Abstract
Loricrin is a terminally differentiating structural protein comprising more than 70% of the cornified envelope. It contributes to the protective barrier function of the stratum corneum. In vivo, loricrin is expressed inall mammalian stratified epithelia with the highest levels of expression in humid tissues such as newborn epidermis, the epithelia of oral and anal mucosa, esophagus, foreskin, vagina and the epidermal parts of sweat ducts. Loricrin is not expressed in non keratinizing epithelia and its expression at these sites actually represents a defensive or protective mechanismof the body. An insight into this protein- “Loricrin” can shed light to its potential as a marker in the early stages of potentially malignant disorders like oral sub mucous fibrosis and leukoplakia. This compilation has been done by taking into account the existing literature, reviews and original studies on loricrin, a major component of the cornifiedcell envelope, its structure and the alterations that result due to its absence or presence of both the epidermis and the oral mucosa.
Keywords: Cornified cell envelope, loricrin, loricrin keratoderma, oral sub mucous fibrosis
INTRODUCTION
Epithelial tissues are the main appendages that protect the body's internal organs from environmental stress, chemical damage and bacterial infection. The stratified epithelia seen in the skin and oral mucosa are one of the toughest and the most protective epithelia as they have to withstand severe physical and chemical forces and do so by producing a toughened structure - the cornified cell envelope (CE).[1] Loricrin is a major component of the CE keratins. These keratins are structural proteins and constitute about 85% of a fully differentiated keratinocyte. They belong to a multi-gene family coded by more than 30 intermediate filament genes and form the cytoskeleton of the vertebrate epithelial cells. A disturbance in these filaments results in a fragile cell which ruptures upon physical stress and is the cause for many a blistering disease.[2] Progression of many potentially malignant lesions show epithelial changes with altered morphology. This occurs due to changes in the normal proliferative and differentiating capacity of the keratinocytes and could be seen as disturbance in the CE of the cells.
The Cornified Cell Envelope (CE)
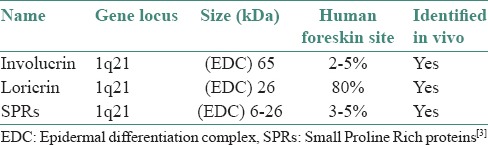
The cell envelope starts its formation in the most superficial granular or transitional cells and its assembly is catalyzed by transglutaminases which forms a protein-protein (gamma-glutamyl) lysine cross-link. Envelope proteins included in CE are involucrin, loricrin, small proline-rich proteins (SPRs), elafin, keratin filaments, filaggrin, cystatin-A and desmosomal proteins. [Table 1].[3]
Table 1.
Loci of CE precursor proteins
Loricrin and CE
The superficial spinous cells express involucrin which cross-links to form the envelope scaffolding. Loricrin in turn is cross linked to involucrin and is expressed in the superficial granular cells forming composite keratohyaline granules or L granules along with profillagrin.[4] The localization of loricrin in the stratum granulosum has been confirmed with immunohistochemistry by the authors in the laboratory. [Figure 1a and b shows the localization of loricrin in the granular layer of the human foreskin].
Figure 1.
