Abstract
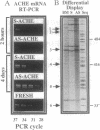
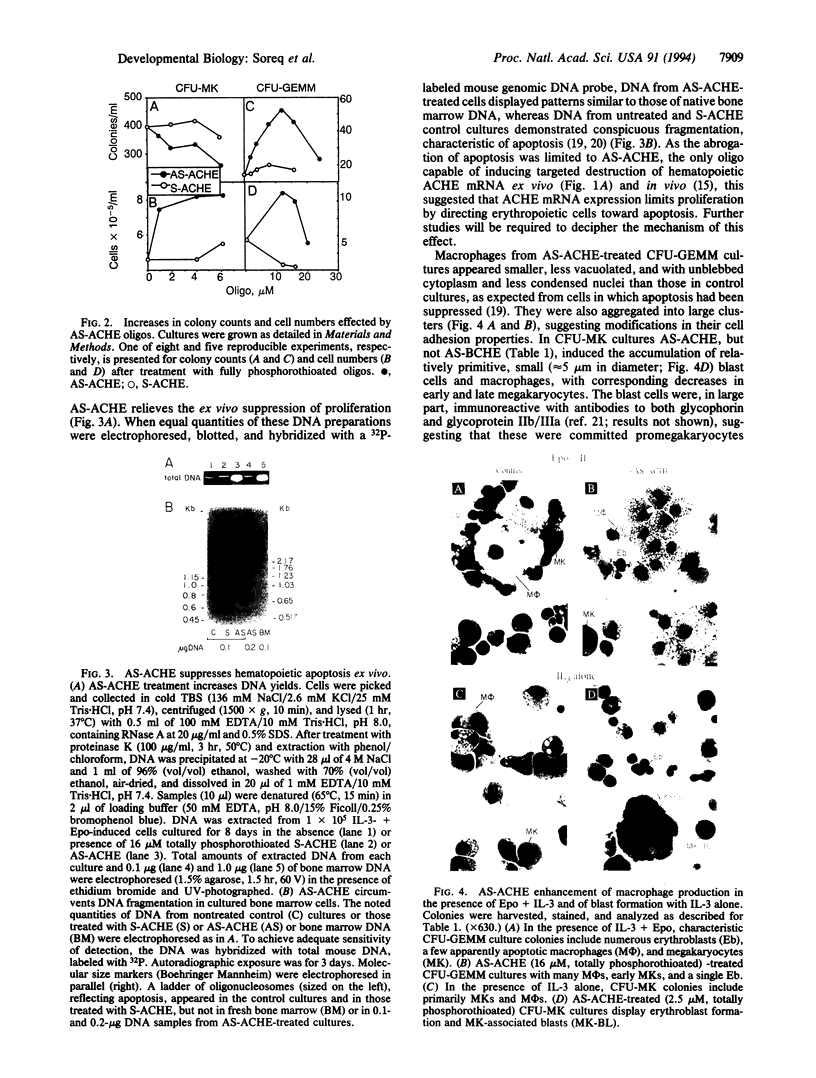
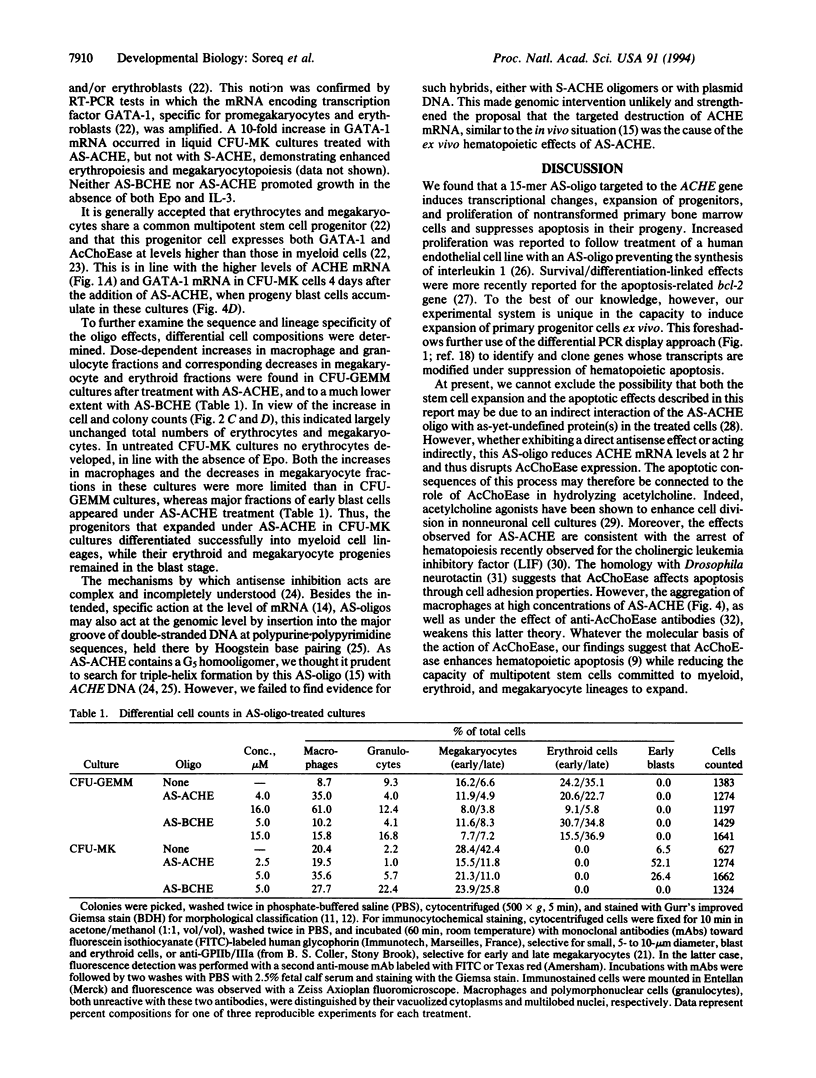
To examine the role of acetylcholinesterase (EC 3.1.1.7) in hematopoietic cell proliferation and differentiation, we administered a 15-mer phosphorothioate oligonucleotide, antisense to the corresponding ACHE gene (AS-ACHE), to primary mouse bone marrow cultures. Within 2 hr of AS-ACHE addition to the culture, ACHE mRNA levels dropped by approximately 90%, as compared with those in cells treated with the "sense" oligomer, S-ACHE. Four days after AS-ACHE treatment, ACHE mRNA increased to levels 10-fold higher than in S-ACHE cultures or in fresh bone marrow. At this later time point, differential PCR display revealed significant differences between cellular mRNA transcripts in bone marrow and those in AS-ACHE- or S-ACHE-treated cultures. These oligonucleotide-triggered effects underlay considerable alterations at the cellular level: AS-ACHE but not S-ACHE increased cell counts, reflecting enhanced proliferation. In the presence of erythropoietin it also enhanced colony counts, reflecting expansion of progenitors. AS-ACHE further suppressed apoptosis-related fragmentation of cellular DNA in the progeny cells, and it diverted hematopoiesis toward production of primitive blasts and macrophages in a dose-dependent manner promoted by erythropoietin. These findings suggest that the hematopoietic role of acetylcholinesterase, anticipated to be inverse to the observed antisense effects, is to reduce proliferation of the multipotent stem cells committed to erythropoiesis and megakaryocytopoiesis and macrophage production and to promote apoptosis in their progeny. Moreover, these findings may explain the tumorigenic association of perturbations in ACHE gene expression with leukemia.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashkenazi A., Ramachandran J., Capon D. J. Acetylcholine analogue stimulates DNA synthesis in brain-derived cells via specific muscarinic receptor subtypes. Nature. 1989 Jul 13;340(6229):146–150. doi: 10.1038/340146a0. [DOI] [PubMed] [Google Scholar]
- Brimijoin S., Moser V., Hammond P., Oka N., Lennon V. A. Death of intermediolateral spinal cord neurons follows selective, complement-mediated destruction of peripheral preganglionic sympathetic terminals by acetylcholinesterase antibodies. Neuroscience. 1993 May;54(1):201–223. doi: 10.1016/0306-4522(93)90394-u. [DOI] [PubMed] [Google Scholar]
- Brown L. M., Blair A., Gibson R., Everett G. D., Cantor K. P., Schuman L. M., Burmeister L. F., Van Lier S. F., Dick F. Pesticide exposures and other agricultural risk factors for leukemia among men in Iowa and Minnesota. Cancer Res. 1990 Oct 15;50(20):6585–6591. [PubMed] [Google Scholar]
