Abstract
Evidence on the association between dietary fiber intake and pancreatic cancer risk has been controversial. Therefore, we carried out this meta-analysis to summarize available evidence from epidemiologic studies on this point. Relevant studies were identified by searching PubMed, Embase and Web of Science databases as well as by reviewing the rence lists of relevant articles. Random or fixed-effects model was used to calculate the summary risk estimates and 95% confidence intervals (CIs). This meta-analysis included one cohort and thirteen case-control studies which involving a total of 3287 subjects with pancreatic cancer. After summarizing the risk estimates of these studies, we yielded a significant association between dietary fiber intake and pancreatic cancer risk among case-control studies (odds ratio = 0.54; 95%CI = 0.44–0.67; I2 = 41.4%; P = 0.043) but a non-significant result in cohort study (hazard ratio = 1.01; 95%CI = 0.59–1.74). Additionally, significant inverse associations were observed when we carried out the stratify analyses by the study characteristics and adjustment for potential confounders among case-control studies. Given only one cohort study included in the present meta-analysis, further prospective-designed studies should validate our findings and report more detail results, including those for subtypes of fiber, the risk estimates which corrected the impact of measurement errors and fully adjust for the potential confounders.
As one of the most fatal types of cancer, about 0.3 million new cases of pancreatic cancer were diagnosed and nearly the same number of patients dead from this disease in 2012 worldwide1. Prognosis of this disease is extremely poor, with a 1-year survival rate of 25% and a 5-year survival rate below 5%2. Given no effective screening at present, priority should be given to identification of more modifiable risk factors and prevention of the disease.
Since dietary factors may partly play a potential role in the etiology of pancreatic cancer3,4, understanding this role would bring substantial clinical and public health benefits. Nevertheless, no convincing dietary risk factors for pancreatic cancer have been established by the report from the World Cancer Research Fund and the American Institute for Cancer Research (WCRF/AICR) reported in 20074.The possibility of an association between dietary fiber intake and pancreatic cancer risk has received considerable interest and has been investigated intensively during the recent two decades. Although several plausible biologic mechanisms have been hypothesized to underlie the possible protective effect of fiber intake, the evidence from epidemiologic studies has been inconclusive. Additionally, besides total fiber, several studies suggested that the beneficial effects might be only limited in soluble and insoluble fiber5,6. To our knowledge, a systematical and comprehensive assessment of the association between dietary fiber intake and pancreatic cancer risk has not been reported before. Thus, to clarify the aforementioned issues, we carried out the present meta-analysis based on published epidemiologic studies.
Results
Search results, study characteristics, and quality assessment
The search identified a total of 1,953 articles for which the titles and abstracts were scanned to determine potential eligibility for inclusion. After a selection process (Fig. 1), 14 studies3,5,6,7,8,9,10,11,12,13,14,15,16,17 fulfilled our inclusion criteria and were included in this meta-analysis (1 cohort study and 13 case-control studies). Although Ji et al.10 and Lyon et al.12 have provided the risk estimates separately by gender, they were treated as two studies in all. These 14 studies cumulatively reported 3,287 pancreatic cancer cases.
Figure 1. Flow-chart of study selection.
Table 1 summarizes the characteristics of these included studies which were conducted in the North America (n = 6), Europe (n = 5), and others (including Asia and Australia) (n = 3). All studies conducted multivariable analyses, adjusted for age and cigarette smoking, and most studies also adjusted for gender (n = 13) and energy intake (n = 13). Fewer studies controlled for body mass index (n = 3), alcohol drinking (n = 5), and history of diabetes (n = 3). The dietary habits of majority of included studies were investigated to the period 1-5 years before cancer diagnosis for cases or interview for controls, except for the study reported by Lyon et al.12. Seven case-control studies10,12,13,14,15,16,17 used proxy respondents of cases or controls when collecting the information of diet habits.
Table 1. Characteristics of studies of fiber intake and pancreatic cancer risk.
