aging Education: Geriatrics for Specialists Initiative.
References
- 1.Winkel E, Piccione W. Coronary artery bypass surgery in patients with left ventricular dysfunction: candidate selection and perioperative care. J Heart Lung Transplant. 1997;16:S19–S24. [PubMed] [Google Scholar]
- 2.Higgins TL, Yared JP, Ryan T. Immediate postoperative care of cardiac surgical patients. J Cardiothorac Vasc Anesth. 1996;10:643–658. doi: 10.1016/s1053-0770(96)80145-5. [DOI] [PubMed] [Google Scholar]
- 3.Rao V, Ivanov J, Weisel RD, et al. Predictors of low cardiac output syndrome after coronary artery bypass. J Thorac Cardiovasc Surg. 1996;112:38–51. doi: 10.1016/s0022-5223(96)70176-9. [DOI] [PubMed] [Google Scholar]
- 4.Bernard F, Denault A, Babin D, et al. Diastolic dysfunction is predictive of difficult weaning from cardiopulmonary bypass. Anesth Analg. 2001;92:291–298. doi: 10.1097/00000539-200102000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Denault AY, Couture P, Buithieu J, et al. Left and right ventricular diastolic dysfunction as predictors of difficult separation from cardiopulmonary bypass. Can J Anaesth. 2006;53:1020–1029. doi: 10.1007/BF03022532. [DOI] [PubMed] [Google Scholar]
- 6.Liu J, Tanaka N, Murata K, et al. Prognostic value of pseudonormal and restrictive filling patterns on left ventricular remodeling and cardiac events after coronary artery bypass grafting. Am J Cardiol. 2003;91:550–554. doi: 10.1016/s0002-9149(02)03304-0. [DOI] [PubMed] [Google Scholar]
- 7.Merello L, Riesle E, Alburquerque J, et al. Risk scores do not predict high mortality after coronary artery bypass surgery in the presence of diastolic dysfunction. Ann Thorac Surg. 2008;85:1247–1255. doi: 10.1016/j.athoracsur.2007.12.068. [DOI] [PubMed] [Google Scholar]
- 8.Smith SA, Stoner JE, Russell AE, et al. Transmitral velocities measured by pulsed Doppler in healthy volunteers: effects of acute changes in blood pressure and heart rate. Br Heart J. 1989;61:344–347. doi: 10.1136/hrt.61.4.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sohn DW, Song JM, Zo JH, et al. Mitral annulus velocity in the evaluation of left ventricular diastolic function in atrial fibrillation. J Am Soc Echocardiogr. 1999;12:927–931. doi: 10.1016/s0894-7317(99)70145-8. [DOI] [PubMed] [Google Scholar]
- 10.Chamoun AJ, Xie T, McCullough M, et al. Color M-mode flow propagation velocity and conventional doppler indices in the assessment of diastolic left ventricular function during isometric exercise. Echocardiography. 2005;22:380–388. doi: 10.1111/j.1540-8175.2005.04061.x. [DOI] [PubMed] [Google Scholar]
- 11.Choong CY, Herrmann HC, Weyman AE, et al. Preload dependence of Doppler-derived indexes of left ventricular diastolic function in humans. J Am Coll Cardiol. 1987;10:800–808. doi: 10.1016/s0735-1097(87)80273-5. [DOI] [PubMed] [Google Scholar]
- 12.Chamoun AJ, Xie T, Trough M, et al. Color M-mode flow propagation velocity versus conventional Doppler indices in the assessment of diastolic left ventricular function in patients on chronic hemodialysis. Echocardiography. 2002;19:467–474. doi: 10.1046/j.1540-8175.2002.00467.x. [DOI] [PubMed] [Google Scholar]
- 13.Bruch C, Stypmann J, Gradaus R, et al. Usefulness of tissue Doppler imaging for estimation of filling pressures in patients with primary or secondary pure mitral regurgitation. Am J Cardiol. 2004;93:324–328. doi: 10.1016/j.amjcard.2003.10.012. [DOI] [PubMed] [Google Scholar]
- 14.Stoddard MF, Pearson AC, Kern MJ, et al. Influence of alteration in preload on the pattern of left ventricular diastolic filling as assessed by Doppler echocardiography in humans. Circulation. 1989;79:1226–1236. doi: 10.1161/01.cir.79.6.1226. [DOI] [PubMed] [Google Scholar]