- Burstein S. A., Adamson J. W., Harker L. A. Megakaryocytopoiesis in culture: modulation by cholinergic mechanisms. J Cell Physiol. 1980 May;103(2):201–208. doi: 10.1002/jcp.1041030205. [DOI] [PubMed] [Google Scholar]
- Coller B. S., Beer J. H., Scudder L. E., Steinberg M. H. Collagen-platelet interactions: evidence for a direct interaction of collagen with platelet GPIa/IIa and an indirect interaction with platelet GPIIb/IIIa mediated by adhesive proteins. Blood. 1989 Jul;74(1):182–192. [PubMed] [Google Scholar]
- Collins M. K., Lopez Rivas A. The control of apoptosis in mammalian cells. Trends Biochem Sci. 1993 Aug;18(8):307–309. doi: 10.1016/0968-0004(93)90042-l. [DOI] [PubMed] [Google Scholar]
- Ehrlich G., Viegas-Pequignot E., Ginzberg D., Sindel L., Soreq H., Zakut H. Mapping the human acetylcholinesterase gene to chromosome 7q22 by fluorescent in situ hybridization coupled with selective PCR amplification from a somatic hybrid cell panel and chromosome-sorted DNA libraries. Genomics. 1992 Aug;13(4):1192–1197. doi: 10.1016/0888-7543(92)90037-s. [DOI] [PubMed] [Google Scholar]
- Escary J. L., Perreau J., Duménil D., Ezine S., Brûlet P. Leukaemia inhibitory factor is necessary for maintenance of haematopoietic stem cells and thymocyte stimulation. Nature. 1993 May 27;363(6427):361–364. doi: 10.1038/363361a0. [DOI] [PubMed] [Google Scholar]
- Fairbairn L. J., Cowling G. J., Reipert B. M., Dexter T. M. Suppression of apoptosis allows differentiation and development of a multipotent hemopoietic cell line in the absence of added growth factors. Cell. 1993 Sep 10;74(5):823–832. doi: 10.1016/0092-8674(93)90462-y. [DOI] [PubMed] [Google Scholar]
- Fauser A. A., Messner H. A. Granuloerythropoietic colonies in human bone marrow, peripheral blood, and cord blood. Blood. 1978 Dec;52(6):1243–1248. [PubMed] [Google Scholar]
- Karpel R., Ben Aziz-Aloya R., Sternfeld M., Ehrlich G., Ginzberg D., Tarroni P., Clementi F., Zakut H., Soreq H. Expression of three alternative acetylcholinesterase messenger RNAs in human tumor cell lines of different tissue origins. Exp Cell Res. 1994 Feb;210(2):268–277. doi: 10.1006/excr.1994.1039. [DOI] [PubMed] [Google Scholar]
- Kelley L. L., Koury M. J., Bondurant M. C., Koury S. T., Sawyer S. T., Wickrema A. Survival or death of individual proerythroblasts results from differing erythropoietin sensitivities: a mechanism for controlled rates of erythrocyte production. Blood. 1993 Oct 15;82(8):2340–2352. [PubMed] [Google Scholar]
- Koury M. J. Programmed cell death (apoptosis) in hematopoiesis. Exp Hematol. 1992 May;20(4):391–394. [PubMed] [Google Scholar]