| First Author, (Reference), Year, Country, Study design | Sex | Study Period | Case/Control (Cohort size) | Fiber categories (dietary assessment) | Risk Estimates (95% CI) | Matched/Adjusted factors |
|---|---|---|---|---|---|---|
| Bidoli et al.5, 2011, Italy, HB | M/F | 1991-2008 | 326/652 | Total: Q5 versus Q1 | 0.4 (0.2–0.7) | Age, sex, center, period of interview, BMI, education, cigarette smoking, alcohol consumption, self-reported history of diabetes, dietary folate and total energy intake |
| Soluble: Q5 versus Q1 | 0.4 (0.2–0.7) | |||||
| Insoluble: Q5 versus Q1 | 0.5 (0.3–0.8) | |||||
| (Validated FFQ) | Odds ratio | |||||
| Jansen et al.6, 2011, USA, HB | M/F | 2004-2009 | 384/983 | Total: Q5 versus Q1 | 0.47 (0.32–0.70) | Age, sex, region of residence, energy, cigarette smoking, BMI, and drinks of alcohol |
| Soluble: Q5 versus Q1 | 0.58 (0.39–0.86) | |||||
| Insoluble: Q5 versus Q1 | 0.48 (0.33–0.71) | |||||
| (Validated DHQ) | Odds ratio | |||||
| Zhang et al.7, 2009, USA, PB | M/F | 1994-1998 | 186/554 | Total: Q4 versus Q1 | 0.52 (0.21–1.30) | Age, sex, race, education, cigarette smoking, alcohol, physical activity and intakes of all other dietary factors |
| (Validated FFQ) | Odds ratio | |||||
| Chan et al.8, 2007, USA, PB | M/F | 1995-1999 | 532/1701 | Total: Q4 versus Q1 | 0.65 (0.47–0.89) | Age, sex, BMI, race, education, cigarette smoking, history of diabetes, and energy intake |
| Crude: Q4 versus Q1 | 0.70 (0.51–0.97) | |||||
| (Validated FFQ) | Odds ratio | |||||
| Lin et al.3, 2005, Japan, PB | M/F | 2000-2002 | 109/218 | Total: T3 versus T1 | 0.54 (0.28–1.06) | Age, cigarette smoking, and energy intake |
| (Validated FFQ) | Odds ratio | |||||
| Stolzenberg-Solomon et al.9, 2002, Finland, Cohort | M | 1985-1997 | 163/27,111 | Total: Q5 versus Q1 | 1.01 (0.59–1.74) | Age, years of cigarette smoking and energy intake |
| Soluble: Q5 versus Q1 | 1.02 (0.56–1.63) | |||||
| Insoluble: Q5 versus Q1 | 0.95 (0.57–1.60) | |||||
| (Validated DHQ) | Hazard ratio | |||||
| Ji et al.10, 1995, China, PB | M/F | 1970-1990 | 451/1552 | Total: Q4 versus Q1 (M) | 0.53 (0.32–0.89) | Age, income, cigarette smoking, green tea drinking (females only), response status, and total calories intake |
| Total: Q4 versus Q1 (F) | 0.67 (0.36–1.30) | |||||
| (FFQ) | Odds ratio | |||||
| Lyon et al.12, 1993, USA, PB | M/F | 1984-1987 | 149/363 | Total: High versus Low (M) | 1.44 (0.70–2.95) | Age, cigarette smoking, and intake of coffee and alcohol |
| Total: High versus Low (F) | 0.28 (0.12–0.67) | |||||
| (FFQ) | Odds ratio | |||||
| Kalapothaki et al.11,* 1993, Greece, HB | M/F | 1991-1992 | 181/181 | Crude: Q5 versus Q1 | 0.26 (0.12–0.57) | Age, sex, hospital, past residence, years of schooling, cigarette smoking, diabetes mellitus, total energy, carbohydrate, protein, and fat intake |
| (DHQ) | Odds ratio | |||||
| Zatonski et al.16, 1991, Poland, PB | M/F | 1985-1988 | 110/195 | Total: Q4 versus Q1 | 0.74 (0.24–2.30) | Age, sex, residence, cigarette smoking and energy intake |
| (DHQ) | Relative Risk | |||||
| Ghadrian et al.15, 1991, Canada, PB | M/F | 1984-1988 | 179/239 | Total: Q4 versus Q1 | 0.74 (0.31–1.73) | Age, sex, cigarette smoking status, response and energy intake |
| Crude: Q4 versus Q1 | 0.81 (0.35–1.85) | |||||
| (FFQ) | Odds ratio | |||||
| Mesquita et al.14, 1991, Netherlands, PB | M/F | 1984-1988 | 164/480 | Total: Q5 versus Q1 | 0.54 (0.29–1.02) | Age, sex, response status, cigarette smoking status and energy intake |
| (Validated FFQ) | Odds ratio | |||||
| Baghurst et al.13, 1991, Australia, PB | M/F | 1984-1987 | 104/253 | Total: Q4 versus Q1 | 0.26 (0.12–0.58) | Age, sex, total energy intake, alcohol and cigarette usage |
| (Validated | Relative | |||||
| FFQ) | Risk | |||||
| Howe et al.17, 1990, Canada, PB | M/F | 1983-1986 | 249/505 | Total: Q5 versus Q1 | 0.42 (0.22–0.78) | Age, sex, caloric intake, and cigarette smoking |
| (Validated DHQ) | Relative Risk |
BMI, body mass index; DHQ, dietary history questionnaire; F, female; FFQ, food frequency questionnaire; HB, hospital based; M, male; PB, population based.