- 15.Kronzon I. PRO: Intraoperative Doppler tissue imaging is a valuable addition to cardiac anesthesiologists' armamentarium. Anesth Analg. 2009;108:37–40. doi: 10.1213/ane.0b013e31818a6f4b. [DOI] [PubMed] [Google Scholar]
- 16.Skubas N. Intraoperative Doppler tissue imaging is a valuable addition to cardiac anesthesiologists' armamentarium: a core review. Anesth Analg. 2009;108:48–66. doi: 10.1213/ane.0b013e31818a6c4c. [DOI] [PubMed] [Google Scholar]
- 17.Dumesnil JG, Paulin C, Pibarot P, et al. Mitral annulus velocities by Doppler tissue imaging: practical implications with regard to preload alterations, sample position, and normal values. J Am Soc Echocardiogr. 2002;15:1226–1231. doi: 10.1067/mje.2002.123396. [DOI] [PubMed] [Google Scholar]
- 18.Ommen SR, Nishimura RA, Appleton CP, et al. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular filling pressures: A comparative simultaneous Doppler-catheterization study. Circulation. 2000;102:1788–1794. doi: 10.1161/01.cir.102.15.1788. [DOI] [PubMed] [Google Scholar]
- 19.Hillis GS, Møller JE, Pellikka PA, et al. Noninvasive estimation of left ventricular filling pressure by E/e' is a powerful predictor of survival after acute myocardial infarction. J Am Coll Cardiol. 2004;43:360–367. doi: 10.1016/j.jacc.2003.07.044. [DOI] [PubMed] [Google Scholar]
- 20.Møller JE, Pellikka PA, Hillis GS, et al. Prognostic importance of diastolic function and filling pressure in patients with acute myocardial infarction. Circulation. 2006;114:438–444. doi: 10.1161/CIRCULATIONAHA.105.601005. [DOI] [PubMed] [Google Scholar]
- 21.Nagueh SF, Kopelen HA, Quiñones MA. Assessment of left ventricular filling pressures by Doppler in the presence of atrial fibrillation. Circulation. 1996;94:2138–2145. doi: 10.1161/01.cir.94.9.2138. [DOI] [PubMed] [Google Scholar]
- 22.Nagueh SF, Mikati I, Kopelen HA, et al. Doppler estimation of left ventricular filling pressure in sinus tachycardia. A new application of tissue doppler imaging. Circulation. 1998;98:1644–1650. doi: 10.1161/01.cir.98.16.1644. [DOI] [PubMed] [Google Scholar]
- 23.Nagueh SF, Lakkis NM, Middleton KJ, et al. Doppler estimation of left ventricular filling pressures in patients with hypertrophic cardiomyopathy. Circulation. 1999;99:254–261. doi: 10.1161/01.cir.99.2.254. [DOI] [PubMed] [Google Scholar]
- 24.Royster RL, Butterworth JF, IV, Prough DS, et al. Preoperative and intraoperative predictors of inotropic support and long-term outcome in patients having coronary artery bypass grafting. Anesth Analg. 1991;72:729–736. doi: 10.1213/00000539-199106000-00002. [DOI] [PubMed] [Google Scholar]
- 25.Salem R, Denault AY, Couture P, et al. Left ventricular end-diastolic pressure is a predictor of mortality in cardiac surgery independently of left ventricular ejection fraction. Br J Anaesth. 2006;97:292–297. doi: 10.1093/bja/ael140. [DOI] [PubMed] [Google Scholar]
- 26.Okura H, Takada Y, Kubo T, et al. Tissue Doppler-derived index of left ventricular filling pressure, E/E', predicts survival of patients with non-valvular atrial fibrillation. Heart. 2006;92:1248–1252. doi: 10.1136/hrt.2005.082594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nagueh SF, Middleton KJ, Kopelen HA, et al. Doppler tissue imaging: a noninvasive technique for evaluation of left ventricular relaxation and estimation of filling pressures. J Am Coll Cardiol. 1997;30:1527–1533. doi: 10.1016/s0735-1097(97)00344-6. [DOI] [PubMed] [Google Scholar]
- 28.Nagueh SF, Appleton CP, Gillebert TC, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography. Eur J Echocardiogr. 2009;10:165–193. doi: 10.1093/ejechocard/jep007. [DOI] [PubMed] [Google Scholar]