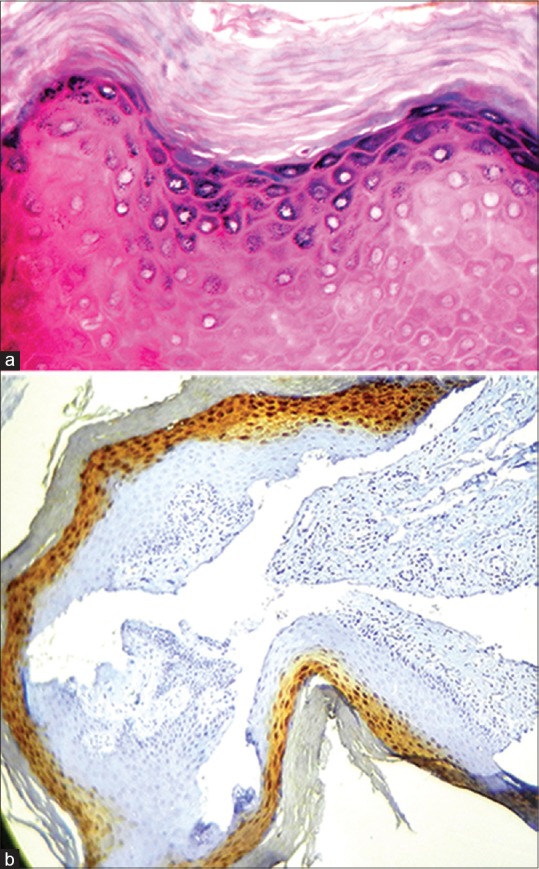
(a) The histopathological image shows Hyperkeratosis of the human foreskin (H&E stain, x40); (Courtesy: Department of Oral Pathology and Microbiology, Ragas Dental College and Hospital, Chennai). (b) Hyperkeratosis without epithelial dysplasia showing loricrin positivity in the stratum granulosum of Human foreskin (IHC stain, x10); (Courtesy: Department of Oral Pathology and Microbiology, Ragas Dental College and Hospital, Chennai)
Keratins and CE
The process of keratinization is characterized by a series of morphological changes in the keratinocytes. The basal cells show loss of adhesion to the basement membrane and progress into spinous cells which in turn forms a granular layer with keratohyaline granules. This terminally differentiates to finally form a cornified cell envelope.[4]
Keratins, the final differentiated end product of an epithelial cell are structural proteins belonging to a multigene family whose function is to form a keratin filament assembly coded by genes of intermediate filament genes. Common glycine motifs are present in both loricrin and the keratin intermediate filaments and interactions between these proteins help stabilize cellular structure. The keratin filaments thus forming the cytoskeleton of the vertebrate epithelial cells contribute to their mechanical strength.[2,5,6]
Lipids and CE
Lipids are an integral part of the cornified envelope. Covalent binding between ceramide lipids and proteins in epidermis are responsible for the orderly arrangement of extra cellular lipids into the lamellae which contributes to the barrier protection function of the CE.
Relation of Loricrin to lipids
The lipid and lamellae complex are reinforced by the binding of transglutaminases enzymes with proteins like loricrin, small proline rich proteins, tricohyaline and repetin. The binding alters the biomechanical structure of the envelope based on its local physical requirement. The lipid lamella thus becomes the medium where the dead corneocyte made up of intermediate filaments is finally embedded providing controlled mechanical and water permeability.[7,8]
Loricrin keratodermas (LK) show cellular fragility, water loss and accelerated barrier recovery. The water loss in LK has been found to be due to alterations in the organisation of the lipid lamellar bilayers due to CE scaffold discontinuities. While amplified lamellar secretions accelerated barrier recovery.[5,9]
Structure and distribution of loricrin
Loricrin is an insoluble polypeptide with a molecular weight of 26 kDa. It has a conserved epitope and is a major protein of the cornified envelope seen in the cytoskeleton of stratified parakeratinized epithelium. Being a late differentiation protein it is introduced into the scaffold of the cornified envelope because of its cross linking and binding property. It enhances the protective barrier function of the corneocyte in terminally differentiated keratinocytes.[10]
Loricrin has a simple structure - a single intron of 1188 base pairs in the untranslated region with none in coding regions. Loricrin is highly conserved and can be mapped to chromosome lq21.[5] It is initially sequestered into loricrin granules with a unique amino acid sequence rich in glycine, serine and cysteine residues. Glutamine or glutamine/lysine residues may also be seen within the sequence.[11] The glycine content of loricrin is higher than that of any known protein in biology and is the reason for its insolubility.[5,6] Owing to its high rate of expression and low solubility, loricrin forms spherical inclusions, called L-bodies in human foreskin and acrosyringium and is also diffusely distributed in the cytoplasm of adult epithelia.[12]
Expression patterns of Loricrin and factors influencing it
Loricrin occupies a major portion [70%] of the epidermal cornified envelope. Its concentration is reduced to about 30-50% in certain areas like palate and esophagus while it is not expressed in many internal epithelia like buccal mucosa. In vivo, loricrin is expressed in all mammalian stratified epithelia with the highest levels of expression in humid tissues such as newborn epidermis, the epithelia of oral and anal mucosa, esophagus, foreskin, vagina and the epidermal parts of sweat ducts. Loricrin is thus seen in the cornified layers and stratum granulosum in the normal keratinized oral epithelium.[6]