*Risk estimate was first recalculated by the method proposed by Danesh et al.30 and then summarized by the inverse-variance method.
The information of study quality are summarized in Supplementary Table S1 and Supplementary Table S2. Briefly, three case-control studies5,6,11 were not assigned a star in the column of “selection of control subjects” because these studies utilized hospital-based controls. One cohort study and five case-control studies5,6,8,9,11,13 were assigned two stars in the column of “control for important factor or additional factor” because they adjusted for more than two important confounders in the primary analyses. Six case-control studies10,11,12,15,16,17 were not assigned a star in the column of “exposure assessment” because their questionnaires were not validated. Nine case-control studies3,6,10,12,13,14,15,16,17 were not assigned a star in the column of “non-response rate” because the response rate between cases and controls were significantly differenced.
Dietary fiber and pancreatic cancer risk
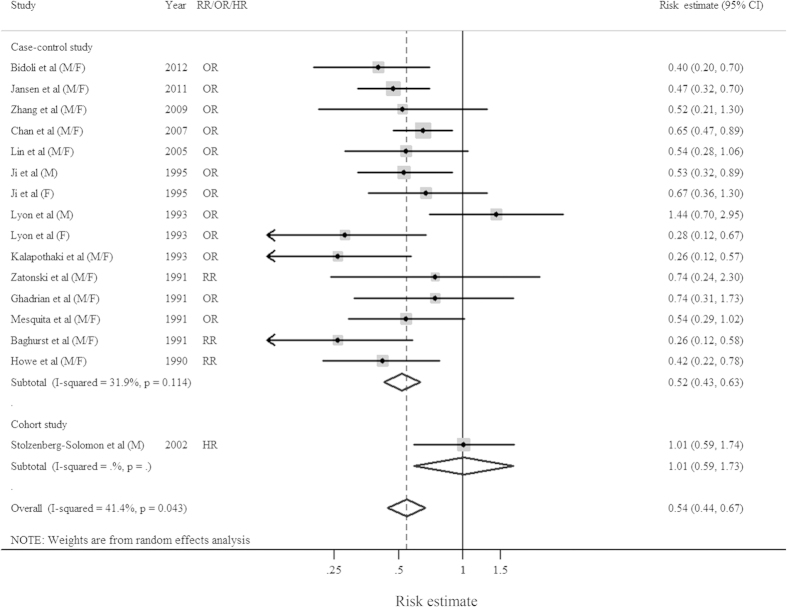
The multivariable-adjusted risk estimates for each study and the summary risk estimates for the highest versus the lowest categories of dietary fiber intake by study design are shown in Fig. 2. Diet high in fiber was associated with a statistically significant reduction in pancreatic cancer risk among case-control studies, with the corresponding summary risk estimate of 0.52 (95%CI = 0.43–0.63; I2 = 31.9%, P = 0.114). However, there is no association between fiber intake and pancreatic cancer risk in cohort study, though only one study was included (Table 2 and Fig. 2). There was no evidence of publication bias, both quantitatively (P = 0.495 for Egger’s test and P = 0.787 for Begg’s test) and qualitatively, on visual inspection of the funnel plot.
Figure 2. Forest plots (random effect model) of meta-analysis on the relationship between fiber intake and pancreatic cancer risk by study design.
Squares indicate study-specific risk estimates (size of the square reflects the study-specific statistical weight); horizontal lines indicate 95% CIs; diamond indicates the summary risk estimate with its 95% CI. F: female; HR: hazard ratio; M: male; OR: odds ratio; RR: relative risk.
Table 2. Summary risk estimates of the association between dietary fiber intake and pancreatic cancer risk.