- 29.Sohn DW, Kim YJ, Kim HC, et al. Evaluation of left ventricular diastolic function when mitral E and A waves are completely fused: role of assessing mitral annulus velocity. J Am Soc Echocardiogr. 1999;12:203–208. doi: 10.1016/s0894-7317(99)70136-7. [DOI] [PubMed] [Google Scholar]
- 30.Firstenberg MS, Greenberg NL, Main ML, et al. Determinants of diastolic myocardial tissue Doppler velocities: influences of relaxation and preload. J Appl Physiol. 2001;90:299–307. doi: 10.1152/jappl.2001.90.1.299. [DOI] [PubMed] [Google Scholar]
- 31.Jacques DC, Pinsky MR, Severyn D, et al. Influence of alterations in loading on mitral annular velocity by tissue Doppler echocardiography and its associated ability to predict filling pressures. Chest. 2004;126:1910–1918. doi: 10.1378/chest.126.6.1910. [DOI] [PubMed] [Google Scholar]
- 32.Oğuzhan A, Arinç H, Abaci A, et al. Preload dependence of Doppler tissue imaging derived indexes of left ventricular diastolic function. Echocardiography. 2005;22:320–325. doi: 10.1111/j.1540-8175.2005.03177.x. [DOI] [PubMed] [Google Scholar]
- 33.De Sutter J, De Backer J, Van de Veire N, et al. Effects of age, gender, and left ventricular mass on septal mitral annulus velocity (E') and the ratio of transmitral early peak velocity to E' (E/E') Am J Cardiol. 2005;95:1020–1023. doi: 10.1016/j.amjcard.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 34.Wierzbowska-Drabik K, Krzemińska-Pakuła M, Chrzanowski L, et al. Age-dependency of classic and new parameters of diastolic function. Echocardiography. 2008;25:149–155. doi: 10.1111/j.1540-8175.2007.00569.x. [DOI] [PubMed] [Google Scholar]
- 35.Hadano Y, Murata K, Tanaka N, et al. Ratio of early transmitral velocity to lateral mitral annular early diastolic velocity has the best correlation with wedge pressure following cardiac surgery. Circ J. 2007;71:1274–1278. doi: 10.1253/circj.71.1274. [DOI] [PubMed] [Google Scholar]
- 36.Lim HS, Kang SJ, Choi JH, et al. Is E/E' reliable in patients with regional wall motion abnormalities to estimate left ventricular filling pressure? Int J Cardiovasc Imaging. 2009;25:33–39. doi: 10.1007/s10554-008-9340-2. [DOI] [PubMed] [Google Scholar]
- 37.Rivas-Gotz C, Manolios M, Thohan V, et al. Impact of left ventricular ejection fraction on estimation of left ventricular filling pressures using tissue Doppler and flow propagation velocity. Am J Cardiol. 2003;91:780–784. doi: 10.1016/s0002-9149(02)03433-1. [DOI] [PubMed] [Google Scholar]
- 38.Bruch C, Stypmann J, Grude M, et al. Tissue Doppler imaging in patients with moderate to severe aortic valve stenosis: clinical usefulness and diagnostic accuracy. Am Heart J. 2004;148:696–702. doi: 10.1016/j.ahj.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 39.Olson JJ, Costa SP, Young CE, Palac RT. Early mitral filling/diastolic mitral annular velocity ratio is not a reliable predictor of left ventricular filling pressure in the setting of severe mitral regurgitation. J Am Soc Echocardiogr. 2006;19:83–87. doi: 10.1016/j.echo.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 40.Pu M, Gao Z, Zhang X, et al. Impact of mitral regurgitation on left ventricular anatomic and molecular remodeling and systolic function: implication for outcome. Am J Physiol Heart Circ Physiol. 2009;296:H1727–H1732. doi: 10.1152/ajpheart.00882.2008. [DOI] [PubMed] [Google Scholar]
- 41.Sevimli S, Arslan S, Gundogdu F, et al. Can transesophageal pulse-wave tissue Doppler imaging be used to evaluate left ventricular function? Echocardiography. 2007;24:946–954. doi: 10.1111/j.1540-8175.2007.00499.x. [DOI] [PubMed] [Google Scholar]
- 42.Couture P, Denault AY, Shi Y, et al. Effects of anesthetic induction in patients with diastolic dysfunction. Can J Anaesth. 2009;56:357–365. doi: 10.1007/s12630-009-9068-z. [DOI] [PubMed] [Google Scholar]