This varied expression is found to be influenced by a number of factors like cell confluence, calcium, vitamin A depletion, transglutaminase activity and Nectin-1. These factors induce terminal epidermal differentiation and there is expression of loricrin through signals acting on certain transcription factors like Activator protein (AP1). Expression of loricrin isnegatively regulated on application of Retinoic acid (RA) and CE is not formed. Calcium increases the transcriptional activity of loricrin and the CE formed when the level of calcium is less than 0.10 mM is immature and fragile. High Cell density at a calcium level of 0.05 mM does not express loricrin.[13] Nectin-1 knockout mice showed defective expression of loricrin with a fragile CE sensitive to mechanical stress.[14]
Functions of loricrin
The major function of the protein loricrin is, to reinforce the CE and to enhance its defensive barrier function. It helps us in understanding the CEs biological significance.[13] Interaction of Loricrin with the keratin intermediate filaments provides flexibility to the CE.[6] Loricrin also protects against mechanical stress by its association with nectin and calcium induction levels.[14]
Mutation of Loricrin
Mutation of loricrin could be either loss or gain of function or insertional mutations. These unique mutations in the glycine-rich domain of the mutant loricrin form arginine-rich nuclear localization sequences (NLSs) that disrupt differentiation of keratinocytes. NLS is an amino acid sequence which ‘tags’ a protein exposed on the cell surface, for import into the cell nucleus by nuclear transport. Loricrin is a small molecule (26 kDa in human and 38 kDa in mouse) localized in both the cytoplasm and nucleoplasm. It has a functional peptide as part of a nucleolar targeting element in signal recognition particles and can cross the nuclear pore complex by diffusion due to its smaller molecular mass.[11,15]
Mutant loricrin alters the nuclear/nucleolar functions instead of directly affecting the CE and might disturb other functions of nucleolus including non ribosomal RNA processing and growth factor signal transduction. Loricrin mutation deranges the keratinocyte differentiation/cell death pathway by affecting nucleolus as a target of apoptotic cellular changes and delays the cell death process in conditions like loricrin keratoderma.[15]
Skin lesions and loricrin
Loricrin Keratoderma
Loss or gain of function mutations of loricrin produces only modest skin phenotypes. Insertional mutations resulting in a frame shift in the C-terminal domain of loricrin however, produce ichthyosis of loricrin keratoderma with varying phenotypes in certain congenital skin abnormalities. The patients affected are diagnosed as suffering from an “Ichthyotic variant of Vohwinkel's syndrome”,“Progressive symmetric erythrokeratoderma,” or “congenital ichthyosiform erythroderma” born as a collodion baby- a condition where the baby is encased in a thin membrane resembling, plastic wrap.
Clinical features include hyperkeratosis of the palms and soles with digital constriction. Histologic characteristics include parakeratotic hyperkeratosis with hypergranulosis and nuclear accumulation of mutant loricrin. This group of unique genodermatoses caused by distinct loricrin mutations is collectively termed as LK.[15,16,17,18]
Immunohistochemistry of LK epidermis demonstrated that mutant loricrin was localized in the differentiated keratinocytesin a predicted, tissue and differentiation dependent manner and was detected up to the cornified layer. This detection of the mutant loricrin in scraped horny layer either by immunoblotting or immunohistochemistry might offer simple non-invasive screening tests for loricrin keratoderma.[6,11,19]
Frame shift mutations result in lack of the C-terminal of the glutamine- lysine rich domain which plays an important role in cross linking CE. Immunoreactivity to involucrin rather than loricrin is seen in such mutations. This is due to continuous cross-linking of involucrin which masks wild type loricrin epitopes even after loricrin cross-linking.[9] In LK epidermis, nucleoli from the basal to the lower granular layer were not apparently different from those in normal skin, but those in the upper layer were distinct. There was deposition of mutant loricrin within and around the nucleoli clusters indicating their pathologic role upon nucleolar functions. Mutant loricrin expressed in a very late stage of terminal differentiation where nucleolus is no more active does not hamper the completion of differentiation and is the reason for limited skin lesion distribution.[9,17,18]
Palmo plantar keratoderma
Palmo plantar keratoderma (PPK) includes a heterogeneous group of disorders exhibiting hyperkeratosis of the palms and soles.[17] They are differentiated by their inheritance pattern and associated clinical and histological features. In the palmo-plantar skin loricrin-expressing cells are several layers thick and show insertional mutation of a single nucleotide in the loricrin gene.