| No. of Studies | Summary risk estimate | 95% CI | I2 (%) | Ph† | Ph‡ | |
|---|---|---|---|---|---|---|
| Overall | 14 | 0.54 | (0.44–0.67) | 41.4 | 0.043 | |
| Study design | 0.093 | |||||
| Cohort study | 1 | 1.01 | (0.59–1.74) | N/A | N/A | |
| Case-control study | 13 | 0.53 | (0.46–0.62) | 31.9 | 0.114 | |
| Fiber type* | 0.570 | |||||
| Soluble fiber | 2 | 0.52 | (0.37–0.73) | 0 | 0.326 | |
| Insoluble fiber | 2 | 0.49 | (0.36–0.66) | 0 | 0.898 | 0.796§ |
| Crude fiber | 3 | 0.55 | (0.30–1.02) | 65.0 | 0.057 | 0.792§ |
| Subgroup analyses* | ||||||
| Type of control subjects | 0.144 | |||||
| PB-CC | 10 | 0.57 | (0.45–0.71) | 28.2 | 0.168 | |
| HB-CC | 3 | 0.41 | (0.30–0.56) | 0 | 0.410 | |
| Proxy respondent | 0.578 | |||||
| Yes | 7 | 0.55 | (0.40–0.76) | 44.9 | 0.069 | |
| No | 6 | 0.52 | (0.42–0.63) | 14.6 | 0.321 | |
| Exposure assessment | 0.440 | |||||
| Validated FFQ/DHQ | 8 | 0.51 | (0.42–0.61) | 0 | 0.517 | |
| Others | 5 | 0.58 | (0.37–0.89) | 56.4 | 0.032 | |
| Geographic location | 0.942 | |||||
| North America | 6 | 0.57 | (0.42–0.79) | 49.4 | 0.065 | |
| Europe | 4 | 0.43 | (0.30–0.62) | 1.4 | 0.385 | 0.342¶ |
| Asia | 2 | 0.57 | (0.40–0.80) | 0 | 0.841 | 0.982¶ |
| Oceania | 1 | 0.26 | (0.12–0.58) | N/A | N/A | 0.208¶ |
| Adjustment for confounders or risk factors* | ||||||
| BMI | 0.976 | |||||
| Yes | 3 | 0.53 | (0.40–0.70) | 24.7 | 0.265 | |
| No | 10 | 0.52 | (0.39–0.68) | 38.2 | 0.086 | |
| Sex | 0.924 | |||||
| Yes | 12 | 0.52 | (0.42–0.64) | 36.7 | 0.082 | |
| No | 1 | 0.54 | (0.28–1.06) | N/A | N/A | |
| Diabetes | 0.620 | |||||
| Yes | 3 | 0.45 | (0.26–0.76) | 64.0 | 0.062 | |
| No | 10 | 0.53 | (0.44–0.64) | 26.6 | 0.183 | |
| Total energy consumption | 0.329 | |||||
| Yes | 12 | 0.52 | (0.44–0.61) | 0 | 0.537 | |
| No | 1 | 0.65 | (0.13–3.22) | N/A | N/A | |
| Alcohol consumption | 0.533 | |||||
| Yes | 5 | 0.47 | (0.30–0.74) | 62.0 | 0.022 | |
| No | 8 | 0.56 | (0.46–0.68) | 0 | 0.607 |
BMI, body mass index; CI, confidence interval; FFQ, food frequency questionnaire; HB-CC, hospital-based case-control study; N/A, not available; PB-CC, population-based case-control study.
*Cohort study was excluded from the analysis.
†P value for heterogeneity within each subgroup.
‡P-value for heterogeneity between subgroups.
§The result of soluble fiber was treated as the reference group.
¶The result of North America was treated as the reference group.
Subgroup and sensitivity analysis
Since cohort study and case-control study were two different study designs and only one cohort study was found in the literature search, we excluded this cohort study in the subgroup analyses. When we carried out the stratified analysis by fiber type, significant results were observed in soluble fiber and insoluble fiber but the result showed borderline significance in crude fiber. Furthermore, table 2 shows the associations between fiber intake and pancreatic cancer risk in pre-planned subgroup meta-analyses stratified by type of control subjects, proxy respondent, exposure assessment method, geographic location, and adjustment for potential confounders. Significant inverse associations of fiber intake on pancreatic cancer were observed among almost all the strata of between-study subgroup analyses.
In a sensitivity analysis in which we removed one study at a time and analyzed the rest, the summary risk estimates ranged from 0.53 (95%CI = 0.46–0.62, I2 = 31.9%) after excluding the study by Stolzenberg-Solomon et al.9 to 0.57 (95%CI = 0.46–0.69, I2 = 36.0%) after excluding the study by Baghurst et al.13. Additionally, we excluded the study by Kalapthaki et al.11 in which risk estimate and 95%CI was recalculated. The summary risk estimate from this sensitivity analysis was 0.57 (95%CI = 0.46–0.69, I2 = 35.8%) which was similar to the main finding.
Discussion
To the best of our knowledge, this is the first meta-analysis to summarize the evidence between total and different types of fiber intake and risk of pancreatic cancer. In our meta-analysis, increased fiber intake is associated with a reduced risk of pancreatic cancer in case-control studies but not in cohort study. The findings partly support the hypothesis that diet high in fiber may provide protection against pancreatic cancer.
The report of WCRF/AICR in 2007 concluded that there was “limited-no conclusion” evidence between foods containing dietary fiber and pancreatic cancer risk which was based on five case-control studies and one cohort study4. After that, several additional case-control studies5,6,7,8 were published in the recent years. Studies by Bidoli et al.5, Jansen et al.6, Zhang et al.7, and Chan et al.8, including a total of 1428 pancreatic cancer cases, accounted for over 43% of the patients in the studies included in this meta-analysis. Compared with the report of WCRF/AICR, this meta-analysis includes more pancreatic cancer cases and updates the evidence between dietary fiber intake and risk of pancreatic cancer.