- Lapidot-Lifson Y., Patinkin D., Prody C. A., Ehrlich G., Seidman S., Ben-Aziz R., Benseler F., Eckstein F., Zakut H., Soreq H. Cloning and antisense oligodeoxynucleotide inhibition of a human homolog of cdc2 required in hematopoiesis. Proc Natl Acad Sci U S A. 1992 Jan 15;89(2):579–583. doi: 10.1073/pnas.89.2.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lapidot-Lifson Y., Prody C. A., Ginzberg D., Meytes D., Zakut H., Soreq H. Coamplification of human acetylcholinesterase and butyrylcholinesterase genes in blood cells: correlation with various leukemias and abnormal megakaryocytopoiesis. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4715–4719. doi: 10.1073/pnas.86.12.4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layer P. G., Weikert T., Willbold E. Chicken retinospheroids as developmental and pharmacological in vitro models: acetylcholinesterase is regulated by its own and by butyrylcholinesterase activity. Cell Tissue Res. 1992 Jun;268(3):409–418. doi: 10.1007/BF00319147. [DOI] [PubMed] [Google Scholar]
- Lev-Lehman E., Ginzberg D., Hornreich G., Ehrlich G., Meshorer A., Eckstein F., Soreq H., Zakut H. Antisense inhibition of acetylcholinesterase gene expression causes transient hematopoietic alterations in vivo. Gene Ther. 1994 Mar;1(2):127–135. [PubMed] [Google Scholar]
- Li Y., Camp S., Taylor P. Tissue-specific expression and alternative mRNA processing of the mammalian acetylcholinesterase gene. J Biol Chem. 1993 Mar 15;268(8):5790–5797. [PubMed] [Google Scholar]
- Liang P., Pardee A. B. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science. 1992 Aug 14;257(5072):967–971. doi: 10.1126/science.1354393. [DOI] [PubMed] [Google Scholar]
- Maier J. A., Voulalas P., Roeder D., Maciag T. Extension of the life-span of human endothelial cells by an interleukin-1 alpha antisense oligomer. Science. 1990 Sep 28;249(4976):1570–1574. doi: 10.1126/science.2218499. [DOI] [PubMed] [Google Scholar]
- Nellen W., Lichtenstein C. What makes an mRNA anti-sense-itive? Trends Biochem Sci. 1993 Nov;18(11):419–423. doi: 10.1016/0968-0004(93)90137-c. [DOI] [PubMed] [Google Scholar]
- Okumura N., Tsuji K., Nakahata T. Changes in cell surface antigen expressions during proliferation and differentiation of human erythroid progenitors. Blood. 1992 Aug 1;80(3):642–650. [PubMed] [Google Scholar]
- Paoletti F., Mocali A., Vannucchi A. M. Acetylcholinesterase in murine erythroleukemia (Friend) cells: evidence for megakaryocyte-like expression and potential growth-regulatory role of enzyme activity. Blood. 1992 Jun 1;79(11):2873–2879. [PubMed] [Google Scholar]
- Patinkin D., Seidman S., Eckstein F., Benseler F., Zakut H., Soreq H. Manipulations of cholinesterase gene expression modulate murine megakaryocytopoiesis in vitro. Mol Cell Biol. 1990 Nov;10(11):6046–6050. doi: 10.1128/mcb.10.11.6046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Glynn J. M., Guilbert L. J., Cotter T. G., Bissonnette R. P., Green D. R. Role for c-myc in activation-induced apoptotic cell death in T cell hybridomas. Science. 1992 Jul 10;257(5067):212–214. doi: 10.1126/science.1378649. [DOI] [PubMed] [Google Scholar]