When the cells start to express mutant loricrin, the late keratinization processes is delayed. The reason for variation in the extra-palmoplantar lesions like “Progresive symmetric erythrokeratoderma” and “Ichthyocytic variant of vohwinkel syndrome” is not clear. It has been found that the patho-mechanisms of dominantly inherited disorders are explained as dominant negative effects of mutant molecules and attributed to haplo insufficiency. In LK, experiments with loricrin null mice has shown that though haploinsufficiency occurs it is the dominant negative effects of mutant loricrin that disrupts assembly of CE.[6,11,16,20,21]
Psoriasis and loricrin
Psoriasis is an immune mediated inflammatory disease involving skin and joints. Experiments have shown that a susceptibility locus PSORS4 in psoriasis localized to chromosome 1q21. This contains a cluster of genes of which, loricrin is an integral component. Though Loricrin gene (LOR) mRNA down regulation is seen in psoriasis, sequencing of LOR gene and genotyping its variants in families linked to PSORS4 locus, were not disease susceptible but could be affected by variants in other locus.[21] Katou F et al., found that loricrin in parakeratotic inflammatory diseases like psoriasis showed a down regulation in its expression due to diminished advanced terminally differentiating products like loricrin, keratins 1, 10 and filaggrin.[22]
Oral lesions and loricrin
Oral sub mucous fibrosis
Oral sub mucous fibrosis [OSF] may be defined as “an insidious, chronic disease affecting any part of the oral cavity and sometimes the pharynx. Although occasionally preceded by and/or associated with vesicle formation, it is always associated with juxtaepithelial inflammatory reaction followed by a fibroelastic change of the lamina propria, with epithelial atrophy leading to stiffness of the oral mucosa and causing trismus and inability to eat”.[23,24] Areca nut chewing plays a major role in the aetiology and pathogenesis of OSF. Chewing hard areca causes both mechanical and chemical stress and creates a environment similar to that of a dry epithelia leading to expression of loricrin and formation of a CE. Areca nut is usually taken along with lime (Calcium hydroxide) and the increased calcium concentration aids the barrier recovery process with loricrin being expressed.[13,25] Early and moderate stages of OSF tend to show an significant difference in expression while this is not evident between moderate and advanced stages which could be due to limitation in its capacity to withstand against the continuous mechanical stress caused by areca nut chewing.[26] The intrinsic property of the epithelia, changes by adapting to the new stimulus and could be the reason for expression of loricrin in nonkeratinized mucosa which lacks stratum corneum.[25] Variation in the different stages of oral sub mucous fibrosis could be due to the adapting capacity of the epithelia towards a new stimuli and can be useful in early identification of any transformation potential.
Loricrin and leukoplakia
Leukoplakia has been defined as “a predominantly white lesion of the oral mucosa that cannot be characterized as any other definable lesion; some oral leukoplakia will transform into cancer” (Axell T, 1996).[25]
When cases of normal mucosa, leukoplakia and OSCC (Oral squamous cell carcinoma) were assessed for the presence of Loricrin gene using Genechip microarray technology, it turned out to be one of the 8 upregulated genes analyzed among 8,800 genes. The signal intensity of loricrin along with other epithelia specific upregulated genes like K10 (Cytokeratin -10), K2e (epidermal ichthyosis bullosa of siemens) and CLSP (Calmodulin like skin protein) were weakly expressed or absent in normal mucosa while they were significantly expressed in leukoplakia. These genes however were found to be downregulated in OSCC transformed from the leukoplakic lesion. These differences in loricrin expression could be detected using transcription assays like Reverse transcriptase polymerase chain reaction (RT-PCR) and could be an early predictor of malignant transformation.[27]
Itoiz et al., has found that fillagrin, another component of the CE has a strong reaction in leukoplakic epithelia in the granular and horny layers with more intense staining seen when hyperkeratosis prevailed. These variations in expression of the genes could be used as an indicator in assessing abnormalities in the cytoskeleton network components and could shed light on the transforming potential of leukoplakia.[28]
CONCLUSION
Given the various implications of mutated loricrin in skin conditions like loricrin keratoderma, a similar correlation can be made to oral conditions exhibiting hyperkeratosis like leukoplakia and OSF. The expression of this loricrin in a mutant form could be appreciated using immunohistochemistry and could result in a proper evaluation of the diseased state. While there are good numbers of research in the field of dermatology, further studies are needed to potentiate the importance of loricrin, especially the significance of its mutation with respect to the oral cavity.