Quality scoring might not only submerge important information by combining disparate study features into a single score but introduce somewhat arbitrary subjective element into the analysis18,19,20. Therefore, although the Newcastle-Ottawa Scale (NOS) was used to assess the quality of included studies, we did not score these included studies or categorize them into high or low quality according to the scores. The results of quality assessment demonstrated that compared with prospective study, case-control studies including in this meta-analysis were less likely to use validated food frequency questionnaire and adjust for potential confounders but more likely to have significantly difference in non-response rate between cases and controls (Supplementary Table 1 and 2). Besides, because information on exposures is collected before the diagnosis of the disease, cohort studies are less susceptible to recall bias than case-control studies. Given this, several biases (e.g. selection bias, information bias, or confounding bias) might be introduced in their primary analyses. Additionally, since the majority of included studies were case-control studies (13/14), we carried out stratified analyses to explore the sources of heterogeneity by excluding the only one cohort study. Therefore, the findings of the stratified analyses on the basis of case-control studies should be interpreted with cautious. Population-based control subjects were more likely to provide a relatively exact estimate of the exposure than hospital-based controls3. We found a slightly attenuated point estimate among the population-based case-control studies compared with the hospital-based case-control studies (Table 2). Furthermore, when stratified by the geographic location, we found that the point estimate for Europeans was slightly attenuated than North Americans and Asians, which might be partly attributed to the different amount of fiber intake. However, restricted by the limited included studies in the stratified analysis, this issue need further investigation.
Although the exact biologic mechanisms underlying the aforementioned inverse association are not fully understood, several biologic plausible reasons might have been proposed to partly explain the protective role of dietary fiber. Foods rich in fiber are known to have several anti-carcinogenic properties, such as the ability to lower levels of circulating markers of inflammation which may be involved in pancreatic cancer initiation and progression21,22, and the ability to improve insulin metabolism by modulating hormonal pathways linked to pancreatic carcinogenesis which have been associated with cancer promotion23,24. Several experimental studies suggested that inositol hexaphosphate, a naturally occurring molecule found in high-fiber foods, was compound that has been shown to demonstrate anti-proliferative effects, resulting in the inhibition of pancreatic cancer cell growth25,26. Since these biologic mechanisms are speculative and there are limited experimental data available, further in vivo and in vitro studies are warranted to shed light on the underlying mechanisms between total and different types of fiber intake and pancreatic cancer risk.
Our study has several strengths. This is the first meta-analysis focused on the relationship between dietary fiber intake and risk of pancreatic cancer. All studies ascertain outcomes using histological findings. Compared to these included studies, the present meta-analysis includes a total of 3,287 cases, which significantly increase the statistical power of the main analysis. Additionally, the summary risk estimate remains stable and robust in the subgroup and sensitivity analyses.
Despite the clear strengths of this meta-analysis, limitations of the present meta-analysis also require considerations. Diets high in fiber may be related to other behaviors including smoking, alcohol drinking, overweight and obesity, diabetes mellitus, and intake of total energy, which could possibly confound the observed associations. However, the summary risk estimates did not change materially in subgroup analyses whether adjusted for major potential confounders, such as body mass index5,6,8, diabetes mellitus5,8,11,alcohol drinking5,6,7,12,13 and total energy intake3,5,6,7,8,9,10,11,13,14,15,16,17. Since limited studies were included in some of the analyses (Table 2), prudence still should be used when interpreting these findings. Second, accurate assessment of dietary fiber intake and other food constituents is a challenge which may bias effect estimates27. A previous meta-analysis focused on fiber intake and colorectal cancer suggested that the different definition of dietary fiber between included studies might be concerned when exploring the source of the heterogeneity. However, only one study5 mentioned the Englyst definition of fiber in their methods. Compared to the Association of Official Analytical Chemists method which includes some starch as dietary fibre, the Englyst definition distinguishes non-starch polysaccharides from starch27. In addition, although slightly stronger risk estimates without heterogeneity was observed among these studies using validated food frequency questionnaire when we carried out the stratified analysis by exposure assessment (Table 2), none of the studies included in the present meta-analysis made any corrections for measurement errors. Measurement errors would, however, most likely result in bias toward the null which suggests that our result for fiber intake and pancreatic cancer risk is likely to be underestimation of the true underlying risk. Further studies should consider correction for measurement error in the analyses. Third, only Stolzenberg-Solomon et al.9 reported the results of fiber intake and