- Smith C. A., Williams G. T., Kingston R., Jenkinson E. J., Owen J. J. Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature. 1989 Jan 12;337(6203):181–184. doi: 10.1038/337181a0. [DOI] [PubMed] [Google Scholar]
- Soreq H., Ben-Aziz R., Prody C. A., Seidman S., Gnatt A., Neville L., Lieman-Hurwitz J., Lev-Lehman E., Ginzberg D., Lipidot-Lifson Y. Molecular cloning and construction of the coding region for human acetylcholinesterase reveals a G + C-rich attenuating structure. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9688–9692. doi: 10.1073/pnas.87.24.9688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soreq H., Lapidot-Lifson Y., Zakut H. A role for cholinesterases in tumorigenesis? Cancer Cells. 1991 Dec;3(12):511–516. [PubMed] [Google Scholar]
- Stein C. A., Cheng Y. C. Antisense oligonucleotides as therapeutic agents--is the bullet really magical? Science. 1993 Aug 20;261(5124):1004–1012. doi: 10.1126/science.8351515. [DOI] [PubMed] [Google Scholar]
- Strobel S. A., Dervan P. B. Site-specific cleavage of a yeast chromosome by oligonucleotide-directed triple-helix formation. Science. 1990 Jul 6;249(4964):73–75. doi: 10.1126/science.2195655. [DOI] [PubMed] [Google Scholar]
- Visvader J. E., Elefanty A. G., Strasser A., Adams J. M. GATA-1 but not SCL induces megakaryocytic differentiation in an early myeloid line. EMBO J. 1992 Dec;11(12):4557–4564. doi: 10.1002/j.1460-2075.1992.tb05557.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaswen P., Stampfer M. R., Ghosh K., Cohen J. S. Effects of sequence of thioated oligonucleotides on cultured human mammary epithelial cells. Antisense Res Dev. 1993 Spring;3(1):67–77. doi: 10.1089/ard.1993.3.67. [DOI] [PubMed] [Google Scholar]
- Zakut H., Ehrlich G., Ayalon A., Prody C. A., Malinger G., Seidman S., Ginzberg D., Kehlenbach R., Soreq H. Acetylcholinesterase and butyrylcholinesterase genes coamplify in primary ovarian carcinomas. J Clin Invest. 1990 Sep;86(3):900–908. doi: 10.1172/JCI114791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakut H., Lapidot-Lifson Y., Beeri R., Ballin A., Soreq H. In vivo gene amplification in non-cancerous cells: cholinesterase genes and oncogenes amplify in thrombocytopenia associated with lupus erythematosus. Mutat Res. 1992 May;276(3):275–284. doi: 10.1016/0165-1110(92)90013-y. [DOI] [PubMed] [Google Scholar]
- de la Escalera S., Bockamp E. O., Moya F., Piovant M., Jiménez F. Characterization and gene cloning of neurotactin, a Drosophila transmembrane protein related to cholinesterases. EMBO J. 1990 Nov;9(11):3593–3601. doi: 10.1002/j.1460-2075.1990.tb07570.x. [DOI] [PMC free article] [PubMed] [Google Scholar]