ACKNOWLEDGEMENT
The authors thank Dr. P.K. Palaniraj M.D, (Paed) for providing the human foreskin sample and also wish to thank and acknowledge the Department of Oral Pathology and Microbiology at Ragas Dental College and Hospital where the tissue was processed and stained as part of the first author's dissertation work done there.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Presland RB, Jurevic RJ. Making sense of the epithelial barrier: What molecular biology and genetics tell us about the functions of oral mucosal and epidermal tissues. J Dent Educ. 2002;66:564–74. [PubMed] [Google Scholar]
- 2.Fuchs E. Keratins and the skin. Annu Rev Cell Dev Biol. 1995;11:123–53. doi: 10.1146/annurev.cb.11.110195.001011. [DOI] [PubMed] [Google Scholar]
- 3.Nemes Z, Steinert PM. Bricks and mortar of the epidermal barrier. Exp Mol Med. 1999;31:5–19. doi: 10.1038/emm.1999.2. [DOI] [PubMed] [Google Scholar]
- 4.Ishida-Yamamoto A, Takahashi H, Lizuka H. Immunoelectron microscopy links molecules and morphology in the studies of keratinisation. Eur J Dermatol. 2000;10:429–35. [PubMed] [Google Scholar]
- 5.Yoneda K, Hohl D, McBride OW, Wang M, Cehrs KU, Idler WW, et al. The human loricrin gene. J Biol Chem. 1992;267:18060–6. [PubMed] [Google Scholar]
- 6.Yoneda K, Steinert PM. Overexpression of human loricrin in transgenic mice produces a normal phenotype. Proc Natl Acad Sci U S A. 1993;90:10754–8. doi: 10.1073/pnas.90.22.10754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalinin A, Marekov LN, Seinert PM. Assembly of the epidermal cornified cell envelope. J Cell Sci. 2001;114:3069–70. doi: 10.1242/jcs.114.17.3069. [DOI] [PubMed] [Google Scholar]
- 8.Kypriotou M, Huber M, Hohl D. The human epidermal differentiation complex: Cornified envelope precursors, S100 proteins and the ‘fused genes’ family. Exp Dermatol. 2012;21:643–9. doi: 10.1111/j.1600-0625.2012.01472.x. [DOI] [PubMed] [Google Scholar]
- 9.Steinert PM, Marekov LN. The proteins elafin, filaggrin, keratin intermediate filaments, loricrin and small proline-rich proteins 1 and 2 are isodipeptide cross-linked components of the human epidermal cornified cell envelope. J Biol Chem. 1995;270:17702–11. doi: 10.1074/jbc.270.30.17702. [DOI] [PubMed] [Google Scholar]
- 10.Jarnik M, de Viragh PA, Scharer E, Bundman D, Simon MN, Roop DR, et al. Quasi-normal cornified cell envelopes in loricrin knockout mice imply the existence of a loricrin backup system. J Invest Dermatol. 2002;118:102–9. doi: 10.1046/j.0022-202x.2001.01661.x. [DOI] [PubMed] [Google Scholar]
- 11.Ishida-Yamamoto A. Loricrin keratoderma: A novel disease entity characterized by nuclear accumulation of mutant loricrin. J Dermatol Sci. 2002;31:3–8. doi: 10.1016/s0923-1811(02)00143-3. [DOI] [PubMed] [Google Scholar]
- 12.Hohl D, Mehrelg T, Lichtig U, Turner ML, Roop DR, Steinert PM. Characterization of human loricrin. Structure and function of a new class of epidermal cell envelope proteins. J Biol Chem. 1991;266:6626–36. [PubMed] [Google Scholar]
- 13.Hohl D, Lichti U, Breitkreutz D, Steinert PM, Roop DR. Transcription of the human loricrin gene in vitro is induced by calcium and cell density and suppressed by retinoic acid. J Invest Dermatol. 1991;96:414–8. doi: 10.1111/1523-1747.ep12469779. [DOI] [PubMed] [Google Scholar]
- 14.Wakamatsu K, Ogita H, Okabe N, Irie K, Okamoto MT, Ishizaki H, et al. Up-regulation of loricrin expression by cell adhesion molecule nectin-1 through Rap1-ERK signaling in keratinocytes. J Biol Chem. 2007;282:18173–81. doi: 10.1074/jbc.M611159200. [DOI] [PubMed] [Google Scholar]