pancreatic cancer risk based on prospective design. Although the results of older male heavy smokers may not be generalizable to nonsmoking populations, compared to case-control study, prospective cohort study are less susceptible to some biases (e.g., selection bias and recall bias)28. Preclinical symptoms of pancreatic cancer (e.g., anorexia and indigestion) may influence the dietary habits such as fiber intake, which may lead to reverse causality in epidemiological studies. However, since the short latency of pancreatic cancer and diet pattern was evaluated for 1-5 years before cancer diagnosis among the majority of included studies which have already concerned about the possibility of diet change caused by the preclinical symptoms5. Therefore, reverse causality may not be a major problem in this study. Besides, given the poor survival of pancreatic cancer, the questionnaires or interviews of some early studies12,13,14,15,16,17 were completed by proxy respondents which may also bias the risk estimates. For example, Lyon et al.12 found that the response rate for cases was greater than that for controls which might result from the different willingness of the next-of-kin of the cases to participate in a study compared with proxy respondents of randomly selected controls. Additionally, most proxy respondents of their study were spouses and recall of spouses diet may differ by sex of the surrogate respondent. This might lead to the different risk estimates of dietary intake between men and women including fiber. Although the result of meta-regression showed no difference between studies whether using proxy respondents (Table 2), notably, we found a significant heterogeneity among studies using proxy respondent which might contribute heterogeneity to the main result. Last, we found the results of trend analysis of many included studies were statistical significant5,6,8,10,11,13,17, but due to only four included studies provided enough information for a dose-response meta-analysis5,6,7,8, future studies and pooled analysis are warranted to investigate whether there is a non-linear relationship between fiber intake and pancreatic cancer risk.
In conclusion, this meta-analysis suggests that a high intake of dietary fiber is associated with a reduced risk of pancreatic cancer in case-control studies but not in cohort study. Further studies, especially prospective-designed studies should validate our finding and report more detail results, including those for subtypes of fiber, the risk estimates which corrected the impact of measurement errors, and fully adjust for the potential confounders in the future.
Materials and Methods
Search Strategy
In this meta-analysis, we followed the guidelines developed by the Meta-analysis Of Observational Studies in Epidemiology group (MOOSE)29. Two authors (C-HW and CQ) performed a systematic literature search in the MEDLINE (PubMed; http://www.ncbi.nlm.nih.gov/pubmed), Embase and Web of Science databases through August, 2014 without limitations by using the following search key words: (diet or dietary or fiber or fibre) and (pancreatic or pancreas) and (cancer or neoplasm). Furthermore, bibliographies of relevant studies were hand-searched for additional publications.
Study Selection
The title and abstract of studies identified in the search were reviewed by two authors (C-HW and CQ) to exclude studies that did not answer the research question of interest. Studies considered in this meta-analysis should meet the following inclusion criteria: the study (1) had a observational study design (e.g., cohort, case-cohort, nested case-control, or case-control study); (2) clearly defined dietary fiber as the exposure of interest; (3) reported pancreatic cancer as the outcome of interest; and (4) reported relative risks (RRs), odds ratios (ORs), and hazard ratios (HRs) with 95% confidence intervals (CIs) or provided data for their calculation. Inclusion was not otherwise restricted by study size, language, or publication type. If multiple articles were on the same study population, the one with more informative data was selected.
Data Extraction
Data were independently abstracted onto a standardized form by two authors (C-HW and CQ). Conflicts in data abstraction were resolved by consensus, referring back to the original article. The following data were collected from each study: first author’s last name, year of publication, country of the study population, study design, sex, study period, number of cases and controls or cohort size, fiber intake categories and methods of dietary assessment, risk estimates with their 95% CIs for the highest versus lowest category, and factors matched by or adjusted for in the design or data analysis. From each study, we extracted the risk estimates that reflected the greatest degree of control for potential confounders. Only two studies10,12 provided information stratified by sex and so each subgroup is included separately. For study reported by Kalapothaki et al.11 that only reported the results by 1 standard deviation increment of dietary fiber intake, we converted the reported risk estimates into a standard scale of effect to compare persons with dietary fiber intakes in the top quintile with persons whose intakes were in the bottom quintile30,31, which was the most common in the included studies5,8,9,14,17. Subsequently, the inverse-variance method was used to summary the converted risk estimates of the different control populations of this study32.
Quality assessment
The NOS includes 3 quality parameters for case-control or cohort studies: the selection of study groups, comparability of groups and ascertainment of either the exposure or outcome of interest was used by two independent researchers (C-HW and CQ) to assess study quality32,33,34.