- 15.Ishida-Yamamoto A, Kato H, Kiyama H, Armstrong DK, Munro CS, Eady RA, et al. Mutant loricrin is not cross linked into the cornified cell envelope but is translocated into the nucleus in loricrin keratoderma. J Invest Dermatol. 2000;115:1088–94. doi: 10.1046/j.1523-1747.2000.00163.x. [DOI] [PubMed] [Google Scholar]
- 16.Ishida-Yamamoto A, Takahashi H, Presland RB, Dale BA, Iizuka H. Translocation of profilaggrin N-terminal domain into keratinocyte nuclei with fragmented DNA in normal human skin and loricrin keratoderma. Lab Invest. 1998;78:1245–53. [PubMed] [Google Scholar]
- 17.Schmuth M, Fluhr JW, Crumrine DC, Uchida Y, Hachem JP, Behne M, et al. Structural and functional consequences of loricrin mutations in human loricrin keratoderma (Vohwinkel syndrome with ichthyosis) J Invest Dermatol. 2004;122:909–22. doi: 10.1111/j.0022-202X.2004.22431.x. [DOI] [PubMed] [Google Scholar]
- 18.Ishida-Yamamoto A, Takahashi H, Lizuka H. Loricrin and human skin diseases: Molecular basis of loricrin keratodermas. Histol Histopathol. 1998;13:819–26. doi: 10.14670/HH-13.819. [DOI] [PubMed] [Google Scholar]
- 19.Kim BE, Howell MD, Guttman-Yassky E, Gilleaudeau PM, Cardinale IR, Boguniewicz M, et al. TNF-α downregulates filaggrin and loricrin through c-Jun N-terminal kinase: Role for TNF-α antagonists to improve skin. J Invest Dermatol. 2011;131:1272–9. doi: 10.1038/jid.2011.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sybert VP, Dale BA, Holbrook KA. Palmar-plantar keratoderma. A clinical, ultratructural, and biochemical study. J Am Acad Dermatol. 1988;18:75–86. [PubMed] [Google Scholar]
- 21.Giardinal E, Capon F, De Rosa MC, Mango R, Zambruno G, Orecchia A, et al. Characterization of the loricrin (LOR) gene as a positional candidate for the PSORS4 psoriasis susceptibility locus. Ann Hum Genet. 2004;68:639–45. doi: 10.1046/j.1529-8817.2004.00118.x. [DOI] [PubMed] [Google Scholar]
- 22.Katou F, Shirai N, Kamakura S, Tagami H, Nagura H, Motegi K, et al. Differential expression of cornified cell envelope precursors in normal skin, intraorally transplanted skin and normal oral mucosa. Br J Dermatol. 2003;148:898–905. doi: 10.1046/j.1365-2133.2003.05288.x. [DOI] [PubMed] [Google Scholar]
- 23.Pindborg JJ, Sirsat MS. Oral submucous fibrosis. Oral Surg Oral Med Oral Pathol. 1966;22:764–79. doi: 10.1016/0030-4220(66)90367-7. [DOI] [PubMed] [Google Scholar]
- 24.Rajendran R, Sivapathasundaram B, editors, editors. Shafer's Text Book of Oral Pathology. 7th ed. New-Delhi: Elsevier Ltd; 2012. pp. 97–101. [Google Scholar]
- 25.Li N, Jian XC, Xu CJ. Expression of loricrin and cytochrome P450 3A5 in oral sub mucous fibrosis and their significance. Hua Xi Kou Qiang Yi Xue Za Zhi. 2009;27:29–33. [PubMed] [Google Scholar]
- 26.Li N, Jian XC, Hu Y, Xu C, Yao Z, Zhong X. Discovery of novel biomarkers in oral sub mucous fibrosis by microarray analysis. Cancer Epidemiol Biomarkers Prev. 2008;17:2249–59. doi: 10.1158/1055-9965.EPI-07-2908. [DOI] [PubMed] [Google Scholar]
- 27.Odani T, Ito D, Li MH, Kawamata A, Isobe T, Iwase M, et al. Gene expression profiles of oral leukoplakia and carcinoma: Genome-wide comparison analysis using oligonucleotide microarray technology. Int J Oncol. 2006;28:619–24. [PubMed] [Google Scholar]
- 28.Itoiz ME, Conti CJ, Lanfranchi HE, Mamrack M, Klein-Szanto AJ. Immunohistochemical detection of filaggrin in preneoplastic and neoplastic lesions of the human oral mucosa. Am J Pathol. 1985;119:456–61. [PMC free article] [PubMed] [Google Scholar]