Statistical analysis
As the absolute risk of pancreatic cancer is low, as well as only three included case-control studies13,16,17 which reported the risk estimate as RR, therefore, we reported the risk estimates from case-control studies as the OR for simplicity. For studies10,12 that reported the results separately by gender, we combined these results with the rest of the studies instead of using a fixed-effect model to obtain an overall combined estimate aforehand32,35. We used the fixed or random-effects model36,37 to calculate summary risk estimates and 95% CIs for the highest versus lowest categories of dietary fiber according to the result of heterogeneity test. In assessing heterogeneity among studies, we used the I2 statistics which values represent the amount of total variation explained by variation among studies, with a value of greater than 50% considered to indicate severe heterogeneity and a value of less than 25% indicating the absence of significant heterogeneity37. Small study bias, such as publication bias (publication bias considered present if P < 0.1), was evaluated via Egger’s linear regression38, Begg’s rank-correlation methods39, and funnel plots. In order to investigate possible sources of heterogeneity among studies, we not only carried out the stratified analyses by study design and fiber type, but also conducted subgroups analyses according to potentially relevant factors: type of control subjects (population-based versus hospital-based), proxy respondent (yes versus no), exposure assessment (validated questionnaire versus others), geographic location (North America, Europe, Asia, and Oceania), and confounders that were adjusted for the following: body mass index, sex, diabetes mellitus, total energy intake, and alcohol drinking. Heterogeneity between subgroups was evaluated by meta-regression. At last, we conducted sensitivity analyses by deleting each study in turn to reflect the influence of individual data sets on the overall estimate. For all tests, a probability level <0.05 was considered statistically significant. All statistical analyses were conducted by using Stata software (version 11.2; StataCorp).
Additional Information
How to cite this article: Wang, C.-H. et al. Dietary fiber intake and pancreatic cancer risk: a meta-analysis of epidemiologic studies. Sci. Rep. 5, 10834; doi: 10.1038/srep10834 (2015).
Supplementary Material
Acknowledgments
C-HW and CQ contributed equally to this work. This study was supported by Liaoning Provincial Natural Science Foundation of China (Grant number: No.20082096 and No. 2014020061 for C-HW). We would like to thank Dr. Qi-Jun Wu (Division of Clinical Epidemiology, Shengjing Hospital of China Medical University) for assistance in the interpretation of the results and revision of this manuscript.
Footnotes
Author Contributions C.-H.W. and C.Q. designed research; C.-H.W. and C.Q. conducted research; C.-H.W., C.Q. and R.-C.W. analyzed data; C.-H.W., C.Q., R.-C.W. and W.-P.Z. wrote the draft; All authors read, reviewed and approved the final manuscript. C-HW had primary responsibility for final content.
References
- Ferlay J. et al. GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11 [Internet]. Lyon, France: International Agency for Research on Cancer; 2013. Available from: http://globocan.iarc.fr. (Date of access: 1/September/2014).
- Siegel R., Ma J., Zou Z. & Jemal A. Cancer statistics, 2014. CA Cancer J. Clin. 64, 9–29 (2014). [DOI] [PubMed] [Google Scholar]
- Lin Y. et al. Nutritional factors and risk of pancreatic cancer: a population-based case-control study based on direct interview in Japan. J. Gastroenterol. 40, 297–301 (2005). [DOI] [PubMed] [Google Scholar]
- World Cancer Research Fund/American Institute for Cancer Research. Food, nutrition, physical activity and the prevention of cancer: a global perspective. Washington, DC. (AICR, 2007).
- Bidoli E. et al. Fiber intake and pancreatic cancer risk: a case-control study. Ann Oncol. 23, 264–268 (2012). [DOI] [PubMed] [Google Scholar]
- Jansen R. J. et al. Fruit and vegetable consumption is inversely associated with having pancreatic cancer. Cancer Causes Control 22, 1613–1625 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. et al. Physical activity, diet, and pancreatic cancer: a population-based, case-control study in Minnesota. Nutr. Cancer 61, 457–465 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan J. M., Wang F. & Holly E. A. Whole grains and risk of pancreatic cancer in a large population-based case-control study in the San Francisco Bay Area, California. Am J. Epidemiol. 166, 1174–1185 (2007). [DOI] [PubMed] [Google Scholar]
- Stolzenberg-Solomon R. Z. et al. Prospective study of diet and pancreatic cancer in male smokers. Am J Epidemiol 155, 783–792 (2002). [DOI] [PubMed] [Google Scholar]
- Ji B. T. et al. Dietary factors and the risk of pancreatic cancer: a case-control study in Shanghai China. Cancer Epidemiol Biomarkers Prev. 4, 885–893 (1995). [PubMed] [Google Scholar]
- Kalapothaki V. et al. Nutrient intake and cancer of the pancreas: a case-control study in Athens, Greece. Cancer Causes Control 4, 383–389 (1993). [DOI] [PubMed] [Google Scholar]
- Lyon J. L., Slattery M. L., Mahoney A. W. & Robison L. M. Dietary intake as a risk factor for cancer of the exocrine pancreas. Cancer Epidemiol Biomarkers Prev. 2, 513–518 (1993). [PubMed] [Google Scholar]
- Baghurst P. A. et al. A case-control study of diet and cancer of the pancreas. Am J. Epidemiol. 134, 167–179 (1991). [DOI] [PubMed] [Google Scholar]
- Bueno D. M. H., Maisonneuve P., Runia S. & Moerman C. J. Intake of foods and nutrients and cancer of the exocrine pancreas: a population-based case-control study in The Netherlands. Int. J. Cancer 48, 540–549 (1991). [DOI] [PubMed] [Google Scholar]
- Ghadirian P. et al. Nutritional factors and pancreatic cancer in the francophone community in Montreal, Canada. Int. J. Cancer 47, 1–6 (1991). [DOI] [PubMed] [Google Scholar]
- Zatonski W. et al. Nutritional factors and pancreatic cancer: a case-control study from south-west Poland. Int. J. Cancer 48, 390–394 (1991). [DOI] [PubMed] [Google Scholar]
- Howe G. R., Jain M. & Miller A. B. Dietary factors and risk of pancreatic cancer: results of a Canadian population-based case-control study. Int. J. Cancer 45, 604–608 (1990). [DOI] [PubMed] [Google Scholar]
- Greenland S. & O’Rourke K. On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics 2, 463–471 (2001). [DOI] [PubMed] [Google Scholar]
- Greenland S. Invited commentary: a critical look at some popular meta-analytic methods. Am J. Epidemiol. 140, 290–296 (1994). [DOI] [PubMed] [Google Scholar]
- Greenland S. & O’ Rourke K. Meta-analysis. In: Rothman K. J., Greenland S. & Lash T. L., eds. Modern Epidemiology, 3rd edn. Lippincott Williams & Wilkins, Philadelphia, USA. 2008. 652–682. [Google Scholar]
- Ma Y. et al. Association between dietary fiber and markers of systemic inflammation in the Women’s Health Initiative Observational Study. Nutrition 24, 941–949 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gukovsky I. et al. Inflammation, autophagy, and obesity: common features in the pathogenesis of pancreatitis and pancreatic cancer. Gastroenterology 144, 1199–1209 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallfrisch J., Facn & Behall K. M. Mechanisms of the effects of grains on insulin and glucose responses. J. Am. Coll. Nutr. 19, 320S–325S (2000). [DOI] [PubMed] [Google Scholar]
- Jensen M. K. et al. Whole grains, bran, and germ in relation to homocysteine and markers of glycemic control, lipids, and inflammation 1. Am J. Clin. Nutr. 83, 275–283 (2006). [DOI] [PubMed] [Google Scholar]
- McMillan B. et al. Dietary influence on pancreatic cancer growth by catechin and inositol hexaphosphate. J. Surg. Res. 141, 115–119 (2007). [DOI] [PubMed] [Google Scholar]
- Somasundar P. et al. Inositol hexaphosphate (IP6): a novel treatment for pancreatic cancer. J. Surg. Res. 126, 199–203 (2005). [DOI] [PubMed] [Google Scholar]
- Aune D. et al. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ 343, d6617 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luan N. N. et al. Breastfeeding and ovarian cancer risk: a meta-analysis of epidemiologic studies. Am J. Clin. Nutr. 98, 1020–1031 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stroup D. F. et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA 283, 2008–2012 (2000). [DOI] [PubMed] [Google Scholar]
- Danesh J., Collins R., Appleby P. & Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA 279, 1477–1482 (1998). [DOI] [PubMed] [Google Scholar]
- Yang Y. et al. Association between dietary fiber and lower risk of all-cause mortality: a meta-analysis of cohort studies. Am J. Epidemiol. 181, 83–91 (2015). [DOI] [PubMed] [Google Scholar]
- Wu Q. J. et al. Cruciferous vegetables intake and the risk of colorectal cancer: a meta-analysis of observational studies. Ann Oncol. 24, 1079–1087 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G. A. et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. (Date of access: 1/September/2014).
- Gong T. T. et al. Age at menarche and risk of ovarian cancer: a meta-analysis of epidemiological studies. Int. J. Cancer 132, 2894–2900 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Q. J. et al. Cruciferous vegetable consumption and gastric cancer risk: a meta-analysis of epidemiological studies. Cancer Sci. 104, 1067–1073 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- DerSimonian R. & Laird N. Meta-analysis in clinical trials. Control Clin. Trials 7, 177–188 (1986). [DOI] [PubMed] [Google Scholar]
- Higgins J. P. & Thompson S. G. Quantifying heterogeneity in a meta-analysis. Stat. Med. 21, 1539–1558 (2002). [DOI] [PubMed] [Google Scholar]
- Egger M., Davey S. G., Schneider M. & Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315, 629–634 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begg C. B. & Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 50, 1088–1101 (1994). [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.